Evaluating Bezlotoxumab-Fidaxomicin Combination Therapy in Clostridioides Infection: A Single-Center Retrospective Study from Aichi Prefecture, Japan
Abstract
1. Introduction
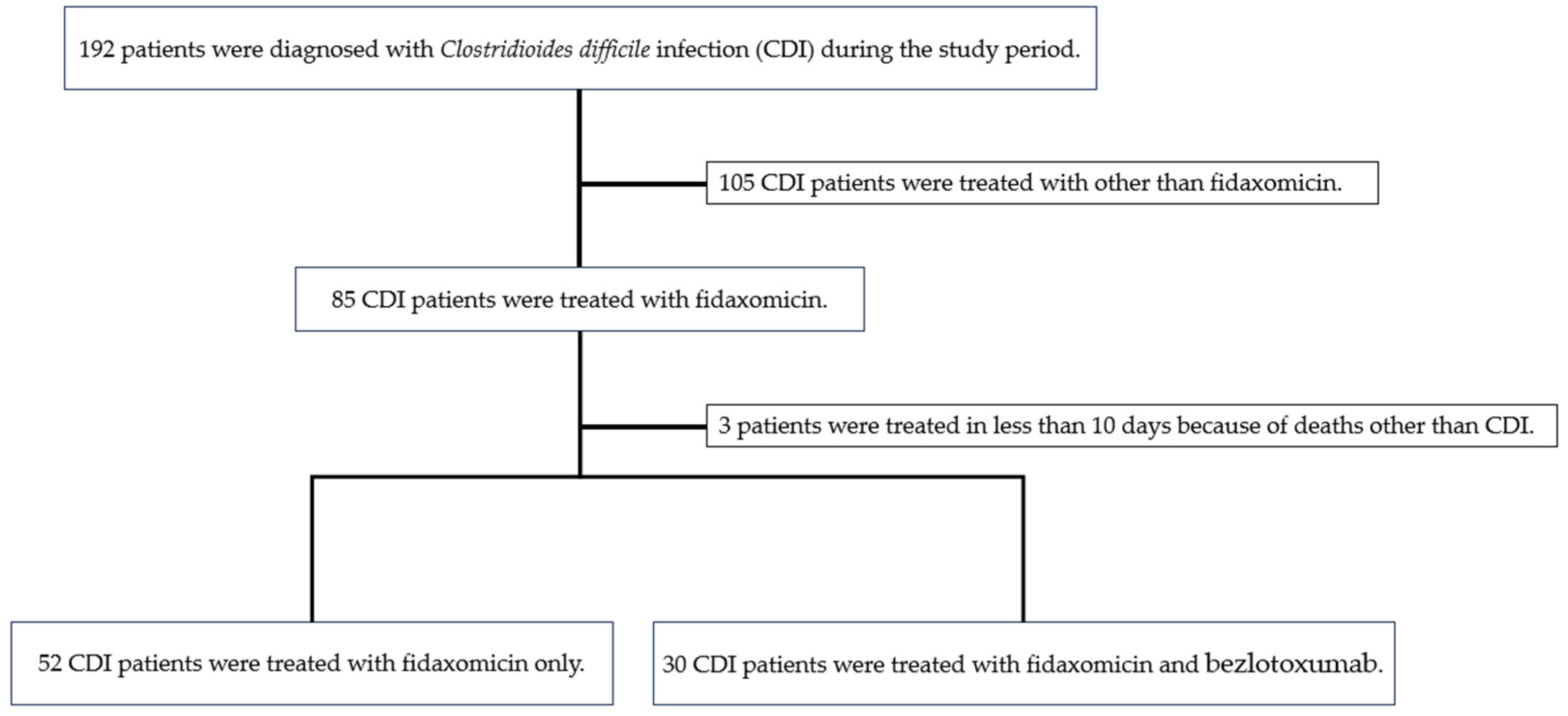
2. Results
3. Discussion
4. Materials and Methods
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lessa, F.C.; Mu, Y.; Bamberg, W.M.; Beldavs, Z.G.; Dumyati, G.K.; Dunn, J.R.; Farley, M.M.; Holzbauer, S.M.; Meek, J.I.; Phipps, E.C.; et al. Burden of Clostridium difficile infection in the United States. N. Engl. J. Med. 2015, 372, 825–834. [Google Scholar] [CrossRef] [PubMed]
- Salvati, F.; Catania, F.; Murri, R.; Fantoni, M.; Torti, C. Clostridioides difficile infection: An update. Infez. Med. 2024, 32, 280–291. [Google Scholar] [PubMed]
- Bouza, E.; Cobo, J.; Rodríguez-Hernández, M.J.; Salavert, M.; Horcajada, J.P.; Iribarren, J.A.; Obi, E.; Lozano, V.; Maratia, S.; Cuesta, M.; et al. Economic burden of recurrent Clostridioides difficile infection in adults admitted to Spanish hospitals. A multicentre retrospective observational study. Rev. Esp. Quimioter. 2021, 34, 126–135. [Google Scholar] [CrossRef] [PubMed]
- Mullish, B.H.; Williams, H.R. Clostridium difficile infection and antibiotic-associated diarrhoea. Clin. Med. 2018, 18, 237–241. [Google Scholar] [CrossRef]
- Wilcox, M.H.; Gerding, D.N.; Poxton, I.R.; Kelly, C.; Nathan, R.; Birch, T.; Cornely, O.A.; Rahav, G.; Bouza, E.; Lee, C.; et al. Bezlotoxumab for Prevention of Recurrent Clostridium difficile Infection. N. Engl. J. Med. 2017, 376, 305–317. [Google Scholar] [CrossRef]
- Louie, T.J.; Miller, M.A.; Mullane, K.M.; Weiss, K.; Lentnek, A.; Golan, Y.; Gorbach, S.; Sears, P.; Shue, Y.K.; OPT-80-003 Clinical Study Group. Fidaxomicin versus vancomycin for Clostridium difficile infection. N. Engl. J. Med. 2011, 364, 422–431. [Google Scholar] [CrossRef]
- Gerding, D.N.; Kelly, C.P.; Rahav, G.; Lee, C.; Dubberke, E.R.; Kumar, P.N.; Yacyshyn, B.; Kao, D.; Eves, K.; Ellison, M.C.; et al. Bezlotoxumab for prevention of recurrent Clostridium difficile infection in patients at increased risk for recurrence. Clin. Infect. Dis. 2018, 67, 649–656. [Google Scholar] [CrossRef]
- Mikamo, H.; Aoyama, N.; Sawata, M.; Fujimoto, G.; Dorr, M.B.; Yoshinari, T. The effect of bezlotoxumab for prevention of recurrent Clostridium difficile infection (CDI) in Japanese patients. J. Infect. Chemother. 2018, 24, 123–129. [Google Scholar] [CrossRef]
- Cornely, O.A.; Crook, D.W.; Esposito, R.; Poirier, A.; Somero, M.S.; Weiss, K.; Sears, P.; Gorbach, S.; OPT-80-004 Clinical Study Group. Fidaxomicin versus vancomycin for infection with Clostridium difficile in Europe, Canada, and the USA: A double-blind, non-inferiority, randomised controlled trial. Lancet Infect. Dis. 2012, 12, 281–289. [Google Scholar] [CrossRef]
- Okumura, H.; Fukushima, A.; Taieb, V.; Shoji, S.; English, M. Fidaxomicin compared with vancomycin and metronidazole for the treatment of Clostridioides (Clostridium) difficile infection: A network meta-analysis. J. Infect. Chemother. 2020, 26, 43–50. [Google Scholar] [CrossRef]
- Giacobbe, D.R.; Vena, A.; Falcone, M.; Menichetti, F.; Bassetti, M. Fidaxomicin for the treatment of Clostridioides difficile infection in adult patients: An update on results from randomized controlled trials. Antibiotics 2022, 11, 1365. [Google Scholar] [CrossRef] [PubMed]
- van Prehn, J.; Reigadas, E.; Vogelzang, E.H.; Bouza, E.; Hristea, A.; Guery, B.; Krutova, M.; Norén, T.; Allerberger, F.; Coia, J.E.; et al. European Society of Clinical Microbiology and Infectious Diseases: 2021 update on the treatment guidance document for Clostridioides difficile infection in adults. Clin. Microbiol. Infect. 2021, 27 (Suppl. S2), S1–S21. [Google Scholar] [CrossRef] [PubMed]
- Hengel, R.L.; Ritter, T.E.; Nathan, R.V.; Van Anglen, L.J.; Schroeder, C.P.; Dillon, R.J.; Marcella, S.W.; Garey, K.W. Real-world experience of bezlotoxumab for prevention of Clostridioides difficile infection: A retrospective multicenter cohort study. Open Forum Infect. Dis. 2020, 7, ofaa097. [Google Scholar] [CrossRef] [PubMed]
- Dubberke, E.R.; Gerding, D.N.; Kelly, C.P.; Garey, K.W.; Rahav, G.; Mosley, A.; Tipping, R.; Dorr, M.B. Efficacy of bezlotoxumab in participants receiving metronidazole, vancomycin, or fidaxomicin for treatment of Clostridioides (Clostridium) difficile infection. Open Forum Infect. Dis. 2020, 7, ofaa157. [Google Scholar] [CrossRef]
- Johnson, T.M.; Molina, K.C.; Howard, A.H.; Schwarz, K.; Allen, L.; Huang, M.; Bajrovic, V.; Miller, M.A. Real-world comparison of bezlotoxumab to standard of care therapy for prevention of recurrent Clostridioides difficile infection in patients at high risk for recurrence. Clin. Infect. Dis. 2022, 74, 1572–1578. [Google Scholar] [CrossRef]
- Polivkova, S.; Krutova, M.; Capek, V.; Sykorova, B.; Benes, J. Fidaxomicin versus metronidazole, vancomycin and their combination for initial episode, first recurrence and severe Clostridioides difficile infection—An observational cohort study. Int. J. Infect. Dis. 2021, 103, 226–233. [Google Scholar] [CrossRef]
- Di Bella, S.; Sanson, G.; Monticelli, J.; Zerbato, V.; Principe, L.; Giuffrè, M.; Pipitone, G.; Luzzati, R. Clostridioides difficile infection: History, epidemiology, risk factors, prevention, clinical manifestations, treatment, and future options. Clin. Microbiol. Rev. 2024, 37, e0013523. [Google Scholar] [CrossRef]
- Pettit, N.N.; Lew, A.K.; Nguyen, C.T.; Bell, E.; Lehmann, C.J.; Pisano, J. Fidaxomicin versus oral vancomycin for Clostridioides difficile infection among patients at high risk for recurrence based on real-world experience. Infect. Control Hosp. Epidemiol. 2024, 45, 1286–1292. [Google Scholar] [CrossRef]
- Mori, N.; Hirai, J.; Ohashi, W.; Asai, N.; Shibata, Y.; Mikamo, H. Clinical efficacy of fidaxomicin and oral metronidazole for treating Clostridioides difficile infection and the associated recurrence rate: A retrospective cohort study. Antibiotics 2023, 12, 1323. [Google Scholar] [CrossRef]
- Czepiel, J.; Dróżdż, M.; Pituch, H.; Kuijper, E.J.; Perucki, W.; Mielimonka, A.; Goldman, S.; Wultańska, D.; Garlicki, A.; Biesiada, G. Clostridium difficile infection: Review. Eur. J. Clin. Microbiol. Infect. Dis. 2019, 38, 1211–1221. [Google Scholar] [CrossRef]
- Johnson, S.; Gerding, D.N. Bezlotoxumab. Clin. Infect. Dis. 2019, 68, 699–704. [Google Scholar] [CrossRef]
- Kunishima, H.; Ichiki, K.; Ohge, H.; Sakamoto, F.; Sato, Y.; Suzuki, H.; Nakamura, A.; Fujimura, S.; Matsumoto, K.; Mikamo, H.; et al. Japanese Society for infection prevention and control guide to Clostridioides difficile infection prevention and control. J. Infect. Chemother. 2024, 30, 673–715. [Google Scholar] [CrossRef] [PubMed]
- Johnson, S.; Lavergne, V.; Skinner, A.M.; Gonzales-Luna, A.J.; Garey, K.W.; Kelly, C.P.; Wilcox, M.H. Clinical practice guideline by the Infectious Diseases Society of America (IDSA) and Society for Healthcare Epidemiology of America (SHEA): 2021 focused update guidelines on management of Clostridioides difficile infection in adults. Clin. Infect. Dis. 2021, 73, e1029–e1044. [Google Scholar] [CrossRef] [PubMed]
- Carter, G.P.; Rood, J.I.; Lyras, D. The role of toxin A and toxin B in Clostridium difficile-associated disease: Past and present perspectives: Past and present perspectives. Gut Microbes 2010, 1, 58–64. [Google Scholar] [CrossRef] [PubMed]
- Ooijevaar, R.E.; van Beurden, Y.H.; Terveer, E.M.; Goorhuis, A.; Bauer, M.P.; Keller, J.J.; Mulder, C.J.J.; Kuijper, E.J. Update of treatment algorithms for Clostridium difficile infection. Clin. Microbiol. Infect. 2018, 24, 452–462. [Google Scholar] [CrossRef]
- Kelly, C.R.; Fischer, M.; Allegretti, J.R.; LaPlante, K.; Stewart, D.B.; Limketkai, B.N.; Stollman, N.H. ACG clinical guidelines: Prevention, diagnosis, and treatment of Clostridioides difficile infections. Am. J. Gastroenterol. 2021, 116, 1124–1147. [Google Scholar] [CrossRef]
- Kachrimanidou, M.; Tsintarakis, E. Insights into the Role of Human Gut Microbiota in Clostridioides difficile Infection. Microorganisms 2020, 8, 200. [Google Scholar] [CrossRef]
- Schubert, A.M.; Rogers, M.A.M.; Ring, C.; Mogle, J.; Petrosino, J.P.; Young, V.B.; Aronoff, D.M.; Schloss, P.D. Microbiome data distinguish patients with Clostridium difficile infection and non-C. difficile-associated diarrhea from healthy controls. MBio 2014, 5, e01021-14. [Google Scholar] [CrossRef]
- Babakhani, F.; Bouillaut, L.; Gomez, A.; Sears, P.; Nguyen, L.; Sonenshein, A.L. Fidaxomicin inhibits spore production in Clostridium difficile. Clin. Infect. Dis. 2012, 55 (Suppl. S2), S162–S169. [Google Scholar] [CrossRef]
- Pillai, A.; Nelson, R. Probiotics for treatment of Clostridium difficile-associated colitis in adults. Cochrane Database Syst. Rev. 2008, CD004611. [Google Scholar] [CrossRef]
- Wullt, M.; Hagslätt, M.-L.J.; Odenholt, I. Lactobacillus plantarum 299v for the treatment of recurrent Clostridium difficile-associated diarrhoea: A double-blind, placebo-controlled trial. Scand. J. Infect. Dis. 2003, 35, 365–367. [Google Scholar] [CrossRef] [PubMed]
- McFarland, L.V. Meta-analysis of probiotics for the prevention of antibiotic associated diarrhea and the treatment of Clostridium difficile disease. Am. J. Gastroenterol. 2006, 101, 812–822. [Google Scholar] [CrossRef] [PubMed]
- Sartelli, M.; Di Bella, S.; McFarland, L.V.; Khanna, S.; Furuya-Kanamori, L.; Abuzeid, N.; Abu-Zidan, F.M.; Ansaloni, L.; Augustin, G.; Bala, M.; et al. 2019 update of the WSES guidelines for management of Clostridioides (Clostridium) difficile infection in surgical patients. World J. Emerg. Surg. 2019, 14, 8. [Google Scholar] [CrossRef]
- Abdelfatah, M.; Nayfe, R.; Nijim, A.; Enriquez, K.; Ali, E.; Watkins, R.R.; Kandil, H. Factors predicting recurrence of Clostridium difficile infection (CDI) in hospitalized patients: Retrospective study of more than 2000 patients: Retrospective study of more than 2000 patients. J. Investig. Med. 2015, 63, 747–751. [Google Scholar] [CrossRef] [PubMed]
- Senoh, M.; Kato, H. Molecular epidemiology of endemic Clostridioides difficile infection in Japan. Anaerobe 2022, 74, 102510. [Google Scholar] [CrossRef]
- Kunishima, H.; Ohge, H.; Suzuki, H.; Nakamura, A.; Matsumoto, K.; Mikamo, H.; Mori, N.; Morinaga, Y.; Yanagihara, K.; Yamagishi, Y.; et al. Japanese Clinical Practice Guidelines for Management of Clostridioides (Clostridium) difficile infection. J. Infect. Chemother. 2022, 28, 1045–1083. [Google Scholar] [CrossRef]
- Hu, M.Y.; Katchar, K.; Kyne, L.; Maroo, S.; Tummala, S.; Dreisbach, V.; Xu, H.; Leffler, D.A.; Kelly, C.P. Prospective derivation and validation of a clinical prediction rule for recurrent Clostridium difficile infection. Gastroenterology 2009, 136, 1206–1214. [Google Scholar] [CrossRef]
- Drekonja, D.M.; Amundson, W.H.; Decarolis, D.D.; Kuskowski, M.A.; Lederle, F.A.; Johnson, J.R. Antimicrobial use and risk for recurrent Clostridium difficile infection. Am. J. Med. 2011, 124, e1–e7. [Google Scholar] [CrossRef]
- Abou Chakra, C.N.; Pepin, J.; Sirard, S.; Valiquette, L. Risk factors for recurrence, complications and mortality in Clostridium difficile infection: A systematic review. PLoS ONE 2014, 9, e98400. [Google Scholar] [CrossRef]
- Deshpande, A.; Pasupuleti, V.; Thota, P.; Pant, C.; Rolston, D.D.K.; Hernandez, A.V.; Donskey, C.J.; Fraser, T.G. Risk factors for recurrent Clostridium difficile infection: A systematic review and meta-analysis. Infect. Control Hosp. Epidemiol. 2015, 36, 452–460. [Google Scholar] [CrossRef]
- van Rossen, T.M.; Ooijevaar, R.E.; Vandenbroucke-Grauls, C.M.J.E.; Dekkers, O.M.; Kuijper, E.J.; Keller, J.J.; van Prehn, J. Prognostic factors for severe and recurrent Clostridioides difficile infection: A systematic review. Clin. Microbiol. Infect. 2022, 28, 321–331. [Google Scholar] [CrossRef] [PubMed]
- Tariq, R.; Singh, S.; Gupta, A.; Pardi, D.S.; Khanna, S. Association of gastric acid suppression with recurrent Clostridium difficile infection: A systematic review and meta-analysis. JAMA Intern. Med. 2017, 177, 784–791. [Google Scholar] [CrossRef] [PubMed]
- Riley, T.V.; Kimura, T. The epidemiology of Clostridium difficile infection in Japan: A systematic review. Infect. Dis. Ther. 2018, 7, 39–70. [Google Scholar] [CrossRef] [PubMed]
- Mikamo, H.; Kondo, T.; Okuyama, K.; Marcella, S.W.; Ruzicka, D.J. Incidence of and risk factors for recurrent Clostridioidesdifficile infection in Japan using a claims database: A retrospective cohort study. Anaerobe 2020, 61, 102139. [Google Scholar] [CrossRef]
- Larrainzar-Coghen, T.; Rodriguez-Pardo, D.; Puig-Asensio, M.; Rodríguez, V.; Ferrer, C.; Bartolomé, R.; Pigrau, C.; Fernández-Hidalgo, N.; Pumarola, T.; Almirante, B. First recurrence of Clostridium difficile infection: Clinical relevance, risk factors, and prognosis. Eur. J. Clin. Microbiol. Infect. Dis. 2016, 35, 371–378. [Google Scholar] [CrossRef]
- Razik, R.; Rumman, A.; Bahreini, Z.; McGeer, A.; Nguyen, G.C. Recurrence of Clostridium difficile infection in patients with inflammatory bowel disease: The RECIDIVISM study. Am. J. Gastroenterol. 2016, 111, 1141–1146. [Google Scholar] [CrossRef]
- Appaneal, H.J.; Caffrey, A.R.; LaPlante, K.L. What is the role for metronidazole in the treatment of Clostridium difficile infection? Results from a national cohort study of Veterans with initial mild disease. Clin. Infect. Dis. 2019, 69, 1288–1295. [Google Scholar] [CrossRef]
- Asaoka, M.; Horita, Y.; Wachino, C.; Kondo, S.; Hotta, Y.; Kataoka, T.; Sanagawa, A.; Hayakawa, T.; Nakamura, A.; Kimura, K. Clinical usefulness of the ‘MN criteria’—The Clostridioides difficile infection severity scoring system—In the Japanese setting. Intern. Med. 2023, 62, 59–67. [Google Scholar] [CrossRef]
| Variables | FDX (n = 52) | FDX + BEZ (n = 30) | p Value |
|---|---|---|---|
| Age (years), median (IQR) | 73 (32–93) | 77 (30–90) | 0.1560 |
| Age ≥ 65 years, no. (%) | 34 (65.4) | 25 (83.3) | 0.0810 |
| Age ≥ 75 years, no. (%) | 25 (48.1) | 18 (60.0) | 0.2980 |
| Age ≥ 80 years, no. (%) | 13 (25.0) | 13 (43.3) | 0.0860 |
| Female sex, no. (%) | 25 (48.1) | 17 (56.7) | 0.4540 |
| Body mass index < 18.5, no. (%) | 22 (42.3) | 12 (40.0) | 0.8380 |
| Past medical history of CDI, no. (%) | 4 (7.7) | 3 (10.0) | 0.7190 |
| Past hospitalization within 3 months before onset of CDI, no. (%) | 24 (46.2) | 9 (30.0) | 0.1510 |
| Past hospitalization < 1 year, no. (%) | 38 (73.1) | 15 (50.0) | 0.0350 |
| Temperature > 37.8 °C at diagnosis of CDI, no. (%) | 23 (44.2) | 14 (46.7) | 0.8310 |
| Abdominal pain at diagnosis of CDI, no. (%) | 23 (44.2) | 16 (53.3) | 0.4270 |
| Number of diarrhea per day > 5 at diagnosis of CDI, no. (%) | 22 (42.3) | 17 (56.7) | 0.2100 |
| Bloody stool, no. (%) | 5 (9.6) | 2 (6.7) | 0.6450 |
| Fasting, no. (%) | 7 (13.5) | 2 (6.7) | 0.3430 |
| Enteral feeding, no. (%) | 5 (9.6) | 7 (23.3) | 0.0900 |
| Hospitalization for more than 1 month before onset of CDI, no. (%) | 16 (30.8) | 11 (36.7) | 0.5840 |
| Use of antimicrobials for at least 3 days after CDI diagnosis, no. (%) | 23 (44.2) | 11 (36.7) | 0.5030 |
| Comorbidities, no. (%) | |||
| Diabetes mellitus | 19 (36.5) | 11 (36.7) | 0.9910 |
| Chronic kidney disease | 19 (36.5) | 23 (76.7) | <0.01 |
| Hemodialysis | 10 (19.2) | 3 (10.0) | 0.2700 |
| Heart failure/ischemic heart disease | 12 (23.1) | 9 (30.0) | 0.4890 |
| Chronic liver disease | 2 (3.8) | 0 (0) | 0.2770 |
| Chronic obstructive pulmonary disease | 4 (7.7) | 2 (6.7) | 0.8640 |
| Cerebrovascular disease | 6 (11.5) | 5 (16.7) | 0.5120 |
| Inflammatory bowel disease | 4 (7.7) | 1 (3.3) | 0.4270 |
| Solid malignancy | 16 (30.8) | 9 (30.0) | 0.9420 |
| Hematologic malignancy | 3 (5.8) | 3 (10.0) | 0.4790 |
| History of abdominal surgery, no. (%) | 15 (28.8) | 4 (13.3) | 0.1090 |
| Community onset CDI, no. (%) | 7 (13.5) | 2 (6.7) | 0.3430 |
| Intensive care unit at diagnosis of CDI, no. (%) | 3 (5.8) | 2 (6.7) | 0.8700 |
| Any severe CDI, no. (%) | |||
| IDSA/SHEA criteria severe | 24 (46.2) | 23 (76.7) | <0.01 |
| MN criteria mild | 5 (9.6) | 0 (0) | 0.0800 |
| MN criteria moderate | 35 (67.3) | 13 (43.3) | 0.0340 |
| MN criteria severe | 10 (19.2) | 15 (50.0) | <0.01 |
| MN criteria super severe | 2 (3.8) | 2 (6.7) | 0.5680 |
| Laboratory data | |||
| White blood cell count > 15,000/mm3, no. (%) | 11 (21.2) | 8 (26.7) | 0.5690 |
| Albumin < 3, no. (%) | 37 (71.2) | 25 (83.3) | 0.2160 |
| Creatinine > 1.5 mg/dL, no. (%) | 14 (26.9) | 20 (66.7) | <0.01 |
| C reactive protein ≥ 10 mg/dL, no. (%) | 11 (21.2) | 9 (30.0) | 0.3690 |
| C reactive protein ≥ 15 mg/dL, no. (%) | 7 (13.5) | 5 (16.7) | 0.6920 |
| Antibiotics administration at CDI onset, no. (%) | |||
| Penicillin | 2 (3.8) | 3 (10.0) | 0.2620 |
| Cephalosporin | 27 (51.9) | 16 (53.3) | 0.9020 |
| Carbapenem | 12 (23.1) | 5 (16.7) | 0.4904 |
| Fluoroquinolone | 8 (15.4) | 1 (3.3) | 0.0930 |
| Clindamycin | 0 (0) | 0 (0) | - |
| β-lactam/β-lactamase inhibitor | 20 (38.5) | 8 (26.7) | 0.2780 |
| Anti-viral agents | 0 (0) | 1 (3.3) | 0.1853 |
| Anti-fungal agents | 0 (0) | 2 (6.7) | 0.0594 |
| None | 10 (19.2) | 9 (30.0) | 0.2660 |
| Concomitant medication use, no. (%) | |||
| PPIs | 17 (32.7) | 15 (50.0) | 0.1220 |
| H2RAs | 2 (3.8) | 4 (13.3) | 0.1120 |
| P-CAB | 16 (30.8) | 4 (13.3) | 0.0770 |
| Steroid | 12 (23.1) | 7 (23.3) | 0.9790 |
| Immunosuppressants other than steroids | 15 (28.8) | 10 (33.3) | 0.6707 |
| Anti-cancer chemotherapy | 7 (13.5) | 3 (10.0) | 0.6450 |
| Risk factors for recurrence of CDI | |||
| Age ≥ 65 years, no. (%) | 34 (65.4) | 25 (83.3) | 0.08 |
| Use of antimicrobials for at least 3 days after CDI diagnosis, no. (%) | 23 (44.2) | 11 (36.7) | 0.50 |
| Chronic kidney disease, no. (%) | 19 (36.5) | 23 (76.7) | <0.01 |
| Past medical history of CDI, no. (%) | 4 (7.7) | 3 (10.0) | 0.71 |
| Use of proton pump inhibitors, no. (%) | 17 (32.7) | 15 (50.0) | 0.12 |
| Use of H2 blocker, no. (%) | 2 (3.8) | 4 (13.3) | 0.11 |
| Use of potassium-competitive acid blocker, no. (%) | 16 (30.8) | 4 (13.3) | 0.07 |
| Past hospitalization within 3 months before onset of CDI, no. (%) | 24 (46.2) | 9 (30.0) | 0.15 |
| Use of fluoroquinolone within one month of onset of CDI, no. (%) | 8 (15.4) | 1 (3.3) | 0.09 |
| Use of carbapenem within one month of onset of CDI, no. (%) | 12 (23.1) | 5 (16.7) | 0.49 |
| Solid malignancy, no. (%) | 16 (30.8) | 9 (30.0) | 0.94 |
| Hematologic cancer, no. (%) | 3 (5.8) | 3 (10.0) | 0.48 |
| ICU at diagnosis of CDI, no. (%) | 3 (5.8) | 2 (6.7) | 0.87 |
| History of cephalosporin use prior to CDI diagnosis, no. (%) | 27 (51.9) | 16 (53.3) | 0.90 |
| History of abdominal surgery, no. (%) | 15 (28.8) | 4 (13.3) | 0.10 |
| Using steroid, no. (%) | 12 (23.1) | 7 (23.3) | 0.98 |
| Enteral feeding, no. (%) | 5 (9.6) | 7 (23.3) | 0.09 |
| Inflammatory bowel disease, no. (%) | 4 (7.7) | 1 (3.3) | 0.43 |
| Severe CDI by IDSA/SHEA criteria, no. (%) | 24 (46.2) | 23 (76.7) | <0.01 |
| Severe and super severe CDI by MN criteria, no. (%) | 12 (23.1) | 17 (56.7) | <0.01 |
| Number of risk factors of CDI recurrence, median (range) | 5 (2–11) | 6 (3–10) | 0.2720 |
| Probiotics before CDI diagnosis, no. (%) | 15 (28.8) | 14 (46.7) | 0.1040 |
| Probiotics after CDI diagnosis, no. (%) | 36 (69.2) | 23 (76.7) | 0.4700 |
| Global cure | 34 (65.4) | 26 (86.7) | <0.05 |
| Clinical cure | 36 (69.2) | 27 (90.0) | <0.05 |
| Recurrence | 6 (11.5) | 1 (3.3) | 0.2000 |
| Adverse effect, no. (%) | 0 (0) | 0 (0) | - |
| All-cause 30-day mortality after CDI treatment | 2 (3.8) | 0 (0) | 0.2770 |
| Variable | Clinical Cure (n = 63) | Clinical Failure (n = 19) | p Value | Adjusted Odds Ratio (95% CI) | p Value |
|---|---|---|---|---|---|
| Chronic kidney disease | 35 (55.6%) | 7 (36.8%) | 0.121 | ||
| Combination with BEZ | 27 (42.9%) | 3 (15.8%) | 0.027 | 4.167 (1.029–16.885) | 0.046 |
| Probiotics after CDI diagnosis | 49 (77.8%) | 10 (52.6%) | 0.035 | 3.403 (1.045–11.079) | 0.042 |
| Steroid use | 11 (17.5%) | 8 (42.1%) | 0.031 | 0.236 (0.068–0.815) | 0.022 |
| Variables | FDX (n = 24) | FDX + BEZ (n = 23) | p Value |
|---|---|---|---|
| Age (years), median (IQR) | 76 (44–86) | 76 (36–90) | 0.6480 |
| Age ≥ 65 years, no. (%) | 18 (75.0) | 19 (82.6) | 0.5240 |
| Age ≥ 75 years, no. (%) | 14 (58.3) | 13 (56.5) | 0.9000 |
| Age ≥ 80 years, no. (%) | 7 (29.1) | 9 (39.1) | 0.4710 |
| Female sex, no. (%) | 8 (33.3) | 11 (47.8) | 0.3120 |
| Body mass index < 18.5, no. (%) | 9 (37.5) | 7 (30.4) | 0.6090 |
| Past medical history of CDI, no. (%) | 2 (8.3) | 1 (4.3) | 0.5760 |
| Past hospitalization within 3 months before onset of CDI, no. (%) | 13 (54.1) | 6 (26.0) | 0.0500 |
| Past hospitalization < 1 year, no. (%) | 18 (75.0) | 10 (43.4) | 0.028 |
| Temperature > 37.8 °C at diagnosis of CDI, no. (%) | 11 (45.8) | 10 (43.4) | 0.8710 |
| Abdominal pain at diagnosis of CDI, no. (%) | 11 (45.8) | 12 (52.1) | 0.4270 |
| Bowel movements > 5 at diagnosis of CDI, no. (%) | 11 (45.8) | 11 (47.8) | 0.6640 |
| Bloody stool, no. (%) | 2 (8.3) | 1 (4.3) | 0.5760 |
| Fasting, no. (%) | 5 (20.8) | 2 (8.6) | 0.2430 |
| Enteral feeding, no. (%) | 1 (4.1) | 7 (30.4) | 0.0170 |
| Hospitalization for more than 1 month before onset of CDI, no. (%) | 11 (45.8) | 9 (39.1) | 0.6420 |
| Use of antimicrobials for at least 3 days after CDI diagnosis, no. (%) | 13 (54.1) | 8 (34.7) | 0.1810 |
| Comorbidities, no. (%) | |||
| Diabetes mellitus | 13 (54.1) | 10 (43.4) | 0.4640 |
| Chronic kidney disease | 16 (66.6) | 20 (86.9) | 0.1010 |
| HD | 10 (41.6) | 3 (13.0) | 0.0280 |
| Heart failure/ischemic heart disease | 7 (29.1) | 9 (39.1) | 0.4710 |
| Chronic liver disease | 2 (8.3) | 0 (0) | 0.1570 |
| Chronic obstructive pulmonary disease | 1 (4.1) | 2 (8.6) | 0.5250 |
| Cerebrovascular disease | 2 (8.3) | 5 (21.7) | 0.1970 |
| Inflammatory bowel disease | 1 (4.1) | 0 (0) | 0.3220 |
| Solid malignancy | 6 (25.0) | 8 (34.7) | 0.4640 |
| Hematologic malignancy | 3 (12.5) | 2 (8.6) | 0.6720 |
| History of abdominal surgery, no. (%) | 7 (29.1) | 3 (13.0) | 0.1770 |
| Community onset CDI, no. (%) | 3 (12.5) | 2 (8.6) | 0.3430 |
| ICU at diagnosis of CDI, no. (%) | 1 (4.1) | 2 (8.6) | 0.5250 |
| Any severe CDI, no. (%) | |||
| MN criteria mild | 0 (0) | 0 (0) | - |
| MN criteria moderate | 15 (62.5) | 9 (39.1) | 0.1090 |
| MN criteria severe | 7 (29.1) | 12 (52.1) | 0.1080 |
| MN criteria super severe | 2 (8.3) | 2 (8.6) | 0.9650 |
| Laboratory data | |||
| White blood cell count > 15,000/mm3, no. (%) | 11 (45.8) | 8 (34.7) | 0.4400 |
| Albumin < 3, no. (%) | 16 (66.6) | 20 (86.9) | 0.1010 |
| Creatinine > 1.5 mg/dL, no. (%) | 14 (58.3) | 20 (86.9) | 0.0280 |
| C reactive protein ≥ 10 mg/dL, no. (%) | 5 (20.8) | 8 (34.7) | 0.2850 |
| C reactive protein ≥ 15 mg/dL, no. (%) | 3 (12.5) | 4 (17.3) | 0.6380 |
| Antibiotics administration at CDI onset, no. (%) | |||
| Penicillin | 1 (4.1) | 3 (13.0) | 0.2760 |
| Cephalosporin | 12 (50.0) | 14 (60.8) | 0.4540 |
| Carbapenem | 7 (29.1) | 4 (17.3) | 0.3410 |
| Fluoroquinolone | 2 (8.3) | 1 (4.3) | 0.5760 |
| Clindamycin | 0 (0) | 0 (0) | - |
| β-lactam/β-lactamase inhibitor | 7 (29.1) | 6 (26.0) | 0.8130 |
| Anti-viral agents | 0 (0) | 1 (4.3) | 0.3020 |
| Anti-fungal agents | 0 (0) | 2 (8.6) | 0.1400 |
| None | 2 (8.3) | 6 (26.0) | 0.1050 |
| Concomitant medication use, no. (%) | |||
| PPIs | 7 (29.1) | 13 (56.5) | 0.0580 |
| H2RAs | 0 (0) | 2 (8.6) | 0.1400 |
| P-CAB | 10 (41.6) | 3 (13.0) | 0.0280 |
| Steroid | 8 (33.3) | 3 (13.0) | 0.1010 |
| Immunosuppressants other than steroids | 8 (33.3) | 5 (21.7) | 0.3740 |
| Anti-cancer chemotherapy | 2 (8.3) | 2 (8.6) | 0.9650 |
| Probiotics before CDI diagnosis, no. (%) | 10 (41.6) | 12 (52.1) | 0.4710 |
| Probiotics after CDI diagnosis, no. (%) | 18 (75.0) | 17 (73.9) | 0.9320 |
| Global cure | 15 (62.5) | 21 (91.3) | <0.05 |
| Clinical cure | 15 (62.5) | 22 (95.6) | <0.01 |
| Recurrence | 2 (8.3) | 1 (4.3) | 0.5760 |
| Adverse effect, no. (%) | 0 (0) | 0 (0) | - |
| All-cause 30-day mortality after CDI treatment | 2 (8.3) | 0 (0) | 0.1570 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hirai, J.; Mori, N.; Hanai, Y.; Asai, N.; Hagihara, M.; Mikamo, H. Evaluating Bezlotoxumab-Fidaxomicin Combination Therapy in Clostridioides Infection: A Single-Center Retrospective Study from Aichi Prefecture, Japan. Antibiotics 2025, 14, 228. https://doi.org/10.3390/antibiotics14030228
Hirai J, Mori N, Hanai Y, Asai N, Hagihara M, Mikamo H. Evaluating Bezlotoxumab-Fidaxomicin Combination Therapy in Clostridioides Infection: A Single-Center Retrospective Study from Aichi Prefecture, Japan. Antibiotics. 2025; 14(3):228. https://doi.org/10.3390/antibiotics14030228
Chicago/Turabian StyleHirai, Jun, Nobuaki Mori, Yuki Hanai, Nobuhiro Asai, Mao Hagihara, and Hiroshige Mikamo. 2025. "Evaluating Bezlotoxumab-Fidaxomicin Combination Therapy in Clostridioides Infection: A Single-Center Retrospective Study from Aichi Prefecture, Japan" Antibiotics 14, no. 3: 228. https://doi.org/10.3390/antibiotics14030228
APA StyleHirai, J., Mori, N., Hanai, Y., Asai, N., Hagihara, M., & Mikamo, H. (2025). Evaluating Bezlotoxumab-Fidaxomicin Combination Therapy in Clostridioides Infection: A Single-Center Retrospective Study from Aichi Prefecture, Japan. Antibiotics, 14(3), 228. https://doi.org/10.3390/antibiotics14030228






