Mechanical Stretch Control of Adipocyte AKT Signaling and the Role of FAK and ROCK Mechanosensors
Abstract
1. Introduction
2. Materials and Methods
2.1. Adipocyte Culture and Mechanical Stretch Loading
2.2. Immunoblotting
2.3. Inhibitors
2.4. Cell Surface Biotinylation
2.5. Immunofluorescence and Analysis
2.6. Statistics
3. Results
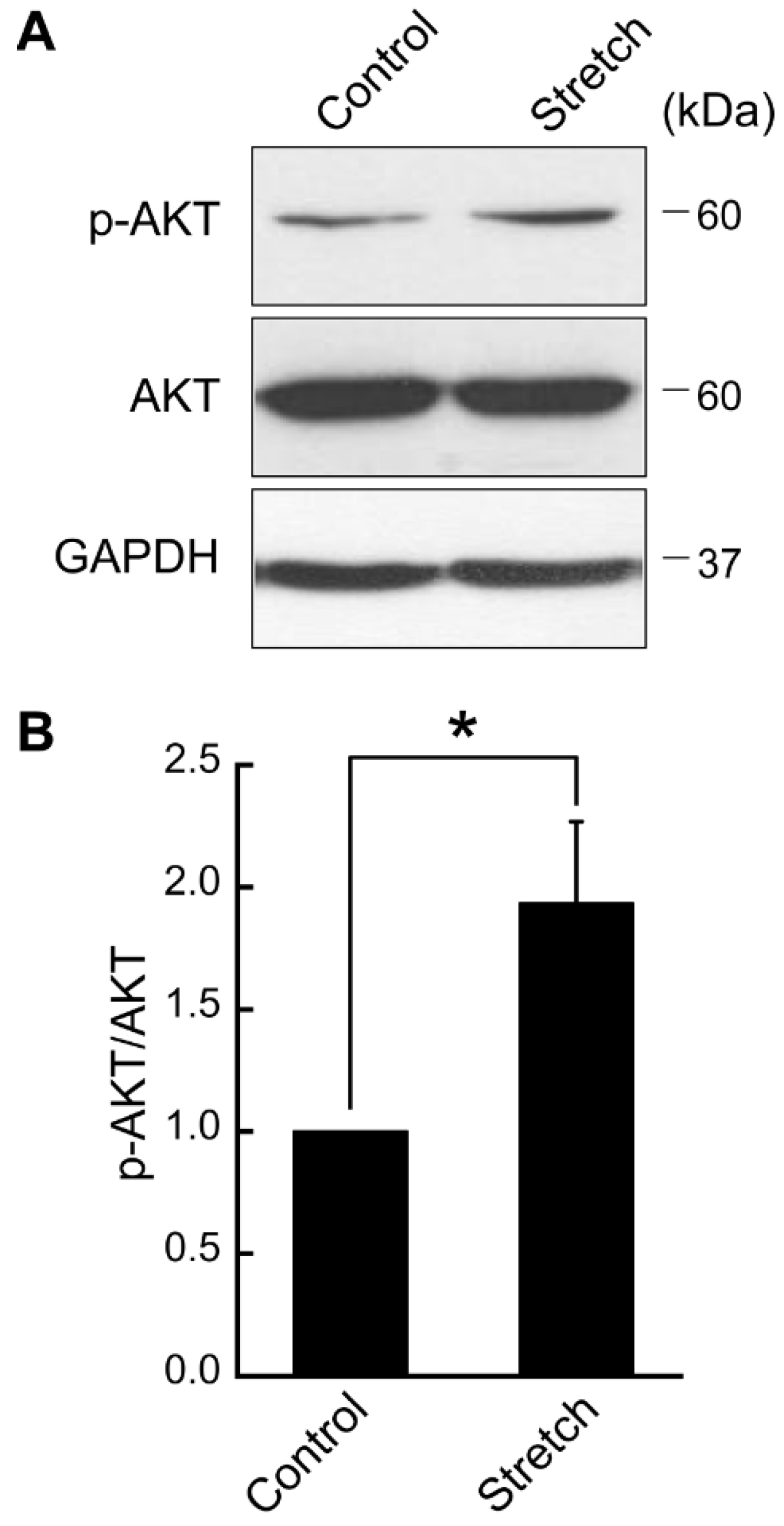
3.1. Cyclic Stretch Loading Upregulates AKT Phosphorylation in Differentiated Adipocytes
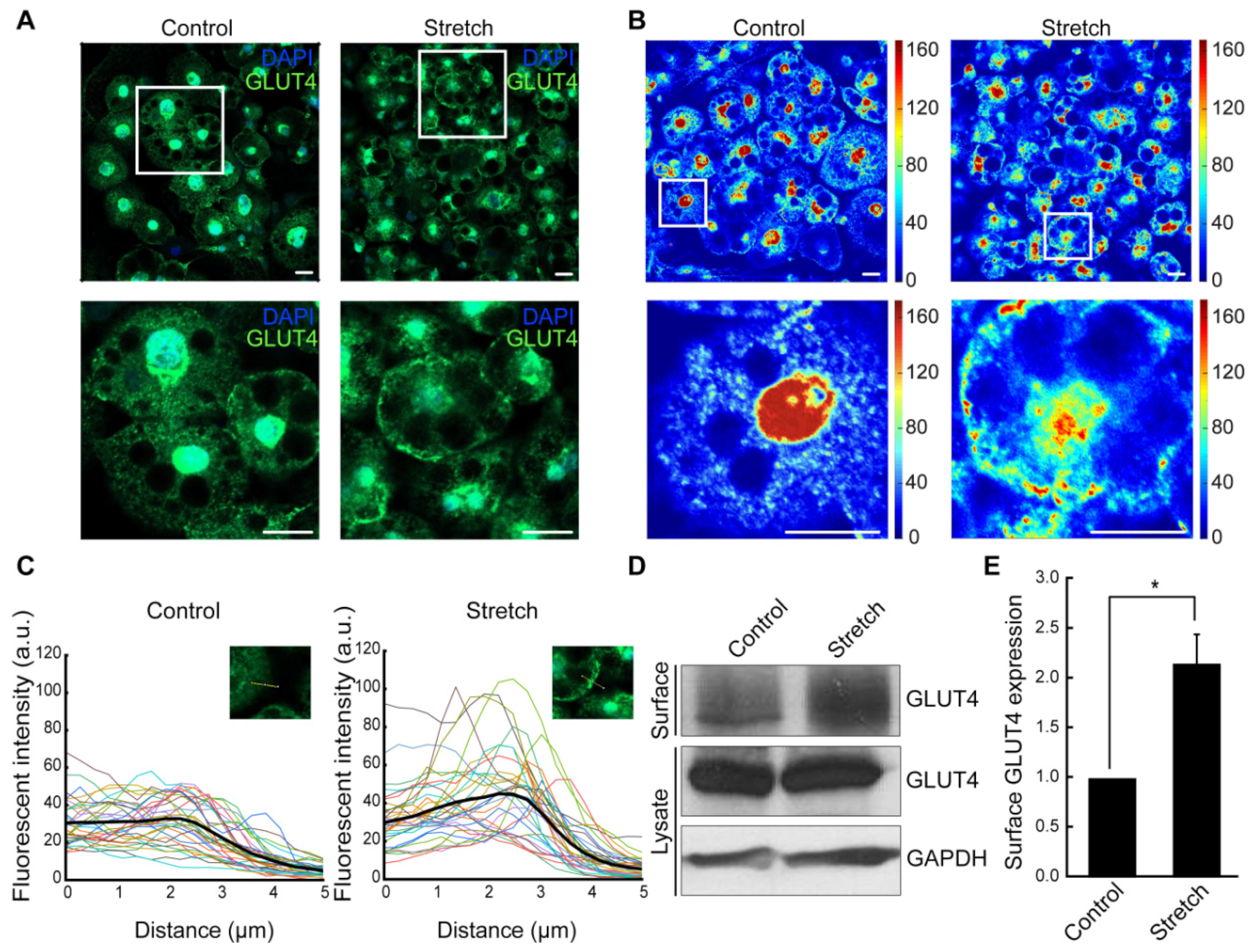
3.2. Cyclic Stretching Induces GLUT4 Translocation to the Plasma Membrane in Adipocytes
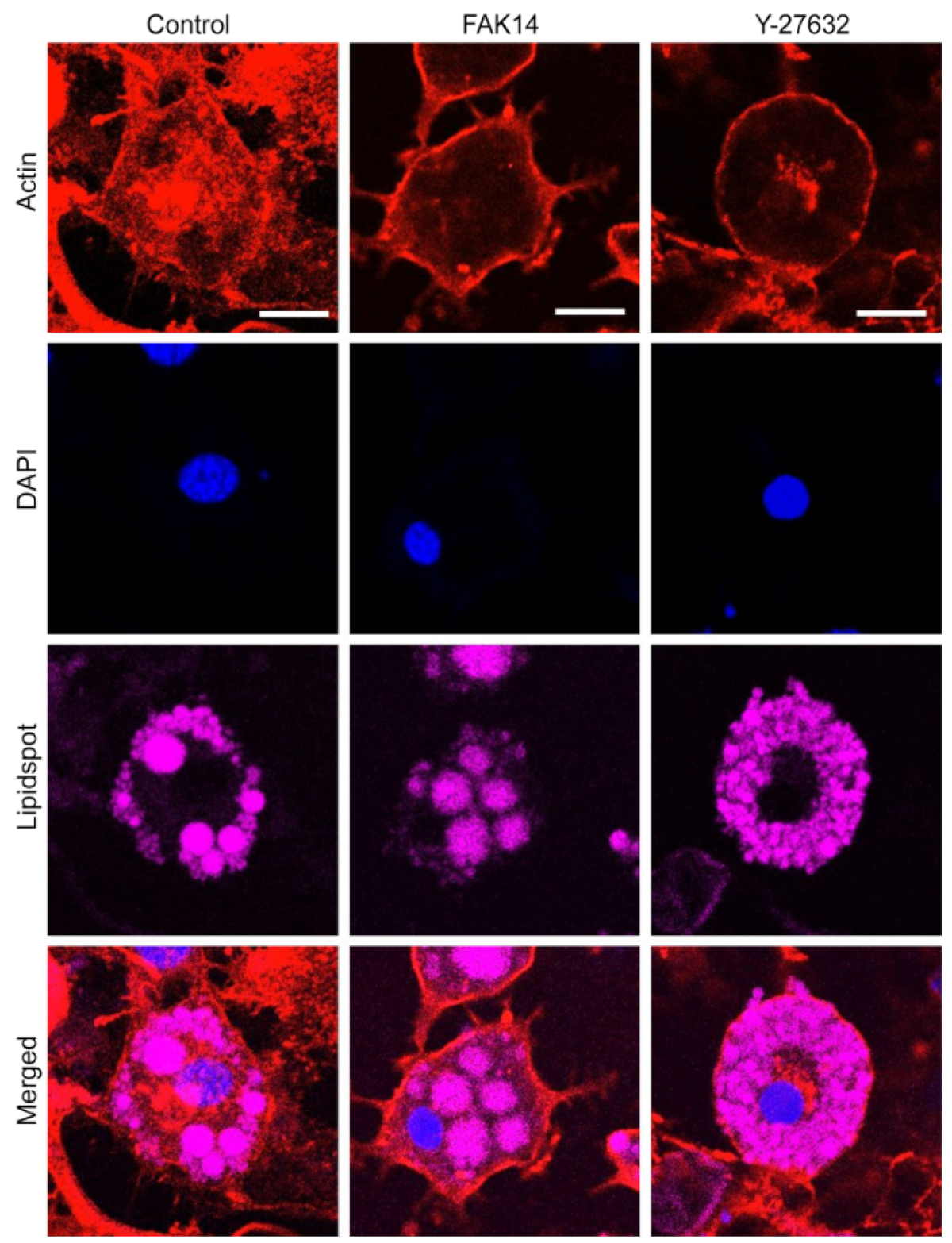
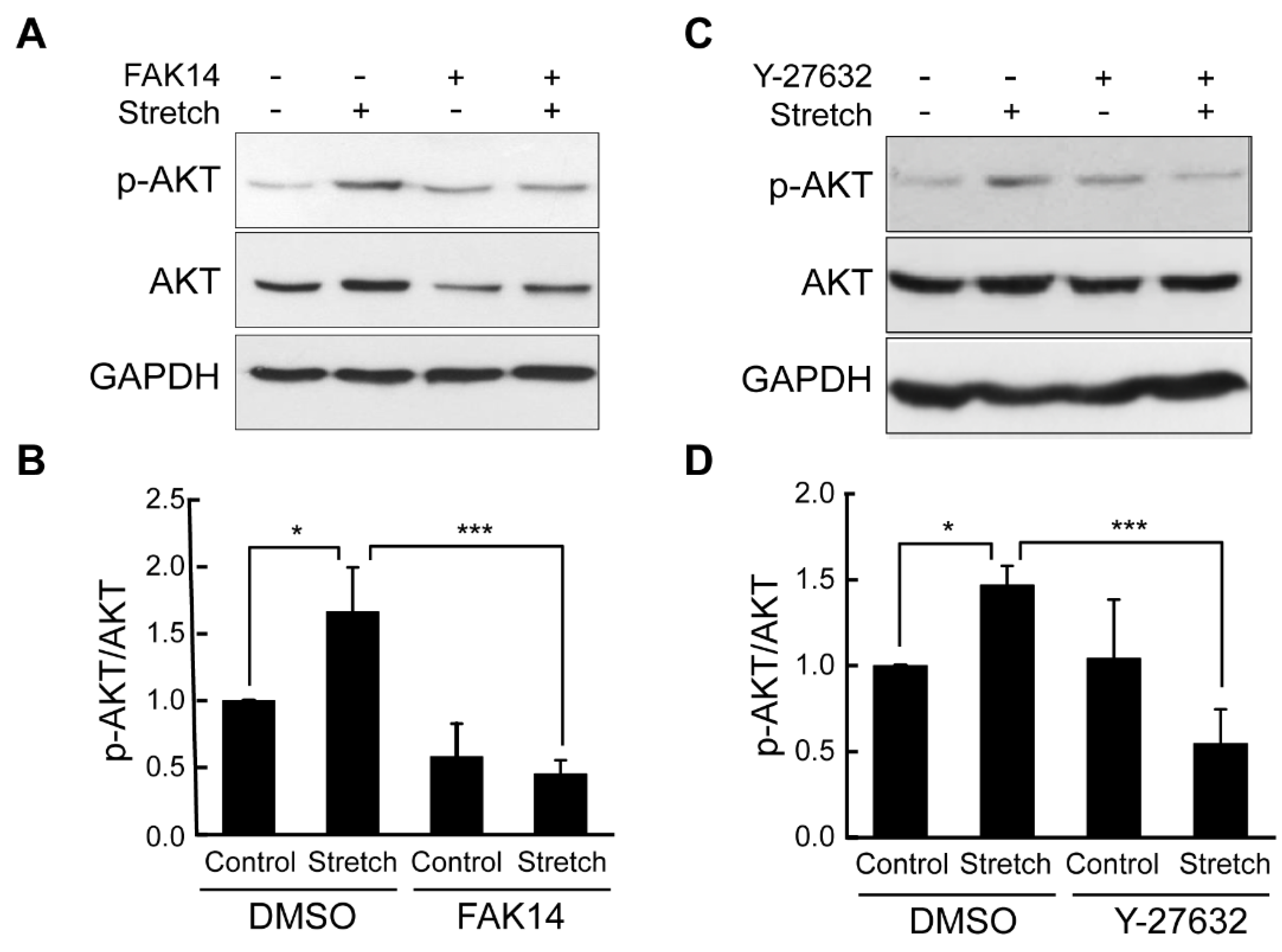
3.3. FAK and ROCK Regulate Actin Development and Mechanical Stretch Loading-Induced AKT Activation in Adipocytes
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Centers for Disease Control and Prevention. National Diabetes Statistics Report. 2020. Available online: https://www.cdc.gov/diabetes/php/data-research/index.html (accessed on 1 March 2020).
- Smushkin, G.; Vella, A. What is type 2 diabetes? Medicine 2010, 38, 597–601. [Google Scholar] [CrossRef] [PubMed]
- Cho, H.; Thorvaldsen, J.L.; Chu, Q.; Feng, F.; Birnbaum, M.J. Akt1/PKBalpha is required for normal growth but dispensable for maintenance of glucose homeostasis in mice. J. Biol. Chem. 2001, 276, 38349–38352. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.; Lee, H.; Cho, Y.M.; Kwon, O.J.; Kim, W.; Lee, E.K. TNFα-induced miR-130 resulted in adipocyte dysfunction during obesity-related inflammation. FEBS Lett. 2013, 587, 3853–3858. [Google Scholar] [CrossRef] [PubMed]
- Sun, K.; Tordjman, J.; Clément, K.; Scherer, P.E. Fibrosis and adipose tissue dysfunction. Cell Metab. 2013, 18, 470–477. [Google Scholar] [CrossRef]
- Shoham, N.; Gefen, A. Mechanotransduction in adipocytes. J. Biomech. 2012, 45, 1–8. [Google Scholar] [CrossRef]
- David, V.; Martin, A.; Lafage-Proust, M.H.; Malaval, L.; Peyroche, S.; Jones, D.B.; Vico, L.; Guignandon, A. Mechanical loading down-regulates peroxisome proliferator-activated receptor gamma in bone marrow stromal cells and favors osteoblastogenesis at the expense of adipogenesis. Endocrinology 2007, 148, 2553–2562. [Google Scholar] [CrossRef]
- Lee, J.S.; Ha, L.; Park, J.H.; Lim, J.Y. Mechanical stretch suppresses BMP4 induction of stem cell adipogenesis via upregulating ERK but not through downregulating Smad or p38. Biochem. Biophys. Res. Commun. 2012, 418, 278–283. [Google Scholar] [CrossRef]
- Linder-Ganz, E.; Shabshin, N.; Itzchak, Y.; Gefen, A. Assessment of mechanical conditions in sub-dermal tissues during sitting: A combined experimental-MRI and finite element approach. J. Biomech. 2007, 40, 1443–1454. [Google Scholar] [CrossRef]
- Bouzid, T.; Esfahani, A.M.; Safa, B.T.; Kim, E.; Saraswathi, V.; Kim, J.K.; Yang, R.; Lim, J.Y. Rho/ROCK mechanosensor in adipocyte stiffness and traction force generation. Biochem. Biophys. Res. Commun. 2022, 606, 42–48. [Google Scholar] [CrossRef]
- Dutta, D.; Ziemke, M.; Sindelar, P.; Vargas, H.; Lim, J.Y.; Chandra, S. Cytoarchitecture of breast cancer cells under diabetic conditions: Role of regulatory kinases—Rho kinase and focal adhesion kinase. Cancers 2024, 16, 3166. [Google Scholar] [CrossRef]
- Choi, Y.O.; Ryu, H.J.; Kim, H.R.; Song, Y.S.; Kim, C.; Lee, W.; Choe, H.; Leem, C.H.; Jang, Y.J. Implication of phosphorylation of the myosin II regulatory light chain in insulin-stimulated GLUT4 translocation in 3T3-F442A adipocytes. Exp. Mol. Med. 2006, 38, 180–189. [Google Scholar] [CrossRef] [PubMed]
- Guilherme, A.; Emoto, M.; Buxton, J.M.; Bose, S.; Sabini, R.; Theurkauf, W.E.; Leszyk, J.; Czech, M.P. Perinuclear localization and insulin responsiveness of GLUT4 requires cytoskeletal integrity in 3T3-L1 adipocytes. J. Biol. Chem. 2000, 275, 38151–38159. [Google Scholar] [CrossRef] [PubMed]
- Olson, A.L. Regulation of GLUT4 and insulin-dependent glucose flux. ISRN Mol. Biol. 2012, 2012, 856987. [Google Scholar] [CrossRef] [PubMed]
- Mackenzie, R.W.; Elliott, B.T. Akt/PKB activation and insulin signaling: A novel insulin signaling pathway in the treatment of type 2 diabetes. Diabetes Metab. Syndr. Obes. 2014, 7, 55–64. [Google Scholar] [CrossRef]
- Sasai, N.; Agata, N.; Inoue-Miyazu, M.; Kawakami, K.; Kobayashi, K.; Sokabe, M.; Hayakawa, K. Involvement of PI3K/Akt/TOR pathway in stretch-induced hypertrophy of myotubes. Muscle Nerve 2010, 41, 100–106. [Google Scholar] [CrossRef]
- Sakamoto, K.; Aschenbach, W.G.; Hirshman, M.F.; Goodyear, L.J. Akt signaling in skeletal muscle: Regulation by exercise and passive stretch. Am. J. Physiol. Endocrinol. Metab. 2003, 285, E1081–E1088. [Google Scholar] [CrossRef]
- Wei, B.; Chen, Z.; Zhang, X.; Feldman, M.; Dong, X.Z.; Doran, R.; Zhao, B.L.; Yin, W.X.; Kotlikoff, M.I.; Ji, G. Nitric oxide mediates stretch-induced Ca2+ release via activation of phosphatidylinositol 3-kinase-Akt pathway in smooth muscle. PLoS ONE 2008, 3, e2526. [Google Scholar] [CrossRef]
- Sen, B.; Guilluy, C.; Xie, Z.; Case, N.; Styner, M.; Thomas, J.; Oguz, I.; Rubin, C.; Burridge, K.; Rubin, J. Mechanically induced focal adhesion assembly amplifies anti-adipogenic pathways in mesenchymal stem cells. Stem Cells 2011, 29, 1829–1836. [Google Scholar] [CrossRef]
- Liu, X.M.; Ensenat, D.; Wang, H.; Schafer, A.I.; Durante, W. Physiologic cyclic stretch inhibits apoptosis in vascular endothelium. FEBS Lett. 2003, 541, 52–56. [Google Scholar] [CrossRef]
- Mao, P.; Li, J.; Huang, Y.; Wu, S.; Pang, X.; He, W.; Liu, X.; Slutsky, A.S.; Zhang, H.; Li, Y. MicroRNA-19b mediates lung epithelial-mesenchymal transition via phosphatidylinositol-3,4,5-trisphosphate 3-phosphatase in response to mechanical stretch. Am. J. Respir. Cell Mol. Biol. 2017, 56, 11–19. [Google Scholar] [CrossRef]
- Tsakiridis, T.; Wang, Q.; Taha, C.; Grinstein, S.; Downey, G.; Klip, A. Involvement of the actin network in insulin signalling. Soc. Gen. Physiol. Ser. 1997, 52, 257–271. [Google Scholar] [PubMed]
- Wang, Q.; Bilan, P.J.; Tsakiridis, T.; Hinek, A.; Klip, A. Actin filaments participate in the relocalization of phosphatidylinositol3-kinase to glucose transporter-containing compartments and in the stimulation of glucose uptake in 3T3-L1 adipocytes. Biochem. J. 1998, 331, 917–928. [Google Scholar] [CrossRef] [PubMed]
- Peyrollier, K.; Hajduch, E.; Gray, A.; Litherland, G.J.; Prescott, A.R.; Leslie, N.R.; Hundal, H.S. A role for the actin cytoskeleton in the hormonal and growth-factor-mediated activation of protein kinase B. Biochem. J. 2000, 352, 617–622. [Google Scholar] [CrossRef] [PubMed]
- Park, E.H.; Kang, S.S.; Lee, Y.S.; Kim, S.J.; Jin, E.J.; Tak, E.N.; Sonn, J.K. Integrity of the cortical actin ring is required for activation of the PI3K/Akt and p38 MAPK signaling pathways in redifferentiation of chondrocytes on chitosan. Cell Biol. Int. 2008, 32, 1272–1278. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.S.; Ha, L.; Kwon, I.K.; Lim, J.Y. The role of focal adhesion kinase in BMP4 induction of mesenchymal stem cell adipogenesis. Biochem. Biophys. Res. Commun. 2013, 435, 696–701. [Google Scholar] [CrossRef]
- Huang, D.; Khoe, M.; Ilic, D.; Bryer-Ash, M. Reduced expression of focal adhesion kinase disrupts insulin action in skeletal muscle cells. Endocrinology 2006, 147, 3333–3343. [Google Scholar] [CrossRef]
- Bisht, B.; Srinivasan, K.; Dey, C.S. In vivo inhibition of focal adhesion kinase causes insulin resistance. J. Physiol. 2008, 586, 3825–3837. [Google Scholar] [CrossRef]
- Gupta, A.; Bisht, B.; Dey, C.S. Focal adhesion kinase negatively regulates neuronal insulin resistance. Biochim. Biophys. Acta 2012, 1822, 1030–1037. [Google Scholar] [CrossRef]
- Luk, C.T.; Shi, S.Y.; Cai, E.P.; Sivasubramaniyam, T.; Krishnamurthy, M.; Brunt, J.J.; Schroer, S.A.; Winer, D.A.; Woo, M. FAK signalling controls insulin sensitivity through regulation of adipocyte survival. Nat. Commun. 2017, 8, 14360. [Google Scholar] [CrossRef]
- Ruiz-Ojeda, F.J.; Wang, J.; Bäcker, T.; Krueger, M.; Zamani, S.; Rosowski, S.; Gruber, T.; Onogi, Y.; Feuchtinger, A.; Schulz, T.J.; et al. Active integrins regulate white adipose tissue insulin sensitivity and brown fat thermogenesis. Mol. Metab. 2021, 45, 101147. [Google Scholar] [CrossRef]
- Andalib, M.N.; Lee, J.S.; Ha, L.; Dzenis, Y.; Lim, J.Y. The role of RhoA kinase (ROCK) in cell alignment on nanofibers. Acta Biomater. 2013, 9, 7737–7745. [Google Scholar] [CrossRef] [PubMed]
- Furukawa, N.; Ongusaha, P.; Jahng, W.J.; Araki, K.; Choi, C.S.; Kim, H.J.; Lee, Y.H.; Kaibuchi, K.; Kahn, B.B.; Masuzaki, H.; et al. Role of Rho-kinase in regulation of insulin action and glucose homeostasis. Cell Metab. 2005, 2, 119–129. [Google Scholar] [CrossRef] [PubMed]
- Duong, K.H.M.; Chun, K.H. Regulation of glucose transport by RhoA in 3T3-L1 adipocytes and L6 myoblasts. Biochem. Biophys. Res. Commun. 2019, 519, 880–886. [Google Scholar] [CrossRef] [PubMed]
- Kao, Y.H.; Chen, P.H.; Sun, C.K.; Chang, Y.C.; Lin, Y.C.; Tsai, M.S.; Lee, P.H.; Cheng, C.I. Cyclic mechanical stretch up-regulates hepatoma-derived growth factor expression in cultured rat aortic smooth muscle cells. Biosci. Rep. 2018, 38, BSR20171398. [Google Scholar] [CrossRef] [PubMed]
- Teramura, T.; Takehara, T.; Onodera, Y.; Nakagawa, K.; Hamanishi, C.; Fukuda, K. Mechanical stimulation of cyclic tensile strain induces reduction of pluripotent related gene expressions via activation of Rho/ROCK and subsequent decreasing of AKT phosphorylation in human induced pluripotent stem cells. Biochem. Biophys. Res. Commun. 2012, 417, 836–841. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bouzid, T.; Kim, E.; Riehl, B.D.; Yang, R.; Saraswathi, V.; Kim, J.K.; Lim, J.Y. Mechanical Stretch Control of Adipocyte AKT Signaling and the Role of FAK and ROCK Mechanosensors. Bioengineering 2024, 11, 1279. https://doi.org/10.3390/bioengineering11121279
Bouzid T, Kim E, Riehl BD, Yang R, Saraswathi V, Kim JK, Lim JY. Mechanical Stretch Control of Adipocyte AKT Signaling and the Role of FAK and ROCK Mechanosensors. Bioengineering. 2024; 11(12):1279. https://doi.org/10.3390/bioengineering11121279
Chicago/Turabian StyleBouzid, Tasneem, Eunju Kim, Brandon D. Riehl, Ruiguo Yang, Viswanathan Saraswathi, Jason K. Kim, and Jung Yul Lim. 2024. "Mechanical Stretch Control of Adipocyte AKT Signaling and the Role of FAK and ROCK Mechanosensors" Bioengineering 11, no. 12: 1279. https://doi.org/10.3390/bioengineering11121279
APA StyleBouzid, T., Kim, E., Riehl, B. D., Yang, R., Saraswathi, V., Kim, J. K., & Lim, J. Y. (2024). Mechanical Stretch Control of Adipocyte AKT Signaling and the Role of FAK and ROCK Mechanosensors. Bioengineering, 11(12), 1279. https://doi.org/10.3390/bioengineering11121279




