Molecular Changes Induced by Oxidative Stress that Impair Human Sperm Motility
Abstract
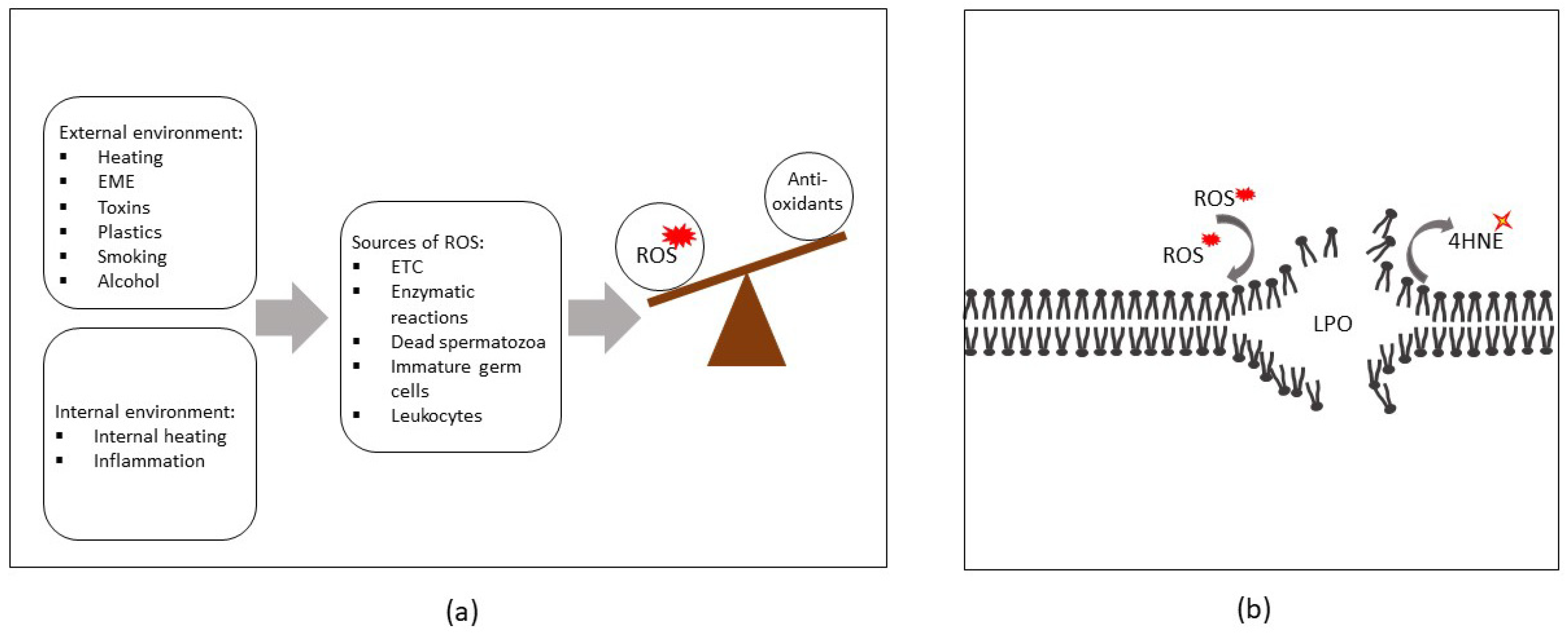
1. Introduction
2. Sources of ROS in Semen
2.1. Endogenous Sources of ROS
2.1.1. Spermatozoa
2.1.2. Immature Germ Cells
2.1.3. Leukocytes
2.1.4. Varicocele
3. Exogenous Sources of ROS
3.1. Physical Factors
3.2. Chemical Factors
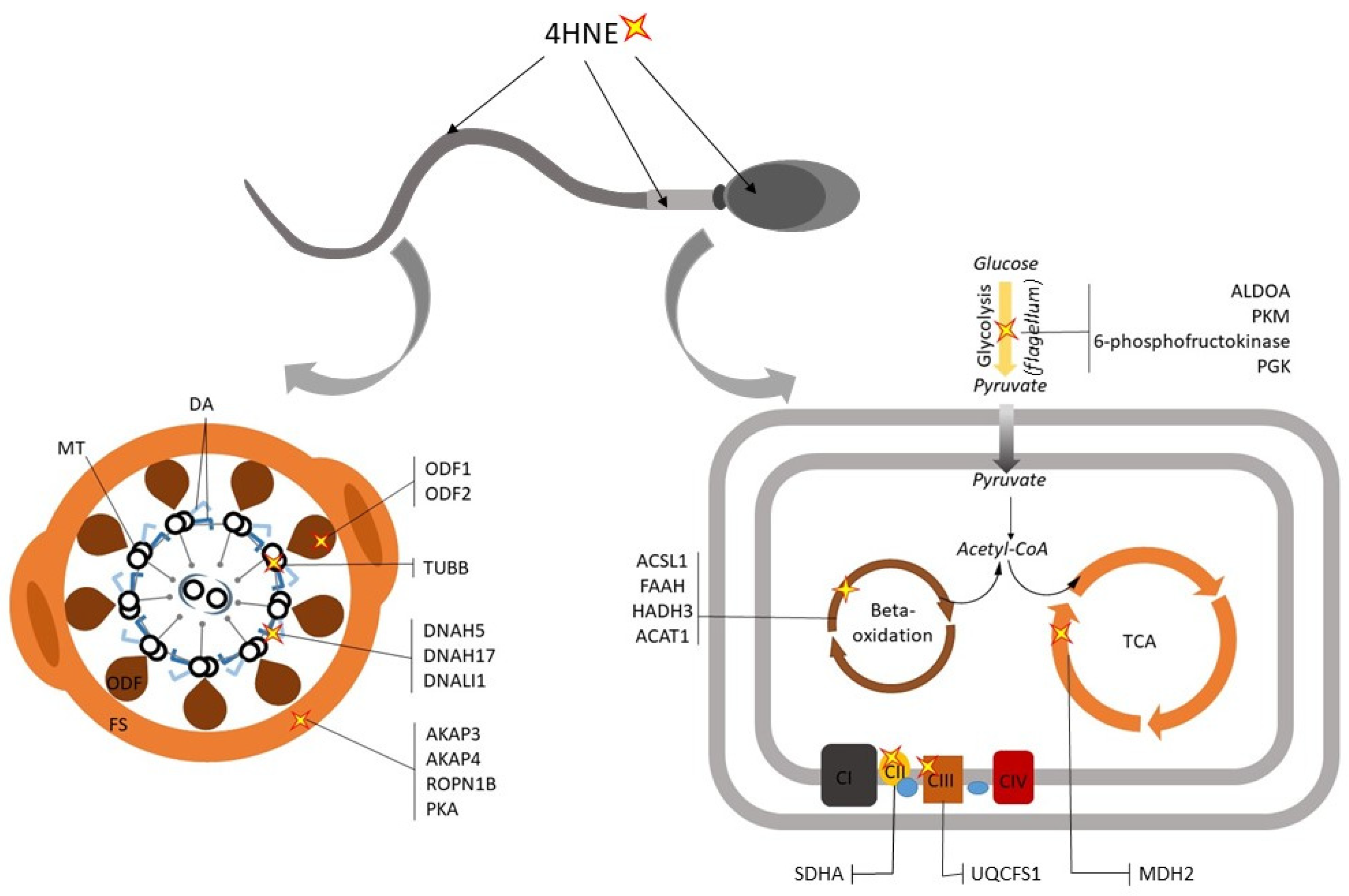
4. ROS-Induced Lesions Detected in Low-Motility Spermatozoa
4.1. Compromised Sperm Membrane Integrity
4.2. Dysregualtion of Sperm Metabolic Enzymes
4.3. Defects in the Sperm Motility Apparatus and Signaling Pathways
4.4. Sperm DNA Modifications
5. Antioxidant Systems in the Male Reproductive Tract
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Brugh, V.M.; Lipshultz, L.I. Male factor infertility: Evaluation and management. Clin. North Am. 2004, 88, 367–385. [Google Scholar] [CrossRef]
- Lotti, F.; Maggi, M. Ultrasound of the male genital tract in relation to male reproductive health. Hum. Reprod. Update 2015, 21, 56–83. [Google Scholar] [CrossRef]
- Vergallo, A.; Giampietri, L.; Baldacci, F.; Volpi, L.; Chico, L.; Pagni, C.; Giorgi, F.S.; Ceravolo, R.; Tognoni, G.; Siciliano, G.; et al. Oxidative Stress Assessment in Alzheimer’s Disease: A Clinic Setting Study. Am. J. Alzheimers Dis. Other Demen. 2018, 33, 35–41. [Google Scholar] [CrossRef] [PubMed]
- Ma-On, C.; Sanpavat, A.; Whongsiri, P.; Suwannasin, S.; Hirankarn, N.; Tangkijvanich, P.; Boonla, C. Oxidative stress indicated by elevated expression of Nrf2 and 8-OHdG promotes hepatocellular carcinoma progression. Med. Oncol. 2017, 34, 57. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Chi, R.F.; Qin, F.Z.; Guo, X.F. Distinct changes of myocyte autophagy during myocardial hypertrophy and heart failure: Association with oxidative stress. Exp. Physiol. 2016, 101, 1050–1063. [Google Scholar] [CrossRef] [PubMed]
- Dursun, E.; Akalin, F.A.; Genc, T.; Cinar, N.; Erel, O.; Yildiz, B.O. Oxidative Stress and Periodontal Disease in Obesity. Medicine (Baltimore) 2016, 95, e3136. [Google Scholar] [CrossRef]
- Lanzafame, F.M.; La Vignera, S.; Vicari, E.; Calogero, A.E. Oxidative stress and medical antioxidant treatment in male infertility. Reprod. Biomed. Online 2009, 19, 638–659. [Google Scholar] [CrossRef]
- Agarwal, A.; Prabakaran, S.; Allamaneni, S.S. Relationship between oxidative stress, varicocele and infertility: A meta-analysis. Reprod. Biomed. Online 2006, 12, 630–633. [Google Scholar] [CrossRef]
- Fraczek, M.; Sanocka, D.; Kamieniczna, M.; Kurpisz, M. Proinflammatory cytokines as an intermediate factor enhancing lipid sperm membrane peroxidation in in vitro conditions. J. Androl. 2008, 29, 85–92. [Google Scholar] [CrossRef]
- Kurfurstova, D.; Bartkova, J.; Vrtel, R.; Mickova, A.; Burdova, A.; Majera, D.; Mistrik, M.; Kral, M.; Santer, F.R.; Bouchal, J.; et al. DNA damage signalling barrier, oxidative stress and treatment-relevant DNA repair factor alterations during progression of human prostate cancer. Mol. Oncol. 2016, 10, 879–894. [Google Scholar] [CrossRef]
- Walters, J.L.H.; De Iuliis, G.N.; Nixon, B.; Bromfield, E.G. Oxidative Stress in the Male Germline: A Review of Novel Strategies to Reduce 4-Hydroxynonenal Production. Antioxidants 2018, 7, 132. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, A.; Mulgund, A.; Sharma, R.; Sabanegh, E. Mechanisms of oligozoospermia: An oxidative stress perspective. Syst. Biol. Reprod. Med. 2014, 60, 206–216. [Google Scholar] [CrossRef] [PubMed]
- Muratori, M.; Tamburrino, L.; Marchiani, S.; Cambi, M.; Olivito, B.; Azzari, C.; Forti, G.; Baldi, E. Investigation on the Origin of Sperm DNA Fragmentation: Role of Apoptosis, Immaturity and Oxidative Stress. Mol. Med. 2015, 21, 109–122. [Google Scholar] [CrossRef] [PubMed]
- Koppers, A.J.; Mitchell, L.A.; Wang, P.; Lin, M.; Aitken, R.J. Phosphoinositide 3-kinase signalling pathway involvement in a truncated apoptotic cascade associated with motility loss and oxidative DNA damage in human spermatozoa. Biochem J. 2011, 436, 687–698. [Google Scholar] [CrossRef]
- Aitken, R.J.; Smith, T.B.; Jobling, M.S.; Baker, M.A.; De Iuliis, G.N. Oxidative stress and male reproductive health. Asian J. Androl. 2014, 16, 31–38. [Google Scholar] [CrossRef]
- World Health Organization, Department of Reproductive Health and Research. WHO Laboratory Manual for the Examination and Processing of Human Semen, 5th ed.; World Health Organization: Geneva, Switzerland, 2010; Volume 1, p. 224. [Google Scholar]
- Liu, F.J.; Liu, X.; Han, J.L.; Wang, Y.W.; Jin, S.H.; Liu, X.X.; Liu, J.; Wang, W.T.; Wang, W.J. Aged men share the sperm protein PATE1 defect with young asthenozoospermia patients. Hum. Reprod. 2015, 30, 861–869. [Google Scholar] [CrossRef]
- Nowicka-Bauer, K.; Lepczynski, A.; Ozgo, M.; Kamieniczna, M.; Fraczek, M.; Stanski, L.; Olszewska, M.; Malcher, A.; Skrzypczak, W.; Kurpisz, M.K. Sperm mitochondrial dysfunction and oxidative stress as possible reasons for isolated asthenozoospermia. J. Physiol. Pharmacol. 2018, 69, 403–417. [Google Scholar] [CrossRef]
- Kumar, R.; Venkatesh, S.; Kumar, M.; Tanwar, M.; Shasmsi, M.B.; Kumar, R.; Gupta, N.P.; Sharma, R.K.; Talwar, P.; Dada, R. Oxidative stress and sperm mitochondrial DNA mutation in idiopathic oligoasthenozoospermic men. Indian J. Biochem. Biophys. 2009, 46, 172–177. [Google Scholar]
- Bonanno, O.; Romeo, G.; Asero, P.; Pezzino, F.M.; Castiglione, R.; Burrello, N.; Sidoti, G.; Frajese, G.V.; Vicari, E.; D’Agata, R. Sperm of patients with severe asthenozoospermia show biochemical, molecular and genomic alterations. Reproduction 2016, 152, 695–704. [Google Scholar] [CrossRef]
- Chen, Y.; Liao, T.; Zhu, L.; Lin, X.; Wu, R.; Jin, L. Seminal plasma cell-free mitochondrial DNA copy number is associated with human semen quality. Eur. J. Obstet. Gynecol. Reprod. Biol. 2018, 231, 164–168. [Google Scholar] [CrossRef]
- Baker, M.A.; Weinberg, A.; Hetherington, L.; Villaverde, A.I.; Velkov, T.; Baell, J.; Gordon, C.P. Defining the mechanisms by which the reactive oxygen species by-product, 4-hydroxynonenal, affects human sperm cell function. Biol. Reprod. 2015, 92, 108. [Google Scholar] [CrossRef] [PubMed]
- Gavella, M.; Lipovac, V. NADH-dependent oxidoreductase (diaphorase) activity and isozyme pattern of sperm in infertile men. Arch. Androl. 1992, 28, 135–141. [Google Scholar] [CrossRef] [PubMed]
- Jastroch, M.; Divakaruni, A.S.; Mookerjee, S.; Treberg, J.R.; Brand, M.D. Mitochondrial proton and electron leaks. Essays Biochem. 2010, 47, 53–67. [Google Scholar] [CrossRef] [PubMed]
- O’Flaherty, C.M.; Beorlegui, N.B.; Beconi, M.T. Reactive oxygen species requirements for bovine sperm capacitation and acrosome reaction. Theriogenology 1999, 52, 289–301. [Google Scholar] [CrossRef]
- Koppenol, W.H. The Haber-Weiss cycle—70 years later. Redox Rep. 2001, 6, 229–234. [Google Scholar] [CrossRef]
- Buzadzic, B.; Vucetic, M.; Jankovic, A.; Stancic, A.; Korac, A.; Korac, B.; Otasevic, V. New insights into male (in)fertility: The importance of NO. Br. J. Pharmacol. 2015, 172, 1455–1467. [Google Scholar] [CrossRef]
- Otasevic, V.; Stancic, A.; Korac, A.; Jankovic, A.; Korac, B. Reactive oxygen, nitrogen, and sulfur species in human male fertility. A crossroad of cellular signaling and pathology. Biofactors 2019. [Google Scholar] [CrossRef]
- Chen, S.J.; Allam, J.P.; Duan, Y.G.; Haidl, G. Influence of reactive oxygen species on human sperm functions and fertilizing capacity including therapeutical approaches. Arch. Gynecol. Obstet. 2013, 288, 191–199. [Google Scholar] [CrossRef]
- Alomar, M.; Alzoabi, M.; Zarkawi, M. Kinetics of hydrogen peroxide generated from live and dead ram spermatozoa and the effects of catalase and oxidase substrates addition. Czech, J. Anim. Sci. 2016, 61, 1–7. [Google Scholar] [CrossRef]
- Gao, W.; Pu, Y.; Luo, K.Q.; Chang, D.C. Temporal relationship between cytochrome c release and mitochondrial swelling during UV-induced apoptosis in living HeLa cells. J. Cell Sci. 2001, 114, 2855–2862. [Google Scholar]
- Patil, P.S.; Humbarwadi, R.S.; Patil, A.D.; Gune, A.R. Immature germ cells in semen-correlation with total sperm count and sperm motility. J. Cytol. 2013, 30, 185–189. [Google Scholar] [CrossRef]
- Hermo, L.; Pelletier, R.M.; Cyr, D.G.; Smith, C.E. Surfing the wave, cycle, life history, and genes/proteins expressed by testicular germ cells. Part 1: Background to spermatogenesis, spermatogonia, and spermatocytes. Microsc. Res. Tech. 2010, 73, 241–278. [Google Scholar] [CrossRef]
- Gomez, E.; Buckingham, D.W.; Brindle, J.; Lanzafame, F.; Irvine, D.S.; Aitken, R.J. Development of an image analysis system to monitor the retention of residual cytoplasm by human spermatozoa: Correlation with biochemical markers of the cytoplasmic space, oxidative stress, and sperm function. J. Androl. 1996, 17, 276–287. [Google Scholar]
- Agarwal, A.; Rana, M.; Qiu, E.; AlBunni, H.; Bui, A.D.; Henkel, R. Role of oxidative stress, infection and inflammation in male infertility. Andrologia 2018, 50, e13126. [Google Scholar] [CrossRef]
- Plante, M.; de Lamirande, E.; Gagnon, C. Reactive oxygen species released by activated neutrophils, but not by deficient spermatozoa, are sufficient to affect normal sperm motility. Fertil. Steril. 1994, 62, 387–393. [Google Scholar] [CrossRef]
- Wang, A.; Fanning, L.; Anderson, D.J.; Loughlin, K.R. Generation of reactive oxygen species by leukocytes and sperm following exposure to urogenital tract infection. Arch. Androl. 1997, 39, 11–17. [Google Scholar] [CrossRef]
- Tamura, M.; Tamura, T.; Tyagi, S.R.; Lambeth, J.D. The superoxide-generating respiratory burst oxidase of human neutrophil plasma membrane. Phosphatidylserine as an effector of the activated enzyme. J. Biol. Chem. 1988, 263, 17621–17626. [Google Scholar] [PubMed]
- Bostwick, D.G.; Ma, J. Spermatic Cord and Testicular Adnexa. In Urologic Surgical Pathology, 4th ed.; Elsevier—Health Sciences Division: Philadelphia, PA, USA, 2020; pp. 834–852. [Google Scholar]
- Practice Committee of the American Society for Reproductive Medicine; Society for Male Reproduction and Urology. Report on varicocele and infertility: A committee opinion. Fertil. Steril. 2014, 102, 1556–1560. [Google Scholar] [CrossRef]
- Hendin, B.N.; Kolettis, P.N.; Sharma, R.K.; Thomas, A.J., Jr.; Agarwal, A. Varicocele is associated with elevated spermatozoal reactive oxygen species production and diminished seminal plasma antioxidant capacity. J. Urol. 1999, 161, 1831–1834. [Google Scholar] [CrossRef]
- Sakamoto, Y.; Ishikawa, T.; Kondo, Y.; Yamaguchi, K.; Fujisawa, M. The assessment of oxidative stress in infertile patients with varicocele. BJU Int. 2008, 101, 1547–1552. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, A.; Hamada, A.; Esteves, S.C. Insight into oxidative stress in varicocele-associated male infertility: Part 1. Nat. Rev. Urol. 2012, 9, 678–690. [Google Scholar] [CrossRef] [PubMed]
- Mostafa, T.; Rashed, L.; Taymour, M. Seminal cyclooxygenase relationship with oxidative stress in infertile oligoasthenoteratozoospermic men with varicocele. Andrologia 2016, 48, 137–142. [Google Scholar] [CrossRef] [PubMed]
- Gul, M.; Bugday, M.S.; Erel, O. Thiol-disulphide homoeostasis as an oxidative stress marker in men with varicocele. Andrologia 2018. [Google Scholar] [CrossRef] [PubMed]
- Erfani Majd, N.; Sadeghi, N.; Tavalaee, M.; Tabandeh, M.R.; Nasr-Esfahani, M.H. Evaluation of Oxidative Stress in Testis and Sperm of Rat Following Induced Varicocele. Urol. J. 2019, 16, 300–306. [Google Scholar] [CrossRef]
- Cho, C.L.; Esteves, S.C.; Agarwal, A. Novel insights into the pathophysiology of varicocele and its association with reactive oxygen species and sperm DNA fragmentation. Asian J. Androl. 2016, 18, 186–193. [Google Scholar] [CrossRef]
- Bedford, J.M. Human spermatozoa and temperature: The elephant in the room. Biol. Reprod. 2015, 93, 97. [Google Scholar] [CrossRef]
- Ikeda, M.; Kodama, H.; Fukuda, J.; Shimizu, Y.; Murata, M.; Kumagai, J.; Tanaka, T. Role of radical oxygen species in rat testicular germ cell apoptosis induced by heat stress. Biol. Reprod. 1999, 61, 393–399. [Google Scholar] [CrossRef]
- Paul, C.; Murray, A.A.; Spears, N.; Saunders, P.T. A single, mild, transient scrotal heat stress causes DNA damage, subfertility and impairs formation of blastocysts in mice. Reproduction 2008, 136, 73–84. [Google Scholar] [CrossRef]
- Saikhun, J.; Kitiyanant, Y.; Vanadurongwan, V.; Pavasuthipaisit, K. Effects of sauna on sperm movement characteristics of normal men measured by computer-assisted sperm analysis. Int. J. Androl. 1998, 21, 358–363. [Google Scholar] [CrossRef]
- Shefi, S.; Tarapore, P.E.; Walsh, T.J.; Croughan, M.; Turek, P.J. Wet heat exposure: A potentially reversible cause of low semen quality in infertile men. Int. Braz. J. Urol. 2007, 33, 50–56. [Google Scholar] [CrossRef]
- Garolla, A.; Torino, M.; Sartini, B.; Cosci, I.; Patassini, C.; Carraro, U.; Foresta, C. Seminal and molecular evidence that sauna exposure affects human spermatogenesis. Hum. Reprod. 2013, 28, 877–885. [Google Scholar] [CrossRef]
- Houston, B.J.; Nixon, B.; Martin, J.H.; De Iuliis, G.N.; Trigg, N.A.; Bromfield, E.G.; McEwan, K.E.; Aitken, R.J. Heat exposure induces oxidative stress and DNA damage in the male germ line. Biol. Reprod. 2018, 98, 593–606. [Google Scholar] [CrossRef]
- Houston, B.J.; Nixon, B.; King, B.V.; De Iuliis, G.N.; Aitken, R.J. The effects of radiofrequency electromagnetic radiation on sperm function. Reproduction 2016, 152, R263–R276. [Google Scholar] [CrossRef]
- Agarwal, A.; Singh, A.; Hamada, A.; Kesari, K. Cell phones and male infertility: A review of recent innovations in technology and consequences. Int. Braz. J. Urol. 2011, 37, 432–454. [Google Scholar] [CrossRef] [PubMed]
- Wdowiak, A.; Mazurek, P.A.; Wdowiak, A.; Bojar, I. Effect of electromagnetic waves on human reproduction. Ann. Agric. Environ. Med. 2017, 24, 13–18. [Google Scholar] [CrossRef]
- Koppers, A.J.; De Iuliis, G.N.; Finnie, J.M.; McLaughlin, E.A.; Aitken, R.J. Significance of mitochondrial reactive oxygen species in the generation of oxidative stress in spermatozoa. J. Clin. Endocrinol. Metab. 2008, 93, 3199–3207. [Google Scholar] [CrossRef] [PubMed]
- De Iuliis, G.N.; Newey, R.J.; King, B.V.; Aitken, R.J. Mobile phone radiation induces reactive oxygen species production and DNA damage in human spermatozoa in vitro. PLoS ONE 2009, 4, e6446. [Google Scholar] [CrossRef] [PubMed]
- Feng, B.; Qiu, L.; Ye, C.; Chen, L.; Fu, Y.; Sun, W. Exposure to a 50-Hz magnetic field induced mitochondrial permeability transition through the ROS/GSK-3β signaling pathway. Int. J. Radiat. Biol. 2016, 92, 148–155. [Google Scholar] [CrossRef] [PubMed]
- Wijesekara, G.U.; Fernando, D.M.; Wijerathna, S.; Bandara, N. Environmental and occupational exposures as a cause of male infertility. Ceylon Med. J. 2015, 60, 52–56. [Google Scholar] [CrossRef] [PubMed]
- Saad, A.B.; Rjeibi, I.; Alimi, H.; Ncib, S.; Smida, A.; Zouari, N.; Zourgui, L. Lithium induced, oxidative stress and related damages in testes and heart in male rats: The protective effects of Malva sylvestris extract. Biomed. Pharmacother. 2017, 86, 127–135. [Google Scholar] [CrossRef]
- Kaur, S.; Saluja, M.; Bansal, M.P. Bisphenol A induced oxidative stress and apoptosis in mice testes: Modulation by selenium. Andrologia 2018, 50. [Google Scholar] [CrossRef] [PubMed]
- Du, J.; Xiong, D.; Zhang, Q.; Li, X.; Liu, X.; You, H.; Ding, S.; Yang, X.; Yuan, J. Mono-butyl phthalate-induced mouse testis injury is associated with oxidative stress and down-regulated expression of Sox9 and Dazl. J. Toxicol. Sci. 2017, 42, 319–328. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.X.; Zeng, Q.; Sun, Y.; You, L.; Wang, P.; Li, M.; Yang, P.; Li, J.; Huang, Z.; Wang, C.; et al. Phthalate exposure in association with serum hormone levels, sperm DNA damage and spermatozoa apoptosis: A cross-sectional study in China. Environ. Res. 2016, 150, 557–565. [Google Scholar] [CrossRef] [PubMed]
- Saleh, R.A.; Agarwal, A.; Sharma, R.K.; Nelson, D.R.; Thomas, A.J., Jr. Effect of cigarette smoking on levels of seminal oxidative stress in infertile men: A prospective study. Fertil. Steril. 2002, 78, 491–499. [Google Scholar] [CrossRef]
- Hamad, M.F.; Shelko, N.; Kartarius, S.; Montenarh, M.; Hammadeh, M.E. Impact of cigarette smoking on histone (H2B) to protamine ratio in human spermatozoa and its relation to sperm parameters. Andrology 2014, 2, 666–677. [Google Scholar] [CrossRef]
- Oh, S.I.; Lee, M.S.; Kim, C.I.; Song, K.Y.; Park, S.C. Aspartate modulates the ethanol-induced oxidative stress and glutathione utilizing enzymes in rat testes. Exp. Mol. Med. 2002, 34, 47–52. [Google Scholar] [CrossRef][Green Version]
- Siervo, G.E.; Vieira, H.R.; Ogo, F.M.; Fernandez, C.D.; Gonçalves, G.D.; Mesquita, S.F.; Anselmo-Franci, J.A.; Cecchini, R.; Guarnier, F.A.; Fernandes, G.S. Spermatic and testicular damages in rats exposed to ethanol: Influence of lipid peroxidation but not testosterone. Toxicology 2015, 330, 1–8. [Google Scholar] [CrossRef]
- Aitken, R.J.; Baker, M.A.; De Iuliis, G.N.; Nixon, B. New Insights into Sperm Physiology and Pathology. In Fertility Control. Handbook of Experimental Pharmacology; Habenicht, U.F., Aitken, R., Eds.; Springer: Berlin/Heidelberg, Germany, 2010; pp. 99–115. [Google Scholar]
- Lenzi, A.; Gandini, L.; Picardo, M.; Tramer, F.; Sandri, G.; Panfili, E. Lipoperoxidation damage of spermatozoa polyunsaturated fatty acids (PUFA): Scavenger mechanisms and possible scavenger therapies. Front. Biosci. 2000, 5, E1–E15. [Google Scholar]
- Hauck, A.K.; Bernlohr, D.A. Oxidative stress and lipotoxicity. J. Lipid Res. 2016, 57, 1976–1986. [Google Scholar] [CrossRef]
- Bromfield, E.G.; Aitken, R.J.; McLaughlin, E.A.; Nixon, B. Proteolytic degradation of heat shock protein A2 occurs in response to oxidative stress in male germ cells of the mouse. Mol. Hum. Reprod. 2017, 23, 91–105. [Google Scholar] [CrossRef]
- Badouard, C.; Ménézo, Y.; Panteix, G.; Ravanat, J.L.; Douki, T.; Cadet, J.; Favier, A. Determination of new types of DNA lesions in human sperm. Zygote 2008, 16, 9–13. [Google Scholar] [CrossRef] [PubMed]
- Dalleau, S.; Baradat, M.; Guéraud, F.; Huc, L. Cell death and diseases related to oxidative stress: 4-hydroxynonenal (HNE) in the balance. Cell Death Differ. 2013, 20, 1615–1630. [Google Scholar] [CrossRef] [PubMed]
- LoPachin, R.M.; Gavin, T.; Petersen, D.R.; Barber, D.S. Molecular mechanisms of 4-hydroxy-2-nonenal and acrolein toxicity: Nucleophilic targets and adduct formation. Chem. Res. Toxicol. 2009, 22, 1499–1508. [Google Scholar] [CrossRef] [PubMed]
- Schaur, R.J.; Siems, W.; Bresgen, N.; Eckl, P.M. 4-Hydroxy-nonenal-A Bioactive Lipid Peroxidation Product. Biomolecules 2015, 5, 2247–2337. [Google Scholar] [CrossRef]
- Doorn, J.A.; Petersen, D.R. Covalent adduction of nucleophilic amino acids by 4-hydroxynonenal and 4-oxononenal. Chem. Biol. Interact. 2003, 143, 93–100. [Google Scholar] [CrossRef]
- Bromfield, E.G.; Aitken, R.J.; Anderson, A.L.; McLaughlin, E.A.; Nixon, B. The impact of oxidative stress on chaperone-mediated human sperm-egg interaction. Hum. Reprod. 2015, 30, 2597–2613. [Google Scholar] [CrossRef]
- Morielli, T.; O’Flaherty, C. Oxidative stress impairs function and increases redox protein modifications in human spermatozoa. Reproduction 2015, 149, 113–123. [Google Scholar] [CrossRef]
- Salvolini, E.; Buldreghini, E.; Lucarini, G.; Vignini, A.; Di Primio, R.; Balercia, G. Nitric oxide synthase and tyrosine nitration in idiopathic asthenozoospermia: An immunohistochemical study. Fertil. Steril. 2012, 97, 554–560. [Google Scholar] [CrossRef]
- Vignini, A.; Nanetti, L.; Buldreghini, E.; Moroni, C.; Ricciardo-Lamonica, G.; Mantero, F.; Boscaro, M.; Mazzanti, L.; Balercia, G. The production of peroxynitrite by human spermatozoa may affect sperm motility through the formation of protein nitrotyrosine. Fertil. Steril. 2006, 85, 947–953. [Google Scholar] [CrossRef]
- Ramirez, J.P.; Carreras, A.; Mendoza, C. Sperm plasma membrane integrity in fertile and infertile men. Andrologia 1992, 24, 141–144. [Google Scholar] [CrossRef]
- Karimfar, M.H.; Niazvand, F.; Haghani, K.; Ghafourian, S.; Shirazi, R.; Bakhtiyari, S. The protective effects of melatonin against cryopreservation-induced oxidative stress in human sperm. Int. J. Immunopathol. Pharmacol. 2015, 28, 69–76. [Google Scholar] [CrossRef] [PubMed]
- Ghorbani, M.; Vatannejad, A.; Khodadadi, I.; Amiri, I.; Tavilani, H. Protective effects of glutathione supplementation against oxidative stress during cryopreservation of human spermatozoa. Cryo. Letters 2016, 37, 34–40. [Google Scholar] [PubMed]
- Najafi, A.; Daghigh Kia, H.; Mehdipour, M.; Shamsollahi, M.; Miller, D.J. Does fennel extract ameliorate oxidative stress frozen-thawed ram sperm? Cryobiology 2019, 87, 47–51. [Google Scholar] [CrossRef] [PubMed]
- Varela, E.; Rojas, M.; Restrepo, G. Membrane stability and mitochondrial activity of bovine sperm frozen with low-density lipoproteins and trehalose. Reprod. Domest. Anim. 2019. [Google Scholar] [CrossRef] [PubMed]
- Dashtestani, F.; Ghourchian, H.; Najafi, A. Silver-gold-apoferritin nanozyme for suppressing oxidative stress during cryopreservation. Mater. Sci. Eng. C. Mater. Biol. Appl. 2019, 94, 831–840. [Google Scholar] [CrossRef]
- Mishra, A.K.; Kumar, A.; Swain, D.K.; Yadav, S.; Nigam, R. Insights into pH regulatory mechanisms in mediating spermatozoa functions. Vet. World 2018, 11, 852–858. [Google Scholar] [CrossRef] [PubMed]
- Elinder, F.; Liin, S.I. Actions and Mechanisms of Polyunsaturated Fatty Acids on Voltage-Gated Ion Channels. Front. Physiol. 2017, 8, 43. [Google Scholar] [CrossRef]
- Hosseinzadeh Colagar, A.; Karimi, F.; Jorsaraei, S.G. Correlation of sperm parameters with semen lipid peroxidation and total antioxidants levels in astheno- and oligoasheno- teratospermic men. Iran. Red. Crescent. Med. J. 2013, 15, 780–785. [Google Scholar] [CrossRef]
- Tamburrino, L.; Marchiani, S.; Vicini, E.; Muciaccia, B.; Cambi, M.; Pellegrini, S.; Forti, G.; Muratori, M.; Baldi, E. Quantification of CatSper1 expression in human spermatozoa and relation to functional parameters. Hum. Reprod. 2015, 30, 1532–1544. [Google Scholar] [CrossRef]
- Liu, S.W.; Li, Y.; Zou, L.L.; Guan, Y.T.; Peng, S.; Zheng, L.X.; Deng, S.M.; Zhu, L.Y.; Wang, L.W.; Chen, L.X. Chloride channels are involved in sperm motility and are downregulated in spermatozoa from patients with asthenozoospermia. Asian J. Androl. 2017, 19, 418–424. [Google Scholar] [CrossRef]
- Sinha, A.; Singh, V.; Singh, S.; Yadav, S. Proteomic analyses reveal lower expression of TEX40 and ATP6V0A2 proteins related to calcium ion entry and acrosomal acidification in asthenozoospermic males. Life Sci. 2019, 218, 81–88. [Google Scholar] [CrossRef]
- Hashemitabar, M.; Sabbagh, S.; Orazizadeh, M.; Ghadiri, A.; Bahmanzadeh, M. A proteomic analysis on human sperm tail: Comparison between normozoospermia and asthenozoospermia. J. Assist. Reprod. Genet. 2015, 32, 853–863. [Google Scholar] [CrossRef]
- Agarwal, A.; Sharma, R.; Durairajanayagam, D.; Ayaz, A.; Cui, Z.; Willard, B.; Gopalan, B.; Sabanegh, E. Major protein alterations in spermatozoa from infertile men with unilateral varicocele. Reprod. Biol. Endocrinol. 2015, 13, 8. [Google Scholar] [CrossRef] [PubMed]
- Ursini, F.; Heim, S.; Kiess, M.; Maiorino, M.; Roveri, A.; Wissing, J.; Flohe, L. Dual function of the selenoprotein PHGPx during sperm maturation. Science 1999, 285, 1393–1396. [Google Scholar] [CrossRef] [PubMed]
- Bahr, G.F.; Engler, W.F. Considerations of volume, mass, DNA, and arrangement of mitochondria in the midpiece of bull spermatozoa. Exp. Cell Res. 1970, 60, 338–340. [Google Scholar] [CrossRef]
- Parker, N.; Vidal-Puig, A.; Brand, M.D. Stimulation of mitochondrial proton conductance by hydroxynonenal requires a high membrane potential. Biosci. Rep. 2008, 28, 83–88. [Google Scholar] [CrossRef]
- Hanukoglu, I.; Rapoport, R.; Weiner, L.; Sklan, D. Electron leakage from the mitochondrial NADPH–adrenodoxin reductase–adrenodoxin–P450scc (cholesterol side chain cleavage) system. Arch. Biochem. Biophys. 1993, 305, 489–498. [Google Scholar] [CrossRef]
- Amaral, A.; Ramalho-Santos, J. Assessment of mitochondrial potential: Implications for the correct monitoring of human sperm function. Int. J. Androl. 2010, 33, 180–186. [Google Scholar] [CrossRef]
- Zhang, W.D.; Zhang, Z.; Jia, L.T.; Zhang, L.L.; Fu, T.; Li, Y.S.; Wang, P.; Sun, L.; Shi, Y.; Zhang, H.Z. Oxygen free radicals and mitochondrial signaling in oligospermia and asthenospermia. Mol. Med. Rep. 2014, 10, 1875–1880. [Google Scholar] [CrossRef][Green Version]
- Amaral, A.; Castillo, J.; Estanyol, J.M.; Ballesca, J.L.; Ramalho-Santos, J.; Oliva, R. Human sperm tail proteome suggests new endogenous metabolic pathways. Mol. Cell Proteom. 2013, 12, 330–342. [Google Scholar] [CrossRef]
- Amaral, A.; Castillo, J.; Ramalho-Santos, J.; Oliva, R. The combined human sperm proteome: Cellular pathways and implications for basic and clinical science. Hu. Reprod. Update 2014, 20, 40–62. [Google Scholar] [CrossRef] [PubMed]
- Moscatelli, N.; Lunetti, P.; Braccia, C.; Armirotti, A.; Pisanello, F.; De Vittorio, M.; Zara, V.; Ferramosca, A. Comparative Proteomic Analysis of Proteins Involved in Bioenergetics Pathways Associated with Human Sperm Motility. Int. J. Mol. Sci. 2019, 20, 3000. [Google Scholar] [CrossRef] [PubMed]
- Aitken, R.J.; Whiting, S.; De Iuliis, G.N.; McClymont, S.; Mitchell, L.A.; Baker, M.A. Electrophilic aldehydes generated by sperm metabolism activate mitochondrial reactive oxygen species generation and apoptosis by targeting succinate dehydrogenase. J. Biol. Chem. 2012, 287, 33048–33060. [Google Scholar] [CrossRef] [PubMed]
- Carbone, D.L.; Doorn, J.A.; Kiebler, Z.; Sampey, B.P.; Petersen, D.R. Inhibition of Hsp72-mediated protein refolding by 4-hydroxy-2-nonenal. Chem. Res. Toxicol. 2004, 17, 1459–1467. [Google Scholar] [CrossRef]
- Carbone, D.L.; Doorn, J.A.; Petersen, D.R. 4-Hydroxynonenal regulates 26S proteasomal degradation of alcohol dehydrogenase. Free Radic. Biol. Med. 2004, 37, 1430–1439. [Google Scholar] [CrossRef]
- Amaral, A.; Paiva, C.; Attardo Parrinello, C.; Estanyol, J.M.; Ballescà, J.L.; Ramalho-Santos, J.; Oliva, R. Identification of proteins involved in human sperm motility using highthroughput differential proteomics. J. Proteome Res. 2014, 13, 5670–5684. [Google Scholar] [CrossRef]
- Guo, Y.; Jiang, W.; Yu, W.; Niu, X.; Liu, F.; Zhou, T.; Zhang, H.; Li, Y.; Zhu, H.; Zhou, Z.; et al. Proteomics analysis of asthenozoospermia and identification of glucose-6-phosphate isomerase as an important enzyme for sperm motility. J. Proteom. 2019, 208, 103478. [Google Scholar] [CrossRef]
- Miki, K.; Qu, W.; Goulding, E.H.; Willis, W.D.; Bunch, D.O.; Strader, L.F.; Perreault, S.D.; Eddy, E.M.; O’Brien, D.A. Glyceraldehyde 3-phosphate dehydrogenase-S, a sperm-specific glycolytic enzyme, is required for sperm motility and male fertility. Proc. Natl. Acad. Sci. USA 2004, 101, 16501–16506. [Google Scholar] [CrossRef]
- Elkina, Y.L.; Atroshchenko, M.M.; Bragina, E.E.; Muronetz, V.I.; Schmalhausen, E.V. Oxidation of glyceraldehyde-3-phosphate dehydrogenase decreases sperm motility. Biochemistry 2011, 76, 268–272. [Google Scholar] [CrossRef]
- Liu, J.; Wang, Y.; Gong, L.; Sun, C. Oxidation of glyceraldehyde-3-phosphate dehydrogenase decreases sperm motility in diabetes mellitus. Biochem. Biophys. Res. Commun. 2015, 465, 245–248. [Google Scholar] [CrossRef]
- Yang, Y.; Cheng, L.; Wang, Y.; Han, Y.; Liu, J.; Deng, X.; Chao, L. Expression of NDUFA13 in asthenozoospermia and possible pathogenesis. Reprod. Biomed. Online 2017, 34, 66–74. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Roberts, A.J.; Kon, T.; Knight, P.J.; Sutoh, K.; Burgess, S.A. Functions and mechanics of dynein motor proteins. Nat. Rev. Mol. Cell Biol. 2013, 14, 713–726. [Google Scholar] [CrossRef] [PubMed]
- Brokaw, C.J. Flagellar movement: A sliding filament model. Science 1972, 178, 455–462. [Google Scholar] [CrossRef] [PubMed]
- Zhao, W.; Li, Z.; Ping, P.; Wang, G.; Yuan, X.; Sun, F. Outer dense fibers stabilize the axoneme to maintain sperm motility. J. Cell Mol. Med. 2018, 22, 1755–1768. [Google Scholar] [CrossRef]
- Linck, R.W.; Chemes, H.; Albertini, D.F. The axoneme: The propulsive engine of spermatozoa and cilia and associated ciliopathies leading to infertility. J. Assist. Reprod. Genet. 2016, 33, 141–156. [Google Scholar] [CrossRef]
- Eddy, E.M.; Toshimori, K.; O’Brien, D.A. Fibrous sheath of mammalian spermatozoa. Microsc. Res. Tech. 2003, 61, 103–115. [Google Scholar] [CrossRef]
- Brown, P.R.; Miki, K.; Harper, D.B.; Eddy, E.M. A-kinase anchoring protein 4 binding proteins in the fibrous sheath of the sperm flagellum. Biol. Reprod. 2003, 68, 2241–2248. [Google Scholar] [CrossRef]
- Nixon, B.; Bernstein, I.; Cafe, S.L.; Delehedde, M.; Sergeant, N.; Eamens, A.L.; Lord, T.; Dun, M.D.; De Iuliis, G.N.; Bromfield, E.G. A Kinase Anchor Protein 4 is vulnerable to oxidative adduction in male germ cells. Front. Cell Dev. Biol. 2019. In press. [Google Scholar] [CrossRef]
- Miki, K.; Willis, W.D.; Brown, P.R.; Goulding, E.H.; Fulcher, K.D.; Eddy, E.M. Targeted disruption of the Akap4 gene causes defects in sperm flagellum and motility. Dev. Biol. 2002, 248, 331–342. [Google Scholar] [CrossRef]
- Ayaz, A.; Agarwal, A.; Sharma, R.; Kothandaraman, N.; Cakar, Z.; Sikka, S. Proteomic analysis of sperm proteins in infertile men with high levels of reactive oxygen species. Andrologia 2018, 50, e13015. [Google Scholar] [CrossRef]
- Nassar, A.; Mahony, M.; Morshedi, M.; Lin, M.H.; Srisombut, C.; Oehninger, S. Modulation of sperm tail protein tyrosine phosphorylation by pentoxifylline and its correlation with hyperactivated motility. Fertil. Steril. 1999, 71, 919–923. [Google Scholar] [CrossRef]
- Miyata, H.; Satouh, Y.; Mashiko, D.; Muto, M.; Nozawa, K.; Shiba, K.; Fujihara, Y.; Isotani, A.; Inaba, K.; Ikawa, M. Sperm calcineurin inhibition prevents mouse fertility with implications for male contraceptive. Science 2015, 350, 442–445. [Google Scholar] [CrossRef] [PubMed]
- Santiago, J.; Silva, J.V.; Fardilha, M. First Insights on the Presence of the Unfolded Protein Response in Human Spermatozoa. Int. J. Mol. Sci. 2019, 20, 5518. [Google Scholar] [CrossRef] [PubMed]
- Jovaisaite, V.; Mouchiroud, L.; Auwerx, J. The mitochondrial unfolded protein response, a conserved stress response pathway with implications in health and disease. J. Exp. Biol. 2014, 217, 137–143. [Google Scholar] [CrossRef]
- Zhang, G.; Ling, X.; Liu, K.; Wang, Z.; Zou, P.; Gao, J.; Cao, J.; Ao, L. The p-eIF2α/ATF4 pathway links endoplasmic reticulum stress to autophagy following the production of reactive oxygen species in mouse spermatocyte-derived cells exposed to dibutyl phthalate. Free Radic. Res. 2016, 50, 698–707. [Google Scholar] [CrossRef]
- Cocuzza, M.; Sikka, S.C.; Athayde, K.S.; Agarwal, A. Clinical relevance of oxidative stress and sperm chromatin damage in male infertility: An evidence based analysis. Int. Braz. J. Urol. 2007, 33, 603–621. [Google Scholar] [CrossRef]
- Xavier, M.J.; Nixon, B.; Roman, S.D.; Scott, R.J.; Drevet, J.R.; Aitken, R.J. Paternal impacts on development: Identification of genomic regions vulnerable to oxidative DNA damage in human spermatozoa. Hum. Reprod. 2019, 34, 1876–1890. [Google Scholar] [CrossRef]
- Xavier, M.J.; Nixon, B.; Roman, S.D.; Aitken, R.J. Improved methods of DNA extraction from human spermatozoa that mitigate experimentally-induced oxidative DNA damage. PLoS ONE 2018, 13, e0195003. [Google Scholar] [CrossRef]
- Kodama, H.; Yamaguchi, R.; Fukuda, J.; Kasai, H.; Tanaka, T. Increased oxidative deoxyribonucleic acid damage in the spermatozoa of infertile male patients. Fertil. Steril. 1997, 68, 519–524. [Google Scholar] [CrossRef]
- Piasecka, M.; Gaczarzewicz, D.; Laszczyńska, M.; Starczewski, A.; Brodowska, A. Flow cytometry application in the assessment of sperm DNA integrity of men with asthenozoospermia. Folia Histochem. Cytobiol. 2007, 45, S127–S136. [Google Scholar]
- Yakes, F.M.; Van Houten, B. Mitochondrial DNA damage is more extensive and persists longer than nuclear DNA damage in human cells following oxidative stress. Proc. Natl. Acad. Sci. USA 1997, 94, 514–519. [Google Scholar] [CrossRef] [PubMed]
- Salazar, J.J.; Van Houten, B. Preferential mitochondrial DNA injury caused by glucose oxidase as a steady generator of hydrogen peroxide in human fibroblasts. Mutat. Res. 1997, 385, 139–149. [Google Scholar] [CrossRef]
- Sawyer, D.E.; Mercer, B.G.; Wiklendt, A.M.; Aitken, R.J. Quantitative analysis of gene-specific DNA damage in human spermatozoa. Mutat. Res. 2003, 529, 21–34. [Google Scholar] [CrossRef]
- Díez-Sánchez, C.; Ruiz-Pesini, E.; Lapeña, A.C.; Montoya, J.; Pérez-Martos, A.; Enríquez, J.A.; López-Pérez, M.J. Mitochondrial DNA content of human spermatozoa. Biol. Reprod. 2003, 68, 180–185. [Google Scholar] [CrossRef]
- Amaral, A.; Ramalho-Santos, J.; St John, J.C. The expression of polymerase gamma and mitochondrial transcription factor A and the regulation of mitochondrial DNA content in mature human sperm. Hum. Reprod. 2007, 22, 1585–1596. [Google Scholar] [CrossRef]
- Song, G.J.; Lewis, V. Mitochondrial DNA integrity and copy number in sperm from infertile men. Fertil. Steril. 2008, 90, 2238–2244. [Google Scholar] [CrossRef]
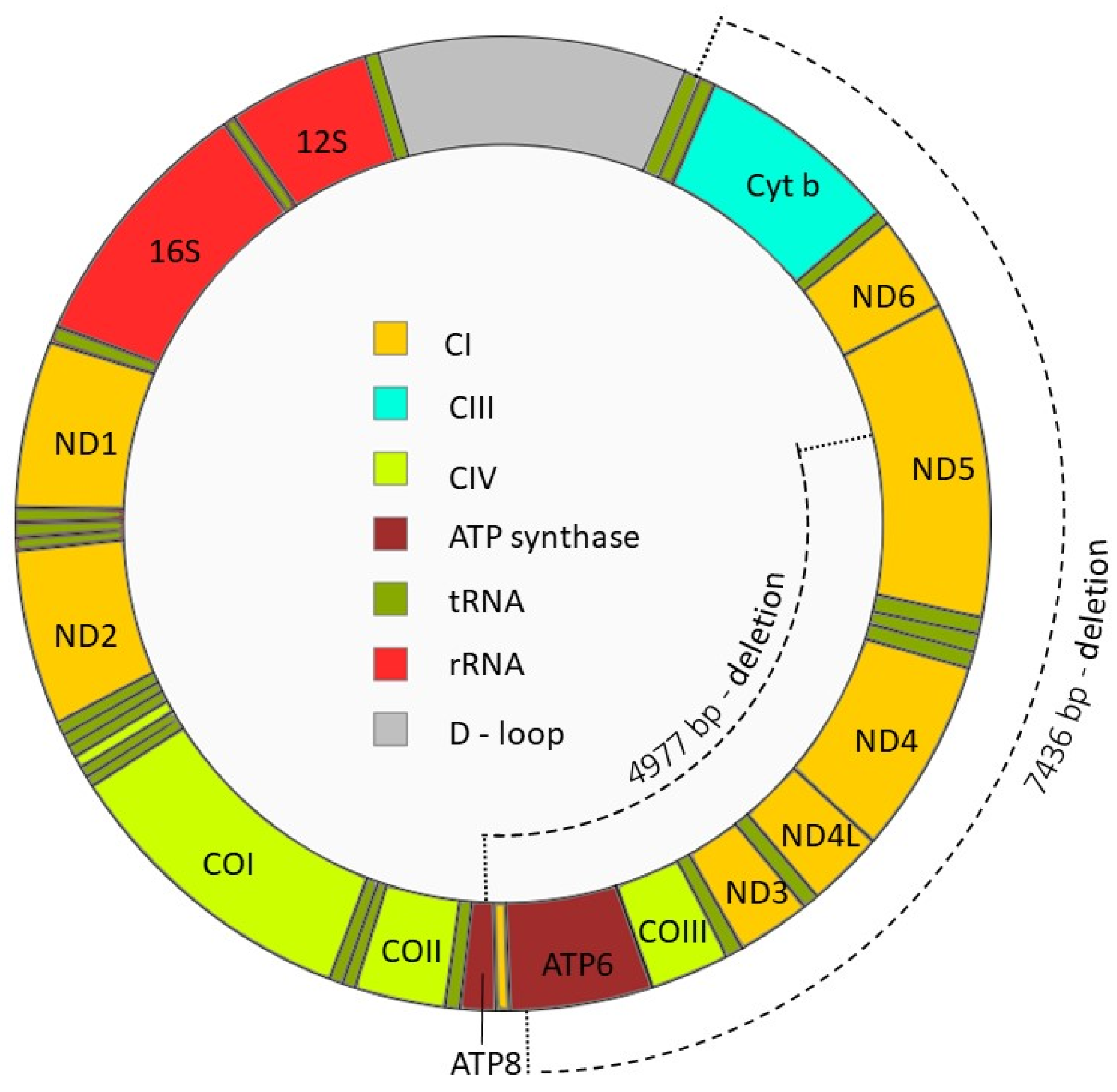
- Kao, S.; Chao, H.T.; Wei, Y.H. Mitochondrial deoxyribonucleic acid 4977-bp deletion is associated with diminished fertility and motility of human sperm. Biol. Reprod. 1995, 52, 729–736. [Google Scholar] [CrossRef]
- Gashti, N.G.; Salehi, Z.; Madani, A.H.; Dalivandan, S.T. 4977-bp mitochondrial DNA deletion in infertile patients with varicocele. Andrologia 2014, 46, 258–262. [Google Scholar] [CrossRef]
- Lin, P.H.; Lee, S.H.; Su, C.P.; Wei, Y.H. Oxidative damage to mitochondrial DNA in atrial muscle of patients with atrial fibrillation. Free Radic. Biol. Med. 2003, 35, 1310–1318. [Google Scholar] [CrossRef]
- Vecoli, C.; Borghini, A.; Pulignani, S.; Mercuri, A.; Turchi, S.; Carpeggiani, C.; Picano, E.; Andreassi, M.G. Prognostic value of mitochondrial DNA4977 deletion and mitochondrial DNA copy number in patients with stable coronary artery disease. Atherosclerosis 2018, 276, 91–97. [Google Scholar] [CrossRef]
- Dimberg, J.; Hong, T.T.; Nguyen, L.T.T.; Skarstedt, M.; Löfgren, S.; Matussek, A. Common 4977 bp deletion and novel alterations in mitochondrial DNA in Vietnamese patients with breast cancer. Springerplus 2015, 4, 58. [Google Scholar] [CrossRef][Green Version]
- Guo, Z.S.; Jin, C.L.; Yao, Z.J.; Wang, Y.M.; Xu, B.T. Analysis of the Mitochondrial 4977 Bp Deletion in Patients with Hepatocellular Carcinoma. Balkan J. Med. Genet. 2017, 20, 81–86. [Google Scholar] [CrossRef]
- Zhang, Y.; Ma, Y.; Bu, D.; Liu, H.; Xia, C.; Zhang, Y.; Zhu, S.; Pan, H.; Pei, P.; Zheng, X.; et al. Deletion of a 4977-bp Fragment in the Mitochondrial Genome Is Associated with Mitochondrial Disease Severity. PLoS ONE 2015, 10, e0128624. [Google Scholar] [CrossRef]
- Lee, H.C.; Pang, C.Y.; Hsu, H.S.; Wei, Y.H. Differential accumulations of 4,977 bp deletion in mitochondrial DNA of various tissues in human ageing. Biochim. Biophys. Acta. 1994, 1226, 37–43. [Google Scholar] [CrossRef]
- Ambulkar, P.S.; Chuadhari, A.R.; Pal, A.K. Association of large scale 4977-bp “common” deletions in sperm mitochondrial DNA with asthenozoospermia and oligoasthenoteratozoospermia. J. Hum. Reprod Sci. 2016, 9, 35–40. [Google Scholar] [CrossRef]
- Bahrehmand Namaghi, I.; Vaziri, H. Sperm mitochondrial DNA deletion in Iranian infertiles with asthenozoospermia. Andrologia 2017, 49. [Google Scholar] [CrossRef]
- Ambulkar, P.S.; Waghmare, J.E.; Chaudhari, A.R.; Wankhede, V.R.; Tarnekar, A.M.; Shende, M.R.; Pal, A.K. Large Scale 7436-bp Deletions in Human Sperm Mitochondrial DNA with Spermatozoa Dysfunction and Male Infertility. J. Clin. Diagn. Res. 2016, 10, GC09–GC12. [Google Scholar] [CrossRef]
- Cummins, J.M.; Jequier, A.M.; Martin, R.; Mehmet, D.; Goldblatt, J. Semen levels of mitochondrial DNA deletions in men attending an infertility clinic do not correlate with phenotype. Int. J. Androl. 1998, 21, 47–52. [Google Scholar] [CrossRef]
- St John, J.C.; Jokhi, R.P.; Barratt, C.L. Men with oligoasthenoteratozoospermia harbour higher numbers of multiple mitochondrial DNA deletions in their spermatozoa, but individual deletions are not indicative of overall aetiology. Mol. Hum. Reprod. 2001, 7, 103–111. [Google Scholar] [CrossRef]
- O’Flaherty, C. Orchestrating the antioxidant defenses in the epididymis. Andrology 2019, 7, 662–668. [Google Scholar] [CrossRef]
- Schneider, M.; Förster, H.; Boersma, A.; Seiler, A.; Wehnes, H.; Sinowatz, F.; Neumüller, C.; Deutsch, M.J.; Walch, A.; Hrabé de Angelis, M.; et al. Mitochondrial glutathione peroxidase 4 disruption causes male infertility. FASEB J. 2009, 23, 3233–3242. [Google Scholar] [CrossRef]
- Chabory, E.; Damon, C.; Lenoir, A.; Kauselmann, G.; Kern, H.; Zevnik, B.; Garrel, C.; Saez, F.; Cadet, R.; Henry-Berger, J.; et al. Epididymis seleno-independent glutathione peroxidase 5 maintains sperm DNA integrity in mice. J. Clin. Invest. 2009, 119, 2074–2085. [Google Scholar] [CrossRef]
- O’Flaherty, C.; de Souza, A.R. Hydrogen peroxide modifies human sperm peroxiredoxins in a dose-dependent manner. Biol. Reprod. 2011, 84, 238–247. [Google Scholar] [CrossRef]
- Dubuisson, M.; Vander Stricht, D.; Clippe, A.; Etienne, F.; Nauser, T.; Kissner, R.; Koppenol, W.H.; Rees, J.F.; Knoops, B. Human peroxiredoxin 5 is a peroxynitrite reductase. FEBS Lett. 2004, 571, 161–165. [Google Scholar] [CrossRef]
- Liu, Y.; O’Flaherty, C. In vivo oxidative stress alters thiol redox status of peroxiredoxin 1 and 6 and impairs rat sperm quality. Asian. J. Androl. 2017, 19, 73–79. [Google Scholar] [CrossRef]
- Fisher, A.B. Peroxiredoxin 6 in the repair of peroxidized cell membranes and cell signaling. Arch. Biochem. Biophys. 2017, 617, 68–83. [Google Scholar] [CrossRef]
- Fernandez, M.C.; O’Flaherty, C. Peroxiredoxin 6 is the primary antioxidant enzyme for the maintenance of viability and DNA integrity in human spermatozoa. Hum. Reprod. 2018, 33, 1394–1407. [Google Scholar] [CrossRef]
- Bansal, A.K.; Bilaspuri, G.S. Impacts of oxidative stress and antioxidants on semen functions. Vet. Med. Int. 2010, 2010, 686137. [Google Scholar] [CrossRef]
- Gong, S.; San Gabriel, M.C.; Zini, A.; Chan, P.; O’Flaherty, C. Low amounts and high thiol oxidation of peroxiredoxins in spermatozoa from infertile men. J. Androl. 2012, 33, 1342–1351. [Google Scholar] [CrossRef]
- Wichmann, L.; Vaalasti, A.; Vaalasti, T.; Tuohimaa, P. Localization of lactoferrin in the male reproductive tract. Int. J. Androl. 1989, 12, 179–186. [Google Scholar] [CrossRef]
- Pearl, C.A.; Roser, J.F. Expression of lactoferrin in the boar epididymis: Effects of reduced estrogen. Domest. Anim. Endocrinol. 2008, 34, 153–159. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Liu, B.; Wang, Z.; Niu, X.; Su, S.; Zhang, W.; Wang, X. Characterization of lactoferrin receptor on human spermatozoa. Reprod. Biomed. Online 2011, 22, 155–161. [Google Scholar] [CrossRef] [PubMed]
- Hamada, A.; Sharma, R.; du Plessis, S.S.; Willard, B.; Yadav, S.P.; Sabanegh, E.; Agarwal, A. Two-dimensional differential in-gel electrophoresis-based proteomics of male gametes in relation to oxidative stress. Fertil. Steril. 2013, 99, 1216–1226. [Google Scholar] [CrossRef]
- Montecinos, V.; Guzmán, P.; Barra, V.; Villagrán, M.; Muñoz-Montesino, C.; Sotomayor, K.; Escobar, E.; Godoy, A.; Mardones, L.; Sotomayor, P.; et al. Vitamin C is an essential antioxidant that enhances survival of oxidatively stressed human vascular endothelial cells in the presence of a vast molar excess of glutathione. J. Biol. Chem. 2007, 282, 15506–15515. [Google Scholar] [CrossRef]
- Nouri, M.; Ghasemzadeh, A.; Farzadi, L.; Shahnazi, V.; Ghaffari-Novin, M. Vitamins C, E and lipid peroxidation levels in sperm and seminal plasma of asthenoteratozoospermic and normozoospermic men. Iran. J. Reprod. Med. 2008, 6, 1–5. [Google Scholar]
- Micheli, L.; Cerretani, D.; Collodel, G.; Menchiari, A.; Moltoni, L.; Fiaschi, A.I.; Moretti, E. Evaluation of enzymatic and non-enzymatic antioxidants in seminal plasma of men with genitourinary infections, varicocele and idiopathic infertility. Andrology 2016, 4, 456–464. [Google Scholar] [CrossRef]
- Huang, C.; Cao, X.; Pang, D.; Li, C.; Luo, Q.; Zou, Y.; Feng, B.; Li, L.; Cheng, A.; Chen, Z. Is male infertility associated with increased oxidative stress in seminal plasma? A-meta analysis. Oncotarget 2018, 9, 24494–24513. [Google Scholar] [CrossRef]
- O’Flaherty, C.; Matsushita-Fournier, D. Reactive oxygen species and protein modifications in spermatozoa. Biol. Reprod. 2017, 97, 577–585. [Google Scholar] [CrossRef]
- Piomboni, P.; Gambera, L.; Serafini, F.; Campanella, G.; Morgante, G.; De Leo, V. Sperm quality improvement after natural anti-oxidant treatment of asthenoteratospermic men with leukocytospermia. Asian J. Androl. 2008, 10, 201–206. [Google Scholar] [CrossRef]
- Akmal, M.; Qadri, J.Q.; Al-Waili, N.S.; Thangal, S.; Haq, A.; Saloom, K.Y. Improvement in human semen quality after oral supplementation of vitamin C. J. Med. Food. 2006, 9, 440–442. [Google Scholar] [CrossRef]
- Omu, A.E.; Al-Azemi, M.K.; Kehinde, E.O.; Anim, J.T.; Oriowo, M.A.; Mathew, T.C. Indications of the mechanisms involved in improved sperm parameters by zinc therapy. Med. Princ. Pract. 2008, 17, 108–116. [Google Scholar] [CrossRef] [PubMed]
- Garolla, A.; Maiorino, M.; Roverato, A.; Roveri, A.; Ursini, F.; Foresta, C. Oral carnitine supplementation increases sperm motility in asthenozoospermic men with normal sperm phospholipid hydroperoxide glutathione peroxidase levels. Fertil. Steril. 2005, 83, 355–361. [Google Scholar] [CrossRef] [PubMed]
- Sigman, M.; Glass, S.; Campagnone, J.; Pryor, J.L. Carnitine for the treatment of idiopathic asthenospermia: A randomized, double-blind, placebo-controlled trial. Fertil. Steril. 2006, 85, 1409–1414. [Google Scholar] [CrossRef] [PubMed]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nowicka-Bauer, K.; Nixon, B. Molecular Changes Induced by Oxidative Stress that Impair Human Sperm Motility. Antioxidants 2020, 9, 134. https://doi.org/10.3390/antiox9020134
Nowicka-Bauer K, Nixon B. Molecular Changes Induced by Oxidative Stress that Impair Human Sperm Motility. Antioxidants. 2020; 9(2):134. https://doi.org/10.3390/antiox9020134
Chicago/Turabian StyleNowicka-Bauer, Karolina, and Brett Nixon. 2020. "Molecular Changes Induced by Oxidative Stress that Impair Human Sperm Motility" Antioxidants 9, no. 2: 134. https://doi.org/10.3390/antiox9020134
APA StyleNowicka-Bauer, K., & Nixon, B. (2020). Molecular Changes Induced by Oxidative Stress that Impair Human Sperm Motility. Antioxidants, 9(2), 134. https://doi.org/10.3390/antiox9020134




