Non-Enzymatic Antioxidants against Alzheimer’s Disease: Prevention, Diagnosis and Therapy
Abstract
:1. Introduction
2. Results
2.1. Carotenoids
2.2. Vitamins
2.3. Flavonoids
2.4. Non-flavonoids
2.5. Organosulfur Compounds
2.6. Mitochondria-Targeted Antioxidants
2.7. Minerals
3. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- DeTure, M.A.; Dickson, D.W. The neuropathological diagnosis of Alzheimer’s disease. Mol. Neurodegener. 2019, 14, 32. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hodson, R. Alzheimer’s disease. Nature 2018, 559, S1. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Husna Ibrahim, N.; Yahaya, M.F.; Mohamed, W.; Teoh, S.L.; Hui, C.K.; Kumar, J. Pharmacotherapy of Alzheimer’s Disease: Seeking Clarity in a Time of Uncertainty. Front. Pharmacol. 2020, 11, 261. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Breijyeh, Z.; Karaman, R. Comprehensive Review on Alzheimer’s Disease: Causes and Treatment. Molecules 2020, 25, 5789. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.-J.; Zhang, X.; Chen, W.-W. Role of oxidative stress in Alzheimer’s disease. Biomed. Rep. 2016, 4, 519–522. [Google Scholar] [CrossRef] [Green Version]
- Nunomura, A.; Castellani, R.J.; Zhu, X.; Moreira, P.I.; Perry, G.; Smith, M.A. Involvement of Oxidative Stress in Alzheimer Disease. J. Neuropathol. Exp. Neurol. 2006, 65, 631–641. [Google Scholar] [CrossRef] [Green Version]
- Buccellato, F.R.; D’Anca, M.; Fenoglio, C.; Scarpini, E.; Galimberti, D. Role of Oxidative Damage in Alzheimer’s Disease and Neurodegeneration: From Pathogenic Mechanisms to Biomarker Discovery. Antioxidants 2021, 10, 1353. [Google Scholar] [CrossRef]
- Baldeiras, I.; Santana, I.; Proença, M.T.; Garrucho, M.H.; Pascoal, R.; Rodrigues, A.; Duro, D.; Oliveira, C.R. Peripheral Oxidative Damage in Mild Cognitive Impairment and Mild Alzheimer’s Disease. J. Alzheimers Dis. 2008, 15, 117–128. [Google Scholar] [CrossRef]
- Greilberger, J.; Koidl, C.; Greilberger, M.; Lamprecht, M.; Schroecksnadel, K.; Leblhuber, F.; Fuchs, D.; Oettl, K. Malondialdehyde, carbonyl proteins and albumin-disulphide as useful oxidative markers in mild cognitive impairment and Alzheimer’s disease. Free Radic. Res. 2008, 42, 633–638. [Google Scholar] [CrossRef]
- Melo, J.B.; Agostinho, P.; Oliveira, C.R. Involvement of oxidative stress in the enhancement of acetylcholinesterase activity induced by amyloid beta-peptide. Neurosci. Res. 2003, 45, 117–127. [Google Scholar] [CrossRef]
- A Massaad, C. Neuronal and Vascular Oxidative Stress in Alzheimers Disease. Curr. Neuropharmacol. 2011, 9, 662–673. [Google Scholar] [CrossRef] [Green Version]
- Manoharan, S.; Guillemin, G.J.; Abiramasundari, R.S.; Essa, M.M.; Akbar, M.; Akbar, M.D. The Role of Reactive Oxygen Species in the Pathogenesis of Alzheimer’s Disease, Parkinson’s Disease, and Huntington’s Disease: A Mini Review. Oxid. Med. Cell. Longev. 2016, 2016, 8590578. [Google Scholar] [CrossRef]
- Kim, T.-S.; Pae, C.-U.; Yoon, S.-J.; Jang, W.-Y.; Lee, N.J.; Kim, J.-J.; Lee, S.-J.; Lee, C.; Paik, I.-H.; Lee, C.-U. Decreased plasma antioxidants in patients with Alzheimer’s disease. Int. J. Geriatr. Psychiatry 2006, 21, 344–348. [Google Scholar] [CrossRef]
- Tönnies, E.; Trushina, E. Oxidative Stress, Synaptic Dysfunction, and Alzheimer’s Disease. J. Alzheimers Dis. 2017, 57, 1105–1121. [Google Scholar] [CrossRef] [Green Version]
- Rahman, T.; Hosen, I.; Islam, M.M.T.; Shekhar, H.U. Oxidative stress and human health. Adv. Biosci. Biotechnol. 2012, 03, 997–1019. [Google Scholar] [CrossRef] [Green Version]
- Cheignon, C.; Tomas, M.; Bonnefont-Rousselot, D.; Faller, P.; Hureau, C.; Collin, F. Oxidative stress and the amyloid beta peptide in Alzheimer’s disease. Redox Biol. 2018, 14, 450–464. [Google Scholar] [CrossRef]
- Montaser, A.; Huttunen, J.; Ibrahim, S.A.; Huttunen, K.M. Astrocyte-Targeted Transporter-Utilizing Derivatives of Ferulic Acid Can Have Multifunctional Effects Ameliorating Inflammation and Oxidative Stress in the Brain. Oxid. Med. Cell. Longev. 2019, 2019, 3528148. [Google Scholar] [CrossRef] [Green Version]
- Butterfield, D.A.; Halliwell, B. Oxidative stress, dysfunctional glucose metabolism and Alzheimer disease. Nat. Rev. Neurosci. 2019, 20, 148–160. [Google Scholar] [CrossRef]
- Elfawy, H.A.; Das, B. Crosstalk between mitochondrial dysfunction, oxidative stress, and age related neurodegenerative disease: Etiologies and therapeutic strategies. Life Sci. 2019, 218, 165–184. [Google Scholar] [CrossRef]
- Papagiouvannis, G.; Theodosis-Nobelos, P.; Kourounakis, P.N.; Rekka, E.A. Multi-Target Directed Compounds with Antioxidant and/or Anti-Inflammatory Properties as Potent Agents for Alzheimer’s Disease. Med. Chem. 2021, 17, 1086–1103. [Google Scholar] [CrossRef]
- Winiarska-Mieczan, A.; Baranowska-Wójcik, E.; Kwiecień, M.; Grela, E.R.; Szwajgier, D.; Kwiatkowska, K.; Kiczorowska, B. The Role of Dietary Antioxidants in the Pathogenesis of Neurodegenerative Diseases and Their Impact on Cerebral Oxidoreductive Balance. Nutrients 2020, 12, 435. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zheng, C.; Zhou, X.-W.; Wang, J.-Z. The dual roles of cytokines in Alzheimer’s disease: Update on interleukins, TNF-α, TGF-β and IFN-γ. Transl. Neurodegener. 2016, 5, 7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, H.-A.; Hayden, M.M.; Bannerman, S.; Jansen, J.; Crowe-White, K.M. Anti-Apoptotic Effects of Carotenoids in Neurodegeneration. Molecules 2020, 25, 3453. [Google Scholar] [CrossRef] [PubMed]
- Young, A.; Lowe, G. Carotenoids—Antioxidant Properties. Antioxidants 2018, 7, 28. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leermakers, E.T.; Darweesh, S.K.; Baena, C.P.; Moreira, E.M.; Melo van Lent, D.; Tielemans, M.J.; Muka, T.; Vitezova, A.; Chowdhury, R.; Bramer, W.M.; et al. The effects of lutein on cardiometabolic health across the life course: A systematic review and meta-analysis1,2. Am. J. Clin. Nutr. 2016, 103, 481–494. [Google Scholar] [CrossRef] [Green Version]
- Tan, B.L.; Norhaizan, M.E. Carotenoids: How Effective Are They to Prevent Age-Related Diseases? Molecules 2019, 24, 1801. [Google Scholar] [CrossRef] [Green Version]
- Craft, N.E.; Haitema, T.B.; Garnett, K.M.; Fitch, K.A.; Dorey, C.K. Carotenoid, tocopherol, and retinol concentrations in elderly human brain. J. Nutr. Health Aging 2004, 8, 156–162. [Google Scholar]
- Kabir, M.T.; Rahman, M.H.; Shah, M.; Jamiruddin, M.R.; Basak, D.; Al-Harrasi, A.; Bhatia, S.; Ashraf, G.M.; Najda, A.; El-kott, A.F.; et al. Therapeutic promise of carotenoids as antioxidants and anti-inflammatory agents in neurodegenerative disorders. Biomed. Pharmacother. 2022, 146, 112610. [Google Scholar] [CrossRef]
- Mullan, K.; Williams, M.A.; Cardwell, C.R.; McGuinness, B.; Passmore, P.; Silvestri, G.; Woodside, J.V.; McKay, G.J. Serum concentrations of vitamin E and carotenoids are altered in Alzheimer’s disease: A case-control study. Alzheimers Dement. Transl. Res. Clin. Interv. 2017, 3, 432–439. [Google Scholar] [CrossRef] [Green Version]
- Polidori, M.C.; Mecocci, P. Plasma susceptibility to free radical-induced antioxidant consumption and lipid peroxidation is increased in very old subjects with Alzheimer disease. J. Alzheimers Dis. 2002, 4, 517–522. [Google Scholar] [CrossRef]
- Yuan, C.; Chen, H.; Wang, Y.; Schneider, J.A.; Willett, W.C.; Morris, M.C. Dietary carotenoids related to risk of incident Alzheimer dementia (AD) and brain AD neuropathology: A community-based cohort of older adults. Am. J. Clin. Nutr. 2021, 113, 200–208. [Google Scholar] [CrossRef]
- Beydoun, M.A.; Beydoun, H.A.; Fanelli-Kuczmarski, M.T.; Weiss, J.; Hossain, S.; Canas, J.A.; Evans, M.K.; Zonderman, A.B. Association of Serum Antioxidant Vitamins and Carotenoids with Incident Alzheimer Disease and All-Cause Dementia Among US Adults. Neurology 2022, 98, e2150–e2162. [Google Scholar] [CrossRef]
- Feart, C.; Letenneur, L.; Helmer, C.; Samieri, C.; Schalch, W.; Etheve, S.; Delcourt, C.; Dartigues, J.-F.; Barberger-Gateau, P. Plasma Carotenoids Are Inversely Associated With Dementia Risk in an Elderly French Cohort. J. Gerontol. Ser. A 2016, 71, 683–688. [Google Scholar] [CrossRef] [Green Version]
- Min, J.; Min, K. Serum Lycopene, Lutein and Zeaxanthin, and the Risk of Alzheimer’s Disease Mortality in Older Adults. Dement. Geriatr. Cogn. Disord. 2014, 37, 246–256. [Google Scholar] [CrossRef]
- Qu, M.; Shi, H.; Wang, K.; Wang, X.; Yu, N.; Guo, B. The Associations of Plasma/Serum Carotenoids with Alzheimer’s Disease: A Systematic Review and Meta-Analysis. J. Alzheimers Dis. 2021, 82, 1055–1066. [Google Scholar] [CrossRef]
- Nolan, J.M.; Loskutova, E.; Howard, A.N.; Moran, R.; Mulcahy, R.; Stack, J.; Bolger, M.; Dennison, J.; Akuffo, K.O.; Owens, N.; et al. Macular Pigment, Visual Function, and Macular Disease among Subjects with Alzheimer’s Disease: An Exploratory Study. J. Alzheimers Dis. 2014, 42, 1191–1202. [Google Scholar] [CrossRef] [Green Version]
- Wang, W.; Shinto, L.; Connor, W.E.; Quinn, J.F. Nutritional Biomarkers in Alzheimer’s Disease: The Association between Carotenoids, n-3 Fatty Acids, and Dementia Severity. J. Alzheimers Dis. 2008, 13, 31–38. [Google Scholar] [CrossRef]
- Palacios, N.; Lee, J.S.; Scott, T.; Kelly, R.S.; Bhupathiraju, S.N.; Bigornia, S.J.; Tucker, K.L. Circulating Plasma Metabolites and Cognitive Function in a Puerto Rican Cohort. J. Alzheimers Dis. 2020, 76, 1267–1280. [Google Scholar] [CrossRef]
- Boccardi, V.; Arosio, B.; Cari, L.; Bastiani, P.; Scamosci, M.; Casati, M.; Ferri, E.; Bertagnoli, L.; Ciccone, S.; Rossi, P.D.; et al. Beta-carotene, telomerase activity and Alzheimer’s disease in old age subjects. Eur. J. Nutr. 2020, 59, 119–126. [Google Scholar] [CrossRef]
- Jimenez-Jimenez, F.J.; Molina, J.A.; Bustos, F.; Orti-Pareja, M.; Benito-Leon, J.; Tallon-Barranco, A.; Gasalla, T.; Porta, J.; Arenas, J. Serum levels of beta-carotene, alpha-carotene and vitamin A in patients with Alzheimer’s disease. Eur. J. Neurol. 1999, 6, 495–497. [Google Scholar] [CrossRef]
- Li, F.-J.; Shen, L.; Ji, H.-F. Dietary Intakes of Vitamin E, Vitamin C, and β-Carotene and Risk of Alzheimer’s Disease: A Meta-Analysis. J. Alzheimers Dis. 2012, 31, 253–258. [Google Scholar] [CrossRef] [PubMed]
- Schippling, S.; Kontush, A.; Arlt, S.; Buhmann, C.; Stürenburg, H.-J.; Mann, U.; Müller-Thomsen, T.; Beisiegel, U. Increased lipoprotein oxidation in alzheimer’s disease. Free Radic. Biol. Med. 2000, 28, 351–360. [Google Scholar] [CrossRef] [PubMed]
- Stuerenburg, H.J.; Ganzer, S.; Müller-Thomsen, T. Plasma beta carotene in Alzheimer’s disease. Association with cerebrospinal fluid beta-amyloid 1-40, (Abeta40), beta-amyloid 1-42 (Abeta42) and total Tau. Neuro Endocrinol. Lett. 2005, 26, 696–698. [Google Scholar] [PubMed]
- Koch, M.; Furtado, J.D.; Cronjé, H.T.; DeKosky, S.T.; Fitzpatrick, A.L.; Lopez, O.L.; Kuller, L.H.; Mukamal, K.J.; Jensen, M.K. Plasma antioxidants and risk of dementia in older adults. Alzheimers Dement. Transl. Res. Clin. Interv. 2021, 7, e12208. [Google Scholar] [CrossRef] [PubMed]
- Hira, S.; Saleem, U.; Anwar, F.; Sohail, M.F.; Raza, Z.; Ahmad, B. β-Carotene: A Natural Compound Improves Cognitive Impairment and Oxidative Stress in a Mouse Model of Streptozotocin-Induced Alzheimer’s Disease. Biomolecules 2019, 9, 441. [Google Scholar] [CrossRef] [Green Version]
- Butler, M.; Nelson, V.A.; Davila, H.; Ratner, E.; Fink, H.A.; Hemmy, L.S.; McCarten, J.R.; Barclay, T.R.; Brasure, M.; Kane, R.L. Over-the-Counter Supplement Interventions to Prevent Cognitive Decline, Mild Cognitive Impairment, and Clinical Alzheimer-Type Dementia: A Systematic Review. Ann. Intern. Med. 2018, 168, 52. [Google Scholar] [CrossRef]
- Liu, X.; Dhana, K.; Furtado, J.D.; Agarwal, P.; Aggarwal, N.T.; Tangney, C.; Laranjo, N.; Carey, V.; Barnes, L.L.; Sacks, F.M. Higher circulating α-carotene was associated with better cognitive function: An evaluation among the MIND trial participants. J. Nutr. Sci. 2021, 10, e64. [Google Scholar] [CrossRef]
- Ratto, F.; Franchini, F.; Musicco, M.; Caruso, G.; Di Santo, S.G. A narrative review on the potential of tomato and lycopene for the prevention of Alzheimer’s disease and other dementias. Crit. Rev. Food Sci. Nutr. 2022, 62, 4970–4981. [Google Scholar] [CrossRef]
- Chen, W.; Mao, L.; Xing, H.; Xu, L.; Fu, X.; Huang, L.; Huang, D.; Pu, Z.; Li, Q. Lycopene attenuates Aβ1–42 secretion and its toxicity in human cell and Caenorhabditis elegans models of Alzheimer disease. Neurosci. Lett. 2015, 608, 28–33. [Google Scholar] [CrossRef]
- Liu, C.-B.; Wang, R.; Yi, Y.-F.; Gao, Z.; Chen, Y.-Z. Lycopene mitigates β-amyloid induced inflammatory response and inhibits NF-κB signaling at the choroid plexus in early stages of Alzheimer’s disease rats. J. Nutr. Biochem. 2018, 53, 66–71. [Google Scholar] [CrossRef]
- Sachdeva, A.K.; Chopra, K. Lycopene abrogates Aβ(1–42)-mediated neuroinflammatory cascade in an experimental model of Alzheimer’s disease. J. Nutr. Biochem. 2015, 26, 736–744. [Google Scholar] [CrossRef]
- Wang, J.; Li, L.; Wang, Z.; Cui, Y.; Tan, X.; Yuan, T.; Liu, Q.; Liu, Z.; Liu, X. Supplementation of lycopene attenuates lipopolysaccharide-induced amyloidogenesis and cognitive impairments via mediating neuroinflammation and oxidative stress. J. Nutr. Biochem. 2018, 56, 16–25. [Google Scholar] [CrossRef]
- Xu, X.; Teng, Y.; Zou, J.; Ye, Y.; Song, H.; Wang, Z. Effects of lycopene on vascular remodeling through the LXR–PI3K–AKT signaling pathway in APP/PS1 mice. Biochem. Biophys. Res. Commun. 2020, 526, 699–705. [Google Scholar] [CrossRef]
- Yu, L.; Wang, W.; Pang, W.; Xiao, Z.; Jiang, Y.; Hong, Y. Dietary Lycopene Supplementation Improves Cognitive Performances in Tau Transgenic Mice Expressing P301L Mutation via Inhibiting Oxidative Stress and Tau Hyperphosphorylation. J. Alzheimers Dis. 2017, 57, 475–482. [Google Scholar] [CrossRef]
- Fang, Y.; Ou, S.; Wu, T.; Zhou, L.; Tang, H.; Jiang, M.; Xu, J.; Guo, K. Lycopene alleviates oxidative stress via the PI3K/Akt/Nrf2pathway in a cell model of Alzheimer’s disease. PeerJ 2020, 8, e9308. [Google Scholar] [CrossRef]
- Huang, C.; Wen, C.; Yang, M.; Gan, D.; Fan, C.; Li, A.; Li, Q.; Zhao, J.; Zhu, L.; Lu, D. Lycopene protects against t-BHP-induced neuronal oxidative damage and apoptosis via activation of the PI3K/Akt pathway. Mol. Biol. Rep. 2019, 46, 3387–3397. [Google Scholar] [CrossRef]
- Lim, S.; Hwang, S.; Yu, J.H.; Lim, J.W.; Kim, H. Lycopene inhibits regulator of calcineurin 1-mediated apoptosis by reducing oxidative stress and down-regulating Nucling in neuronal cells. Mol. Nutr. Food Res. 2017, 61, 1600530. [Google Scholar] [CrossRef]
- Qu, M.; Jiang, Z.; Liao, Y.; Song, Z.; Nan, X. Lycopene Prevents Amyloid [Beta]-Induced Mitochondrial Oxidative Stress and Dysfunctions in Cultured Rat Cortical Neurons. Neurochem. Res. 2016, 41, 1354–1364. [Google Scholar] [CrossRef]
- Qu, M.; Li, L.; Chen, C.; Li, M.; Pei, L.; Chu, F.; Yang, J.; Yu, Z.; Wang, D.; Zhou, Z. Protective effects of lycopene against amyloid β-induced neurotoxicity in cultured rat cortical neurons. Neurosci. Lett. 2011, 505, 286–290. [Google Scholar] [CrossRef]
- Hwang, S.; Lim, J.; Kim, H. Inhibitory Effect of Lycopene on Amyloid-β-Induced Apoptosis in Neuronal Cells. Nutrients 2017, 9, 883. [Google Scholar] [CrossRef] [Green Version]
- Huang, C.; Gan, D.; Fan, C.; Wen, C.; Li, A.; Li, Q.; Zhao, J.; Wang, Z.; Zhu, L.; Lu, D. The Secretion from Neural Stem Cells Pretreated with Lycopene Protects against tert-Butyl Hydroperoxide-Induced Neuron Oxidative Damage. Oxid. Med. Cell. Longev. 2018, 2018, 5490218. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Liu, C.; Wang, R.; Gao, X.; Hao, C.; Liu, C. A combination of lycopene and human amniotic epithelial cells can ameliorate cognitive deficits and suppress neuroinflammatory signaling by choroid plexus in Alzheimer’s disease rat. J. Nutr. Biochem. 2021, 88, 108558. [Google Scholar] [CrossRef] [PubMed]
- Bun, S.; Ikejima, C.; Kida, J.; Yoshimura, A.; Lebowitz, A.J.; Kakuma, T.; Asada, T. A Combination of Supplements May Reduce the Risk of Alzheimer’s Disease in Elderly Japanese with Normal Cognition. J. Alzheimers Dis. 2015, 45, 15–25. [Google Scholar] [CrossRef] [PubMed]
- Crowe-White, K.M.; Phillips, T.A.; Ellis, A.C. Lycopene and cognitive function. J. Nutr. Sci. 2019, 8, e20. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ning, W.; Lv, R.; Xu, N.; Hou, X.; Shen, C.; Guo, Y.; Fan, Z.; Cao, N.; Liu, X.-P. Lycopene-Loaded Microemulsion Regulates Neurogenesis in Rats with Aβ-Induced Alzheimer’s Disease Rats Based on the Wnt/β-catenin Pathway. Neural Plast. 2021, 2021, 5519330. [Google Scholar] [CrossRef]
- Ashok, A.; Singh, N.; Chaudhary, S.; Bellamkonda, V.; Kritikos, A.E.; Wise, A.S.; Rana, N.; McDonald, D.; Ayyagari, R. Retinal Degeneration and Alzheimer’s Disease: An Evolving Link. Int. J. Mol. Sci. 2020, 21, 7290. [Google Scholar] [CrossRef]
- Hammond, B.R.; Renzi-Hammond, L. The influence of the macular carotenoids on women’s eye and brain health. Nutr. Neurosci. 2022, 1–7. [Google Scholar] [CrossRef]
- Wang, H.; Wang, G.; Billings, R.; Li, D.; Haase, S.R.; Wheeler, P.F.; Vance, D.E.; Li, W. Can Diet Supplements of Macular Pigment of Lutein, Zeaxanthin, and Meso-zeaxanthin Affect Cognition? J. Alzheimers Dis. 2022, 87, 1079–1087. [Google Scholar] [CrossRef]
- Ademowo, O.S.; Dias, I.H.K.; Diaz-Sanchez, L.; Sanchez-Aranguren, L.; Stahl, W.; Griffiths, H.R. Partial Mitigation of Oxidized Phospholipid-Mediated Mitochondrial Dysfunction in Neuronal Cells by Oxocarotenoids. J. Alzheimers Dis. 2020, 74, 113–126. [Google Scholar] [CrossRef] [Green Version]
- Singhrang, N.; Tocharus, C.; Thummayot, S.; Sutheerawattananonda, M.; Tocharus, J. Protective effects of silk lutein extract from Bombyx mori cocoons on β-Amyloid peptide-induced apoptosis in PC12 cells. Biomed. Pharmacother. 2018, 103, 582–587. [Google Scholar] [CrossRef]
- Li, X.; Zhang, P.; Li, H.; Yu, H.; Xi, Y. The Protective Effects of Zeaxanthin on Amyloid-β Peptide 1–42-Induced Impairment of Learning and Memory Ability in Rats. Front. Behav. Neurosci. 2022, 16, 912896. [Google Scholar] [CrossRef]
- Zhang, L.-N.; Li, M.-J.; Shang, Y.-H.; Liu, Y.-R.; Han-Chang, H.; Lao, F.-X. Zeaxanthin Attenuates the Vicious Circle Between Endoplasmic Reticulum Stress and Tau Phosphorylation: Involvement of GSK-3β Activation. J. Alzheimers Dis. 2022, 86, 191–204. [Google Scholar] [CrossRef]
- Power, R.; Coen, R.F.; Beatty, S.; Mulcahy, R.; Moran, R.; Stack, J.; Howard, A.N.; Nolan, J.M. Supplemental Retinal Carotenoids Enhance Memory in Healthy Individuals with Low Levels of Macular Pigment in A Randomized, Double-Blind, Placebo-Controlled Clinical Trial. J. Alzheimers Dis. 2018, 61, 947–961. [Google Scholar] [CrossRef] [Green Version]
- Stringham, N.T.; Holmes, P.V.; Stringham, J.M. Effects of macular xanthophyll supplementation on brain-derived neurotrophic factor, pro-inflammatory cytokines, and cognitive performance. Physiol. Behav. 2019, 211, 112650. [Google Scholar] [CrossRef]
- Chew, E.Y.; Clemons, T.E.; Agrón, E.; Launer, L.J.; Grodstein, F.; Bernstein, P.S. Effect of Omega-3 Fatty Acids, Lutein/Zeaxanthin, or Other Nutrient Supplementation on Cognitive Function: The AREDS2 Randomized Clinical Trial. JAMA 2015, 314, 791. [Google Scholar] [CrossRef]
- Nolan, J.M.; Loskutova, E.; Howard, A.; Mulcahy, R.; Moran, R.; Stack, J.; Bolger, M.; Coen, R.F.; Dennison, J.; Akuffo, K.O.; et al. The Impact of Supplemental Macular Carotenoids in Alzheimer’s Disease: A Randomized Clinical Trial. J. Alzheimers Dis. 2015, 44, 1157–1169. [Google Scholar] [CrossRef] [Green Version]
- Ademowo, O.S.; Dias, H.K.I.; Milic, I.; Devitt, A.; Moran, R.; Mulcahy, R.; Howard, A.N.; Nolan, J.M.; Griffiths, H.R. Phospholipid oxidation and carotenoid supplementation in Alzheimer’s disease patients. Free Radic. Biol. Med. 2017, 108, 77–85. [Google Scholar] [CrossRef] [Green Version]
- Nolan, J.M.; Mulcahy, R.; Power, R.; Moran, R.; Howard, A.N. Nutritional Intervention to Prevent Alzheimer’s Disease: Potential Benefits of Xanthophyll Carotenoids and Omega-3 Fatty Acids Combined. J. Alzheimers Dis. 2018, 64, 367–378. [Google Scholar] [CrossRef] [Green Version]
- Power, R.; Nolan, J.M.; Prado-Cabrero, A.; Roche, W.; Coen, R.; Power, T.; Mulcahy, R. Omega-3 fatty acid, carotenoid and vitamin E supplementation improves working memory in older adults: A randomised clinical trial. Clin. Nutr. 2022, 41, 405–414. [Google Scholar] [CrossRef]
- Dhas, N.; Mehta, T. Cationic biopolymer functionalized nanoparticles encapsulating lutein to attenuate oxidative stress in effective treatment of Alzheimer’s disease: A non-invasive approach. Int. J. Pharm. 2020, 586, 119553. [Google Scholar] [CrossRef]
- Grundman, M. Vitamin E and Alzheimer disease: The basis for additional clinical trials. Am. J. Clin. Nutr. 2000, 71, 630S–636S. [Google Scholar] [CrossRef] [PubMed]
- Boothby, L.A.; Doering, P.L. Vitamin C and Vitamin E for Alzheimer’s Disease. Ann. Pharmacother. 2005, 39, 2073–2080. [Google Scholar] [CrossRef] [PubMed]
- Aisen, P.S.; Schneider, L.S.; Sano, M.; Diaz-Arrastia, R.; van Dyck, C.H.; Weiner, M.F.; Bottiglieri, T.; Jin, S.; Stokes, K.S.; Thomas, R.G.; et al. High-Dose B Vitamin Supplementation and Cognitive Decline in Alzheimer Disease: A Randomized Controlled Trial. JAMA 2008, 300, 1774. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.-M.; Ye, J.-X.; Mu, J.-S.; Cui, X.-P. Efficacy of Vitamin B Supplementation on Cognition in Elderly Patients with Cognitive-Related Diseases: A Systematic Review and Meta-Analysis. J. Geriatr. Psychiatry Neurol. 2017, 30, 50–59. [Google Scholar] [CrossRef] [PubMed]
- Mielech, A.; Puścion-Jakubik, A.; Markiewicz-Żukowska, R.; Socha, K. Vitamins in Alzheimer’s Disease—Review of the Latest Reports. Nutrients 2020, 12, 3458. [Google Scholar] [CrossRef]
- Fei, H.-X.; Qian, C.-F.; Wu, X.-M.; Wei, Y.-H.; Huang, J.-Y.; Wei, L.-H. Role of micronutrients in Alzheimer’s disease: Review of available evidence. World J. Clin. Cases 2022, 10, 7631–7641. [Google Scholar] [CrossRef]
- Browne, D.; McGuinness, B.; Woodside, J.V.; McKay, G.J. Vitamin E and Alzheimer’s disease: What do we know so far? Clin. Interv. Aging 2019, 14, 1303–1317. [Google Scholar] [CrossRef] [Green Version]
- Shahidi, F.; Pinaffi-Langley, A.C.C.; Fuentes, J.; Speisky, H.; de Camargo, A.C. Vitamin E as an essential micronutrient for human health: Common, novel, and unexplored dietary sources. Free Radic. Biol. Med. 2021, 176, 312–321. [Google Scholar] [CrossRef]
- Jiang, Q. Natural forms of vitamin E: Metabolism, antioxidant, and anti-inflammatory activities and their role in disease prevention and therapy. Free Radic. Biol. Med. 2014, 72, 76–90. [Google Scholar] [CrossRef] [Green Version]
- Gugliandolo, A.; Bramanti, P.; Mazzon, E. Role of Vitamin E in the Treatment of Alzheimer’s Disease: Evidence from Animal Models. Int. J. Mol. Sci. 2017, 18, 2504. [Google Scholar] [CrossRef] [Green Version]
- Dorey, C.K.; Gierhart, D.; Fitch, K.A.; Crandell, I.; Craft, N.E. Low Xanthophylls, Retinol, Lycopene, and Tocopherols in Grey and White Matter of Brains with Alzheimer’s Disease. J. Alzheimers Dis. 2022, 1–16. [Google Scholar] [CrossRef]
- Singh, U.; Jialal, I. Anti-inflammatory Effects of α-Tocopherol. Ann. N. Y. Acad. Sci. 2004, 1031, 195–203. [Google Scholar] [CrossRef]
- Singh, U.; Devaraj, S.; Jialal, I. Vitamin E, Oxidative Stress, and Inflammation. Annu. Rev. Nutr. 2005, 25, 151–174. [Google Scholar] [CrossRef]
- Ciarcià, G.; Bianchi, S.; Tomasello, B.; Acquaviva, R.; Malfa, G.A.; Naletova, I.; La Mantia, A.; Di Giacomo, C. Vitamin E and Non-Communicable Diseases: A Review. Biomedicines 2022, 10, 2473. [Google Scholar] [CrossRef]
- Donnelly, J.; Appathurai, A.; Yeoh, H.-L.; Driscoll, K.; Faisal, W. Vitamin E in Cancer Treatment: A Review of Clinical Applications in Randomized Control Trials. Nutrients 2022, 14, 4329. [Google Scholar] [CrossRef]
- Di Donato, I.; Bianchi, S.; Federico, A. Ataxia with vitamin E deficiency: Update of molecular diagnosis. Neurol. Sci. 2010, 31, 511–515. [Google Scholar] [CrossRef]
- Ulatowski, L.M.; Manor, D. Vitamin E and neurodegeneration. Neurobiol. Dis. 2015, 84, 78–83. [Google Scholar] [CrossRef]
- Nishida, Y.; Ito, S.; Ohtsuki, S.; Yamamoto, N.; Takahashi, T.; Iwata, N.; Jishage, K.; Yamada, H.; Sasaguri, H.; Yokota, S.; et al. Depletion of Vitamin E Increases Amyloid β Accumulation by Decreasing Its Clearances from Brain and Blood in a Mouse Model of Alzheimer Disease. J. Biol. Chem. 2009, 284, 33400–33408. [Google Scholar] [CrossRef] [Green Version]
- Grimm, M.O.W.; Stahlmann, C.P.; Mett, J.; Haupenthal, V.J.; Zimmer, V.C.; Lehmann, J.; Hundsdörfer, B.; Endres, K.; Grimm, H.S.; Hartmann, T. Vitamin E: Curse or benefit in Alzheimer’s disease? A systematic investigation of the impact of α-, γ- and δ-tocopherol on Aβ generation and degradation in neuroblastoma cells. J. Nutr. Health Aging 2015, 19, 646–654. [Google Scholar] [CrossRef]
- Grimm, M.; Regner, L.; Mett, J.; Stahlmann, C.; Schorr, P.; Nelke, C.; Streidenberger, O.; Stoetzel, H.; Winkler, J.; Zaidan, S.; et al. Tocotrienol Affects Oxidative Stress, Cholesterol Homeostasis and the Amyloidogenic Pathway in Neuroblastoma Cells: Consequences for Alzheimer’s Disease. Int. J. Mol. Sci. 2016, 17, 1809. [Google Scholar] [CrossRef] [Green Version]
- Chin, K.-Y.; Tay, S. A Review on the Relationship between Tocotrienol and Alzheimer Disease. Nutrients 2018, 10, 881. [Google Scholar] [CrossRef] [PubMed]
- Rinaldi, P.; Polidori, M.C.; Metastasio, A.; Mariani, E.; Mattioli, P.; Cherubini, A.; Catani, M.; Cecchetti, R.; Senin, U.; Mecocci, P. Plasma antioxidants are similarly depleted in mild cognitive impairment and in Alzheimer’s disease. Neurobiol. Aging 2003, 24, 915–919. [Google Scholar] [CrossRef] [PubMed]
- Sung, S.; Yao, Y.; Uryu, K.; Yang, H.; Lee, V.M.-Y.; Trojanowski, J.Q.; Praticò, D. Early Vitamin E supplementation in young but not aged mice reduces Aβ levels and amyloid deposition in a transgenic model of Alzheimer’s disease. FASEB J. 2004, 18, 323–325. [Google Scholar] [CrossRef] [PubMed]
- Sinha, M.; Bir, A.; Banerjee, A.; Bhowmick, P.; Chakrabarti, S. Multiple mechanisms of age-dependent accumulation of amyloid beta protein in rat brain: Prevention by dietary supplementation with N-acetylcysteine, α-lipoic acid and α-tocopherol. Neurochem. Int. 2016, 95, 92–99. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Yang, S.; Liu, W.; Zhang, Y.; Xu, P.; Wang, T.; Ling, T.; Liu, R. Alpha-tocopherol quinine ameliorates spatial memory deficits by reducing beta-amyloid oligomers, neuroinflammation and oxidative stress in transgenic mice with Alzheimer’s disease. Behav. Brain Res. 2016, 296, 109–117. [Google Scholar] [CrossRef]
- Qi, X.-L.; Xiu, J.; Shan, K.-R.; Xiao, Y.; Gu, R.; Liu, R.-Y.; Guan, Z.-Z. Oxidative stress induced by beta-amyloid peptide1–42 is involved in the altered composition of cellular membrane lipids and the decreased expression of nicotinic receptors in human SH-SY5Y neuroblastoma cells. Neurochem. Int. 2005, 46, 613–621. [Google Scholar] [CrossRef]
- Dai, X.; Sun, Y.; Jiang, Z. Protective Effects of Vitamin E against Oxidative Damage Induced by Aβ1–40 Cu(II) Complexes. Acta Biochim. Biophys. Sin. 2007, 39, 123–130. [Google Scholar] [CrossRef] [Green Version]
- Rota, C.; Rimbach, G.; Minihane, A.-M.; Stoecklin, E.; Barella, L. Dietary vitamin E modulates differential gene expression in the rat hippocampus: Potential implications for its neuroprotective properties. Nutr. Neurosci. 2005, 8, 21–29. [Google Scholar] [CrossRef]
- Gohil, K.; Schock, B.C.; Chakraborty, A.A.; Terasawa, Y.; Raber, J.; Farese, R.V.; Packer, L.; Cross, C.E.; Traber, M.G. Gene expression profile of oxidant stress and neurodegeneration in transgenic mice deficient in α-tocopherol transfer protein. Free Radic. Biol. Med. 2003, 35, 1343–1354. [Google Scholar] [CrossRef]
- Block, M.L. NADPH oxidase as a therapeutic target in Alzheimer’s disease. BMC Neurosci. 2008, 9, S8. [Google Scholar] [CrossRef] [Green Version]
- Chu, J.; Praticò, D. 5-lipoxygenase as an endogenous modulator of amyloid beta formation in vivo. Ann. Neurol. 2011, 69, 34–46. [Google Scholar] [CrossRef]
- Voronkov, M.; Braithwaite, S.P.; Stock, J.B. Phosphoprotein phosphatase 2A: A novel druggable target for Alzheimer’s disease. Future Med. Chem. 2011, 3, 821–833. [Google Scholar] [CrossRef] [Green Version]
- Nohturfft, A.; Yabe, D.; Goldstein, J.L.; Brown, M.S.; Espenshade, P.J. Regulated Step in Cholesterol Feedback Localized to Budding of SCAP from ER Membranes. Cell 2000, 102, 315–323. [Google Scholar] [CrossRef] [Green Version]
- Fassbender, K.; Simons, M.; Bergmann, C.; Stroick, M.; Lütjohann, D.; Keller, P.; Runz, H.; Kühl, S.; Bertsch, T.; von Bergmann, K.; et al. Simvastatin strongly reduces levels of Alzheimer’s disease β-amyloid peptides Aβ42 and Aβ40 in vitro and in vivo. Proc. Natl. Acad. Sci. USA 2001, 98, 5856–5861. [Google Scholar] [CrossRef] [Green Version]
- Maulik, M.; Westaway, D.; Jhamandas, J.H.; Kar, S. Role of Cholesterol in APP Metabolism and Its Significance in Alzheimer’s Disease Pathogenesis. Mol. Neurobiol. 2013, 47, 37–63. [Google Scholar] [CrossRef]
- Refolo, L.M.; Pappolla, M.A.; Malester, B.; LaFrancois, J.; Bryant-Thomas, T.; Wang, R.; Tint, G.S.; Sambamurti, K.; Duff, K. Hypercholesterolemia Accelerates the Alzheimer’s Amyloid Pathology in a Transgenic Mouse Model. Neurobiol. Dis. 2000, 7, 321–331. [Google Scholar] [CrossRef]
- Grimm, M.O.W.; Grimm, H.S.; Tomic, I.; Beyreuther, K.; Hartmann, T.; Bergmann, C. Independent Inhibition of Alzheimer Disease β- and γ-Secretase Cleavage by Lowered Cholesterol Levels. J. Biol. Chem. 2008, 283, 11302–11311. [Google Scholar] [CrossRef] [Green Version]
- Osenkowski, P.; Ye, W.; Wang, R.; Wolfe, M.S.; Selkoe, D.J. Direct and Potent Regulation of γ-Secretase by Its Lipid Microenvironment. J. Biol. Chem. 2008, 283, 22529–22540. [Google Scholar] [CrossRef] [Green Version]
- Cossec, J.-C.; Simon, A.; Marquer, C.; Moldrich, R.X.; Leterrier, C.; Rossier, J.; Duyckaerts, C.; Lenkei, Z.; Potier, M.-C. Clathrin-dependent APP endocytosis and Aβ secretion are highly sensitive to the level of plasma membrane cholesterol. Biochim. Biophys. Acta BBA—Mol. Cell Biol. Lipids 2010, 1801, 846–852. [Google Scholar] [CrossRef]
- Giraldo, E.; Lloret, A.; Fuchsberger, T.; Viña, J. Aβ and tau toxicities in Alzheimer’s are linked via oxidative stress-induced p38 activation: Protective role of vitamin E. Redox Biol. 2014, 2, 873–877. [Google Scholar] [CrossRef] [Green Version]
- Nakashima, H.; Ishihara, T.; Yokota, O.; Terada, S.; Trojanowski, J.Q.; Lee, V.M.-Y.; Kuroda, S. Effects of α-tocopherol on an animal model of tauopathies. Free Radic. Biol. Med. 2004, 37, 176–186. [Google Scholar] [CrossRef] [PubMed]
- Bourdel-Marchasson, I.; Delmas-Beauvieux, M.C.; Peuchant, E.; Richard-Harston, S.; Decamps, A.; Reignier, B.; Emeriau, J.P.; Rainfray, M. Antioxidant defences and oxidative stress markers in erythrocytes and plasma from normally nourished elderly Alzheimer patients. Age Ageing 2001, 30, 235–241. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mangialasche, F.; Xu, W.; Kivipelto, M.; Costanzi, E.; Ercolani, S.; Pigliautile, M.; Cecchetti, R.; Baglioni, M.; Simmons, A.; Soininen, H.; et al. Tocopherols and tocotrienols plasma levels are associated with cognitive impairment. Neurobiol. Aging 2012, 33, 2282–2290. [Google Scholar] [CrossRef] [PubMed]
- Lopes da Silva, S.; Vellas, B.; Elemans, S.; Luchsinger, J.; Kamphuis, P.; Yaffe, K.; Sijben, J.; Groenendijk, M.; Stijnen, T. Plasma nutrient status of patients with Alzheimer’s disease: Systematic review and meta-analysis. Alzheimers Dement. 2014, 10, 485–502. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morris, M.C.; Evans, D.A.; Tangney, C.C.; Bienias, J.L.; Wilson, R.S.; Aggarwal, N.T.; Scherr, P.A. Relation of the tocopherol forms to incident Alzheimer disease and to cognitive change. Am. J. Clin. Nutr. 2005, 81, 508–514. [Google Scholar] [CrossRef] [Green Version]
- Mangialasche, F.; Kivipelto, M.; Mecocci, P.; Rizzuto, D.; Palmer, K.; Winblad, B.; Fratiglioni, L. High Plasma Levels of Vitamin E Forms and Reduced Alzheimer’s Disease Risk in Advanced Age. J. Alzheimers Dis. 2010, 20, 1029–1037. [Google Scholar] [CrossRef] [Green Version]
- Dysken, M.W.; Sano, M.; Asthana, S.; Vertrees, J.E.; Pallaki, M.; Llorente, M.; Love, S.; Schellenberg, G.D.; McCarten, J.R.; Malphurs, J.; et al. Effect of Vitamin E and Memantine on Functional Decline in Alzheimer Disease: The TEAM-AD VA Cooperative Randomized Trial. JAMA 2014, 311, 33. [Google Scholar] [CrossRef]
- Petersen, R.C.; Thomas, R.G.; Grundman, M.; Bennett, D.; Doody, R.; Ferris, S.; Galasko, D.; Jin, S.; Kaye, J.; Levey, A.; et al. Vitamin E and Donepezil for the Treatment of Mild Cognitive Impairment. N. Engl. J. Med. 2005, 352, 2379–2388. [Google Scholar] [CrossRef] [Green Version]
- Galasko, D.R.; Peskind, E.; Clark, C.M.; Quinn, J.F.; Ringman, J.M.; Jicha, G.A.; Cotman, C.; Cottrell, B.; Montine, T.J.; Thomas, R.G.; et al. Antioxidants for Alzheimer Disease: A Randomized Clinical Trial With Cerebrospinal Fluid Biomarker Measures. Arch. Neurol. 2012, 69, 836–841. [Google Scholar] [CrossRef] [Green Version]
- Wołoszynowska-Fraser, M.U.; Kouchmeshky, A.; McCaffery, P. Vitamin A and Retinoic Acid in Cognition and Cognitive Disease. Annu. Rev. Nutr. 2020, 40, 247–272. [Google Scholar] [CrossRef]
- Ono, K.; Yoshiike, Y.; Takashima, A.; Hasegawa, K.; Naiki, H.; Yamada, M. Vitamin A exhibits potent antiamyloidogenic and fibril-destabilizing effects in vitro. Exp. Neurol. 2004, 189, 380–392. [Google Scholar] [CrossRef]
- Ono, K.; Yamada, M. Vitamin A potently destabilizes preformed α-synuclein fibrils in vitro: Implications for Lewy body diseases. Neurobiol. Dis. 2007, 25, 446–454. [Google Scholar] [CrossRef]
- Ono, K.; Yamada, M. Vitamin A and Alzheimer’s disease: Vitamin A and Alzheimer’s disease. Geriatr. Gerontol. Int. 2012, 12, 180–188. [Google Scholar] [CrossRef]
- Takasaki, J.; Ono, K.; Yoshiike, Y.; Hirohata, M.; Ikeda, T.; Morinaga, A.; Takashima, A.; Yamada, M. Vitamin A has Anti-Oligomerization Effects on Amyloid-β In Vitro. J. Alzheimers Dis. 2011, 27, 271–280. [Google Scholar] [CrossRef] [Green Version]
- Huang, S.-H.; Fang, S.-T.; Chen, Y.-C. Molecular Mechanism of Vitamin K2 Protection against Amyloid-β-Induced Cytotoxicity. Biomolecules 2021, 11, 423. [Google Scholar] [CrossRef]
- Booth, S.L.; Shea, M.K.; Barger, K.; Leurgans, S.E.; James, B.D.; Holland, T.M.; Agarwal, P.; Fu, X.; Wang, J.; Matuszek, G.; et al. Association of vitamin K with cognitive decline and neuropathology in community-dwelling older persons. Alzheimers Dement. Transl. Res. Clin. Interv. 2022, 8, e12255. [Google Scholar] [CrossRef]
- Emekli-Alturfan, E.; Alturfan, A.A. The emerging relationship between vitamin K and neurodegenerative diseases: A review of current evidence. Mol. Biol. Rep. 2022. [Google Scholar] [CrossRef]
- Wimalawansa, S.J. Vitamin D Deficiency: Effects on Oxidative Stress, Epigenetics, Gene Regulation, and Aging. Biology 2019, 8, 30. [Google Scholar] [CrossRef] [Green Version]
- Mantle, D.; Hargreaves, I.P. Mitochondrial Dysfunction and Neurodegenerative Disorders: Role of Nutritional Supplementation. Int. J. Mol. Sci. 2022, 23, 12603. [Google Scholar] [CrossRef]
- Ricca, C.; Aillon, A.; Bergandi, L.; Alotto, D.; Castagnoli, C.; Silvagno, F. Vitamin D Receptor Is Necessary for Mitochondrial Function and Cell Health. Int. J. Mol. Sci. 2018, 19, 1672. [Google Scholar] [CrossRef] [Green Version]
- Le Floch, M.; Gautier, J.; Annweiler, C. Vitamin D Concentration and Motoric Cognitive Risk in Older Adults: Results from the Gait and Alzheimer Interactions Tracking (GAIT) Cohort. Int. J. Environ. Res. Public. Health 2022, 19, 13086. [Google Scholar] [CrossRef] [PubMed]
- Patel, P.; Shah, J. Vitamin D3 supplementation ameliorates cognitive impairment and alters neurodegenerative and inflammatory markers in scopolamine induced rat model. Metab. Brain Dis. 2022, 37, 2653–2667. [Google Scholar] [CrossRef] [PubMed]
- Malouf, R.; Grimley Evans, J.; Areosa Sastre, A. Folic acid with or without vitamin B12 for cognition and dementia. In The Cochrane Database of Systematic Reviews; The Cochrane Collaboration, Ed.; John Wiley & Sons, Ltd.: Chichester, UK, 2003; p. CD004514. [Google Scholar]
- Malouf, R.; Grimley Evans, J. Folic acid with or without vitamin B12 for the prevention and treatment of healthy elderly and demented people. Cochrane Database Syst. Rev. 2008, 8, CD004514. [Google Scholar] [CrossRef] [PubMed]
- Jager, C.A.; Oulhaj, A.; Jacoby, R.; Refsum, H.; Smith, A.D. Cognitive and clinical outcomes of homocysteine-lowering B-vitamin treatment in mild cognitive impairment: A randomized controlled trial: Treatment of mild cognitive impairment. Int. J. Geriatr. Psychiatry 2012, 27, 592–600. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Quan, M.; Li, T.; Jia, J. Serum Homocysteine, Vitamin B12, Folate, and Their Association with Mild Cognitive Impairment and Subtypes of Dementia. J. Alzheimers Dis. 2022, 90, 681–691. [Google Scholar] [CrossRef]
- Li, M.-M.; Yu, J.-T.; Wang, H.-F.; Jiang, T.; Wang, J.; Meng, X.-F.; Tan, C.-C.; Wang, C.; Tan, L. Efficacy of vitamins B supplementation on mild cognitive impairment and Alzheimer’s disease: A systematic review and meta-analysis. Curr. Alzheimer Res. 2014, 11, 844–852. [Google Scholar]
- Li, S.; Guo, Y.; Men, J.; Fu, H.; Xu, T. The preventive efficacy of vitamin B supplements on the cognitive decline of elderly adults: A systematic review and meta-analysis. BMC Geriatr. 2021, 21, 367. [Google Scholar] [CrossRef]
- Monacelli, F.; Acquarone, E.; Giannotti, C.; Borghi, R.; Nencioni, A. Vitamin C, Aging and Alzheimer’s Disease. Nutrients 2017, 9, 670. [Google Scholar] [CrossRef] [Green Version]
- Hamid, M.; Mansoor, S.; Amber, S.; Zahid, S. A quantitative meta-analysis of vitamin C in the pathophysiology of Alzheimer’s disease. Front. Aging Neurosci. 2022, 14, 970263. [Google Scholar] [CrossRef]
- Consoli, D.C.; Brady, L.J.; Bowman, A.B.; Calipari, E.S.; Harrison, F.E. Ascorbate deficiency decreases dopamine release in gulo −/− and APP/PSEN1 mice. J. Neurochem. 2021, 157, 656–665. [Google Scholar] [CrossRef]
- Stefaniak, O.; Dobrzyńska, M.; Drzymała-Czyż, S.; Przysławski, J. Diet in the Prevention of Alzheimer’s Disease: Current Knowledge and Future Research Requirements. Nutrients 2022, 14, 4564. [Google Scholar] [CrossRef]
- Nishiumi, S.; Miyamoto, S.; Kawabata, K.; Ohnishi, K.; Mukai, R.; Murakami, A.; Ashida, H.; Terao, J. Dietary flavonoids as cancer-preventive and therapeutic biofactors. Front. Biosci. 2011, S3, 1332. [Google Scholar] [CrossRef]
- Shahidi, F.; Ambigaipalan, P. Phenolics and polyphenolics in foods, beverages and spices: Antioxidant activity and health effects—A review. J. Funct. Foods 2015, 18, 820–897. [Google Scholar] [CrossRef]
- Cho, I.; Song, H.; Cho, J.H. Flavonoids mitigate neurodegeneration in aged Caenorhabditis elegans by mitochondrial uncoupling. Food Sci. Nutr. 2020, 8, 6633–6642. [Google Scholar] [CrossRef]
- Commenges, D.; Scotet, V.; Renaud, S.; Jacqmin-Gadda, H.; Barberger-Gateau, P.; Dartigues, J.-F. Intake of flavonoids and risk of dementia. Eur. J. Epidemiol. 2000, 16, 357–363. [Google Scholar] [CrossRef]
- Bondonno, C.P.; Bondonno, N.P.; Dalgaard, F.; Murray, K.; Gardener, S.L.; Martins, R.N.; Rainey-Smith, S.R.; Cassidy, A.; Lewis, J.R.; Croft, K.D.; et al. Flavonoid intake and incident dementia in the Danish Diet, Cancer, and Health cohort. Alzheimers Dement. Transl. Res. Clin. Interv. 2021, 7, e12175. [Google Scholar] [CrossRef]
- Kuriyama, S.; Hozawa, A.; Ohmori, K.; Shimazu, T.; Matsui, T.; Ebihara, S.; Awata, S.; Nagatomi, R.; Arai, H.; Tsuji, I. Green tea consumption and cognitive function: A cross-sectional study from the Tsurugaya Project. Am. J. Clin. Nutr. 2006, 83, 355–361. [Google Scholar] [CrossRef] [Green Version]
- Nurk, E.; Refsum, H.; Drevon, C.A.; Tell, G.S.; Nygaard, H.A.; Engedal, K.; Smith, A.D. Intake of Flavonoid-Rich Wine, Tea, and Chocolate by Elderly Men and Women Is Associated with Better Cognitive Test Performance. J. Nutr. 2009, 139, 120–127. [Google Scholar] [CrossRef] [Green Version]
- Manach, C.; Scalbert, A.; Morand, C.; Rémésy, C.; Jiménez, L. Polyphenols: Food sources and bioavailability. Am. J. Clin. Nutr. 2004, 79, 727–747. [Google Scholar] [CrossRef] [Green Version]
- Naoi, M.; Wu, Y.; Shamoto-Nagai, M.; Maruyama, W. Mitochondria in Neuroprotection by Phytochemicals: Bioactive Polyphenols Modulate Mitochondrial Apoptosis System, Function and Structure. Int. J. Mol. Sci. 2019, 20, 2451. [Google Scholar] [CrossRef] [Green Version]
- Dragicevic, N.; Smith, A.; Lin, X.; Yuan, F.; Copes, N.; Delic, V.; Tan, J.; Cao, C.; Shytle, R.D.; Bradshaw, P.C. Green Tea Epigallocatechin-3-Gallate (EGCG) and Other Flavonoids Reduce Alzheimer’s Amyloid-Induced Mitochondrial Dysfunction. J. Alzheimers Dis. 2011, 26, 507–521. [Google Scholar] [CrossRef] [PubMed]
- Kozłowska, A.; Szostak-Wegierek, D. Flavonoids--food sources and health benefits. Rocz. Panstw. Zakl. Hig. 2014, 65, 79–85. [Google Scholar] [PubMed]
- Bernatoniene, J.; Kopustinskiene, D. The Role of Catechins in Cellular Responses to Oxidative Stress. Molecules 2018, 23, 965. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bae, J.; Kim, N.; Shin, Y.; Kim, S.-Y.; Kim, Y.-J. Activity of catechins and their applications. Biomed. Dermatol. 2020, 4, 8. [Google Scholar] [CrossRef] [Green Version]
- Kocahan, S.; Doğan, Z. Mechanisms of Alzheimer’s Disease Pathogenesis and Prevention: The Brain, Neural Pathology, N-methyl-D-aspartate Receptors, Tau Protein and Other Risk Factors. Clin. Psychopharmacol. Neurosci. 2017, 15, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Lou, H.; Fan, P.; Perez, R.G.; Lou, H. Neuroprotective effects of linarin through activation of the PI3K/Akt pathway in amyloid-β-induced neuronal cell death. Bioorg. Med. Chem. 2011, 19, 4021–4027. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Xu, B.; Nie, L.; He, K.; Zhou, L.; Huang, X.; Spencer, P.; Yang, X.; Liu, J. Flavanol-rich lychee fruit extract substantially reduces progressive cognitive and molecular deficits in a triple-transgenic animal model of Alzheimer disease. Nutr. Neurosci. 2021, 24, 720–734. [Google Scholar] [CrossRef]
- Sheng, C.; Xu, P.; Zhou, K.; Deng, D.; Zhang, C.; Wang, Z. Icariin Attenuates Synaptic and Cognitive Deficits in an A β 1–42-Induced Rat Model of Alzheimer’s Disease. BioMed Res. Int. 2017, 2017, 7464872. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.; Varghese, M.; Ono, K.; Yamada, M.; Levine, S.; Tzavaras, N.; Gong, B.; Hurst, W.J.; Blitzer, R.D.; Pasinetti, G.M. Cocoa Extracts Reduce Oligomerization of Amyloid-β: Implications for Cognitive Improvement in Alzheimer’s Disease. J. Alzheimers Dis. 2014, 41, 643–650. [Google Scholar] [CrossRef]
- Spencer, J.P.E. The interactions of flavonoids within neuronal signalling pathways. Genes Nutr. 2007, 2, 257–273. [Google Scholar] [CrossRef] [Green Version]
- Ehrnhoefer, D.E.; Bieschke, J.; Boeddrich, A.; Herbst, M.; Masino, L.; Lurz, R.; Engemann, S.; Pastore, A.; Wanker, E.E. EGCG redirects amyloidogenic polypeptides into unstructured, off-pathway oligomers. Nat. Struct. Mol. Biol. 2008, 15, 558–566. [Google Scholar] [CrossRef]
- Bieschke, J.; Russ, J.; Friedrich, R.P.; Ehrnhoefer, D.E.; Wobst, H.; Neugebauer, K.; Wanker, E.E. EGCG remodels mature α-synuclein and amyloid-β fibrils and reduces cellular toxicity. Proc. Natl. Acad. Sci. USA 2010, 107, 7710–7715. [Google Scholar] [CrossRef]
- Walker, J.M.; Klakotskaia, D.; Ajit, D.; Weisman, G.A.; Wood, W.G.; Sun, G.Y.; Serfozo, P.; Simonyi, A.; Schachtman, T.R. Beneficial Effects of Dietary EGCG and Voluntary Exercise on Behavior in an Alzheimer’s Disease Mouse Model. J. Alzheimers Dis. 2015, 44, 561–572. [Google Scholar] [CrossRef]
- Ettcheto, M.; Cano, A.; Manzine, P.R.; Busquets, O.; Verdaguer, E.; Castro-Torres, R.D.; García, M.L.; Beas-Zarate, C.; Olloquequi, J.; Auladell, C.; et al. Epigallocatechin-3-Gallate (EGCG) Improves Cognitive Deficits Aggravated by an Obesogenic Diet Through Modulation of Unfolded Protein Response in APPswe/PS1dE9 Mice. Mol. Neurobiol. 2020, 57, 1814–1827. [Google Scholar] [CrossRef]
- Nan, S.; Wang, P.; Zhang, Y.; Fan, J. Epigallocatechin-3-Gallate Provides Protection Against Alzheimer’s Disease-Induced Learning and Memory Impairments in Rats. Drug Des. Dev. Ther. 2021, 15, 2013–2024. [Google Scholar] [CrossRef]
- Rezai-Zadeh, K.; Arendash, G.W.; Hou, H.; Fernandez, F.; Jensen, M.; Runfeldt, M.; Shytle, R.D.; Tan, J. Green tea epigallocatechin-3-gallate (EGCG) reduces β-amyloid mediated cognitive impairment and modulates tau pathology in Alzheimer transgenic mice. Brain Res. 2008, 1214, 177–187. [Google Scholar] [CrossRef]
- Sonawane, S.K.; Chidambaram, H.; Boral, D.; Gorantla, N.V.; Balmik, A.A.; Dangi, A.; Ramasamy, S.; Marelli, U.K.; Chinnathambi, S. EGCG impedes human Tau aggregation and interacts with Tau. Sci. Rep. 2020, 10, 12579. [Google Scholar] [CrossRef] [PubMed]
- Du, K.; Liu, M.; Zhong, X.; Yao, W.; Xiao, Q.; Wen, Q.; Yang, B.; Wei, M. Epigallocatechin Gallate Reduces Amyloid β-Induced Neurotoxicity via Inhibiting Endoplasmic Reticulum Stress-Mediated Apoptosis. Mol. Nutr. Food Res. 2018, 62, 1700890. [Google Scholar] [CrossRef]
- Zhong, X.; Liu, M.; Yao, W.; Du, K.; He, M.; Jin, X.; Jiao, L.; Ma, G.; Wei, B.; Wei, M. Epigallocatechin-3-Gallate Attenuates Microglial Inflammation and Neurotoxicity by Suppressing the Activation of Canonical and Noncanonical Inflammasome via TLR4/NF-κB Pathway. Mol. Nutr. Food Res. 2019, 63, 1801230. [Google Scholar] [CrossRef]
- Liu, M.; Chen, F.; Sha, L.; Wang, S.; Tao, L.; Yao, L.; He, M.; Yao, Z.; Liu, H.; Zhu, Z.; et al. (−)-Epigallocatechin-3-Gallate Ameliorates Learning and Memory Deficits by Adjusting the Balance of TrkA/p75NTR Signaling in APP/PS1 Transgenic Mice. Mol. Neurobiol. 2014, 49, 1350–1363. [Google Scholar] [CrossRef] [Green Version]
- Jiang, Z.; Dong, X.; Yan, X.; Liu, Y.; Zhang, L.; Sun, Y. Nanogels of dual inhibitor-modified hyaluronic acid function as a potent inhibitor of amyloid β-protein aggregation and cytotoxicity. Sci. Rep. 2018, 8, 3505. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mori, T.; Koyama, N.; Tan, J.; Segawa, T.; Maeda, M.; Town, T. Combination therapy with octyl gallate and ferulic acid improves cognition and neurodegeneration in a transgenic mouse model of Alzheimer’s disease. J. Biol. Chem. 2017, 292, 11310–11325. [Google Scholar] [CrossRef] [PubMed]
- Forcano, L.; Fauria, K.; Soldevila-Domenech, N.; Minguillón, C.; Lorenzo, T.; Cuenca-Royo, A.; Menezes-Cabral, S.; Pizarro, N.; Boronat, A.; Molinuevo, J.L.; et al. Prevention of cognitive decline in subjective cognitive decline APOE ε4 carriers after EGCG and a multimodal intervention (PENSA): Study design. Alzheimers Dement. Transl. Res. Clin. Interv. 2021, 7, e12155. [Google Scholar] [CrossRef] [PubMed]
- Thenmozhi, A.J.; Raja, T.R.W.; Janakiraman, U.; Manivasagam, T. Neuroprotective Effect of Hesperidin on Aluminium Chloride Induced Alzheimer’s Disease in Wistar Rats. Neurochem. Res. 2015, 40, 767–776. [Google Scholar] [CrossRef] [PubMed]
- Evans, J.A.; Mendonca, P.; Soliman, K.F.A. Neuroprotective Effects and Therapeutic Potential of the Citrus Flavonoid Hesperetin in Neurodegenerative Diseases. Nutrients 2022, 14, 2228. [Google Scholar] [CrossRef]
- Hajialyani, M.; Hosein Farzaei, M.; Echeverría, J.; Nabavi, S.; Uriarte, E.; Sobarzo-Sánchez, E. Hesperidin as a Neuroprotective Agent: A Review of Animal and Clinical Evidence. Molecules 2019, 24, 648. [Google Scholar] [CrossRef] [Green Version]
- Ikram, M.; Muhammad, T.; Rehman, S.U.; Khan, A.; Jo, M.G.; Ali, T.; Kim, M.O. Hesperetin Confers Neuroprotection by Regulating Nrf2/TLR4/NF-κB Signaling in an Aβ Mouse Model. Mol. Neurobiol. 2019, 56, 6293–6309. [Google Scholar] [CrossRef]
- Kuşi, M.; Becer, E.; Vatansever, H.S.; Yücecan, S. Neuroprotective Effects of Hesperidin and Naringin in SK-N-AS Cell as an in vitro Model for Alzheimer’s Disease. J. Am. Nutr. Assoc. 2022, 1–9. [Google Scholar] [CrossRef]
- Khan, A.; Ikram, M.; Hahm, J.R.; Kim, M.O. Antioxidant and Anti-Inflammatory Effects of Citrus Flavonoid Hesperetin: Special Focus on Neurological Disorders. Antioxidants 2020, 9, 609. [Google Scholar] [CrossRef]
- Li, C.; Zug, C.; Qu, H.; Schluesener, H.; Zhang, Z. Hesperidin ameliorates behavioral impairments and neuropathology of transgenic APP/PS1 mice. Behav. Brain Res. 2015, 281, 32–42. [Google Scholar] [CrossRef]
- Luo, Y.; Fan, H.; Tan, X.; Li, Z. Hesperetin rescues emotional memory and synaptic plasticity deficit in aged rats. Behav. Neurosci. 2021, 135, 721–731. [Google Scholar] [CrossRef] [PubMed]
- Justin Thenmozhi, A.; William Raja, T.R.; Manivasagam, T.; Janakiraman, U.; Essa, M.M. Hesperidin ameliorates cognitive dysfunction, oxidative stress and apoptosis against aluminium chloride induced rat model of Alzheimer’s disease. Nutr. Neurosci. 2017, 20, 360–368. [Google Scholar] [CrossRef] [PubMed]
- Justin-Thenmozhi, A.; Dhivya Bharathi, M.; Kiruthika, R.; Manivasagam, T.; Borah, A.; Essa, M.M. Attenuation of Aluminum Chloride-Induced Neuroinflammation and Caspase Activation Through the AKT/GSK-3β Pathway by Hesperidin in Wistar Rats. Neurotox. Res. 2018, 34, 463–476. [Google Scholar] [CrossRef] [PubMed]
- Abou Baker, D.H.; Ibrahim, B.M.M.; Hassan, N.S.; Yousuf, A.F.; Gengaihi, S.E. Exploiting Citrus aurantium seeds and their secondary metabolites in the management of Alzheimer disease. Toxicol. Rep. 2020, 7, 723–729. [Google Scholar] [CrossRef] [PubMed]
- Mandour, D.A.; Bendary, M.A.; Alsemeh, A.E. Histological and imunohistochemical alterations of hippocampus and prefrontal cortex in a rat model of Alzheimer like-disease with a preferential role of the flavonoid “hesperidin” . J. Mol. Histol. 2021, 52, 1043–1065. [Google Scholar] [CrossRef]
- Wang, D.; Liu, L.; Zhu, X.; Wu, W.; Wang, Y. Hesperidin Alleviates Cognitive Impairment, Mitochondrial Dysfunction and Oxidative Stress in a Mouse Model of Alzheimer’s Disease. Cell. Mol. Neurobiol. 2014, 34, 1209–1221. [Google Scholar] [CrossRef]
- Hong, Y.; An, Z. Hesperidin attenuates learning and memory deficits in APP/PS1 mice through activation of Akt/Nrf2 signaling and inhibition of RAGE/NF-κB signaling. Arch. Pharm. Res. 2018, 41, 655–663. [Google Scholar] [CrossRef]
- Javed, H.; Vaibhav, K.; Ahmed, M.E.; Khan, A.; Tabassum, R.; Islam, F.; Safhi, M.M.; Islam, F. Effect of hesperidin on neurobehavioral, neuroinflammation, oxidative stress and lipid alteration in intracerebroventricular streptozotocin induced cognitive impairment in mice. J. Neurol. Sci. 2015, 348, 51–59. [Google Scholar] [CrossRef]
- Kean, R.J.; Lamport, D.J.; Dodd, G.F.; Freeman, J.E.; Williams, C.M.; Ellis, J.A.; Butler, L.T.; Spencer, J.P. Chronic consumption of flavanone-rich orange juice is associated with cognitive benefits: An 8-wk, randomized, double-blind, placebo-controlled trial in healthy older adults. Am. J. Clin. Nutr. 2015, 101, 506–514. [Google Scholar] [CrossRef] [Green Version]
- Zhang, S.; Tomata, Y.; Sugiyama, K.; Sugawara, Y.; Tsuji, I. Citrus consumption and incident dementia in elderly Japanese: The Ohsaki Cohort 2006 Study. Br. J. Nutr. 2017, 117, 1174–1180. [Google Scholar] [CrossRef] [Green Version]
- Ahsan, A.U.; Sharma, V.L.; Wani, A.; Chopra, M. Naringenin Upregulates AMPK-Mediated Autophagy to Rescue Neuronal Cells From β-Amyloid (1–42) Evoked Neurotoxicity. Mol. Neurobiol. 2020, 57, 3589–3602. [Google Scholar] [CrossRef]
- Wu, L.-H.; Lin, C.; Lin, H.-Y.; Liu, Y.-S.; Wu, C.Y.-J.; Tsai, C.-F.; Chang, P.-C.; Yeh, W.-L.; Lu, D.-Y. Naringenin Suppresses Neuroinflammatory Responses Through Inducing Suppressor of Cytokine Signaling 3 Expression. Mol. Neurobiol. 2016, 53, 1080–1091. [Google Scholar] [CrossRef]
- Yang, Z.; Kuboyama, T.; Tohda, C. Naringenin promotes microglial M2 polarization and Aβ degradation enzyme expression. Phytother. Res. 2019, 33, 1114–1121. [Google Scholar] [CrossRef]
- Md, S.; Gan, S.Y.; Haw, Y.H.; Ho, C.L.; Wong, S.; Choudhury, H. In vitro neuroprotective effects of naringenin nanoemulsion against β-amyloid toxicity through the regulation of amyloidogenesis and tau phosphorylation. Int. J. Biol. Macromol. 2018, 118, 1211–1219. [Google Scholar] [CrossRef]
- Khan, M.B.; Khan, M.M.; Khan, A.; Ahmed, M.E.; Ishrat, T.; Tabassum, R.; Vaibhav, K.; Ahmad, A.; Islam, F. Naringenin ameliorates Alzheimer’s disease (AD)-type neurodegeneration with cognitive impairment (AD-TNDCI) caused by the intracerebroventricular-streptozotocin in rat model. Neurochem. Int. 2012, 61, 1081–1093. [Google Scholar] [CrossRef]
- Ghofrani, S.; Joghataei, M.-T.; Mohseni, S.; Baluchnejadmojarad, T.; Bagheri, M.; Khamse, S.; Roghani, M. Naringenin improves learning and memory in an Alzheimer’s disease rat model: Insights into the underlying mechanisms. Eur. J. Pharmacol. 2015, 764, 195–201. [Google Scholar] [CrossRef] [Green Version]
- Haider, S.; Liaquat, L.; Ahmad, S.; Batool, Z.; Siddiqui, R.A.; Tabassum, S.; Shahzad, S.; Rafiq, S.; Naz, N. Naringenin protects AlCl3/D-galactose induced neurotoxicity in rat model of AD via attenuation of acetylcholinesterase levels and inhibition of oxidative stress. PLoS ONE 2020, 15, e0227631. [Google Scholar] [CrossRef] [Green Version]
- Zhou, T.; Liu, L.; Wang, Q.; Gao, Y. Naringenin alleviates cognition deficits in high-fat diet-fed SAMP8 mice. J. Food Biochem. 2020, 44, e13375. [Google Scholar] [CrossRef]
- Rebello, C.J.; Beyl, R.A.; Lertora, J.J.L.; Greenway, F.L.; Ravussin, E.; Ribnicky, D.M.; Poulev, A.; Kennedy, B.J.; Castro, H.F.; Campagna, S.R.; et al. Safety and pharmacokinetics of naringenin: A randomized, controlled, single-ascending-dose clinical trial. Diabetes Obes. Metab. 2020, 22, 91–98. [Google Scholar] [CrossRef]
- Nakajima, A.; Ohizumi, Y. Potential Benefits of Nobiletin, A Citrus Flavonoid, against Alzheimer’s Disease and Parkinson’s Disease. Int. J. Mol. Sci. 2019, 20, 3380. [Google Scholar] [CrossRef] [Green Version]
- Nakajima, A.; Aoyama, Y.; Nguyen, T.-T.L.; Shin, E.-J.; Kim, H.-C.; Yamada, S.; Nakai, T.; Nagai, T.; Yokosuka, A.; Mimaki, Y.; et al. Nobiletin, a citrus flavonoid, ameliorates cognitive impairment, oxidative burden, and hyperphosphorylation of tau in senescence-accelerated mouse. Behav. Brain Res. 2013, 250, 351–360. [Google Scholar] [CrossRef] [PubMed]
- Qi, G.; Mi, Y.; Fan, R.; Li, R.; Liu, Z.; Liu, X. Nobiletin Protects against Systemic Inflammation-Stimulated Memory Impairment via MAPK and NF-κB Signaling Pathways. J. Agric. Food Chem. 2019, 67, 5122–5134. [Google Scholar] [CrossRef] [PubMed]
- Youn, K.; Lee, S.; Jun, M. Discovery of Nobiletin from Citrus Peel as a Potent Inhibitor of β-Amyloid Peptide Toxicity. Nutrients 2019, 11, 2648. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chai, W.; Zhang, J.; Xiang, Z.; Zhang, H.; Mei, Z.; Nie, H.; Xu, R.; Zhang, P. Potential of nobiletin against Alzheimer’s disease through inhibiting neuroinflammation. Metab. Brain Dis. 2022, 37, 1145–1154. [Google Scholar] [CrossRef] [PubMed]
- Ghasemi-Tarie, R.; Kiasalari, Z.; Fakour, M.; Khorasani, M.; Keshtkar, S.; Baluchnejadmojarad, T.; Roghani, M. Nobiletin prevents amyloid β1-40-induced cognitive impairment via inhibition of neuroinflammation and oxidative/nitrosative stress. Metab. Brain Dis. 2022, 37, 1337–1349. [Google Scholar] [CrossRef]
- Wirianto, M.; Wang, C.; Kim, E.; Koike, N.; Gomez-Gutierrez, R.; Nohara, K.; Escobedo, G.; Choi, J.M.; Han, C.; Yagita, K.; et al. The clock modulator Nobiletin mitigates astrogliosis-associated neuroinflammation and disease hallmarks in an Alzheimer’s disease model. FASEB J. 2022, 36, e22186. [Google Scholar] [CrossRef]
- Matsuzaki, K.; Yamakuni, T.; Hashimoto, M.; Haque, A.M.; Shido, O.; Mimaki, Y.; Sashida, Y.; Ohizumi, Y. Nobiletin restoring β-amyloid-impaired CREB phosphorylation rescues memory deterioration in Alzheimer’s disease model rats. Neurosci. Lett. 2006, 400, 230–234. [Google Scholar] [CrossRef]
- Onozuka, H.; Nakajima, A.; Matsuzaki, K.; Shin, R.-W.; Ogino, K.; Saigusa, D.; Tetsu, N.; Yokosuka, A.; Sashida, Y.; Mimaki, Y.; et al. Nobiletin, a Citrus Flavonoid, Improves Memory Impairment and Aβ Pathology in a Transgenic Mouse Model of Alzheimer’s Disease. J. Pharmacol. Exp. Ther. 2008, 326, 739–744. [Google Scholar] [CrossRef]
- Yamada, S.; Shirai, M.; Ono, K.; Teruya, T.; Yamano, A.; Tae Woo, J. Beneficial effects of a nobiletin-rich formulated supplement of Sikwasa (C. depressa) peel on cognitive function in elderly Japanese subjects; A multicenter, randomized, double-blind, placebo-controlled study. Food Sci. Nutr. 2021, 9, 6844–6853. [Google Scholar] [CrossRef]
- Meguro, K.; Yamaguchi, S. Decreased Behavioral Abnormalities After Treatment with Combined Donepezil and Yokukansankachimpihange in Alzheimer Disease: An Observational Study. The Osaki-Tajiri Project. Neurol. Ther. 2018, 7, 333–340. [Google Scholar] [CrossRef] [Green Version]
- Seki, T.; Kamiya, T.; Furukawa, K.; Azumi, M.; Ishizuka, S.; Takayama, S.; Nagase, S.; Arai, H.; Yamakuni, T.; Yaegashi, N. Nobiletin-rich Citrus reticulata peels, a kampo medicine for Alzheimer’s disease: A case series: Letters to the Editor. Geriatr. Gerontol. Int. 2013, 13, 236–238. [Google Scholar] [CrossRef]
- Nabavi, S.F.; Khan, H.; D’onofrio, G.; Šamec, D.; Shirooie, S.; Dehpour, A.R.; Argüelles, S.; Habtemariam, S.; Sobarzo-Sanchez, E. Apigenin as neuroprotective agent: Of mice and men. Pharmacol. Res. 2018, 128, 359–365. [Google Scholar] [CrossRef]
- Salehi, B.; Venditti, A.; Sharifi-Rad, M.; Kręgiel, D.; Sharifi-Rad, J.; Durazzo, A.; Lucarini, M.; Santini, A.; Souto, E.; Novellino, E.; et al. The Therapeutic Potential of Apigenin. Int. J. Mol. Sci. 2019, 20, 1305. [Google Scholar] [CrossRef] [Green Version]
- Zhao, F.; Dang, Y.; Zhang, R.; Jing, G.; Liang, W.; Xie, L.; Li, Z. Apigenin attenuates acrylonitrile-induced neuro-inflammation in rats: Involved of inactivation of the TLR4/NF-κB signaling pathway. Int. Immunopharmacol. 2019, 75, 105697. [Google Scholar] [CrossRef]
- Dourado, N.S.; Souza, C.d.S.; de Almeida, M.M.A.; Bispo da Silva, A.; dos Santos, B.L.; Silva, V.D.A.; De Assis, A.M.; da Silva, J.S.; Souza, D.O.; Costa, M.d.F.D.; et al. Neuroimmunomodulatory and Neuroprotective Effects of the Flavonoid Apigenin in in vitro Models of Neuroinflammation Associated with Alzheimer’s Disease. Front. Aging Neurosci. 2020, 12, 119. [Google Scholar] [CrossRef]
- Zhao, L.; Wang, J.-L.; Liu, R.; Li, X.-X.; Li, J.-F.; Zhang, L. Neuroprotective, Anti-Amyloidogenic and Neurotrophic Effects of Apigenin in an Alzheimer’s Disease Mouse Model. Molecules 2013, 18, 9949–9965. [Google Scholar] [CrossRef] [Green Version]
- Alsadat, A.M.; Nikbakht, F.; Hossein Nia, H.; Golab, F.; Khadem, Y.; Barati, M.; Vazifekhah, S. GSK-3β as a target for apigenin-induced neuroprotection against Aβ 25–35 in a rat model of Alzheimer’s disease. Neuropeptides 2021, 90, 102200. [Google Scholar] [CrossRef]
- Malar, D.S.; Prasanth, M.I.; Jeyakumar, M.; Balamurugan, K.; Devi, K.P. Vitexin prevents Aβ proteotoxicity in transgenic Caenorhabditis elegans model of Alzheimer’s disease by modulating unfolded protein response. J. Biochem. Mol. Toxicol. 2021, 35, e22632. [Google Scholar] [CrossRef]
- Balez, R.; Steiner, N.; Engel, M.; Muñoz, S.S.; Lum, J.S.; Wu, Y.; Wang, D.; Vallotton, P.; Sachdev, P.; O’Connor, M.; et al. Neuroprotective effects of apigenin against inflammation, neuronal excitability and apoptosis in an induced pluripotent stem cell model of Alzheimer’s disease. Sci. Rep. 2016, 6, 31450. [Google Scholar] [CrossRef] [Green Version]
- Panche, A.N.; Diwan, A.D.; Chandra, S.R. Flavonoids: An overview. J. Nutr. Sci. 2016, 5, e47. [Google Scholar] [CrossRef] [Green Version]
- Anand David, A.; Arulmoli, R.; Parasuraman, S. Overviews of biological importance of quercetin: A bioactive flavonoid. Pharmacogn. Rev. 2016, 10, 84. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, D.-M.; Li, S.-Q.; Wu, W.-L.; Zhu, X.-Y.; Wang, Y.; Yuan, H.-Y. Effects of Long-Term Treatment with Quercetin on Cognition and Mitochondrial Function in a Mouse Model of Alzheimer’s Disease. Neurochem. Res. 2014, 39, 1533–1543. [Google Scholar] [CrossRef] [PubMed]
- Xu, M.; Huang, H.; Mo, X.; Zhu, Y.; Chen, X.; Li, X.; Peng, X.; Xu, Z.; Chen, L.; Rong, S.; et al. Quercetin-3-O-Glucuronide Alleviates Cognitive Deficit and Toxicity in Aβ 1-42-Induced AD-Like Mice and SH-SY5Y Cells. Mol. Nutr. Food Res. 2021, 65, 2000660. [Google Scholar] [CrossRef] [PubMed]
- Paula, P.-C.; Angelica Maria, S.-G.; Luis, C.-H.; Gloria Patricia, C.-G. Preventive Effect of Quercetin in a Triple Transgenic Alzheimer’s Disease Mice Model. Molecules 2019, 24, 2287. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, X.-W.; Chen, J.-Y.; Ouyang, D.; Lu, J.-H. Quercetin in Animal Models of Alzheimer’s Disease: A Systematic Review of Preclinical Studies. Int. J. Mol. Sci. 2020, 21, 493. [Google Scholar] [CrossRef] [Green Version]
- Nakagawa, T.; Itoh, M.; Ohta, K.; Hayashi, Y.; Hayakawa, M.; Yamada, Y.; Akanabe, H.; Chikaishi, T.; Nakagawa, K.; Itoh, Y.; et al. Improvement of memory recall by quercetin in rodent contextual fear conditioning and human early-stage Alzheimer’s disease patients. NeuroReport 2016, 27, 671–676. [Google Scholar] [CrossRef]
- Holland, T.M.; Agarwal, P.; Wang, Y.; Leurgans, S.E.; Bennett, D.A.; Booth, S.L.; Morris, M.C. Dietary flavonols and risk of Alzheimer dementia. Neurology 2020, 94, e1749–e1756. [Google Scholar] [CrossRef]
- Hou, Y.; Aboukhatwa, M.A.; Lei, D.-L.; Manaye, K.; Khan, I.; Luo, Y. Anti-depressant natural flavonols modulate BDNF and beta amyloid in neurons and hippocampus of double TgAD mice. Neuropharmacology 2010, 58, 911–920. [Google Scholar] [CrossRef] [Green Version]
- Choi, R.C.Y.; Zhu, J.T.T.; Leung, K.W.; Chu, G.K.Y.; Xie, H.Q.; Chen, V.P.; Zheng, K.Y.Z.; Lau, D.T.W.; Dong, T.T.X.; Chow, P.C.Y.; et al. A Flavonol Glycoside, Isolated from Roots of Panax notoginseng, Reduces Amyloid-β-Induced Neurotoxicity in Cultured Neurons: Signaling Transduction and Drug Development for Alzheimer’s Disease. J. Alzheimers Dis. 2010, 19, 795–811. [Google Scholar] [CrossRef]
- Bhullar, K.S.; Rupasinghe, H.P.V. Partridgeberry polyphenols protect primary cortical and hippocampal neurons against β-amyloid toxicity. Food Res. Int. 2015, 74, 237–249. [Google Scholar] [CrossRef]
- Huebbe, P.; Wagner, A.E.; Boesch-Saadatmandi, C.; Sellmer, F.; Wolffram, S.; Rimbach, G. Effect of dietary quercetin on brain quercetin levels and the expression of antioxidant and Alzheimer’s disease relevant genes in mice. Pharmacol. Res. 2010, 61, 242–246. [Google Scholar] [CrossRef]
- Pinheiro, R.G.R.; Granja, A.; Loureiro, J.A.; Pereira, M.C.; Pinheiro, M.; Neves, A.R.; Reis, S. RVG29-Functionalized Lipid Nanoparticles for Quercetin Brain Delivery and Alzheimer’s Disease. Pharm. Res. 2020, 37, 139. [Google Scholar] [CrossRef]
- Pinheiro, R.G.R.; Granja, A.; Loureiro, J.A.; Pereira, M.C.; Pinheiro, M.; Neves, A.R.; Reis, S. Quercetin lipid nanoparticles functionalized with transferrin for Alzheimer’s disease. Eur. J. Pharm. Sci. 2020, 148, 105314. [Google Scholar] [CrossRef]
- Moreno, L.C.G.e.I.; Puerta, E.; Suárez-Santiago, J.E.; Santos-Magalhães, N.S.; Ramirez, M.J.; Irache, J.M. Effect of the oral administration of nanoencapsulated quercetin on a mouse model of Alzheimer’s disease. Int. J. Pharm. 2017, 517, 50–57. [Google Scholar] [CrossRef]
- Maher, P. Preventing and Treating Neurological Disorders with the Flavonol Fisetin. Brain Plast. 2021, 6, 155–166. [Google Scholar] [CrossRef]
- Ahmad, A.; Ali, T.; Park, H.Y.; Badshah, H.; Rehman, S.U.; Kim, M.O. Neuroprotective Effect of Fisetin Against Amyloid-Beta-Induced Cognitive/Synaptic Dysfunction, Neuroinflammation, and Neurodegeneration in Adult Mice. Mol. Neurobiol. 2017, 54, 2269–2285. [Google Scholar] [CrossRef]
- Kim, S.; Choi, K.J.; Cho, S.-J.; Yun, S.-M.; Jeon, J.-P.; Koh, Y.H.; Song, J.; Johnson, G.V.W.; Jo, C. Fisetin stimulates autophagic degradation of phosphorylated tau via the activation of TFEB and Nrf2 transcription factors. Sci. Rep. 2016, 6, 24933. [Google Scholar] [CrossRef] [Green Version]
- Currais, A.; Prior, M.; Dargusch, R.; Armando, A.; Ehren, J.; Schubert, D.; Quehenberger, O.; Maher, P. Modulation of p25 and inflammatory pathways by fisetin maintains cognitive function in A lzheimer’s disease transgenic mice. Aging Cell 2014, 13, 379–390. [Google Scholar] [CrossRef]
- Currais, A.; Farrokhi, C.; Dargusch, R.; Armando, A.; Quehenberger, O.; Schubert, D.; Maher, P. Fisetin Reduces the Impact of Aging on Behavior and Physiology in the Rapidly Aging SAMP8 Mouse. J. Gerontol. Ser. A 2018, 73, 299–307. [Google Scholar] [CrossRef] [Green Version]
- Jung, H.A.; Jin, S.E.; Park, J.-S.; Choi, J.S. Antidiabetic complications and anti-alzheimer activities of sophoflavescenol, a prenylated flavonol from Sophora flavescens, and its structure-activity relationship: Diabetic complications inhibitory activity of sophoflavescenol. Phytother. Res. 2011, 25, 709–715. [Google Scholar] [CrossRef]
- Yu, H.; Shi, J.; Lin, Y.; Zhang, Y.; Luo, Q.; Huang, S.; Wang, S.; Wei, J.; Huang, J.; Li, C.; et al. Icariin Ameliorates Alzheimer’s Disease Pathology by Alleviating Myelin Injury in 3 × Tg-AD Mice. Neurochem. Res. 2022, 47, 1049–1059. [Google Scholar] [CrossRef] [PubMed]
- Calderón-Garcidueñas, L.; Mora-Tiscareño, A.; Franco-Lira, M.; Cross, J.V.; Engle, R.; Aragón-Flores, M.; Gómez-Garza, G.; Jewells, V.; Medina-Cortina, H.; Solorio, E.; et al. Flavonol-rich dark cocoa significantly decreases plasma endothelin-1 and improves cognition in urban children. Front. Pharmacol. 2013, 4, 104. [Google Scholar] [CrossRef] [PubMed]
- Augustin, S.; Huebbe, P.; Matzner, N.; Augustin, K.; Schliebs, R.; Cermak, R.; Wolffram, S.; Rimbach, G. Ginkgo biloba Extract and its Flavonol and Terpenelactone Fractions do not Affect β-Secretase mRNA and Enzyme Activity Levels in Cultured Neurons and in Mice. Planta Med. 2008, 74, 6–13. [Google Scholar] [CrossRef] [PubMed]
- Augustin, S.; Rimbach, G.; Augustin, K.; Cermak, R.; Wolffram, S. Gene Regulatory Effects of Ginkgo biloba Extract and Its Flavonol and Terpenelactone Fractions in Mouse Brain. J. Clin. Biochem. Nutr. 2009, 45, 315–321. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, X.; Wang, J.; Hong, C.; Luo, W.; Wang, C. Design, synthesis and evaluation of genistein-polyamine conjugates as multi-functional anti-Alzheimer agents. Acta Pharm. Sin. B 2015, 5, 67–73. [Google Scholar] [CrossRef] [Green Version]
- Mas-Bargues, C.; Borrás, C.; Viña, J. The multimodal action of genistein in Alzheimer’s and other age-related diseases. Free Radic. Biol. Med. 2022, 183, 127–137. [Google Scholar] [CrossRef]
- Castillo, W.O.; Palomino, N.V.; Takahashi, C.S.; Giuliatti, S. Genistein and Galantamine Combinations Decrease β-Amyloid Peptide (1–42)–Induced Genotoxicity and Cell Death in SH-SY5Y Cell Line: An In Vitro and In Silico Approach for Mimic of Alzheimer’s Disease. Neurotox. Res. 2020, 38, 691–706. [Google Scholar] [CrossRef]
- Petry, F.d.S.; Coelho, B.P.; Gaelzer, M.M.; Kreutz, F.; Guma, F.T.C.R.; Salbego, C.G.; Trindade, V.M.T. Genistein protects against amyloid-beta-induced toxicity in SH-SY5Y cells by regulation of Akt and Tau phosphorylation. Phytother. Res. 2020, 34, 796–807. [Google Scholar] [CrossRef]
- Shentu, Y.-P.; Hu, W.-T.; Liang, J.-W.; Liuyang, Z.-Y.; Wei, H.; Qun, W.; Wang, X.-C.; Wang, J.-Z.; Westermarck, J.; Liu, R. Genistein Decreases APP/tau Phosphorylation and Ameliorates Aβ Overproduction Through Inhibiting CIP2A. Curr. Alzheimer Res. 2019, 16, 732–740. [Google Scholar] [CrossRef]
- Chatterjee, G.; Roy, D.; Kumar Khemka, V.; Chattopadhyay, M.; Chakrabarti, S. Genistein, the Isoflavone in Soybean, Causes Amyloid Beta Peptide Accumulation in Human Neuroblastoma Cell Line: Implications in Alzheimer’s Disease. Aging Dis. 2015, 6, 456. [Google Scholar] [CrossRef] [Green Version]
- Mirahmadi, S.-M.-S.; Shahmohammadi, A.; Rousta, A.-M.; Azadi, M.-R.; Fahanik-Babaei, J.; Baluchnejadmojarad, T.; Roghani, M. Soy isoflavone genistein attenuates lipopolysaccharide-induced cognitive impairments in the rat via exerting anti-oxidative and anti-inflammatory effects. Cytokine 2018, 104, 151–159. [Google Scholar] [CrossRef]
- Wang, Y.; Xia, Z.; Jiang, X.; Li, L.; Wang, H.; An, D.; Liu, Y. Genistein inhibits amyloid peptide 25-35-induced neuronal death by modulating estrogen receptors, choline acetyltransferase and glutamate receptors. Arch. Biochem. Biophys. 2020, 693, 108561. [Google Scholar] [CrossRef]
- Bagheri, M.; Roghani, M.; Joghataei, M.-T.; Mohseni, S. Genistein inhibits aggregation of exogenous amyloid-beta1–40 and alleviates astrogliosis in the hippocampus of rats. Brain Res. 2012, 1429, 145–154. [Google Scholar] [CrossRef] [Green Version]
- Pierzynowska, K.; Podlacha, M.; Gaffke, L.; Majkutewicz, I.; Mantej, J.; Węgrzyn, A.; Osiadły, M.; Myślińska, D.; Węgrzyn, G. Autophagy-dependent mechanism of genistein-mediated elimination of behavioral and biochemical defects in the rat model of sporadic Alzheimer’s disease. Neuropharmacology 2019, 148, 332–346. [Google Scholar] [CrossRef]
- Liu, J.Y.H.; Sun, M.Y.Y.; Sommerville, N.; Ngan, M.P.; Ponomarev, E.D.; Lin, G.; Rudd, J.A. Soy flavonoids prevent cognitive deficits induced by intra-gastrointestinal administration of beta-amyloid. Food Chem. Toxicol. 2020, 141, 111396. [Google Scholar] [CrossRef]
- Bonet-Costa, V.; Herranz-Pérez, V.; Blanco-Gandía, M.; Mas-Bargues, C.; Inglés, M.; Garcia-Tarraga, P.; Rodriguez-Arias, M.; Miñarro, J.; Borras, C.; Garcia-Verdugo, J.M.; et al. Clearing Amyloid-β through PPARγ/ApoE Activation by Genistein is a Treatment of Experimental Alzheimer’s Disease. J. Alzheimers Dis. 2016, 51, 701–711. [Google Scholar] [CrossRef]
- Petry, F.d.S.; Hoppe, J.B.; Klein, C.P.; dos Santos, B.G.; Hözer, R.M.; Bifi, F.; Matté, C.; Salbego, C.G.; Trindade, V.M.T. Genistein attenuates amyloid-beta-induced cognitive impairment in rats by modulation of hippocampal synaptotoxicity and hyperphosphorylation of Tau. J. Nutr. Biochem. 2021, 87, 108525. [Google Scholar] [CrossRef]
- Bagheri, M.; Joghataei, M.-T.; Mohseni, S.; Roghani, M. Genistein ameliorates learning and memory deficits in amyloid β(1–40) rat model of Alzheimer’s disease. Neurobiol. Learn. Mem. 2011, 95, 270–276. [Google Scholar] [CrossRef] [Green Version]
- Gleason, C.E.; Fischer, B.L.; Dowling, N.M.; Setchell, K.D.R.; Atwood, C.S.; Carlsson, C.M.; Asthana, S. Cognitive Effects of Soy Isoflavones in Patients with Alzheimer’s Disease. J. Alzheimers Dis. 2015, 47, 1009–1019. [Google Scholar] [CrossRef] [Green Version]
- Guo, J.; Yang, G.; He, Y.; Xu, H.; Fan, H.; An, J.; Zhang, L.; Zhang, R.; Cao, G.; Hao, D.; et al. Involvement of α7nAChR in the Protective Effects of Genistein Against β-Amyloid-Induced Oxidative Stress in Neurons via a PI3K/Akt/Nrf2 Pathway-Related Mechanism. Cell. Mol. Neurobiol. 2021, 41, 377–393. [Google Scholar] [CrossRef]
- Zhou, X.; Yuan, L.; Zhao, X.; Hou, C.; Ma, W.; Yu, H.; Xiao, R. Genistein antagonizes inflammatory damage induced by β-amyloid peptide in microglia through TLR4 and NF-κB. Nutrition 2014, 30, 90–95. [Google Scholar] [CrossRef] [PubMed]
- Duro-Castano, A.; Borrás, C.; Herranz-Pérez, V.; Blanco-Gandía, M.C.; Conejos-Sánchez, I.; Armiñán, A.; Mas-Bargues, C.; Inglés, M.; Miñarro, J.; Rodríguez-Arias, M.; et al. Targeting Alzheimer’s disease with multimodal polypeptide-based nanoconjugates. Sci. Adv. 2021, 7, eabf9180. [Google Scholar] [CrossRef] [PubMed]
- Hong, C.; Guo, H.; Chen, S.; Lv, J.; Zhang, X.; Yang, Y.; Huang, K.; Zhang, Y.; Tian, Z.; Luo, W.; et al. Synthesis and biological evaluation of genistein-O-alkylamine derivatives as potential multifunctional anti-Alzheimer agents. Chem. Biol. Drug Des. 2019, 93, 188–200. [Google Scholar] [CrossRef]
- Sang, Z.; Shi, J.; Zhou, Y.; Wang, K.; Zhao, Y.; Li, Q.; Qiao, Z.; Wu, A.; Tan, Z.; Liu, W. Development of genistein-O-alkylamines derivatives as multifunctional agents for the treatment of Alzheimer’s disease. Bioorganic Chem. 2021, 107, 104602. [Google Scholar] [CrossRef] [PubMed]
- Afzal, M.; Redha, A.; AlHasan, R. Anthocyanins Potentially Contribute to Defense against Alzheimer’s Disease. Molecules 2019, 24, 4255. [Google Scholar] [CrossRef] [Green Version]
- Zhang, J.; Wu, J.; Liu, F.; Tong, L.; Chen, Z.; Chen, J.; He, H.; Xu, R.; Ma, Y.; Huang, C. Neuroprotective effects of anthocyanins and its major component cyanidin-3-O-glucoside (C3G) in the central nervous system: An outlined review. Eur. J. Pharmacol. 2019, 858, 172500. [Google Scholar] [CrossRef]
- Suresh, S.; Begum, R.F.; Singh, S.A.; Chitra, V. Anthocyanin as a therapeutic in Alzheimer’s disease: A systematic review of preclinical evidences. Ageing Res. Rev. 2022, 76, 101595. [Google Scholar] [CrossRef]
- Shishtar, E.; Rogers, G.T.; Blumberg, J.B.; Au, R.; Jacques, P.F. Long-term dietary flavonoid intake and risk of Alzheimer disease and related dementias in the Framingham Offspring Cohort. Am. J. Clin. Nutr. 2020, 112, 343–353. [Google Scholar] [CrossRef]
- Rosli, H.; Shahar, S.; Rajab, N.F.; Che Din, N.; Haron, H. The effects of polyphenols-rich tropical fruit juice on cognitive function and metabolomics profile—A randomized controlled trial in middle-aged women. Nutr. Neurosci. 2022, 25, 1577–1593. [Google Scholar] [CrossRef]
- Li, J.; Zhao, R.; Jiang, Y.; Xu, Y.; Zhao, H.; Lyu, X.; Wu, T. Bilberry anthocyanins improve neuroinflammation and cognitive dysfunction in APP/PSEN1 mice via the CD33/TREM2/TYROBP signaling pathway in microglia. Food Funct. 2020, 11, 1572–1584. [Google Scholar] [CrossRef]
- Khan, M.S.; Ali, T.; Kim, M.W.; Jo, M.H.; Chung, J.I.; Kim, M.O. Anthocyanins Improve Hippocampus-Dependent Memory Function and Prevent Neurodegeneration via JNK/Akt/GSK3β Signaling in LPS-Treated Adult Mice. Mol. Neurobiol. 2019, 56, 671–687. [Google Scholar] [CrossRef]
- Wen, H.; Cui, H.; Tian, H.; Zhang, X.; Ma, L.; Ramassamy, C.; Li, J. Isolation of Neuroprotective Anthocyanins from Black Chokeberry (Aronia melanocarpa) against Amyloid-β-Induced Cognitive Impairment. Foods 2020, 10, 63. [Google Scholar] [CrossRef]
- Rehman, S.U.; Shah, S.A.; Ali, T.; Chung, J.I.; Kim, M.O. Anthocyanins Reversed D-Galactose-Induced Oxidative Stress and Neuroinflammation Mediated Cognitive Impairment in Adult Rats. Mol. Neurobiol. 2017, 54, 255–271. [Google Scholar] [CrossRef]
- Fan, Z.; Wen, H.; Zhang, X.; Li, J.; Zang, J. Cyanidin 3-O-β-Galactoside Alleviated Cognitive Impairment in Mice by Regulating Brain Energy Metabolism During Aging. J. Agric. Food Chem. 2022, 70, 1111–1121. [Google Scholar] [CrossRef]
- El-Shiekh, R.A.; Ashour, R.M.; Abd El-Haleim, E.A.; Ahmed, K.A.; Abdel-Sattar, E. Hibiscus sabdariffa L.: A potent natural neuroprotective agent for the prevention of streptozotocin-induced Alzheimer’s disease in mice. Biomed. Pharmacother. 2020, 128, 110303. [Google Scholar] [CrossRef]
- Gutierres, J.M.; Carvalho, F.B.; Schetinger, M.R.C.; Marisco, P.; Agostinho, P.; Rodrigues, M.; Rubin, M.A.; Schmatz, R.; da Silva, C.R.; Cognato, G.D.P.; et al. Anthocyanins restore behavioral and biochemical changes caused by streptozotocin-induced sporadic dementia of Alzheimer’s type. Life Sci. 2014, 96, 7–17. [Google Scholar] [CrossRef] [Green Version]
- Shimada, M.; Maeda, H.; Nanashima, N.; Yamada, K.; Nakajima, A. Anthocyanin-rich blackcurrant extract improves long-term memory impairment and emotional abnormality in senescence-accelerated mice. J. Food Biochem. 2022, 46, e14295. [Google Scholar] [CrossRef]
- Ma, H.; Johnson, S.; Liu, W.; DaSilva, N.; Meschwitz, S.; Dain, J.; Seeram, N. Evaluation of Polyphenol Anthocyanin-Enriched Extracts of Blackberry, Black Raspberry, Blueberry, Cranberry, Red Raspberry, and Strawberry for Free Radical Scavenging, Reactive Carbonyl Species Trapping, Anti-Glycation, Anti-β-Amyloid Aggregation, and Microglial Neuroprotective Effects. Int. J. Mol. Sci. 2018, 19, 461. [Google Scholar] [CrossRef] [Green Version]
- Navarro-Hortal, M.D.; Romero-Márquez, J.M.; Esteban-Muñoz, A.; Sánchez-González, C.; Rivas-García, L.; Llopis, J.; Cianciosi, D.; Giampieri, F.; Sumalla-Cano, S.; Battino, M.; et al. Strawberry (Fragaria × ananassa cv. Romina) methanolic extract attenuates Alzheimer’s beta amyloid production and oxidative stress by SKN-1/NRF and DAF-16/FOXO mediated mechanisms in C. elegans. Food Chem. 2022, 372, 131272. [Google Scholar] [CrossRef]
- Sun, C.; Zhang, S.; Ba, S.; Dang, J.; Ren, Q.; Zhu, Y.; Liu, K.; Jin, M. Eucommia ulmoides Olive Male Flower Extracts Ameliorate Alzheimer’s Disease-Like Pathology in Zebrafish via Regulating Autophagy, Acetylcholinesterase, and the Dopamine Transporter. Front. Mol. Neurosci. 2022, 15, 901953. [Google Scholar] [CrossRef]
- Badshah, H.; Kim, T.H.; Kim, M.O. Protective effects of Anthocyanins against Amyloid beta-induced neurotoxicity in vivo and in vitro. Neurochem. Int. 2015, 80, 51–59. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.J.; Rehman, S.U.; Amin, F.U.; Kim, M.O. Enhanced neuroprotection of anthocyanin-loaded PEG-gold nanoparticles against Aβ1-42-induced neuroinflammation and neurodegeneration via the NF-KB/JNK/GSK3β signaling pathway. Nanomed. Nanotechnol. Biol. Med. 2017, 13, 2533–2544. [Google Scholar] [CrossRef] [PubMed]
- Ali, T.; Kim, T.; Rehman, S.U.; Khan, M.S.; Amin, F.U.; Khan, M.; Ikram, M.; Kim, M.O. Natural Dietary Supplementation of Anthocyanins via PI3K/Akt/Nrf2/HO-1 Pathways Mitigate Oxidative Stress, Neurodegeneration, and Memory Impairment in a Mouse Model of Alzheimer’s Disease. Mol. Neurobiol. 2018, 55, 6076–6093. [Google Scholar] [CrossRef] [PubMed]
- Pacheco, S.M.; Soares, M.S.P.; Gutierres, J.M.; Gerzson, M.F.B.; Carvalho, F.B.; Azambuja, J.H.; Schetinger, M.R.C.; Stefanello, F.M.; Spanevello, R.M. Anthocyanins as a potential pharmacological agent to manage memory deficit, oxidative stress and alterations in ion pump activity induced by experimental sporadic dementia of Alzheimer’s type. J. Nutr. Biochem. 2018, 56, 193–204. [Google Scholar] [CrossRef] [PubMed]
- Ochiishi, T.; Kaku, M.; Kajsongkram, T.; Thisayakorn, K. Mulberry fruit extract alleviates the intracellular amyloid-β oligomer-induced cognitive disturbance and oxidative stress in Alzheimer’s disease model mice. Genes Cells 2021, 26, 861–873. [Google Scholar] [CrossRef]
- Li, H.; Zheng, T.; Lian, F.; Xu, T.; Yin, W.; Jiang, Y. Anthocyanin-rich blueberry extracts and anthocyanin metabolite protocatechuic acid promote autophagy-lysosomal pathway and alleviate neurons damage in in vivo and in vitro models of Alzheimer’s disease. Nutrition 2022, 93, 111473. [Google Scholar] [CrossRef]
- Ali, T.; Kim, M.J.; Rehman, S.U.; Ahmad, A.; Kim, M.O. Anthocyanin-Loaded PEG-Gold Nanoparticles Enhanced the Neuroprotection of Anthocyanins in an Aβ1–42 Mouse Model of Alzheimer’s Disease. Mol. Neurobiol. 2017, 54, 6490–6506. [Google Scholar] [CrossRef]
- Singla, R.K.; Dubey, A.K.; Garg, A.; Sharma, R.K.; Fiorino, M.; Ameen, S.M.; Haddad, M.A.; Al-Hiary, M. Natural Polyphenols: Chemical Classification, Definition of Classes, Subcategories, and Structures. J. AOAC Int. 2019, 102, 1397–1400. [Google Scholar] [CrossRef]
- Varesi, A.; Chirumbolo, S.; Campagnoli, L.I.M.; Pierella, E.; Piccini, G.B.; Carrara, A.; Ricevuti, G.; Scassellati, C.; Bonvicini, C.; Pascale, A. The Role of Antioxidants in the Interplay between Oxidative Stress and Senescence. Antioxidants 2022, 11, 1224. [Google Scholar] [CrossRef]
- Marino, A.; Battaglini, M.; Moles, N.; Ciofani, G. Natural Antioxidant Compounds as Potential Pharmaceutical Tools against Neurodegenerative Diseases. ACS Omega 2022, 7, 25974–25990. [Google Scholar] [CrossRef]
- Mori, T.; Koyama, N.; Guillot-Sestier, M.-V.; Tan, J.; Town, T. Ferulic Acid Is a Nutraceutical β-Secretase Modulator That Improves Behavioral Impairment and Alzheimer-like Pathology in Transgenic Mice. PLoS ONE 2013, 8, e55774. [Google Scholar] [CrossRef] [Green Version]
- Pi, R.; Mao, X.; Chao, X.; Cheng, Z.; Liu, M.; Duan, X.; Ye, M.; Chen, X.; Mei, Z.; Liu, P.; et al. Tacrine-6-Ferulic Acid, a Novel Multifunctional Dimer, Inhibits Amyloid-β-Mediated Alzheimer’s Disease-Associated Pathogenesis In Vitro and In Vivo. PLoS ONE 2012, 7, e31921. [Google Scholar] [CrossRef]
- Sul, D.; Kim, H.-S.; Lee, D.; Joo, S.S.; Hwang, K.W.; Park, S.-Y. Protective effect of caffeic acid against beta-amyloid-induced neurotoxicity by the inhibition of calcium influx and tau phosphorylation. Life Sci. 2009, 84, 257–262. [Google Scholar] [CrossRef]
- Kim, J.H.; Wang, Q.; Choi, J.M.; Lee, S.; Cho, E.J. Protective role of caffeic acid in an Aβ 25-35-induced Alzheimer’s disease model. Nutr. Res. Pract. 2015, 9, 480. [Google Scholar] [CrossRef] [Green Version]
- Morroni, F.; Sita, G.; Graziosi, A.; Turrini, E.; Fimognari, C.; Tarozzi, A.; Hrelia, P. Neuroprotective Effect of Caffeic Acid Phenethyl Ester in A Mouse Model of Alzheimer’s Disease Involves Nrf2/HO-1 Pathway. Aging Dis. 2018, 9, 605. [Google Scholar] [CrossRef] [Green Version]
- Yoon, J.-H.; Youn, K.; Ho, C.-T.; Karwe, M.V.; Jeong, W.-S.; Jun, M. p-Coumaric Acid and Ursolic Acid from Corni fructus Attenuated β-Amyloid 25–35-Induced Toxicity through Regulation of the NF-κB Signaling Pathway in PC12 Cells. J. Agric. Food Chem. 2014, 62, 4911–4916. [Google Scholar] [CrossRef]
- Daroi, P.A.; Dhage, S.N.; Juvekar, A.R. p-Coumaric acid protects against D-galactose induced neurotoxicity by attenuating neuroinflammation and apoptosis in mice brain. Metab. Brain Dis. 2022, 37, 2569–2579. [Google Scholar] [CrossRef]
- Shin, K.Y.; Lee, G.H.; Park, C.H.; Kim, H.J.; Park, S.-H.; Kim, S.; Kim, H.-S.; Lee, K.-S.; Won, B.Y.; Lee, H.G.; et al. A novel compound, maltolylp-coumarate, attenuates cognitive deficits and shows neuroprotective effects in vitro and in vivo dementia models. J. Neurosci. Res. 2007, 85, 2500–2511. [Google Scholar] [CrossRef]
- Ogunlade, B.; Adelakun, S.A.; Agie, J.A. Nutritional supplementation of gallic acid ameliorates Alzheimer-type hippocampal neurodegeneration and cognitive impairment induced by aluminum chloride exposure in adult Wistar rats. Drug Chem. Toxicol. 2022, 45, 651–662. [Google Scholar] [CrossRef]
- Yu, M.; Chen, X.; Liu, J.; Ma, Q.; Zhuo, Z.; Chen, H.; Zhou, L.; Yang, S.; Zheng, L.; Ning, C.; et al. Gallic acid disruption of Aβ1–42 aggregation rescues cognitive decline of APP/PS1 double transgenic mouse. Neurobiol. Dis. 2019, 124, 67–80. [Google Scholar] [CrossRef]
- Zhong, L.; Liu, H.; Zhang, W.; Liu, X.; Jiang, B.; Fei, H.; Sun, Z. Ellagic acid ameliorates learning and memory impairment in APP/PS1 transgenic mice via inhibition of β-amyloid production and tau hyperphosphorylation. Exp. Ther. Med. 2018, 16, 4951–4958. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kiasalari, Z.; Heydarifard, R.; Khalili, M.; Afshin-Majd, S.; Baluchnejadmojarad, T.; Zahedi, E.; Sanaierad, A.; Roghani, M. Ellagic acid ameliorates learning and memory deficits in a rat model of Alzheimer’s disease: An exploration of underlying mechanisms. Psychopharmacology 2017, 234, 1841–1852. [Google Scholar] [CrossRef] [PubMed]
- Ramadan, W.S.; Alkarim, S. Ellagic Acid Modulates the Amyloid Precursor Protein Gene via Superoxide Dismutase Regulation in the Entorhinal Cortex in an Experimental Alzheimer’s Model. Cells 2021, 10, 3511. [Google Scholar] [CrossRef] [PubMed]
- Porquet, D.; Casadesús, G.; Bayod, S.; Vicente, A.; Canudas, A.M.; Vilaplana, J.; Pelegrí, C.; Sanfeliu, C.; Camins, A.; Pallàs, M.; et al. Dietary resveratrol prevents Alzheimer’s markers and increases life span in SAMP8. AGE 2013, 35, 1851–1865. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gu, J.; Li, Z.; Chen, H.; Xu, X.; Li, Y.; Gui, Y. Neuroprotective Effect of Trans-Resveratrol in Mild to Moderate Alzheimer Disease: A Randomized, Double-Blind Trial. Neurol. Ther. 2021, 10, 905–917. [Google Scholar] [CrossRef]
- Kong, D.; Yan, Y.; He, X.-Y.; Yang, H.; Liang, B.; Wang, J.; He, Y.; Ding, Y.; Yu, H. Effects of Resveratrol on the Mechanisms of Antioxidants and Estrogen in Alzheimer’s Disease. BioMed Res. Int. 2019, 2019, 8983752. [Google Scholar] [CrossRef] [Green Version]
- Lim, G.P.; Chu, T.; Yang, F.; Beech, W.; Frautschy, S.A.; Cole, G.M. The Curry Spice Curcumin Reduces Oxidative Damage and Amyloid Pathology in an Alzheimer Transgenic Mouse. J. Neurosci. 2001, 21, 8370–8377. [Google Scholar] [CrossRef] [Green Version]
- Sadegh Malvajerd, S.; Izadi, Z.; Azadi, A.; Kurd, M.; Derakhshankhah, H.; Sharifzadeh, M.; Akbari Javar, H.; Hamidi, M. Neuroprotective Potential of Curcumin-Loaded Nanostructured Lipid Carrier in an Animal Model of Alzheimer’s Disease: Behavioral and Biochemical Evidence. J. Alzheimers Dis. 2019, 69, 671–686. [Google Scholar] [CrossRef]
- Ringman, J.M.; Frautschy, S.A.; Teng, E.; Begum, A.N.; Bardens, J.; Beigi, M.; Gylys, K.H.; Badmaev, V.; Heath, D.D.; Apostolova, L.G.; et al. Oral curcumin for Alzheimer’s disease: Tolerability and efficacy in a 24-week randomized, double blind, placebo-controlled study. Alzheimers Res. Ther. 2012, 4, 43. [Google Scholar] [CrossRef] [Green Version]
- Mostafa, N.M.; Mostafa, A.M.; Ashour, M.L.; Elhady, S.S. Neuroprotective Effects of Black Pepper Cold-Pressed Oil on Scopolamine-Induced Oxidative Stress and Memory Impairment in Rats. Antioxidants 2021, 10, 1993. [Google Scholar] [CrossRef]
- Mao, X.; Liao, Z.; Guo, L.; Xu, X.; Wu, B.; Xu, M.; Zhao, X.; Bi, K.; Jia, Y. Schisandrin C Ameliorates Learning and Memory Deficits by Aβ 1-42-induced Oxidative Stress and Neurotoxicity in Mice: AD.; SCH-C.; AΒ1-42; Neuroprotective; Intracerebroventricular Injection. Phytother. Res. 2015, 29, 1373–1380. [Google Scholar] [CrossRef]
- Jeong, E.J.; Lee, H.K.; Lee, K.Y.; Jeon, B.J.; Kim, D.H.; Park, J.-H.; Song, J.-H.; Huh, J.; Lee, J.-H.; Sung, S.H. The effects of lignan-riched extract of Shisandra chinensis on amyloid-β-induced cognitive impairment and neurotoxicity in the cortex and hippocampus of mouse. J. Ethnopharmacol. 2013, 146, 347–354. [Google Scholar] [CrossRef]
- Kiokias, S.; Proestos, C.; Oreopoulou, V. Phenolic Acids of Plant Origin—A Review on Their Antioxidant Activity In Vitro (O/W Emulsion Systems) Along with Their in Vivo Health Biochemical Properties. Foods 2020, 9, 534. [Google Scholar] [CrossRef] [Green Version]
- Kiokias, S.; Oreopoulou, V. A Review of the Health Protective Effects of Phenolic Acids against a Range of Severe Pathologic Conditions (Including Coronavirus-Based Infections). Molecules 2021, 26, 5405. [Google Scholar] [CrossRef]
- Chandrasekara, A. Phenolic Acids. In Encyclopedia of Food Chemistry; Elsevier: Amsterdam, The Netherlands, 2019; pp. 535–545. ISBN 978-0-12-814045-1. [Google Scholar]
- Szwajgier, D.; Borowiec, K.; Pustelniak, K. The Neuroprotective Effects of Phenolic Acids: Molecular Mechanism of Action. Nutrients 2017, 9, 477. [Google Scholar] [CrossRef] [Green Version]
- Stevenson, D.E.; Hurst, R.D. Polyphenolic phytochemicals—Just antioxidants or much more? Cell. Mol. Life Sci. 2007, 64, 2900–2916. [Google Scholar] [CrossRef]
- Phadke, A.V.; Tayade, A.A.; Khambete, M.P. Therapeutic potential of ferulic acid and its derivatives in Alzheimer’s disease—A systematic review. Chem. Biol. Drug Des. 2021, 98, 713–721. [Google Scholar] [CrossRef]
- Zduńska, K.; Dana, A.; Kolodziejczak, A.; Rotsztejn, H. Antioxidant Properties of Ferulic Acid and Its Possible Application. Skin Pharmacol. Physiol. 2018, 31, 332–336. [Google Scholar] [CrossRef]
- Liu, Y.; Shi, L.; Qiu, W.; Shi, Y. Ferulic acid exhibits anti-inflammatory effects by inducing autophagy and blocking NLRP3 inflammasome activation. Mol. Cell. Toxicol. 2022, 18, 509–519. [Google Scholar] [CrossRef]
- Mishra, T.; Nagarajan, K.; Dixit, P.K.; Kumar, V. Neuroprotective potential of ferulic acid against cyclophosphamide-induced neuroinflammation and behavioral changes. J. Food Biochem. 2022, 46, e14436. [Google Scholar] [CrossRef]
- Ren, Z.; Zhang, R.; Li, Y.; Li, Y.; Yang, Z.; Yang, H. Ferulic acid exerts neuroprotective effects against cerebral ischemia/reperfusion-induced injury via antioxidant and anti-apoptotic mechanisms in vitro and in vivo. Int. J. Mol. Med. 2017, 40, 1444–1456. [Google Scholar] [CrossRef] [Green Version]
- Long, T.; Wu, Q.; Wei, J.; Tang, Y.; He, Y.-N.; He, C.-L.; Chen, X.; Yu, L.; Yu, C.-L.; Law, B.Y.-K.; et al. Ferulic Acid Exerts Neuroprotective Effects via Autophagy Induction in C. elegans and Cellular Models of Parkinson’s Disease. Oxid. Med. Cell. Longev. 2022, 2022, 3723567. [Google Scholar] [CrossRef] [PubMed]
- Haque, E.; Javed, H.; Azimullah, S.; Abul Khair, S.B.; Ojha, S. Neuroprotective potential of ferulic acid in the rotenone model of Parkinson’s disease. Drug Des. Dev. Ther. 2015, 2015, 5499–5518. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ghobadi, M.; Arji Roodsari, B.; Yadegari, M.; Homayouni-Moghadam, F.; Rezvani, M.E. The Effect of Ferulic Acid on Cognitive Disorder and Brain Structural Deficit in Mice in Cuprizone Induced Animal Model of Multiple Sclerosis. J. Rafsanjan Univ. Med. Sci. 2017, 16, 541–552. [Google Scholar]
- Wang, E.-J.; Wu, M.-Y.; Lu, J.-H. Ferulic Acid in Animal Models of Alzheimer’s Disease: A Systematic Review of Preclinical Studies. Cells 2021, 10, 2653. [Google Scholar] [CrossRef]
- Yan, J.-J.; Jung, J.-S.; Kim, T.-K.; Hasan, M.A.; Hong, C.-W.; Nam, J.-S.; Song, D.-K. Protective Effects of Ferulic Acid in Amyloid Precursor Protein Plus Presenilin-1 Transgenic Mouse Model of Alzheimer Disease. Biol. Pharm. Bull. 2013, 36, 140–143. [Google Scholar] [CrossRef] [Green Version]
- Ono, K.; Hirohata, M.; Yamada, M. Ferulic acid destabilizes preformed β-amyloid fibrils in vitro. Biochem. Biophys. Res. Commun. 2005, 336, 444–449. [Google Scholar] [CrossRef]
- Wang, Q. Effect of Ferulic Acid on Learning and Memory Impairment by the Repairing of Mitochondrial Fission-Fusion Imbalance in AD Mice. Chin. Pharm. J. 2019, 24, 703–710. [Google Scholar]
- Picone, P.; Bondi, M.L.; Picone, P.; Bondi, M.L.; Montana, G.; Bruno, A.; Pitarresi, G.; Giammona, G.; Di Carlo, M. Ferulic acid inhibits oxidative stress and cell death induced by Ab oligomers: Improved delivery by solid lipid nanoparticles. Free Radic. Res. 2009, 43, 1133–1145. [Google Scholar] [CrossRef]
- Zafeer, M.F.; Firdaus, F.; Anis, E.; Mobarak Hossain, M. Prolong treatment with Trans-ferulic acid mitigates bioenergetics loss and restores mitochondrial dynamics in streptozotocin-induced sporadic dementia of Alzheimer’s type. NeuroToxicology 2019, 73, 246–257. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, C.; Wang, X.; Yu, S. Effects of Ferulic Acid on Oxidative Stress and Apoptosis Related Proteins in Alzheimer’s Disease Transgenic Mice. Nat. Prod. Res. Dev. 2017, 29, 762. [Google Scholar] [CrossRef]
- Wu, Y.; Shi, Y.; Zheng, X.; Dang, Y.; Zhu, C.; Zhang, R.; Fu, Y.; Zhou, T.; Li, J. Lipophilic ferulic acid derivatives protect PC12 cells against oxidative damage via modulating β-amyloid aggregation and activating Nrf2 enzymes. Food Funct. 2020, 11, 4707–4718. [Google Scholar] [CrossRef]
- Rui, M.; Yi-qing, C.; Qin, C. Effects of ferulic acid on glial activation and inflammatory cytokines expression in the cerebral cortex of Alzheimer’s disease like model mice. Chin. Hosp. Pharm. J. 2018, 38, 50–53. [Google Scholar]
- Wang, Y.; Wang, Y.; Li, J.; Hua, L.; Han, B.; Zhang, Y.; Yang, X.; Zeng, Z.; Bai, H.; Yin, H.; et al. Effects of caffeic acid on learning deficits in a model of Alzheimer’s disease. Int. J. Mol. Med. 2016, 38, 869–875. [Google Scholar] [CrossRef] [Green Version]
- Paciello, F.; Di Pino, A.; Rolesi, R.; Troiani, D.; Paludetti, G.; Grassi, C.; Fetoni, A.R. Anti-oxidant and anti-inflammatory effects of caffeic acid: In vivo evidences in a model of noise-induced hearing loss. Food Chem. Toxicol. 2020, 143, 111555. [Google Scholar] [CrossRef]
- Kanimozhi, G.; Prasad, N.R. Anticancer Effect of Caffeic Acid on Human Cervical Cancer Cells. In Coffee in Health and Disease Prevention; Elsevier: Amsterdam, The Netherlands, 2015; pp. 655–661. ISBN 978-0-12-409517-5. [Google Scholar]
- Kilani-Jaziri, S.; Mokdad-Bzeouich, I.; Krifa, M.; Nasr, N.; Ghedira, K.; Chekir-Ghedira, L. Immunomodulatory and cellular anti-oxidant activities of caffeic, ferulic, and p -coumaric phenolic acids: A structure–activity relationship study. Drug Chem. Toxicol. 2017, 40, 416–424. [Google Scholar] [CrossRef]
- Damasceno, S.S.; Dantas, B.B.; Ribeiro-Filho, J.; Araújo, D.A.M.; Costa, J.G.M. Chemical Properties of Caffeic and Ferulic Acids in Biological System: Implications in Cancer Therapy. A Review. Curr. Pharm. Des. 2017, 23, 3015–3023. [Google Scholar] [CrossRef]
- Gulcin, I. Antioxidant activity of caffeic acid (3,4-dihydroxycinnamic acid). Toxicology 2006, 217, 213–220. [Google Scholar] [CrossRef]
- Liang, G.; Shi, B.; Luo, W.; Yang, J. The protective effect of caffeic acid on global cerebral ischemia-reperfusion injury in rats. Behav. Brain Funct. 2015, 11, 18. [Google Scholar] [CrossRef] [Green Version]
- Zhang, L.; Zhang, W.-P.; Chen, K.-D.; Qian, X.-D.; Fang, S.-H.; Wei, E.-Q. Caffeic acid attenuates neuronal damage, astrogliosis and glial scar formation in mouse brain with cryoinjury. Life Sci. 2007, 80, 530–537. [Google Scholar] [CrossRef]
- Zhang, Y.; Wu, Q.; Zhang, L.; Wang, Q.; Yang, Z.; Liu, J.; Feng, L. Caffeic acid reduces A53T α-synuclein by activating JNK/Bcl-2-mediated autophagy in vitro and improves behaviour and protects dopaminergic neurons in a mouse model of Parkinson’s disease. Pharmacol. Res. 2019, 150, 104538. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Wang, J.; Chang, Y.; Li, R.; Sun, X.; Peng, L.; Zheng, W.; Qiu, W. Caffeic Acid Phenethyl Ester Protects against Experimental Autoimmune Encephalomyelitis by Regulating T Cell Activities. Oxid. Med. Cell. Longev. 2020, 2020, 7274342. [Google Scholar] [CrossRef] [PubMed]
- Wan, T.; Wang, Z.; Luo, Y.; Zhang, Y.; He, W.; Mei, Y.; Xue, J.; Li, M.; Pan, H.; Li, W.; et al. FA-97, a New Synthetic Caffeic Acid Phenethyl Ester Derivative, Protects against Oxidative Stress-Mediated Neuronal Cell Apoptosis and Scopolamine-Induced Cognitive Impairment by Activating Nrf2/HO-1 Signaling. Oxid. Med. Cell. Longev. 2019, 2019, 8239642. [Google Scholar] [CrossRef] [PubMed]
- Deshmukh, R.; Kaundal, M.; Bansal, V. Samardeep Caffeic acid attenuates oxidative stress, learning and memory deficit in intra-cerebroventricular streptozotocin induced experimental dementia in rats. Biomed. Pharmacother. 2016, 81, 56–62. [Google Scholar] [CrossRef] [PubMed]
- Haam, J.; Yakel, J.L. Cholinergic modulation of the hippocampal region and memory function. J. Neurochem. 2017, 142, 111–121. [Google Scholar] [CrossRef]
- Pei, K.; Ou, J.; Huang, J.; Ou, S. p -Coumaric acid and its conjugates: Dietary sources, pharmacokinetic properties and biological activities: P -Coumaric acid and its conjugates. J. Sci. Food Agric. 2016, 96, 2952–2962. [Google Scholar] [CrossRef]
- Boz, H. p -Coumaric acid in cereals: Presence, antioxidant and antimicrobial effects. Int. J. Food Sci. Technol. 2015, 50, 2323–2328. [Google Scholar] [CrossRef]
- Abdel-Wahab, M.; El-Mahdy, M.A.; Abd-Ellah, M.F.; Helal, G.K.; Khalifa, F.; Hamada, F.M.A. Influence of p-coumaric acid on doxorubicin-induced oxidative stress in rat’s heart. Pharmacol. Res. 2003, 48, 461–465. [Google Scholar] [CrossRef]
- Pragasam, S.J.; Venkatesan, V.; Rasool, M. Immunomodulatory and Anti-inflammatory Effect of p-Coumaric Acid, a Common Dietary Polyphenol on Experimental Inflammation in Rats. Inflammation 2013, 36, 169–176. [Google Scholar] [CrossRef]
- Kong, C.-S.; Jeong, C.-H.; Choi, J.-S.; Kim, K.-J.; Jeong, J.-W. Antiangiogenic Effects of P -Coumaric Acid in Human Endothelial Cells: P -COUMARIC ACID INHIBITS TUMOR ANGIOGENESIS. Phytother. Res. 2013, 27, 317–323. [Google Scholar] [CrossRef]
- Hudson, E.A.; Dinh, P.A.; Kokubun, T.; Simmonds, M.S.; Gescher, A. Characterization of potentially chemopreventive phenols in extracts of brown rice that inhibit the growth of human breast and colon cancer cells. Cancer Epidemiol. Biomark. Prev. Publ. Am. Assoc. Cancer Res. Cosponsored Am. Soc. Prev. Oncol. 2000, 9, 1163–1170. [Google Scholar]
- Kiliç, I.; Yeşiloğlu, Y. Spectroscopic studies on the antioxidant activity of p-coumaric acid. Spectrochim. Acta. A. Mol. Biomol. Spectrosc. 2013, 115, 719–724. [Google Scholar] [CrossRef]
- Roychoudhury, S.; Sinha, B.; Choudhury, B.P.; Jha, N.K.; Palit, P.; Kundu, S.; Mandal, S.C.; Kolesarova, A.; Yousef, M.I.; Ruokolainen, J.; et al. Scavenging Properties of Plant-Derived Natural Biomolecule Para-Coumaric Acid in the Prevention of Oxidative Stress-Induced Diseases. Antioxidants 2021, 10, 1205. [Google Scholar] [CrossRef]
- Guven, M.; Bozkurt Aras, A.; Akman, T.; Murat Sen, H.; Ozkan, A.; Salis, O.; Sehitoglu, I.; Kalkan, Y.; Silan, C.; Deniz, M.; et al. Neuroprotective effect of p-coumaric acid in rat model of embolic cerebral ischemia. Iran. J. Basic Med. Sci. 2015, 18, 356–363. [Google Scholar] [CrossRef]
- Sakamula, R.; Thong-asa, W. Neuroprotective effect of p-coumaric acid in mice with cerebral ischemia reperfusion injuries. Metab. Brain Dis. 2018, 33, 765–773. [Google Scholar] [CrossRef]
- Daroi, P.A.; Dhage, S.N.; Juvekar, A.R. p-Coumaric acid mitigates lipopolysaccharide induced brain damage via alleviating oxidative stress, inflammation and apoptosis. J. Pharm. Pharmacol. 2022, 74, 556–564. [Google Scholar] [CrossRef]
- Khan, M.S.; Althobaiti, M.S.; Almutairi, G.S.; Alokail, M.S.; Altwaijry, N.; Alenad, A.M.; Al-Bagmi, M.S.; Alafaleq, N.O. Elucidating the binding and inhibitory potential of p-coumaric acid against amyloid fibrillation and their cytotoxicity: Biophysical and docking analysis. Biophys. Chem. 2022, 291, 106823. [Google Scholar] [CrossRef]
- Ghaderi, S.; Gholipour, P.; Komaki, A.; Salehi, I.; Rashidi, K.; Esmaeil Khoshnam, S.; Rashno, M. p-Coumaric acid ameliorates cognitive and non-cognitive disturbances in a rat model of Alzheimer’s disease: The role of oxidative stress and inflammation. Int. Immunopharmacol. 2022, 112, 109295. [Google Scholar] [CrossRef]
- Bai, J.; Zhang, Y.; Tang, C.; Hou, Y.; Ai, X.; Chen, X.; Zhang, Y.; Wang, X.; Meng, X. Gallic acid: Pharmacological activities and molecular mechanisms involved in inflammation-related diseases. Biomed. Pharmacother. 2021, 133, 110985. [Google Scholar] [CrossRef]
- Daglia, M.; Lorenzo, A.; Nabavi, S.; Talas, Z.; Nabavi, S. Polyphenols: Well Beyond The Antioxidant Capacity: Gallic Acid and Related Compounds as Neuroprotective Agents: You are What You Eat! Curr. Pharm. Biotechnol. 2014, 15, 362–372. [Google Scholar] [CrossRef]
- Kahkeshani, N.; Farzaei, F.; Fotouhi, M.; Alavi, S.S.; Bahramsoltani, R.; Naseri, R.; Momtaz, S.; Abbasabadi, Z.; Rahimi, R.; Farzaei, M.H.; et al. Pharmacological effects of gallic acid in health and disease: A mechanistic review. Iran. J. Basic Med. Sci. 2019, 22, 225–237. [Google Scholar] [CrossRef] [PubMed]
- Punithavathi, V.R.; Prince, P.S.M.; Kumar, R.; Selvakumari, J. Antihyperglycaemic, antilipid peroxidative and antioxidant effects of gallic acid on streptozotocin induced diabetic Wistar rats. Eur. J. Pharmacol. 2011, 650, 465–471. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.-J. Antimelanogenic and Antioxidant Properties of Gallic Acid. Biol. Pharm. Bull. 2007, 30, 1052–1055. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, M.-J.; Seong, A.-R.; Yoo, J.-Y.; Jin, C.-H.; Lee, Y.-H.; Kim, Y.J.; Lee, J.; Jun, W.J.; Yoon, H.-G. Gallic acid, a histone acetyltransferase inhibitor, suppresses β-amyloid neurotoxicity by inhibiting microglial-mediated neuroinflammation. Mol. Nutr. Food Res. 2011, 55, 1798–1808. [Google Scholar] [CrossRef]
- Verma, S.; Singh, A.; Mishra, A. Gallic acid: Molecular rival of cancer. Environ. Toxicol. Pharmacol. 2013, 35, 473–485. [Google Scholar] [CrossRef]
- Kratz, J.M.; Andrighetti-Fröhner, C.R.; Kolling, D.J.; Leal, P.C.; Cirne-Santos, C.C.; Yunes, R.A.; Nunes, R.J.; Trybala, E.; Bergström, T.; Frugulhetti, I.C.; et al. Anti-HSV-1 and anti-HIV-1 activity of gallic acid and pentyl gallate. Mem. Inst. Oswaldo Cruz 2008, 103, 437–442. [Google Scholar] [CrossRef]
- Mirshekar, M.A.; Sarkaki, A.; Farbood, Y.; Gharib Naseri, M.K.; Badavi, M.; Mansouri, M.T.; Haghparast, A. Neuroprotective effects of gallic acid in a rat model of traumatic brain injury: Behavioral, electrophysiological and molecular studies. Iran. J. Basic Med. Sci. 2018, 21, 1056–1063. [Google Scholar] [CrossRef]
- Mansouri, M.T.; Farbood, Y.; Sameri, M.J.; Sarkaki, A.; Naghizadeh, B.; Rafeirad, M. Neuroprotective effects of oral gallic acid against oxidative stress induced by 6-hydroxydopamine in rats. Food Chem. 2013, 138, 1028–1033. [Google Scholar] [CrossRef]
- Maya, S.; Prakash, T.; Goli, D. Evaluation of neuroprotective effects of wedelolactone and gallic acid on aluminium-induced neurodegeneration: Relevance to sporadic amyotrophic lateral sclerosis. Eur. J. Pharmacol. 2018, 835, 41–51. [Google Scholar] [CrossRef]
- Shabani, S.; Rabiei, Z.; Amini-Khoei, H. Exploring the multifaceted neuroprotective actions of gallic acid: A review. Int. J. Food Prop. 2020, 23, 736–752. [Google Scholar] [CrossRef]
- Mori, T.; Koyama, N.; Yokoo, T.; Segawa, T.; Maeda, M.; Sawmiller, D.; Tan, J.; Town, T. Gallic acid is a dual α/β-secretase modulator that reverses cognitive impairment and remediates pathology in Alzheimer mice. J. Biol. Chem. 2020, 295, 16251–16266. [Google Scholar] [CrossRef]
- Ban, J.Y.; Nguyen, H.T.T.; Lee, H.-J.; Cho, S.O.; Ju, H.S.; Kim, J.Y.; Bae, K.; Song, K.-S.; Seong, Y.H. Neuroprotective Properties of Gallic Acid from Sanguisorbae Radix on Amyloid. BETA. Protein (25-35)-Induced Toxicity in Cultured Rat Cortical Neurons. Biol. Pharm. Bull. 2008, 31, 149–153. [Google Scholar] [CrossRef] [Green Version]
- Gerzson, M.F.B.; Bona, N.P.; Soares, M.S.P.; Teixeira, F.C.; Rahmeier, F.L.; Carvalho, F.B.; da Cruz Fernandes, M.; Onzi, G.; Lenz, G.; Gonçales, R.A.; et al. Tannic Acid Ameliorates STZ-Induced Alzheimer’s Disease-Like Impairment of Memory, Neuroinflammation, Neuronal Death and Modulates Akt Expression. Neurotox. Res. 2020, 37, 1009–1017. [Google Scholar] [CrossRef]
- Ardah, M.T.; Bharathan, G.; Kitada, T.; Haque, M.E. Ellagic Acid Prevents Dopamine Neuron Degeneration from Oxidative Stress and Neuroinflammation in MPTP Model of Parkinson’s Disease. Biomolecules 2020, 10, 1519. [Google Scholar] [CrossRef]
- Ahmed, T.; Setzer, W.N.; Fazel Nabavi, S.; Erdogan Orhan, I.; Braidy, N.; Sobarzo-Sanchez, E.; Mohammad Nabavi, S. Insights into Effects of Ellagic Acid on the Nervous System: A Mini Review. Curr. Pharm. Des. 2016, 22, 1350–1360. [Google Scholar] [CrossRef]
- Wang, Y.; Wu, Y.; Liang, C.; Tan, R.; Tan, L.; Tan, R. Pharmacodynamic Effect of Ellagic Acid on Ameliorating Cerebral Ischemia/Reperfusion Injury. Pharmacology 2019, 104, 320–331. [Google Scholar] [CrossRef]
- Aslan, A.; Gok, O.; Beyaz, S.; Arslan, E.; Erman, O.; Ağca, C.A. The preventive effect of ellagic acid on brain damage in rats via regulating of Nrf-2, NF-kB and apoptotic pathway. J. Food Biochem. 2020, 44, e13217. [Google Scholar] [CrossRef]
- Lee, W.-J.; Ou, H.-C.; Hsu, W.-C.; Chou, M.-M.; Tseng, J.-J.; Hsu, S.-L.; Tsai, K.-L.; Sheu, W.H.-H. Ellagic acid inhibits oxidized LDL-mediated LOX-1 expression, ROS generation, and inflammation in human endothelial cells. J. Vasc. Surg. 2010, 52, 1290–1300. [Google Scholar] [CrossRef] [Green Version]
- Dalvi, L.T.; Moreira, D.C.; Andrade, R.; Ginani, J.; Alonso, A.; Hermes-Lima, M. Ellagic acid inhibits iron-mediated free radical formation. Spectrochim. Acta. A Mol. Biomol. Spectrosc. 2017, 173, 910–917. [Google Scholar] [CrossRef]
- Ríos, J.-L.; Giner, R.; Marín, M.; Recio, M. A Pharmacological Update of Ellagic Acid. Planta Med. 2018, 84, 1068–1093. [Google Scholar] [CrossRef] [Green Version]
- Gomes, B.A.Q.; Silva, J.P.B.; Romeiro, C.F.R.; dos Santos, S.M.; Rodrigues, C.A.; Gonçalves, P.R.; Sakai, J.T.; Mendes, P.F.S.; Varela, E.L.P.; Monteiro, M.C. Neuroprotective Mechanisms of Resveratrol in Alzheimer’s Disease: Role of SIRT1. Oxid. Med. Cell. Longev. 2018, 2018, 8152373. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rocha-González, H.I.; Ambriz-Tututi, M.; Granados-Soto, V. Resveratrol: A Natural Compound with Pharmacological Potential in Neurodegenerative Diseases. CNS Neurosci. Ther. 2008, 14, 234–247. [Google Scholar] [CrossRef] [PubMed]
- Rossi, M.; Caruso, F.; Antonioletti, R.; Viglianti, A.; Traversi, G.; Leone, S.; Basso, E.; Cozzi, R. Scavenging of hydroxyl radical by resveratrol and related natural stilbenes after hydrogen peroxide attack on DNA. Chem. Biol. Interact. 2013, 206, 175–185. [Google Scholar] [CrossRef] [PubMed]
- Sadi, G.; Konat, D. Resveratrol regulates oxidative biomarkers and antioxidant enzymes in the brain of streptozotocin-induced diabetic rats. Pharm. Biol. 2015, 54, 1156–1163. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tong, W.; Chen, X.; Song, X.; Chen, Y.; Jia, R.; Zou, Y.; Li, L.; Yin, L.; He, C.; Liang, X.; et al. Resveratrol inhibits LPS-induced inflammation through suppressing the signaling cascades of TLR4-NF-κB/MAPKs/IRF3. Exp. Ther. Med. 2019, 19, 1824–1834. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moussa, C.; Hebron, M.; Huang, X.; Ahn, J.; Rissman, R.A.; Aisen, P.S.; Turner, R.S. Resveratrol regulates neuro-inflammation and induces adaptive immunity in Alzheimer’s disease. J. Neuroinflamm. 2017, 14, 1. [Google Scholar] [CrossRef] [PubMed]
- Turner, R.S.; Thomas, R.G.; Craft, S.; van Dyck, C.H.; Mintzer, J.; Reynolds, B.A.; Brewer, J.B.; Rissman, R.A.; Raman, R.; Aisen, P.S.; et al. A randomized, double-blind, placebo-controlled trial of resveratrol for Alzheimer disease. Neurology 2015, 85, 1383–1391. [Google Scholar] [CrossRef] [PubMed]
- Frozza, R.L.; Bernardi, A.; Hoppe, J.B.; Meneghetti, A.B.; Matté, A.; Battastini, A.M.O.; Pohlmann, A.R.; Guterres, S.S.; Salbego, C. Neuroprotective Effects of Resveratrol Against Aβ Administration in Rats are Improved by Lipid-Core Nanocapsules. Mol. Neurobiol. 2013, 47, 1066–1080. [Google Scholar] [CrossRef]
- Zhang, W.; Kung, M.-P.; Oya, S.; Hou, C.; Kung, H.F. 18F-labeled styrylpyridines as PET agents for amyloid plaque imaging. Nucl. Med. Biol. 2007, 34, 89–97. [Google Scholar] [CrossRef]
- Zhu, L.-N.; Mei, X.; Zhang, Z.-G.; Xie, Y.; Lang, F. Curcumin intervention for cognitive function in different types of people: A systematic review and meta-analysis: Curcumin intervention for cognitive function. Phytother. Res. 2019, 33, 524–533. [Google Scholar] [CrossRef] [Green Version]
- Hanai, H.; Sugimoto, K. Curcumin has Bright Prospects for the Treatment of Inflammatory Bowel Disease. Curr. Pharm. Des. 2009, 15, 2087–2094. [Google Scholar] [CrossRef]
- Jackson, J.K.; Higo, T.; Hunter, W.L.; Burt, H.M. The antioxidants curcumin and quercetin inhibit inflammatory processes associated with arthritis. Inflamm. Res. 2006, 55, 168–175. [Google Scholar] [CrossRef]
- Nebrisi, E.E. Neuroprotective Activities of Curcumin in Parkinson’s Disease: A Review of the Literature. Int. J. Mol. Sci. 2021, 22, 11248. [Google Scholar] [CrossRef]
- Wu, S.; Guo, T.; Qi, W.; Li, Y.; Gu, J.; Liu, C.; Sha, Y.; Yang, B.; Hu, S.; Zong, X. Curcumin ameliorates ischemic stroke injury in rats by protecting the integrity of the blood-brain barrier. Exp. Ther. Med. 2021, 22, 783. [Google Scholar] [CrossRef]
- Labanca, F.; Ullah, H.; Khan, H.; Milella, L.; Xiao, J.; Dajic-Stevanovic, Z.; Jeandet, P. Therapeutic and Mechanistic Effects of Curcumin in Huntington’s Disease. Curr. Neuropharmacol. 2021, 19, 1007–1018. [Google Scholar] [CrossRef]
- Alisi, I.O.; Uzairu, A.; Abechi, S.E.; Idris, S.O. Evaluation of the antioxidant properties of curcumin derivatives by genetic function algorithm. J. Adv. Res. 2018, 12, 47–54. [Google Scholar] [CrossRef]
- Jurenka, J.S. Anti-inflammatory properties of curcumin, a major constituent of Curcuma longa: A review of preclinical and clinical research. Altern. Med. Rev. J. Clin. Ther. 2009, 14, 141–153. [Google Scholar]
- Zheng, K.; Dai, X.; Xiao, N.; Wu, X.; Wei, Z.; Fang, W.; Zhu, Y.; Zhang, J.; Chen, X. Curcumin Ameliorates Memory Decline via Inhibiting BACE1 Expression and β-Amyloid Pathology in 5×FAD Transgenic Mice. Mol. Neurobiol. 2017, 54, 1967–1977. [Google Scholar] [CrossRef]
- Xiong, Z.; Hongmei, Z.; Lu, S.; Yu, L. Curcumin mediates presenilin-1 activity to reduce β-amyloid production in a model of Alzheimer’s disease. Pharmacol. Rep. 2011, 63, 1101–1108. [Google Scholar] [CrossRef]
- Ono, K.; Hasegawa, K.; Naiki, H.; Yamada, M. Curcumin has potent anti-amyloidogenic effects for Alzheimer’s?-amyloid fibrils in vitro. J. Neurosci. Res. 2004, 75, 742–750. [Google Scholar] [CrossRef]
- Huang, H.-C.; Tang, D.; Xu, K.; Jiang, Z.-F. Curcumin attenuates amyloid-β-induced tau hyperphosphorylation in human neuroblastoma SH-SY5Y cells involving PTEN/Akt/GSK-3β signaling pathway. J. Recept. Signal Transduct. 2014, 34, 26–37. [Google Scholar] [CrossRef] [PubMed]
- Cianciulli, A.; Calvello, R.; Porro, C.; Trotta, T.; Salvatore, R.; Panaro, M.A. PI3k/Akt signalling pathway plays a crucial role in the anti-inflammatory effects of curcumin in LPS-activated microglia. Int. Immunopharmacol. 2016, 36, 282–290. [Google Scholar] [CrossRef] [PubMed]
- Shi, X.; Zheng, Z.; Li, J.; Xiao, Z.; Qi, W.; Zhang, A.; Wu, Q.; Fang, Y. Curcumin inhibits Aβ-induced microglial inflammatory responses in vitro: Involvement of ERK1/2 and p38 signaling pathways. Neurosci. Lett. 2015, 594, 105–110. [Google Scholar] [CrossRef] [PubMed]
- Voulgaropoulou, S.D.; van Amelsvoort, T.A.M.J.; Prickaerts, J.; Vingerhoets, C. The effect of curcumin on cognition in Alzheimer’s disease and healthy aging: A systematic review of pre-clinical and clinical studies. Brain Res. 2019, 1725, 146476. [Google Scholar] [CrossRef] [PubMed]
- Baum, L.; Lam, C.W.K.; Cheung, S.K.-K.; Kwok, T.; Lui, V.; Tsoh, J.; Lam, L.; Leung, V.; Hui, E.; Ng, C.; et al. Six-Month Randomized, Placebo-Controlled, Double-Blind, Pilot Clinical Trial of Curcumin in Patients with Alzheimer Disease. J. Clin. Psychopharmacol. 2008, 28, 110–113. [Google Scholar] [CrossRef] [Green Version]
- Tang, M.; Taghibiglou, C. The Mechanisms of Action of Curcumin in Alzheimer’s Disease. J. Alzheimers Dis. 2017, 58, 1003–1016. [Google Scholar] [CrossRef]
- Ruan, Y.; Xiong, Y.; Fang, W.; Yu, Q.; Mai, Y.; Cao, Z.; Wang, K.; Lei, M.; Xu, J.; Liu, Y.; et al. Highly sensitive Curcumin-conjugated nanotheranostic platform for detecting amyloid-beta plaques by magnetic resonance imaging and reversing cognitive deficits of Alzheimer’s disease via NLRP3-inhibition. J. Nanobiotechnol. 2022, 20, 322. [Google Scholar] [CrossRef]
- Lazar, A.N.; Mourtas, S.; Youssef, I.; Parizot, C.; Dauphin, A.; Delatour, B.; Antimisiaris, S.G.; Duyckaerts, C. Curcumin-conjugated nanoliposomes with high affinity for Aβ deposits: Possible applications to Alzheimer disease. Nanomedicine Nanotechnol. Biol. Med. 2013, 9, 712–721. [Google Scholar] [CrossRef]
- Goozee, K.G.; Shah, T.M.; Sohrabi, H.R.; Rainey-Smith, S.R.; Brown, B.; Verdile, G.; Martins, R.N. Examining the potential clinical value of curcumin in the prevention and diagnosis of Alzheimer’s disease. Br. J. Nutr. 2016, 115, 449–465. [Google Scholar] [CrossRef]
- Szopa, A.; Dziurka, M.; Warzecha, A.; Kubica, P.; Klimek-Szczykutowicz, M.; Ekiert, H. Targeted Lignan Profiling and Anti-Inflammatory Properties of Schisandra rubriflora and Schisandra chinensis Extracts. Molecules 2018, 23, 3103. [Google Scholar] [CrossRef] [Green Version]
- Eklund, P.C.; Långvik, O.K.; Wärnå, J.P.; Salmi, T.O.; Willför, S.M.; Sjöholm, R.E. Chemical studies on antioxidant mechanisms and free radical scavenging properties of lignans. Org. Biomol. Chem. 2005, 3, 3336. [Google Scholar] [CrossRef]
- Laura Esquivel-Campos, A.; Pérez-Gutiérrez, S.; Sánchez-Pérez, L.; Campos-Xolalpa, N.; Pérez-Ramos, J. Cytotoxicity and Antitumor Action of Lignans and Neolignans. In Secondary Metabolites—Trends and Reviews; Vijayakumar, R., Selvapuram Sudalaimuthu Raja, S., Eds.; IntechOpen: London, UK, 2022; ISBN 978-1-80355-207-1. [Google Scholar]
- Sowndhararajan, K.; Deepa, P.; Kim, M.; Park, S.J.; Kim, S. An overview of neuroprotective and cognitive enhancement properties of lignans from Schisandra chinensis. Biomed. Pharmacother. 2018, 97, 958–968. [Google Scholar] [CrossRef]
- Hsieh, M.-T.; Wu, C.-R.; Wang, W.-H.; Lin, L.-W. The ameliorating effect of the water layer of fructusschisandrae on cycloheximide-induced amnesia in rats: Interaction with drugs acting at neurotransmitter receptors. Pharmacol. Res. 2001, 43, 17–22. [Google Scholar] [CrossRef]
- Yu, J.; Kwon, H.; Cho, E.; Jeon, J.; Kang, R.H.; Youn, K.; Jun, M.; Lee, Y.C.; Ryu, J.H.; Kim, D.H. The effects of pinoresinol on cholinergic dysfunction-induced memory impairments and synaptic plasticity in mice. Food Chem. Toxicol. 2019, 125, 376–382. [Google Scholar] [CrossRef]
- Xu, M.; Dong, Y.; Wan, S.; Yan, T.; Cao, J.; Wu, L.; Bi, K.; Jia, Y. Schisantherin B ameliorates Aβ 1–42 -induced cognitive decline via restoration of GLT-1 in a mouse model of Alzheimer’s disease. Physiol. Behav. 2016, 167, 265–273. [Google Scholar] [CrossRef]
- Song, J.-X.; Lin, X.; Wong, R.N.-S.; Sze, S.C.-W.; Tong, Y.; Shaw, P.-C.; Zhang, Y.-B. Protective effects of dibenzocyclooctadiene lignans from Schisandra chinensis against beta-amyloid and homocysteine neurotoxicity in PC12 cells. Phytother. Res. 2010, 25, 435–443. [Google Scholar] [CrossRef]
- Zhao, X.; Liu, C.; Xu, M.; Li, X.; Bi, K.; Jia, Y. Total Lignans of Schisandra chinensis Ameliorates Aβ1-42-Induced Neurodegeneration with Cognitive Impairment in Mice and Primary Mouse Neuronal Cells. PLoS ONE 2016, 11, e0152772. [Google Scholar] [CrossRef]
- Hu, D.; Cao, Y.; He, R.; Han, N.; Liu, Z.; Miao, L.; Yin, J. Schizandrin, an Antioxidant Lignan from Schisandra chinensis, Ameliorates A β1–42 -Induced Memory Impairment in Mice. Oxid. Med. Cell. Longev. 2012, 2012, 721721. [Google Scholar] [CrossRef] [Green Version]
- Liu, Q.; Xie, T.; Xi, Y.; Li, L.; Mo, F.; Liu, X.; Liu, Z.; Gao, J.-M.; Yuan, T. Sesamol Attenuates Amyloid Peptide Accumulation and Cognitive Deficits in APP/PS1 Mice: The Mediating Role of the Gut–Brain Axis. J. Agric. Food Chem. 2021, 69, 12717–12729. [Google Scholar] [CrossRef]
- Liu, Q.; Wang, J.; Lin, B.; Cheng, Z.-Y.; Bai, M.; Shi, S.; Huang, X.-X.; Song, S.-J. Phenylpropanoids and lignans from Prunus tomentosa seeds as efficient β-amyloid (Aβ) aggregation inhibitors. Bioorganic Chem. 2019, 84, 269–275. [Google Scholar] [CrossRef]
- Gaucher, C.; Boudier, A.; Bonetti, J.; Clarot, I.; Leroy, P.; Parent, M. Glutathione: Antioxidant Properties Dedicated to Nanotechnologies. Antioxidants 2018, 7, 62. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lv, H.; Zhen, C.; Liu, J.; Yang, P.; Hu, L.; Shang, P. Unraveling the Potential Role of Glutathione in Multiple Forms of Cell Death in Cancer Therapy. Oxid. Med. Cell. Longev. 2019, 2019, 3150145. [Google Scholar] [CrossRef] [Green Version]
- Traverso, N.; Ricciarelli, R.; Nitti, M.; Marengo, B.; Furfaro, A.L.; Pronzato, M.A.; Marinari, U.M.; Domenicotti, C. Role of Glutathione in Cancer Progression and Chemoresistance. Oxid. Med. Cell. Longev. 2013, 2013, 972913. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, G.; Fang, Y.-Z.; Yang, S.; Lupton, J.R.; Turner, N.D. Glutathione Metabolism and Its Implications for Health. J. Nutr. 2004, 134, 489–492. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, J.J.; Thiyagarajah, M.; Song, J.; Chen, C.; Herrmann, N.; Gallagher, D.; Rapoport, M.J.; Black, S.E.; Ramirez, J.; Andreazza, A.C.; et al. Altered central and blood glutathione in Alzheimer’s disease and mild cognitive impairment: A meta-analysis. Alzheimers Res. Ther. 2022, 14, 23. [Google Scholar] [CrossRef]
- Lin, C.-H.; Lane, H.-Y. Plasma Glutathione Levels Decreased with Cognitive Decline among People with Mild Cognitive Impairment (MCI): A Two-Year Prospective Study. Antioxidants 2021, 10, 1839. [Google Scholar] [CrossRef]
- Mandal, P.K.; Tripathi, M.; Sugunan, S. Brain oxidative stress: Detection and mapping of anti-oxidant marker ‘Glutathione’ in different brain regions of healthy male/female, MCI and Alzheimer patients using non-invasive magnetic resonance spectroscopy. Biochem. Biophys. Res. Commun. 2012, 417, 43–48. [Google Scholar] [CrossRef]
- Duffy, S.L.; Lagopoulos, J.; Hickie, I.B.; Diamond, K.; Graeber, M.B.; Lewis, S.J.G.; Naismith, S.L. Glutathione relates to neuropsychological functioning in mild cognitive impairment. Alzheimers Dement. 2014, 10, 67–75. [Google Scholar] [CrossRef]
- Mandal, P.K.; Saharan, S.; Tripathi, M.; Murari, G. Brain Glutathione Levels—A Novel Biomarker for Mild Cognitive Impairment and Alzheimer’s Disease. Biol. Psychiatry 2015, 78, 702–710. [Google Scholar] [CrossRef]
- Shukla, D.; Mandal, P.K.; Mishra, R.; Punjabi, K.; Dwivedi, D.; Tripathi, M.; Badhautia, V. Hippocampal Glutathione Depletion and pH Increment in Alzheimer’s Disease: An in vivo MRS Study. J. Alzheimers Dis. 2021, 84, 1139–1152. [Google Scholar] [CrossRef]
- Izumi, H.; Sato, K.; Kojima, K.; Saito, T.; Saido, T.C.; Fukunaga, K. Oral glutathione administration inhibits the oxidative stress and the inflammatory responses in AppNL−G-F/NL−G-F knock-in mice. Neuropharmacology 2020, 168, 108026. [Google Scholar] [CrossRef]
- Christopher Kwon, Y.I.; Xie, W.; Zhu, H.; Xie, J.; Shinn, K.; Juckel, N.; Vince, R.; More, S.S.; Lee, M.K. γ-Glutamyl-Transpeptidase-Resistant Glutathione Analog Attenuates Progression of Alzheimer’s Disease-like Pathology and Neurodegeneration in a Mouse Model. Antioxidants 2021, 10, 1796. [Google Scholar] [CrossRef]
- Li, Q.; Cui, J.; Fang, C.; Liu, M.; Min, G.; Li, L. S-Adenosylmethionine Attenuates Oxidative Stress and Neuroinflammation Induced by Amyloid-β Through Modulation of Glutathione Metabolism. J. Alzheimers Dis. 2017, 58, 549–558. [Google Scholar] [CrossRef]
- Yang, H.; Xie, Z.; Wei, L.; Ding, M.; Wang, P.; Bi, J. Glutathione-mimetic D609 alleviates memory deficits and reduces amyloid-β deposition in an AβPP/PS1 transgenic mouse model. NeuroReport 2018, 29, 833–838. [Google Scholar] [CrossRef]
- Braidy, N.; Zarka, M.; Jugder, B.-E.; Welch, J.; Jayasena, T.; Chan, D.K.Y.; Sachdev, P.; Bridge, W. The Precursor to Glutathione (GSH), γ-Glutamylcysteine (GGC), Can Ameliorate Oxidative Damage and Neuroinflammation Induced by Aβ40 Oligomers in Human Astrocytes. Front. Aging Neurosci. 2019, 11, 177. [Google Scholar] [CrossRef] [Green Version]
- Barkats, M.; Millecamps, S.; Abrioux, P.; Geoffroy, M.-C.; Mallet, J. Overexpression of Glutathione Peroxidase Increases the Resistance of Neuronal Cells to Aβ-Mediated Neurotoxicity. J. Neurochem. 2002, 75, 1438–1446. [Google Scholar] [CrossRef]
- Shin, E.-J.; Chung, Y.H.; Sharma, N.; Nguyen, B.T.; Lee, S.H.; Kang, S.W.; Nah, S.-Y.; Wie, M.B.; Nabeshima, T.; Jeong, J.H.; et al. Glutathione Peroxidase-1 Knockout Facilitates Memory Impairment Induced by β-Amyloid (1–42) in Mice via Inhibition of PKC βII-Mediated ERK Signaling; Application with Glutathione Peroxidase-1 Gene-Encoded Adenovirus Vector. Neurochem. Res. 2020, 45, 2991–3002. [Google Scholar] [CrossRef]
- Song, J.; Kang, S.M.; Lee, W.T.; Park, K.A.; Lee, K.M.; Lee, J.E. Glutathione Protects Brain Endothelial Cells from Hydrogen Peroxide-Induced Oxidative Stress by Increasing Nrf2 Expression. Exp. Neurobiol. 2014, 23, 93–103. [Google Scholar] [CrossRef] [Green Version]
- Ansary, J.; Forbes-Hernández, T.Y.; Gil, E.; Cianciosi, D.; Zhang, J.; Elexpuru-Zabaleta, M.; Simal-Gandara, J.; Giampieri, F.; Battino, M. Potential Health Benefit of Garlic Based on Human Intervention Studies: A Brief Overview. Antioxidants 2020, 9, 619. [Google Scholar] [CrossRef]
- Qu, Z.; Mossine, V.V.; Cui, J.; Sun, G.Y.; Gu, Z. Protective Effects of AGE and Its Components on Neuroinflammation and Neurodegeneration. NeuroMolecular Med. 2016, 18, 474–482. [Google Scholar] [CrossRef]
- Ray, B.; Chauhan, N.B.; Lahiri, D.K. The “aged garlic extract:” (AGE) and one of its active ingredients S-allyl-L-cysteine (SAC) as potential preventive and therapeutic agents for Alzheimer’s disease (AD). Curr. Med. Chem. 2011, 18, 3306–3313. [Google Scholar] [CrossRef] [PubMed]
- Tedeschi, P.; Nigro, M.; Travagli, A.; Catani, M.; Cavazzini, A.; Merighi, S.; Gessi, S. Therapeutic Potential of Allicin and Aged Garlic Extract in Alzheimer’s Disease. Int. J. Mol. Sci. 2022, 23, 6950. [Google Scholar] [CrossRef] [PubMed]
- Griffin, B.; Selassie, M.; Gwebu, E.T. Effect of Aged Garlic Extract on the Cytotoxicity of Alzheimer β-Amyloid Peptide in Neuronal PC12 Cells. Nutr. Neurosci. 2000, 3, 139–142. [Google Scholar] [CrossRef] [PubMed]
- Gupta, V.B.; Indi, S.S.; Rao, K.S.J. Garlic extract exhibits antiamyloidogenic activity on amyloid-beta fibrillogenesis: Relevance to Alzheimer’s disease. Phytother. Res. 2009, 23, 111–115. [Google Scholar] [CrossRef] [PubMed]
- Gupta, V.B.; Rao, K.S.J. Anti-amyloidogenic activity of S-allyl-l-cysteine and its activity to destabilize Alzheimer’s β-amyloid fibrils in vitro. Neurosci. Lett. 2007, 429, 75–80. [Google Scholar] [CrossRef]
- Jeong, J.H.; Jeong, H.R.; Jo, Y.N.; Kim, H.J.; Shin, J.H.; Heo, H.J. Ameliorating effects of aged garlic extracts against Aβ-induced neurotoxicity and cognitive impairment. BMC Complement. Altern. Med. 2013, 13, 268. [Google Scholar] [CrossRef] [Green Version]
- Luo, J.-F.; Dong, Y.; Chen, J.-Y.; Lu, J.-H. The effect and underlying mechanisms of garlic extract against cognitive impairment and Alzheimer’s disease: A systematic review and meta-analysis of experimental animal studies. J. Ethnopharmacol. 2021, 280, 114423. [Google Scholar] [CrossRef]
- Chauhan, N.B.; Sandoval, J. Amelioration of early cognitive deficits by aged garlic extract in Alzheimer’s transgenic mice. Phytother. Res. 2007, 21, 629–640. [Google Scholar] [CrossRef]
- Elosta, A.; Slevin, M.; Rahman, K.; Ahmed, N. Aged garlic has more potent antiglycation and antioxidant properties compared to fresh garlic extract in vitro. Sci. Rep. 2017, 7, 39613. [Google Scholar] [CrossRef]
- Nillert, N.; Pannangrong, W.; Welbat, J.; Chaijaroonkhanarak, W.; Sripanidkulchai, K.; Sripanidkulchai, B. Neuroprotective Effects of Aged Garlic Extract on Cognitive Dysfunction and Neuroinflammation Induced by β-Amyloid in Rats. Nutrients 2017, 9, 24. [Google Scholar] [CrossRef] [Green Version]
- Thorajak, P.; Pannangrong, W.; Welbat, J.U.; Chaijaroonkhanarak, W.; Sripanidkulchai, K.; Sripanidkulchai, B. Effects of Aged Garlic Extract on Cholinergic, Glutamatergic and GABAergic Systems with Regard to Cognitive Impairment in Aβ-Induced Rats. Nutrients 2017, 9, 686. [Google Scholar] [CrossRef] [Green Version]
- Burbaeva, G.S.; Boksha, I.S.; Tereshkina, E.B.; Savushkina, O.K.; Prokhorova, T.A.; Vorobyeva, E.A. Glutamate and GABA-Metabolizing Enzymes in Post-mortem Cerebellum in Alzheimer’s Disease: Phosphate-Activated Glutaminase and Glutamic Acid Decarboxylase. The Cerebellum 2014, 13, 607–615. [Google Scholar] [CrossRef]
- Rodriguez-Perdigon, M.; Tordera, R.M.; Gil-Bea, F.J.; Gerenu, G.; Ramirez, M.J.; Solas, M. Down-regulation of glutamatergic terminals (VGLUT1) driven by Aβ in Alzheimer’s disease: Aβ and VGLUT1 in Alzheimer’s Disease. Hippocampus 2016, 26, 1303–1312. [Google Scholar] [CrossRef]
- Ray, B.; Chauhan, N.B.; Lahiri, D.K. Oxidative insults to neurons and synapse are prevented by aged garlic extract and S-allyl-l-cysteine treatment in the neuronal culture and APP-Tg mouse model: Oxidative insults to neurons and synapse: Role of AGE and SAC. J. Neurochem. 2011, 117, 388–402. [Google Scholar] [CrossRef] [Green Version]
- Javed, H.; Khan, M.M.; Khan, A.; Vaibhav, K.; Ahmad, A.; Khuwaja, G.; Ahmed, M.E.; Raza, S.S.; Ashafaq, M.; Tabassum, R.; et al. S-allyl cysteine attenuates oxidative stress associated cognitive impairment and neurodegeneration in mouse model of streptozotocin-induced experimental dementia of Alzheimer’s type. Brain Res. 2011, 1389, 133–142. [Google Scholar] [CrossRef]
- Chauhan, N.B. Effect of aged garlic extract on APP processing and tau phosphorylation in Alzheimer’s transgenic model Tg2576. J. Ethnopharmacol. 2006, 108, 385–394. [Google Scholar] [CrossRef]
- Nadeem, M.S.; Kazmi, I.; Ullah, I.; Muhammad, K.; Anwar, F. Allicin, an Antioxidant and Neuroprotective Agent, Ameliorates Cognitive Impairment. Antioxidants 2021, 11, 87. [Google Scholar] [CrossRef]
- Li, X.-H.; Li, C.-Y.; Lu, J.-M.; Tian, R.-B.; Wei, J. Allicin ameliorates cognitive deficits ageing-induced learning and memory deficits through enhancing of Nrf2 antioxidant signaling pathways. Neurosci. Lett. 2012, 514, 46–50. [Google Scholar] [CrossRef]
- Kaur, S.; Raj, K.; Gupta, Y.K.; Singh, S. Allicin ameliorates aluminium- and copper-induced cognitive dysfunction in Wistar rats: Relevance to neuro-inflammation, neurotransmitters and Aβ(1–42) analysis. JBIC J. Biol. Inorg. Chem. 2021, 26, 495–510. [Google Scholar] [CrossRef]
- Li, X.-H.; Li, C.-Y.; Xiang, Z.-G.; Zhong, F.; Chen, Z.-Y.; Lu, J.-M. Allicin can reduce neuronal death and ameliorate the spatial memory impairment in Alzheimer’s disease models. Neurosci. Riyadh Saudi Arab. 2010, 15, 237–243. [Google Scholar]
- Zhang, H.; Wang, P.; Xue, Y.; Liu, L.; Li, Z.; Liu, Y. Allicin ameliorates cognitive impairment in APP/PS1 mice via Suppressing oxidative stress by Blocking JNK Signaling Pathways. Tissue Cell 2018, 50, 89–95. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.-F.; Li, X.-H.; Yuan, Z.-P.; Li, C.-Y.; Tian, R.-B.; Jia, W.; Xiao, Z.-P. Allicin improves endoplasmic reticulum stress-related cognitive deficits via PERK/Nrf2 antioxidative signaling pathway. Eur. J. Pharmacol. 2015, 762, 239–246. [Google Scholar] [CrossRef] [PubMed]
- de Sousa, C.N.S.; da Silva Leite, C.M.G.; da Silva Medeiros, I.; Vasconcelos, L.C.; Cabral, L.M.; Patrocínio, C.F.V.; Patrocínio, M.L.V.; Mouaffak, F.; Kebir, O.; Macedo, D.; et al. Alpha-lipoic acid in the treatment of psychiatric and neurological disorders: A systematic review. Metab. Brain Dis. 2019, 34, 39–52. [Google Scholar] [CrossRef] [PubMed]
- Lyu, M.; Liu, H.; Ye, Y.; Yin, Z. Inhibition effect of thiol-type antioxidants on protein oxidative aggregation caused by free radicals. Biophys. Chem. 2020, 260, 106367. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.-H.; Wang, D.-W.; Xu, S.-F.; Zhang, S.; Fan, Y.-G.; Yang, Y.-Y.; Guo, S.-Q.; Wang, S.; Guo, T.; Wang, Z.-Y.; et al. α-Lipoic acid improves abnormal behavior by mitigation of oxidative stress, inflammation, ferroptosis, and tauopathy in P301S Tau transgenic mice. Redox Biol. 2018, 14, 535–548. [Google Scholar] [CrossRef]
- Memudu, A.E.; Adewumi, A.E. Alpha lipoic acid ameliorates scopolamine induced memory deficit and neurodegeneration in the cerebello-hippocampal cortex. Metab. Brain Dis. 2021, 36, 1729–1745. [Google Scholar] [CrossRef]
- Farr, S.A.; Price, T.O.; Banks, W.A.; Ercal, N.; Morley, J.E. Effect of Alpha-Lipoic Acid on Memory, Oxidation, and Lifespan in SAMP8 Mice. J. Alzheimers Dis. 2012, 32, 447–455. [Google Scholar] [CrossRef]
- Cho, J.Y.; Um, H.S.; Kang, H.S.; Cho, I.H.; Kim, C.H.; Cho, J.S.; Hwang, D.Y. The combination of exercise training and α-lipoic acid treatment has therapeutic effects on the pathogenic phenotypes of Alzheimer’s disease in NSE/APPsw-transgenic mice. Int. J. Mol. Med. 2010, 25, 337–346. [Google Scholar] [CrossRef]
- Quinn, J.F.; Bussiere, J.R.; Hammond, R.S.; Montine, T.J.; Henson, E.; Jones, R.E.; Stackman, R.W. Chronic dietary α-lipoic acid reduces deficits in hippocampal memory of aged Tg2576 mice. Neurobiol. Aging 2007, 28, 213–225. [Google Scholar] [CrossRef]
- Sharma, M.; Gupta, Y.K. Effect of alpha lipoic acid on intracerebroventricular streptozotocin model of cognitive impairment in rats. Eur. Neuropsychopharmacol. 2003, 13, 241–247. [Google Scholar] [CrossRef]
- Di Stefano, A.; Sozio, P.; Cerasa, L.S.; Iannitelli, A.; Cataldi, A.; Zara, S.; Giorgioni, G.; Nasuti, C. Ibuprofen and Lipoic Acid Diamide as Co-Drug with Neuroprotective Activity: Pharmacological Properties and Effects in β-Amyloid (1–40) Infused Alzheimer’s Disease Rat Model. Int. J. Immunopathol. Pharmacol. 2010, 23, 589–599. [Google Scholar] [CrossRef] [Green Version]
- Hager, K.; Kenklies, M.; McAfoose, J.; Engel, J.; Münch, G. α-Lipoic acid as a new treatment option for Alzheimer’s disease—A 48 months follow-up analysis. In Neuropsychiatric Disorders An Integrative Approach; Gerlach, M., Deckert, J., Double, K., Koutsilieri, E., Eds.; Springer: Vienna, Austria, 2007; pp. 189–193. ISBN 978-3-211-73573-2. [Google Scholar]
- Shinto, L.; Quinn, J.; Montine, T.; Dodge, H.H.; Woodward, W.; Baldauf-Wagner, S.; Waichunas, D.; Bumgarner, L.; Bourdette, D.; Silbert, L.; et al. A Randomized Placebo-Controlled Pilot Trial of Omega-3 Fatty Acids and Alpha Lipoic Acid in Alzheimer’s Disease. J. Alzheimers Dis. 2013, 38, 111–120. [Google Scholar] [CrossRef] [Green Version]
- López-Otín, C.; Blasco, M.A.; Partridge, L.; Serrano, M.; Kroemer, G. Hallmarks of aging: An expanding universe. Cell 2023, S0092867422013770. [Google Scholar] [CrossRef]
- Calkins, M.; Manczak, M.; Reddy, P. Mitochondria-Targeted Antioxidant SS31 Prevents Amyloid Beta-Induced Mitochondrial Abnormalities and Synaptic Degeneration in Alzheimer’s Disease. Pharmaceuticals 2012, 5, 1103–1119. [Google Scholar] [CrossRef] [Green Version]
- Du, F.; Yu, Q.; Kanaan, N.M.; Yan, S.S. Mitochondrial oxidative stress contributes to the pathological aggregation and accumulation of tau oligomers in Alzheimer’s disease. Hum. Mol. Genet. 2022, 31, 2498–2507. [Google Scholar] [CrossRef]
- Lin, M.T.; Beal, M.F. Mitochondrial dysfunction and oxidative stress in neurodegenerative diseases. Nature 2006, 443, 787–795. [Google Scholar] [CrossRef]
- Lanzillotta, C.; Di Domenico, F.; Perluigi, M.; Butterfield, D.A. Targeting Mitochondria in Alzheimer Disease: Rationale and Perspectives. CNS Drugs 2019, 33, 957–969. [Google Scholar] [CrossRef]
- Reddy, P.H.; Manczak, M.; Yin, X.; Reddy, A.P. Synergistic Protective Effects of Mitochondrial Division Inhibitor 1 and Mitochondria-Targeted Small Peptide SS31 in Alzheimer’s Disease. J. Alzheimers Dis. 2018, 62, 1549–1565. [Google Scholar] [CrossRef]
- Soeda, Y.; Saito, M.; Maeda, S.; Ishida, K.; Nakamura, A.; Kojima, S.; Takashima, A. Methylene Blue Inhibits Formation of Tau Fibrils but not of Granular Tau Oligomers: A Plausible Key to Understanding Failure of a Clinical Trial for Alzheimer’s Disease. J. Alzheimers Dis. 2019, 68, 1677–1686. [Google Scholar] [CrossRef]
- Young, M.L.; Franklin, J.L. The mitochondria-targeted antioxidant MitoQ inhibits memory loss, neuropathology, and extends lifespan in aged 3xTg-AD mice. Mol. Cell. Neurosci. 2019, 101, 103409. [Google Scholar] [CrossRef]
- McManus, M.J.; Murphy, M.P.; Franklin, J.L. The Mitochondria-Targeted Antioxidant MitoQ Prevents Loss of Spatial Memory Retention and Early Neuropathology in a Transgenic Mouse Model of Alzheimer’s Disease. J. Neurosci. 2011, 31, 15703–15715. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ng, L.F.; Gruber, J.; Cheah, I.K.; Goo, C.K.; Cheong, W.F.; Shui, G.; Sit, K.P.; Wenk, M.R.; Halliwell, B. The mitochondria-targeted antioxidant MitoQ extends lifespan and improves healthspan of a transgenic Caenorhabditis elegans model of Alzheimer disease. Free Radic. Biol. Med. 2014, 71, 390–401. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rudnitskaya, E.A.; Burnyasheva, A.O.; Kozlova, T.A.; Peunov, D.A.; Kolosova, N.G.; Stefanova, N.A. Changes in Glial Support of the Hippocampus during the Development of an Alzheimer’s Disease-like Pathology and Their Correction by Mitochondria-Targeted Antioxidant SkQ1. Int. J. Mol. Sci. 2022, 23, 1134. [Google Scholar] [CrossRef] [PubMed]
- Stefanova, N.A.; Muraleva, N.A.; Maksimova, K.Y.; Rudnitskaya, E.A.; Kiseleva, E.; Telegina, D.V.; Kolosova, N.G. An antioxidant specifically targeting mitochondria delays progression of Alzheimer’s disease-like pathology. Aging 2016, 8, 2713–2733. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Muraleva, N.A.; Stefanova, N.A.; Kolosova, N.G. SkQ1 Suppresses the p38 MAPK Signaling Pathway Involved in Alzheimer’s Disease-Like Pathology in OXYS Rats. Antioxidants 2020, 9, 676. [Google Scholar] [CrossRef]
- Stefanova, N.A.; Fursova, A.Z.; Kolosova, N.G. Behavioral Effects Induced by Mitochondria-Targeted Antioxidant SkQ1 in Wistar and Senescence-Accelerated OXYS Rats. J. Alzheimers Dis. 2010, 21, 479–491. [Google Scholar] [CrossRef] [Green Version]
- Kapay, N.A.; Popova, O.V.; Isaev, N.K.; Stelmashook, E.V.; Kondratenko, R.V.; Zorov, D.B.; Skrebitsky, V.G.; Skulachev, V.P. Mitochondria-Targeted Plastoquinone Antioxidant SkQ1 Prevents Amyloid-β-Induced Impairment of Long-Term Potentiation in Rat Hippocampal Slices. J. Alzheimers Dis. 2013, 36, 377–383. [Google Scholar] [CrossRef]
- Attia, H.; Albuhayri, S.; Alaraidh, S.; Alotaibi, A.; Yacoub, H.; Mohamad, R.; Al-Amin, M. Biotin, coenzyme Q10, and their combination ameliorate aluminium chloride-induced Alzheimer’s disease via attenuating neuroinflammation and improving brain insulin signaling. J. Biochem. Mol. Toxicol. 2020, 34, e22519. [Google Scholar] [CrossRef]
- Ibrahim Fouad, G. Combination of Omega 3 and Coenzyme Q10 Exerts Neuroprotective Potential Against Hypercholesterolemia-Induced Alzheimer’s-Like Disease in Rats. Neurochem. Res. 2020, 45, 1142–1155. [Google Scholar] [CrossRef]
- Wadsworth, T.L.; Bishop, J.A.; Pappu, A.S.; Woltjer, R.L.; Quinn, J.F. Evaluation of Coenzyme Q as an Antioxidant Strategy for Alzheimer’s Disease. J. Alzheimers Dis. 2008, 14, 225–234. [Google Scholar] [CrossRef]
- Dumont, M.; Kipiani, K.; Yu, F.; Wille, E.; Katz, M.; Calingasan, N.Y.; Gouras, G.K.; Lin, M.T.; Beal, M.F. Coenzyme Q10 Decreases Amyloid Pathology and Improves Behavior in a Transgenic Mouse Model of Alzheimer’s Disease. J. Alzheimers Dis. 2011, 27, 211–223. [Google Scholar] [CrossRef]
- Muthukumaran, K.; Kanwar, A.; Vegh, C.; Marginean, A.; Elliott, A.; Guilbeault, N.; Badour, A.; Sikorska, M.; Cohen, J.; Pandey, S. Ubisol-Q10 (a Nanomicellar Water-Soluble Formulation of CoQ10) Treatment Inhibits Alzheimer-Type Behavioral and Pathological Symptoms in a Double Transgenic Mouse (TgAPEswe, PSEN1dE9) Model of Alzheimer’s Disease. J. Alzheimers Dis. 2017, 61, 221–236. [Google Scholar] [CrossRef]
- Lee, H.; Jeong, H.-R.; Park, J.-H.; Hoe, H.-S. Idebenone Decreases Aβ Pathology by Modulating RAGE/Caspase-3 Signaling and the Aβ Degradation Enzyme NEP in a Mouse Model of AD. Biology 2021, 10, 938. [Google Scholar] [CrossRef]
- Komaki, H.; Faraji, N.; Komaki, A.; Shahidi, S.; Etaee, F.; Raoufi, S.; Mirzaei, F. Investigation of protective effects of coenzyme Q10 on impaired synaptic plasticity in a male rat model of Alzheimer’s disease. Brain Res. Bull. 2019, 147, 14–21. [Google Scholar] [CrossRef]
- Vegh, C.; Pupulin, S.; Wear, D.; Culmone, L.; Huggard, R.; Ma, D.; Pandey, S. Resumption of Autophagy by Ubisol-Q 10 in Presenilin-1 Mutated Fibroblasts and Transgenic AD Mice: Implications for Inhibition of Senescence and Neuroprotection. Oxid. Med. Cell. Longev. 2019, 2019, 7404815. [Google Scholar] [CrossRef] [Green Version]
- Yang, X.; Yang, Y.; Li, G.; Wang, J.; Yang, E.S. Coenzyme Q10 Attenuates β-Amyloid Pathology in the Aged Transgenic Mice with Alzheimer Presenilin 1 Mutation. J. Mol. Neurosci. 2008, 34, 165–171. [Google Scholar] [CrossRef]
- Singh, A.; Kumar, A. Microglial Inhibitory Mechanism of Coenzyme Q10 Against Aβ (1-42) Induced Cognitive Dysfunctions: Possible Behavioral, Biochemical, Cellular, and Histopathological Alterations. Front. Pharmacol. 2015, 6, 268. [Google Scholar] [CrossRef] [Green Version]
- Sheykhhasan, M.; Amini, R.; Soleimani Asl, S.; Saidijam, M.; Hashemi, S.M.; Najafi, R. Neuroprotective effects of coenzyme Q10-loaded exosomes obtained from adipose-derived stem cells in a rat model of Alzheimer’s disease. Biomed. Pharmacother. 2022, 152, 113224. [Google Scholar] [CrossRef]
- Yang, X.; Dai, G.; Li, G.; Yang, E.S. Coenzyme Q10 Reduces β-Amyloid Plaque in an APP/PS1 Transgenic Mouse Model of Alzheimer’s Disease. J. Mol. Neurosci. 2010, 41, 110–113. [Google Scholar] [CrossRef]
- Hargreaves, I.; Heaton, R.A.; Mantle, D. Disorders of Human Coenzyme Q10 Metabolism: An Overview. Int. J. Mol. Sci. 2020, 21, 6695. [Google Scholar] [CrossRef]
- Garrido-Maraver, J.; Cordero, M.D.; Oropesa-Avila, M.; Vega, A.F.; de la Mata, M.; Delgado Pavon, A.; Alcocer-Gomez, E.; Perez Calero, C.; Villanueva Paz, M.; Alanis, M.; et al. Clinical applications of coenzyme Q10. Front. Biosci. 2014, 19, 619. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Isobe, C.; Abe, T.; Terayama, Y. Levels of reduced and oxidized coenzyme Q-10 and 8-hydroxy-2′-deoxyguanosine in the CSF of patients with Alzheimer’s disease demonstrate that mitochondrial oxidative damage and/or oxidative DNA damage contributes to the neurodegenerative process. J. Neurol. 2010, 257, 399–404. [Google Scholar] [CrossRef] [PubMed]
- the ActiFE Ulm study group; von Arnim, C.A.F.; Herbolsheimer, F.; Nikolaus, T.; Peter, R.; Biesalski, H.K.; Ludolph, A.C.; Riepe, M.; Nagel, G. Dietary Antioxidants and Dementia in a Population-Based Case-Control Study among Older People in South Germany. J. Alzheimers Dis. 2012, 31, 717–724. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McCarthy, S.; Somayajulu, M.; Sikorska, M.; Borowy-Borowski, H.; Pandey, S. Paraquat induces oxidative stress and neuronal cell death; neuroprotection by water-soluble Coenzyme Q10. Toxicol. Appl. Pharmacol. 2004, 201, 21–31. [Google Scholar] [CrossRef] [PubMed]
- Choi, H.; Park, H.-H.; Lee, K.-Y.; Choi, N.-Y.; Yu, H.-J.; Lee, Y.J.; Park, J.; Huh, Y.-M.; Lee, S.-H.; Koh, S.-H. Coenzyme Q10 Restores Amyloid Beta-Inhibited Proliferation of Neural Stem Cells by Activating the PI3K Pathway. Stem Cells Dev. 2013, 22, 2112–2120. [Google Scholar] [CrossRef]
- Li, L.; Xu, D.; Lin, J.; Zhang, D.; Wang, G.; Sui, L.; Ding, H.; Du, J. Coenzyme Q10 attenuated β-amyloid 25–35 –induced inflammatory responses in PC12 cells through regulation of the NF–κB signaling pathway. Brain Res. Bull. 2017, 131, 192–198. [Google Scholar] [CrossRef]
- Yang, M.; Lian, N.; Yu, Y.; Wang, Y.; Xie, K.; Yu, Y. Coenzyme Q10 alleviates sevoflurane-induced neuroinflammation by regulating the levels of apolipoprotein E and phosphorylated tau protein in mouse hippocampal neurons. Mol. Med. Rep. 2020, 22, 445–453. [Google Scholar] [CrossRef]
- Ali, A.A.; Abo El-Ella, D.M.; El-Emam, S.Z.; Shahat, A.S.; El-Sayed, R.M. Physical & mental activities enhance the neuroprotective effect of vinpocetine & coenzyme Q10 combination against Alzheimer & bone remodeling in rats. Life Sci. 2019, 229, 21–35. [Google Scholar] [CrossRef]
- Wainwright, L.; Hargreaves, I.P.; Georgian, A.R.; Turner, C.; Dalton, R.N.; Abbott, N.J.; Heales, S.J.R.; Preston, J.E. CoQ10 Deficient Endothelial Cell Culture Model for the Investigation of CoQ10 Blood–Brain Barrier Transport. J. Clin. Med. 2020, 9, 3236. [Google Scholar] [CrossRef]
- Wear, D.; Vegh, C.; Sandhu, J.K.; Sikorska, M.; Cohen, J.; Pandey, S. Ubisol-Q10, a Nanomicellar and Water-Dispersible Formulation of Coenzyme-Q10 as a Potential Treatment for Alzheimer’s and Parkinson’s Disease. Antioxidants 2021, 10, 764. [Google Scholar] [CrossRef]
- Wani, W.Y.; Gudup, S.; Sunkaria, A.; Bal, A.; Singh, P.P.; Kandimalla, R.J.L.; Sharma, D.R.; Gill, K.D. Protective efficacy of mitochondrial targeted antioxidant MitoQ against dichlorvos induced oxidative stress and cell death in rat brain. Neuropharmacology 2011, 61, 1193–1201. [Google Scholar] [CrossRef]
- Xiao, L.; Xu, X.; Zhang, F.; Wang, M.; Xu, Y.; Tang, D.; Wang, J.; Qin, Y.; Liu, Y.; Tang, C.; et al. The mitochondria-targeted antioxidant MitoQ ameliorated tubular injury mediated by mitophagy in diabetic kidney disease via Nrf2/PINK1. Redox Biol. 2017, 11, 297–311. [Google Scholar] [CrossRef]
- Manczak, M.; Mao, P.; Calkins, M.J.; Cornea, A.; Reddy, A.P.; Murphy, M.P.; Szeto, H.H.; Park, B.; Reddy, P.H. Mitochondria-Targeted Antioxidants Protect Against Amyloid-β Toxicity in Alzheimer’s Disease Neurons. J. Alzheimers Dis. 2010, 20, S609–S631. [Google Scholar] [CrossRef] [Green Version]
- Samluk, L.; Ostapczuk, P.; Dziembowska, M. Long-term mitochondrial stress induces early steps of Tau aggregation by increasing reactive oxygen species levels and affecting cellular proteostasis. Mol. Biol. Cell 2022, 33, ar67. [Google Scholar] [CrossRef]
- Dudek, J. Role of Cardiolipin in Mitochondrial Signaling Pathways. Front. Cell Dev. Biol. 2017, 5, 90. [Google Scholar] [CrossRef] [Green Version]
- Ma, T.; Hoeffer, C.A.; Wong, H.; Massaad, C.A.; Zhou, P.; Iadecola, C.; Murphy, M.P.; Pautler, R.G.; Klann, E. Amyloid -Induced Impairments in Hippocampal Synaptic Plasticity Are Rescued by Decreasing Mitochondrial Superoxide. J. Neurosci. 2011, 31, 5589–5595. [Google Scholar] [CrossRef] [Green Version]
- Skulachev, V.P. Mitochondria-Targeted Antioxidants as Promising Drugs for Treatment of Age-Related Brain Diseases. J. Alzheimers Dis. 2012, 28, 283–289. [Google Scholar] [CrossRef] [Green Version]
- Kolosova, N.G.; Tyumentsev, M.A.; Muraleva, N.A.; Kiseleva, E.; Vitovtov, A.O.; Stefanova, N.A. Antioxidant SkQ1 Alleviates Signs of Alzheimer’s Disease-like Pathology in Old OXYS Rats by Reversing Mitochondrial Deterioration. Curr. Alzheimer Res. 2017, 14, 1283–1292. [Google Scholar] [CrossRef]
- Muraleva, N.A.; Kozhevnikova, O.S.; Fursova, A.Z.; Kolosova, N.G. Suppression of AMD-Like Pathology by Mitochondria-Targeted Antioxidant SkQ1 Is Associated with a Decrease in the Accumulation of Amyloid β and in mTOR Activity. Antioxidants 2019, 8, 177. [Google Scholar] [CrossRef] [Green Version]
- Adlard, P.A.; Bush, A.I. Metals and Alzheimer’s Disease: How Far Have We Come in the Clinic? J. Alzheimers Dis. 2018, 62, 1369–1379. [Google Scholar] [CrossRef] [Green Version]
- Kim, G.H.; Kim, J.E.; Rhie, S.J.; Yoon, S. The Role of Oxidative Stress in Neurodegenerative Diseases. Exp. Neurobiol. 2015, 24, 325–340. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Socha, K.; Klimiuk, K.; Naliwajko, S.K.; Soroczyńska, J.; Puścion-Jakubik, A.; Markiewicz-Żukowska, R.; Kochanowicz, J. Dietary Habits, Selenium, Copper, Zinc and Total Antioxidant Status in Serum in Relation to Cognitive Functions of Patients with Alzheimer’s Disease. Nutrients 2021, 13, 287. [Google Scholar] [CrossRef] [PubMed]
- Vural, H.; Demirin, H.; Kara, Y.; Eren, I.; Delibas, N. Alterations of plasma magnesium, copper, zinc, iron and selenium concentrations and some related erythrocyte antioxidant enzyme activities in patients with Alzheimer’s disease. J. Trace Elem. Med. Biol. 2010, 24, 169–173. [Google Scholar] [CrossRef] [PubMed]
- Cardoso, B.R.; Roberts, B.R.; Malpas, C.B.; Vivash, L.; Genc, S.; Saling, M.M.; Desmond, P.; Steward, C.; Hicks, R.J.; Callahan, J.; et al. Supranutritional Sodium Selenate Supplementation Delivers Selenium to the Central Nervous System: Results from a Randomized Controlled Pilot Trial in Alzheimer’s Disease. Neurotherapeutics 2019, 16, 192–202. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gholamigeravand, B.; Shahidi, S.; Amiri, I.; Samzadeh-kermani, A.; Abbasalipourkabir, R.; Soleimani Asl, S. Administration of Selenium Nanoparticles Reverses Streptozotocin-Induced Neurotoxicity in the male rats. Metab. Brain Dis. 2021, 36, 1259–1266. [Google Scholar] [CrossRef]
- Ishrat, T.; Parveen, K.; Khan, M.M.; Khuwaja, G.; Khan, M.B.; Yousuf, S.; Ahmad, A.; Shrivastav, P.; Islam, F. Selenium prevents cognitive decline and oxidative damage in rat model of streptozotocin-induced experimental dementia of Alzheimer’s type. Brain Res. 2009, 1281, 117–127. [Google Scholar] [CrossRef]
- Balaban, H.; Nazıroğlu, M.; Demirci, K.; Övey, İ.S. The Protective Role of Selenium on Scopolamine-Induced Memory Impairment, Oxidative Stress, and Apoptosis in Aged Rats: The Involvement of TRPM2 and TRPV1 Channels. Mol. Neurobiol. 2017, 54, 2852–2868. [Google Scholar] [CrossRef]
- Cansev, M.; Turkyilmaz, M.; Sijben, J.W.C.; Sevinc, C.; Broersen, L.M.; van Wijk, N. Synaptic Membrane Synthesis in Rats Depends on Dietary Sufficiency of Vitamin C, Vitamin E, and Selenium: Relevance for Alzheimer’s Disease. J. Alzheimers Dis. 2017, 59, 301–311. [Google Scholar] [CrossRef]
- Zhang, Z.-H.; Chen, C.; Jia, S.-Z.; Cao, X.-C.; Liu, M.; Tian, J.; Hoffmann, P.R.; Xu, H.-X.; Ni, J.-Z.; Song, G.-L. Selenium Restores Synaptic Deficits by Modulating NMDA Receptors and Selenoprotein K in an Alzheimer’s Disease Model. Antioxid. Redox Signal. 2021, 35, 863–884. [Google Scholar] [CrossRef]
- Song, G.; Zhang, Z.; Wen, L.; Chen, C.; Shi, Q.; Zhang, Y.; Ni, J.; Liu, Q. Selenomethionine Ameliorates Cognitive Decline, Reduces Tau Hyperphosphorylation, and Reverses Synaptic Deficit in the Triple Transgenic Mouse Model of Alzheimer’s Disease. J. Alzheimers Dis. 2014, 41, 85–99. [Google Scholar] [CrossRef]
- Zhang, Z.-H.; Wu, Q.-Y.; Zheng, R.; Chen, C.; Chen, Y.; Liu, Q.; Hoffmann, P.R.; Ni, J.-Z.; Song, G.-L. Selenomethionine Mitigates Cognitive Decline by Targeting Both Tau Hyperphosphorylation and Autophagic Clearance in an Alzheimer’s Disease Mouse Model. J. Neurosci. 2017, 37, 2449–2462. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Z.-H.; Wu, Q.-Y.; Chen, C.; Zheng, R.; Chen, Y.; Ni, J.-Z.; Song, G.-L. Comparison of the effects of selenomethionine and selenium-enriched yeast in the triple-transgenic mouse model of Alzheimer’s disease. Food Funct. 2018, 9, 3965–3973. [Google Scholar] [CrossRef]
- Van der Jeugd, A.; Parra-Damas, A.; Baeta-Corral, R.; Soto-Faguás, C.M.; Ahmed, T.; LaFerla, F.M.; Giménez-Llort, L.; D’Hooge, R.; Saura, C.A. Reversal of memory and neuropsychiatric symptoms and reduced tau pathology by selenium in 3xTg-AD mice. Sci. Rep. 2018, 8, 6431. [Google Scholar] [CrossRef] [Green Version]
- Iqbal, J.; Zhang, K.; Jin, N.; Zhao, Y.; Liu, Q.; Ni, J.; Shen, L. Selenium positively affects the proteome of 3 × Tg-AD mice cortex by altering the expression of various key proteins: Unveiling the mechanistic role of selenium in AD prevention. J. Neurosci. Res. 2018, 96, 1798–1815. [Google Scholar] [CrossRef]
- Abozaid, O.A.R.; Sallam, M.W.; El-Sonbaty, S.; Aziza, S.; Emad, B.; Ahmed, E.S.A. Resveratrol-Selenium Nanoparticles Alleviate Neuroinflammation and Neurotoxicity in a Rat Model of Alzheimer’s Disease by Regulating Sirt1/miRNA-134/GSK3β Expression. Biol. Trace Elem. Res. 2022, 200, 5104–5114. [Google Scholar] [CrossRef]
- Cosín-Tomàs, M.; Senserrich, J.; Arumí-Planas, M.; Alquézar, C.; Pallàs, M.; Martín-Requero, A.; Suñol, C.; Kaliman, P.; Sanfeliu, C. Role of Resveratrol and Selenium on Oxidative Stress and Expression of Antioxidant and Anti-Aging Genes in Immortalized Lymphocytes from Alzheimer’s Disease Patients. Nutrients 2019, 11, 1764. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Z.H.; Cao, X.-C.; Peng, J.-Y.; Huang, S.-L.; Chen, C.; Jia, S.-Z.; Ni, J.-Z.; Song, G.-L. Reversal of Lipid Metabolism Dysregulation by Selenium and Folic Acid Co-Supplementation to Mitigate Pathology in Alzheimer’s Disease. Antioxidants 2022, 11, 829. [Google Scholar] [CrossRef]
- Tamtaji, O.R.; Heidari-soureshjani, R.; Mirhosseini, N.; Kouchaki, E.; Bahmani, F.; Aghadavod, E.; Tajabadi-Ebrahimi, M.; Asemi, Z. Probiotic and selenium co-supplementation, and the effects on clinical, metabolic and genetic status in Alzheimer’s disease: A randomized, double-blind, controlled trial. Clin. Nutr. 2019, 38, 2569–2575. [Google Scholar] [CrossRef]
- Cuajungco, M.P.; Goldstein, L.E.; Nunomura, A.; Smith, M.A.; Lim, J.T.; Atwood, C.S.; Huang, X.; Farrag, Y.W.; Perry, G.; Bush, A.I. Evidence that the β-Amyloid Plaques of Alzheimer’s Disease Represent the Redox-silencing and Entombment of Aβ by Zinc. J. Biol. Chem. 2000, 275, 19439–19442. [Google Scholar] [CrossRef] [Green Version]
- Donnelly, P.S.; Caragounis, A.; Du, T.; Laughton, K.M.; Volitakis, I.; Cherny, R.A.; Sharples, R.A.; Hill, A.F.; Li, Q.-X.; Masters, C.L.; et al. Selective Intracellular Release of Copper and Zinc Ions from Bis(thiosemicarbazonato) Complexes Reduces Levels of Alzheimer Disease Amyloid-β Peptide. J. Biol. Chem. 2008, 283, 4568–4577. [Google Scholar] [CrossRef] [Green Version]
- Zafar, R.; Zubair, M.; Ali, S.; Shahid, K.; Waseem, W.; Naureen, H.; Haider, A.; Jan, M.S.; Ullah, F.; Sirajuddin, M.; et al. Zinc metal carboxylates as potential anti-Alzheimer’s candidate: In vitro anticholinesterase, antioxidant and molecular docking studies. J. Biomol. Struct. Dyn. 2021, 39, 1044–1054. [Google Scholar] [CrossRef] [PubMed]
- Vilella, A.; Belletti, D.; Sauer, A.K.; Hagmeyer, S.; Sarowar, T.; Masoni, M.; Stasiak, N.; Mulvihill, J.J.E.; Ruozi, B.; Forni, F.; et al. Reduced plaque size and inflammation in the APP23 mouse model for Alzheimer’s disease after chronic application of polymeric nanoparticles for CNS targeted zinc delivery. J. Trace Elem. Med. Biol. 2018, 49, 210–221. [Google Scholar] [CrossRef] [PubMed]
- Singla, N.; Dhawan, D.K. Zinc Improves Cognitive and Neuronal Dysfunction During Aluminium-Induced Neurodegeneration. Mol. Neurobiol. 2017, 54, 406–422. [Google Scholar] [CrossRef] [PubMed]
- Harris, C.J.; Voss, K.; Murchison, C.; Ralle, M.; Frahler, K.; Carter, R.; Rhoads, A.; Lind, B.; Robinson, E.; Quinn, J.F. Oral Zinc Reduces Amyloid Burden in Tg2576 Mice. J. Alzheimers Dis. 2014, 41, 179–192. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brewer, G.J. Alzheimer’s disease causation by copper toxicity and treatment with zinc. Front. Aging Neurosci. 2014, 6, 92. [Google Scholar] [CrossRef]
- Farbood, Y.; Sarkaki, A.; Mahdavinia, M.; Ghadiri, A.; Teimoori, A.; Seif, F.; Dehghani, M.A.; Navabi, S.P. Protective Effects of Co-administration of Zinc and Selenium Against Streptozotocin-Induced Alzheimer’s Disease: Behavioral, Mitochondrial Oxidative Stress, and GPR39 Expression Alterations in Rats. Neurotox. Res. 2020, 38, 398–407. [Google Scholar] [CrossRef]
- Fu, C.; Dai, L.; Yuan, X.; Xu, Y. Effects of Fish Oil Combined with Selenium and Zinc on Learning and Memory Impairment in Aging Mice and Amyloid Precursor Protein Processing. Biol. Trace Elem. Res. 2021, 199, 1855–1863. [Google Scholar] [CrossRef]
- Cherny, R.A.; Atwood, C.S.; Xilinas, M.E.; Gray, D.N.; Jones, W.D.; McLean, C.A.; Barnham, K.J.; Volitakis, I.; Fraser, F.W.; Kim, Y.-S.; et al. Treatment with a Copper-Zinc Chelator Markedly and Rapidly Inhibits β-Amyloid Accumulation in Alzheimer’s Disease Transgenic Mice. Neuron 2001, 30, 665–676. [Google Scholar] [CrossRef]
- Tian, Z.-Y.; Wang, C.-Y.; Wang, T.; Li, Y.-C.; Wang, Z.-Y. Glial S100A6 Degrades β-amyloid Aggregation through Targeting Competition with Zinc Ions. Aging Dis. 2019, 10, 756. [Google Scholar] [CrossRef] [Green Version]
- Guo, C.; Wang, T.; Zheng, W.; Shan, Z.-Y.; Teng, W.-P.; Wang, Z.-Y. Intranasal deferoxamine reverses iron-induced memory deficits and inhibits amyloidogenic APP processing in a transgenic mouse model of Alzheimer’s disease. Neurobiol. Aging 2013, 34, 562–575. [Google Scholar] [CrossRef]
- Zhaba, W.-D.; Deji, Q.-Z.; Gao, S.-Q.; Han, Y.-L.; Gao, C.-C.; Deng, H.-J.; Liu, X.-L.; Li, T.; Zhou, M.-L. Deferoxamine reduces amyloid-beta peptides genesis and alleviates neural apoptosis after traumatic brain injury. NeuroReport 2021, 32, 472–478. [Google Scholar] [CrossRef]
- Fawzi, S.F.; Menze, E.T.; Tadros, M.G. Deferiprone ameliorates memory impairment in Scopolamine-treated rats: The impact of its iron-chelating effect on β-amyloid disposition. Behav. Brain Res. 2020, 378, 112314. [Google Scholar] [CrossRef]
- Rao, S.S.; Portbury, S.D.; Lago, L.; Bush, A.I.; Adlard, P.A. The Iron Chelator Deferiprone Improves the Phenotype in a Mouse Model of Tauopathy1. J. Alzheimers Dis. 2020, 77, 753–771. [Google Scholar] [CrossRef]
- Aalikhani, M.; Safdari, Y.; Jahanshahi, M.; Alikhani, M.; Khalili, M. Comparison Between Hesperidin, Coumarin, and Deferoxamine Iron Chelation and Antioxidant Activity Against Excessive Iron in the Iron Overloaded Mice. Front. Neurosci. 2022, 15, 811080. [Google Scholar] [CrossRef]
- Jahanshahi, M.; Khalili, M.; Margedari, A. Naringin Chelates Excessive Iron and Prevents the Formation of Amyloid-Beta Plaques in the Hippocampus of Iron-Overloaded Mice. Front. Pharmacol. 2021, 12, 651156. [Google Scholar] [CrossRef]
- Dairam, A.; Fogel, R.; Daya, S.; Limson, J.L. Antioxidant and Iron-Binding Properties of Curcumin, Capsaicin, and S -Allylcysteine Reduce Oxidative Stress in Rat Brain Homogenate. J. Agric. Food Chem. 2008, 56, 3350–3356. [Google Scholar] [CrossRef]
- Kupershmidt, L.; Amit, T.; Bar-Am, O.; Youdim, M.B.H.; Weinreb, O. The Novel Multi-Target Iron Chelating-Radical Scavenging Compound M30 Possesses Beneficial Effects on Major Hallmarks of Alzheimer’s Disease. Antioxid. Redox Signal. 2012, 17, 860–877. [Google Scholar] [CrossRef]
- Xiao, Y.; Gong, X.; Deng, R.; Liu, W.; Yang, Y.; Wang, X.; Wang, J.; Bao, J.; Shu, X. Iron Chelation Remits Memory Deficits Caused by the High-Fat Diet in a Mouse Model of Alzheimer’s Disease. J. Alzheimers Dis. 2022, 86, 1959–1971. [Google Scholar] [CrossRef]
- Wu, Z.; Palanimuthu, D.; Braidy, N.; Salikin, N.H.; Egan, S.; Huang, M.L.H.; Richardson, D.R. Novel multifunctional iron chelators of the aroyl nicotinoyl hydrazone class that markedly enhance cellular NAD+/NADH ratios. Br. J. Pharmacol. 2020, 177, 1967–1987. [Google Scholar] [CrossRef]
- Bailey, D.K.; Clark, W.; Kosman, D.J. The iron chelator, PBT434, modulates transcellular iron trafficking in brain microvascular endothelial cells. PLoS ONE 2021, 16, e0254794. [Google Scholar] [CrossRef]
- Liu, G.; Men, P.; Harris, P.L.R.; Rolston, R.K.; Perry, G.; Smith, M.A. Nanoparticle iron chelators: A new therapeutic approach in Alzheimer disease and other neurologic disorders associated with trace metal imbalance. Neurosci. Lett. 2006, 406, 189–193. [Google Scholar] [CrossRef] [PubMed]
- Shen, X.; Liu, J.; Fujita, Y.; Liu, S.; Maeda, T.; Kikuchi, K.; Obara, T.; Takebe, A.; Sayama, R.; Takahashi, T.; et al. Iron treatment inhibits Aβ42 deposition in vivo and reduces Aβ42/Aβ40 ratio. Biochem. Biophys. Res. Commun. 2019, 512, 653–658. [Google Scholar] [CrossRef] [PubMed]
- Ceccom, J.; Coslédan, F.; Halley, H.; Francès, B.; Lassalle, J.M.; Meunier, B. Copper Chelator Induced Efficient Episodic Memory Recovery in a Non-Transgenic Alzheimer’s Mouse Model. PLoS ONE 2012, 7, e43105. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, J.; Shi, Q.; Tian, H.; Li, Y.; Liu, Y.; Xu, Z.; Robert, A.; Liu, Q.; Meunier, B. TDMQ20, a Specific Copper Chelator, Reduces Memory Impairments in Alzheimer’s Disease Mouse Models. ACS Chem. Neurosci. 2021, 12, 140–149. [Google Scholar] [CrossRef] [PubMed]
- Maher, P. Modulation of the Neuroprotective and Anti-inflammatory Activities of the Flavonol Fisetin by the Transition Metals Iron and Copper. Antioxidants 2020, 9, 1113. [Google Scholar] [CrossRef]
- Harris, C.J.; Gray, N.E.; Caruso, M.; Hunter, M.; Ralle, M.; Quinn, J.F. Copper Modulation and Memory Impairment due to Hippocampal Tau Pathology. J. Alzheimers Dis. 2020, 78, 49–60. [Google Scholar] [CrossRef] [Green Version]
- Sestito, S.; Wang, S.; Chen, Q.; Lu, J.; Bertini, S.; Pomelli, C.; Chiellini, G.; He, X.; Pi, R.; Rapposelli, S. Multi-targeted ChEI-copper chelating molecules as neuroprotective agents. Eur. J. Med. Chem. 2019, 174, 216–225. [Google Scholar] [CrossRef]
- Hamulakova, S.; Poprac, P.; Jomova, K.; Brezova, V.; Lauro, P.; Drostinova, L.; Jun, D.; Sepsova, V.; Hrabinova, M.; Soukup, O.; et al. Targeting copper(II)-induced oxidative stress and the acetylcholinesterase system in Alzheimer’s disease using multifunctional tacrine-coumarin hybrid molecules. J. Inorg. Biochem. 2016, 161, 52–62. [Google Scholar] [CrossRef]
- Solovyev, N.D. Importance of selenium and selenoprotein for brain function: From antioxidant protection to neuronal signalling. J. Inorg. Biochem. 2015, 153, 1–12. [Google Scholar] [CrossRef]
- Reddy, V.S.; Bukke, S.; Dutt, N.; Rana, P.; Pandey, A.K. A systematic review and meta-analysis of the circulatory, erythrocellular and CSF selenium levels in Alzheimer’s disease: A metal meta-analysis (AMMA Study-I). J. Trace Elem. Med. Biol. 2017, 42, 68–75. [Google Scholar] [CrossRef]
- Chmatalova, Z.; Vyhnalek, M.; Laczo, J.; Hort, J.; Pospisilova, R.; Pechova, M.; Skoumalova, A. Relation of Plasma Selenium and Lipid Peroxidation End Products in Patients with Alzheimer’s Disease. Physiol. Res. 2017, 66, 1049–1056. [Google Scholar] [CrossRef]
- Varikasuvu, S.R.; Prasad, V.S.; Kothapalli, J.; Manne, M. Brain Selenium in Alzheimer’s Disease (BRAIN SEAD Study): A Systematic Review and Meta-Analysis. Biol. Trace Elem. Res. 2019, 189, 361–369. [Google Scholar] [CrossRef]
- Yan, X.; Liu, K.; Sun, X.; Qin, S.; Wu, M.; Qin, L.; Wang, Y.; Li, Z.; Zhong, X.; Wei, X. A cross-sectional study of blood selenium concentration and cognitive function in elderly Americans: National Health and Nutrition Examination Survey 2011–2014. Ann. Hum. Biol. 2020, 47, 610–619. [Google Scholar] [CrossRef]
- Nazıroğlu, M.; Muhamad, S.; Pecze, L. Nanoparticles as potential clinical therapeutic agents in Alzheimer’s disease: Focus on selenium nanoparticles. Expert Rev. Clin. Pharmacol. 2017, 10, 773–782. [Google Scholar] [CrossRef]
- Iqbal, J.; Zhang, K.; Jin, N.; Zhao, Y.; Liu, Q.; Ni, J.; Shen, L. Effect of Sodium Selenate on Hippocampal Proteome of 3×Tg-AD Mice—Exploring the Antioxidant Dogma of Selenium against Alzheimer’s Disease. ACS Chem. Neurosci. 2018, 9, 1637–1651. [Google Scholar] [CrossRef]
- Tamtaji, O.R.; Taghizadeh, M.; Daneshvar Kakhaki, R.; Kouchaki, E.; Bahmani, F.; Borzabadi, S.; Oryan, S.; Mafi, A.; Asemi, Z. Clinical and metabolic response to probiotic administration in people with Parkinson’s disease: A randomized, double-blind, placebo-controlled trial. Clin. Nutr. 2019, 38, 1031–1035. [Google Scholar] [CrossRef]
- Kiyohara, A.C.P.; Torres, D.J.; Hagiwara, A.; Pak, J.; Rueli, R.H.L.H.; Shuttleworth, C.W.R.; Bellinger, F.P. Selenoprotein P Regulates Synaptic Zinc and Reduces Tau Phosphorylation. Front. Nutr. 2021, 8, 683154. [Google Scholar] [CrossRef]
- Squitti, R.; Pal, A.; Picozza, M.; Avan, A.; Ventriglia, M.; Rongioletti, M.C.; Hoogenraad, T. Zinc Therapy in Early Alzheimer’s Disease: Safety and Potential Therapeutic Efficacy. Biomolecules 2020, 10, 1164. [Google Scholar] [CrossRef]
- Craven, K.M.; Kochen, W.R.; Hernandez, C.M.; Flinn, J.M. Zinc Exacerbates Tau Pathology in a Tau Mouse Model. J. Alzheimers Dis. 2018, 64, 617–630. [Google Scholar] [CrossRef]
- Wang, C.-Y.; Wang, T.; Zheng, W.; Zhao, B.-L.; Danscher, G.; Chen, Y.-H.; Wang, Z.-Y. Zinc Overload Enhances APP Cleavage and Aβ Deposition in the Alzheimer Mouse Brain. PLoS ONE 2010, 5, e15349. [Google Scholar] [CrossRef]
- Fasae, K.D.; Abolaji, A.O.; Faloye, T.R.; Odunsi, A.Y.; Oyetayo, B.O.; Enya, J.I.; Rotimi, J.A.; Akinyemi, R.O.; Whitworth, A.J.; Aschner, M. Metallobiology and therapeutic chelation of biometals (copper, zinc and iron) in Alzheimer’s disease: Limitations, and current and future perspectives. J. Trace Elem. Med. Biol. 2021, 67, 126779. [Google Scholar] [CrossRef] [PubMed]
- Akiyama, H.; Hosokawa, M.; Kametani, F.; Kondo, H.; Chiba, M.; Fukushima, M.; Tabira, T. Long-term oral intake of aluminium or zinc does not accelerate Alzheimer pathology in AβPP and AβPP/tau transgenic mice: Aluminium, zinc and Alzheimer’s disease. Neuropathology 2012, 32, 390–397. [Google Scholar] [CrossRef] [PubMed]
- Joppe, K.; Roser, A.-E.; Maass, F.; Lingor, P. The Contribution of Iron to Protein Aggregation Disorders in the Central Nervous System. Front. Neurosci. 2019, 13, 15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, D.; Liu, Y.; Zeng, X.; Xiong, Z.; Yao, Y.; Liang, D.; Qu, H.; Xiang, H.; Yang, Z.; Nie, L.; et al. Quantitative Study of the Changes in Cerebral Blood Flow and Iron Deposition During Progression of Alzheimer’s Disease. J. Alzheimers Dis. 2020, 78, 439–452. [Google Scholar] [CrossRef] [PubMed]
- Meadowcroft, M.D.; Connor, J.R.; Yang, Q.X. Cortical iron regulation and inflammatory response in Alzheimer’s disease and APPSWE/PS1ΔE9 mice: A histological perspective. Front. Neurosci. 2015, 9, 255. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Bai, X.; Zhang, Y.; Yao, S.; Cui, Y.; You, L.-H.; Yu, P.; Chang, Y.-Z.; Gao, G. Hippocampal Iron Accumulation Impairs Synapses and Memory via Suppressing Furin Expression and Downregulating BDNF Maturation. Mol. Neurobiol. 2022, 59, 5574–5590. [Google Scholar] [CrossRef]
- Singh, S.K.; Balendra, V.; Obaid, A.A.; Esposto, J.; Tikhonova, M.A.; Gautam, N.K.; Poeggeler, B. Copper-mediated β-amyloid toxicity and its chelation therapy in Alzheimer’s disease. Metallomics 2022, 14, mfac018. [Google Scholar] [CrossRef]
- Zubčić, K.; Hof, P.R.; Šimić, G.; Jazvinšćak Jembrek, M. The Role of Copper in Tau-Related Pathology in Alzheimer’s Disease. Front. Mol. Neurosci. 2020, 13, 572308. [Google Scholar] [CrossRef]
- Chen, C.; Jiang, X.; Li, Y.; Yu, H.; Li, S.; Zhang, Z.; Xu, H.; Yang, Y.; Liu, G.; Zhu, F.; et al. Low-dose oral copper treatment changes the hippocampal phosphoproteomic profile and perturbs mitochondrial function in a mouse model of Alzheimer’s disease. Free Radic. Biol. Med. 2019, 135, 144–156. [Google Scholar] [CrossRef]
- Redman, L.M.; Smith, S.R.; Burton, J.H.; Martin, C.K.; Il’yasova, D.; Ravussin, E. Metabolic Slowing and Reduced Oxidative Damage with Sustained Caloric Restriction Support the Rate of Living and Oxidative Damage Theories of Aging. Cell Metab. 2018, 27, 805–815.e4. [Google Scholar] [CrossRef] [Green Version]
- Mazzotti, D.R.; Guindalini, C.; Moraes, W.A.d.S.; Andersen, M.L.; Cendoroglo, M.S.; Ramos, L.R.; Tufik, S. Human longevity is associated with regular sleep patterns, maintenance of slow wave sleep, and favorable lipid profile. Front. Aging Neurosci. 2014, 6, 134. [Google Scholar] [CrossRef] [Green Version]
- Epel, E.S.; Lithgow, G.J. Stress Biology and Aging Mechanisms: Toward Understanding the Deep Connection Between Adaptation to Stress and Longevity. J. Gerontol. A. Biol. Sci. Med. Sci. 2014, 69, S10–S16. [Google Scholar] [CrossRef] [Green Version]
- Jenkinson, C.E.; Dickens, A.P.; Jones, K.; Thompson-Coon, J.; Taylor, R.S.; Rogers, M.; Bambra, C.L.; Lang, I.; Richards, S.H. Is volunteering a public health intervention? A systematic review and meta-analysis of the health and survival of volunteers. BMC Public Health 2013, 13, 773. [Google Scholar] [CrossRef] [Green Version]
- Arab, L.; Sabbagh, M.N. Are Certain Lifestyle Habits Associated with Lower Alzheimer’s Disease Risk? J. Alzheimers Dis. 2010, 20, 785–794. [Google Scholar] [CrossRef] [Green Version]
- Bartolotti, N.; Lazarov, O. Lifestyle and Alzheimer’s Disease. In Genes, Environment and Alzheimer’s Disease; Elsevier: Amsterdam, The Netherlands, 2016; pp. 197–237. ISBN 978-0-12-802851-3. [Google Scholar]
- Dhana, K.; Evans, D.A.; Rajan, K.B.; Bennett, D.A.; Morris, M.C. Healthy lifestyle and the risk of Alzheimer dementia: Findings from 2 longitudinal studies. Neurology 2020, 95, e374–e383. [Google Scholar] [CrossRef]
- Kivipelto, M.; Mangialasche, F.; Ngandu, T. Lifestyle interventions to prevent cognitive impairment, dementia and Alzheimer disease. Nat. Rev. Neurol. 2018, 14, 653–666. [Google Scholar] [CrossRef]
- Ngandu, T.; Lehtisalo, J.; Solomon, A.; Levälahti, E.; Ahtiluoto, S.; Antikainen, R.; Bäckman, L.; Hänninen, T.; Jula, A.; Laatikainen, T.; et al. A 2 year multidomain intervention of diet, exercise, cognitive training, and vascular risk monitoring versus control to prevent cognitive decline in at-risk elderly people (FINGER): A randomised controlled trial. Lancet 2015, 385, 2255–2263. [Google Scholar] [CrossRef]
- Ionescu-Tucker, A.; Cotman, C.W. Emerging roles of oxidative stress in brain aging and Alzheimer’s disease. Neurobiol. Aging 2021, 107, 86–95. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhao, B. Oxidative Stress and the Pathogenesis of Alzheimer’s Disease. Oxid. Med. Cell. Longev. 2013, 2013, 316523. [Google Scholar] [CrossRef] [Green Version]
- Ferruzzi, M.G.; Lobo, J.K.; Janle, E.M.; Cooper, B.; Simon, J.E.; Wu, Q.-L.; Welch, C.; Ho, L.; Weaver, C.; Pasinetti, G.M. Bioavailability of Gallic Acid and Catechins from Grape Seed Polyphenol Extract is Improved by Repeated Dosing in Rats: Implications for Treatment in Alzheimer’s Disease. J. Alzheimers Dis. 2009, 18, 113–124. [Google Scholar] [CrossRef] [Green Version]
- Mangoni, A.A.; Jackson, S.H.D. Age-related changes in pharmacokinetics and pharmacodynamics: Basic principles and practical applications: Age-related changes in pharmacokinetics and pharmacodynamics. Br. J. Clin. Pharmacol. 2003, 57, 6–14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Erdoğan, M.E.; Aydın, S.; Yanar, K.; Mengi, M.; Kansu, A.D.; Cebe, T.; Belce, A.; Çelikten, M.; Çakatay, U. The effects of lipoic acid on redox status in brain regions and systemic circulation in streptozotocin-induced sporadic Alzheimer’s disease model. Metab. Brain Dis. 2017, 32, 1017–1031. [Google Scholar] [CrossRef] [PubMed]
- Sharman, M.J.; Gyengesi, E.; Liang, H.; Chatterjee, P.; Karl, T.; Li, Q.-X.; Wenk, M.R.; Halliwell, B.; Martins, R.N.; Münch, G. Assessment of diets containing curcumin, epigallocatechin-3-gallate, docosahexaenoic acid and α-lipoic acid on amyloid load and inflammation in a male transgenic mouse model of Alzheimer’s disease: Are combinations more effective? Neurobiol. Dis. 2019, 124, 505–519. [Google Scholar] [CrossRef] [PubMed]
- Sneideris, T.; Sakalauskas, A.; Sternke-Hoffmann, R.; Peduzzo, A.; Ziaunys, M.; Buell, A.K.; Smirnovas, V. The Environment Is a Key Factor in Determining the Anti-Amyloid Efficacy of EGCG. Biomolecules 2019, 9, 855. [Google Scholar] [CrossRef]
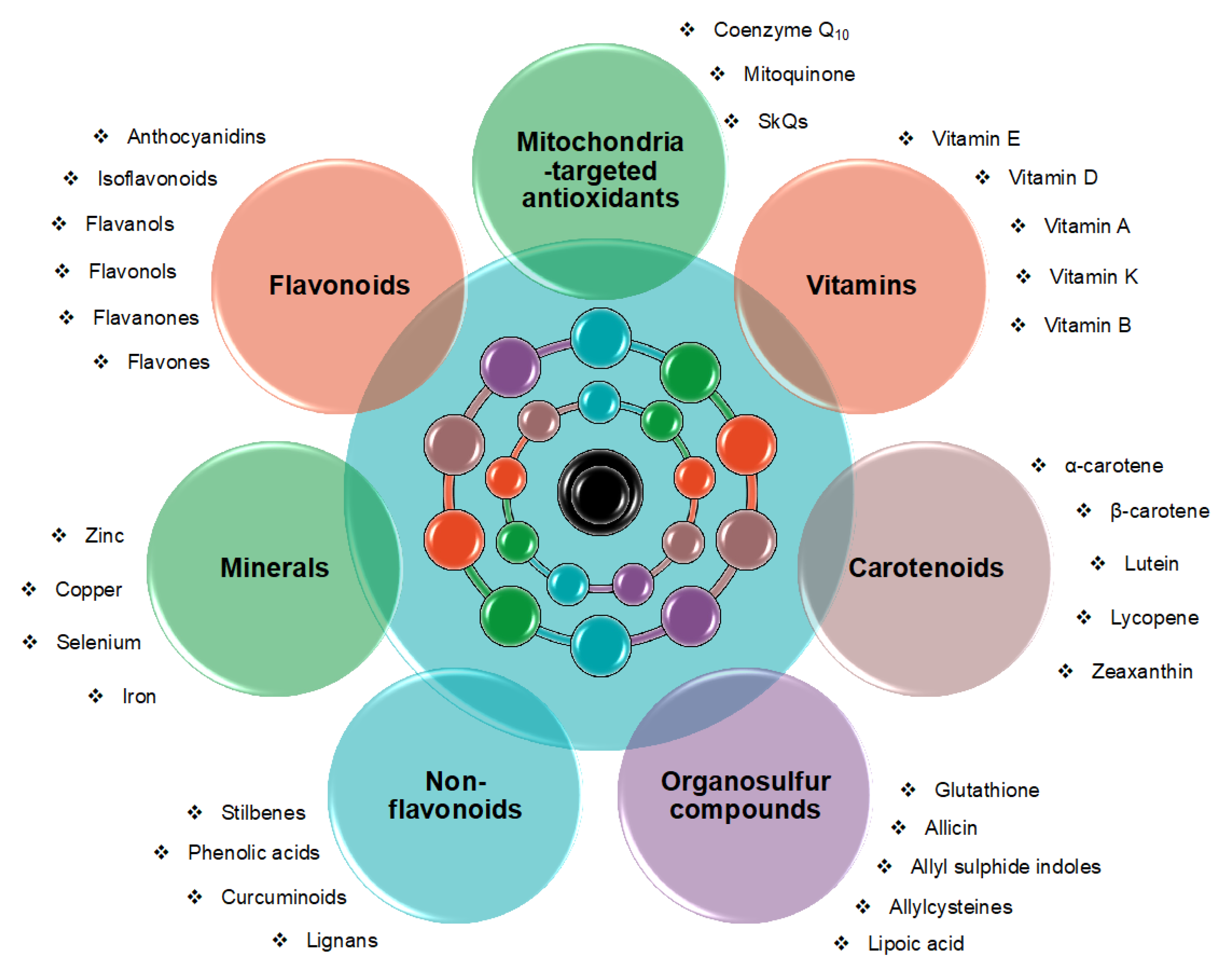
| Non-Flavonoid | Experimental Model | Treatment Duration | Treatment Effects | Reference |
|---|---|---|---|---|
| Ferulic acid | In vivo—PSAPP transgenic mouse model | 6 months | Amelioration of behavioral performance Reduction in Aβ deposits, as well as in Aβ1–42 and Aβ1–40 abundance by the inhibition of BACE1 activity Attenuation of neuroinflammation (↓GFAP and Iba1 levels, as well as ↓TNFα and IL-1β mRNA expression) and OS (↑SOD1, CAT and GPx1 mRNA expression) | [302] |
| In vitro—Aβ1–40 -damaged PC12 cells | 30 min | Prevention of cell death Reduction in intracellular ROS Inhibition of Aβ1–40 aggregation | [303] | |
| In vivo—Aβ1–40 -induced mouse model | 21 days | Improvement in cognitive abilities by increasing SOD and ChAT activity and by decreasing AChE activity Attenuation of lipid peroxidation (↓MDA levels) | ||
| Caffeic acid | In vitro—Aβ25–35 -damaged PC12 cells | 1 h | Protection against Aβ-induced toxicity by inhibiting OS, calcium influx, and tau hyperphosphorylation | [304] |
| In vivo—Aβ25–35 -induced mouse model | 2 weeks | Improvement in spatial cognitive and memory functions Inhibition of lipid peroxidation (↓MDA levels) and NO formation | [305] | |
| In vivo—Aβ1–42 -induced mouse model | 10 days | Decreasing in neuronal apoptosis (↓caspase 9) and neuroinflammation (↓GFAP and Iba1 expression) Improvement in learning and memory Attenuation of OS by inducing the Nrf2/HO-1 signaling pathway | [306] | |
| p-Coumaric acid | In vitro—Aβ25–35 -damaged PC12 cells | 1 h | Attenuation of Aβ25–35 -induced toxicity, through reduction of neuroinflammation (↓iNOS and COX-2), via downregulation of NF-kB and MAPKs pathways | [307] |
| In vivo—D-galactose mouse model | 42 days | Amelioration of cognitive performance by decreasing AChE levels Attenuation of OS (↑SOD and GSH) and neuronal apoptosis (↓Caspase3) Reduction in NF-kB and BACE1 levels | [308] | |
| In vitro—SH-SY5Y cells | 4 h | Attenuation of apoptotic cell death (↓Caspase3) Reduction in ROS accumulation, as well as in cytochrome c release into the cytosol | [309] | |
| In vivo—Aβ1–42 -induced rat model | 2 weeks | Amelioration of learning and memory deficits and neuronal apoptosis | ||
| Gallic acid | In vivo—AlCl3 -induced rat model | 60 days | Amelioration of spatial memory and learning deficits Reduction in neurofibrillary tangles and amyloid plaques Increase in CAT, GSH, and SOD activity, and a decrease in MDA and NO contents | [310] |
| In vivo—APP/PS1 transgenic mouse model | 30 days | Amelioration of spatial memory and learning impairments Inhibition of Aβ aggregation Reduction in neuroinflammation (↓GFAP) and increase in synaptic strength | [311] | |
| Ellagic acid | In vivo—APP/PS1 transgenic mouse model | 60 days | Improvement in learning and memory abilities Decrease in Aβ production by reduction of BACE1 levels Inhibition of tau phosphorylation by modulation of Akt/GSK-3β signaling pathway | [312] |
| In vivo—Aβ25–35 -induced rat model | 1 week | Amelioration of learning and memory deficits Attenuation of OS, by increasing antioxidant defense (CAT, GSH), and neuroinflammation (↓NF-kB) Reduction in AChE activity | [313] | |
| In vivo—AlCl3 -induced rat model | 4 weeks | Increase in SOD and GSH levels Attenuation of lipid peroxidation Reduction in neurofibrillary tangles and neuritic plaques | [314] | |
| Resveratrol | In vivo—SAMP8 mouse model | 7 months | Increase in lifespan Activation of AMPK signaling pathways and increase in SIRT1 levels Reduction in cognitive impairments as well as in Aβ burden and tau phosphorylation | [315] |
| In vivo—SAMP8 mouse model | 15 days | Improvement in learning abilities Increase in the activity of SOD, GSH-Px, and CAT through the Nrf2/HO-1 signaling pathway Decrease in MDA content | [316] | |
| In vivo—30 AD patients | 52 weeks | Not statistically significant changes in Aβ1–40 levels in blood and CSF Reduction in brain volume at 52 weeks Decrease by 46% in MMP-9 levels in CSF | [317] | |
| Curcumin | In vivo—Transgenic mouse model APPSw | 6 months | Suppression of inflammation (↓GFAP, and IL-1β) and oxidative damage Decrease in insoluble and soluble amyloid as well as in plaque burden | [318] |
| In vivo—Aβ-induced rat model | 4 days | Improvement in learning and memory performance Reduction in OS parameters (ROS formation, lipid peroxidation, and ADP/ATP ratio) as well as in amyloid plaques | [319] | |
| In vivo—48 AD patients | 24 weeks | Not effective on CSF and plasma AD markers, including Aβ1–42, tau, and p-tau | [320] | |
| Lignans | In vivo—Scopolamine-induced rat model | 2 weeks | Improvement in rat behaviors Alleviation of OS (↑CAT and SOD) and lipid peroxidation (↓MDA) Decrease in AChE levels | [321] |
| In vivo—Aβ1–42 -induced mouse model | 5 days | Improvement in learning and memory abilities by reduction of ChE total levels and increase of SOD, GSH-Px activity as well as GSH content Attenuation of memory impairment | [322] | |
| In vivo—Aβ1–42 -induced mouse model | 4 days | Reduction in Aβ1–42 levels by inhibition of β-secretase activity Inhibition of AChE activity and reduction of GSH levels | [323] |
| Compound | Experimental Model | Treatment | Results | Ref. |
|---|---|---|---|---|
| MitoQ | 3xTg-AD female mice | 1: 4 mix of 100 μM MitoQ + β-cyclodextrin for 5 months in drinking water | ↑ memory, lifespan ↓ brain OS, astrogliosis, Aβ accumulation, tau hyperphosphorylation, microglial proliferation, caspase activation | [495,496] |
| Caenorhabditis elegans overexpressing human Aβ | 1 µM MitoQ in NGM agar and Escherichia coli OP50-1 | ↑ lifespan, healthspan, electron transport chain function | [497] | |
| SkQ1 | OXYS male rats (12-month-old) | 250 nM SkQ1/kg daily for 6 months | ↑ resting/activated microglia ratio, learning, memory, synaptic function, neurotrophic supply, locomotor, and exploratory functions ↓ inflammation, neurodegeneration, neuronal loss, synaptic damage, p38 MAPK signaling, AD progression, tau hyperphosphorylation, Aβ1–42 | [498,499,500,501] |
| Male Wistar rats | One i.p. injection of 250 nM SkQ1/kg | ↑ neuroprotection ↓ Aβ-induced OS | [502] | |
| CoQ10 | AlCl3 treated rats | Biotin (2 mg/kg), CoQ10 (10 mg/kg) for 60 days | ↑ insulin signaling ↓ inflammation | [503] |
| Hypercholesterolemic rats | 10 mg/kg for 30 days (oral) | ↑ memory, cholinergic function ↓ brain OS and inflammation, amyloidosis | [504] | |
| C65/Bl6 mice | 10 g/kg for one month | ↓ brain OS measured by protein carbonyls | [505] | |
| Tg19959 mice | 3 months of 0.4% CoQ10 in chow or 5 months of 2.4% CoQ10 in chow | ↑ cognitive function (Morris water maze test) ↓ amyloid pathology, brain OS measured by protein carbonyls | [506] | |
| Male Wistar rats | 50 mg/kg of CoQ10 daily for 6 weeks (3 before and 3 after AD induction) | ↑ EPSP slope and population spike amplitude ↓ serum malondialdehyde, OS | [509] | |
| Female mice overexpressing presenilin 1-L235P | 1200 mg/kg of CoQ10 daily for 60 days | ↑ SOD activity ↓ MDA levels, cortex Aβ burden | [511] | |
| Male Sprague–Dawley rats | 20 and 40 mg/kg for 21 days | ↑ SOD, CAT, GSH, mitochondrial respiration ↓ transfer latency, AChE activity, TNFα, LPO, nitrite | [512] | |
| Wistar rats | CoQ10-loaded ADSCs-exosomes | ↑ Cognition, memory, hippocampal BDNF and SOX2 | [513] | |
| APP/PS1 transgenic mice | 1200 mg/kg CoQ10 daily for 60 days | ↓ Aβ plaque burden | [514] | |
| Ubisol-Q10 | TgAPEswe, PSEN1dE9 mouse | 6 mg/kg Ubisol-Q10 daily for 18 months | ↑ long-term and working spatial memory ↓ circulating Aβ, Aβ plaque formation | [507] |
| Male APP/PS-1 mice | 200 μg/mL of Ubisol-Q10 in drinking water for 18 months | ↑ cortical beclin-1 and JNK1, autophagy | [510] | |
| Idebenone | 5xFAD mice | i.p. injection of 100 mg/kg/day for 14 days | ↑ NEP, α-secretase ADAM17, tau hyperphosphorylation, total tau ↓ Aβ plaque number, RAGE/caspase 3 signaling | [508] |
| Study Design | Treatment | Results | Conclusion | Reference |
|---|---|---|---|---|
| CSF of AD patients | 0.32 or 10 mg sodium selenate oral supplementation 3 times daily | ↑ Se CSF levels | Sodium selenate as a possible therapeutic tool against AD | [539] |
| STZ-induced male rats | 0.4 mg/kg Se nanoparticles oral administration daily for one month | ↓ ROS ↓ Aβ deposition | Selenium nanoparticles to contrast AD pathogenesis | [540] |
| ICV-STZ rats | 0.1 mg/kg intraperitoneal sodium selenite for 7 days | ↓ reduction of GPx | Sodium selenite as a possible supportive approach to treat SDAT | [541] |
| Hippocampal and dorsal root ganglion neuronal cultures from 1 or 1.5 mg/kg/day scopolamine-treated aged rats | 1.5 mg/kg intraperitoneal Se supplementation for 14 days | ↓ membrane permeability to Ca2+ ↑ membrane phospholipids ↓ reduction of GPx | Se as a neuroprotective factor | [542] |
| Brain tissue from rats treated with DHA + EPA + uridine (fish oil) | 1600 mg/kg vitamin C, 1600 mg/kg E and 1.2 mg/kg Se diet for 6 weeks | ↑ membrane phospholipids | Co-supplementation of Se, vitamins, and fish oil promotes synaptogenesis | [543] |
| Triple transgenic AD mice | 6 μg/mL selenomethionine supplementation through drinking water for 12 weeks | ↓ extrasynaptic NMDARs activity ↑ synaptic NMDARs activity ↓ membrane permeability to Ca2+ | Selenomethionine improves synaptic plasticity and cognitive functioning | [544] |
| Triple transgenic AD mice | 6 μg/mL selenomethionine supplementation through drinking water for 12 weeks | ↓ total tau and phosphorylated tau ↓ synaptic protein loss | Selenomethionine to restore synapses | [545] |
| Triple transgenic AD mice | 6 μg/mL selenomethionine supplementation through drinking water for 12 weeks | ↓ total tau and hyperphosphorylated tau ↓ autophagic dysfunction | Selenomethionine to restore synapses | [546] |
| Triple transgenic AD mice | 6 μg/mL selenomethionine supplementation through drinking water for 12 weeks | ↓ tau pathologies | Se supplementation, as a potential tool to improve cognitive deficits related to AD | [547] |
| Triple transgenic AD mice | 12 μg/mL sodium selenate chronic dietary supplementation | ↓ tau aggregation | Sodium selenate, as a promising supportive therapy against AD | [548] |
| iTRAQ proteomics technology in hippocampus of triple transgenic AD mice | 9–12 μg sodium selenate per day in drinking water for 4 months | ↓ expression of cortical proteins involved in AD pathogenesis | Sodium selenate, as a potential supportive therapeutic agent for AD | [549] |
| Wistar rats intoxicated with aluminum chloride to mimic AD neurodegeneration | 100 mg/kg/day oral resveratrol-Se nanoparticles for 60 days | ↑ antioxidant effect compared to Se alone administration | Se as a promising supplementation against AD when combined with resveratrol | [550] |
| Lymphoblastoid cell lines from AD patients | Resveratrol and Se exposure | ↑ antioxidant effect | Se as a protective agent against AD when combined with resveratrol | [551] |
| APP/Tau/PSEN and APP/PS1 transgenic mouse models | 3 or 1.5 μg/g Se and 36 or 18 μg/g folic acid oral co-supplementation | ↓ Aβ generation ↓ tau hyperphosphorylation | Se as a potential therapy against AD when combined with folic acid | [552] |
| AD patients | Combined probiotics (Lactobacillus acidophilus, Bifidobacterium bifidum, and Bifidobacterium longum, 2 × 109 CFU/day each) and 200 mg/day selenium oral supplementation for 12 weeks | ↑ antioxidant effect | Se as a potential supportive therapy against AD when combined with probiotics | [553] |
| Primary cortical and human embryonic kidney cells exposed to Aβ1–42 | Zn2+ incubation | ↑ antioxidant effect toward H2O2 formation | Zn as a neuroprotective factor against AD | [554] |
| Chinese hamster ovary cells overexpressing amyloid precursor protein | Exposure to Cu or Zn bis (thiosemicarbazonato) therapy | ↓ monomeric Aβ peptide | Zn bis (thiosemicarbazonato) as a potential AD supplementation | [555] |
| Acetylcholinesterase enzymes from Electric eel | Zinc carboxylate derivatives exposure | ↓ acetylcholinesterase enzyme activity | Zinc carboxylate derivatives to treat AD | [556] |
| APP23 mice | Zn nanoparticle injection for 14 days | ↓ plaques deposition | ↑ brain Zn levels to counteract AD | [557] |
| Male Sprague Dawley rats receiving aluminum chloride | 227 mg/L Zinc sulfate in drinking water for 8 weeks | ↓ tau levels ↓ APP levels ↓ α-synuclein levels ↓ alterations in histological architecture | Zn to reverse the effects of aluminum-induced neurodegeneration, which is correlated with AD | [558] |
| Tg2576 mice treated with Cu | 2 g/L Zinc acetate in drinking water for 6 months | ↓ ROS formation ↓ amyloid burden ↓ Cu absorption | Zn to reverse the Cu toxic effects, which are correlated with AD | [559] |
| AD patients | 150 mg Zn supplementation for 6 months | ↑ Zn levels ↓ Cu levels | Zn therapy to lower Cu absorption and to restore Zn levels, with the aim to protect from cognitive impairment | [560] |
| Male Wistar rats treated with STZ | Co-administration of 10 mg/kg Zn and 0.1 mg/kg Se intraperitoneally for 1 week | ↓ oxidative stress ↓ mitochondrial membranes collapse ↑ GPx ↑ superoxide dismutase | Zn and Se co-administration to improve cognitive functions and prevent the development of AD | [561] |
| Male Kunming mice | Zn, Se, and fish-oil (EPA + DHA) co-administration for 7 weeks | ↓ APP cleavage | Zn, Se and fish-oil co-administration to improve cognitive functions in AD models | [562] |
| APP2576 transgenic mice | Oral clioquinol (Cu/Zn chelator) administration for 9 weeks | ↓ Aβ plaques | Cu/Zn chelators as a supportive therapeutic strategy | [563] |
| APP/PS1 mouse brain sections | 300 μg/mL recombinant human S100A6 protein (Zn chelator) incubation for 12 h or culture with human S100A6-expressing cells | ↓ Aβ plaques | Zn sequestration as a supportive therapeutic strategy | [564] |
| APP/PS1 transgenic mice watered with high quantities of iron | 200 mg/kg intranasal deferoxamine (Fe chelator) once every other day for 3 months | ↓ Aβ plaques | Deferoxamine as a supplemental treatment for AD | [565] |
| Traumatic brain injury murine model | Deferoxamine (Fe chelator) intraperitoneal treatment | ↓ Aβ plaques ↓ brain ferritin | Deferoxamine as a potential preventive treatment to avoid neurodegeneration in AD patients | [566] |
| 1.14 mg/kg/day scopolamine-treated rats for 7 days | 5, 10, 20 mg/kg oral deferiprone (Fe chelator) for 14 days | ↓ Aβ plaques | Deferiprone as a potential preventive treatment in AD patients | [567] |
| rTg(tauP301L)4510 tauopathy murine model | 100 mg/kg oral deferiprone (Fe chelator) for 16 weeks | ↓ cognitive deficit | Deferiprone as a potential supportive therapy for tauopathies | [568] |
| Blood samples and brain tissues from NMRI male mice treated with 100 mg/kg/day iron dextran for 4 times a week for 6 weeks | Hesperidin/coumarin/desferal (all Fe chelators) treatment for 4 times a week for 4 weeks | ↓ Fe levels ↑ antioxidant enzymatic activity | Hesperidin and coumarin to enhance antioxidant enzymatic activity | [569] |
| Brain sections from NMRI male mice following treatment with 100 mg/kg/day iron dextran injections for 4 times a week for 4 weeks | 30/60 mg/kg/day naringin (Fe chelator) administration for a month | ↓ Fe levels ↓ Aβ plaques | Naringin as a preventive supportive treatment for AD | [570] |
| Brain homogenates from rats | Curcumin, capsaicin, and S-allylcysteine (Fe chelators) exposure | ↓ Fe levels ↑ antioxidant effect | Curcumin, capsaicin, and S-allylcysteine as possible tools for the prevention and treatment of AD | [571] |
| APP/PS1 double transgenic AD mice | M30 (Fe chelator) oral administration 4 times a week for 9 months | ↓ Fe levels ↓ APP levels ↓ APP and tau phosphorylation ↓ Aβ plaques | M30 as a potential supportive therapy for AD | [572] |
| AD murine model under a high-fat diet | 0.5 mg/kg M30 oral administration once every 2 days for 1 month | ↓ Fe levels ↓ Aβ burden ↓ neuroinflammation ↓ synaptic impairment | High-fat diet as a risk factor for AD and M30 as a potential therapeutic compound | [573] |
| Human cells and Caenorhabditis elegans nematode | 20 multifunctional synthetic compounds based on the nicotinoyl hydrazone scaffold, in particular, SNH6 (Fe chelator and NAD+ donor) | ↓ Fe levels ↓ Aβ burden ↓ oxidative stress ↑ sirtuin | nicotinoyl hydrazone-based compounds, especially SNH6 as a promising supportive therapy for AD | [574] |
| Human brain micro-vascular endothelial cells | PBT434 (Fe chelator) exposure | ↓ Fe reuptake by blood-brain barrier endothelial cells | PBT434, used to prevent Fe-induced cytotoxic effects | [575] |
| Human plasma | Chelator–nanoparticles systems | ↑ blood-brain barrier permeability to Fe chelators | Nanoparticles as a tool to improve chelation treatment for AD | [576] |
| AD murine model | Fe-enriched water administration for 8 months | ↓ Aβ42 burden | Fe as a supplemental treatment for AD | [577] |
| Murine AD model by an ICV injection of Aβ1–42 peptide | Oral administration of 25 mg/kg of bis-8-aminoquinoline PA1637 (Cu chelator) three times per week (8 doses in total) | ↑ functioning of the episodic memory | PA1637 as a possible supportive treatment for AD | [578] |
| AD mouse models | Oral administration of 10 mg kg−1 TDMQ20 (Cu chelator) in 100 μL of solvent every 2 days for 3 months | ↑ cognitive and behavioral performance | TDMQ20 as a possible supplemental treatment for AD | [579] |
| Mouse brain cells | Flavonoid fisetin (Cu and Fe chelator) exposure | ↓ cell death | Fisetin as a neuroprotective compound against AD | [580] |
| PS19 transgenic murine model | Oral zinc acetate (Cu chelator) treatment | ↓ spatial memory deficit in female mice, but not in male ones No significant differences in tau pathology | Cu chelation may improve cognitive symptoms | [581] |
| HT22 cells | MTDLs (Cu chelator) with a rivastigmine skeleton (inhibitor of AChE) | ↓ AChE and BuChE activity ↓ Cu quantities | MTDLs as promising protective compounds for neurons | [582] |
| In vitro assays | Multifunctional tacrine-7-hydroxycoumarin hybrids chelating Cu and inhibiting AChE and BuChE | ↓ AChE and BuChE activity ↓ Cu quantities | Multifunctional agents as promising compounds to treat AD | [583] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Varesi, A.; Campagnoli, L.I.M.; Carrara, A.; Pola, I.; Floris, E.; Ricevuti, G.; Chirumbolo, S.; Pascale, A. Non-Enzymatic Antioxidants against Alzheimer’s Disease: Prevention, Diagnosis and Therapy. Antioxidants 2023, 12, 180. https://doi.org/10.3390/antiox12010180
Varesi A, Campagnoli LIM, Carrara A, Pola I, Floris E, Ricevuti G, Chirumbolo S, Pascale A. Non-Enzymatic Antioxidants against Alzheimer’s Disease: Prevention, Diagnosis and Therapy. Antioxidants. 2023; 12(1):180. https://doi.org/10.3390/antiox12010180
Chicago/Turabian StyleVaresi, Angelica, Lucrezia Irene Maria Campagnoli, Adelaide Carrara, Ilaria Pola, Elena Floris, Giovanni Ricevuti, Salvatore Chirumbolo, and Alessia Pascale. 2023. "Non-Enzymatic Antioxidants against Alzheimer’s Disease: Prevention, Diagnosis and Therapy" Antioxidants 12, no. 1: 180. https://doi.org/10.3390/antiox12010180





