In Vitro Activities and Inoculum Effects of Ceftazidime-Avibactam and Aztreonam-Avibactam against Carbapenem-Resistant Enterobacterales Isolates from South Korea
Abstract
1. Introduction
2. Materials and Methods
2.1. Bacterial Isolates and Study Design
2.2. Antimicrobial Susceptibility Testing and the Inoculum Effect
2.3. Basis of Resistance and Molecular Identification of β-Lactamase Genes
2.4. Statistical Analysis
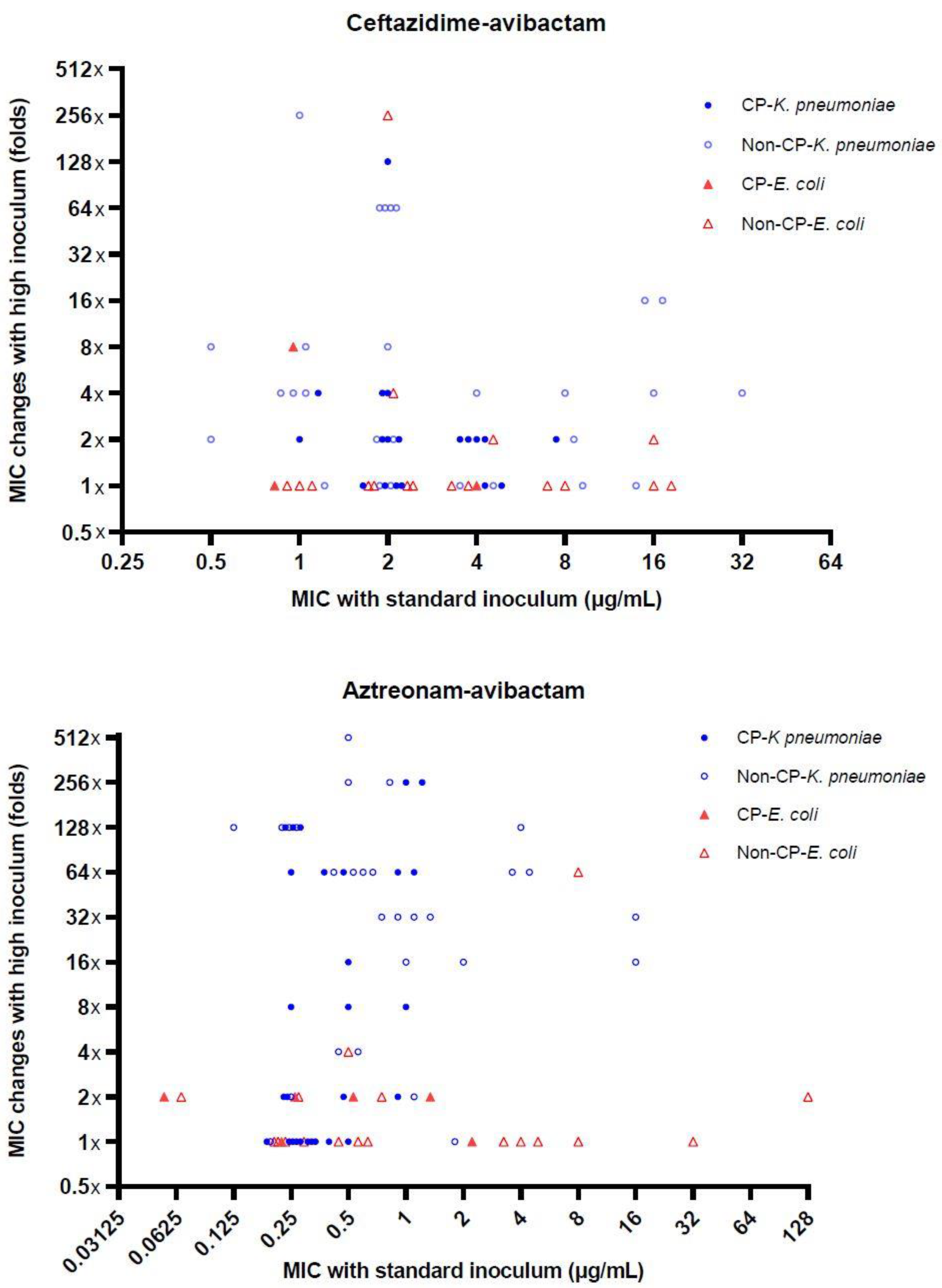
3. Results
4. Discussion
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Logan, L.K.; Weinstein, R.A. The epidemiology of carbapenem-resistant Enterobacteriaceae: The impact and evolution of a global menace. J. Infect. Dis. 2017, 215, S28–S36. [Google Scholar] [CrossRef]
- Akajagbor, D.S.; Wilson, S.L.; Shere-Wolfe, K.D.; Dakum, P.; Charurat, M.E.; Gilliam, B.L. Higher incidence of acute kidney injury with intravenous colistimethate sodium compared with polymyxin B in critically ill patients at a tertiary care medical center. Clin. Infect. Dis. 2013, 57, 1300–1303. [Google Scholar] [CrossRef] [PubMed]
- Ehmann, D.E.; Jahic, H.; Ross, P.L.; Gu, R.F.; Hu, J.; Durand-Réville, T.F.; Lahiri, S.; Thresher, J.; Livchak, S.; Gao, N.; et al. Kinetics of avibactam inhibition against class A, C, and D β-lactamases. J. Biol. Chem. 2013, 288, 27960–27971. [Google Scholar] [CrossRef] [PubMed]
- Sader, H.S.; Castanheira, M.; Shortridge, D.; Mendes, R.E.; Flamm, R.K. Antimicrobial activity of ceftazidime-avibactam tested against multidrug-resistant Enterobacteriaceae and Pseudomonas aeruginosa isolates from U.S. medical centers, 2013 to 2016. Antimicrob. Agents Chemother. 2017, 61. [Google Scholar] [CrossRef] [PubMed]
- Coppi, M.; Di Pilato, V.; Monaco, F.; Giani, T.; Conaldi, P.G.; Rossolini, G.M. Ceftazidime-avibactam resistance associated with increased blaKPC-3 gene copy number mediated by pKpQIL plasmid derivatives in sequence Type 258 Klebsiella pneumoniae. Antimicrob. Agents Chemother. 2020, 64, e01816-19. [Google Scholar] [CrossRef]
- Wu, W.; Feng, Y.; Tang, G.; Qiao, F.; McNally, A.; Zong, Z. NDM metallo-β-lactamases and their bacterial producers in health care settings. Clin. Microbiol. Rev. 2019, 32, e00115-18. [Google Scholar] [CrossRef]
- Marshall, S.; Hujer, A.M.; Rojas, L.J.; Papp-Wallace, K.M.; Humphries, R.M.; Spellberg, B.; Hujer, K.M.; Marshall, E.K.; Rudin, S.D.; Perez, F.; et al. Can ceftazidime-avibactam and aztreonam overcome beta-lactam resistance conferred by metallo-beta-lactamases in Enterobacteriaceae? Antimicrob. Agents Chemother. 2017, 61. [Google Scholar] [CrossRef]
- Lenhard, J.R.; Bulman, Z.P. Inoculum effect of beta-lactam antibiotics. J. Antimicrob. Chemother. 2019, 74, 2825–2843. [Google Scholar] [CrossRef]
- Clinical and Laboratory Standards Institute (CLSI). Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria That Grow Aerobically, 11th ed.; CLSI supplement M07; Wayne, P.A., Ed.; Clinical and Laboratory Standards Institue: Annapolis Junction, MD, USA, 2018. [Google Scholar]
- Sader, H.S.; Flamm, R.K.; Carvalhaes, C.G.; Castanheira, M. Comparison of ceftazidime-avibactam and ceftolozane-tazobactam in vitro activities when tested against gram-negative bacteria isolated from patients hospitalized with pneumonia in United States medical centers (2017–2018). Diagn. Microbiol. Infect. Dis. 2020, 96, 114833. [Google Scholar] [CrossRef]
- Clinical and Laboratory Standards Institute (CLSI). Performance Standards for Antimicrobial Susceptibility Testing, 29th ed.; CLSI Supplement M100; Wayne, P.A., Ed.; Clinical and Laboratory Standards Institue: Annapolis Junction, MD, USA, 2019. [Google Scholar]
- European Committee on Antimicrobial Susceptibility Testing. Breakpoint Tables for Interpretation of MICs and Zone Diameters; Version 9.0.; European Committee on Antimicrobial Susceptibility Testing: Växjö, Sweden, 2019. [Google Scholar]
- Kang, C.I.; Pai, H.; Kim, S.H.; Kim, H.B.; Kim, E.C.; Oh, M.D.; Choe, K.-W. Cefepime and the inoculum effect in tests with Klebsiella pneumoniae producing plasmid-mediated AmpC-type beta-lactamase. J. Antimicrob. Chemother. 2004, 54, 1130–1133. [Google Scholar] [CrossRef]
- Chong, Y.P.; Park, S.J.; Kim, E.S.; Bang, K.M.; Kim, M.N.; Kim, S.H.; Lee, S.-O.; Choi, S.-H.; Jeong, J.-Y.; Woo, J.H.; et al. Prevalence of blaZ gene types and the cefazolin inoculum effect among methicillin-susceptible Staphylococcus aureus blood isolates and their association with multilocus sequence types and clinical outcome. Eur. J. Clin. Microbiol. Infect. Dis. 2015, 34, 349–355. [Google Scholar] [CrossRef] [PubMed]
- Thomson, K.S.; Moland, E.S. Cefepime, piperacillin-tazobactam, and the inoculum effect in tests with extended-spectrum beta-lactamase-producing Enterobacteriaceae. Antimicrob. Agents Chemother. 2001, 45, 3548–3554. [Google Scholar] [CrossRef] [PubMed]
- Pierce, V.M.; Simner, P.J.; Lonsway, D.R.; Roe-Carpenter, D.E.; Johnson, J.K.; Brasso, W.B.; Bobenchik, A.M.; Lockett, Z.C.; Charnot-Katsikas, A.; Ferraro, M.J.; et al. Modified carbapenem inactivation method for phenotypic detection of carbapenemase production among Enterobacteriaceae. J. Clin. Microbiol. 2017, 55, 2321–2333. [Google Scholar] [CrossRef] [PubMed]
- Dallenne, C.; Da Costa, A.; Decre, D.; Favier, C.; Arlet, G. Development of a set of multiplex PCR assays for the detection of genes encoding important beta-lactamases in Enterobacteriaceae. J. Antimicrob. Chemother. 2010, 65, 490–495. [Google Scholar] [CrossRef]
- Du, X.-X.; Wang, J.-F.; Fu, Y.; Zhao, F.; Chen, Y.; Wang, H.-P.; Yu, Y.-S. Genetic characteristics of blaNDM-1-positive plasmid in Citrobacter freundii isolate separated from a clinical infectious patient. J. Med. Microbiol. 2013, 62, 1332–1337. [Google Scholar] [CrossRef] [PubMed]
- Manning, N.; Balabanian, G.; Rose, M.; Landman, D.; Quale, J. Activity of ceftazidime-avibactam against clinical isolates of Klebsiella pneumoniae, including KPC-carrying isolates, endemic to New York City. Microb. Drug Resist. 2018, 24, 35–39. [Google Scholar] [CrossRef]
- Tzelepi, E.; Giakkoupi, P.; Sofianou, D.; Loukova, V.; Kemeroglou, A.; Tsakris, A. Detection of extended-spectrum β-lactamases in clinical isolates of Enterobacter cloacae and Enterobacter aerogenes. J. Clin. Microbiol. 2000, 38, 542–546. [Google Scholar] [CrossRef]
- Paterson, D.L.; Bonomo, R.A. Extended-spectrum beta-lactamases: A clinical update. Clin. Microbiol. Rev. 2005, 18, 657–686. [Google Scholar] [CrossRef]
- Jacoby, G.A. AmpC β-Lactamases. Clin. Microbiol. Rev. 2009, 22, 161–182. [Google Scholar] [CrossRef]
- Tan, T.Y.; Ng, L.S.; He, J.; Koh, T.H.; Hsu, L.Y. Evaluation of screening methods to detect plasmid-mediated AmpC in Escherichia coli, Klebsiella pneumoniae, and Proteus mirabilis. Antimicrob. Agents Chemother. 2009, 53, 146–149. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, F.; Zhao, C.; Wang, Z.; Nichols, W.W.; Testa, R.; Li, H.; Chen, H.; He, W.; Wang, Q.; et al. In vitro activities of ceftazidime-avibactam and aztreonam-avibactam against 372 gram-negative bacilli collected in 2011 and 2012 from 11 teaching hospitals in China. Antimicrob. Agents Chemother. 2014, 58, 1774–1778. [Google Scholar] [CrossRef]
- Alm, R.A.; Johnstone, M.R.; Lahiri, S.D. Characterization of Escherichia coli NDM isolates with decreased susceptibility to aztreonam/avibactam: Role of a novel insertion in PBP3. J. Antimicrob. Chemother. 2015, 70, 1420–1428. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Estabrook, M.; Jacoby, G.A.; Nichols, W.W.; Testa, R.T.; Bush, K. In vitro susceptibility of characterized beta-lactamase-producing strains tested with avibactam combinations. Antimicrob. Agents Chemother. 2015, 59, 1789–1793. [Google Scholar] [CrossRef] [PubMed]
- Papp-Wallace, K.M.; Bajaksouzian, S.; Abdelhamed, A.M.; Foster, A.N.; Winkler, M.L.; Gatta, J.A.; Nichols, W.W.; Testa, R.; Bonomo, R.A.; Jacobs, M.R. Activities of ceftazidime, ceftaroline, and aztreonam alone and combined with avibactam against isogenic Escherichia coli strains expressing selected single beta-lactamases. Diagn. Microbiol. Infect. Dis. 2015, 82, 65–69. [Google Scholar] [CrossRef]
- Yoshizumi, A.; Ishii, Y.; Aoki, K.; Testa, R.; Nichols, W.W.; Tateda, K. In vitro susceptibility of characterized beta-lactamase-producing gram-negative bacteria isolated in Japan to ceftazidime-, ceftaroline-, and aztreonam-avibactam combinations. J. Infect. Chemother. 2015, 21, 148–151. [Google Scholar] [CrossRef] [PubMed]
- Livermore, D.M.; Mushtaq, S.; Warner, M.; Zhang, J.; Maharjan, S.; Doumith, M.; Woodford, N. Activities of NXL104 combinations with ceftazidime and aztreonam against carbapenemase-Producing Enterobacteriaceae. Antimicrob. Agents Chemother. 2011, 55, 390–394. [Google Scholar] [CrossRef] [PubMed]
- Dupont, H.; Gaillot, O.; Goetgheluck, A.S.; Plassart, C.; Emond, J.P.; Lecuru, M.; Gaillard, N.; Derdouri, S.; Lemaire, B.; De Courtilles, M.G.; et al. Molecular characterization of carbapenem-nonsusceptible enterobacterial isolates collected during a prospective interregional survey in France and susceptibility to the novel ceftazidime-avibactam and aztreonam-avibactam combinations. Antimicrob. Agents Chemother. 2016, 60, 215–221. [Google Scholar] [CrossRef]
- Galani, I.; Karaiskos, I.; Karantani, I.; Papoutsaki, V.; Maraki, S.; Papaioannou, V.; Kazila, P.; Tsorlini, H.; Charalampaki, N.; Toutouza, M.; et al. Epidemiology and resistance phenotypes of carbapenemase-producing Klebsiella pneumoniae in Greece, 2014 to 2016. Eurosurveillance 2018, 23, 1700775. [Google Scholar] [CrossRef]
- Suay-Garcia, B.; Perez-Gracia, M.T. Present and future of carbapenem-resistant Enterobacteriaceae (CRE) Infections. Antibiotics 2019, 8, 122. [Google Scholar] [CrossRef]
- Queenan, A.M.; Bush, K. Carbapenemases: The versatile beta-lactamases. Clin. Microbiol. Rev. 2007, 20, 440–458. [Google Scholar] [CrossRef]
- Jayol, A.; Nordmann, P.; Poirel, L.; Dubois, V. Ceftazidime/avibactam alone or in combination with aztreonam against colistin-resistant and carbapenemase-producing Klebsiella pneumoniae. J. Antimicrob. Chemother. 2018, 73, 542–544. [Google Scholar] [CrossRef] [PubMed]
- Sader, H.S.; Mendes, R.E.; Pfaller, M.A.; Shortridge, D.; Flamm, R.K.; Castanheira, M. Antimicrobial activities of aztreonam-avibactam and comparator agents against contemporary (2016) Clinical Enterobacteriaceae Isolates. Antimicrob. Agents Chemother. 2018, 62, e01856-17. [Google Scholar] [CrossRef] [PubMed]
- Zou, H.; Xiong, S.J.; Lin, Q.X.; Wu, M.L.; Niu, S.Q.; Huang, S.F. CP-CRE/non-CP-CRE stratification and CRE resistance mechanism determination help in better managing CRE bacteremia using ceftazidime-avibactam and aztreonam-avibactam. Infect. Drug Resist. 2019, 12, 3017–3027. [Google Scholar] [CrossRef] [PubMed]
- Brook, I. Inoculum effect. Rev. Infect. Dis. 1989, 11, 361–368. [Google Scholar] [CrossRef]
- Harada, Y.; Morinaga, Y.; Kaku, N.; Nakamura, S.; Uno, N.; Hasegawa, H.; Izumikawa, K.; Kohno, S.; Yanagihara, K. In vitro and in vivo activities of piperacillin-tazobactam and meropenem at different inoculum sizes of ESBL-producing Klebsiella pneumoniae. Clin. Microbiol. Infect. 2014, 20, O831–O839. [Google Scholar] [CrossRef]
| Species | Antimicrobial Agent | Inoculum Size | Cumulative% of Isolates with Indicated MICs (μg/mL) | MIC (μg/mL) | S a | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0.06 | 0.125 | 0.25 | 0.5 | 1 | 2 | 4 | 8 | 16 | 32 | 64 | 128 | 256 | ≥512 | MIC50 | MIC90 | ||||
| Non-CP-E. coli (18) | CAZ | Standard | 5.6 | 11.1 | 16.7 | 38.9 | 100 | ≥512 | ≥512 | 5.6 | |||||||||
| High | 5.6 | 11.1 | 100 | ≥512 | ≥512 | 0 | |||||||||||||
| CAZ-AVI | Standard | 16.7 | 50.0 | 66.7 | 77.8 | 94.4 | 100 | 2 | 16 | 77.8 | |||||||||
| High | 16.7 | 38.9 | 50.0 | 72.2 | 83.3 | 88.9 | 100 | 4 | ≥512 | 72.2 | |||||||||
| ATM | Standard | 5.6 | 22.2 | 27.8 | 100 | ≥512 | ≥512 | 0 | |||||||||||
| High | 5.6 | 11.1 | 100 | ≥512 | ≥512 | 0 | |||||||||||||
| ATM-AVI | Standard | 5.6 | 33.3 | 55.6 | 61.1 | 77.8 | 88.9 | 94.4 | 100 | 0.5 | 32 | NA b | |||||||
| High | 5.6 | 27.8 | 50.0 | 61.1 | 77.8 | 83.3 | 88.9 | 94.4 | 100 | 0.5 | 256 | NA | |||||||
| MEM | Standard | 5.6 | 16.7 | 22.2 | 27.8 | 66.7 | 88.9 | 100 | 8 | 32 | 16.7 | ||||||||
| High | 5.6 | 11.1 | 22.2 | 27.8 | 61.1 | 83.3 | 94.4 | 100 | 8 | 32 | 11.1 | ||||||||
| CST | Standard | 55.6 | 94.4 | 100 | 0.25 | 0.5 | 94.4 | ||||||||||||
| TGC | Standard | 11.1 | 38.9 | 66.7 | 83.3 | 88.9 | 94.4 | 100 | 0.5 | 16 | 66.7 | ||||||||
| CP-E. coli (7) | CAZ | Standard | 14.3 | 28.6 | 100 | ≥512 | ≥512 | 0 | |||||||||||
| High | 28.6 | 100 | ≥512 | ≥512 | 0 | ||||||||||||||
| CAZ-AVI | Standard | 28.6 | 42.9 | 100 | ≥512 | ≥512 | 42.9 | ||||||||||||
| High | 14.3 | 28.6 | 42.9 | 100 | ≥512 | ≥512 | 42.9 | ||||||||||||
| ATM | Standard | 14.3 | 28.6 | 42.9 | 57.1 | 100 | 256 | ≥512 | 28.6 | ||||||||||
| High | 14.3 | 28.6 | 42.9 | 100 | ≥512 | ≥512 | 28.6 | ||||||||||||
| ATM-AVI | Standard | 28.6 | 57.1 | 71.4 | 85.7 | 100 | 0.25 | 2 | NA | ||||||||||
| High | 14.3 | 28.6 | 42.9 | 57.1 | 85.7 | 100 | 1 | 32 | NA | ||||||||||
| MEM | Standard | 14.3 | 28.6 | 42.9 | 71.4 | 85.7 | 100 c | 64 | 256 | 0 | |||||||||
| High | 14.3 | 28.6 | 57.1 | 100 c | 32 | ≥256 | 0 | ||||||||||||
| CST | Standard | 14.3 | 100 | 0.5 | 0.5 | 100 | |||||||||||||
| TGC | Standard | 57.1 | 85.7 | 100 | 0.5 | 4 | 85.7 | ||||||||||||
| Non-CP-K. pneumoniae | CAZ | Standard | 3.6 | 7.1 | 10.7 | 14.3 | 32.1 | 100 | ≥512 | ≥512 | 7.1 | ||||||||
| High | 3.6 | 7.1 | 10.7 | 100 | ≥512 | ≥512 | 3.6 | ||||||||||||
| (28) | CAZ-AVI | Standard | 7.1 | 28.6 | 60.7 | 71.4 | 82.1 | 96.4 | 100 | 2 | ≥512 | 82.1 | |||||||
| High | 7.1 | 14.3 | 42.9 | 50.0 | 64.3 | 67.9 | 71.4 | 89.3 | 100 | 8 | ≥512 | 50.0 | |||||||
| ATM | Standard | 10.7 | 14.3 | 17.9 | 100 | ≥512 | ≥512 | 10.7 | |||||||||||
| High | 3.6 | 7.1 | 10.7 | 100 | ≥512 | ≥512 | 3.6 | ||||||||||||
| ATM-AVI | Standard | 3.6 | 21.4 | 50.0 | 75.0 | 82.1 | 92.9 | 100 | 0.5 | 4 | NA | ||||||||
| High | 13.6 | 7.1 | 21.4 | 28.6 | 71.4 | 75.0 | 92.9 | 100 | 32 | 256 | NA | ||||||||
| MEM | Standard | 7.1 | 17.9 | 21.4 | 28.6 | 42.9 | 75.0 | 92.9 | 100 | 16 | 32 | 17.9 | |||||||
| High | 3.6 | 10.7 | 14.3 | 28.6 | 53.6 | 71.4 | 78.6 | 92.9 | 100 c | 16 | 128 | 3.6 | |||||||
| CST | Standard | 14.3 | 64.3 | 67.9 | 75.0 | 85.7 | 96.4 | 100 c | 0.5 | 128 | 64.3 | ||||||||
| TGC | Standard | 7.1 | 42.9 | 64.3 | 89.3 | 96.4 | 100 | 1 | 8 | 7.1 | |||||||||
| CP-K. pneumoniae | CAZ | Standard | 32.1 | 57.1 | 100 | ≥512 | ≥512 | 0 | |||||||||||
| High | 3.6 | 100 | ≥512 | ≥512 | 0 | ||||||||||||||
| (28) | CAZ-AVI | Standard | 7.1 | 42.9 | 64.3 | 67.9 | 100 | 4 | ≥512 | 67.9 | |||||||||
| High | 17.9 | 39.3 | 60.7 | 64.3 | 67.9 | 100 | 8 | ≥512 | 60.7 | ||||||||||
| ATM | Standard | 17.9 | 100 | ≥512 | ≥512 | 0 | |||||||||||||
| High | 3.6 | 100 | ≥512 | ≥512 | 0 | ||||||||||||||
| ATM-AVI | Standard | 53.6 | 78.6 | 100 | 0.25 | 1 | NA | ||||||||||||
| High | 28.6 | 42.9 | 46.4 | 53.6 | 57.1 | 64.3 | 67.9 | 85.7 | 92.9 | 100 | 2 | 64 | NA | ||||||
| MEM | Standard | 3.6 | 7.1 | 10.7 | 25.0 | 53.6 | 64.3 | 100 c | 64 | ≥256 | 0 | ||||||||
| High | 3.6 | 7.1 | 10.7 | 25.0 | 46.4 | 100 c | ≥256 | ≥256 | 0 | ||||||||||
| CST | Standard | 14.3 | 100 | 0.5 | 0.5 | 100 | |||||||||||||
| TGC | Standard | 17.9 | 42.9 | 75.0 | 92.9 | 96.4 | 100 | 4 | 8 | 0 | |||||||||
| Antimicrobial Agent (Resistance Mechanism) | No. of Isolates (%) with Inoculum Effect a | p Value | ||
|---|---|---|---|---|
| Total | E. coli | K. pneumoniae | ||
| Ceftazidime-avibactam b | 12/67 (17.9) | 2/20 (10) | 10/47 (21.3) | 0.27 |
| in CP-CRE | 2/22 (9.1) | 1/3 (33.3) | 1/19 (5.3) | 0.26 |
| in non-CP-CRE | 10/45 (22.2) | 1/17 (5.9) | 9/28 (32.1) | 0.04 |
| Aztreonam-avibactam | 38/81 (46.9) | 2/25 (8.0) | 36/56 (64.3) | <0.001 |
| in CP-CRE | 15/35 (42.9) | 1/7 (14.3) | 14/28 (50) | 0.10 |
| in non-CP-CRE | 23/46 (50) | 1/18 (5.6) | 22/28 (78.6) | <0.001 |
| Mechanism (n) | Antimicrobial Agent | Inoculum Size | Cumulative% with Indicated MICs (μg/mL) | MIC (μg/mL) | S a | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0.06 | 0.125 | 0.25 | 0.5 | 1 | 2 | 4 | 8 | 16 | 32 | 64 | 128 | 256 | ≥512 | MIC50 | MIC90 | ||||
| Non-CP-CRE(46) b | CAZ-AVI | Standard | 4.3 | 23.9 | 56.5 | 69.6 | 80.4 | 95.7 | 97.8 | 100 | 2 | 16 | 80.4 | ||||||
| High | 10.9 | 23.9 | 45.7 | 58.7 | 71.7 | 76.1 | 78.3 | 89.1 | 95.7 | 100 | 8 | 256 | 58.7 | ||||||
| ATM-AVI | Standard | 2.2 | 4.3 | 26.1 | 52.2 | 69.6 | 73.9 | 87.0 | 91.3 | 95.7 | 97.8 | 100 | 0.5 | 8 | NA c | ||||
| High | 2.2 | 13 | 23.9 | 37 | 43.5 | 45.7 | 50 | 78.3 | 80.4 | 93.5 | 100 | 16 | 256 | NA | |||||
| ESBL (30) | CAZ-AVI | Standard | 6.7 | 16.7 | 50.0 | 63.3 | 80.0 | 93.3 | 96.7 | 100 | 2 | 16 | 80.0 | ||||||
| High | 10.0 | 23.3 | 33.3 | 53.3 | 66.7 | 73.3 | 76.7 | 90.0 | 93.3 | 100 | 8 | 128 | 53.3 | ||||||
| ATM-AVI | Standard | 3.3 | 6.7 | 30.0 | 60.0 | 73.3 | 86.7 | 93.3 | 100 | 0.5 | 8 | NA | |||||||
| High | 3.3 | 16.7 | 30 | 33.3 | 40 | 43.3 | 50 | 73.3 | 76.7 | 93.3 | 100 | 16 | 256 | NA | |||||
| AmpC (2) | CAZ-AVI | Standard | 100 | - | - | 100 | |||||||||||||
| High | 50.0 | 100 | - | - | 50.0 | ||||||||||||||
| ATM-AVI | Standard | 50.0 | 100 | - | - | NA | |||||||||||||
| High | 50.0 | ‘ | 100 | - | - | NA | |||||||||||||
| ESBL + AmpC (7) | CAZ-AVI | Standard | 28.6 | 85.7 | 100 | 2 | 4 | 100 | |||||||||||
| High | 14.3 | 85.7 | 100 | 4 | 128 | ||||||||||||||
| ATM-AVI | Standard | 14.3 | 42.9 | 71.4 | 100 | 1 | 2 | NA | |||||||||||
| High | 42.9 | 100 | 32 | 32 | NA | ||||||||||||||
| CP-CRE(35) | CAZ-AVI | Standard | 11.4 | 40.0 | 60.0 | 62.9 | 100 | 4 | ≥512 | 62.9 | |||||||||
| High | 2.9 | 17.1 | 37.1 | 57.1 | 60.0 | 62.9 | 100 | 8 | ≥512 | 57.1 | |||||||||
| ATM-AVI | Standard | 5.7 | 54.3 | 77.1 | 97.1 | 100 | 0.25 | 1 | NA | ||||||||||
| High | 2.9 | 28.6 | 42.9 | 48.6 | 60.0 | 62.9 | 68.6 | 71.4 | 88.6 | 94.3 | 100 | 2 | 64 | NA | |||||
| KPC (17) | CAZ-AVI | Standard | 11.8 | 58.8 | 82.4 | 100 | 2 | ≥512 | 82.4 | ||||||||||
| High | 11.8 | 41.2 | 76.5 | 82.4 | 100 | 8 | ≥512 | 76.5 | |||||||||||
| ATM-AVI | Standard | 5.9 | 58.8 | 82.4 | 100 | 0.25 | 1 | NA | |||||||||||
| High | 35.3 | 47.1 | 58.8 | 64.7 | 70.6 | 94.1 | 100 | 1 | 32 | NA | |||||||||
| NDM (11) | CAZ-AVI | Standard | 9.1 | 18.2 | 27.3 | 100 | ≥512 | ≥512 | 27.3 | ||||||||||
| High | 9.1 | 18.2 | 27.3 | 100 | ≥512 | ≥512 | 18.2 | ||||||||||||
| ATM-AVI | Standard | 9.1 | 54.5 | 63.6 | 90.9 | 100 | 0.25 | 1 | NA | ||||||||||
| High | 9.1 | 36.4 | 54.5 | 81.8 | 100 | 0.5 | 64 | NA | |||||||||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, T.; Lee, S.C.; Bae, M.; Sung, H.; Kim, M.-N.; Jung, J.; Kim, M.J.; Kim, S.-H.; Lee, S.-O.; Choi, S.-H.; et al. In Vitro Activities and Inoculum Effects of Ceftazidime-Avibactam and Aztreonam-Avibactam against Carbapenem-Resistant Enterobacterales Isolates from South Korea. Antibiotics 2020, 9, 912. https://doi.org/10.3390/antibiotics9120912
Kim T, Lee SC, Bae M, Sung H, Kim M-N, Jung J, Kim MJ, Kim S-H, Lee S-O, Choi S-H, et al. In Vitro Activities and Inoculum Effects of Ceftazidime-Avibactam and Aztreonam-Avibactam against Carbapenem-Resistant Enterobacterales Isolates from South Korea. Antibiotics. 2020; 9(12):912. https://doi.org/10.3390/antibiotics9120912
Chicago/Turabian StyleKim, Taeeun, Seung Cheol Lee, Moonsuk Bae, Heungsup Sung, Mi-Na Kim, Jiwon Jung, Min Jae Kim, Sung-Han Kim, Sang-Oh Lee, Sang-Ho Choi, and et al. 2020. "In Vitro Activities and Inoculum Effects of Ceftazidime-Avibactam and Aztreonam-Avibactam against Carbapenem-Resistant Enterobacterales Isolates from South Korea" Antibiotics 9, no. 12: 912. https://doi.org/10.3390/antibiotics9120912
APA StyleKim, T., Lee, S. C., Bae, M., Sung, H., Kim, M.-N., Jung, J., Kim, M. J., Kim, S.-H., Lee, S.-O., Choi, S.-H., Kim, Y. S., & Chong, Y. P. (2020). In Vitro Activities and Inoculum Effects of Ceftazidime-Avibactam and Aztreonam-Avibactam against Carbapenem-Resistant Enterobacterales Isolates from South Korea. Antibiotics, 9(12), 912. https://doi.org/10.3390/antibiotics9120912





