Scoping Meta-Review of Methods Used to Assess Artificial Intelligence-Based Medical Devices for Heart Failure
Abstract
:1. Introduction
2. Methods
2.1. PICO and Eligibility Criteria
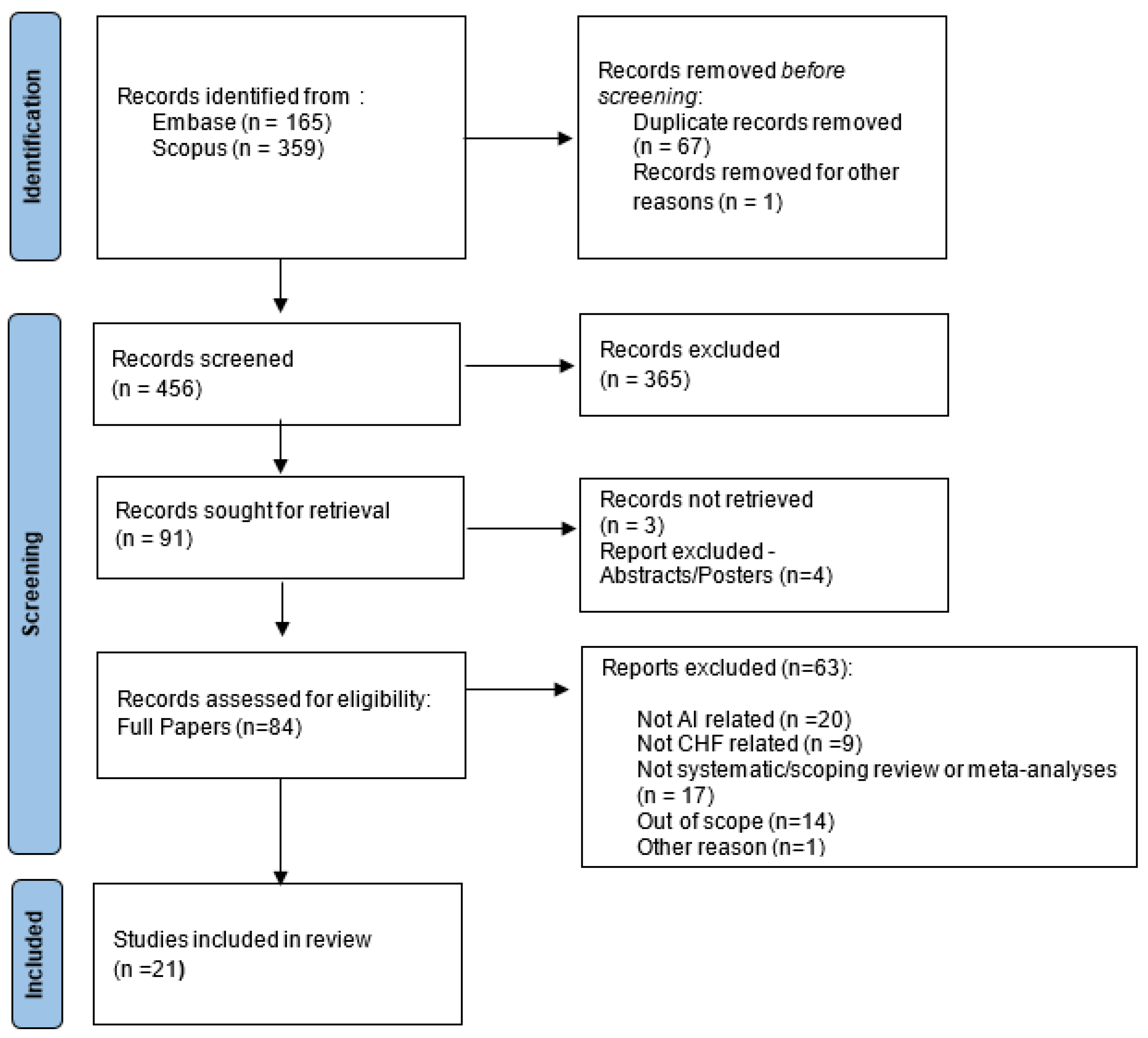
2.2. Identification and Screening
2.3. Data Extraction and Analysis
3. Results
3.1. Selected Papers
3.2. Clinical Aspects
3.3. AI/ML Algorithms
3.4. Performance, Effectiveness, and Safety
4. Discussion
4.1. Key Findings
- Generalisability and representativeness. The majority of the retrieved systematic reviews mainly considered cases in developed countries (Figure 2), with an associated risk of discrimination and lack of representativeness. From an HTA perspective, this has consequences on the generalisability of both the trial results to other geographical areas and the performance of AI/ML algorithms to other populations of patients, not included in the data sources employed to develop the algorithms. This limits not only the recommendation an HTA can provide but also its transferability to other settings [53].
- Quality of available evidence. Guidelines for reporting trials that evaluate interventions are increasingly used when it comes to modelling the impact of AI-driven technologies. For instance, TRIPOD-AI [27] was developed to predict models, STARD-AI [28] was developed for diagnostic accuracy studies, and SPIRIT-AI [11] and CONSORT-AI [29] were developed for randomised controlled studies. Recently, the Developmental and Exploratory Clinical Investigations of DEcision support systems driven by Artificial Intelligence (DECIDE-AI) approach [30] was proposed. This approach aims to improve the reporting of early-stage clinical evaluations of AI-based technologies, independently of the study design chosen. We encountered both a lack of attention and variability in reporting quality assessment in reviews on AI/ML algorithms for HF, as well as a lack of agreement on which criteria/scale should be adopted to investigate quality. This is in line with the observation by Shazad et al. [54], who highlighted that the quality of reporting of randomised controlled trials in AI is suboptimal. It is also in line with the finding of Plana et al. [55], who reported high variability in adherence to reporting standards. At the same time, available tools adapted to AI are not yet fully able to capture the peculiarities of AI/ML algorithms and trials. As an immediate consequence, practitioners should interpret with caution the findings of studies regarding AI/ML algorithms for HF.
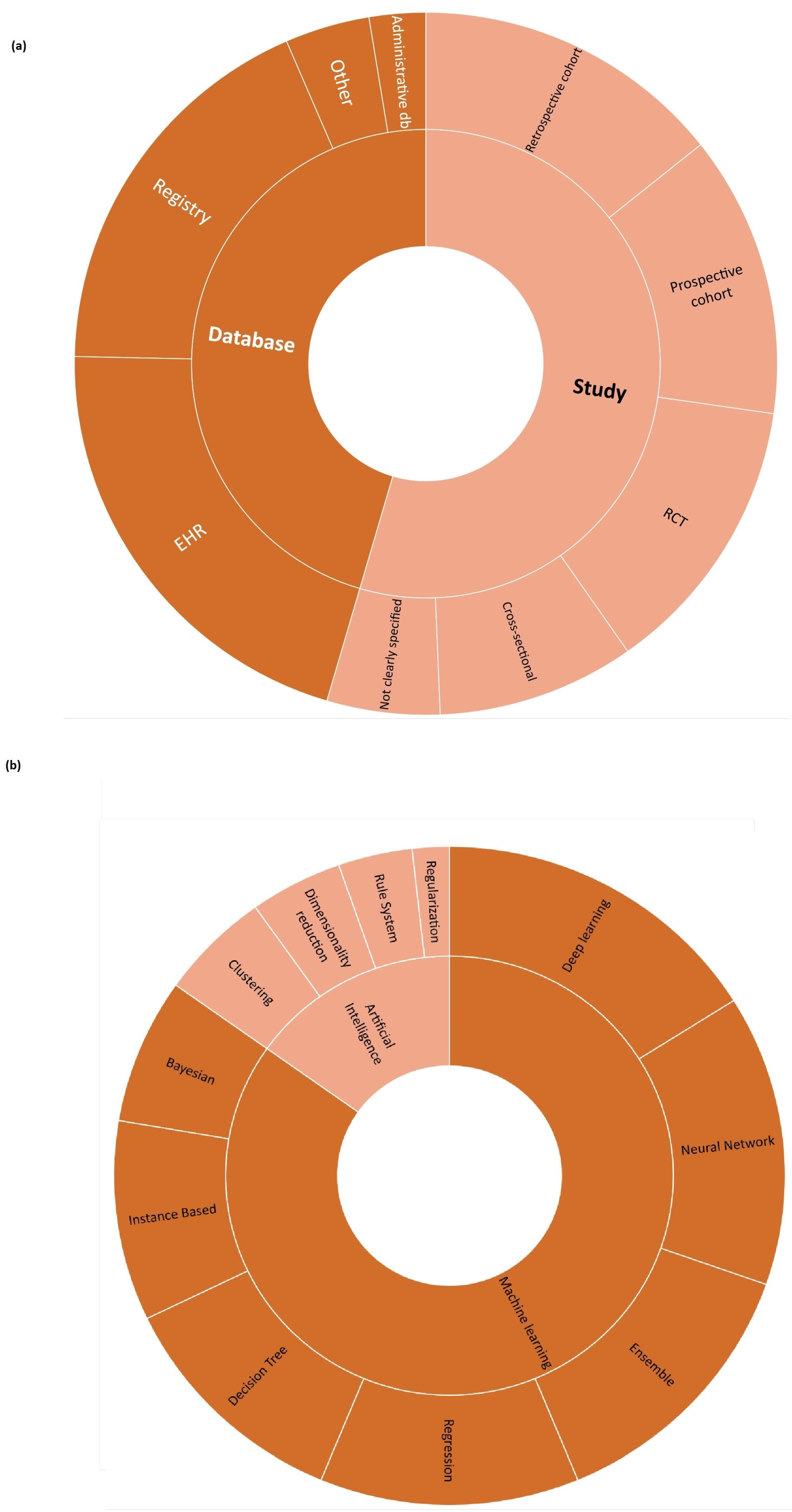
- AI/ML methods. Different models are currently being developed to manage HF, but no guidelines are available for assessors to investigate in detail the reliability of each algorithm and capture the added value of one AI/ML model in comparison to others. Given the long list of methods currently used, as shown in Figure 4, the HTA is neither able to select the most appropriate comparators nor conduct a comparative assessment of AI/ML algorithms.
- Comparative evidence. Only a small proportion of studies evaluated AI/ML algorithms without conducting any kind of comparison. This is a promising result (Figure 5). However, the preferred comparator was not current clinical practice, as requested by the HTA, but rather other AI/ML models or other statistical methods. As occurs with any expected disruptive technology, the choice of the comparator is not easy. It is not just a new active principle or MD, AI/ML promises to be a new paradigm, able to redefine clinical pathways. In this case, direct or indirect comparisons with current clinical practice are even more important and necessary.
- Data sources. Last but not least, the data at the core of AI/ML algorithms are crucial. They are usually real-world data/evidence (RWD/RWE), which are becoming more and more relevant for the HTA and decision makers. While investigating the complexity of AI for the HTA, Alami et al. [14] mentioned not only data quality and representativeness but also fragmented and unstructured data coming from different sources. It becomes clear how that adds complexity to a scenario where the role of RWD/RWE and issues such as real-world data availability, governance, and quality are not fully addressed [56].
4.2. Strengths and Limitations
4.3. Further Development
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| AI/ML | Artificial intelligence/machine learning |
| AUC/ROC | Area under the ROC curve |
| EHR | Electronic Health Records |
| HF | Heart failure |
| HTA | Health Technology Assessment |
| MD | Medical device |
| MDSW | Medical device software |
| RCT | Randomised controlled trial |
Appendix A
Appendix A.1. Search String—Embase
- 1 “heart failure”/
- 2 cardiomyopathy, dilated/
- 3 shock, cardiogenic/
- 4 exp ventricular dysfunction/
- 5 cardiac output, low/
- 6 ((heart or cardiac or coronary or myocardial) adj2 (failure or decompensation or death or incompetence or insufficiency)).ti,ab.
- 7 ((dilated or congestive) adj2 cardiomyopath*).ti,ab.
- 8 cardiogenic shock.ti,ab.
- 9 ((ventricular or ventricle*) adj2 (failure or insufficien* or dysfunction*)).ti,ab.
- 10 ((left ventricular or left ventricle) adj2 (failure or insufficien* or dysfunction*)).ti,ab.
- 11 lvsd.ti,ab.
- 12 scd.ti,ab.
- 13 scd.ti,ab.
- 14 hf.ti,ab.
- 15 chf.ti,ab.
- 16 or/1–15
- 17 artificial intelligence/
- 18 model,neural network/
- 19 models, neural network/
- 20 neural network model/
- 21 neural network models/
- 22 neural networks computer/
- 23 neural network computer/
- 24 Computational Intelligence/
- 25 Natural Language Processing/
- 26 (deep learn* or machine learn* or continuous learn*).ti,ab.
- 27 “neural network*”.ti,ab.
- 28 ((Artificial or Comput* or Machine) adj1 Intelligence).ti,ab.
- 29 Natural Language Processing*.ti,ab.
- 30 Computer Vision*.ti,ab.
- 31 or/17–30
- 32 16 and 31
- 33 (systematic review or meta-analysis).pt.
- 34 meta-analysis/ or systematic review/ or systematic reviews as topic/ or meta-analysis as topic/ or “meta analysis (topic)”/ or “systematic review (topic)”/ or exp technology assessment, biomedical/ or network meta-analysis/
- 35 ((systematic* adj3 (review* or overview*)) or (methodologic* adj3 (review* or overview*))). ti,ab,kf,kw.
- 36 ((quantitative adj3 (review* or overview* or synthes*)) or (research adj3 (integrati* or overview*))).ti,ab,kf,kw.
- 37 ((integrative adj3 (review* or overview*)) or (collaborative adj3 (review* or overview*)) or (pool* adj3 analy*)).ti,ab,kf,kw.
- 38 (data synthes* or data extraction* or data abstraction*).ti,ab,kf,kw.
- 39 (handsearch* or hand search*).ti,ab,kf,kw.
- 40 (mantel haenszel or peto or der simonian or dersimonian or fixed effect* or latin square*).ti,ab,kf,kw.
- 41 (met analy* or metanaly* or technology assessment* or HTA or HTAs or technology overview* or technology appraisal*).ti,ab,kf,kw.
- 42 (meta regression* or metaregression*).ti,ab,kf,kw.
- 43 (meta-analy* or metaanaly* or systematic review* or biomedical technology assessment* or bio-medical technology assessment*).mp,hw.
- 44 (medline or cochrane or pubmed or medlars or embase or cinahl).ti,ab,hw.
- 45 (cochrane or (health adj2 technology assessment) or evidence report).jw.
- 46 (comparative adj3 (efficacy or effectiveness)).ti,ab,kf,kw.
- 47 (outcomes research or relative effectiveness).ti,ab,kf,kw.
- 48 ((indirect or indirect treatment or mixed-treatment or bayesian) adj3 comparison*).ti,ab,kf,kw.
- 49 [(meta-analysis or systematic review).md.]
- 50 (multi* adj3 treatment adj3 comparison*).ti,ab,kf,kw.
- 51 (mixed adj3 treatment adj3 (meta-analy* or metaanaly*)).ti,ab,kf,kw.
- 52 umbrella review*.ti,ab,kf,kw.
- 53 (multi* adj2 paramet* adj2 evidence adj2 synthesis).ti,ab,kw,kf.
- 54 (multiparamet* adj2 evidence adj2 synthesis).ti,ab,kw,kf.
- 55 (multi-paramet* adj2 evidence adj2 synthesis).ti,ab,kw,kf.
- 56 or/33–55
- 57 56 and 32
- 58 remove duplicates from 57
- 59 limit 58 to yr=”2015 -Current”
Appendix A.2. Search String—Scopus
- 1 TITLE-ABS-KEY (“heart failure” OR “Cardiac Failure” OR “Heart Decompensation” OR “Decompensation, Heart” OR “Heart Failure, Right-Sided” OR “Heart Failure, Right Sided” OR “Right-Sided Heart Failure” OR “Right Sided Heart Failure” OR “Myocardial Failure” OR “Congestive Heart Failure” OR “Heart Failure, Congestive” OR “Heart Failure, Left-Sided” OR “Heart Failure, Left Sided” OR “Left-Sided Heart Failure” OR “Left Sided Heart Failure”)
- 2 TITLE-ABS-KEY (“cardiomyopathy, dilated” OR “Cardiomyopathies, Dilated” OR “Dilated Cardiomyopathies” OR “Dilated Cardiomyopathy” OR “Cardiomyopathy, Familial Idiopathic” OR “Cardiomyopathies, Familial Idiopathic” OR “Familial Idiopathic Cardiomyopathies” OR “Familial Idiopathic Cardiomyopathy” OR “Idiopathic Cardiomyopathies, Familial” OR “Idiopathic Cardiomyopathy, Familial” OR “Congestive Cardiomyopathy” OR “Cardiomyopathies, Congestive” OR “Congestive Cardiomyopathies” OR “Cardiomyopathy, Congestive” OR “Cardiomyopathy, Idiopathic Dilated” OR “Cardiomyopathies, Idiopathic Dilated” OR “Dilated Cardiomyopathies, Idiopathic” OR “Dilated Cardiomyopathy, Idiopathic” OR “Idiopathic Dilated Cardiomyopathies” OR “Idiopathic Dilated Cardiomyopathy” OR “Cardiomyopathy, Dilated, LMNA” OR “Cardiomyopathy, Dilated, Autosomal Recessive” OR “Cardiomyopathy, Dilated, 1a” OR “Cardiomyopathy, Dilated, With Conduction Defect 1” OR “Cardiomyopathy, Dilated, with Conduction Deffect1” OR “Cardiomyopathy, Dilated, CMD1A”)
- 3 TITLE-ABS-KEY (“shock, cardiogenic” OR “Cardiogenic Shock”)
- 4 TITLE-ABS-KEY (“ventricular dysfunction” OR “Dysfunction, Ventricular” OR “Dysfunctions, Ventricular” OR “Ventricular Dysfunctions” OR “Right Ventricular Dysfunction” OR “Dysfunction, Right Ventricular” OR “Dysfunctions, Right Ventricular” OR “Right Ventricular Dysfunctions” OR “Ventricular Dysfunctions, Right” OR “Left Ventricular Dysfunction” OR “Dysfunction, Left Ventricular” OR “Dysfunctions, Left Ventricular” OR “Left Ventricular Dysfunctions” OR “Ventricular Dysfunctions, Left”)
- 5 TITLE-ABS-KEY (“cardiac output, low” OR “Output, Low Cardiac” OR “Low Cardiac Output” OR “Low Cardiac Output Syndrome”)
- 6 TITLE-ABS ((heart or cardiac or coronary or myocardial) W/2 (failure or decompensation or death or incompetence or insufficiency))
- 7 TITLE-ABS ((dilated or congestive) W/2 cardiomyopath!)
- 8 TITLE-ABS (“cardiogenic shock”)
- 9 TITLE-ABS ((ventricular or ventricle!) W/2 (failure or insufficien! or dysfunction!))
- 10 TITLE-ABS ((“left ventricular” or “left ventricle”) W/2 (failure or insufficien! or dysfunction!))
- 11 TITLE-ABS (lvsd)
- 12 TITLE-ABS (scd)
- 13 TITLE-ABS (scd)
- 14 TITLE-ABS (hf)
- 15 TITLE-ABS (chf)
- 16 or/1–15
- 17 TITLE-ABS-KEY (“artificial intelligence” OR “Intelligence, Artificial” OR “Computational Intelligence” OR “Intelligence, Computational” OR “Machine Intelligence” OR “Intelligence, Machine” OR “Computer Reasoning” OR “Reasoning, Computer” OR “AI (Artificial Intelligence)” OR “Computer Vision Systems” OR “Computer Vision System” OR “System, Computer Vision” OR “Systems, Computer Vision” OR “Vision System, Computer” OR “Vision Systems, Computer” OR “Knowledge Acquisition (Computer)” OR “Acquisition, Knowledge (Computer)” OR “Knowledge Representation (Computer)” OR “Knowledge Representations (Computer)” OR “ Representation, Knowledge (Computer)”)
- 18 TITLE-ABS-KEY (“model,neural network” OR “Computer Neural Network” OR “Computer Neural Networks” OR “Network, Computer Neural” OR “Networks, Computer Neural” OR “Neural Network, Computer” OR “Models, Neural Network” OR “Model, Neural Network” OR “Network Model, Neural” OR “Network Models, Neural” OR “Neural Network Model” OR “Neural Network Models” OR “Computational Neural Networks” OR “Computational Neural Network” OR “Network, Computational Neural” OR “Networks, Computational Neural” OR “Neural Network, Computational” OR “Neural Networks, Computational” OR “Perceptrons” OR “Perceptron” OR “Connectionist Models” OR “Connectionist Model” OR “Model, Connectionist” OR “Models, Connectionist” OR “Neural Networks (Computer)” OR “Network, Neural (Computer)” OR “ Networks, Neural (Computer)” OR “Neural Network (Computer)”)
- 19 TITLE-ABS-KEY (“Natural Language Processing” OR “Language Processing, Natural” OR “Language Processings, Natural” OR “Natural Language Processings” OR “Processing, Natural Language” OR “Processings, Natural Language” )
- 20 TITLE-ABS (“deep learn!” or “machine learn!” or “continuous learn!”)
- 21 TITLE-ABS (“neural network!”)
- 22 TITLE-ABS ((Artificial or Comput! or Machine) W/1 Intelligence)
- 23 TITLE-ABS (“Natural Language Processing!”)
- 24 TITLE-ABS (“Computer Vision!”)
- 25 or/17–24
- 26 16 and 25
- 27 (“systematic review” or “meta-analysis”)
- 28 TITLE-ABS-KEY ((“meta-analysis”) or (“systematic review” OR “Review, Systematic”) or (“systematic reviews as topic” OR “Systematic Review as Topic” OR “Reviews Systematic as Topic”) or (“meta-analysis as topic” OR “ Meta Analysis as Topic” OR “Data Pooling” OR “Data Poolings” OR “Overviews, Clinical Trial” OR “Clinical Trial Overviews” OR “Clinical Trial Overview” OR “Overview, Clinical Trial”) OR (“technology assessment, biomedical” OR “Biomedical Technology Assessment” OR “Technology Assessment, Health” OR “Assessment, Health Technology” OR “Assessments, Health Technology” OR “Health Technology Assessment” OR “Health Technology Assessments” OR “Technology Assessments, Health” OR “Assessment, Biomedical Technology” OR “Assessments, Biomedical Technology” OR “Biomedical Technology Assessments” OR “Technology Assessments, Biomedical” OR “Technology Assessment” OR “Assessment, Technology” OR “Assessments, Technology” OR “Technology Assessments” )OR (“network meta-analysis” OR “Meta-Analyses, Network” OR “Meta-Analysis, Network” OR “Network Meta Analysis” OR “Network Meta-Analyses” OR “Multiple Treatment Comparison Meta-Analysis” OR “Multiple Treatment Comparison Meta Analysis” OR “Mixed Treatment Meta-Analysis” OR “Meta-Analyses, Mixed Treatment” OR “Meta-Analysis, Mixed Treatment” OR “Mixed Treatment Meta Analysis” OR “Mixed Treatment Meta-Analyses”))
- 29 TITLE-ABS-KEY ((systematic! W/3 (review! or overview!)) or (methodologic! W/3 (review! or overview!)))
- 30 TITLE-ABS-KEY ((quantitative W/3 (review! or overview! or synthes!)) or (research W/3 (integrati! or overview!)))
- 31 TITLE-ABS-KEY ((integrative W/3 (review! or overview!)) or (collaborative W/3 (review! or overview!)) or (pool! W/3 analy!))
- 32 TITLE-ABS-KEY (“data synthes!” or “data extraction!” or “data abstraction!”)
- 33 TITLE-ABS-KEY (handsearch! or “hand search!”)
- 34 TITLE-ABS-KEY (“mantel haenszel” or peto or “der simonian” or dersimonian or fixed effect! or latin square!)
- 35 TITLE-ABS-KEY ((met AND analy!) or “metanaly!” or “technology assessment!” or HTA or HTAs or “technology overview!” or “technology appraisal!”)
- 36 TITLE-ABS-KEY (“meta regression!” or metaregression!)
- 37 TITLE-ABS-KEY (“meta-analy!” or “metaanaly!” or “systematic review!” or “biomedical technology assessment!” or “bio-medical technology assessment!”)
- 38 TITLE-ABS-KEY (comparative W/3 (efficacy or effectiveness))
- 39 TITLE-ABS-KEY (“outcomes research” or “relative effectiveness”)
- 40 TITLE-ABS-KEY ((indirect or “indirect treatment” or “mixed-treatment” or bayesian) W/3 comparison!)
- 41 TITLE-ABS-KEY (multi! W/3 treatment W/3 comparison!)
- 42 TITLE-ABS-KEY (mixed W/3 treatment W/3 (“meta-analy!” or “metaanaly!”))
- 43 TITLE-ABS-KEY (“umbrella review!”)
- 44 TITLE-ABS-KEY (multi! W/2 paramet! W/2 evidence W/2 synthesis)
- 45 TITLE-ABS-KEY (multiparamet! W/2 evidence W/2 synthesis)
- 46 TITLE-ABS-KEY (multi-paramet! W/2 evidence W/2 synthesis)
- 47 or/27–46
- 48 47 and 26
- 50 48 and (limit-to (pubyear, 2021) or limit-to (pubyear, 2020) or limit-to (pubyear, 2019) or limit-to (pubyear, 2018) or limit-to (pubyear, 2017) or limit-to (pubyear, 2016) or limit-to (pubyear, 2015)
| Type | Sub-Type | N |
|---|---|---|
| Database | Electronic Health Records | 16 |
| Registry | 14 | |
| Administrative Database | 2 | |
| Other | 3 | |
| Study | Retrospective Cohort | 11 |
| Randomised Controlled Trial | 10 | |
| Prospective Cohort | 10 | |
| Cross-sectional | 7 | |
| Not Clearly Specified | 4 |
| Category | Type | N |
|---|---|---|
| Machine Learning | Deep Learning | 18 |
| Neural Network | 16 | |
| Ensemble | 15 | |
| Regression | 14 | |
| Decision Tree | 13 | |
| Instance-Based | 11 | |
| Bayesian | 8 | |
| Artificial Intelligence | Clustering | 6 |
| Dimensionality Reduction | 5 | |
| Rule System | 4 | |
| Regularisation | 2 |
References
- GBD 2017 Disease and Injury Incidence and Prevalence Collaborators. Global, regional, and national incidence, prevalence, and years lived with disability for 354 diseases and injuries for 195 countries and territories, 1990–2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet 2018, 392, 1789–1858. [Google Scholar] [CrossRef] [PubMed]
- Savarese, G.; Becher, P.M.; Lund, L.H.; Seferovic, P.; Rosano, G.M.; Coats, A.J. Global burden of heart failure: A comprehensive and updated review of epidemiology. Cardiovasc. Res. 2022, 118, 3272–3287. [Google Scholar] [CrossRef] [PubMed]
- Bozkurt, B.; Coats, A.J.; Tsutsui, H.; Abdelhamid, M.; Adamopoulos, S.; Albert, N.; Anker, S.D.; Atherton, J.; Böhm, M.; Butler, J.; et al. Universal Definition and Classification of Heart Failure: A Report of the Heart Failure Society of America, Heart Failure Association of the European Society of Cardiology, Japanese Heart Failure Society and Writing Committee of the Universal Definition of Heart Failure. J. Card. Fail. 2021, 27, 387–413. [Google Scholar] [CrossRef]
- Averbuch, T.; Sullivan, K.; Sauer, A.; Mamas, M.A.; Voors, A.A.; Gale, C.P.; Metra, M.; Ravindra, N.; Van Spall, H.G. Applications of artificial intelligence and machine learning in heart failure. J. Eur. Heart J. Digit. Health 2022, 3, 311–322. [Google Scholar] [CrossRef]
- Guidi, G.; Pettenati, M.; Miniati, R.; Iadanza, E. Heart Failure analysis Dashboard for patient’s remote monitoring combining multiple artificial intelligence technologies. In Proceedings of the 2012 Annual International Conference of the IEEE Engineering in Medicine and Biology Society, San Diego, CA, USA, 10 November 2012; pp. 2210–2213. [Google Scholar] [CrossRef]
- Guidi, G.; Melillo, P.; Pettenati, M.; Milli, M.; Iadanza, E. Performance assessment of a Clinical Decision Support System for analysis of Heart Failure. In XIII Mediterranean Conference on Medical and Biological Engineering and Computing 2013. IFMBE Proceedings; Roa Romero, L., Ed.; Springer: Cham, Switzerland, 2014; Volume 41, pp. 1354–1357. [Google Scholar] [CrossRef]
- Goretti, F.; Oronti, B.; Milli, M.; Iadanza, E. Deep Learning for Predicting Congestive Heart Failure. Electronics 2022, 11, 3996. [Google Scholar] [CrossRef]
- Kijo, A.; Leotsakos, A.; Sands, A. WHO Strengthens Medical Device Regulation as Machine Learning-Enabled Medical Devices Gather Pace. Health Manag. 2023, 23, 79–82. [Google Scholar]
- Pecchia, L.; Pallikarakis, N.; Magjarevic, R.; Iadanza, E. Health Technology Assessment and Biomedical Engineering: Global trends, gaps and opportunities. Med. Eng. Phys. 2019, 72, 19–26. [Google Scholar] [CrossRef]
- Van Spall, H.G.; Averbuch, T.; Damman, K.; Voors, A.A. Risk and risk reduction in trials of heart failure with reduced ejection fraction: Absolute or relative? Eur. J. Heart Fail. 2021, 23, 1437–1444. [Google Scholar] [CrossRef]
- Rivera, S.C.; Liu, X.; Chan, A.W.; Denniston, A.K.; Calvert, M.J.; Ashrafian, H.; Beam, A.L.; Collins, G.S.; Darzi, A.; Deeks, J.J.; et al. Guidelines for clinical trial protocols for interventions involving artificial intelligence: The SPIRIT-AI extension. Lancet Digit. Health 2020, 2, e549–e560. [Google Scholar] [CrossRef]
- Murali, K.M.; Mullan, J.; Chen, J.H.; Roodenrys, S.; Lonergan, M. Medication adherence in randomized controlled trials evaluating cardiovascular or mortality outcomes in dialysis patients: A systematic review. BMC Nephrol. 2017, 18, 1–11. [Google Scholar] [CrossRef]
- O’Rourke, B.; Oortwijn, W.; Schuller, T. Announcing the new definition of health technology assessment. Value Health 2020, 23, 824–825. [Google Scholar] [CrossRef] [PubMed]
- Alami, H.; Lehoux, P.; Auclair, Y.; de Guise, M.; Gagnon, M.P.; Shaw, J.; Roy, D.; Fleet, R.; Ag Ahmed, M.A.; Fortin, J.P. Artificial Intelligence and Health Technology Assessment: Anticipating a New Level of Complexity. J. Med. Internet Res. 2020, 22, e17707. [Google Scholar] [CrossRef] [PubMed]
- Kidholm, K.; Ekel, A.G.; Jensen, L.K.; Rasmussen, J.; Pedersen, C.D.; Bowes, A.; Flottorp, S.A.; Bech, M. A model for assessment of telemedicine applications: MAST. Int. J. Technol. Assess. Health Care 2012, 28, 44–51. [Google Scholar] [CrossRef] [PubMed]
- National Institute for Health and Care Excellence (NICE). Evidence Standards Framework (ESF) for Digital Health Technologies. 2022. Available online: https://www.nice.org.uk/about/what-we-do/our-programmes/evidence-standards-framework-for-digital-health-technologies (accessed on 20 September 2023).
- Fasterholdt, I.; Kjølhede, T.; Naghavi-Behzad, M.; Schmidt, T.; Rautalammi, Q.T.; Hildebrandt, M.G.; Gerdes, A.; Barkler, A.; Kidholm, K.; Rac, V.E.; et al. Model for ASsessing the value of Artificial Intelligence in medical imaging (MAS-AI). Int. J. Technol. Assess. Health Care 2022, 38, e74. [Google Scholar] [CrossRef]
- McCarthy, J. From here to human-level AI. Artif. Intell. 2007, 171, 1174–1182. [Google Scholar] [CrossRef]
- Kristensen, F.B.; Lampe, K.; Wild, C.; Cerbo, M.; Goettsch, W.; Becla, L. The HTA Core Model®—10 years of developing an international framework to share multidimensional value assessment. Value Health 2017, 20, 244–250. [Google Scholar] [CrossRef]
- Estévez Almenzar, M.; Fernández Llorca, D.; Gómez, E.; Martinez Plumed, F. Glossary of Human-Centric Artificial Intelligence; Technical report; Joint Research Centre (Seville Site): Sevilla, Spain, 2022. [Google Scholar]
- Medical Device Coordination Group (MDCG). Guidance on Qualification and Classification of Software in Regulation (EU) 2017/745-MDR and Regulation (EU) 2017/746-IVDR. 2019. Available online: https://health.ec.europa.eu/system/files/2020-09/md_mdcg_2019_11_guidance_qualification_classification_software_en_0.pdf (accessed on 20 September 2023).
- Hunt, H.; Pollock, A.; Campbell, P.; Estcourt, L.; Brunton, G. An introduction to overviews of reviews: Planning a relevant research question and objective for an overview. Syst. Rev. 2018, 7, 39. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Tricco, A.C.; Lillie, E.; Zarin, W.; O’Brien, K.K.; Colquhoun, H.; Levac, D.; Moher, D.; Peters, M.D.; Horsley, T.; Weeks, L.; et al. PRISMA extension for scoping reviews (PRISMA-ScR): Checklist and explanation. Ann. Intern. Med. 2018, 169, 467–473. [Google Scholar] [CrossRef]
- Graili, P.; Ieraci, L.; Hosseinkhah, N.; Argent-Katwala, M. Artificial intelligence in outcomes research: A systematic scoping review. Expert Rev. Pharmacoecon. Outcomes Res. 2021, 21, 601–623. [Google Scholar] [CrossRef]
- Brownlee, J. Supervised and Unsupervised Machine Learning Algorithms. 2020. Available online: https://machinelearningmastery.com/supervised-and-unsupervised-machine-learning-algorithms/ (accessed on 20 September 2023).
- Collins, G.S.; Dhiman, P.; Navarro, C.L.; Ma, J.; Hooft, L.; Reitsma, J.B.; Logullo, P.; Beam, A.L.; Peng, L.; Van Calster, B.; et al. Protocol for development of a reporting guideline (TRIPOD-AI) and risk of bias tool (PROBAST-AI) for diagnostic and prognostic prediction model studies based on artificial intelligence. BMJ Open 2021, 11, e048008. [Google Scholar] [CrossRef] [PubMed]
- Sounderajah, V.; Ashrafian, H.; Golub, R.M.; Shetty, S.; De Fauw, J.; Hooft, L.; Moons, K.; Collins, G.; Moher, D.; Bossuyt, P.M.; et al. Developing a reporting guideline for artificial intelligence-centred diagnostic test accuracy studies: The STARD-AI protocol. BMJ Open 2021, 11, e047709. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, H.; Liu, X.; Rivera, S.C.; Moher, D.; Chan, A.W.; Sydes, M.R.; Calvert, M.J.; Denniston, A.K. Reporting guidelines for clinical trials of artificial intelligence interventions: The SPIRIT-AI and CONSORT-AI guidelines. Trials 2021, 22, 11. [Google Scholar] [CrossRef] [PubMed]
- Vasey, B.; Novak, A.; Ather, S.; Ibrahim, M.; McCulloch, P. DECIDE-AI: A new reporting guideline and its relevance to artificial intelligence studies in radiology. Clin. Radiol. 2023, 78, 130–136. [Google Scholar] [CrossRef]
- European network for Health Technology Assessment (EUnetHTA). Comparators & Comparisons: Criteria for the Choice of the Most Appropriate Comparator(s), Adapted version; European network for Health Technology Assessment: Vienna, Austria, 2015. [Google Scholar]
- Grün, D.; Rudolph, F.; Gumpfer, N.; Hannig, J.; Elsner, L.K.; von Jeinsen, B.; Hamm, C.W.; Rieth, A.; Guckert, M.; Keller, T. Identifying Heart Failure in ECG Data With Artificial Intelligence—A Meta-Analysis. Front. Digit. Health 2021, 2, 584555. [Google Scholar] [CrossRef]
- Bazoukis, G.; Stavrakis, S.; Zhou, J.; Bollepalli, S.C.; Tse, G.; Zhang, Q.; Singh, J.P.; Armoundas, A.A. Machine learning versus conventional clinical methods in guiding management of heart failure patients—A systematic review. Heart Fail. Rev. 2021, 26, 23–34. [Google Scholar] [CrossRef]
- Krittanawong, C.; Virk, H.U.; Bangalore, S.; Wang, Z.; Johnson, K.W.; Pinotti, R.; Zhang, H.; Kaplin, S.; Narasimhan, B.; Kitai, T.; et al. Machine learning prediction in cardiovascular diseases: A meta-analysis. Sci. Rep. 2020, 10, 16057. [Google Scholar] [CrossRef]
- Nadarajah, R.; Alsaeed, E.; Hurdus, B.; Aktaa, S.; Hogg, D.; Bates, M.G.; Cowan, C.; Wu, J.; Gale, C.P. Prediction of incident atrial fibrillation in community-based electronic health records: A systematic review with meta-analysis. Heart 2022, 108, 1020–1029. [Google Scholar] [CrossRef]
- Lee, S.; Chu, Y.; Ryu, J.; Park, Y.; Yang, S.; Koh, S. Artificial Intelligence for Detection of Cardiovascular-Related Diseases from Wearable Devices: A Systematic Review and Meta-Analysis. Yonsei Med. J. 2022, 63, S93–S107. [Google Scholar] [CrossRef]
- Mahajan, S.; Heidenreich, P.; Abbott, B.; Newton, A.; Ward, D. Predictive models for identifying risk of readmission after index hospitalization for heart failure: A systematic review. Eur. J. Cardiovasc. Nurs. 2018, 17, 675–689. [Google Scholar] [CrossRef]
- Medic, G.; Klieb, M.K.; Atallah, L.; Weichert, J.; Panda, S.; Postma, M.; Amer, E.K. Evidence-based Clinical Decision Support Systems for the prediction and detection of three disease states in critical care: A systematic literature review [version 2; peer review: 2 approved]. F1000Research 2019, 8, 1728. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, A.; Chen, S.; Fatemifar, G.; Zeina, M.; Lumbers, R.T.; Mielke, J.; Gill, S.; Kotecha, D.; Freitag, D.F.; Denaxas, S.; et al. Machine learning for subtype definition and risk prediction in heart failure, acute coronary syndromes and atrial fibrillation: Systematic review of validity and clinical utility. BMC Med. 2021, 19, 85. [Google Scholar] [CrossRef] [PubMed]
- Mpanya, D.; Celik, T.; Klug, E.; Ntsinjana, H. Predicting mortality and hospitalization in heart failure using machine learning: A systematic literature review. Int. J. Cardiol. Heart Vasc. 2021, 34, 100773. [Google Scholar] [CrossRef] [PubMed]
- Reading Turchioe, M.; Volodarskiy, A.; Pathak, J.; Wright, D.; Tcheng, J.; Slotwiner, D. Systematic review of current natural language processing methods and applications in cardiology. Int. Heart 2022, 8, 909–916. [Google Scholar] [CrossRef]
- Shin, S.; Austin, P.C.; Ross, H.J.; Abdel-Qadir, H.; Freitas, C.; Tomlinson, G.; Chicco, D.; Mahendiran, M.; Lawler, P.R.; Billia, F.; et al. Machine learning vs. conventional statistical models for predicting heart failure readmission and mortality. Int. ESC Heart Fail. 2021, 8, 106–115. [Google Scholar] [CrossRef]
- Wu, Q.; Lu, Z.; Liu, Y.; Xu, Y.; Zhang, J.; Xiao, W.; Yang, M. Machine learning for early warning of cardiac arrest: A systematic review. Chin. J. Evid.-Based Med. 2021, 21, 942–952. [Google Scholar] [CrossRef]
- Błaziak, M.; Urban, S.; Wietrzyk, W.; Jura, M.; Iwanek, G.; Stańczykiewicz, B.; Kuliczkowski, W.; Zymliński, R.; Pondel, M.; Berka, P.; et al. An Artificial Intelligence Approach to Guiding the Management of Heart Failure Patients Using Predictive Models: A Systematic Review. Int. Biomed. 2022, 10, 2188. [Google Scholar] [CrossRef]
- Javeed, A.; Khan, S.; Ali, L.; Ali, S.; Imrana, Y.; Rahman, A. Machine Learning-Based Automated Diagnostic Systems Developed for Heart Failure Prediction Using Different Types of Data Modalities: A Systematic Review and Future Directions. Int. Comput. Math. Methods Med. 2022, 2022, 9288452. [Google Scholar] [CrossRef]
- Sun, Z.; Dong, W.; Shi, H.; Ma, H.; Cheng, L.; Huang, Z. Comparing Machine Learning Models and Statistical Models for Predicting Heart Failure Events: A Systematic Review and Meta-Analysis. Int. Front. Cardiovasc. Med. 2022, 9, 812276. [Google Scholar] [CrossRef]
- Sun, J.; Guo, H.; Wang, W.; Wang, X.; Ding, J.; He, K.; Guan, X. Identifying novel subgroups in heart failure patients with unsupervised machine learning: A scoping review. Int. Front. Cardiovasc. Med. 2022, 9, 895836. [Google Scholar] [CrossRef]
- Tripoliti, E.E.; Papadopoulos, T.G.; Karanasiou, G.S.; Naka, K.K.; Fotiadis, D.I. Heart Failure: Diagnosis, Severity Estimation and Prediction of Adverse Events Through Machine Learning Techniques. Int. Comput. Struct. Biotechnol. J. 2016, 15, 26–47. [Google Scholar] [CrossRef]
- Safdar, S.; Zafar, S.; Zafar, N.; Khan, N.F. Machine learning based decision support systems (DSS) for heart disease diagnosis: A review. Int. Artif. Intell. Rev. 2018, 50, 597–623. [Google Scholar] [CrossRef]
- Kilic, A. Artificial Intelligence and Machine Learning in Cardiovascular Health Care. Int. Ann. Thorac. Surg. 2020, 109, 1323–1329. [Google Scholar] [CrossRef]
- Maurya, M.R.; Riyaz, N.U.; Reddy, M.S.; Yalcin, H.C.; Ouakad, H.M.; Bahadur, I.; Al-Maadeed, S.; Sadasivuni, K.K. A review of smart sensors coupled with Internet of Things and Artificial Intelligence approach for heart failure monitoring. Int. Med. Biol. Eng. Comput. 2021, 59, 2185–2203. [Google Scholar] [CrossRef]
- Shu, S.; Ren, J.; Song, J. Clinical Application of Machine Learning-Based Artificial Intelligence in the Diagnosis, Prediction, and Classification of Cardiovascular Diseases. Int. J. Circumpolar Health 2021, 85, 1416–1425. [Google Scholar] [CrossRef] [PubMed]
- Heupink, L.F.; Peacocke, E.F.; Sæterdal, I.; Chola, L.; Frønsdal, K. Considerations for transferability of health technology assessments: A scoping review of tools, methods, and practices. Int. J. Technol. Assess. Health Care 2022, 38, e78. [Google Scholar] [CrossRef] [PubMed]
- Shahzad, R.; Ayub, B.; Siddiqui, M.R. Quality of reporting of randomised controlled trials of artificial intelligence in healthcare: A systematic review. BMJ Open 2022, 12, e061519. [Google Scholar] [CrossRef] [PubMed]
- Plana, D.; Shung, D.L.; Grimshaw, A.A.; Saraf, A.; Sung, J.J.; Kann, B.H. Randomized clinical trials of machine learning interventions in health care: A systematic review. JAMA Netw. Open 2022, 5, e2233946. [Google Scholar] [CrossRef]
- Capkun, G.; Corry, S.; Dowling, O.; Kolaei, F.A.Z.V.; Takyar, S.; Furtado, C.; Jónsson, P.; Kleinermans, D.; Lambert, L.; Schiel, A.; et al. Can we use existing guidance to support the development of robust real-world evidence for health technology assessment/payer decision-making? Int. J. Technol. Assess. Health Care 2022, 38, e79. [Google Scholar] [CrossRef]
- Sharma, M.; Savage, C.; Nair, M.; Larsson, I.; Svedberg, P.; Nygren, J.M. Artificial Intelligence Applications in Health Care Practice: Scoping Review. J. Med. Internet Res. 2022, 24, e40238. [Google Scholar] [CrossRef]
- Yin, J.; Ngiam, K.Y.; Teo, H.H. Role of artificial intelligence applications in real-life clinical practice: Systematic review. J. Med. Internet Res. 2021, 23, e25759. [Google Scholar] [CrossRef] [PubMed]



| Type | Study ID | Citations * | Years Covered | No. Studies | Clinical Indication ** |
|---|---|---|---|---|---|
| Meta-analysis | Gruen et al., 2020 [32] | 10 | 2017–2020 | 5 | HF |
| Krittanawong et al., 2020 [34] | 78 | 1966–2019 | 55 | HF. ACS | |
| Nadarajah et al., 2021 [35] | 2 | Till March 2021 | 11 | HF, AI, stroke | |
| Lee et al., 2022 [36] | 6 | 1970-2021 | 102 | HF, AI, Other | |
| Systematic reviews | Mahajan et al., 2018 [37] | 40 | 1948–2018 | 25 | HF |
| Medic et al., 2019 [38] | 25 | 2013–2018 | 20 | HF | |
| Banerjee et al., 2021 [39] | 17 | 2000–2019 | 97 | HF, ACS, AF | |
| Bazoukis et al., 2021 [33] | 31 | 2005–2019 | 122 | HF | |
| Mpanya et al., 2021 [40] | 4 | 1993–2007 | 30 | HF | |
| Reading Turchioe et al., 2021 [41] | 4 | 2015–2020 | 37 | HF, ACS, Other | |
| Shin et al., 2021 [42] | 35 | 2000–2020 | 20 | HF | |
| Wu et al., 2021 [43] | 0 | 2015–2021 | 38 | HF, Other | |
| Blaziak et al., 2022 [44] | 1 | Till March 2022 | 9 | HF, ACS, AF | |
| Javeed et al., 2022 [45] | 9 | 1995–2021 | 105 | HF, other | |
| Sun et al., 2022 [46] | 2 | 2010–2021 | 116 | HF | |
| Scoping reviews | Sun et al., 2022 [47] | 0 | Till December 2021 | 47 | HF, Other |
| Narrative reviews | Tripoliti et al., 2017 [48] | 167 | 2000–2017 | N/A | HF |
| Safdar et al., 2018 [49] | 96 | Till–2015 | 20 | HF, Other | |
| Kilic, 2020 [50] | 74 | until 2019 | N/A | HF, Other | |
| Maurya et al., 2021 [51] | 1 | N/A | N/A | HF | |
| Shu et al., 2021 [52] | 1 | N/A | 16 | HF, Other |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Di Bidino, R.; Piaggio, D.; Andellini, M.; Merino-Barbancho, B.; Lopez-Perez, L.; Zhu, T.; Raza, Z.; Ni, M.; Morrison, A.; Borsci, S.; et al. Scoping Meta-Review of Methods Used to Assess Artificial Intelligence-Based Medical Devices for Heart Failure. Bioengineering 2023, 10, 1109. https://doi.org/10.3390/bioengineering10101109
Di Bidino R, Piaggio D, Andellini M, Merino-Barbancho B, Lopez-Perez L, Zhu T, Raza Z, Ni M, Morrison A, Borsci S, et al. Scoping Meta-Review of Methods Used to Assess Artificial Intelligence-Based Medical Devices for Heart Failure. Bioengineering. 2023; 10(10):1109. https://doi.org/10.3390/bioengineering10101109
Chicago/Turabian StyleDi Bidino, Rossella, Davide Piaggio, Martina Andellini, Beatriz Merino-Barbancho, Laura Lopez-Perez, Tianhui Zhu, Zeeshan Raza, Melody Ni, Andra Morrison, Simone Borsci, and et al. 2023. "Scoping Meta-Review of Methods Used to Assess Artificial Intelligence-Based Medical Devices for Heart Failure" Bioengineering 10, no. 10: 1109. https://doi.org/10.3390/bioengineering10101109
APA StyleDi Bidino, R., Piaggio, D., Andellini, M., Merino-Barbancho, B., Lopez-Perez, L., Zhu, T., Raza, Z., Ni, M., Morrison, A., Borsci, S., Fico, G., Pecchia, L., & Iadanza, E. (2023). Scoping Meta-Review of Methods Used to Assess Artificial Intelligence-Based Medical Devices for Heart Failure. Bioengineering, 10(10), 1109. https://doi.org/10.3390/bioengineering10101109









