Towards a Better Understanding of the Human Health Risk of Per- and Polyfluoroalkyl Substances Using Organoid Models
Abstract
1. Introduction
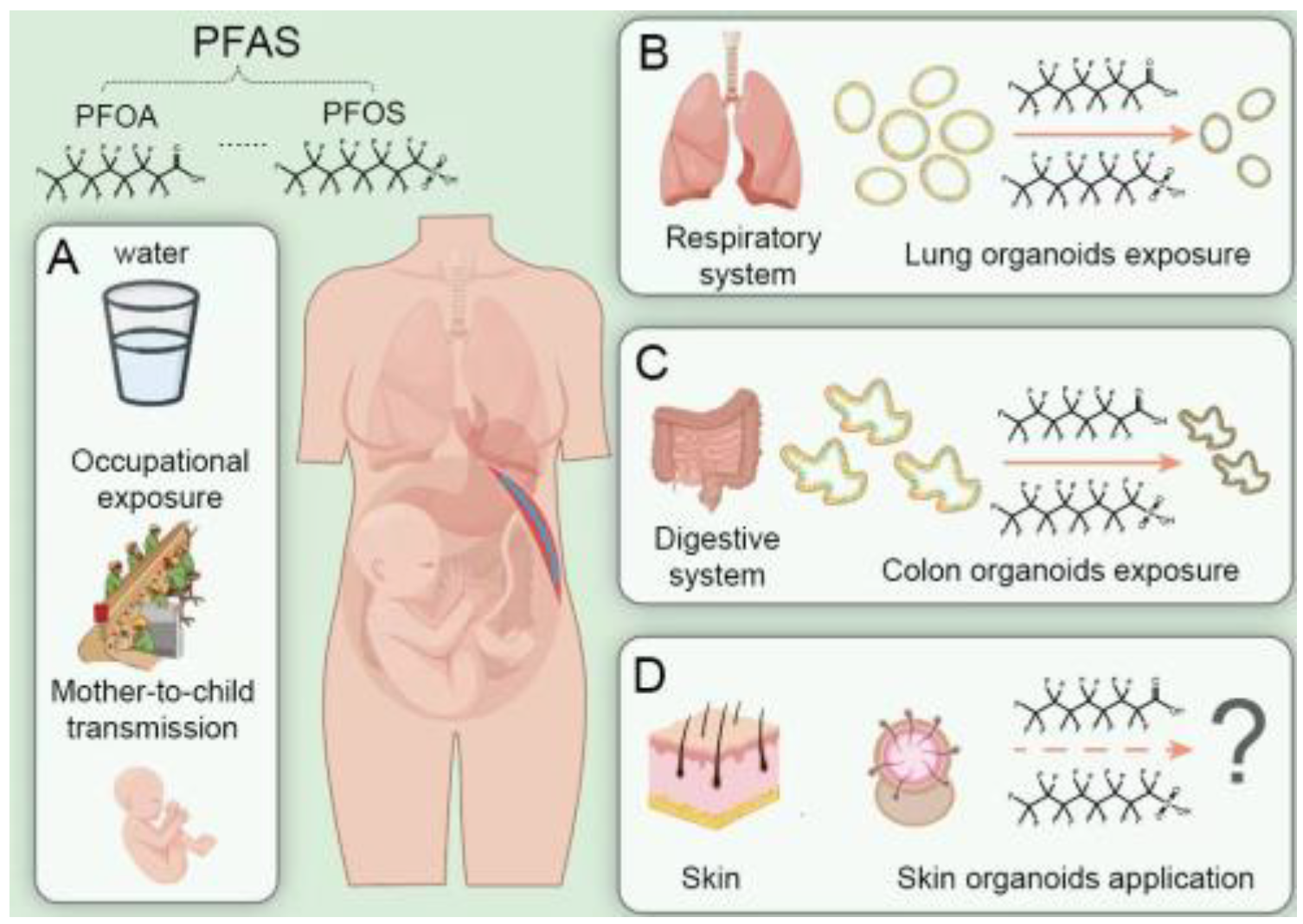
2. PFAS Pose Threats to Human Health
3. Potential of Human Organoids in PFAS Toxicity Assessment
4. Advances and Challenges of Human Organoids
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Cousins, I.T.; DeWitt, J.C.; Gluge, J.; Goldenman, G.; Herzke, D.; Lohmann, R.; Ng, C.A.; Scheringer, M.; Wang, Z. The high persistence of PFAS is sufficient for their management as a chemical class. Environ. Sci. Process. Impacts 2020, 22, 2307–2312. [Google Scholar] [CrossRef] [PubMed]
- Scheringer, M. Innovate beyond PFAS. Science 2023, 381, 251. [Google Scholar] [CrossRef] [PubMed]
- Alazaiza, M.Y.D.; Alzghoul, T.M.; Ramu, M.B.; Abu Amr, S.S.; Abushammala, M.F.M. PFAS contamination and mitigation: A comprehensive analysis of research trends and global contributions. Case Stud. Chem. Environ. Eng. 2025, 11, 101127. [Google Scholar] [CrossRef]
- Sunderland, E.M.; Hu, X.C.; Dassuncao, C.; Tokranov, A.K.; Wagner, C.C.; Allen, J.G. A review of the pathways of human exposure to poly- and perfluoroalkyl substances (PFASs) and present understanding of health effects. J. Expo. Sci. Environ. Epidemiol. 2019, 29, 131–147. [Google Scholar] [CrossRef]
- Ng, C.; Cousins, I.T.; DeWitt, J.C.; Gluge, J.; Goldenman, G.; Herzke, D.; Lohmann, R.; Miller, M.; Patton, S.; Scheringer, M.; et al. Addressing Urgent Questions for PFAS in the 21st Century. Environ. Sci. Technol. 2021, 55, 12755–12765. [Google Scholar] [CrossRef]
- Borghese, M.M.; Ward, A.; MacPherson, S.; Manz, K.E.; Atlas, E.; Fisher, M.; Arbuckle, T.E.; Braun, J.M.; Bouchard, M.F.; Ashley-Martin, J. Serum concentrations of legacy, alternative, and precursor per- and polyfluoroalkyl substances: A descriptive analysis of adult female participants in the MIREC-ENDO study. Environ. Health A Glob. Access Sci. Source 2024, 23, 55. [Google Scholar] [CrossRef] [PubMed]
- Ateia, M.; Scheringer, M. From “forever chemicals” to fluorine-free alternatives. Science 2024, 385, 256–258. [Google Scholar] [CrossRef]
- Hamid, N.; Junaid, M.; Sultan, M.; Yoganandham, S.T.; Chuan, O.M. The untold story of PFAS alternatives: Insights into the occurrence, ecotoxicological impacts, and removal strategies in the aquatic environment. Water Res. 2024, 250, 121044. [Google Scholar] [CrossRef]
- Van Norman, G.A. Limitations of Animal Studies for Predicting Toxicity in Clinical Trials: Is it Time to Rethink Our Current Approach? JACC Basic Transl. Sci. 2019, 4, 845–854. [Google Scholar] [CrossRef]
- Li, M.; Gong, J.; Gao, L.; Zou, T.; Kang, J.; Xu, H. Advanced human developmental toxicity and teratogenicity assessment using human organoid models. Ecotoxicol. Environ. Saf. 2022, 235, 113429. [Google Scholar] [CrossRef]
- Kiani, A.K.; Pheby, D.; Henehan, G.; Brown, R.; Sieving, P.; Sykora, P.; Marks, R.; Falsini, B.; Capodicasa, N.; Miertus, S.; et al. Ethical considerations regarding animal experimentation. J. Prev. Med. Hyg. 2022, 63, E255–E266. [Google Scholar] [CrossRef] [PubMed]
- Borah, A.; Kumar, D.S. Overcoming the barriers of two-dimensional cell culture systems with three-dimensional cell culture systems: Techniques, drug discovery, and biomedical applications. In Biomedical Product and Materials Evaluation; Mohanan, P.V., Ed.; Woodhead Publishing: Sawston, UK, 2022; pp. 179–229. [Google Scholar] [CrossRef]
- Fang, G.; Chen, Y.-C.; Lu, H.; Jin, D. Advances in Spheroids and Organoids on a Chip. Adv. Funct. Mater. 2023, 33, 2215043. [Google Scholar] [CrossRef]
- Law, A.M.K.; Rodriguez de la Fuente, L.; Grundy, T.J.; Fang, G.; Valdes-Mora, F.; Gallego-Ortega, D. Advancements in 3D Cell Culture Systems for Personalizing Anti-Cancer Therapies. Front. Oncol. 2021, 11, 782766. [Google Scholar] [CrossRef]
- Kim, J.; Koo, B.K.; Knoblich, J.A. Human organoids: Model systems for human biology and medicine. Nat. Rev. Mol. Cell Biol. 2020, 21, 571–584. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Zhou, Z.; Zhang, Y.; Zhong, H.; Cai, X.; Guan, R. Recent progress on the organoids: Techniques, advantages and applications. Biomed. Pharmacother. 2025, 185, 117942. [Google Scholar] [CrossRef] [PubMed]
- Fang, Y.; Akhtar, H.; Wang, J. The application of organoids in toxicity test of environmental pollutants. Cell Organoid 2024. [Google Scholar] [CrossRef]
- Kwak, E.; Peng, G. Human-Organoid-Based In Vitro Modeling for Environmental Toxicology. Environ. Sci. Technol. Lett. 2024, 11, 503–510. [Google Scholar] [CrossRef]
- DeLuca, N.M.; Minucci, J.M.; Mullikin, A.; Slover, R.; Cohen Hubal, E.A. Human exposure pathways to poly- and perfluoroalkyl substances (PFAS) from indoor media: A systematic review. Environ. Int. 2022, 162, 107149. [Google Scholar] [CrossRef]
- Holder, C.; DeLuca, N.; Luh, J.; Alexander, P.; Minucci, J.M.; Vallero, D.A.; Thomas, K.; Cohen Hubal, E.A. Systematic Evidence Mapping of Potential Exposure Pathways for Per- and Polyfluoroalkyl Substances Based on Measured Occurrence in Multiple Media. Environ. Sci. Technol. 2023, 57, 5107–5116. [Google Scholar] [CrossRef]
- Ackerman Grunfeld, D.; Gilbert, D.; Hou, J.; Jones, A.M.; Lee, M.J.; Kibbey, T.C.G.; O’Carroll, D.M. Underestimated burden of per- and polyfluoroalkyl substances in global surface waters and groundwaters. Nat. Geosci. 2024, 17, 340–346. [Google Scholar] [CrossRef]
- Andrews, D.Q.; Naidenko, O.V. Population-Wide Exposure to Per- and Polyfluoroalkyl Substances from Drinking Water in the United States. Environ. Sci. Technol. Lett. 2020, 7, 931–936. [Google Scholar] [CrossRef]
- Hu, X.C.; Tokranov, A.K.; Liddie, J.; Zhang, X.; Grandjean, P.; Hart, J.E.; Laden, F.; Sun, Q.; Yeung, L.W.Y.; Sunderland, E.M. Tap Water Contributions to Plasma Concentrations of Poly- and Perfluoroalkyl Substances (PFAS) in a Nationwide Prospective Cohort of U.S. Women. Environ. Health Perspect. 2019, 127, 67006. [Google Scholar] [CrossRef]
- Christensen, B.T.; Calkins, M.M. Occupational exposure to per- and polyfluoroalkyl substances: A scope review of the literature from 1980–2021. J. Expo. Sci. Environ. Epidemiol. 2023, 33, 673–686. [Google Scholar] [CrossRef] [PubMed]
- Schlezinger, J.J.; Gokce, N. Perfluoroalkyl/Polyfluoroalkyl Substances: Links to Cardiovascular Disease Risk. Circ. Res. 2024, 134, 1136–1159. [Google Scholar] [CrossRef]
- Lucas, K.; Gaines, L.G.T.; Paris-Davila, T.; Nylander-French, L.A. Occupational exposure and serum levels of per- and polyfluoroalkyl substances (PFAS): A review. Am. J. Ind. Med. 2023, 66, 379–392. [Google Scholar] [CrossRef]
- Zhao, L.; Cheng, Z.; Zhu, H.; Chen, H.; Yao, Y.; Baqar, M.; Yu, H.; Qiao, B.; Sun, H. Electronic-waste-associated pollution of per- and polyfluoroalkyl substances: Environmental occurrence and human exposure. J. Hazard. Mater. 2023, 451, 131204. [Google Scholar] [CrossRef]
- Fenton, S.E.; Ducatman, A.; Boobis, A.; DeWitt, J.C.; Lau, C.; Ng, C.; Smith, J.S.; Roberts, S.M. Per- and Polyfluoroalkyl Substance Toxicity and Human Health Review: Current State of Knowledge and Strategies for Informing Future Research. Environ. Toxicol. Chem. 2021, 40, 606–630. [Google Scholar] [CrossRef]
- Winquist, A.; Hodge, J.M.; Diver, W.R.; Rodriguez, J.L.; Troeschel, A.N.; Daniel, J.; Teras, L.R. Case-Cohort Study of the Association between PFAS and Selected Cancers among Participants in the American Cancer Society’s Cancer Prevention Study II LifeLink Cohort. Environ. Health Perspect. 2023, 131, 127007. [Google Scholar] [CrossRef]
- Shearer, J.J.; Callahan, C.L.; Calafat, A.M.; Huang, W.-Y.; Jones, R.R.; Sabbisetti, V.S.; Freedman, N.D.; Sampson, J.N.; Silverman, D.T.; Purdue, M.P.; et al. Serum Concentrations of Per- and Polyfluoroalkyl Substances and Risk of Renal Cell Carcinoma. JNCI J. Natl. Cancer Inst. 2021, 113, 580–587. [Google Scholar] [CrossRef]
- Liu, D.; Yan, S.; Wang, P.; Chen, Q.; Liu, Y.; Cui, J.; Liang, Y.; Ren, S.; Gao, Y. Perfluorooctanoic acid (PFOA) exposure in relation to the kidneys: A review of current available literature. Front. Physiol. 2023, 14, 1103141. [Google Scholar] [CrossRef]
- Stanifer, J.W.; Stapleton, H.M.; Souma, T.; Wittmer, A.; Zhao, X.; Boulware, L.E. Perfluorinated Chemicals as Emerging Environmental Threats to Kidney Health: A Scoping Review. Clin. J. Am. Soc. Nephrol. CJASN 2018, 13, 1479–1492. [Google Scholar] [CrossRef]
- Liu, X.; Chen, R.; Peng, Y.; Zhou, Y.; Xia, M.; Wu, X.; Wang, Y.; Yin, W.; Han, Y.; Yu, M. Perfluorooctanoic acid (PFOA) induces cardiotoxicity by activating the Keap1/Nrf2 pathway in zebrafish (Danio rerio) embryos. Ecotoxicol. Environ. Saf. 2024, 285, 117098. [Google Scholar] [CrossRef] [PubMed]
- Zhao, M.; Yin, N.; Yang, R.; Li, S.; Zhang, S.; Faiola, F. Assessment and Comparison of Early Developmental Toxicity of Six Per- and Polyfluoroalkyl Substances with Human Embryonic Stem Cell Models. Environ. Sci. Technol. 2024, 58, 8215–8227. [Google Scholar] [CrossRef]
- Lu, Y.; Meng, L.; Ma, D.; Cao, H.; Liang, Y.; Liu, H.; Wang, Y.; Jiang, G. The occurrence of PFAS in human placenta and their binding abilities to human serum albumin and organic anion transporter 4. Environ. Pollut. 2021, 273, 116460. [Google Scholar] [CrossRef]
- Chen, H.; Wei, S.; Li, J.; Zhong, Z.; Chen, D. Transplacental transport of per- and polyfluoroalkyl substances (PFAS): Mechanism exploration via BeWo cell monolayer model. J. Hazard. Mater. 2024, 466, 133205. [Google Scholar] [CrossRef]
- Du, H.; Song, L.; Zhao, M.; Zhao, X.; Mu, R.; Gao, S.; Zhang, B.; Wang, J. Prenatal Perfluorooctanoic Acid (PFOA) exposure causes reproductive toxicity by disrupting the formation of transzonal projections (TZPs) and down-regulating Wnt4/β-catenin signaling pathway in progeny. Ecotoxicol. Environ. Saf. 2025, 291, 117816. [Google Scholar] [CrossRef]
- Zhao, Z.; Chen, X.; Dowbaj, A.M.; Sljukic, A.; Bratlie, K.; Lin, L.; Fong, E.L.S.; Balachander, G.M.; Chen, Z.; Soragni, A.; et al. Organoids. Nat. Rev. Methods Primers 2022, 2, 94. [Google Scholar] [CrossRef]
- Clevers, H. Modeling Development and Disease with Organoids. Cell 2016, 165, 1586–1597. [Google Scholar] [CrossRef]
- Sato, T.; Vries, R.G.; Snippert, H.J.; van de Wetering, M.; Barker, N.; Stange, D.E.; van Es, J.H.; Abo, A.; Kujala, P.; Peters, P.J.; et al. Single Lgr5 stem cells build crypt-villus structures in vitro without a mesenchymal niche. Nature 2009, 459, 262–265. [Google Scholar] [CrossRef] [PubMed]
- Verstegen, M.M.A.; Coppes, R.P.; Beghin, A.; De Coppi, P.; Gerli, M.F.M.; de Graeff, N.; Pan, Q.; Saito, Y.; Shi, S.; Zadpoor, A.A.; et al. Clinical applications of human organoids. Nat. Med. 2025, 31, 409–421. [Google Scholar] [CrossRef] [PubMed]
- Yao, Q.; Cheng, S.; Pan, Q.; Yu, J.; Cao, G.; Li, L.; Cao, H. Organoids: Development and applications in disease models, drug discovery, precision medicine, and regenerative medicine. MedComm 2024, 5, e735. [Google Scholar] [CrossRef]
- Wu, X.; Ciminieri, C.; Bos, I.S.T.; Woest, M.E.; D’Ambrosi, A.; Wardenaar, R.; Spierings, D.C.J.; Königshoff, M.; Schmidt, M.; Kistemaker, L.E.M.; et al. Diesel exhaust particles distort lung epithelial progenitors and their fibroblast niche. Environ. Pollut. 2022, 305, 119292. [Google Scholar] [CrossRef]
- Wang, R.; Kang, N.; Zhang, W.; Chen, B.; Xu, S.; Wu, L. The developmental toxicity of PM2.5 on the early stages of fetal lung with human lung bud tip progenitor organoids. Environ. Pollut. 2023, 330, 121764. [Google Scholar] [CrossRef]
- Philippat, C.; Coiffier, O.; Lyon-Caen, S.; Boudier, A.; Jovanovic, N.; Quentin, J.; Gioria, Y.; Haug, L.S.; Thomsen, C.; Bayat, S.; et al. In utero exposure to poly- and perfluoroalkyl substances and children respiratory health in the three first years of life. Environ. Res. 2023, 234, 116544. [Google Scholar] [CrossRef]
- Rafiee, A.; Faridi, S.; Sly, P.D.; Stone, L.; Kennedy, L.P.; Mahabee-Gittens, E.M. Asthma and decreased lung function in children exposed to perfluoroalkyl and polyfluoroalkyl substances (PFAS): An updated meta-analysis unveiling research gaps. Environ. Res. 2024, 262, 119827. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Yang, T.; Zhou, R.; Yang, C.; Huang, Q.; Cheng, S. Impact of polyfluoroalkyl chemicals and volatile organic compounds exposure on lung function of American adults. Environ. Pollut. 2024, 363, 125152. [Google Scholar] [CrossRef]
- Zhang, W.; Tian, Y.; Chen, B.; Xu, S.; Wu, L. PFOA/PFOS Facilitated Intestinal Fatty Acid Absorption by Activating the PPARα Pathway: Insights from Organoids Model. Environ. Health 2024, 2, 85–94. [Google Scholar] [CrossRef]
- Hou, Z.; Meng, R.; Chen, G.; Lai, T.; Qing, R.; Hao, S.; Deng, J.; Wang, B. Distinct accumulation of nanoplastics in human intestinal organoids. Sci. Total Environ. 2022, 838, 155811. [Google Scholar] [CrossRef]
- Park, S.B.; Jung, W.H.; Choi, K.J.; Koh, B.; Kim, K.Y. A Comparative Systematic Analysis of The Influence of Microplastics on Colon Cells, Mouse and Colon Organoids. Tissue Eng. Regen. Med. 2023, 20, 49–58. [Google Scholar] [CrossRef]
- Lee, J.; Rabbani, C.C.; Gao, H.; Steinhart, M.R.; Woodruff, B.M.; Pflum, Z.E.; Kim, A.; Heller, S.; Liu, Y.; Shipchandler, T.Z.; et al. Hair-bearing human skin generated entirely from pluripotent stem cells. Nature 2020, 582, 399–404. [Google Scholar] [CrossRef]
- Ragnarsdóttir, O.; Abdallah, M.A.-E.; Harrad, S. Dermal uptake: An important pathway of human exposure to perfluoroalkyl substances? Environ. Pollut. 2022, 307, 119478. [Google Scholar] [CrossRef] [PubMed]
- Lu, S.; Zhu, X.; Zeng, P.; Hu, L.; Huang, Y.; Guo, X.; Chen, Q.; Wang, Y.; Lai, L.; Xue, A.; et al. Exposure to PFOA, PFOS, and PFHxS induces Alzheimer’s disease-like neuropathology in cerebral organoids. Environ. Pollut. 2024, 363, 125098. [Google Scholar] [CrossRef]
- Basaly, V.; Hill, J.; Bihaqi, S.W.; Marques, E.; Slitt, A.L.; Zawia, N.H. Developmental Perfluorooctanesulfonic acid (PFOS) exposure as a potential risk factor for late-onset Alzheimer’s disease in CD-1 mice and SH-SY5Y cells. NeuroToxicology 2021, 86, 26–36. [Google Scholar] [CrossRef] [PubMed]
- Palazzolo, S.; Caligiuri, I.; Sfriso, A.A.; Mauceri, M.; Rotondo, R.; Campagnol, D.; Canzonieri, V.; Rizzolio, F. Early Warnings by Liver Organoids on Short- and Long-Chain PFAS Toxicity. Toxics 2022, 10, 91. [Google Scholar] [CrossRef]
- Shi, W.; Zhang, Z.; Li, M.; Dong, H.; Li, J. Reproductive toxicity of PFOA, PFOS and their substitutes: A review based on epidemiological and toxicological evidence. Environ. Res. 2024, 250, 118485. [Google Scholar] [CrossRef]
- Li, X.; Zheng, M.; Xu, B.; Li, D.; Shen, Y.; Nie, Y.; Ma, L.; Wu, J. Generation of offspring-producing 3D ovarian organoids derived from female germline stem cells and their application in toxicological detection. Biomaterials 2021, 279, 121213. [Google Scholar] [CrossRef]
- Hori, T.; Okae, H.; Shibata, S.; Kobayashi, N.; Kobayashi, E.H.; Oike, A.; Sekiya, A.; Arima, T.; Kaji, H. Trophoblast stem cell-based organoid models of the human placental barrier. Nat. Commun. 2024, 15, 962. [Google Scholar] [CrossRef]
- Xu, C.; Ma, H.; Gao, F.; Zhang, C.; Hu, W.; Jia, Y.; Xu, J.; Hu, J. Screening of Organophosphate Flame Retardants with Placentation-Disrupting Effects in Human Trophoblast Organoid Model and Characterization of Adverse Pregnancy Outcomes in Mice. Environ. Health Perspect. 2022, 130, 057002. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Zeng, Y.; Ge, L.; Gong, J.; Weng, C.; Yang, C.; Yang, J.; Fang, Y.; Li, Q.; Zou, T.; et al. Evaluation of the influences of low dose polybrominated diphenyl ethers exposure on human early retinal development. Environ. Int. 2022, 163, 107187. [Google Scholar] [CrossRef]
- Yang, L.; Zou, J.; Zang, Z.; Wang, L.; Du, Z.; Zhang, D.; Cai, Y.; Li, M.; Li, Q.; Gao, J.; et al. Di-(2-ethylhexyl) phthalate exposure impairs cortical development in hESC-derived cerebral organoids. Sci. Total Environ. 2023, 865, 161251. [Google Scholar] [CrossRef]
- Winkler, J.; Liu, P.; Phong, K.; Hinrichs, J.H.; Ataii, N.; Williams, K.; Hadler-Olsen, E.; Samson, S.; Gartner, Z.J.; Fisher, S.; et al. Bisphenol A replacement chemicals, BPF and BPS, induce protumorigenic changes in human mammary gland organoid morphology and proteome. Proc. Natl. Acad. Sci. USA 2022, 119, e2115308119. [Google Scholar] [CrossRef] [PubMed]
- Xuan, L.; Luo, J.; Qu, C.; Guo, P.; Yi, W.; Yang, J.; Yan, Y.; Guan, H.; Zhou, P.; Huang, R. Predictive metabolomic signatures for safety assessment of three plastic nanoparticles using intestinal organoids. Sci. Total Environ. 2024, 913, 169606. [Google Scholar] [CrossRef]
- Liu, Y.; Chen, L.; Yu, J.; Ye, L.; Hu, H.; Wang, J.; Wu, B. Advances in Single-Cell Toxicogenomics in Environmental Toxicology. Environ. Sci. Technol. 2022, 56, 11132–11145. [Google Scholar] [CrossRef]
- Peng, J.; Cao, S.; Hu, Z.; Zhu, J.; Zhu, Y.; Sheng, X.; Cai, Z.; Bai, R.; Xiong, X.; Sheng, J. Heterogeneity effects of bisphenol A and its substitute, fluorene-9-bisphenol, on intestinal homeostasis. Environ. Int. 2024, 191, 108948. [Google Scholar] [CrossRef] [PubMed]
- Dorgau, B.; Collin, J.; Rozanska, A.; Boczonadi, V.; Moya-Molina, M.; Unsworth, A.; Hussain, R.; Coxhead, J.; Dhanaseelan, T.; Armstrong, L.; et al. Deciphering the spatiotemporal transcriptional and chromatin accessibility of human retinal organoid development at the single-cell level. iScience 2024, 27, 109397. [Google Scholar] [CrossRef]
- Lan, Y.; Gao, X.; Xu, H.; Li, M. 20 years of polybrominated diphenyl ethers on toxicity assessments. Water Res. 2023, 249, 121007. [Google Scholar] [CrossRef]
- Hofer, M.; Lutolf, M.P. Engineering organoids. Nat. Rev. Mater. 2021, 6, 402–420. [Google Scholar] [CrossRef] [PubMed]
- Gjorevski, N.; Sachs, N.; Manfrin, A.; Giger, S.; Bragina, M.E.; Ordóñez-Morán, P.; Clevers, H.; Lutolf, M.P. Designer matrices for intestinal stem cell and organoid culture. Nature 2016, 539, 560–564. [Google Scholar] [CrossRef]
- Li, M.; Gao, L.; Zhao, L.; Zou, T.; Xu, H. Toward the next generation of vascularized human neural organoids. Med. Res. Rev. 2023, 43, 31–54. [Google Scholar] [CrossRef]
- Cakir, B.; Xiang, Y.; Tanaka, Y.; Kural, M.H.; Parent, M.; Kang, Y.-J.; Chapeton, K.; Patterson, B.; Yuan, Y.; He, C.-S.; et al. Engineering of human brain organoids with a functional vascular-like system. Nat. Methods 2019, 16, 1169–1175. [Google Scholar] [CrossRef]
- Schafer, S.T.; Mansour, A.A.; Schlachetzki, J.C.M.; Pena, M.; Ghassemzadeh, S.; Mitchell, L.; Mar, A.; Quang, D.; Stumpf, S.; Ortiz, I.S.; et al. An in vivo neuroimmune organoid model to study human microglia phenotypes. Cell 2023, 186, 2111–2126.e20. [Google Scholar] [CrossRef] [PubMed]
- Leung, C.M.; de Haan, P.; Ronaldson-Bouchard, K.; Kim, G.-A.; Ko, J.; Rho, H.S.; Chen, Z.; Habibovic, P.; Jeon, N.L.; Takayama, S.; et al. A guide to the organ-on-a-chip. Nat. Rev. Methods Primers 2022, 2, 33. [Google Scholar] [CrossRef]
- Park, S.E.; Georgescu, A.; Huh, D. Organoids-on-a-chip. Science 2019, 364, 960–965. [Google Scholar] [CrossRef]
- Zhang, T.; Yang, S.; Ge, Y.; Yin, L.; Pu, Y.; Gu, Z.; Chen, Z.; Liang, G. Unveiling the Heart’s Hidden Enemy: Dynamic Insights into Polystyrene Nanoplastic-Induced Cardiotoxicity Based on Cardiac Organoid-on-a-Chip. ACS Nano 2024, 18, 31569–31585. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, L.; Zhu, Y.; Qin, J. Human brain organoid-on-a-chip to model prenatal nicotine exposure. Lab Chip 2018, 18, 851–860. [Google Scholar] [CrossRef]
- Skardal, A.; Aleman, J.; Forsythe, S.; Rajan, S.; Murphy, S.; Devarasetty, M.; Pourhabibi Zarandi, N.; Nzou, G.; Wicks, R.; Sadri-Ardekani, H.; et al. Drug compound screening in single and integrated multi-organoid body-on-a-chip systems. Biofabrication 2020, 12, 025017. [Google Scholar] [CrossRef]
- Hu, C.; Yang, S.; Zhang, T.; Ge, Y.; Chen, Z.; Zhang, J.; Pu, Y.; Liang, G. Organoids and organoids-on-a-chip as the new testing strategies for environmental toxicology-applications & advantages. Environ. Int. 2024, 184, 108415. [Google Scholar] [CrossRef]
- Zhao, Y.; Landau, S.; Okhovatian, S.; Liu, C.; Lu, R.X.Z.; Lai, B.F.L.; Wu, Q.; Kieda, J.; Cheung, K.; Rajasekar, S.; et al. Integrating organoids and organ-on-a-chip devices. Nat. Rev. Bioeng. 2024, 2, 588–608. [Google Scholar] [CrossRef]
- Lu, R.X.Z.; Radisic, M. Organ-on-a-chip platforms for evaluation of environmental nanoparticle toxicity. Bioact. Mater. 2021, 6, 2801–2819. [Google Scholar] [CrossRef]
- Hu, W.; Lazar, M.A. Modelling metabolic diseases and drug response using stem cells and organoids. Nat. Rev. Endocrinol. 2022, 18, 744–759. [Google Scholar] [CrossRef]
- Yang, J.; Jiang, Y.; Li, M.; Wu, K.; Wei, S.; Zhao, Y.; Shen, J.; Du, F.; Chen, Y.; Deng, S.; et al. Organoid, organ-on-a-chip and traditional Chinese medicine. Chin. Med. 2025, 20, 22. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, H.; Kang, J.; Gao, X.; Lan, Y.; Li, M. Towards a Better Understanding of the Human Health Risk of Per- and Polyfluoroalkyl Substances Using Organoid Models. Bioengineering 2025, 12, 393. https://doi.org/10.3390/bioengineering12040393
Xu H, Kang J, Gao X, Lan Y, Li M. Towards a Better Understanding of the Human Health Risk of Per- and Polyfluoroalkyl Substances Using Organoid Models. Bioengineering. 2025; 12(4):393. https://doi.org/10.3390/bioengineering12040393
Chicago/Turabian StyleXu, Haoan, Jiahui Kang, Xue Gao, Yingying Lan, and Minghui Li. 2025. "Towards a Better Understanding of the Human Health Risk of Per- and Polyfluoroalkyl Substances Using Organoid Models" Bioengineering 12, no. 4: 393. https://doi.org/10.3390/bioengineering12040393
APA StyleXu, H., Kang, J., Gao, X., Lan, Y., & Li, M. (2025). Towards a Better Understanding of the Human Health Risk of Per- and Polyfluoroalkyl Substances Using Organoid Models. Bioengineering, 12(4), 393. https://doi.org/10.3390/bioengineering12040393







