Utilization of Existing Human Kinase Inhibitors as Scaffolds in the Development of New Antimicrobials
Abstract
1. Introduction
2. Repurposing of Human Kinase Inhibitors as Antimicrobials
2.1. Human Kinase Inhibitors Designed as Eukaryotic Competitive Ligands for Protein Kinase ATP Binding Sites
| Bacteria | Eukaryotic Kinase/Inhibitor/Type of Inhibition/Ref. | Bacterial Target of HumKI/ MOA/Ref. | Type of Antibacterial Activity/Adjuvant/Ref. |
|---|---|---|---|
| M. catarrhalis, S. aureus, E. coli, H. influenzae | Compound 4 (PD173074) is an inhibitor of VEGFR2 and FGFR1 kinases. 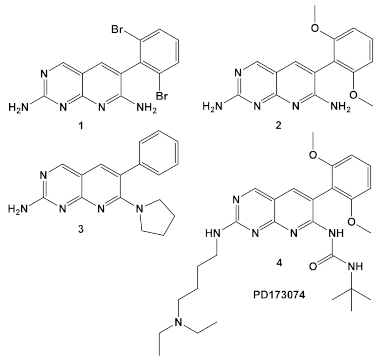 [13] | biotin carboxylase ATP-binding. [13] | Bactericidal alone in vitro & in vivo. Adjuvant activity of compound 1 with triclosan and ciprofloxacin in H. influenzae [fractional inhibitory conc. (FIC) 0.37 and 0.26, respectively)] [13] |
| S. aureus MRSA | Inhibitor of the p38 serine/threonine kinase signal-transducing enzyme. 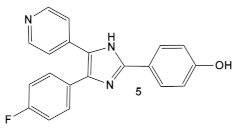 [14,15]  [16] | Phosphory- lation of BlaR1. [16] | Reversing of the MRSA phenotype which makes is susceptible to β-lactam antibiotics [16] 5a–5c potentiate oxacillin at 7 μg/mL against MRSA strains |
| S. aureus Multiple MRSA and MSSA isolates | GW779439X putative human CDK4 inhibitor with low specificity and high toxicity. 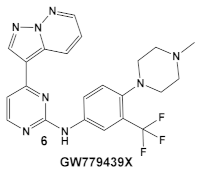 [17] Scaffold modifications of HumKI GW779439X (6) leads to an STK1 inhibitor CAF078 (7) which retains the GW779439X activity. 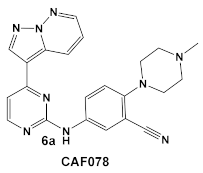 [18] | Inhibition of StK1 in S. aureus both MSSA and MRSA strains [18] | Potentiation of β-lactam antibiotics against MRSA by increasing β-lactam susceptibility 2−512-fold—mainly nafcillin and oxacillin, to a lesser degree ceftriaxone [18] |
| S. aureus | Ceritinib ATP-competitive tyrosine kinase (ALK) inhibitor 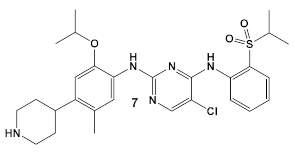 [19] | MOA unknown Disrupts bacterial cell membrane. [20] | Anti-MRSA persisters & antibiofilm mass at a range of 8–16 μg/mL [22] |
| L. monocy- togenes | GSK690693 is an AKT inhibitor. 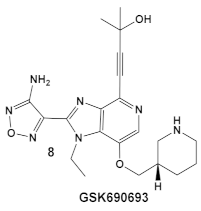 [17,25,26] | Targets L. monocytogenes PASTA kinase PrkA [25] | Antimicrobial activity alone, as well as potentiation of β-lactam antibiotics. [25] |
| Mycobacteria: M. smegmatis, M. bovis, Mtb and Nocardia asteroides | 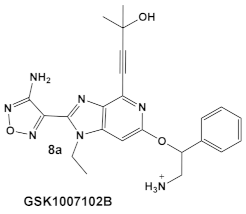 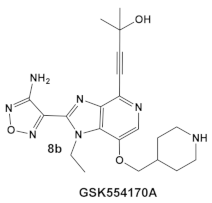 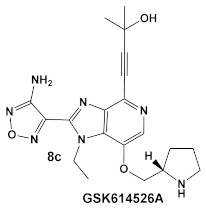 [17,26] | GSK690693 binding to PknB in an ATP-competitive fashion. [26] | Potentiation of β-lactam antibiotics—mainly nafcillin and oxacillin, to a lesser degree ceftriaxone [26] |
| M. tuberculosis | GW779439X putative human CDK4 inhibitor with low specificity and high toxicity.  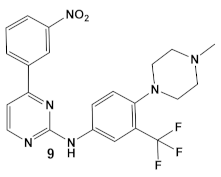 [17,18,27] |
2.2. Human Kinase Inhibitors Designed as Ligands of the Catalytic and Non-Catalytic Cys Residues of Eukaryotic Protein Kinases
| Bacteria | Eukaryotic Kinase/Inhibitor/Type of Inhibition/Ref. | Bacterial Target of HumKI/ MOA/Ref. | Type of Antibacterial Activity/Adjuvant/Ref. |
|---|---|---|---|
| E. coli, P. aeruginosa & Salmonella typhimurium | Afatinib/FDA appr. 2013 EGF receptor and ErbB tyrosine kinase inhibitor 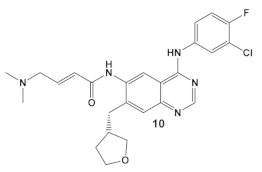 [9,29] | Specific target/MOA unknown. [30] | Bactericidal to biofilm cells. |
| B. subtilis, S. aureus, MSSA, and MRSA strains. | Dacomitinib/FDA appr. 2018 EGFR tyrosine kinase inhibitor 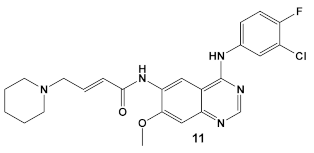 [29] | FtsZ protein (filamentous temperature-sensitive mutant Z) via hydrophobic and H-bonding interactions. [33] | Inhibition of bacterial cell division. MICs 16 μg/mL against B. subtilis 168 and 32–64 μg/mL against S. aureus strains. [33] |
| S. aureus, MSSA, Candida albicans | BAY 11-7085, TNF-α-stimulated IκBα phosphorylation inhibitor  [35,36] | Specific target/MOA unknown. [38] | Antibiofilm as monoculture and polymicrobial co-culture. S. aureus (MRSA, 4 μg/mL) and Candida albicans/anti-biofilm. [38] |
| S. aureus MRSA | BAY 11-7082, IκBα phosphorylation and NF-κB inhibitor. IκB kinase and protein tyrosine phosphatases (PTPs) active site inhibitor 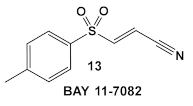 [35,36]  [40] 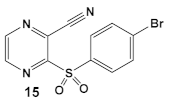 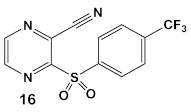 [39] | MOA undetermined [39] | Bactericidal alone and in combination Compound 13 adjunctive for penicillin G. (16-fold reduction in MIC). For compounds 15 and 16 the reduction of penicillin G MIC to 0.39 μM. Modest antimicrobial activity against Gram (−), e.g., P. aeruginosa [39] |
2.3. Human Kinase Inhibitors of Amide and Urea Chemotypes
3. Summary
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AKT | alpha serine/threonine-protein kinase |
| ALK | anaplastic lymphoma kinase |
| ATP | Adenosine triphosphate |
| BLAR1 | cell-surface receptor protein (S. aureus) |
| CDK4 | cyclin-dependent kinase 4 |
| EGF | epidermal growth factor |
| EGFR | epidermal growth factor receptor |
| ErbB | receptor tyrosine kinase |
| FA | Fatty acid(s) |
| FGFR1 | fibroblast growth factor receptor 1 |
| FtsZ | filamenting temperature-sensitive mutant Z |
| HumKIs | Human Kinase Inhibitors |
| IκB-α | nuclear factor of kappa light polypeptide gene enhancer in B-cells inhibitor, alpha |
| IKK-β | inhibitor of nuclear factor kappa-B kinase |
| MBC | minimum bactericidal concentration |
| MDR | multi drug resistant |
| MEK1/2 | Mitogen-Activated protein kinases (also known as MAPKK and MAP2K0 |
| MenG | demethylmenaquinone methyltransferase |
| MIC | minimum inhibitory concentration |
| MOA | mechanism of action |
| Mtb | M. tuberculosis |
| NF-κB | nuclear factor kappa B protein transcription factor |
| NP | Nanoparticles |
| PASTA | serine/threonine kinase-associated kinases |
| PBP2A | penicillin binding protein 2a |
| PknA and PknB | protein kinases A and B (M. tuberculosis) |
| PrkA | putative serine protein kinase |
| PKI | protein kinase inhibitors |
| ROS | reactive oxygen species |
| eSTKs | eukaryotic-like Ser/Thr kinases |
| Stk1 | serum thymidine kinase 1 |
| TNF-α | tumor necrosis factor, alpha |
| TK | Tyrosine kinase |
| VEGFR2 | Vascular endothelial growth factor receptor 2 |
| Bacterial Names | |
| A. baumannii | Acinetobacter baumannii |
| B. subtilis | Bacillus subtilis |
| E. coli | Escherichia coli |
| H. influenzae | Haemophilis influenzae |
| K. pneumoniae | Klebsiella pneumoniae |
| M. catarrhalis | Moraxella catarrhalis |
| Mtb, M. tuberculosis | Mycobacterium tuberculosis |
| M. bovis | Mycobacterium bovis |
| M. smegmatis | Mycobacterium smegmatis |
| MRSA | methicillin-resistant Staphylococcus aureus |
| MSSA | methicillin-susceptible S. aureus |
| N. asteroides | Nocardia asteroides |
| P. aeruginosa | Pseudomonas aeruginosa |
| S. aureus | Staphylococcus aureus |
| VISA | vancomycin-intermediate S. aureus |
| VRSA | vancomycin-resistant S. aureus |
| VSSA | vancomycin-susceptible S. aureus |
| VRE | vancomycin-resistant Enterococcus |
References
- O’Neill, J. Tackling Drug-Resistant Infections Globally: Final Report and Recommendations; The Review on Antimicrobial Resistance; Government of the United Kingdom: London, UK, 2015.
- CDC. Transcript for VitalSigns Teleconference: Antibiotic Resistant Germs; Center for Disease Control and Prevention: Atlanta, GA, USA, 2018. [Google Scholar]
- Plackett, B. Why big pharma has abandoned antibiotics. Nature 2020, 586, S50–S52. [Google Scholar] [CrossRef]
- King, A.; Blackledge, M.S. Evaluation of Small Molecule Kinase Inhibitors as Novel Antimicrobial and Antibiofilm Agents. Chem. Biol. Drug Des. 2021, 98, 1038–1064. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Yu, C.; Wu, H.; Li, G.; Li, C.; Hong, W.; Yang, X.; Wang, H.; You, X. Recent Advances in Histidine Kinase-Targeted Antimicrobial Agents. Front. Chem. 2022, 10, 866392. [Google Scholar] [CrossRef] [PubMed]
- Bem, A.E.; Velikova, N.; Pellicer, M.T.; van Baarlen, P.; Marina, A.; Wells, J.M. Bacterial Histidine Kinases as Novel Antibacterial Drug Targets. ACS Chem. Biol. 2015, 10, 213–224. [Google Scholar] [CrossRef] [PubMed]
- Ayala-Aguilera, C.C.; Valero, T.; Lorente-Macías, Á.; Baillache, D.J.; Croke, S.; Unciti-Broceta, A. Small Molecule Kinase Inhibitor Drugs (1995–2021): Medical Indication, Pharmacology, and Synthesis. J. Med. Chem. 2022, 65, 1047–1131. [Google Scholar] [CrossRef] [PubMed]
- Roskoski, R., Jr. Properties of FDA-approved small molecule protein kinase inhibitors: A 2022 update. Pharmacol. Res. 2022, 175, 106037. [Google Scholar] [CrossRef] [PubMed]
- Roskoski, R., Jr. Properties of FDA-approved small molecule protein kinase inhibitors: A 2023 update. Pharmacol. Res. 2023, 178, 106552. [Google Scholar] [CrossRef]
- Gupta, M.N.; Alam, A.; Hasnain, S.E. Protein promiscuity in drug discovery, drug-repurposing and antibiotic resistance. Biochimie 2020, 175, 50–57. [Google Scholar] [CrossRef]
- Payne, D.J.; Gwynn, M.N.; Holmes, D.J.; Pompliano, D.L. Drugs for Bad Bugs: Confronting the Challenges of Antibacterial Discovery. Nat. Rev. Drug Discov. 2007, 6, 29–40. [Google Scholar] [CrossRef]
- Tommasi, R.; Brown, D.G.; Walkup, G.K.; Manchester, J.I.; Miller, A.A. ESKAPEing the Labyrinth of Antibacterial Discovery. Nat. Rev. Drug Discov. 2015, 14, 529–542. [Google Scholar] [CrossRef]
- Miller, J.R.; Dunham, S.; Mochalkin, I.; Banotai, C.; Bowman, M.; Buist, S.; Dunkle, B.; Hanna, D.; Harwood, H.J.; Huband, M.D.; et al. A class of selective antibacterials derived from a protein kinase inhibitor pharmacophore. Proc. Natl. Acad. Sci. USA 2009, 106, 1737–1742. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.C.; Laydon, J.T.; McDonnell, P.C.; Gallagher, T.F.; Kumar, S.; Green, D.; McNulty, D.; Blumenthal, M.J.; Heys, J.R.; Landvatter, S.W.; et al. A protein kinase involved in the regulation of inflammatory cytokine biosynthesis. Nature 1994, 372, 739–746. [Google Scholar] [CrossRef] [PubMed]
- Frantz, B.; Klatt, T.; Pang, M.; Parsons, J.; Rolando, A.; Williams, H.; Tocci, M.J.; O’Keefe, S.J.; O’Neill, E.A. The activation state of p38 mitogen-activated protein kinase determines the efficiency of ATP competition for pyridinylimidazole inhibitor binding. Biochemistry 1998, 37, 13846–13853. [Google Scholar] [CrossRef] [PubMed]
- Boudreau, M.A.; Fishovitz, J.; Llarrull, L.I.; Xiao, Q.; Mobashery, S. Phosphorylation of BlaR1 in Manifestation of Antibiotic Resistance in Methicillin-Resistant Staphylococcus aureus and Its Abrogation by Small Molecules. ACS Infect. Dis. 2015, 1, 454–459. [Google Scholar] [CrossRef] [PubMed]
- Elkinsns, J.M.; Fedele, V.; Szklarz, M.; Azeez, K.R.A.; Salah, E.; Mikolajczyk, J.; Romanov, S.; Sepetov, N.; Huang, X.-P.; Roth, B.L.; et al. Comprehensive char-acterization of the published kinase inhibitor set. Nat. Biotechnol. 2016, 34, 95–103. [Google Scholar] [CrossRef] [PubMed]
- Schaenzer, A.J.; Wlodarchak, N.; Drewry, D.H.; Zuercher, W.J.; Rose, W.E.; Ferrer, C.A.; Sauer, J.-D.; Striker, R. GW779439X and Its pyrazolopyridazine derivatives Inhibit the serine/threonine kinase Stk1 and act as antibiotic adjuvants against β-Lactam-resistant Staphylococcus aureus. ACS Infect. Dis. 2018, 4, 1508–1518. [Google Scholar] [CrossRef] [PubMed]
- Vornhagen, J.; Burnside, K.; Whidbey, C.; Berry, J.; Qin, X.; Rajagopal, L. Kinase Inhibitors that Increase the sensitivity of methicillin resistant Staphylococcus aureus to beta-lactam antibiotics. Pathogens 2015, 4, 708–721. [Google Scholar] [CrossRef]
- Beltramini, A.M.; Mukhopadhyay, C.D.; Pancholi, V. Modulation of cell wall structure and antimicrobial susceptibility by a Staphylococcus aureus eukaryote-like serine/threonine kinase and phosphatase. Infect. Immun. 2009, 77, 1406–1416. [Google Scholar] [CrossRef]
- Tamber, S.; Schwartzman, J.; Cheung, A.L. Role of PknB kinase in antibiotic resistance and virulence in community-acquired methicillin-resistant Staphylococcus aureus strain USA300. Infect. Immun. 2010, 78, 3637–3646. [Google Scholar] [CrossRef]
- Liu, S.; She, P.; Li, Z.; Yimin Li, Y.; Yang, Y.; Li, L.; Zhou, L.; Wu, Y. Insights into the antimicrobial effects of ceritinib against Staphylococcus aureus in vitro and in vivo by cell membrane disruption. AMB Expr. 2022, 12, 150. [Google Scholar] [CrossRef]
- Pensinger, D.A.; Aliota, M.T.; Schaenzer, A.J.; Boldon, K.M.; Ansari, I.U.; Vincent, W.J.; Knight, B.; Reniere, M.L.; Striker, R.; Sauer, J.D. Selective pharmacologic inhibition of a PASTA kinase increases Listeria monocytogenes susceptibility to beta-lactam antibiotics. Antimicrob. Agents Chemother. 2014, 58, 4486–4494. [Google Scholar] [CrossRef] [PubMed]
- Carol, H.; Morton, C.L.; Gorlick, R.; Kolb, E.A.; Keir, S.T.; Reynolds, C.P.; Kang, M.H.; Maris, J.M.; Billups, C.; Smith, M.A.; et al. Initial Testing (Stage 1) of the Akt Inhibitor GSK690693 by the Pediatric Preclinical Testing Program. Pediatr. Blood Cancer 2010, 55, 1329–1337. [Google Scholar] [CrossRef] [PubMed]
- Schaenzer, A.J.; Wlodarchak, N.; Drewry, D.H.; Zuercher, W.J.; Rose, W.E.; Striker, R.; Sauer, J.-D. A screen for kinase inhibitors identifies antimicrobial imidazopyridine aminofurazans as specific inhibitors of the Listeria monocytogenes PASTA kinase PrkA. Biol. Chem. 2017, 292, 17037–17045. [Google Scholar] [CrossRef] [PubMed]
- Wlodarchak, N.; Teachout, N.; Beczkiewicz, J.; Procknow, R.; Schaenzer, A.J.; Satyshur, K.; Pavelka, M.; Zuercher, W.; Drewry, D.; Sauer, J.-D.; et al. In silico screen and structural analysis identifies bacterial kinase inhibitors which act with β-Lactams to inhibit Mycobacterial Growth. Mol. Pharm. 2018, 15, 5410–5426. [Google Scholar] [CrossRef]
- Wlodarchak, N.; Feltenberger, J.B.; Ye, Z.; Beczkiewicz, J.; Procknow, R.; Yan, G.; King, T.M., Jr.; Golden, J.E.; Striker, R. Engineering selectivity for reduced toxicity of bacterial kinase inhibitors using structure-guided medicinal chemistry. ACS Med. Chem. Lett. 2021, 12, 228–235. [Google Scholar] [CrossRef] [PubMed]
- Gold, B.; Zhang, J.; Quezada, L.L.; Roberts, J.; Ling, Y.; Wood, M.; Shinwari, W.; Goullieux, L.; Roubert, C.; Fraisse, L.; et al. Identification of β-Lactams Active against Mycobacterium tuberculosis by a Consortium of Pharmaceutical Companies and Academic Institutions. ACS Infect. Dis. 2022, 8, 557–573. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.; Smaill, J.B.; Patterson, A.V.; Ding, K. Discovery of Cysteine-targeting Covalent Protein Kinase Inhibitors. J. Med. Chem. 2022, 65, 58–83. [Google Scholar] [CrossRef]
- Yahya, M.F.Z.R.; Alias, Z.; Karsani, S.A. Antibiofilm activity and mode of action of DMSO alone and its combination with afatinib against Gram-negative pathogens. Folia Microbiol. 2018, 63, 23–30. [Google Scholar] [CrossRef]
- Li, X.-H.; Lee, J.-H. Antibiofilm agents: A new perspective for antimicrobial strategy. J. Microbiol. 2017, 55, 753–766. [Google Scholar] [CrossRef]
- Asma, S.T.; Imre, K.; Morar, A.; Herman, V.; Acaroz, U.; Mukhtar, H.; Arslan-Acaroz, D.; Shah, S.R.A.; Gerlach, R. An Overview of Biofilm Formation–Combating Strategies and Mechanisms of Action of Antibiofilm Agents. Life 2022, 12, 1110. [Google Scholar] [CrossRef]
- Du, R.-L.; Sun, N.; Fung, Y.-H.; Zheng, Y.-Y.; Chen, Y.-W.; Chan, P.-H.; Wong, W.-L.; Wong, K.-Y. Discovery of FtsZ inhibitors by virtual screening as antibacterial agents and study of the inhibition mechanism. RSC Med. Chem. 2022, 13, 79. [Google Scholar] [CrossRef]
- Haydon, D.J.; Stokes, N.R.; Ure, R.; Galbraith, G.; Bennett, J.M.; Brown, D.R.; Baker, P.J.; Barynin, V.V.; Rice, D.W.; Sedelnikova, S.E.; et al. An inhibitor of FtsZ with potent and selective anti-staphylococcal activity. Science 2008, 321, 1673–1675. [Google Scholar] [CrossRef] [PubMed]
- Pierce, J.W.; Schoenleber, R.; Jesmok, G.; Jennifer Best, J.; Moore, S.A.; Collins, T.; Gerritsen, M.E. Novel inhibitors of cytokine-induced IkBa phosphorylation and endothelial cell adhesion molecule expression show anti-inflammatory effects in vivo. J. Biol. Chem. 1997, 272, 21096–21103. [Google Scholar] [CrossRef] [PubMed]
- Azevedo, M.M.; Teixeira-Santos, R.; Silva, A.P.; Cruz, L.; Ricardo, E.; Pina-Vaz, C.; Rodrigues, A.G. The effect of antibacterial and non-antibacterial compounds alone or associated with antifungals upon Fungi. Front. Microbiol. 2015, 6, 669. [Google Scholar] [CrossRef] [PubMed]
- Krishnan, N.; Bencze, G.; Philip Cohen, P.; Tonks, N.K. The anti-inflammatory compound BAY 11-7082 is a potent inhibitor of Protein Tyrosine Phosphatases. FEBS J. 2013, 280, 2830–2841. [Google Scholar] [CrossRef] [PubMed]
- Escobar, I.E.; Rossatto, F.C.P.; Kim, S.M.; Kang, M.H.; Kim, W.; Mylonakis, E. Repurposing kinase Inhibitor Bay 11-7085 to combat Staphylococcus aureus and Candida albicans biofilms. Front. Pharmacol. 2021, 12, 675300. [Google Scholar] [CrossRef] [PubMed]
- Coles, V.E.; Darveau, P.; Zhang, X.; Harvey, H.; Henriksbo, B.D.; Yang, A.; Schertzer, J.D.; Magolan, J.; Burrows, L.L. Exploration of BAY 11-7082 as a Potential Antibiotic. ACS Infect. Dis. 2022, 8, 170–182. [Google Scholar] [CrossRef] [PubMed]
- Rajamuthiah, R.; Jayamani, E.; Majed, H.; Conery, A.L.; Kim, W.; Kwon, B.; Fuchs, B.B.; Kelso, M.J.; Ausubel, F.M.; Mylonakis, E. Antibacterial Properties of 3-(Phenylsulfonyl)-2-Pyrazinecarbonitrile. Bioorg. Med. Chem. Lett. 2015, 25, 5203–5207. [Google Scholar] [CrossRef]
- Lang, L. FDA approves sorafenib for patients with inoperable liver cancer. Gastroenterology 2008, 134, 379. [Google Scholar] [CrossRef]
- Le, P.; Kunold, E.; Macsics, R.; Rox, K.; Jennings, M.C.; Ugur, I.; Reinecke, M.; Chaves-Moreno, D.; Hackl, M.W.; Fetzer, C.; et al. Repurposing human kinase inhibitors to create an antibiotic active against drug-resistant Staphylococcus aureus, persisters and biofilms. Nat. Chem. 2020, 12, 145–158. [Google Scholar] [CrossRef]
- Onai, Y.; Suzuki, J.-I.; Kakuta, T.; Maejima, Y.; Haraguchi, G.; Fukasawa, H.; Muto, S.; Itai, A.; Isobe, M. Inhibition of IkappaB phosphorylation in cardiomyocytes attenuates myocardial ischemia/reperfusion injury. Cardiovasc. Res. 2004, 63, 51–59. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Ko, D.; Lee, Y.; Jang, S.; Lee, Y.; Lee, I.Y.; Kim, S. Anti-cancer activity of the novel 2-hydroxydiarylamide derivatives IMD-0354 and KRT1853 through suppression of cancer cell invasion, proliferation, and survival mediated by TMPRSS4. Sci. Rep. 2019, 9, 10003. [Google Scholar] [CrossRef] [PubMed]
- Gomez-Cabrero, A.; Wrasidlo, W.; Reisfeld, R.A. IMD-0354 targets breast cancer stem cells: A novel approach for an adjuvant to chemotherapy to prevent multidrug resistance in a murine model. PLoS ONE 2013, 8, e73607. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Escobar, I.E.; White, A.; Kim, W.; Mylonakis, E. New antimicrobial bioactivity against multidrug-resistant Gram-positive bacteria of kinase inhibitor IMD0354. Antibiotics 2020, 9, 665. [Google Scholar] [CrossRef] [PubMed]
- Lee, T.X.; Packer, M.D.; Huang, J.; Akhmametyeva, E.M.; Kulp, S.K.; Chen, C.S.; Giovannini, M.; Jacob, A.; Welling, D.B.; Chang, L.S. Growth inhibitory and anti-tumour activities of OSU-03012, a novel PDK-1 inhibitor, on vestibular schwannoma and malignant schwannoma cells. Eur. J. Cancer 2009, 45, 1709–1720. [Google Scholar] [CrossRef] [PubMed]
- Barker, W.T.; Nemeth, A.M.; Brackett, S.M.; Basak, A.K.; Chandler, C.E.; Jania, L.A.; Zuercher, W.J.; Melander, R.J.; Koller, B.H.; Ernst, R.K.; et al. Repurposing eukaryotic kinase inhibitors as colistin adjuvants in Gram-negative bacteria. ACS Infect. Dis. 2019, 5, 1764–1771. [Google Scholar] [CrossRef] [PubMed]
- Manning, G.; Whyte, D.B.; Martinez, R.; Hunter, T.; Sudarsanam, S. The Protein Kinase Complement of the Human Genome. Science 2002, 298, 1912–1934. [Google Scholar] [CrossRef]
- Abdeldayem, A.; Raouf, Y.S.; Constantinescu, S.N.; Moriggl, R.; Gunning, P.T. Advances in Covalent Kinase Inhibitors. Chem. Soc. Rev. 2020, 49, 2617–2687. [Google Scholar] [CrossRef]
- Krishnan, S.; Miller, R.M.; Tian, B.; Mullins, R.D.; Jacobson, M.P.; Taunton, J. Design of Reversible, Cysteine-Targeted Michael Acceptors Guided by Kinetic and Computational Analysis. J. Am. Chem. Soc. 2014, 136, 12624–12630. [Google Scholar] [CrossRef]
- Schauperl, M.; Denny, R.A. AI-Based Protein Structure Prediction in Drug Discovery: Impacts and Challenges. J. Chem. Inf. Model. 2022, 62, 3142–3156. [Google Scholar] [CrossRef]
- Fowler, N.J.; Williamson, M.P. The accuracy of protein structures in solution determined by AlphaFold and NMR. Structure 2022, 30, 925–933. [Google Scholar] [CrossRef]
- Yang, Z.; Zeng, X.; Zhao, Y.; Chen, R. AlphaFold2 and its applications in the fields of biology and medicine. Signal Transduct. Target. Ther. 2023, 8, 115. [Google Scholar] [CrossRef]
| Bacteria | Eukaryotic Kinase/Inhibitor/Type of Inhibition/Ref. | Bacterial Target of HumKI /MOA/Ref. | Type of Antibacterial Activity/Adjuvant/Ref. |
|---|---|---|---|
| S. aureus MRSA | Sorafenib, inhibits multiple targets including Raf serine/threonine kinases, vascular endothelial growth factor receptor tyrosine kinases; VEGFR-1, VEGFR-2, VEGFR-3 and platelet-derived growth factor receptor β (PDGFR-β).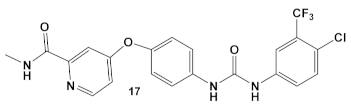 [41]  [42] | Inhibitors of MenG biosynthesis Sorafenib exhibits anti-MRSA, including persisters and biofilm-embedded cells, activity. [42] | Anti-persisters and antibiofilm. PK150 exhibits activity against several pathogenic isolates at submicromolar conc. [42] |
| S. aureus MRSA and VRSA strains | An IKK-β inhibitor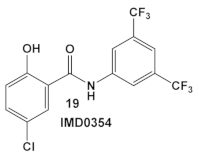 [43,44,45] | MOA at low conc. ≥ 4 µg/mL is membrane permeabilization. MOA at lower conc. is unidentified. [46] | Anti-VRSA activity (MIC 0.06 µg/mL) and inhibition of VRSA adherence and subsequent biofilm formation at sub-MIC levels [46] |
| E. coli, K. pneumoniae, A. baumannii and P. aeruginosa | An IKK-β inhibitor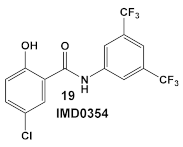 [43,44,45] A pyruvate dehydrogenase kinase-1 (PDK-1)  [47] | Suppression of lipid A modification in colistin-resistant strains, an insight into its MOA. [48] | Potentiation of colistin against Gram (−) bacteria. [48] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Konaklieva, M.I.; Plotkin, B.J. Utilization of Existing Human Kinase Inhibitors as Scaffolds in the Development of New Antimicrobials. Antibiotics 2023, 12, 1418. https://doi.org/10.3390/antibiotics12091418
Konaklieva MI, Plotkin BJ. Utilization of Existing Human Kinase Inhibitors as Scaffolds in the Development of New Antimicrobials. Antibiotics. 2023; 12(9):1418. https://doi.org/10.3390/antibiotics12091418
Chicago/Turabian StyleKonaklieva, Monika I., and Balbina J. Plotkin. 2023. "Utilization of Existing Human Kinase Inhibitors as Scaffolds in the Development of New Antimicrobials" Antibiotics 12, no. 9: 1418. https://doi.org/10.3390/antibiotics12091418
APA StyleKonaklieva, M. I., & Plotkin, B. J. (2023). Utilization of Existing Human Kinase Inhibitors as Scaffolds in the Development of New Antimicrobials. Antibiotics, 12(9), 1418. https://doi.org/10.3390/antibiotics12091418





