Alterations in the Neuromuscular Control Mechanism of the Legs During a Post-Fatigue Landing Make the Lower Limbs More Susceptible to Injury
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
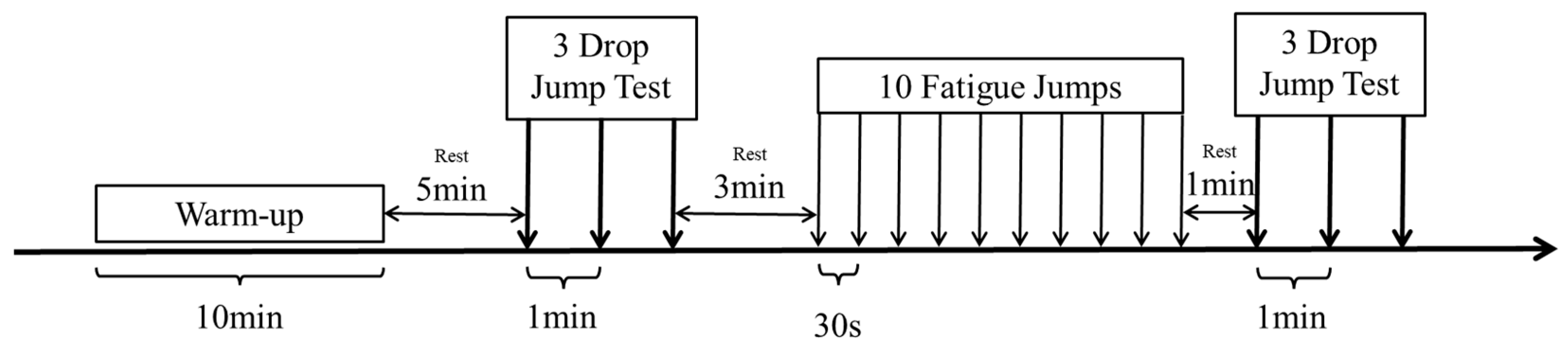
2.2. Experiment Process
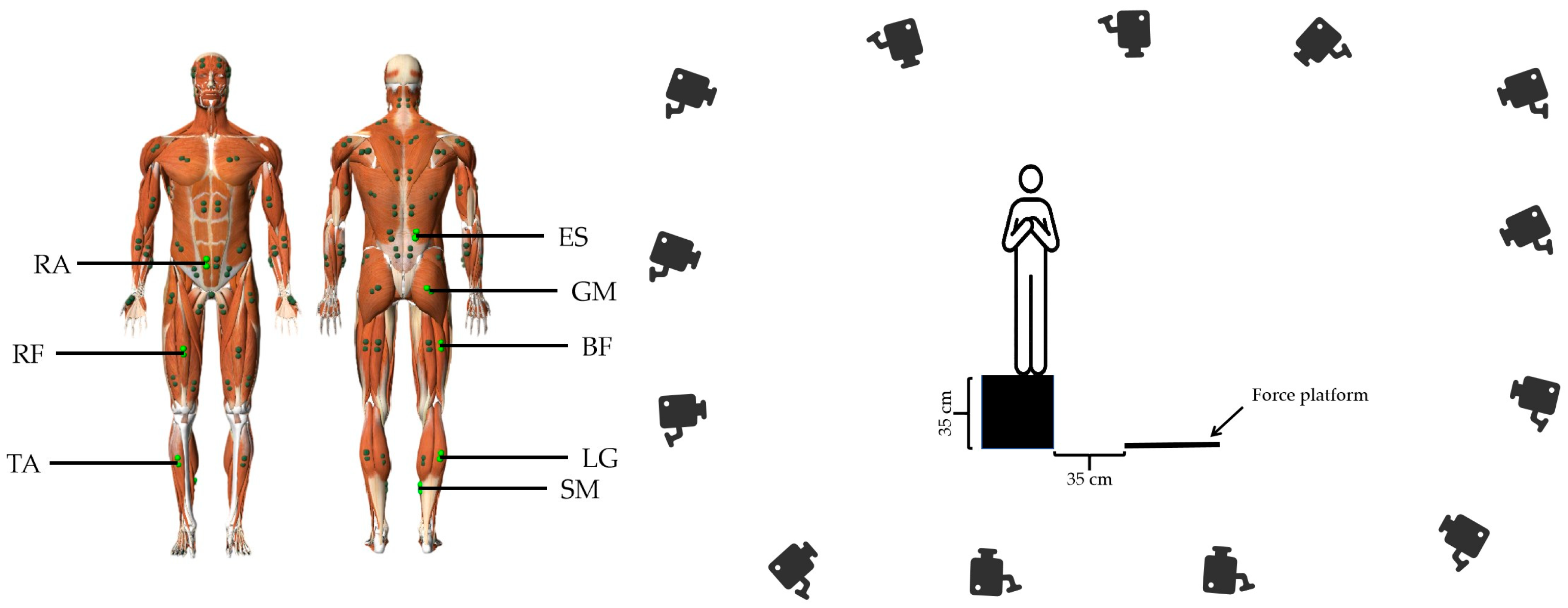
2.3. Landing Test
2.4. Fatigue Intervention Strategies
2.5. Data Collection
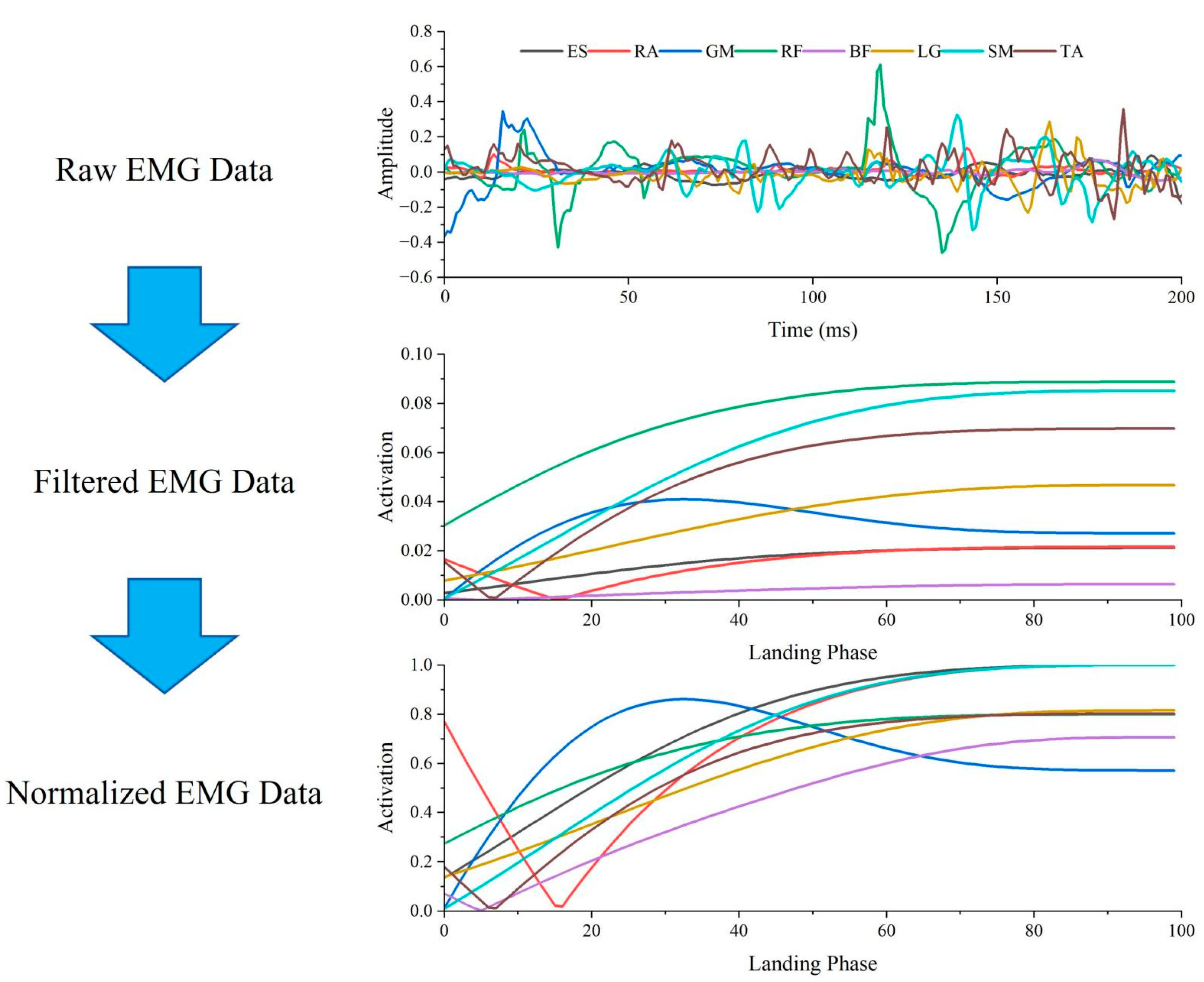
2.6. Data Processing
2.7. Statistical Analysis
3. Results
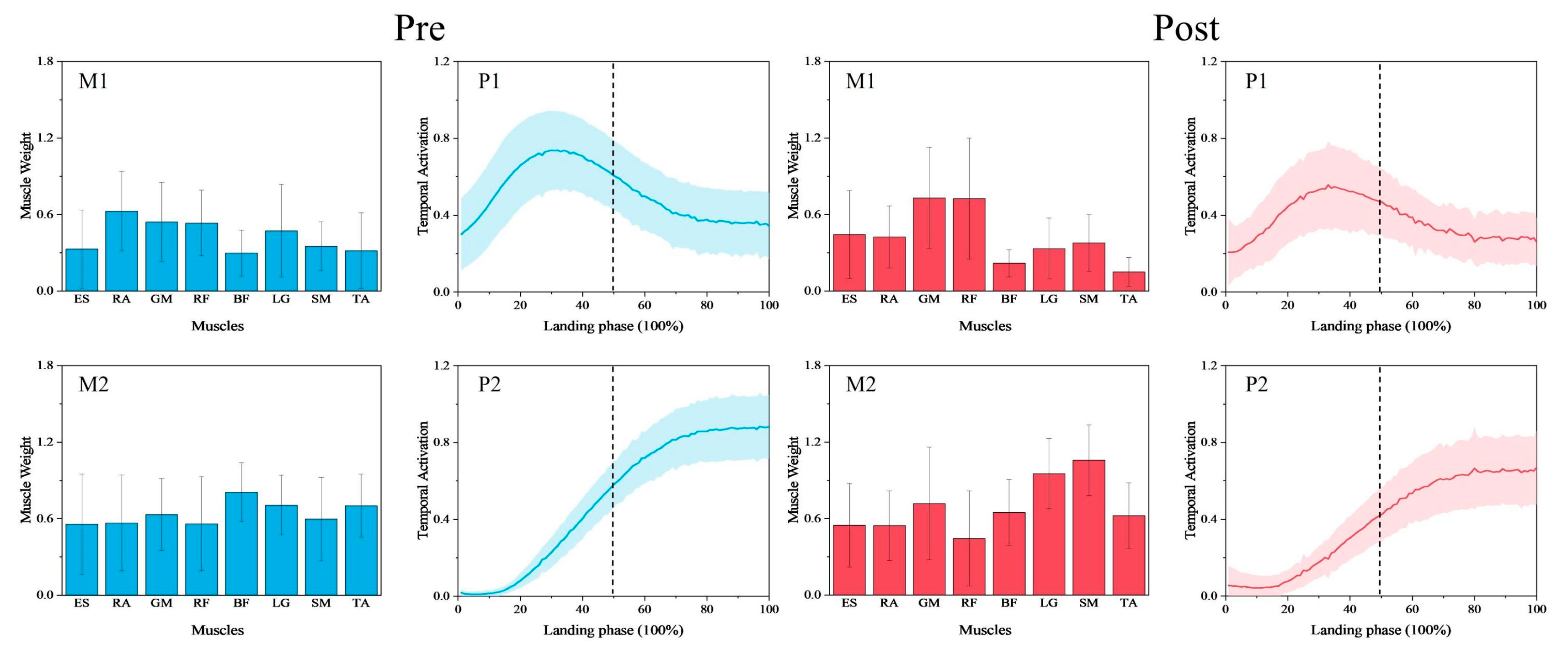
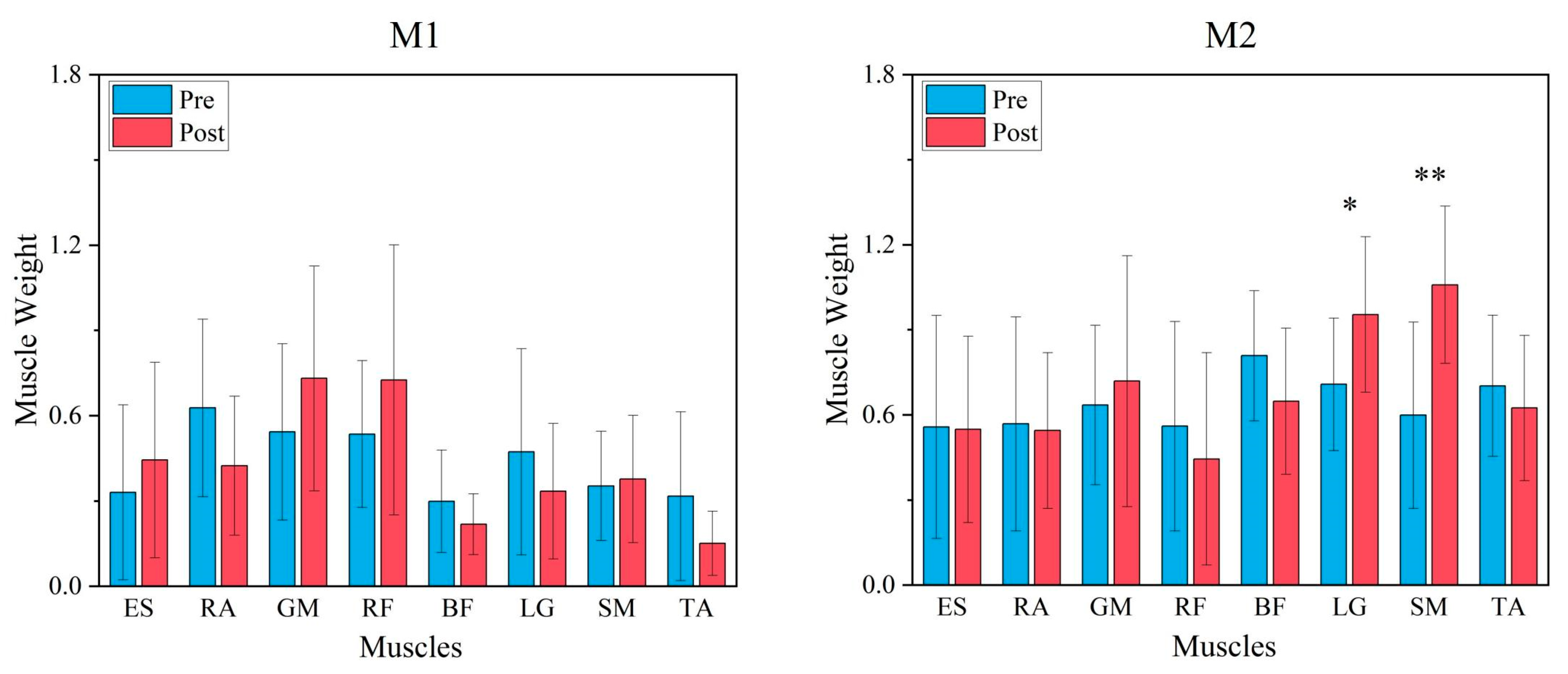
3.1. Comparison of Synergy Modules Pre- and Post-Fatigue Intervention
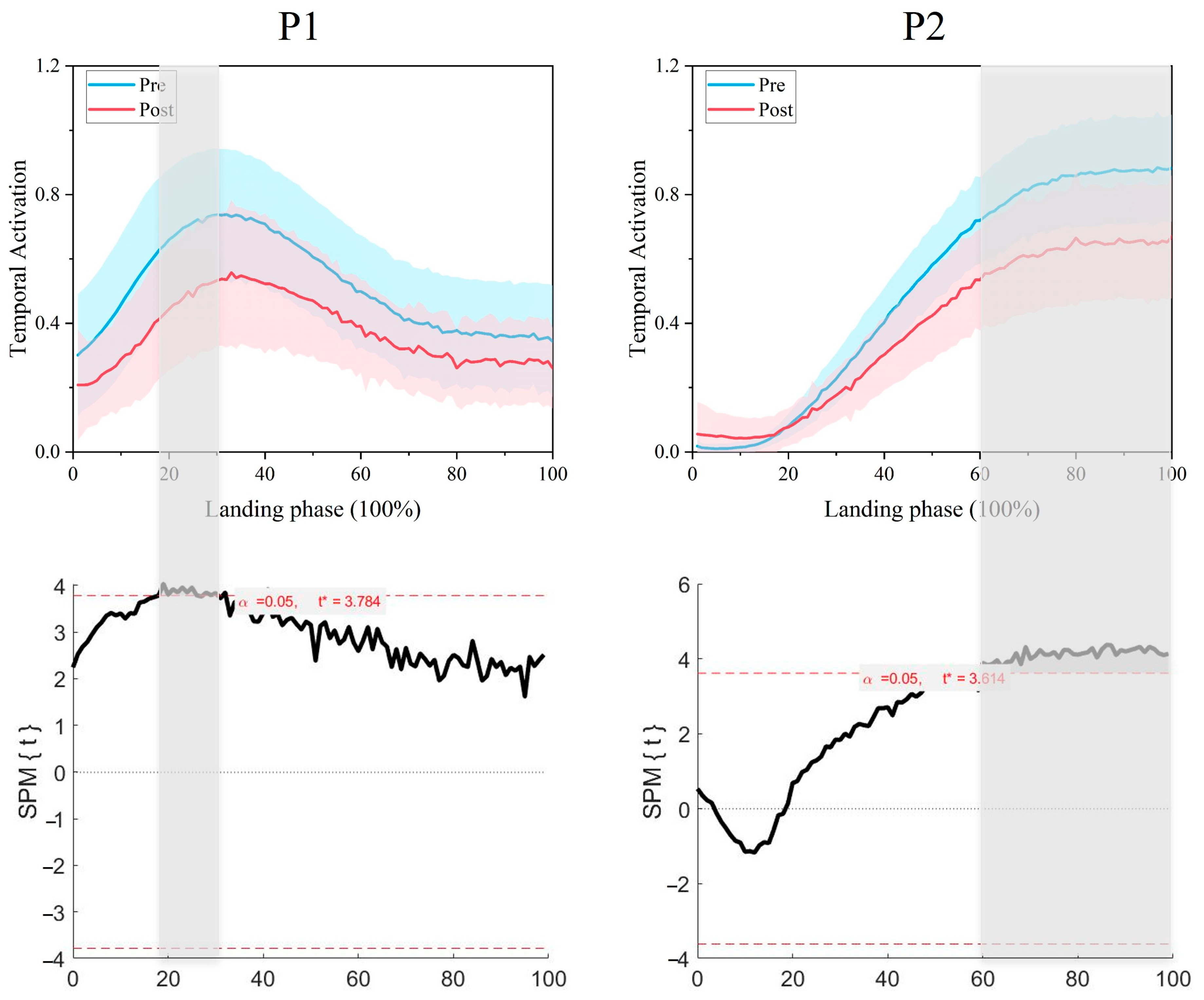
3.2. Comparison of Synergy Primitives Pre- and Post-Fatigue Intervention
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Taylor, J.B.; Ford, K.R.; Nguyen, A.-D.; Terry, L.N.; Hegedus, E.J. Prevention of lower extremity injuries in basketball: A systematic review and meta-analysis. Sports Health 2015, 7, 392–398. [Google Scholar] [CrossRef] [PubMed]
- Cortes, N.; Morrison, S.; Van Lunen, B.L.; Onate, J.A. Landing technique affects knee loading and position during athletic tasks. J. Sci. Med. Sport 2012, 15, 175–181. [Google Scholar] [CrossRef] [PubMed]
- Brazen, D.M.M.; Todd, M.K.; Ambegaonkar, J.P.P.; Wunderlich, R.; Peterson, C.P. The effect of fatigue on landing biomechanics in single-leg drop landings. Am. J. Ther. 2010, 20, 286–292. [Google Scholar] [CrossRef]
- Kernozek, T.W.; Torry, M.R.; Iwasaki, M. Gender Differences in lower extremity landing mechanics caused by neuromuscular fatigue. Am. J. Sports Med. 2008, 36, 554–565. [Google Scholar] [CrossRef] [PubMed]
- Chappell, J.D.; Herman, D.C.; Knight, B.S.; Kirkendall, D.T.; Garrett, W.E.; Yu, B. Effect of fatigue on knee kinetics and kinematics in stop-jump tasks. Am. J. Sports Med. 2005, 33, 1022–1029. [Google Scholar] [CrossRef]
- Verschueren, J.; Tassignon, B.; De Pauw, K.; Proost, M.; Teugels, A.; Van Cutsem, J.; Roelands, B.; Verhagen, E.; Meeusen, R. Does acute fatigue negatively affect intrinsic risk factors of the lower extremity injury risk profile? A systematic and critical review. Sports Med. 2020, 50, 767–784. [Google Scholar] [CrossRef]
- Rozzi, S.L.; Lephart, S.M.; Fu, F.H. Effects of muscular fatigue on knee joint laxity and neuromuscular characteristics of male and female athletes. J. Athl. Train. 1999, 34, 106–114. [Google Scholar]
- Skinner, H.B.; Wyatt, M.P.; Hodgdon, J.A.; Conard, D.W.; Barrack, R.L. Effect of fatigue on joint position sense of the knee. J. Orthop. Res. 1986, 4, 112–118. [Google Scholar] [CrossRef]
- Heil, J.; Loffing, F.; Büsch, D. The Influence of exercise-induced fatigue on inter-limb asymmetries: A systematic review. Sports Med. Open 2020, 6, 39. [Google Scholar] [CrossRef]
- Delextrat, A.; Badiella, A.; Saavedra, V.; Matthew, D.; Schelling, X.; Torres-Ronda, L. Match activity demands of elite Spanish female basketball players by playing position. Int. J. Perform. Anal. Sport 2015, 15, 687–703. [Google Scholar] [CrossRef]
- Yoshida, N.; Hornsby, W.G.; Sole, C.J.; Sato, K.; Stone, M.H. Effect of neuromuscular fatigue on the countermovement jump characteristics: Basketball-related high-intensity exercises. J. Strength Cond. Res. 2024, 38, 164–173. [Google Scholar] [CrossRef] [PubMed]
- Sawers, A.; Allen, J.L.; Ting, L.H. Long-term training modifies the modular structure and organization of walking balance control. J. Neurophysiol. 2015, 114, 3359–3373. [Google Scholar] [CrossRef] [PubMed]
- Santuz, A.; Ekizos, A.; Janshen, L.; Baltzopoulos, V.; Arampatzis, A. On the methodological implications of extracting muscle synergies from human locomotion. Int. J. Neural Syst. 2017, 27, 1750007. [Google Scholar] [CrossRef] [PubMed]
- Bizzi, E.; Cheung, V.; D’Avella, A.; Saltiel, P.; Tresch, M. Combining modules for movement. Brain Res. Rev. 2008, 57, 125–133. [Google Scholar] [CrossRef]
- Safavynia, S.A.; Torres-Oviedo, G.; Ting, L.H. Muscle synergies: Implications for clinical evaluation and rehabilitation of movement. Top. Spinal Cord Inj. Rehabil. 2011, 17, 16–24. [Google Scholar] [CrossRef]
- Hajiloo, B.; Anbarian, M.; Esmaeili, H.; Mirzapour, M. The effects of fatigue on synergy of selected lower limb muscles during running. J. Biomech. 2020, 103, 109692. [Google Scholar] [CrossRef]
- Singh, T.; Latash, M.L. Effects of muscle fatigue on multi-muscle synergies. Exp. Brain Res. 2011, 214, 335–350. [Google Scholar] [CrossRef]
- Matsunaga, N.; Okubo, Y.; Isagawa, S.; Niitsuma, J.; Otsudo, T.; Sawada, Y.; Akasaka, K. Muscle fatigue in the gluteus maximus changes muscle synergies during single-leg landing. J. Bodyw. Mov. Ther. 2021, 27, 493–499. [Google Scholar] [CrossRef]
- James, C.R.; Scheuermann, B.W.; Smith, M.P. Effects of two neuromuscular fatigue protocols on landing performance. J. Electromyogr. Kinesiol. 2010, 20, 667–675. [Google Scholar] [CrossRef]
- Li, M.; Meng, X.; Guan, L.; Kim, Y.; Kim, S. Comparing the effects of static stretching alone and in combination with post-activation performance enhancement on squat jump performance at different knee starting angles. J. Sports Sci. Med. 2023, 22, 769–777. [Google Scholar] [CrossRef]
- Taylor, K.; Terry, M.; Utturkar, G.; Spritzer, C.; Queen, R.; Irribarra, L.; Garrett, W.; DeFrate, L. Measurement of in vivo anterior cruciate ligament strain during dynamic jump landing. J. Biomech. 2011, 44, 365–371. [Google Scholar] [CrossRef] [PubMed]
- Lazaridis, S.; Patikas, D.A.; Bassa, E.; Tsatalas, T.; Hatzikotoulas, K.; Ftikas, C.; Kotzamanidis, C. The acute effects of an intense stretch-shortening cycle fatigue protocol on the neuromechanical parameters of lower limbs in men and prepubescent boys. J. Sports Sci. 2018, 36, 131–139. [Google Scholar] [CrossRef] [PubMed]
- Madigan, M.L.; Pidcoe, P.E. Changes in landing biomechanics during a fatiguing landing activity. J. Electromyogr. Kinesiol. 2003, 13, 491–498. [Google Scholar] [CrossRef] [PubMed]
- Barbero, M.; Merletti, R.; Rainoldi, A. Atlas of Muscle Innervation Zones: Understanding Surface Electromyography and Its Applications; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2012. [Google Scholar] [CrossRef]
- Yoon, S.; Bailey, C.A.; Cohen, N.R.; Côté, J.N. Changes in muscle activation, oxygenation, and morphology following a fatiguing repetitive forward reaching task in young adult males and females. J. Electromyogr. Kinesiol. 2021, 59, 102564. [Google Scholar] [CrossRef]
- Merletti, R.; Cerone, G. Tutorial. Surface EMG detection, conditioning and pre-processing: Best practices. J. Electromyogr. Kinesiol. 2020, 54, 102440. [Google Scholar] [CrossRef]
- Martire, R.L.; Gladh, K.; Westman, A.; Äng, B.O. Neck muscle Emg-force relationship and its reliability during isometric contractions. Sports Med. Open 2017, 3, 16. [Google Scholar] [CrossRef]
- Castillo-Lozano, R.; Cuesta-Vargas, A.I. A comparison land-water environment of maximal voluntary isometric contraction during manual muscle testing through surface electromyography. J. Sports Med. Arthrosc. Rehabil. Ther. Technol. 2013, 5, 28. [Google Scholar] [CrossRef]
- Gholipour Aghdam, G.M.; Alizadeh, M.H.; Minoonejad, H.; Shirzad, E.; Wilke, J. Knee biomechanics during neurocognitively challenged drop landings in male elite soccer players with anterior cruciate ligament reconstruction. Sports Med. Open 2024, 10, 19. [Google Scholar] [CrossRef]
- Kipp, K.; Pfeiffer, R.; Sabick, M.; Harris, C.; Sutter, J.; Kuhlman, S.; Shea, K. muscle synergies during a single-leg drop-landing in boys and girls. J. Appl. Biomech. 2014, 30, 262–268. [Google Scholar] [CrossRef]
- Horita, T.; Komi, P.; Nicol, C.; Kyröläinen, H. Interaction between pre-landing activities and stiffness regulation of the knee joint musculoskeletal system in the drop jump: Implications to performance. Eur. J. Appl. Physiol. 2002, 88, 76–84. [Google Scholar] [CrossRef]
- Koga, H.; Muneta, T. ACL injury mechanisms. In ACL Injury and Its Treatment; Muneta, T., Sekiya, I., Eds.; Springer: Berlin/Heidelberg, Germany, 2016; pp. 113–125. [Google Scholar] [CrossRef]
- Fan, P.; Yang, Z.; Wang, T.; Li, J.; Kim, Y.; Kim, S. Neuromuscular control strategies in basketball shooting: Distance-dependent analysis of muscle synergies. J. Sports Sci. Med. 2024, 23, 571–580. [Google Scholar] [CrossRef] [PubMed]
- Aoyama, T.; Ae, K.; Kohno, Y. Interindividual differences in upper limb muscle synergies during baseball throwing motion in male college baseball players. J. Biomech. 2022, 145, 111384. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.; Seung, H.S. Algorithms for non-negative matrix factorization. In Advances in Neural Information Processing Systems; Dietterich, T.G., Becker, S., Ghahramani, Z., Eds.; MIT Press: Cambridge, MA, USA, 2000; Volume 13, pp. 556–562. [Google Scholar]
- Li, Z.; Zhao, X.; Wang, Z.; Xu, R.; Meng, L.; Ming, D. A hierarchical classification of gestures under two force levels based on muscle synergy. Biomed. Signal Process. Control 2022, 77, 103695. [Google Scholar] [CrossRef]
- Pan, Z.; Liu, L.; Li, X.; Ma, Y. Characteristics of muscle synergy and anticipatory synergy adjustments strategy when cutting in different angles. Gait Posture 2024, 107, 114–120. [Google Scholar] [CrossRef]
- Baumgart, C.; Kurz, E.; Freiwald, J.; Hoppe, M.W. Effects of hip flexion on knee extension and flexion isokinetic angle-specific torques and Hq-ratios. Sports Med. Open 2021, 7, 41. [Google Scholar] [CrossRef]
- Stoloff, R.H.; Zehr, E.P.; Ferris, D.P. Recumbent stepping has similar but simpler neural control compared to walking. Exp. Brain Res. 2006, 178, 427–438. [Google Scholar] [CrossRef]
- Ricotta, J.M.; De, S.D.; Nardon, M.; Benamati, A.; Latash, M.L. Effects of fatigue on intramuscle force-stabilizing synergies. J. Appl. Physiol. 2023, 135, 1023–1035. [Google Scholar] [CrossRef]
- Latash, M.L. Motor Synergies and the equilibrium-point hypothesis. Mot. Control 2010, 14, 294–322. [Google Scholar] [CrossRef]
- Akl, A.-R.; Conceição, F.; Richards, J. An exploration of muscle co-activation during different walking speeds and the association with lower limb joint stiffness. J. Biomech. 2023, 157, 111715. [Google Scholar] [CrossRef]
- Shultz, S.J.; Schmitz, R.J. Effects of transverse and frontal plane knee laxity on hip and knee neuromechanics during drop landings. Am. J. Sports Med. 2009, 37, 1821–1830. [Google Scholar] [CrossRef]
- Orishimo, K.F.; Kremenic, I.J. Effect of fatigue on single-leg hop landing biomechanics. J. Appl. Biomech. 2006, 22, 245–254. [Google Scholar] [CrossRef] [PubMed]
- Gaffney, C.J.; Fomina, E.; Babich, D.; Kitov, V.; Uskov, K.; Green, D.A. The effect of long-term confinement and the efficacy of exercise countermeasures on muscle strength during a simulated mission to Mars: Data from the Mars500 study. Sports Med. Open 2017, 3, 40. [Google Scholar] [CrossRef] [PubMed]
- Edgerton, V.R.; Smith, J.L.; Simpson, D.R. Muscle fibre type populations of human leg muscles. Histochem. J. 1975, 7, 259–266. [Google Scholar] [CrossRef] [PubMed]
- Place, N.; Yamada, T.; Bruton, J.D.; Westerblad, H. Muscle fatigue: From observations in humans to underlying mechanisms studied in intact single muscle fibres. Eur. J. Appl. Physiol. 2010, 110, 1–15. [Google Scholar] [CrossRef]
- Regueme, S.C.; Nicol, C.; Barthèlemy, J.; Grélot, L. Acute and delayed neuromuscular adjustments of the triceps surae muscle group to exhaustive stretch? shortening cycle fatigue. Eur. J. Appl. Physiol. 2005, 93, 398–410. [Google Scholar] [CrossRef]
- Riemann, B.L.; DeMont, R.G.; Ryu, K.; Lephart, S.M. The effects of sex, joint angle, and the gastrocnemius muscle on passive ankle joint complex stiffness. J. Athl. Train. 2001, 36, 369–375. [Google Scholar]
- Kvist, J.; Cunningham, D.; Tigerstrand-Wejlemark, H. Gender differences in post-exercise sagittal knee translation: A comparison between elite volleyball players and swimmers. Knee 2006, 13, 132–136. [Google Scholar] [CrossRef]
- Zhai, H.; Li, C.; Xia, J.; Wei, H.; Qin, S. Integrative neuromuscular training for injury prevention of lower extremity in athletes: A meta-analysis. Chin. J. Tissue Eng. Res. 2022, 26, 2454–2460. [Google Scholar] [CrossRef]
- Belamjahad, A.; Tourny, C.; Jebabli, N.; Clark, C.C.T.; Laher, I.; Hackney, A.C.; Granacher, U.; Zouhal, H. Effects of a preseason neuromuscular training program vs. an endurance-dominated program on physical fitness and injury prevention in female soccer players. Sports Med. Open 2024, 10, 76. [Google Scholar] [CrossRef]
- Zemková, E.; Zapletalová, L. The role of neuromuscular control of postural and core stability in functional movement and athlete performance. Front. Physiol. 2022, 13, 796097. [Google Scholar] [CrossRef]
- Donelon, T.A.; Edwards, J.; Brown, M.; Jones, P.A.; O’driscoll, J.; Dos’santos, T. Differences in biomechanical determinants of Acl injury risk in change of direction tasks between males and females: A systematic review and meta-analysis. Sports Med. Open 2024, 10, 29. [Google Scholar] [CrossRef] [PubMed]
- Mejane, J.; Faubert, J.; Romeas, T.; Labbe, D.R. The combined impact of a perceptual–cognitive task and neuromuscular fatigue on knee biomechanics during landing. Knee 2019, 26, 52–60. [Google Scholar] [CrossRef]
- Steele, K.M.; Tresch, M.C.; Perreault, E.J. The number and choice of muscles impact the results of muscle synergy analyses. Front. Comput. Neurosci. 2013, 7, 105. [Google Scholar] [CrossRef] [PubMed]
- Prodromos, C.C.; Han, Y.; Rogowski, J.; Joyce, B.; Shi, K. A Meta-analysis of the incidence of anterior cruciate ligament tears as a function of gender, sport, and a knee injury–reduction regimen. Arthrosc. J. Arthrosc. Relat. Surg. 2007, 23, 1320–1325.e6. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, N.; Kunugi, S.; Mashimo, S.; Okuma, Y.; Masunari, A.; Miyazaki, S.; Hisajima, T.; Miyakawa, S. Effect of forefoot strike on lower extremity muscle activity and knee joint angle during cutting in female team handball players. Sports Med. Open 2016, 2, 32. [Google Scholar] [CrossRef]
| Age (Years) | Height (m) | Weight (kg) | Body Mass Index (kg/m²) | Body Fat Percentage (%) |
|---|---|---|---|---|
| 20.3 ± 1.5 | 1.81 ± 0.06 | 76.62 ± 5.24 | 23.29 ± 2.14 | 14.3 ± 2.9 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fan, P.; Kim, Y.; Han, D.-W.; Kim, S.; Wang, T. Alterations in the Neuromuscular Control Mechanism of the Legs During a Post-Fatigue Landing Make the Lower Limbs More Susceptible to Injury. Bioengineering 2025, 12, 233. https://doi.org/10.3390/bioengineering12030233
Fan P, Kim Y, Han D-W, Kim S, Wang T. Alterations in the Neuromuscular Control Mechanism of the Legs During a Post-Fatigue Landing Make the Lower Limbs More Susceptible to Injury. Bioengineering. 2025; 12(3):233. https://doi.org/10.3390/bioengineering12030233
Chicago/Turabian StyleFan, Penglei, Youngsuk Kim, Dong-Wook Han, Sukwon Kim, and Ting Wang. 2025. "Alterations in the Neuromuscular Control Mechanism of the Legs During a Post-Fatigue Landing Make the Lower Limbs More Susceptible to Injury" Bioengineering 12, no. 3: 233. https://doi.org/10.3390/bioengineering12030233
APA StyleFan, P., Kim, Y., Han, D.-W., Kim, S., & Wang, T. (2025). Alterations in the Neuromuscular Control Mechanism of the Legs During a Post-Fatigue Landing Make the Lower Limbs More Susceptible to Injury. Bioengineering, 12(3), 233. https://doi.org/10.3390/bioengineering12030233







