TRAP1 in Oxidative Stress and Neurodegeneration
Abstract
1. Introduction
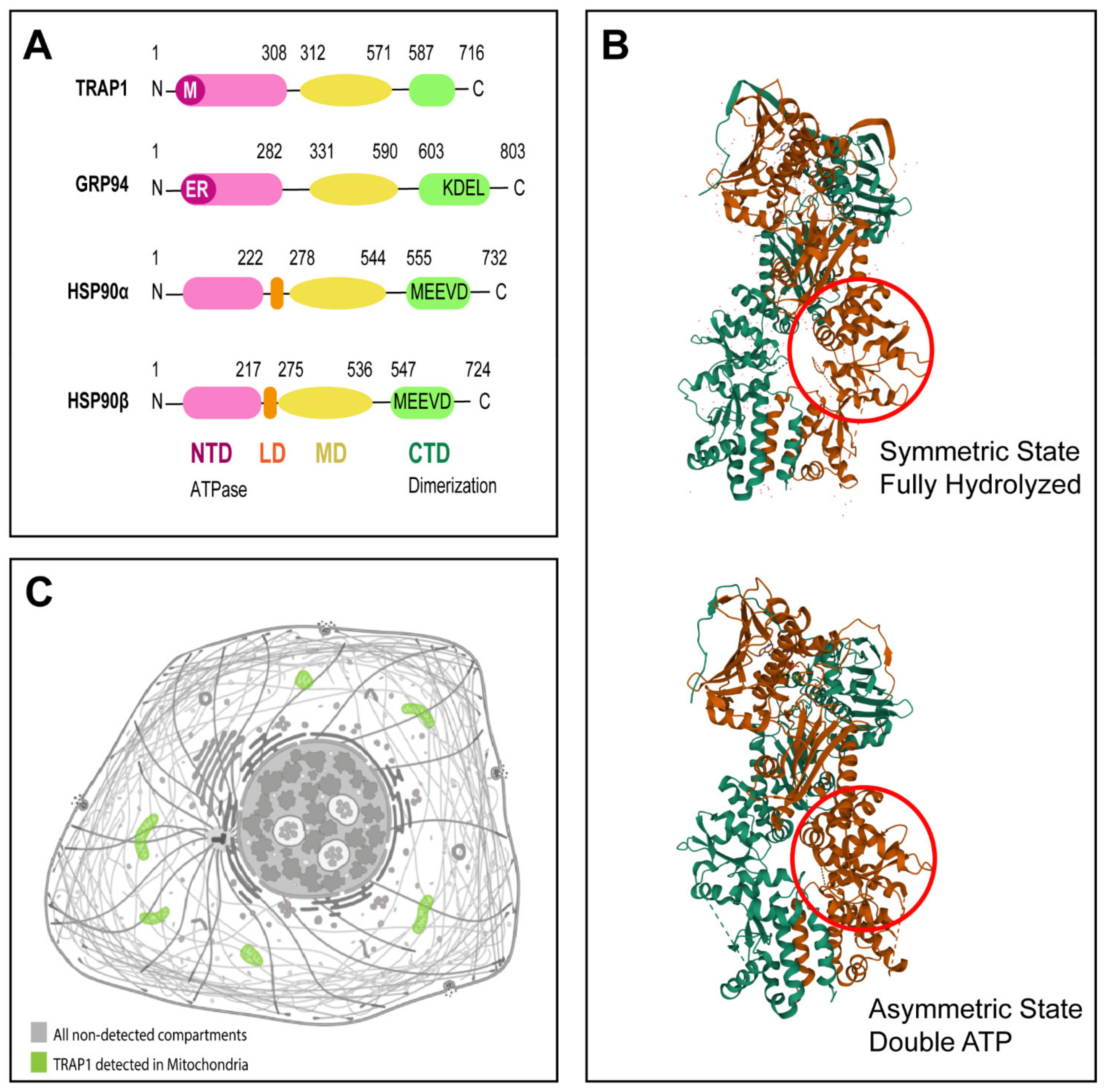
2. TRAP1 Molecular Structure
3. TRAP1 Functions and Signaling Pathways
3.1. Role of TRAP1 in Mitochondria
3.1.1. TRAP1 and Energetic Metabolism Regulation
3.1.2. TRAP1 and Redox Homeostasis: Protection against Oxidative Stress and Apoptosis
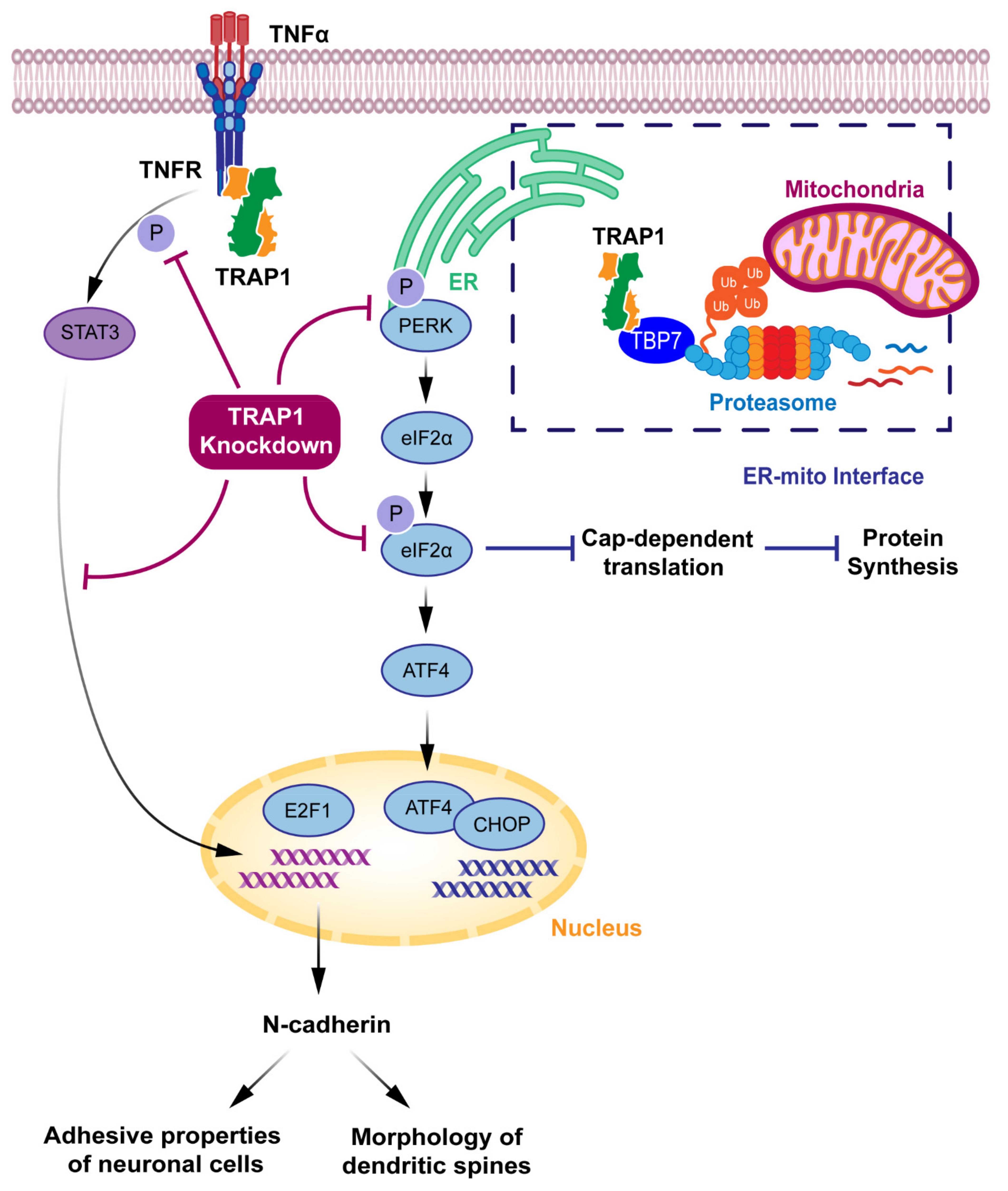
3.2. TRAP1 Suggested Extra-Mitochondrial Roles
4. TRAP1 and Neurodegeneration
5. TRAP1 Inhibitors and Neuroprotection
6. Concluding Remarks and Future Perspectives
Author Contributions
Funding
Conflicts of Interest
References
- Serapian, S.A.; Sanchez-Martín, C.; Moroni, E.; Rasola, A.; Colombo, G. Targeting the Mitochondrial Chaperone TRAP1: Strategies and Therapeutic Perspectives. Trends Pharmacol. Sci. 2021, 42, 566–576. [Google Scholar] [CrossRef] [PubMed]
- Dekker, F.A.; Rüdiger, S.G.D. The Mitochondrial Hsp90 TRAP1 and Alzheimer’s Disease. Front. Mol. Biosci. 2021, 8, 6979138. [Google Scholar] [CrossRef] [PubMed]
- Faienza, F.; Rizza, S.; Giglio, P.; Filomeni, G. TRAP1: A Metabolic Hub Linking Aging Pathophysiology to Mitochondrial S-Nitrosylation. Front. Physiol. 2020, 11, 340. [Google Scholar] [CrossRef] [PubMed]
- Xiang, F.; Huang, Y.S.; Shi, X.H.; Zhang, Q. Mitochondrial Chaperone Tumour Necrosis Factor Receptor-Associated Protein 1 Protects Cardiomyocytes from Hypoxic Injury by Regulating Mitochondrial Permeability Transition Pore Opening. FEBS J. 2010, 277, 1929–1938. [Google Scholar] [CrossRef]
- Wang, Y.; Lin, J.; Chen, Q.Z.; Zhu, N.; Jiang, D.Q.; Li, M.X.; Wang, Y. Overexpression of Mitochondrial Hsp75 Protects Neural Stem Cells against Microglia-Derived Soluble Factor-Induced Neurotoxicity by Regulating Mitochondrial Permeability Transition Pore Opening in Vitro. Int. J. Mol. Med. 2015, 36, 1487–1496. [Google Scholar] [CrossRef]
- Guzzo, G.; Sciacovelli, M.; Bernardi, P.; Rasola, A. Inhibition of Succinate Dehydrogenase by the Mitochondrial Chaperone TRAP1 Has Anti-Oxidant and Anti-Apoptotic Effects on Tumor Cells. Oncotarget 2014, 5, 11897–11908. [Google Scholar] [CrossRef]
- Takemoto, K.; Miyata, S.; Takamura, H.; Katayama, T.; Tohyama, M. Mitochondrial TRAP1 Regulates the Unfolded Protein Response in the Endoplasmic Reticulum. Neurochem. Int. 2011, 58, 880–887. [Google Scholar] [CrossRef]
- Amoroso, M.R.; Matassa, D.S.; Laudiero, G.; Egorova, A.V.; Polishchuk, R.S.; Maddalena, F.; Piscazzi, A.; Paladino, S.; Sarnataro, D.; Garbi, C.; et al. TRAP1 and the proteasome regulatory particle TBP7/Rpt3 interact in the endoplasmic reticulum and control cellular ubiquitination of specific mitochondrial proteins. Cell Death Differ. 2012, 19, 592–604. [Google Scholar] [CrossRef]
- Rasola, A.; Neckers, L.; Picard, D. Mitochondrial Oxidative Phosphorylation TRAP(1)Ped in Tumor Cells. Trends Cell Biol. 2014, 24, 455–463. [Google Scholar] [CrossRef]
- Kang, B.H. TRAP1 regulation of mitochondrial life or death decision in cancer cells and mitochondria-targeted TRAP1 inhibitors. BMB Rep. 2012, 45, 1–6. [Google Scholar] [CrossRef]
- Sehnal, D.; Bittrich, S.; Deshpande, M.; Svobodová, R.; Berka, K.; Bazgier, V.; Velankar, S.; Burley, S.K.; Koča, J.; Rose, A.S. Mol* Viewer: Modern web app for 3D visualization and analysis of large biomolecular structures. Nucleic Acids Res. 2021, 49 (W1), W431–W437. [Google Scholar] [CrossRef]
- Elnatan, D.; Betegon, M.; Agard, D.A. Crystal Structure of Mitochondrial Hsp90 (TRAP1) with ATP in Absence of Mg, Fully Hydrolyzed. 2016. Available online: https://www.rcsb.org/structure/6XG6 (accessed on 29 September 2021). [CrossRef]
- Elnatan, D.; Betegon, M.; Liu, Y.; Ramelot, T.; Kennedy, M.A.; Agard, D.A. Symmetry broken and rebroken during the ATP hydrolysis cycle of the mitochondrial Hsp90 TRAP1. eLife 2017, 6, e25235. [Google Scholar] [CrossRef]
- Liu, Y.X.; Wang, F.; Agard, D.A. Full-Length Human Mitochondrial Hsp90 (TRAP1) with ADP-BeF3. 2020. Available online: https://www.rcsb.org/structure/5TVX (accessed on 29 September 2021). [CrossRef]
- Wang, F.; Liu, Y.; Yu, Z.; Li, S.; Feng, S.; Cheng, Y.; Agard, D.A. General and robust covalently linked graphene oxide affinity grids for high-resolution cryo-EM. Proc. Natl. Acad. Sci. USA 2020, 117, 24269–24273. [Google Scholar] [CrossRef]
- Hoter, A.; El-Sabban, M.E.; Naim, H.Y. The HSP90 Family: Structure, Regulation, Function, and Implications in Health and Disease. Int. J. Mol. Sci. 2018, 19, 2560. [Google Scholar] [CrossRef]
- Song, H.Y.; Dunbar, J.D.; Zhang, Y.X.; Guo, D.; Donner, D.B. Identification of a Protein with Homology to Hsp90 That Binds the Type 1 Tumor Necrosis Factor Receptor. J. Biol. Chem. 1995, 270, 3574–3581. [Google Scholar] [CrossRef]
- Felts, S.J.; Owen, B.A.L.L.; Nguyen, P.M.; Trepel, J.; Donner, D.B.; Toft, D.O. The Hsp90-Related Protein TRAP1 Is a Mitochondrial Protein with Distinct Functional Properties. J. Biol. Chem. 2000, 275, 3305–3312. [Google Scholar] [CrossRef]
- Guo, J.; Zhu, P.; Wu, C.; Yu, L.; Zhao, S.; Gu, X. In Silico Analysis Indicates a Similar Gene Expression Pattern between Human Brain and Testis. Cytogenet. Genome Res. 2003, 103, 58–62. [Google Scholar] [CrossRef]
- Shiau, A.K.; Harris, S.F.; Southworth, D.R.; Agard, D.A. Structural Analysis of E. Coli Hsp90 Reveals Dramatic Nucleotide-Dependent Conformational Rearrangements. Cell 2006, 127, 329–340. [Google Scholar] [CrossRef]
- Cechetto, J.D.; Gupta, R.S. Immunoelectron Microscopy Provides Evidence That Tumor Necrosis Factor Receptor-Associated Protein 1 (TRAP-1) Is a Mitochondrial Protein Which Also Localizes at Specific Extramitochondrial Sites. Exp. Cell Res. 2000, 260, 30–39. [Google Scholar] [CrossRef]
- Zuehlke, A.; Johnson, J.L. Hsp90 and Co-Chaperones Twist the Functions of Diverse Client Proteins. Biopolymers 2010, 93, 211–217. [Google Scholar] [CrossRef]
- Elnatan, D.; Agard, D.A. Calcium Binding to a Remote Site Can Replace Magnesium as Cofactor for Mitochondrial Hsp90 (TRAP1) ATPase Activity. J. Biol. Chem. 2018, 293, 13717–13724. [Google Scholar] [CrossRef] [PubMed]
- Masgras, I.; Laquatra, C.; Cannino, G.; Serapian, S.A.; Colombo, G.; Rasola, A. The Molecular Chaperone TRAP1 in Cancer: From the Basics of Biology to Pharmacological Targeting. Semin. Cancer Biol. 2021, in press. [Google Scholar] [CrossRef] [PubMed]
- Joshi, A.; Dai, L.; Liu, Y.; Lee, J.; Ghahhari, N.M.; Segala, G.; Beebe, K.; Jenkins, L.M.; Lyons, G.C.; Bernasconi, L.; et al. The Mitochondrial HSP90 Paralog TRAP1 Forms an OXPHOS-Regulated Tetramer and Is Involved in Mitochondrial Metabolic Homeostasis. BMC Biol. 2020, 18, 10. [Google Scholar] [CrossRef] [PubMed]
- Sima, S.; Richter, K. Regulation of the Hsp90 System. Biochim. Biophys. Acta. Mol. Cell Res. 2018, 1865, 889–897. [Google Scholar] [CrossRef]
- Leskovar, A.; Wegele, H.; Werbeck, N.D.; Buchner, J.; Reinstein, J. The ATPase Cycle of the Mitochondrial Hsp90 Analog Trap1. J. Biol. Chem. 2008, 283, 11677–11688. [Google Scholar] [CrossRef]
- Matassa, D.S.; Amoroso, M.R.; Agliarulo, I.; Maddalena, F.; Sisinni, L.; Paladino, S.; Romano, S.; Romano, M.F.; Sagar, V.; Loreni, F.; et al. Translational Control in the Stress Adaptive Response of Cancer Cells: A Novel Role for the Heat Shock Protein TRAP1. Cell Death Dis. 2013, 4, e851. [Google Scholar] [CrossRef]
- Lavery, L.A.; Partridge, J.R.; Ramelot, T.A.; Elnatan, D.; Kennedy, M.A.; Agard, D.A. Structural Asymmetry in the Closed State of Mitochondrial Hsp90 (TRAP1) Supports a Two-Step ATP Hydrolysis Mechanism. Mol. Cell 2014, 53, 330–343. [Google Scholar] [CrossRef]
- Sung, N.; Lee, J.; Kim, J.H.; Chang, C.; Tsai, F.T.; Lee, S. 2.4 Å Resolution Crystal Structure of Human TRAP1NM, the Hsp90 Paralog in the Mitochondrial Matrix. Acta Crystallogr. 2016, 72, 904–911. [Google Scholar] [CrossRef]
- Masgras, I.; Sanchez-Martin, C.; Colombo, G.; Rasola, A. The Chaperone TRAP1 As a Modulator of the Mitochondrial Adaptations in Cancer Cells. Front. Oncol. 2017, 7, 58. [Google Scholar] [CrossRef]
- Masgras, I.; Ciscato, F.; Brunati, A.M.; Tibaldi, E.; Indraccolo, S.; Curtarello, M.; Chiara, F.; Cannino, G.; Papaleo, E.; Lambrughi, M.; et al. Absence of Neurofibromin Induces an Oncogenic Metabolic Switch via Mitochondrial ERK-Mediated Phosphorylation of the Chaperone TRAP1. Cell Rep. 2017, 18, 659–672. [Google Scholar] [CrossRef]
- Verba, K.A.; Wang, R.Y.-R.; Arakawa, A.; Liu, Y.; Shirouzu, M.; Yokoyama, S.; Agard, D.A. Atomic structure of Hsp90-Cdc37-Cdk4 reveals that Hsp90 traps and stabilizes an unfolded kinase. Science 2016, 352, 1542–1547. [Google Scholar] [CrossRef]
- Lee, C.; Park, H.K.; Jeong, H.; Lim, J.; Lee, A.J.; Cheon, K.Y.; Kim, C.S.; Thomas, A.P.; Bae, B.; Kim, N.D.; et al. Development of a Mitochondria-Targeted Hsp90 Inhibitor Based on the Crystal Structures of Human TRAP1. J. Am. Chem. Soc. 2015, 137, 4358–4367. [Google Scholar] [CrossRef]
- Yoshida, S.; Tsutsumi, S.; Muhlebach, G.; Sourbier, C.; Lee, M.-J.; Lee, S.; Vartholomaiou, E.; Tatokoro, M.E.; Beebe, K.; Miyajima, N.; et al. Molecular Chaperone TRAP1 Regulates a Metabolic Switch between Mitochondrial Respiration and Aerobic Glycolysis. Proc. Natl. Acad. Sci. USA 2013, 110, E1604–E1612. [Google Scholar] [CrossRef]
- Sciacovelli, M.; Guzzo, G.; Morello, V.; Frezza, C.; Zheng, L.; Nannini, N.; Calabrese, F.; Laudiero, G.; Esposito, F.; Landriscina, M.; et al. The Mitochondrial Chaperone TRAP1 Promotes Neoplastic Growth by Inhibiting Succinate Dehydrogenase. Cell Metab. 2013, 17, 988–999. [Google Scholar] [CrossRef]
- Miyazaki, T.; Neff, L.; Tanaka, S.; Horne, W.C.; Baron, R. Regulation of Cytochrome c Oxidase Activity by C-Src in Osteoclasts. J. Cell Biol. 2003, 160, 709–718. [Google Scholar] [CrossRef]
- Ogura, M.; Yamaki, J.; Homma, M.K.; Homma, Y. Mitochondrial C-Src Regulates Cell Survival through Phosphorylation of Respiratory Chain Components. Biochem. J. 2012, 447, 281–289. [Google Scholar] [CrossRef]
- Laquatra, C.; Sanchez-Martin, C.; Dinarello, A.; Cannino, G.; Minervini, G.; Moroni, E.; Schiavone, M.; Tosatto, S.; Argenton, F.; Colombo, G.; et al. HIF1α-Dependent Induction of the Mitochondrial Chaperone TRAP1 Regulates Bioenergetic Adaptations to Hypoxia. Cell Death Dis. 2021, 12, 434. [Google Scholar] [CrossRef]
- Singh, D.; Arora, R.; Kaur, P.; Singh, B.; Mannan, R.; Arora, S. Overexpression of Hypoxia-Inducible Factor and Metabolic Pathways: Possible Targets of Cancer. Cell Biosci. 2017, 7, 1–9. [Google Scholar] [CrossRef]
- Zhang, Z.; Yao, L.; Yang, J.; Wang, Z.; Du, G. PI3K/Akt and HIF 1 Signaling Pathway in Hypoxia ischemia (Review). Mol. Med. Rep. 2018, 18, 3547–3554. [Google Scholar] [CrossRef]
- Bhattacharya, B.; Mohd Omar, M.F.; Soong, R. The Warburg Effect and Drug Resistance. Br. J. Pharmacol. 2016, 173, 970–979. [Google Scholar] [CrossRef]
- Chae, Y.C.; Caino, M.C.; Lisanti, S.; Ghosh, J.C.; Dohi, T.; Danial, N.N.; Villanueva, J.; Ferrero, S.; Vaira, V.; Santambrogio, L.; et al. Control of Tumor Bioenergetics and Survival Stress Signaling by Mitochondrial HSP90s. Cancer Cell 2012, 22, 331–344. [Google Scholar] [CrossRef]
- Matassa, D.S.; Agliarulo, I.; Avolio, R.; Landriscina, M.; Esposito, F. Trap1 Regulation of Cancer Metabolism: Dual Role as Oncogene or Tumor Suppressor. Genes 2018, 9, 195. [Google Scholar] [CrossRef]
- Lisanti, S.; Tavecchio, M.; Chae, Y.C.; Liu, Q.; Brice, A.K.; Thakur, M.L.; Languino, L.R.; Altieri, D.C. Deletion of the Mitochondrial Chaperone TRAP-1 Uncovers Global Reprogramming of Metabolic Networks. Cell Rep. 2014, 8, 671–677. [Google Scholar] [CrossRef]
- Wang, R.; Shao, F.; Liu, Z.; Zhang, J.; Wang, S.; Liu, J.; Liu, H.; Chen, H.; Liu, K.; Xia, M.; et al. The Hsp90 Inhibitor SNX-2112, Induces Apoptosis in Multidrug Resistant K562/ADR Cells through Suppression of Akt/NF-ΚB and Disruption of Mitochondria-Dependent Pathways. Chem.-Biol. Interact. 2013, 205, 1–10. [Google Scholar] [CrossRef]
- Gaki, G.S.; Papavassiliou, A.G. Oxidative Stress-Induced Signaling Pathways Implicated in the Pathogenesis of Parkinson’s Disease. Neuromol. Med. 2014, 16, 217–230. [Google Scholar] [CrossRef]
- Avolio, R.; Matassa, D.S.; Criscuolo, D.; Landriscina, M.; Esposito, F. Modulation of Mitochondrial Metabolic Reprogramming and Oxidative Stress to Overcome Chemoresistance in Cancer. Biomolecules 2020, 10, 135. [Google Scholar] [CrossRef]
- Amoroso, M.R.; Matassa, D.S.; Sisinni, L.; Lettini, G.; Landriscina, M.; Esposito, F. TRAP1 Revisited: Novel Localizations and Functions of a “next-Generation” Biomarker (Review). Int. J. Oncol. 2014, 45, 969–977. [Google Scholar] [CrossRef]
- Purushottam Dharaskar, S.; Paithankar, K.; Kanugovi Vijayavittal, A.; Shabbir Kara, H.; Amere Subbarao, S. Mitochondrial Chaperone, TRAP1 Modulates Mitochondrial Dynamics and Promotes Tumor Metastasis. Mitochondrion 2020, 54, 92–101. [Google Scholar] [CrossRef]
- Standing, A.S.I.; Hong, Y.; Paisan-Ruiz, C.; Omoyinmi, E.; Medlar, A.; Stanescu, H.; Kleta, R.; Rowcenzio, D.; Hawkins, P.; Lachmann, H.; et al. TRAP1 Chaperone Protein Mutations and Autoinflammation. Life Sci. Alliance 2020, 3, e201900376. [Google Scholar] [CrossRef]
- Sanchez-Martin, C.; Moroni, E.; Ferraro, M.; Laquatra, C.; Cannino, G.; Masgras, I.; Negro, A.; Quadrelli, P.; Rasola, A.; Colombo, G. Rational Design of Allosteric and Selective Inhibitors of the Molecular Chaperone TRAP1. Cell Rep. 2020, 31, 107531. [Google Scholar] [CrossRef]
- Hua, G.; Zhang, Q.; Fan, Z. Heat Shock Protein 75 (TRAP1) Antagonizes Reactive Oxygen Species Generation and Protects Cells from Granzyme M-Mediated Apoptosis. J. Biol. Chem. 2007, 282, 20553–20560. [Google Scholar] [CrossRef] [PubMed]
- Im, C.N.; Lee, J.S.; Zheng, Y.; Seo, J.S. Iron Chelation Study in a Normal Human Hepatocyte Cell Line Suggests That Tumor Necrosis Factor Receptor-Associated Protein 1 (TRAP1) Regulates Production of Reactive Oxygen Species. J. Cell. Biochem. 2007, 100, 474–486. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Lu, Y.; Yu, D.; Zhang, D.; Hu, W. TRAP1 Provides Protection Against Myocardial Ischemia-Reperfusion Injury by Ameliorating Mitochondrial Dysfunction. Cell. Physiol. Biochem. 2015, 36, 2072–2082. [Google Scholar] [CrossRef]
- Montesano, G.N.; Chirico, G.; Pirozzi, G.; Costantino, E.; Landriscina, M.; Esposito, F. Tumor necrosis factor-associated protein 1 (TRAP-1) protects cells from oxidative stress and apoptosis. Stress 2007, 10, 342–350. [Google Scholar]
- Baines, C.P.; Kaiser, R.A.; Purcell, N.H.; Blair, N.S.; Osinska, H.; Hambleton, M.A.; Brunskill, E.W.; Sayen, M.R.; Gottlieb, R.A.; Dorn, G.W.; et al. Loss of Cyclophilin D Reveals a Critical Role for Mitochondrial Permeability Transition in Cell Death. Nature 2005, 434, 658–662. [Google Scholar] [CrossRef] [PubMed]
- Kroemer, G.; Blomgren, K. Mitochondrial Cell Death Control in Familial Parkinson Disease. PLoS Biol. 2007, 5, e206. [Google Scholar] [CrossRef] [PubMed]
- Rasola, A.; Bernardi, P. The Mitochondrial Permeability Transition Pore and Its Involvement in Cell Death and in Disease Pathogenesis. Apoptosis Int. J. Program. Cell Death 2007, 12, 815–833. [Google Scholar] [CrossRef] [PubMed]
- Xie, S.; Wang, X.; Gan, S.; Tang, X.; Kang, X.; Zhu, S. The Mitochondrial Chaperone TRAP1 as a Candidate Target of Oncotherapy. Front. Oncol. 2021, 10, 585047. [Google Scholar] [CrossRef]
- Tsujimoto, Y.; Shimizu, S. Role of the Mitochondrial Membrane Permeability Transition in Cell Death. Apoptosis Int. J. Program. Cell Death 2007, 12, 835–840. [Google Scholar] [CrossRef]
- Kang, B.H.; Plescia, J.; Dohi, T.; Rosa, J.; Doxsey, S.J.; Altieri, D.C. Regulation of Tumor Cell Mitochondrial Homeostasis by an Organelle-Specific Hsp90 Chaperone Network. Cell 2007, 131, 257–270. [Google Scholar] [CrossRef]
- Siegelin, M.D. Inhibition of the Mitochondrial Hsp90 Chaperone Network: A Novel, Efficient Treatment Strategy for Cancer? Cancer Lett. 2013, 333, 133–146. [Google Scholar] [CrossRef]
- Pridgeon, J.W.; Olzmann, J.A.; Chin, L.-S.S.; Li, L. PINK1 Protects against Oxidative Stress by Phosphorylating Mitochondrial Chaperone TRAP1. PLoS Biol. 2007, 5, e172. [Google Scholar] [CrossRef]
- Xiang, F.; Ma, S.; Lv, Y.; Zhang, D.; Song, H.; Huang, Y. Tumor Necrosis Factor Receptor-Associated Protein 1 Regulates Hypoxia-Induced Apoptosis through a Mitochondria-Dependent Pathway Mediated by Cytochrome c Oxidase Subunit II. Burn. Trauma 2019, 7, 16. [Google Scholar] [CrossRef]
- Liu, L.; Zhang, L.; Zhao, J.; Guo, X.; Luo, Y.; Hu, W.; Zhao, T. Tumor Necrosis Factor Receptor-Associated Protein 1 Protects against Mitochondrial Injury by Preventing High Glucose-Induced MPTP Opening in Diabetes. Oxid. Med. Cell. Longev. 2020, 2020, 6431517. [Google Scholar] [CrossRef]
- Zhang, L.; Liu, L.; Li, X.; Zhang, X.; Zhao, J.; Luo, Y.; Guo, X.; Zhao, T. TRAP1 Attenuates H9C2 Myocardial Cell Injury Induced by Extracellular Acidification via the Inhibition of MPTP Opening. Int. J. Mol. Med. 2020, 46, 663–674. [Google Scholar] [CrossRef]
- Kubota, K.; Inoue, K.; Hashimoto, R.; Kumamoto, N.; Kosuga, A.; Tatsumi, M.; Kamijima, K.; Kunugi, H.; Iwata, N.; Ozaki, N.; et al. Tumor Necrosis Factor Receptor-Associated Protein 1 Regulates Cell Adhesion and Synaptic Morphology via Modulation of N-Cadherin Expression. J. Neurochem. 2009, 110, 496–508. [Google Scholar] [CrossRef]
- Hetz, C. The Unfolded Protein Response: Controlling Cell Fate Decisions under ER Stress and Beyond. Nature reviews. Mol. Cell Biol. 2012, 13, 89–102. [Google Scholar] [CrossRef]
- Grimm, S. The ER–Mitochondria Interface: The Social Network of Cell Death. Biochim. Biophys. Acta (BBA) Mol. Cell Res. 2012, 1823, 327–334. [Google Scholar] [CrossRef]
- Park, H.K.; Yoon, N.G.; Lee, J.E.; Hu, S.; Yoon, S.; Kim, S.Y.; Hong, J.H.; Nam, D.; Chae, Y.C.; Park, J.B.; et al. Unleashing the Full Potential of Hsp90 Inhibitors as Cancer Therapeutics through Simultaneous Inactivation of Hsp90, Grp94, and TRAP1. Exp. Mol. Med. 2020, 52, 79–91. [Google Scholar] [CrossRef]
- Landriscina, M.; Laudiero, G.; Maddalena, F.; Amoroso, M.R.; Piscazzi, A.; Cozzolino, F.; Monti, M.; Garbi, C.; Fersini, A.; Pucci, P.; et al. Mitochondrial Chaperone Trap1 and the Calcium Binding Protein Sorcin Interact and Protect Cells against Apoptosis Induced by Antiblastic Agents. Cancer Res. 2010, 70, 6577–6586. [Google Scholar] [CrossRef]
- Maddalena, F.; Sisinni, L.; Lettini, G.; Condelli, V.; Matassa, D.S.; Piscazzi, A.; Amoroso, M.R.; la Torre, G.; Esposito, F.; Landriscina, M. Resistance to Paclitxel in Breast Carcinoma Cells Requires a Quality Control of Mitochondrial Antiapoptotic Proteins by TRAP1. Mol. Oncol. 2013, 7, 895–906. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.F.; Chen, Y.; Dai, K.; Chen, P.L.; Riley, D.J.; Lee, W.H. A New Member of the Hsp90 Family of Molecular Chaperones Interacts with the Retinoblastoma Protein during Mitosis and after Heat Shock. Mol. Cell. Biol. 1996, 16, 4691–4699. [Google Scholar] [CrossRef] [PubMed]
- Romanatto, T.; Cesquini, M.; Amaral, M.E.; Roman, E.A.; Moraes, J.C.; Torsoni, M.A.; Cruz-Neto, A.P.; Velloso, L.A. TNF-Alpha Acts in the Hypothalamus Inhibiting Food Intake and Increasing the Respiratory Quotient—Effects on Leptin and Insulin Signaling Pathways. Peptides 2007, 28, 1050–1058. [Google Scholar] [CrossRef] [PubMed]
- McCoy, M.K.; Tansey, M.G. TNF Signaling Inhibition in the CNS: Implications for Normal Brain Function and Neurodegenerative Disease. J. Neuroinflamm. 2008, 5, 48. [Google Scholar] [CrossRef]
- Casadei, N.; Sood, P.; Ulrich, T.; Fallier-Becker, P.; Kieper, N.; Helling, S.; May, C.; Glaab, E.; Chen, J.; Nuber, S.; et al. Mitochondrial Defects and Neurodegeneration in Mice Overexpressing Wild-Type or G399S Mutant HtrA2. Hum. Mol. Genet. 2016, 25, 459–471. [Google Scholar] [CrossRef]
- Strauss, K.M.; Martins, L.M.; Plun-Favreau, H.; Marx, F.P.; Kautzmann, S.; Berg, D.; Gasser, T.; Wszolek, Z.; Müller, T.; Bornemann, A.; et al. Loss of Function Mutations in the Gene Encoding Omi/HtrA2 in Parkinson’s Disease. Hum. Mol. Genet. 2005, 14, 2099–2111. [Google Scholar] [CrossRef]
- Quinn, P.M.J.; Moreira, P.I.; Ambrósio, A.F.; Alves, C.H. PINK1/PARKIN Signalling in Neurodegeneration and Neuroinflammation. Acta Neuropathol. Commun. 2020, 8, 189. [Google Scholar] [CrossRef]
- Larsen, S.B.; Hanss, Z.; Krüger, R. The Genetic Architecture of Mitochondrial Dysfunction in Parkinson’s Disease. Cell Tissue Res. 2018, 373, 21–37. [Google Scholar] [CrossRef]
- Fitzgerald, J.C.; Zimprich, A.; Berrio, D.A.C.; Schindler, K.M.; Maurer, B.; Schulte, C.; Bus, C.; Hauser, A.-K.; Kübler, M.; Lewin, R.; et al. Metformin Reverses TRAP1 Mutation-Associated Alterations in Mitochondrial Function in Parkinson’s Disease. Brain J. Neurol. 2017, 140, 2444–2459. [Google Scholar] [CrossRef]
- Rai, S.N.; Singh, S.S.; Birla, H.; Zahra, W.; Rathore, A.S.; Singh, P.; Singh, S.P. Commentary: Metformin Reverses TRAP1 Mutation-Associated Alterations in Mitochondrial Function in Parkinson’s Disease. Front. Aging Neurosci. 2018, 10, 221. [Google Scholar] [CrossRef]
- Gaare, J.J.; Nido, G.S.; Sztromwasser, P.; Knappskog, P.M.; Dahl, O.; Lund-Johansen, M.; Alves, G.; Tysnes, O.B.; Johansson, S.; Haugarvoll, K.; et al. No Evidence for Rare TRAP1 Mutations Influencing the Risk of Idiopathic Parkinson’s Disease. Brain J. Neurol. 2018, 141, e16. [Google Scholar] [CrossRef]
- Kim, H.; Yang, J.; Kim, M.J.; Choi, S.; Chung, J.-R.; Kim, J.-M.; Yoo, Y.H.; Chung, J.; Koh, H. Tumor Necrosis Factor Receptor-Associated Protein 1 (TRAP1) Mutation and TRAP1 Inhibitor Gamitrinib-Triphenylphosphonium (G-TPP) Induce a Forkhead Box O (FOXO)-Dependent Cell Protective Signal from Mitochondria. J. Biol. Chem. 2016, 291, 1841–1853. [Google Scholar] [CrossRef]
- Baqri, R.M.; Pietron, A.V.; Gokhale, R.H.; Turner, B.A.; Kaguni, L.S.; Shingleton, A.W.; Kunes, S.; Miller, K.E. Mitochondrial Chaperone TRAP1 Activates the Mitochondrial UPR and Extends Healthspan in Drosophila. Mech. Ageing Dev. 2014, 141–142, 35–45. [Google Scholar] [CrossRef][Green Version]
- Zhang, L.; Karsten, P.; Hamm, S.; Pogson, J.H.; Müller-Rischart, A.K.; Exner, N.; Haass, C.; Whitworth, A.J.; Winklhofer, K.F.; Schulz, J.B.; et al. TRAP1 rescues PINK1 loss-of-function phenotypes. Hum. Mol. Genet. 2013, 22, 2829–2841. [Google Scholar] [CrossRef]
- Costa, A.C.; Loh, S.H.Y.; Martins, L.M. Drosophila Trap1 Protects against Mitochondrial Dysfunction in a PINK1/Parkin Model of Parkinson’s Disease. Cell Death Dis. 2013, 4, e467. [Google Scholar] [CrossRef]
- Butler, E.K.; Voigt, A.; Lutz, A.K.; Toegel, J.P.; Gerhardt, E.; Karsten, P.; Falkenburger, B.; Reinartz, A.; Winklhofer, K.F.; Schulz, J.B. The Mitochondrial Chaperone Protein TRAP1 Mitigates α-Synuclein Toxicity. PLoS Genet. 2012, 8, e1002488. [Google Scholar] [CrossRef]
- Lopez-Crisosto, C.; Díaz-Vegas, A.; Castro, P.F.; Rothermel, B.A.; Bravo-Sagua, R.; Lavandero, S. Endoplasmic Reticulum-Mitochondria Coupling Increases during Doxycycline-Induced Mitochondrial Stress in HeLa Cells. Cell Death Dis. 2021, 12, 657. [Google Scholar] [CrossRef]
- Zheng, T.; Zhang, Z. Activated Microglia Facilitate the Transmission of α-Synuclein in Parkinson’s Disease. Neurochem. Int. 2021, 148, 105094. [Google Scholar] [CrossRef]
- Takamura, H.; Koyama, Y.; Matsuzaki, S.; Yamada, K.; Hattori, T.; Miyata, S.; Takemoto, K.; Tohyama, M.; Katayama, T. TRAP1 Controls Mitochondrial Fusion/Fission Balance through Drp1 and Mff Expression. PLoS ONE 2012, 7, e51912. [Google Scholar] [CrossRef]
- Chien, W.L.; Lee, T.R.; Hung, S.Y.; Kang, K.H.; Lee, M.J.; Fu, W.M. Impairment of Oxidative Stress-Induced Heme Oxygenase-1 Expression by the Defect of Parkinson-Related Gene of PINK1. J. Neurochem. 2011, 117, 643–653. [Google Scholar] [CrossRef]
- Shin, D.; Oh, Y.J. Tumor Necrosis Factor-Associated Protein 1 (TRAP1) Is Released from the Mitochondria Following 6-Hydroxydopamine Treatment. Exp. Neurobiol. 2014, 23, 65–76. [Google Scholar] [CrossRef][Green Version]
- Voloboueva, L.A.; Duan, M.; Ouyang, Y.; Emery, J.F.; Stoy, C.; Giffard, R.G. Overexpression of Mitochondrial Hsp70/Hsp75 Protects Astrocytes against Ischemic Injury in Vitro. J. Cereb. Blood Flow Metab. Off. J. Int. Soc. Cereb. Blood Flow Metab. 2008, 28, 1009–1016. [Google Scholar] [CrossRef]
- Xu, L.; Voloboueva, L.A.; Ouyang, Y.B.; Emery, J.F.; Giffard, R.G.; Emery, J.F.; Giffard, R.G. Overexpression of Mitochondrial Hsp70/Hsp75 in Rat Brain Protects Mitochondria, Reduces Oxidative Stress, and Protects from Focal Ischemia. J. Cereb. Blood Flow Metab. 2009, 29, 365–374. [Google Scholar] [CrossRef]
- Li, X.T.; Li, Y.S.; Shi, Z.Y.; Guo, X.L. New Insights into Molecular Chaperone TRAP1 as a Feasible Target for Future Cancer Treatments. Life Sci. 2020, 254, 117737. [Google Scholar] [CrossRef]
- Costantino, E.; Maddalena, F.; Calise, S.; Piscazzi, A.; Tirino, V.; Fersini, A.; Ambrosi, A.; Neri, V.; Esposito, F.; Landriscina, M. TRAP1, a Novel Mitochondrial Chaperone Responsible for Multi-Drug Resistance and Protection from Apoptotis in Human Colorectal Carcinoma Cells. Cancer Lett. 2009, 279, 39–46. [Google Scholar] [CrossRef]
- Plescia, J.; Salz, W.; Xia, F.; Pennati, M.; Zaffaroni, N.; Daidone, M.G.; Meli, M.; Dohi, T.; Fortugno, P.; Nefedova, Y.; et al. Rational Design of Shepherdin, a Novel Anticancer Agent. Cancer Cell 2005, 7, 457–468. [Google Scholar] [CrossRef]
- Hu, S.; Ferraro, M.; Thomas, A.P.; Chung, J.M.; Yoon, N.G.; Seol, J.H.; Kim, S.; Kim, H.U.; An, M.Y.; Ok, H.; et al. Dual Binding to Orthosteric and Allosteric Sites Enhances the Anticancer Activity of a TRAP1-Targeting Drug. J. Med. Chem. 2020, 63, 2930–2940. [Google Scholar] [CrossRef]
- Park, H.K.; Jeong, H.; Ko, E.; Lee, G.; Lee, J.E.H.E.; Lee, S.K.; Lee, A.J.; Im, J.Y.; Hu, S.; Kim, S.H.; et al. Paralog Specificity Determines Subcellular Distribution, Action Mechanism, and Anticancer Activity of TRAP1 Inhibitors. J. Med. Chem. 2017, 60, 7569–7578. [Google Scholar] [CrossRef]
- Kim, D.D.; Kim, S.Y.Y.; Kim, D.D.; Yoon, N.G.; Yun, J.; Hong, K.B.; Lee, C.; Lee, J.H.; Kang, B.H.; Kang, S. Development of Pyrazolo[3, 4-d]Pyrimidine-6-Amine-Based TRAP1 Inhibitors That Demonstrate in Vivo Anticancer Activity in Mouse Xenograft Models. Bioorg. Chem. 2020, 101, 103901. [Google Scholar] [CrossRef]
- Khong, T.; Spencer, A. Targeting HSP 90 Induces Apoptosis and Inhibits Critical Survival and Proliferation Pathways in Multiple Myeloma. Mol. Cancer Ther. 2011, 10, 1909–1917. [Google Scholar] [CrossRef]
- Menezes, D.L.; Taverna, P.; Jensen, M.R.; Abrams, T.; Stuart, D.; Yu, G.K.; Duhl, D.; Machajewski, T.; Sellers, W.R.; Pryer, N.K.; et al. The Novel Oral Hsp90 Inhibitor NVP-HSP990 Exhibits Potent and Broad-Spectrum Antitumor Activities in Vitro and in Vivo. Mol. Cancer Ther. 2012, 11, 730–739. [Google Scholar] [CrossRef] [PubMed]
- Spreafico, A.; Delord, J.P.; De Mattos-Arruda, L.; Berge, Y.; Rodon, J.; Cottura, E.; Bedard, P.L.; Akimov, M.; Lu, H.; Pain, S.; et al. A First-in-Human Phase I, Dose-Escalation, Multicentre Study of HSP990 Administered Orally in Adult Patients with Advanced Solid Malignancies. Br. J. Cancer 2015, 112, 650–659. [Google Scholar] [CrossRef]
- Sanchez-Martin, C.; Menon, D.; Moroni, E.; Ferraro, M.; Masgras, I.; Elsey, J.; Arbiser, J.L.; Colombo, G.; Rasola, A. Honokiol Bis-Dichloroacetate Is a Selective Allosteric Inhibitor of the Mitochondrial Chaperone TRAP1. Antioxid. Redox Signal. 2021, 34, 505–516. [Google Scholar] [CrossRef] [PubMed]
- Hu, L.; Guo, J.; Zhou, L.; Zhu, S.; Wang, C.; Liu, J.; Hu, S.; Yang, M.; Lin, C. Hydrogen Sulfide Protects Retinal Pigment Epithelial Cells from Oxidative Stress-Induced Apoptosis and Affects Autophagy. Oxidative Med. Cell. Longev. 2020, 2020, 8868564. [Google Scholar] [CrossRef] [PubMed]
- Zhu, A.; Ren, Y.; Wang, N.; Jin, Q.; Zhang, D.; Yang, G.; Wang, Q. Adeno-Associated Virus Mediated Gene Transfer of Shepherdin Inhibits Gallbladder Carcinoma Growth in Vitro and in Vivo. Gene 2015, 572, 87–94. [Google Scholar] [CrossRef]
- Xiaojiang, T.; Jinsong, Z.; Jiansheng, W.; Chengen, P.; Guangxiao, Y.; Quanying, W. Adeno-Associated Virus Harboring Fusion Gene NT4-Ant-Shepherdin Induce Cell Death in Human Lung Cancer Cells. Cancer Investig. 2010, 28, 465–471. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, A.; Ishikawa, K.; Inoshita, T.; Shiba-Fukushima, K.; Saiki, S.; Hatano, T.; Mori, A.; Oji, Y.; Okuzumi, A.; Li, Y.; et al. Identifying Therapeutic Agents for Amelioration of Mitochondrial Clearance Disorder in Neurons of Familial Parkinson Disease. Stem Cell Rep. 2020, 14, 1060–1075. [Google Scholar] [CrossRef] [PubMed]
- Zhou, D.; Liu, Y.; Ye, J.; Ying, W.; Ogawa, L.S.; Inoue, T.; Tatsuta, N.; Wada, Y.; Koya, K.; Huang, Q.; et al. A Rat Retinal Damage Model Predicts for Potential Clinical Visual Disturbances Induced by Hsp90 Inhibitors. Toxicol. Appl. Pharmacol. 2013, 273, 401–409. [Google Scholar] [CrossRef]
- Mendes, H.F.; Cheetham, M.E. Pharmacological Manipulation of Gain-of-Function and Dominant-Negative Mechanisms in Rhodopsin Retinitis Pigmentosa. Hum. Mol. Genet. 2008, 17, 3043–3054. [Google Scholar] [CrossRef]
- Ho, S.W.; Tsui, Y.T.C.; Wong, T.T.; Cheung, S.K.K.; Goggins, W.B.; Yi, L.M.; Cheng, K.K.; Baum, L. Effects of 17-Allylamino-17-Demethoxygeldanamycin (17-AAG) in Transgenic Mouse Models of Frontotemporal Lobar Degeneration and Alzheimer’s Disease. Transl. Neurodegener. 2013, 2, 24. [Google Scholar] [CrossRef]
- Waza, M.; Adachi, H.; Katsuno, M.; Minamiyama, M.; Sang, C.; Tanaka, F.; Inukai, A.; Doyu, M. 17-AAG, an Hsp90 Inhibitor, Ameliorates Polyglutamine-Mediated Motor Neuron Degeneration. Nat. Med. 2005, 11, 1088–1095. [Google Scholar] [CrossRef]
- Zare, N.; Khalifeh, S.; Khodagholi, F.; Shahamati, S.Z.; Motamedi, F.; Maghsoudi, N. Geldanamycin Reduces Aβ-Associated Anxiety and Depression, Concurrent with Autophagy Provocation. J. Mol. Neurosci. 2015, 57, 317–324. [Google Scholar] [CrossRef]
- Li, J.; Yang, F.; Guo, J.; Zhang, R.; Xing, X.; Qin, X. 17-AAG Post-Treatment Ameliorates Memory Impairment and Hippocampal CA1 Neuronal Autophagic Death Induced by Transient Global Cerebral Ischemia. Brain Res. 2015, 1610, 80–88. [Google Scholar] [CrossRef]
- Bradley, E.; Zhao, X.; Wang, R.; Brann, D.; Bieberich, E.; Wang, G. Low Dose Hsp90 Inhibitor 17AAG Protects Neural Progenitor Cells from Ischemia Induced Death. J. Cell Commun. Signal. 2014, 8, 353–362. [Google Scholar] [CrossRef]
- Silva-Fernandes, A.; Duarte-Silva, S.; Neves-Carvalho, A.; Amorim, M.; Soares-Cunha, C.; Oliveira, P.; Thirstrup, K.; Teixeira-Castro, A.; Maciel, P. Chronic Treatment with 17-DMAG Improves Balance and Coordination in a New Mouse Model of Machado-Joseph Disease. Neurother. J. Am. Soc. Exp. Neurother. 2014, 11, 433–449. [Google Scholar] [CrossRef]
- Chen, Y.; Wang, B.; Liu, D.; Li, J.J.; Xue, Y.; Sakata, K.; Zhu, L.Q.; Heldt, S.A.; Xu, H.; Liao, F.F. Hsp90 Chaperone Inhibitor 17-AAG Attenuates Aβ-Induced Synaptic Toxicity and Memory Impairment. J. Neurosci. Off. J. Soc. Neurosci. 2014, 34, 2464–2470. [Google Scholar] [CrossRef]
- Fiesel, F.C.; James, E.D.; Hudec, R.; Springer, W. Mitochondrial Targeted HSP90 Inhibitor Gamitrinib-TPP (G-TPP) Induces PINK1/Parkin-Dependent Mitophagy. Oncotarget 2017, 8, 106233–106248. [Google Scholar] [CrossRef]
- Labbadia, J.; Cunliffe, H.; Weiss, A.; Katsyuba, E.; Sathasivam, K.; Seredenina, T.; Woodman, B.; Moussaoui, S.; Frentzel, S.; Luthi-Carter, R.; et al. Altered Chromatin Architecture Underlies Progressive Impairment of the Heat Shock Response in Mouse Models of Huntington Disease. J. Clin. Investig. 2011, 121, 3306–3319. [Google Scholar] [CrossRef]
- Kojima, M.; Hoshimaru, M.; Aoki, T.; Takahashi, J.B.; Ohtsuka, T.; Asahi, M.; Matsuura, N.; Kikuchi, H. Expression of Heat Shock Proteins in the Developing Rat Retina. Neurosci. Lett. 1996, 205, 215–217. [Google Scholar] [CrossRef]
- Dean, D.O.; Tytell, M. Hsp25 and -90 Immunoreactivity in the Normal Rat Eye. Investig. Ophthalmol. Vis. Sci. 2001, 42, 3031–3040. [Google Scholar]
- Aguilà, M.; Bevilacqua, D.; Mcculley, C.; Schwarz, N.; Athanasiou, D.; Kanuga, N.; Novoselov, S.S.; Lange, C.A.K.K.; Ali, R.R.; Bainbridge, J.W.; et al. Hsp90 Inhibition Protects against Inherited Retinal Degeneration. Hum. Mol. Genet. 2014, 23, 2164–2175. [Google Scholar] [CrossRef] [PubMed]



| Drug | Properties | References |
|---|---|---|
| Gamitrinib-(G1-G4) and Gamitrinib-TPP | TRAP1 and HSP90 inhibitors | [62] |
| Shepherdin | TRAP1 and HSP90 inhibitor | [97,98] |
| SMTIN-P01 | TRAP1 inhibitor | [34] |
| SMTIN-C10 | TRAP1 inhibitor | [99] |
| DN401 (compound 4) | Hsp90 paralogs, including TRAP1 | [71,100] |
| Compounds 47 and 48 | TRAP1 and HSP90 inhibitors | [101] |
| NVP-HSP990 | TRAP1 and HSP90 inhibitor | [102,103,104] |
| HDCA | TRAP1 inhibitor | [105] |
| Compound 1 Source: Vitas-M; Cat#STK031415 | TRAP1 inhibitors | [52] |
| Compound 2 Source: Enamine; Cat#Z1128779798 | ||
| Compound 3 Source: National Cancer Institute (NCI); Cat#NSC338501; CAS: 26988-58-9 | ||
| Compound 4 Source: NCI; Cat#NSC668594 | ||
| Compound 5 Source: Ambinter; Cat#AMB9798487 | ||
| Compound 6 Source: Enamine; Cat#Z363507628 | ||
| Compound 7 Source: NCI; Cat#NSC56914; CAS: 6947-27-9 | ||
| Compound 8 Source: Ambinter; Cat#AMB3429185 | ||
| Compound 9 Souce: Vitas-M; Cat#STL380969 | ||
| Compound 10 Source: NCI; Cat#NSC1032; CAS: 5336-09-4 | ||
| Compound 11 Source: NCI; Cat#NSC151831 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ramos Rego, I.; Santos Cruz, B.; Ambrósio, A.F.; Alves, C.H. TRAP1 in Oxidative Stress and Neurodegeneration. Antioxidants 2021, 10, 1829. https://doi.org/10.3390/antiox10111829
Ramos Rego I, Santos Cruz B, Ambrósio AF, Alves CH. TRAP1 in Oxidative Stress and Neurodegeneration. Antioxidants. 2021; 10(11):1829. https://doi.org/10.3390/antiox10111829
Chicago/Turabian StyleRamos Rego, Inês, Beatriz Santos Cruz, António Francisco Ambrósio, and Celso Henrique Alves. 2021. "TRAP1 in Oxidative Stress and Neurodegeneration" Antioxidants 10, no. 11: 1829. https://doi.org/10.3390/antiox10111829
APA StyleRamos Rego, I., Santos Cruz, B., Ambrósio, A. F., & Alves, C. H. (2021). TRAP1 in Oxidative Stress and Neurodegeneration. Antioxidants, 10(11), 1829. https://doi.org/10.3390/antiox10111829








