Assessing Thresholds for Nerve Activation and Action Potential Block Using a Multielectrode Array to Minimize External Stimulation
Abstract
:1. Introduction
2. Materials and Methods
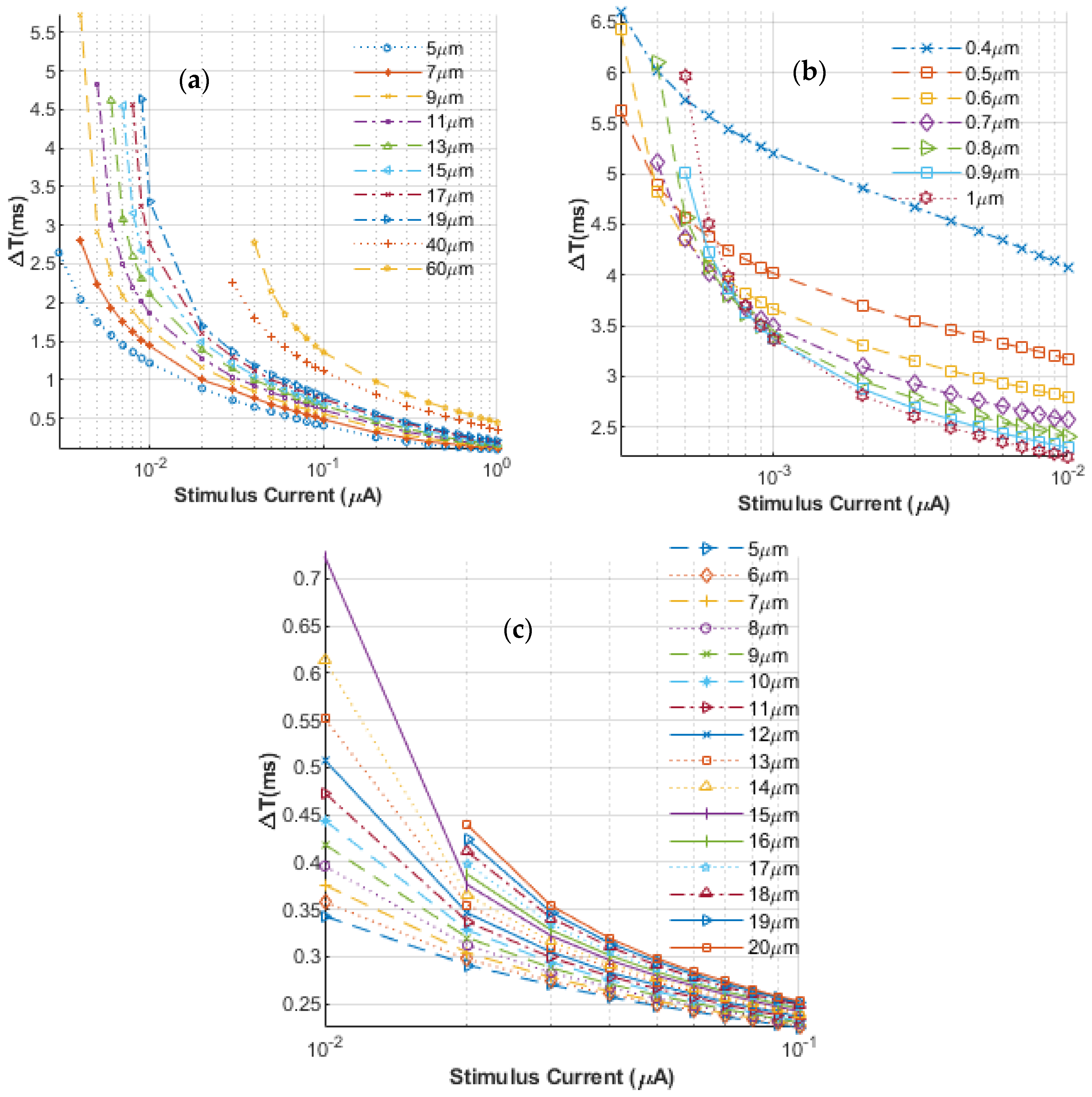
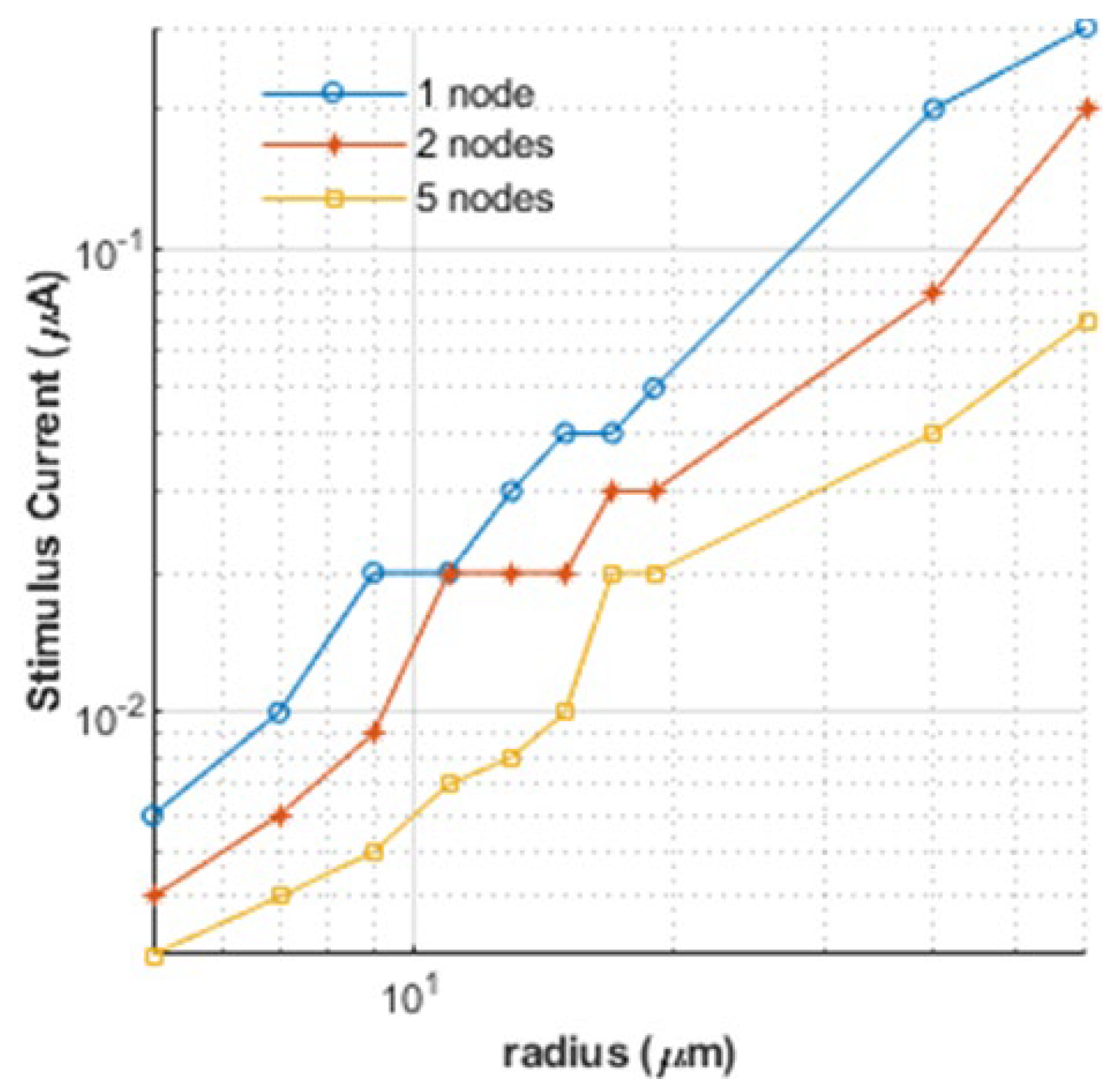
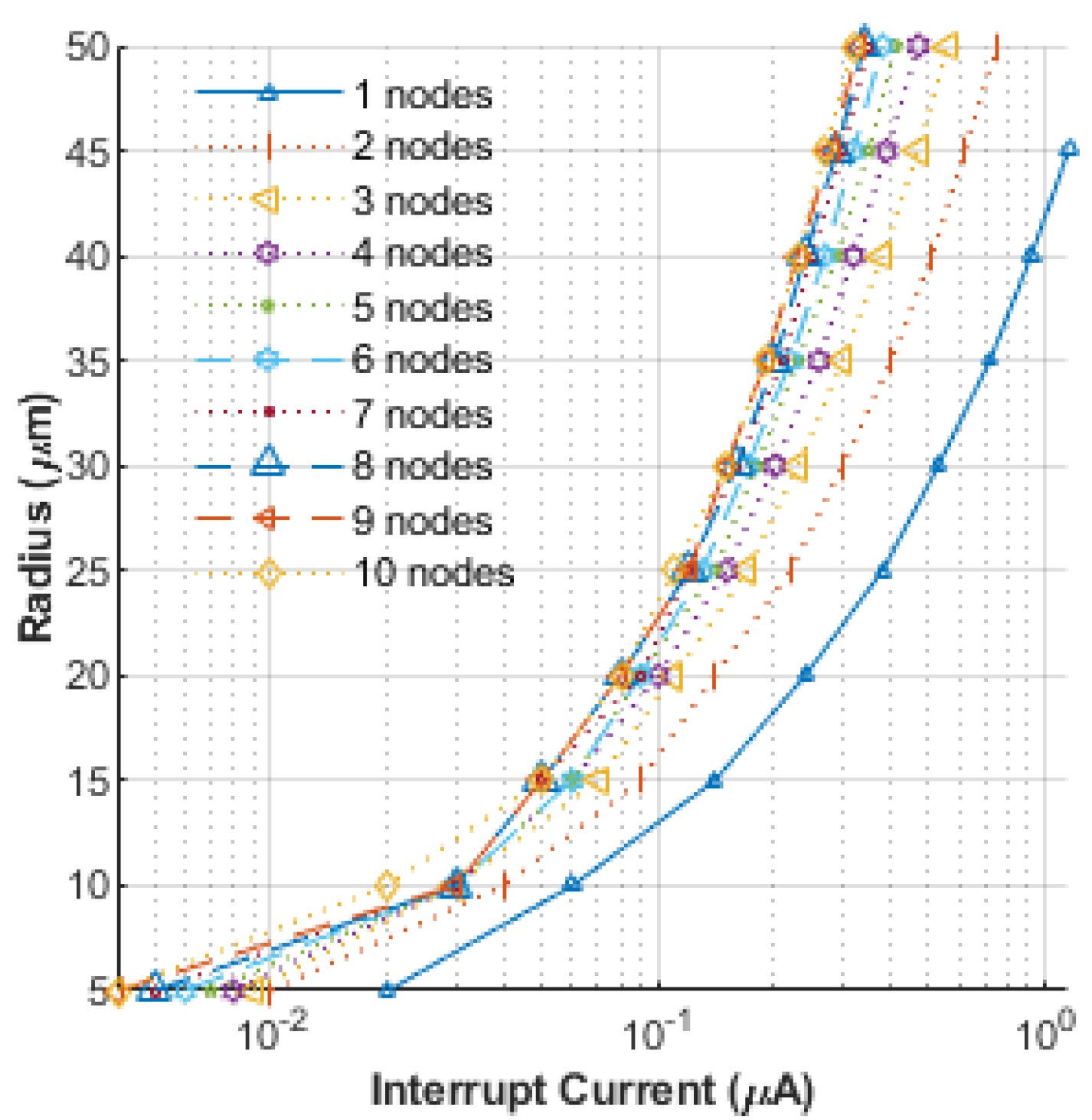
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Waters, R.L.; McNeal, D.R.; Faloon, W.; Clifford, B. Functional electrical stimulation of the peroneal nerve for hemiplegia. Long-term clinical follow-up. J. Bone Jt. Surg. Am. 1985, 67, 792–793. [Google Scholar] [CrossRef]
- Tanagho, E.A.; Schmidt, R.A.; Orvis, B.R. Neural stimulation for control of voiding dysfunction: A preliminary report in 22 patients with serious neuropathic voiding disorders. J. Urol. 1989, 142, 340–345. [Google Scholar] [CrossRef] [PubMed]
- Baker, L.L.; McNeal, D.R.; Benton, L.A.; Bowman, B.R.; Waters, R.L. Neuromuscular Electrical Stimulation: A Practical Guide; Los Amigo Research Education Institute: Downey, CA, USA, 1993. [Google Scholar]
- Stein, R.B.; Chong, S.L.; James, K.B.; Kido, A.; Bell, G.J.; Tubman, L.A.; Belanger, M. Electrical stimulation for therapy and mobility after spinal cord injury. Prog. Brain Res. 2002, 137, 27–34. [Google Scholar]
- Popovic, M.R.; Thrasher, T.A. Neuroprostheses. In Encyclopedia of Biomaterials and Biomedical Engineering; Wnek, G.E., Bowlin, G.L., Eds.; Marcel Dekker Press: New York, NY, USA, 2004; Volume 2, pp. 1056–1065. [Google Scholar]
- Tsai, S.J.; Lew, H.L.; Date, E.; Bih, L.I. Treatment of detrusorsphincter dyssynergia by pudendal nerve block in patients with spinal cord injury. Arch. Phys. Med. Rehabil. 2002, 83, 714–717. [Google Scholar]
- Baratta, R.; Ichie, M.; Hwang, S.K.; Solomonow, M. Orderly stimulation of skeletal muscle motor units with tripolar nerve cuff electrode. IEEE Trans. Biomed. Eng. 1989, 36, 836–843. [Google Scholar] [CrossRef]
- Fang, Z.P.; Mortimer, J.T. A method to effect physiological recruitment order in electrically activated muscle. IEEE Trans. Biomed. Eng. 1991, 38, 175–179. [Google Scholar] [CrossRef]
- Cattell, M.; Gerard, R.W. The inhibitory effect of high-frequency stimulation and the excitation state of nerve. J. Physiol. 1935, 83, 407–415. [Google Scholar] [CrossRef]
- Whitwam, J.G.; Kidd, C. The use of direct current to cause selective block of large fibers in peripheral nerves. Br. J. Anaesth. 1975, 47, 1123–1133. [Google Scholar] [CrossRef]
- Bhadra, N.; Kilgore, K. Direct current electrical conduction block of peripheral nerve. IEEE Trans. Neural Syst. Rehab. Eng. 2004, 12, 313–324. [Google Scholar]
- Tai, C.; de Groat, W.C.; Roppolo, J.R. Simulation analysis of conduction block in unmyelinated axons induced by high-frequency biphasic electrical currents. IEEE Trans. Biomed. Eng. 2005, 52, 1323–1332. [Google Scholar]
- Joshi, R.P.; Mishra, A.; Song, J.; Pakhomov, A.; Schoenbach, K.H. Simulation studies of ultra-short, high-intensity electric pulse induced action potential block in whole-animal nerves. IEEE Trans. Biomed. Eng. 2008, 55, 1391–1398. [Google Scholar] [PubMed]
- Joshi, R.P.; Mishra, A.; Song, J.; Hu, Q.; Schoenbach, K.H.; Pakhomov, A. Self-consistent analyses for potential conduction block in nerves by an ultra-short, high-intensity electric pulse. Phys. Rev. E 2007, 75, 061906. [Google Scholar]
- Tracey, K.J. The revolutionary future of bioelectronic medicine. Bioelectron. Med. 2014, 1, 1–15. [Google Scholar]
- Birmingham, K.; Gradinaru, V.; Anikeeva, P.; Grill, W.M.; Pikov, V.; McLaughlin, B.; Pasricha, P.; Weber, D.; Ludwig, K.; Famm, K. Bioelectronic medicines: A research roadmap. Nat. Rev. Drug Discov. 2014, 13, 399–400. [Google Scholar]
- Pavlov, V.A.; Tracey, K.J. Bioelectronic medicine: Updates, challenges and paths forward. Bioelectron. Med. 2019, 5, 1–4. [Google Scholar]
- Grayden, D.B.; Clark, G.M. Implant design and development. In Cochlear Implants: A Practical Guide; Cooper, H., Craddock, L., Eds.; Whurr: London, UK, 2006; pp. 1–20. [Google Scholar]
- Lewis, P.M.; Ackland, H.M.; Lowery, A.J.; Rosenfeld, J.V. Restoration of vision in blind individuals using bionic devices: A review with a focus on cortical visual prostheses. Brain Res. 2015, 1595, 51–73. [Google Scholar]
- Van De Berg, R.; Guinand, N.; Nguyen, T.K.; Ranieri, M.; Cavuscens, S.; Guyot, J.P.; Stokroos, R.; Kingma, H.; Perez-Fornos, A. The vestibular implant: Frequency-dependency of the electrically evoked vestibulo-ocular reflex in humans. Front. Syst. Neurosci. 2015, 8, 255. [Google Scholar]
- Papageorgiou, P.N.; Deschner, J.; Papageorgiou, S.N. Effectiveness and adverse effects of deep brain stimulation: Umbrella review of meta-analyses. J. Neurol. Surg. A Cent. Eur. Neurosurg. 2017, 78, 180–190. [Google Scholar]
- Greenberg, B.D.; Malone, D.A.; Friehs, G.M.; Rezai, A.R.; Kubu, C.S.; Malloy, P.F.; Salloway, S.P.; Okun, M.S.; Goodman, W.K.; Rasmussen, S.A. Three-year outcomes in deep brain stimulation for highly resistant obsessive-com-pulsive disorder. Neuropsychopharmacology 2006, 31, 2384–2393. [Google Scholar]
- Nagaraj, V.; Lee, S.T.; Krook-Magnuson, E.; Soltesz, I.; Benquet, P.; Irazoqui, P.P.; Netoff, T.I. Future of seizure prediction and intervention: Closing the loop. J. Clin. Neurophysiol. 2015, 32, 194–206. [Google Scholar]
- Schlaepfer, T.E.; Cohen, M.X.; Frick, C.; Kosel, M.; Brodesser, D.; Axmacher, N.; Joe, A.Y.; Kreft, M.; Lenartz, D.; Sturm, V. Deep brain stimulation to reward circuitry alleviates anhedonia in refractory major depression. Neuropsychopharmacology 2008, 33, 368–377. [Google Scholar] [CrossRef] [PubMed]
- Gordon, T.; English, A.W. Strategies to promote peripheral nerve regeneration: Electrical stimulation and/or exercise. Eur. J. Neurosci. 2016, 43, 336–350. [Google Scholar] [CrossRef]
- Hodgkin, A.L.; Huxley, A.F. A quantitative description of membrane current and its application to conduction and excitation in nerve. J. Physiol. 1952, 117, 500–544. [Google Scholar] [CrossRef] [PubMed]
- Tarnaud, T.; Joseph, W.; Martens, L.; Tanghe, E. Dependence of excitability indices on membrane channel dynamics, myelin impedance, electrode location and stimulus waveforms in myelinated and unmyelinated fibre models. Med. Biol. Eng. Comput. 2018, 56, 1595–1613. [Google Scholar] [PubMed]
- Hill, T.L.; Chen, Y.D. On the theory of ion transport across the nerve membrane: Noise from the open-close kinetics of K+ channels. Biophys. J. 1972, 12, 948–959. [Google Scholar] [CrossRef]
- Skaugen, E.; Walløe, L. Firing behaviour in a stochastic nerve membrane model based upon the Hodgkin-Huxley equations. Acta Physiol. Scand. 1979, 107, 343–363. [Google Scholar]
- Strassberg, A.F.; DeFelice, L.J. Limitations of the Hodgkin-Huxley formalism: Effects of single channel kinetics on transmembrane voltage dynamics. Neural Comput. 1993, 5, 843–855. [Google Scholar] [CrossRef]
- White, J.A.; Rubinstein, J.T.; Kay, A.R. Channel noise in neurons. Trends Neurosci. 2000, 23, 131–137. [Google Scholar]
- Sokol, M.; Baker, C.; Baker, M.; Joshi, R.P. Simple model to incorporate statistical noise based on a modified Hodgkin-Huxley approach for external electrical field driven neural responses. Biomed. Phys. Eng. Express 2024, 10, 045037. [Google Scholar] [CrossRef]
- Cooley, J.W.; Dodge, F.A., Jr. Digital computer solutions for excitation and propagation of the nerve impulse. Biophys. J. 1966, 6, 583–599. [Google Scholar] [CrossRef]
- McNeal, D.R. Analysis of a model for excitation of myelinated nerve. IEEE Trans. Biomed. Eng. 1976, 23, 329–337. [Google Scholar] [CrossRef] [PubMed]
- Rattay, F. Analysis of models for external stimulation of axons. IEEE Trans. Biomed. Eng. 1986, 33, 974–977. [Google Scholar]
- Rattay, F. Electrical Nerve Stimulation: Theory, Experiments and Applications; Springer: New York, NY, USA, 1990. [Google Scholar]
- Reilly, J.P. Electrical Stimulation and Electropathology; Cambridge Press: Cambridge, UK, 1982. [Google Scholar]
- Mascagni, M. The backward Euler method for numerical solution of the Hodgkin-Huxley equations of nerve conduction. Siam J. Numer. Anal. 1990, 27, 941–962. [Google Scholar] [CrossRef]
- Benzi, R.; Sutera, A.; Vulpiani, A. The mechanism of stochastic resonance. J. Phys. A Math. Gen. 1981, 14, L453–L457. [Google Scholar]
- Rowat, P. Interspike interval statistics in the stochastic Hodgkin-Huxley model: Coexistence of gamma frequency bursts and highly irregular firing. Neural Comput. 2007, 19, 1215–1250. [Google Scholar] [CrossRef] [PubMed]
- Schmid, G.; Goychuk, I.; Hanggi, P. Effect of channel block on the spiking activity of excitable membranes in a stochastic Hodgkin-Huxley model. Phys. Bio. 2004, 1, 61–66. [Google Scholar] [CrossRef]
- Matias, I.; Maturana, M.I.; Apollo, N.V.; Hadjinicolaou, A.E.; Garrett, D.J.; Cloherty, S.L.; Kameneva, T.; Grayden, D.B.; Ibbotson, M.R.; Meffin, H. A simple and accurate model to predict responses to multi-electrode stimulation in the retina. PLoS Comput. Biol. 2016, 12, e1004849. [Google Scholar]
- Rozman, J.; Pečlin, P.; Ribarič, S.; Godec, M.; Burja, J. An improved method of crafting a multi-electrode spiral cuff for the selective stimulation of peripheral nerves. Sci. Rep. 2018, 8, 915. [Google Scholar]
- Liu, F.; Habibollahi, M.; Wu, Y.; Neshatvar, N.; Zhang, J.; Zinno, C.; Akouissi, O.; Bernini, F.; Alibrandi, L.; Gabisonia, K.; et al. A multi-channel stimulator with an active electrode array implant for vagal-cardiac neuromodulation studies. Bioelectron. Med. 2024, 10, 16. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mishra, A.; Joshi, R.P. Assessing Thresholds for Nerve Activation and Action Potential Block Using a Multielectrode Array to Minimize External Stimulation. Bioengineering 2025, 12, 372. https://doi.org/10.3390/bioengineering12040372
Mishra A, Joshi RP. Assessing Thresholds for Nerve Activation and Action Potential Block Using a Multielectrode Array to Minimize External Stimulation. Bioengineering. 2025; 12(4):372. https://doi.org/10.3390/bioengineering12040372
Chicago/Turabian StyleMishra, Ashutosh, and R. P. Joshi. 2025. "Assessing Thresholds for Nerve Activation and Action Potential Block Using a Multielectrode Array to Minimize External Stimulation" Bioengineering 12, no. 4: 372. https://doi.org/10.3390/bioengineering12040372
APA StyleMishra, A., & Joshi, R. P. (2025). Assessing Thresholds for Nerve Activation and Action Potential Block Using a Multielectrode Array to Minimize External Stimulation. Bioengineering, 12(4), 372. https://doi.org/10.3390/bioengineering12040372







