Alpha-Lipoic Acid as an Antioxidant Strategy for Managing Neuropathic Pain
Abstract
1. Introduction
2. Neuropathic Pain
3. Oxidative Stress and Neuropathic Pain
3.1. Mitochondrial Dysfunction and Pain Processing
3.2. NADPH Oxidases and Pain Processing
4. Antioxidant Potential of Alpha-Lipoic Acid in Neuropathic Pain
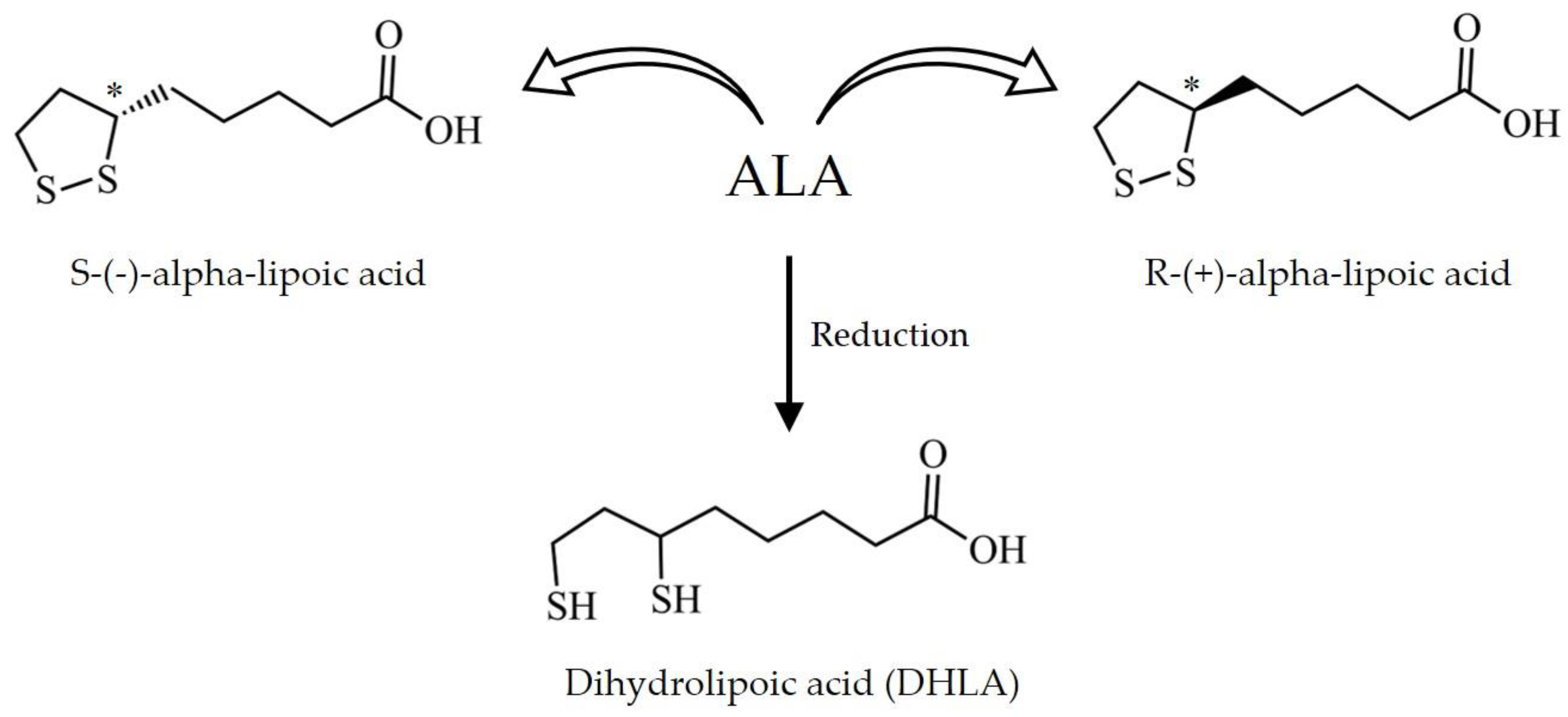
4.1. Chemical Properties and Pharmacokinetics
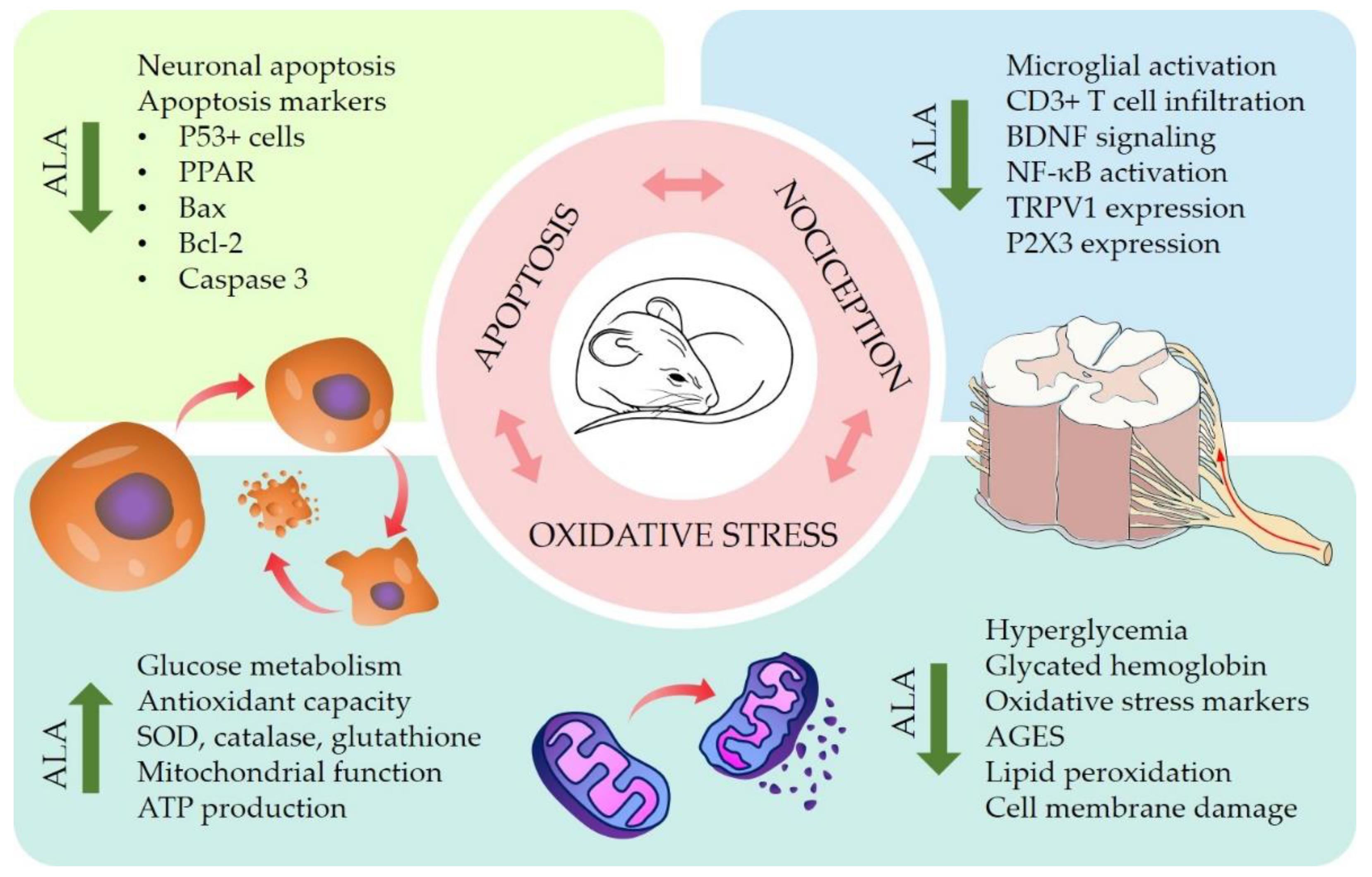
4.2. Pre-Clinical In Vitro and In Vivo Studies
4.3. Clinical Trials
5. Innovation and Alpha-Lipoic Acid-Based Products
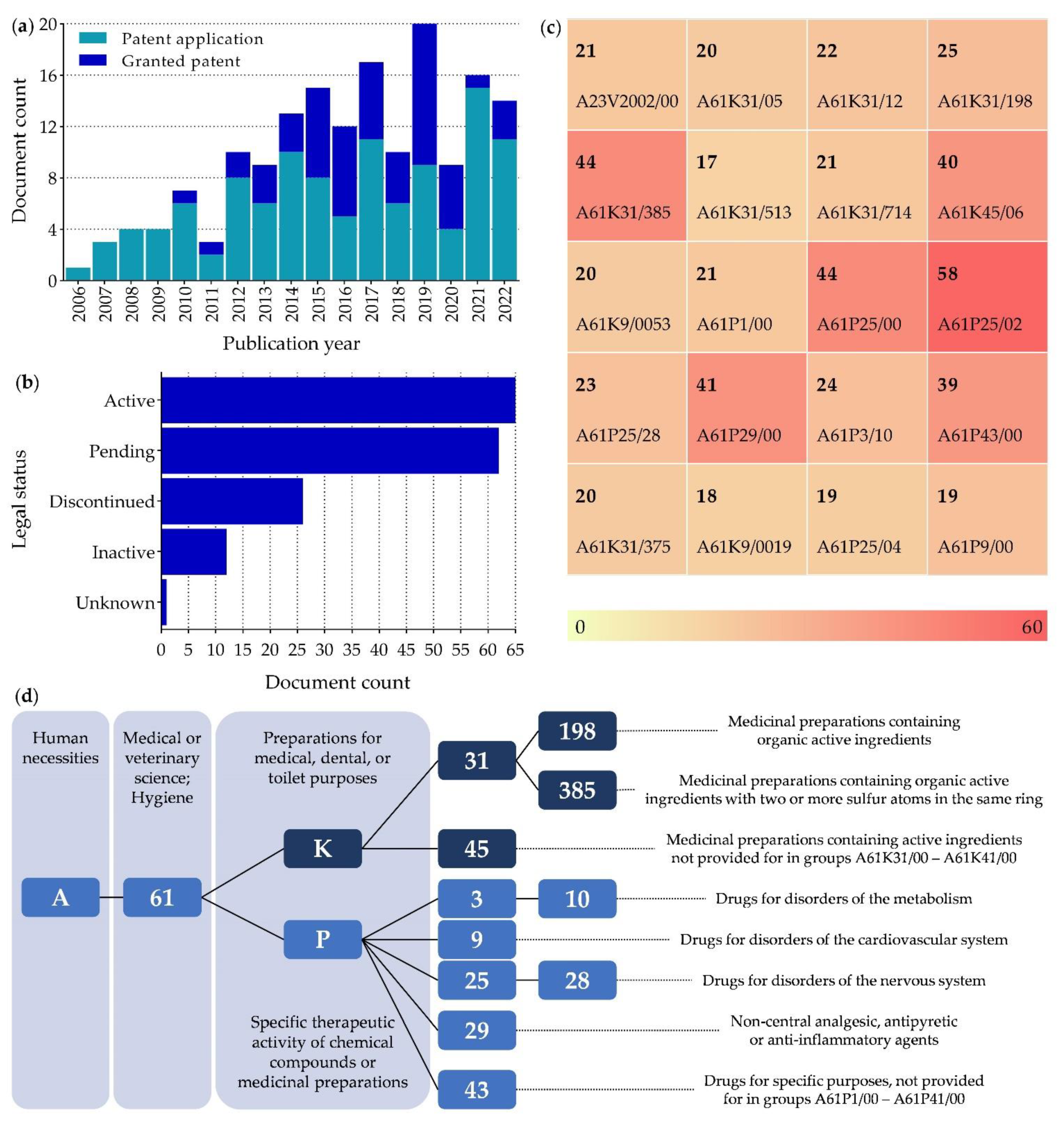
5.1. Patents
5.2. Market
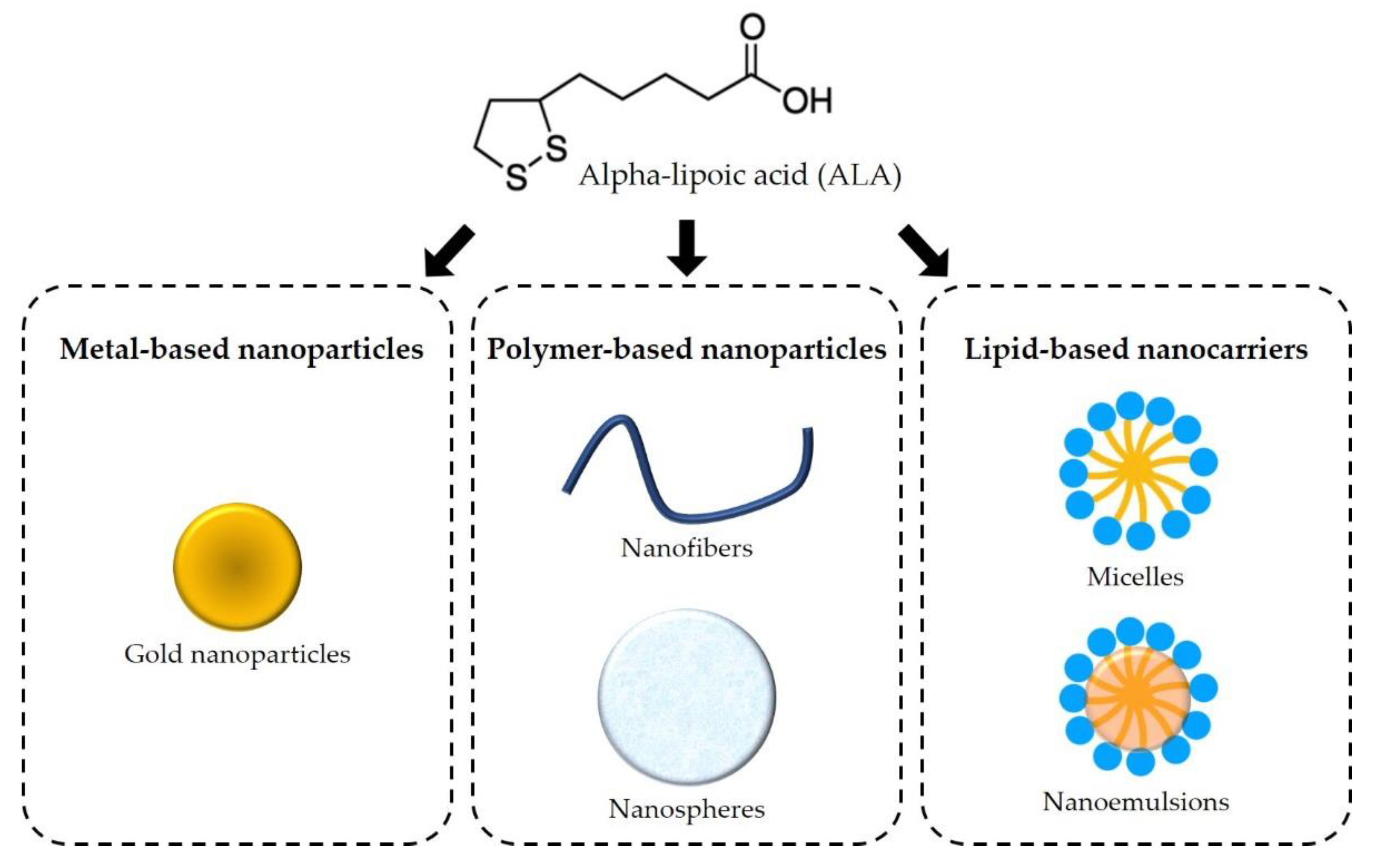
6. Novel Therapeutic Strategies
6.1. Metal-Based Nanoparticles
6.2. Polymer-Based Nanoparticles
6.3. Lipid-Based Nanocarriers
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ADP | Adenosine diphosphate |
| AGES | Advanced glycation end products |
| ALA | Alpha-lipoic acid |
| ASIC | Acid-sensing ion channels |
| ATP | Adenosine triphosphate |
| BDNF | Brain-derived neurotrophic factor |
| CPC | Cooperative patent classification |
| DHLA | Dihydrolipoic acid |
| DRG | Dorsal root ganglia |
| DSPN | Diabetic sensorimotor polyneuropathy |
| GNP | Gold nanoparticles |
| IL-1β | Interleukin 1 beta |
| IPC | International patent classification |
| MPTP | 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine |
| MTT | 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide |
| NADPH | Nicotinamide adenine dinucleotide phosphate |
| NF-κB | Nuclear factor kappa-light-chain-enhancer of activated B cells |
| Nox | Nicotinamide adenine dinucleotide phosphate oxidases |
| NP | Neuropathic pain |
| PPAR | Poly (ADP-ribose) polymerase |
| RNS | Reactive nitrogen species |
| ROS | Reactive oxygen species |
| SOD | Superoxide dismutase |
| STZ | Streptozotocin |
| TNF-α | Tumor necrosis factor alpha |
| TrkB | Tropomyosin receptor kinase B |
| TRPA1 | Transient receptor potential ankyrin subtype 1 |
| TRPV1 | Transient receptor potential vanilloid subtype 1 |
References
- Raja, S.N.; Carr, D.B.; Cohen, M.; Finnerup, N.B.; Flor, H.; Gibson, S.; Keefe, F.J.; Mogil, J.S.; Ringkamp, M.; Sluka, K.A.; et al. The revised International Association for the Study of Pain definition of pain: Concepts, challenges, and compromises. Pain 2020, 161, 1976–1982. [Google Scholar] [CrossRef] [PubMed]
- Finnerup, N.B.; Haroutounian, S.; Kamerman, P.; Baron, R.; Bennett, D.L.H.; Bouhassira, D.; Cruccu, G.; Freeman, R.; Hansson, P.; Nurmikko, T.; et al. Neuropathic pain: An updated grading system for research and clinical practice. Pain 2016, 157, 1599–1606. [Google Scholar] [CrossRef] [PubMed]
- Dworkin, R.H.; O’Connor, A.B.; Audette, J.; Baron, R.; Gourlay, G.K.; Haanpaa, M.L.; Kent, J.L.; Krane, E.J.; Lebel, A.A.; Levy, R.M.; et al. Recommendations for the pharmacological management of neuropathic pain: An overview and literature update. Mayo Clin. Proc. 2010, 85, S3–S14. [Google Scholar] [CrossRef] [PubMed]
- Carrasco, C.; Naziroglu, M.; Rodriguez, A.B.; Pariente, J.A. Neuropathic pain: Delving into the oxidative origin and the possible implication of transient receptor potential channels. Front. Physiol. 2018, 9, 95. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Wu, S.; Wang, J.; Wang, J.; Yan, Y.; Zhu, M.; Zhang, D.; Jiang, C.; Liu, T. Oxidative stress induced by NOX2 contributes to neuropathic pain via plasma membrane translocation of PKCepsilon in rat dorsal root ganglion neurons. J. Neuroinflamm. 2021, 18, 106. [Google Scholar] [CrossRef] [PubMed]
- Pizzino, G.; Irrera, N.; Cucinotta, M.; Pallio, G.; Mannino, F.; Arcoraci, V.; Squadrito, F.; Altavilla, D.; Bitto, A. Oxidative stress: Harms and benefits for human health. Oxidative Med. Cell. Longev. 2017, 2017, 8416763. [Google Scholar] [CrossRef]
- Cassanego, G.; Rodrigues, P.; De Freitas Bauermann, L.; Trevisan, G. Evaluation of the analgesic effect of a-lipoic acid in treating pain disorders: A systematic review and meta-analysis of randomized controlled trials. Pharmacol. Res. 2022, 177, 106075. [Google Scholar] [CrossRef]
- Salehi, B.; Berkay Yilmaz, Y.; Antika, G.; Boyunegmez Tumer, T.; Fawzi Mahomoodally, M.; Lobine, D.; Akram, M.; Riaz, M.; Capanoglu, E.; Sharopov, F.; et al. Insights on the use of alpha-lipoic acid for therapeutic purposes. Biomolecules 2019, 9, 356. [Google Scholar] [CrossRef]
- Rochette, L.; Ghibu, S.; Richard, C.; Zeller, M.; Cottin, Y.; Vergely, C. Direct and indirect antioxidant properties of alpha-lipoic acid and therapeutic potential. Mol. Nutr. Food Res. 2013, 57, 114–125. [Google Scholar] [CrossRef]
- Bouhassira, D. Neuropathic pain: Definition, assessment and epidemiology. Rev. Neurol. 2019, 175, 16–25. [Google Scholar] [CrossRef]
- Abbott, C.A.; Malik, R.A.; van Ross, E.R.; Kulkarni, J.; Boulton, A.J. Prevalence and characteristics of painful diabetic neuropathy in a large community-based diabetic population in the UK. Diabetes Care 2011, 34, 2220–2224. [Google Scholar] [CrossRef]
- Baron, R.; Maier, C.; Attal, N.; Binder, A.; Bouhassira, D.; Cruccu, G.; Finnerup, N.B.; Haanpaa, M.; Hansson, P.; Hullemann, P.; et al. Peripheral neuropathic pain: A mechanism-related organizing principle based on sensory profiles. Pain 2017, 158, 261–272. [Google Scholar] [CrossRef] [PubMed]
- Finnerup, N.B.; Attal, N.; Haroutounian, S.; McNicol, E.; Baron, R.; Dworkin, R.H.; Gilron, I.; Haanpää, M.; Hansson, P.; Jensen, T.S.; et al. Pharmacotherapy for neuropathic pain in adults: A systematic review and meta-analysis. Lancet. Neurol. 2015, 14, 162–173. [Google Scholar] [CrossRef]
- Yalcin, I.; Choucair-Jaafar, N.; Benbouzid, M.; Tessier, L.H.; Muller, A.; Hein, L.; Freund-Mercier, M.J.; Barrot, M. beta(2)-adrenoceptors are critical for antidepressant treatment of neuropathic pain. Ann. Neurol. 2009, 65, 218–225. [Google Scholar] [CrossRef] [PubMed]
- Baron, R. Mechanisms of disease: Neuropathic pain, a clinical perspective. Nat. Clin. Pr. Neurol. 2006, 2, 95–106. [Google Scholar] [CrossRef] [PubMed]
- Attal, N.; Lanteri-Minet, M.; Laurent, B.; Fermanian, J.; Bouhassira, D. The specific disease burden of neuropathic pain: Results of a French nationwide survey. Pain 2011, 152, 2836–2843. [Google Scholar] [CrossRef] [PubMed]
- Doth, A.H.; Hansson, P.T.; Jensen, M.P.; Taylor, R.S. The burden of neuropathic pain: A systematic review and meta-analysis of health utilities. Pain 2010, 149, 338–344. [Google Scholar] [CrossRef]
- Langley, P.C.; Van Litsenburg, C.; Cappelleri, J.C.; Carroll, D. The burden associated with neuropathic pain in Western Europe. J. Med. Econ. 2013, 16, 85–95. [Google Scholar] [CrossRef]
- Park, E.S.; Gao, X.; Chung, J.M.; Chung, K. Levels of mitochondrial reactive oxygen species increase in rat neuropathic spinal dorsal horn neurons. Neurosci. Lett. 2006, 391, 108–111. [Google Scholar] [CrossRef] [PubMed]
- Zhao, R.Z.; Jiang, S.; Zhang, L.; Yu, Z.B. Mitochondrial electron transport chain, ROS generation and uncoupling (Review). Int. J. Mol. Med. 2019, 44, 3–15. [Google Scholar] [CrossRef]
- He, L.; He, T.; Farrar, S.; Ji, L.; Liu, T.; Ma, X. Antioxidants maintain cellular redox homeostasis by elimination of reactive oxygen species. Cell. Physiol. Biochem. Int. J. Exp. Cell. Physiol. Biochem. Pharmacol. 2017, 44, 532–553. [Google Scholar] [CrossRef] [PubMed]
- Ye, D.; Fairchild, T.J.; Vo, L.; Drummond, P.D. Painful diabetic peripheral neuropathy: Role of oxidative stress and central sensitisation. Diabet. Med. A J. Br. Diabet. Assoc. 2022, 39, e14729. [Google Scholar] [CrossRef] [PubMed]
- Negi, G.; Kumar, A.; Joshi, R.P.; Sharma, S.S. Oxidative stress and Nrf2 in the pathophysiology of diabetic neuropathy: Old perspective with a new angle. Biochem. Biophys. Res. Commun. 2011, 408, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Gupta, P.; Makkar, T.K.; Goel, L.; Pahuja, M. Role of inflammation and oxidative stress in chemotherapy-induced neurotoxicity. Immunol. Res. 2022, 70, 725–741. [Google Scholar] [CrossRef]
- Ilari, S.; Giancotti, L.A.; Lauro, F.; Gliozzi, M.; Malafoglia, V.; Palma, E.; Tafani, M.; Russo, M.A.; Tomino, C.; Fini, M.; et al. Natural antioxidant control of neuropathic pain-exploring the role of mitochondrial SIRT3 pathway. Antioxidants 2020, 9, 1103. [Google Scholar] [CrossRef]
- Saifi, G.M.; Szigeti, K.; Snipes, G.J.; Garcia, C.A.; Lupski, J.R. Molecular mechanisms, diagnosis, and rational approaches to management of and therapy for Charcot-Marie-Tooth disease and related peripheral neuropathies. J. Investig. Med. Off. Publ. Am. Fed. Clin. Res. 2003, 51, 261–283. [Google Scholar] [CrossRef]
- Siotto, M.; Aprile, I.; Simonelli, I.; Pazzaglia, C.; Ventriglia, M.; Santoro, M.; Imbimbo, I.; Squitti, R.; Padua, L. An exploratory study of BDNF and oxidative stress marker alterations in subacute and chronic stroke patients affected by neuropathic pain. J. Neural Transm. 2017, 124, 1557–1566. [Google Scholar] [CrossRef]
- Valko, M.; Leibfritz, D.; Moncol, J.; Cronin, M.T.; Mazur, M.; Telser, J. Free radicals and antioxidants in normal physiological functions and human disease. Int. J. Biochem. Cell Biol. 2007, 39, 44–84. [Google Scholar] [CrossRef]
- Salvemini, D.; Little, J.W.; Doyle, T.; Neumann, W.L. Roles of reactive oxygen and nitrogen species in pain. Free Radic. Biol. Med. 2011, 51, 951–966. [Google Scholar] [CrossRef]
- Janes, K.; Doyle, T.; Bryant, L.; Esposito, E.; Cuzzocrea, S.; Ryerse, J.; Bennett, G.J.; Salvemini, D. Bioenergetic deficits in peripheral nerve sensory axons during chemotherapy-induced neuropathic pain resulting from peroxynitrite-mediated post-translational nitration of mitochondrial superoxide dismutase. Pain 2013, 154, 2432–2440. [Google Scholar] [CrossRef]
- Munoz, F.M.; Gao, R.; Tian, Y.; Henstenburg, B.A.; Barrett, J.E.; Hu, H. Neuronal P2X7 receptor-induced reactive oxygen species production contributes to nociceptive behavior in mice. Sci. Rep. 2017, 7, 3539. [Google Scholar] [CrossRef] [PubMed]
- Kallenborn-Gerhardt, W.; Schröder, K.; Schmidtko, A. NADPH oxidases in pain processing. Antioxidants 2022, 11, 1162. [Google Scholar] [CrossRef] [PubMed]
- Doyle, T.M.; Salvemini, D. Mini-Review: Mitochondrial dysfunction and chemotherapy-induced neuropathic pain. Neurosci. Lett. 2021, 760, 136087. [Google Scholar] [CrossRef] [PubMed]
- Román-Pintos, L.M.; Villegas-Rivera, G.; Rodríguez-Carrizalez, A.D.; Miranda-Díaz, A.G.; Cardona-Muñoz, E.G. Diabetic polyneuropathy in type 2 diabetes mellitus: Inflammation, oxidative stress, and mitochondrial function. J. Diabetes Res. 2016, 2016, 3425617. [Google Scholar] [CrossRef]
- Oliveira, A.L.L.; Santos, G.G.L.; Espirito-Santo, R.F.; Silva, G.S.A.; Evangelista, A.F.; Silva, D.N.; Soares, M.B.P.; Villarreal, C.F. Reestablishment of redox homeostasis in the nociceptive primary afferent as a mechanism of antinociception promoted by mesenchymal stem/stromal cells in oxaliplatin-induced chronic peripheral neuropathy. Stem Cells Int. 2021, 2021, 8815206. [Google Scholar] [CrossRef]
- Leo, M.; Schmitt, L.I.; Küsterarent, P.; Kutritz, A.; Rassaf, T.; Kleinschnitz, C.; Hendgen-Cotta, U.B.; Hagenacker, T. Platinum-based drugs cause mitochondrial dysfunction in cultured dorsal root ganglion neurons. Int. J. Mol. Sci. 2020, 21, 8636. [Google Scholar] [CrossRef]
- Flatters, S.J.L.; Bennett, G.J. Studies of peripheral sensory nerves in paclitaxel-induced painful peripheral neuropathy: Evidence for mitochondrial dysfunction. Pain 2006, 122, 245–257. [Google Scholar] [CrossRef]
- Garcia, G.C.; Bartol, T.M.; Phan, S.; Bushong, E.A.; Perkins, G.; Sejnowski, T.J.; Ellisman, M.H.; Skupin, A. Mitochondrial morphology provides a mechanism for energy buffering at synapses. Sci. Rep. 2019, 9, 18306. [Google Scholar] [CrossRef]
- Xiao, W.H.; Bennett, G.J. Effects of mitochondrial poisons on the neuropathic pain produced by the chemotherapeutic agents, paclitaxel and oxaliplatin. Pain 2012, 153, 704–709. [Google Scholar] [CrossRef]
- Ludman, T.; Melemedjian, O.K. Bortezomib-induced aerobic glycolysis contributes to chemotherapy-induced painful peripheral neuropathy. Mol. Pain 2019, 15, 1744806919837429. [Google Scholar] [CrossRef]
- Bedard, K.; Krause, K.H. The NOX family of ROS-generating NADPH oxidases: Physiology and pathophysiology. Physiol. Rev. 2007, 87, 245–313. [Google Scholar] [CrossRef] [PubMed]
- Fabisiak, T.; Patel, M. Crosstalk between neuroinflammation and oxidative stress in epilepsy. Front. Cell Dev. Biol. 2022, 10, 976953. [Google Scholar] [CrossRef] [PubMed]
- Bettiol, A.; Galora, S.; Argento, F.R.; Fini, E.; Emmi, G.; Mattioli, I.; Bagni, G.; Fiorillo, C.; Becatti, M. Erythrocyte oxidative stress and thrombosis. Expert Rev. Mol. Med. 2022, 24, e31. [Google Scholar] [CrossRef]
- De Logu, F.; Nassini, R.; Materazzi, S.; Carvalho Gonçalves, M.; Nosi, D.; Rossi Degl’Innocenti, D.; Marone, I.M.; Ferreira, J.; Li Puma, S.; Benemei, S.; et al. Schwann cell TRPA1 mediates neuroinflammation that sustains macrophage-dependent neuropathic pain in mice. Nat. Commun. 2017, 8, 1887. [Google Scholar] [CrossRef]
- Kallenborn-Gerhardt, W.; Hohmann, S.W.; Syhr, K.M.; Schröder, K.; Sisignano, M.; Weigert, A.; Lorenz, J.E.; Lu, R.; Brüne, B.; Brandes, R.P.; et al. Nox2-dependent signaling between macrophages and sensory neurons contributes to neuropathic pain hypersensitivity. Pain 2014, 155, 2161–2170. [Google Scholar] [CrossRef] [PubMed]
- Geis, C.; Geuss, E.; Sommer, C.; Schmidt, H.H.; Kleinschnitz, C. NOX4 is an early initiator of neuropathic pain. Exp. Neurol. 2017, 288, 94–103. [Google Scholar] [CrossRef] [PubMed]
- Ding, R.; Sun, B.; Liu, Z.; Yao, X.; Wang, H.; Shen, X.; Jiang, H.; Chen, J. Advanced oxidative protein products cause pain hypersensitivity in rats by inducing dorsal root ganglion neurons apoptosis via NADPH oxidase 4/c-Jun N-terminal kinase pathways. Front. Mol. Neurosci. 2017, 10, 195. [Google Scholar] [CrossRef]
- Miao, F.; Wang, R.; Cui, G.; Li, X.; Wang, T.; Li, X. Engagement of microRNA-155 in exaggerated oxidative stress signal and TRPA1 in the dorsal horn of the spinal cord and neuropathic pain during chemotherapeutic oxaliplatin. Neurotox. Res. 2019, 36, 712–723. [Google Scholar] [CrossRef]
- Kallenborn-Gerhardt, W.; Schröder, K.; Del Turco, D.; Lu, R.; Kynast, K.; Kosowski, J.; Niederberger, E.; Shah, A.M.; Brandes, R.P.; Geisslinger, G.; et al. NADPH oxidase-4 maintains neuropathic pain after peripheral nerve injury. J. Neurosci. Off. J. Soc. Neurosci. 2012, 32, 10136–10145. [Google Scholar] [CrossRef]
- Eid, S.A.; El Massry, M.; Hichor, M.; Haddad, M.; Grenier, J.; Dia, B.; Barakat, R.; Boutary, S.; Chanal, J.; Aractingi, S.; et al. Targeting the NADPH oxidase-4 and liver X receptor pathway preserves Schwann cell integrity in diabetic mice. Diabetes 2020, 69, 448–464. [Google Scholar] [CrossRef]
- Zhao, W.C.; Zhang, B.; Liao, M.J.; Zhang, W.X.; He, W.Y.; Wang, H.B.; Yang, C.X. Curcumin ameliorated diabetic neuropathy partially by inhibition of NADPH oxidase mediating oxidative stress in the spinal cord. Neurosci. Lett. 2014, 560, 81–85. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.L.; Lu, J.H.; Xie, C.S.; Shen, Y.J.; Wang, J.W.; Ye, X.Y.; Zhang, M.B.; Jia, G.L.; Tao, Y.X.; Li, J.; et al. Caveolin-1 in spinal cord modulates type-2 diabetic neuropathic pain through the Rac1/NOX2/NR2B signaling pathway. Am. J. Transl. Res. 2020, 12, 1714–1727. [Google Scholar] [CrossRef] [PubMed]
- Kuhad, A.; Sharma, S.; Chopra, K. Lycopene attenuates thermal hyperalgesia in a diabetic mouse model of neuropathic pain. Eur. J. Pain 2008, 12, 624–632. [Google Scholar] [CrossRef] [PubMed]
- Goel, R.; Tyagi, N. Potential contribution of antioxidant mechanism in the defensive effect of lycopene against partial sciatic nerve ligation induced behavioral, biochemical and histopathological modification in Wistar rats. Drug Res. 2016, 66, 633–638. [Google Scholar] [CrossRef]
- Recalde, M.D.; Miguel, C.A.; Noya-Riobó, M.V.; González, S.L.; Villar, M.J.; Coronel, M.F. Resveratrol exerts anti-oxidant and anti-inflammatory actions and prevents oxaliplatin-induced mechanical and thermal allodynia. Brain Res. 2020, 1748, 147079. [Google Scholar] [CrossRef]
- Joseph, E.K.; Chen, X.; Bogen, O.; Levine, J.D. Oxaliplatin acts on IB4-positive nociceptors to induce an oxidative stress-dependent acute painful peripheral neuropathy. J. Pain 2008, 9, 463–472. [Google Scholar] [CrossRef]
- Moura, F.A.; de Andrade, K.Q.; dos Santos, J.C.; Goulart, M.O. Lipoic acid: Its antioxidant and anti-inflammatory role and clinical applications. Curr. Top. Med. Chem. 2015, 15, 458–483. [Google Scholar] [CrossRef]
- Kishimoto-Urata, M.; Urata, S.; Fujimoto, C.; Yamasoba, T. Role of oxidative stress and antioxidants in acquired inner ear disorders. Antioxidants 2022, 11, 1469. [Google Scholar] [CrossRef]
- Brookes, M.H.; Golding, B.T.; Howes, D.A.; Hudson, A.T. Proof that the absolute configuration of natural alpha-lipoic acid is R by the synthesis of its enantiomer [(S)-(–)-alpha-lipoic acid] from (S)-malic acid. J. Chem. Soc. Chem. Commun. 1983, 19, 1051–1053. [Google Scholar] [CrossRef]
- Ghibu, S.; Richard, C.; Vergely, C.; Zeller, M.; Cottin, Y.; Rochette, L. Antioxidant properties of an endogenous thiol: Alpha-lipoic acid, useful in the prevention of cardiovascular diseases. J. Cardiovasc. Pharmacol. 2009, 54, 391–398. [Google Scholar] [CrossRef]
- Szeląg, M.; Mikulski, D.; Molski, M. Quantum-chemical investigation of the structure and the antioxidant properties of α-lipoic acid and its metabolites. J. Mol. Model. 2012, 18, 2907–2916. [Google Scholar] [CrossRef]
- Akiba, S.; Matsugo, S.; Packer, L.; Konishi, T. Assay of protein-bound lipoic acid in tissues by a new enzymatic method. Anal. Biochem. 1998, 258, 299–304. [Google Scholar] [CrossRef] [PubMed]
- Hermann, R.; Niebch, G.D.; Borbe, H.O.; Fieger-Büschges, H.; Ruus, P.; Nowak, H.; Riethmüller-Winzen, H.; Peukert, M.; Blume, H.H. Enantioselective pharmacokinetics and bioavailability of different racemic α-lipoic acid formulations in healthy volunteers. Eur. J. Pharm. Sci. 1996, 4, 167–174. [Google Scholar] [CrossRef]
- Golbidi, S.; Badran, M.; Laher, I. Diabetes and alpha lipoic acid. Front. Pharmacol. 2011, 2, 69. [Google Scholar] [CrossRef] [PubMed]
- Rochette, L.; Ghibu, S.; Muresan, A.; Vergely, C. Alpha-lipoic acid: Molecular mechanisms and therapeutic potential in diabetes. Can. J. Physiol. Pharmacol. 2015, 93, 1021–1027. [Google Scholar] [CrossRef]
- Mignini, F.; Nasuti, C.; Gioventu, G.; Napolioni, V.; Di Martino, P. Human bioavailability and pharmacokinetic profile of different formulations delivering alpha lipoic acid. J. Clin. Cell. Immunol. 2012, 1, 418. [Google Scholar] [CrossRef]
- Brufani, M.; Figliola, R. (R)-α-lipoic acid oral liquid formulation: Pharmacokinetic parameters and therapeutic efficacy. Acta Bio-Med. Atenei Parm. 2014, 85, 108–115. [Google Scholar]
- Gleiter, C.H.; Schug, B.S.; Hermann, R.; Elze, M.; Blume, H.H.; Gundert-Remy, U. Influence of food intake on the bioavailability of thioctic acid enantiomers. Eur. J. Clin. Pharmacol. 1996, 50, 513–514. [Google Scholar] [CrossRef]
- Packer, L.; Kraemer, K.; Rimbach, G. Molecular aspects of lipoic acid in the prevention of diabetes complications. Nutrition 2001, 17, 888–895. [Google Scholar] [CrossRef]
- May, J.M.; Qu, Z.C.; Nelson, D.J. Uptake and reduction of alpha-lipoic acid by human erythrocytes. Clin. Biochem. 2007, 40, 1135–1142. [Google Scholar] [CrossRef]
- Schupke, H.; Hempel, R.; Peter, G.; Hermann, R.; Wessel, K.; Engel, J.; Kronbach, T. New metabolic pathways of alpha-lipoic acid. Drug Metab. Dispos. Biol. Fate Chem. 2001, 29, 855–862. [Google Scholar] [PubMed]
- Bock, E.; Schneeweiss, J. Ein beitrag zur therapie der neuropathia diabetica. Munch. Med. Wochenschr. 1959, 101, 1911–1912. [Google Scholar] [PubMed]
- Packer, L. alpha-Lipoic acid: A metabolic antioxidant which regulates NF-kappa B signal transduction and protects against oxidative injury. Drug Metab. Rev. 1998, 30, 245–275. [Google Scholar] [CrossRef] [PubMed]
- Biewenga, G.P.; Haenen, G.R.; Bast, A. The pharmacology of the antioxidant lipoic acid. Gen. Pharmacol. 1997, 29, 315–331. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, Y.J.; Tsuchiya, M.; Packer, L. Thioctic acid and dihydrolipoic acid are novel antioxidants which interact with reactive oxygen species. Free Radic. Res. Commun. 1991, 15, 255–263. [Google Scholar] [CrossRef]
- Wray, D.W.; Nishiyama, S.K.; Harris, R.A.; Zhao, J.; McDaniel, J.; Fjeldstad, A.S.; Witman, M.A.; Ives, S.J.; Barrett-O’Keefe, Z.; Richardson, R.S. Acute reversal of endothelial dysfunction in the elderly after antioxidant consumption. Hypertension 2012, 59, 818–824. [Google Scholar] [CrossRef]
- Reljanovic, M.; Reichel, G.; Rett, K.; Lobisch, M.; Schuette, K.; Möller, W.; Tritschler, H.J.; Mehnert, H. Treatment of diabetic polyneuropathy with the antioxidant thioctic acid (alpha-lipoic acid): A two year multicenter randomized double-blind placebo-controlled trial (ALADIN II). Alpha Lipoic Acid in Diabetic Neuropathy. Free Radic. Res. 1999, 31, 171–179. [Google Scholar] [CrossRef]
- Akter, N. Diabetic peripheral neuropathy: Epidemiology, physiopathology, diagnosis and treatment. Delta Med. Coll. J. 2019, 7, 35–48. [Google Scholar] [CrossRef]
- Pang, L.; Lian, X.; Liu, H.; Zhang, Y.; Li, Q.; Cai, Y.; Ma, H.; Yu, X. Understanding diabetic neuropathy: Focus on oxidative stress. Oxidative Med. Cell. Longev. 2020, 2020, 9524635. [Google Scholar] [CrossRef]
- Chen, J.; Li, Q. Lipoic acid decreases the expression of poly ADP-ribose polymerase and inhibits apoptosis in diabetic rats. Diabetes Metab. Syndr. Obes. Targets Ther. 2020, 13, 1725–1731. [Google Scholar] [CrossRef]
- Zhang, B.Y.; Zhang, Y.L.; Sun, Q.; Zhang, P.A.; Wang, X.X.; Xu, G.Y.; Hu, J.; Zhang, H.H. Alpha-lipoic acid downregulates TRPV1 receptor via NF-κB and attenuates neuropathic pain in rats with diabetes. CNS Neurosci. Ther. 2020, 26, 762–772. [Google Scholar] [CrossRef] [PubMed]
- Sadeghiyan Galeshkalami, N.; Abdollahi, M.; Najafi, R.; Baeeri, M.; Jamshidzade, A.; Falak, R.; Davoodzadeh Gholami, M.; Hassanzadeh, G.; Mokhtari, T.; Hassani, S.; et al. Alpha-lipoic acid and coenzyme Q10 combination ameliorates experimental diabetic neuropathy by modulating oxidative stress and apoptosis. Life Sci. 2019, 216, 101–110. [Google Scholar] [CrossRef] [PubMed]
- Najafi, R.; Sharifi, A.M.; Hosseini, A. Protective effects of alpha lipoic acid on high glucose-induced neurotoxicity in PC12 cells. Metab. Brain Dis. 2015, 30, 731–738. [Google Scholar] [CrossRef]
- Siniscalco, D.; Fuccio, C.; Giordano, C.; Ferraraccio, F.; Palazzo, E.; Luongo, L.; Rossi, F.; Roth, K.A.; Maione, S.; de Novellis, V. Role of reactive oxygen species and spinal cord apoptotic genes in the development of neuropathic pain. Pharmacol. Res. 2007, 55, 158–166. [Google Scholar] [CrossRef]
- Jain, S.K.; Lim, G. Lipoic acid decreases lipid peroxidation and protein glycosylation and increases (Na+ + K+)- and Ca++-ATPase activities in high glucose-treated human erythrocytes. Free Radic. Biol. Med. 2000, 29, 1122–1128. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Qin, X.; Song, Z.Y.; Yang, P.P.; Feng, Y.; Sun, Q.; Xu, G.Y.; Zhang, H.H. Alpha-lipoic acid suppresses P2X receptor activities and visceral hypersensitivity to colorectal distention in diabetic rats. Sci. Rep. 2017, 7, 3928. [Google Scholar] [CrossRef]
- Fei, X.; He, X.; Tai, Z.; Wang, H.; Qu, S.; Chen, L.; Hu, Q.; Fang, J.; Jiang, Y. Electroacupuncture alleviates diabetic neuropathic pain in rats by suppressing P2X3 receptor expression in dorsal root ganglia. Purinergic Signal. 2020, 16, 491–502. [Google Scholar] [CrossRef] [PubMed]
- Lam, D.; Momeni, Z.; Theaker, M.; Jagadeeshan, S.; Yamamoto, Y.; Ianowski, J.P.; Campanucci, V.A. RAGE-dependent potentiation of TRPV1 currents in sensory neurons exposed to high glucose. PLoS ONE 2018, 13, e0193312. [Google Scholar] [CrossRef] [PubMed]
- Gomes, M.B.; Negrato, C.A. Alpha-lipoic acid as a pleiotropic compound with potential therapeutic use in diabetes and other chronic diseases. Diabetol. Metab. Syndr. 2014, 6, 80. [Google Scholar] [CrossRef]
- Martorana, F.; Foti, M.; Virtuoso, A.; Gaglio, D.; Aprea, F.; Latronico, T.; Rossano, R.; Riccio, P.; Papa, M.; Alberghina, L.; et al. Differential Modulation of NF-κB in Neurons and Astrocytes Underlies Neuroprotection and Antigliosis Activity of Natural Antioxidant Molecules. Oxidative Med. Cell. Longev. 2019, 2019, 8056904. [Google Scholar] [CrossRef]
- Fang, X.X.; Wang, H.; Song, H.L.; Wang, J.; Zhang, Z.J. Neuroinflammation Involved in Diabetes-Related Pain and Itch. Front. Pharmacol. 2022, 13, 921612. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Lou, Z.; Xi, H.; Li, Z.; Li, L.; Li, Z.; Zhang, K.; Asakawa, T. Verification of neuroprotective effects of alpha-lipoic acid on chronic neuropathic pain in a chronic constriction injury rat model. Open Life Sci. 2021, 16, 222–228. [Google Scholar] [CrossRef] [PubMed]
- Khan, N.; Gordon, R.; Woodruff, T.M.; Smith, M.T. Antiallodynic effects of alpha lipoic acid in an optimized RR-EAE mouse model of MS-neuropathic pain are accompanied by attenuation of upregulated BDNF-TrkB-ERK signaling in the dorsal horn of the spinal cord. Pharmacol. Res. Perspect. 2015, 3, e00137. [Google Scholar] [CrossRef] [PubMed]
- Melli, G.; Taiana, M.; Camozzi, F.; Triolo, D.; Podini, P.; Quattrini, A.; Taroni, F.; Lauria, G. Alpha-lipoic acid prevents mitochondrial damage and neurotoxicity in experimental chemotherapy neuropathy. Exp. Neurol. 2008, 214, 276–284. [Google Scholar] [CrossRef] [PubMed]
- Thompson, E. Mouse Sleeping. Available online: https://www.scidraw.io/drawing/285 (accessed on 11 November 2022).
- Georgievckaya, K. Spine Cord. Available online: https://www.scidraw.io/drawing/454 (accessed on 11 November 2022).
- Mijnhout, G.S.; Kollen, B.J.; Alkhalaf, A.; Kleefstra, N.; Bilo, H.J. Alpha lipoic acid for symptomatic peripheral neuropathy in patients with diabetes: A meta-analysis of randomized controlled trials. Int. J. Endocrinol. 2012, 2012, 456279. [Google Scholar] [CrossRef]
- Kulaklı, F. The effect of alpha lipoic acid in the treatment of multiple sclerosis induced neuropathic pain: A case report. Eurasian J. Med. Oncol. 2018, 2, 179–181. [Google Scholar] [CrossRef]
- Ziegler, D.; Papanas, N.; Schnell, O.; Nguyen, B.D.T.; Nguyen, K.T.; Kulkantrakorn, K.; Deerochanawong, C. Current concepts in the management of diabetic polyneuropathy. J. Diabetes Investig. 2021, 12, 464–475. [Google Scholar] [CrossRef]
- Abdelrahman, K.A.; Ibrahim, A.S.; Osman, A.M.; Aly, M.G.; Ali, A.S.; Farrag, W.S. Alpha lipoic acid with pulsed radiofrequency in treatment of chronic lumbosacral radicular pain: A prospective, randomized study. Medicine 2021, 100, e26344. [Google Scholar] [CrossRef]
- Barreto da Silva, L.; Camargo, S.B.; Moraes, R.D.A.; Medeiros, C.F.; Jesus, A.M.; Evangelista, A.; Villarreal, C.F.; Quintans-Júnior, L.J.; Silva, D.F. Antihypertensive effect of carvacrol is improved after incorporation in β-cyclodextrin as a drug delivery system. Clin. Exp. Pharmacol. Physiol. 2020, 47, 1798–1807. [Google Scholar] [CrossRef]
- Crescenzo, A.D.; Cacciatore, I.; Petrini, M.; D’Alessandro, M.; Petragnani, N.; Boccio, P.D.; Profio, P.D.; Boncompagni, S.; Spoto, G.; Turkez, H.; et al. Gold nanoparticles as scaffolds for poor water soluble and difficult to vehiculate antiparkinson codrugs. Nanotechnology 2017, 28, 025102. [Google Scholar] [CrossRef]
- Piersimoni, M.E.; Teng, X.; Cass, A.E.G.; Ying, L. Antioxidant lipoic acid ligand-shell gold nanoconjugates against oxidative stress caused by α-synuclein aggregates. Nanoscale Adv. 2020, 2, 5666–5681. [Google Scholar] [CrossRef] [PubMed]
- Aljaeid, B.M.; El-Moselhy, M.A. Loading of gentamicin and alpha lipoic acid on a biodegradable polymer for more effective and less nephrotoxic formula. Int. J. Pharmacol. 2018, 14, 796–801. [Google Scholar] [CrossRef]
- Haidar, M.K.; Timur, S.S.; Kazanci, A.; Turkoglu, O.F.; Gürsoy, R.N.; Nemutlu, E.; Sargon, M.F.; Bodur, E.; Gök, M.; Ulubayram, K.; et al. Composite nanofibers incorporating alpha lipoic acid and atorvastatin provide neuroprotection after peripheral nerve injury in rats. Eur. J. Pharm. Biopharm. Off. J. Arb. Fur Pharm. Verfahr. 2020, 153, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Kulikova, O.I.; Berezhnoy, D.S.; Stvolinsky, S.L.; Lopachev, A.V.; Orlova, V.S.; Fedorova, T.N. Neuroprotective effect of the carnosine—α-lipoic acid nanomicellar complex in a model of early-stage Parkinson’s disease. Regul. Toxicol. Pharmacol. RTP 2018, 95, 254–259. [Google Scholar] [CrossRef] [PubMed]
- Kubota, Y.; Musashi, M.; Nagasawa, T.; Shimura, N.; Igarashi, R.; Yamaguchi, Y. Novel nanocapsule of α-lipoic acid reveals pigmentation improvement: α-Lipoic acid stimulates the proliferation and differentiation of keratinocyte in murine skin by topical application. Exp. Dermatol. 2019, 28 (Suppl. 1), 55–63. [Google Scholar] [CrossRef] [PubMed]
- Çoban, Ö.; Yıldırım, S.; Bakır, T. Alpha-lipoic acid and cyanocobalamin co-loaded nanoemulsions: Development, characterization, and evaluation of stability. J. Pharm. Innov. 2022, 17, 510–520. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Viana, M.D.M.; Lauria, P.S.S.; Lima, A.A.d.; Opretzka, L.C.F.; Marcelino, H.R.; Villarreal, C.F. Alpha-Lipoic Acid as an Antioxidant Strategy for Managing Neuropathic Pain. Antioxidants 2022, 11, 2420. https://doi.org/10.3390/antiox11122420
Viana MDM, Lauria PSS, Lima AAd, Opretzka LCF, Marcelino HR, Villarreal CF. Alpha-Lipoic Acid as an Antioxidant Strategy for Managing Neuropathic Pain. Antioxidants. 2022; 11(12):2420. https://doi.org/10.3390/antiox11122420
Chicago/Turabian StyleViana, Max Denisson Maurício, Pedro Santana Sales Lauria, Alyne Almeida de Lima, Luiza Carolina França Opretzka, Henrique Rodrigues Marcelino, and Cristiane Flora Villarreal. 2022. "Alpha-Lipoic Acid as an Antioxidant Strategy for Managing Neuropathic Pain" Antioxidants 11, no. 12: 2420. https://doi.org/10.3390/antiox11122420
APA StyleViana, M. D. M., Lauria, P. S. S., Lima, A. A. d., Opretzka, L. C. F., Marcelino, H. R., & Villarreal, C. F. (2022). Alpha-Lipoic Acid as an Antioxidant Strategy for Managing Neuropathic Pain. Antioxidants, 11(12), 2420. https://doi.org/10.3390/antiox11122420





