The Effect of Zero-Valent Iron Nanoparticles (nZVI) on Bacteriophages
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Consumables
2.3. Synthesis of nZVI
2.4. Bacteriophages
2.5. Bacteria
2.6. Double Overlay Titration for Phage Analysis
2.7. Dose-Response Experiments
2.8. Experiments with Fe (II) and Fe (III) Salts
2.9. SEM, STEM, and TEM Analysis
3. Results
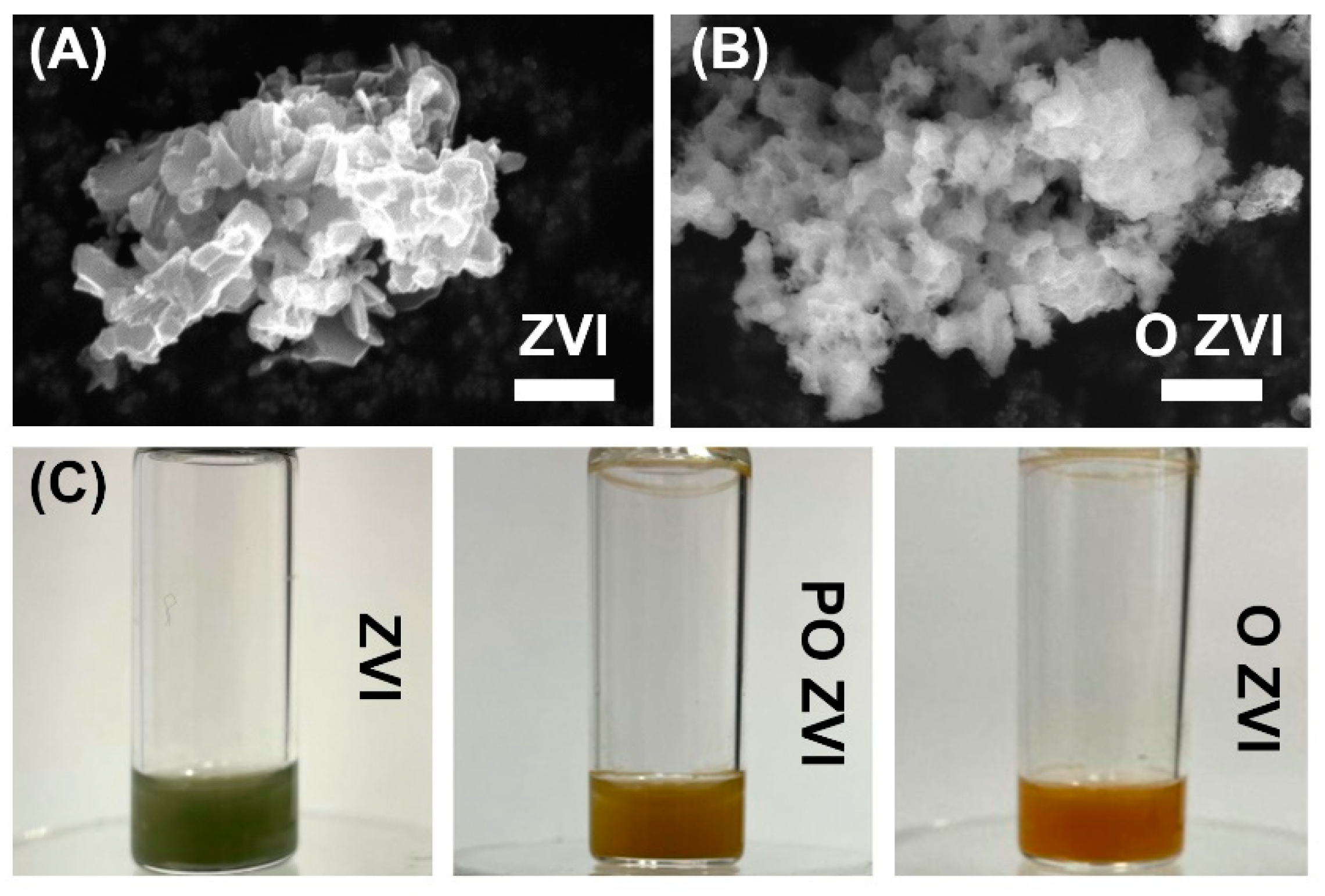
3.1. Characterization of ZVI Nanoparticles
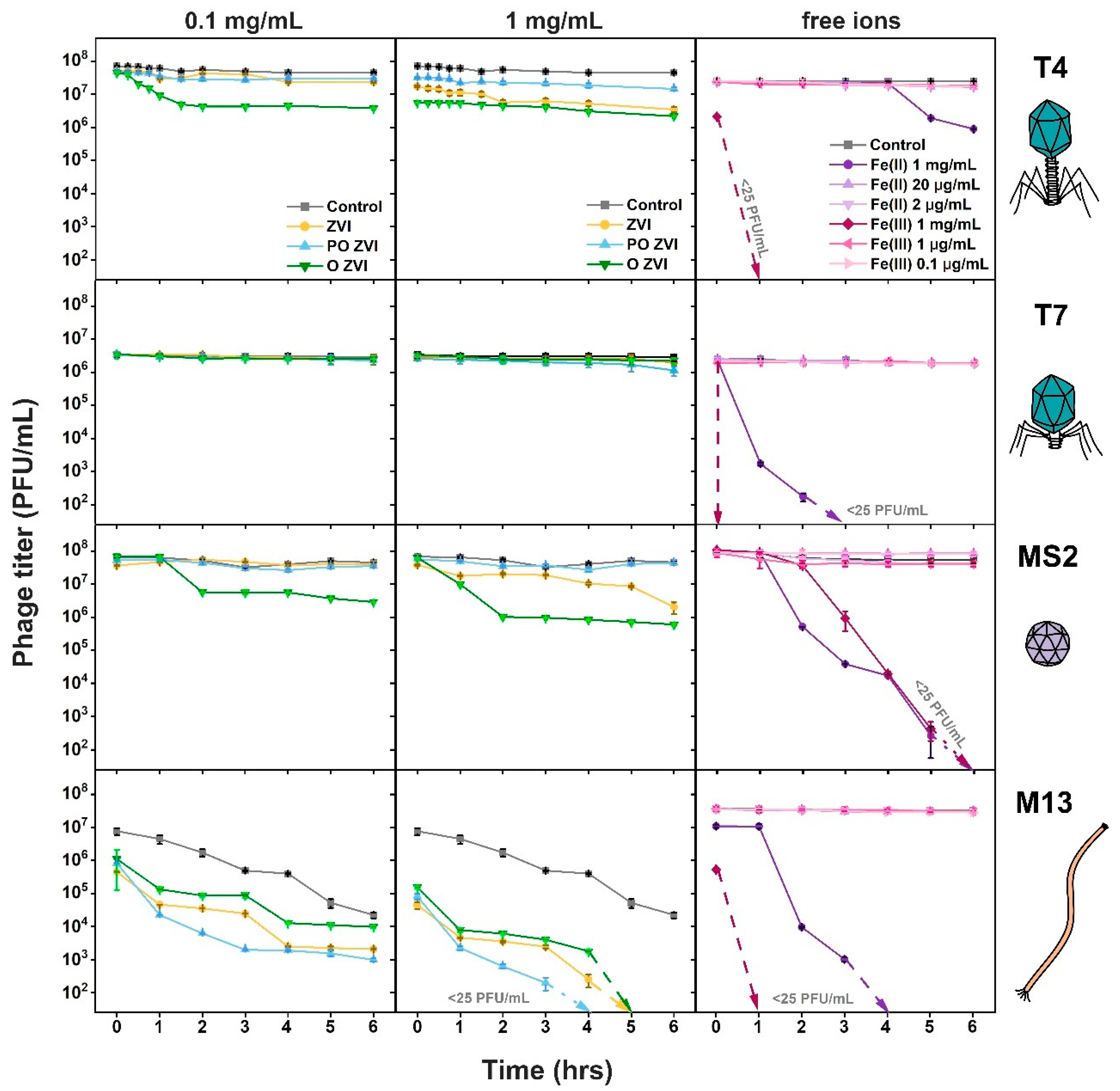
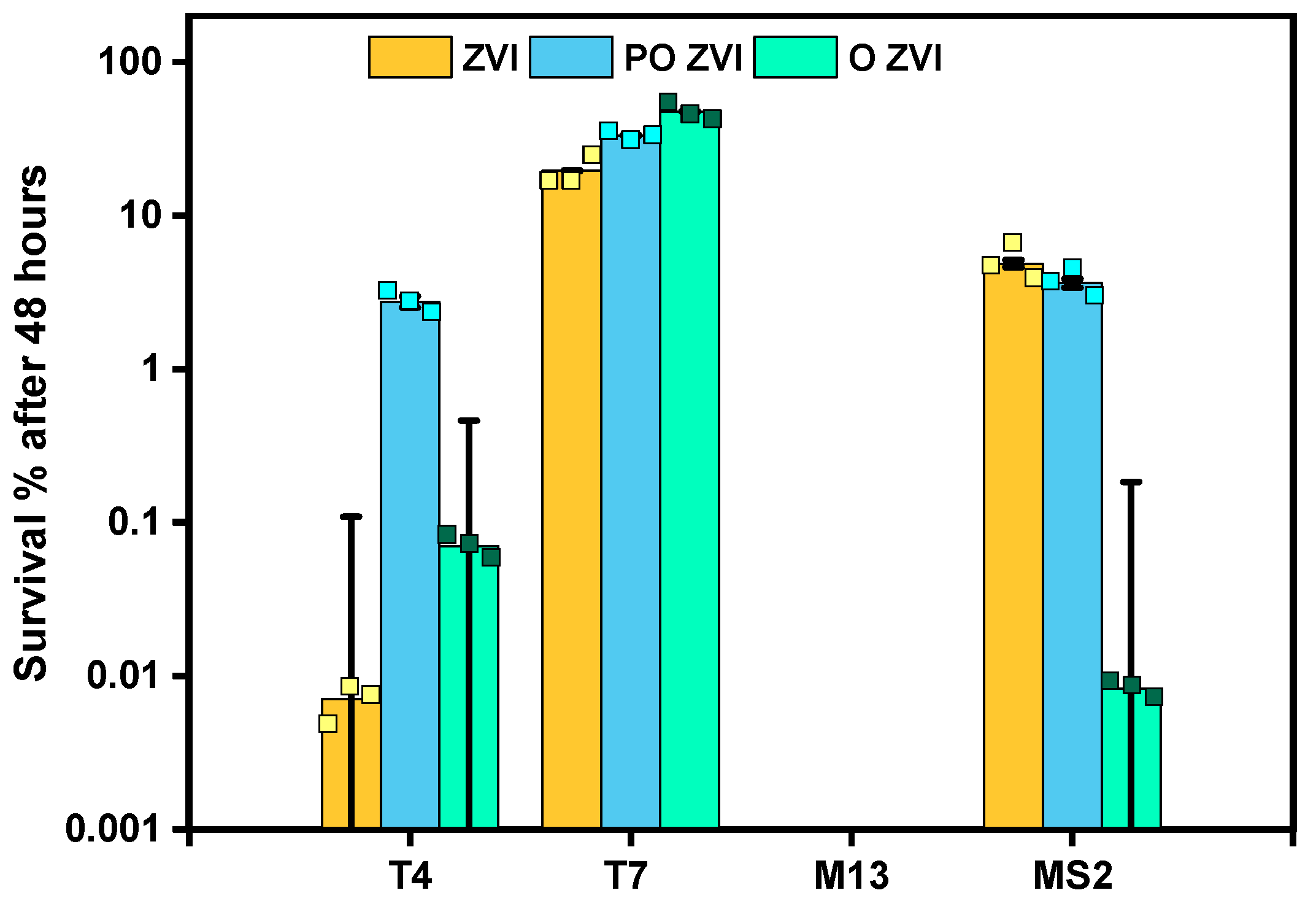
3.2. Influence of nZVI on Bacteriophages
3.3. Influence of Fe(II)/Fe(III) Ions on Bacteriophages
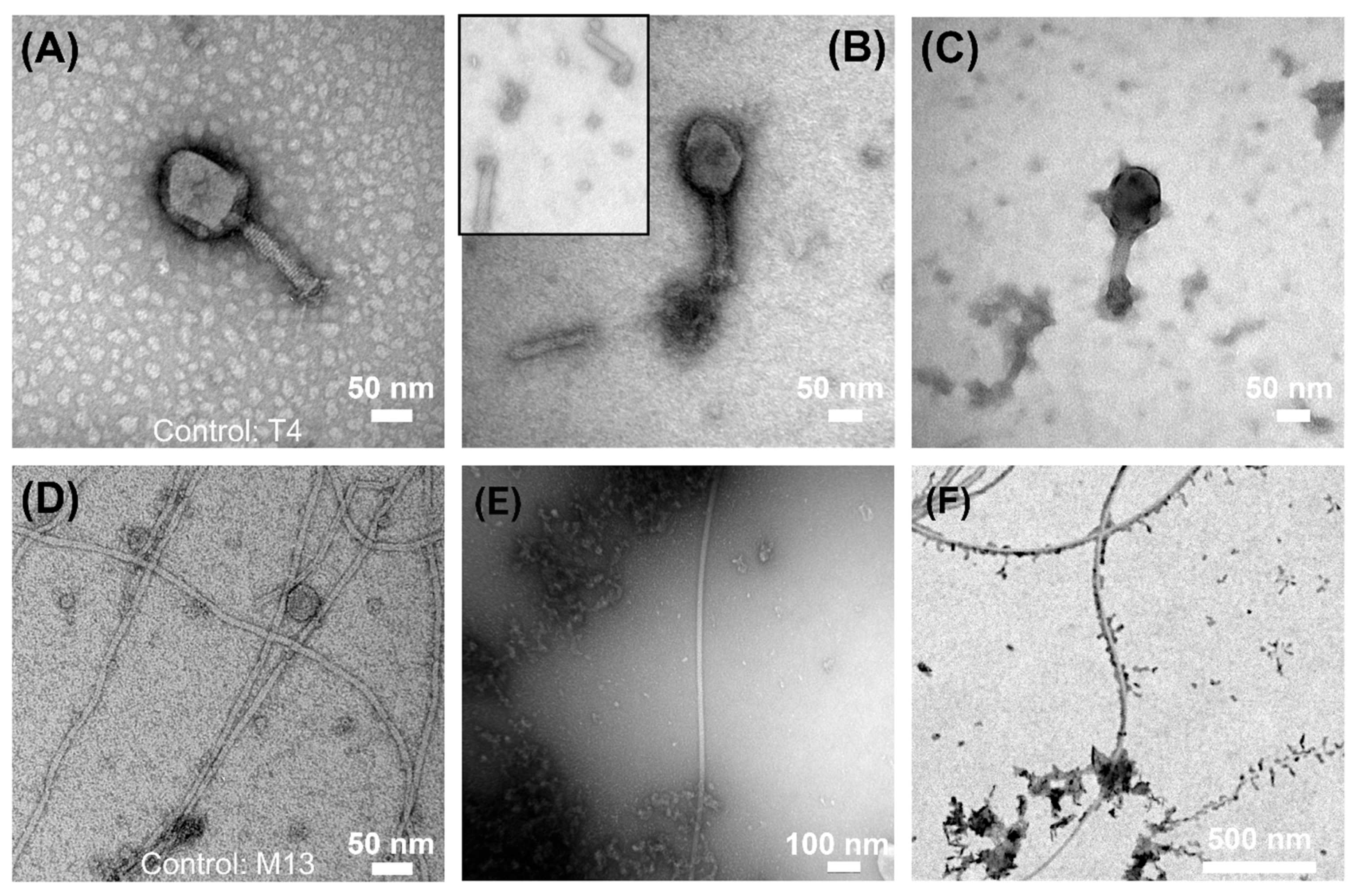
3.4. Electron Microscopy Visualization of Phages Exposed to ZVI
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Naureen, Z.; Dautaj, A.; Anpilogov, K.; Camilleri, G.; Dhuli, K.; Tanzi, B.; Maltese, P.E.; Cristofoli, F.; de Antoni, L.; Beccari, T.; et al. Bacteriophages presence in nature and their role in the natural selection of bacterial populations. Acta Biomed. 2020, 91, e2020024. [Google Scholar] [CrossRef] [PubMed]
- Wegrzyn, G.; Łoś, M.; Neubauer, P. A simple emergency procedure to be used if biotechnological protein production is endangered by bacteriophage infection of Escherichia coli cultures: Effective inhibition of bacteriophage lytic development in infected cultures by removing a carbon source. Microb. Cell Fact. 2006, 5, P81. [Google Scholar] [CrossRef]
- Calendar, R.; Abedon, S. Book, The Bacteriophages, 2nd ed.; Oxford University Press: Oxford, UK, 2006; ISBN 9780195148503. [Google Scholar]
- De Melo, A.G.; Levesque, S.; Moineau, S. Phages as friends and enemies in food processing. Curr. Opin. Biotechnol. 2018, 49, 185–190. [Google Scholar] [CrossRef]
- Ghosh, K.; Kang, H.S.; Hyun, W.B.; Kim, K.P. High prevalence of Bacillus subtilis-infecting bacteriophages in soybean-based fermented foods and its detrimental effects on the process and quality of Cheonggukjang. Food Microbiol. 2018, 76, 196–203. [Google Scholar] [CrossRef] [PubMed]
- Łos, M.; Czyz, A.; Sell, E.; Wȩgrzyn, A.; Neubauer, P.; Wȩgrzyn, G. Bacteriophage contamination: Is there a simple method to reduce its deleterious effects in laboratory cultures and biotechnological factories? J. Appl. Genet. 2004, 45, 111–120. [Google Scholar] [PubMed]
- Fister, S.; Mester, P.; Witte, A.K.; Sommer, J.; Schoder, D.; Rossmanith, P. Part of the problem or the solution? Indiscriminate use of bacteriophages in the food industry can reduce their potential and impair growth-based detection methods. Trends Food Sci. Technol. 2019, 90, 170–174. [Google Scholar] [CrossRef]
- Mayo, J.A. A comparison of methods for detecting bacteriophage contamination of tissue culture sera. In Vitro 1978, 14, 413–417. [Google Scholar] [CrossRef]
- Brown-Jaque, M.; Muniesa, M.; Navarro, F. Bacteriophages in clinical samples can interfere with microbiological diagnostic tools. Sci. Rep. 2016, 6, 33000. [Google Scholar] [CrossRef] [Green Version]
- Łoś, M. Strategies of phage contamination prevention in industry. Open J. Bacteriol. 2020, 4, 020–023. [Google Scholar] [CrossRef]
- Capra, M.L.; Patrignani, F.; del Quiberoni, A.L.; Reinheimer, J.A.; Lanciotti, R.; Guerzoni, M.E. Effect of high pressure homogenization on lactic acid bacteria phages and probiotic bacteria phages. Int. Dairy J. 2009, 19, 336–341. [Google Scholar] [CrossRef]
- Guglielmotti, D.M.; Mercanti, D.J.; Reinheimer, J.A.; del Quiberoni, A.L. Review: Efficiency of physical and chemical treatments on the inactivation of dairy bacteriophages. Front. Microbiol. 2012, 2, 282. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, X.; Liu, Y.; Fan, M.; Wang, Z.; Wu, W.; Wang, J. Thermal and chemical inactivation of Lactobacillus virulent bacteriophage. J. Dairy Sci. 2017, 100, 7041–7050. [Google Scholar] [CrossRef] [PubMed]
- Agún, S.; Fernández, L.; González-Menéndez, E.; Martínez, B.; Rodríguez, A.; García, P. Study of the interactions between bacteriophage phiIPLA-RODI and four chemical disinfectants for the elimination of Staphylococcus aureus contamination. Viruses 2018, 10, 103. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hayes, S.; Murphy, J.; Mahony, J.; Lugli, G.A.; Ventura, M.; Noben, J.P.; Franz, C.M.A.P.; Neve, H.; Nauta, A.; Van Sinderen, D. Biocidal inactivation of Lactococcus lactis bacteriophages: Efficacy and targets of commonly used sanitizers. Front. Microbiol. 2017, 8, 107. [Google Scholar] [CrossRef] [Green Version]
- Yusuf, A.; Al Jitan, S.; Garlisi, C.; Palmisano, G. A review of recent and emerging antimicrobial nanomaterials in wastewater treatment applications. Chemosphere 2021, 278, 130440. [Google Scholar] [CrossRef]
- Wybrańska, K.; Paczesny, J.; Serejko, K.; Sura, K.; Włodyga, K.; Dzięcielewski, I.; Jones, S.T.; Śliwa, A.; Wybrańska, I.; Hołyst, R.; et al. Gold-oxoborate nanocomposites and their biomedical applications. ACS Appl. Mater. Interfaces 2015, 7, 3931–3939. [Google Scholar] [CrossRef]
- Budarz, J.F.; Badireddy, A.R.; Chellam, S.; Wiesner, M.R. Bacteriophage inactivation by UV-A illuminated fullerenes: Role of nanoparticle-virus association. Water Qual. Technol. Conf. Expo. 2012, 46, 5963–5970. [Google Scholar]
- You, J.; Zhang, Y.; Hu, Z. Bacteria and bacteriophage inactivation by silver and zinc oxide nanoparticles. Colloids Surf. B Biointerfaces 2011, 85, 161–167. [Google Scholar] [CrossRef]
- Syngouna, V.I.; Chrysikopoulos, C.V. Inactivation of MS2 bacteriophage by titanium dioxide nanoparticles in the presence of quartz sand with and without ambient light. J. Colloid Interface Sci. 2017, 497, 117–125. [Google Scholar] [CrossRef]
- Abdelsattar, A.S.; Nofal, R.; Makky, S.; Safwat, A.; Taha, A.; El-Shibiny, A. The synergistic effect of biosynthesized silver nanoparticles and phage zcse2 as a novel approach to combat multidrug-resistant salmonella enterica. Antibiotics 2021, 10, 678. [Google Scholar] [CrossRef]
- Cheng, R.; Kang, M.; Zhuang, S.; Wang, S.; Zheng, X.; Pan, X.; Shi, L.; Wang, J. Removal of bacteriophage f2 in water by Fe/Ni nanoparticles: Optimization of Fe/Ni ratio and influencing factors. Sci. Total Environ. 2019, 649, 995–1003. [Google Scholar] [CrossRef] [PubMed]
- Bonnain, C.; Breitbart, M.; Buck, K.N. The Ferrojan horse hypothesis: Iron-virus interactions in the ocean. Front. Mar. Sci. 2016, 3, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Muratore, D.; Weitz, J.S. Infect while the iron is scarce: Nutrient-explicit phage-bacteria games. Theor. Ecol. 2021, 14, 467–487. [Google Scholar] [CrossRef]
- Kim, K.; Narayanan, J.; Sen, A.; Chellam, S. Virus Removal and Inactivation Mechanisms during Iron Electrocoagulation: Capsid and Genome Damages and Electro-Fenton Reactions. Environ. Sci. Technol. 2021, 55, 13198–13208. [Google Scholar] [CrossRef]
- Heffron, J.; McDermid, B.; Mayer, B.K. Bacteriophage inactivation as a function of ferrous iron oxidation. Environ. Sci. Water Res. Technol. 2019, 5, 1309–1317. [Google Scholar] [CrossRef]
- Nieto-Juarez, J.I.; Kohn, T. Virus removal and inactivation by iron (hydr)oxide-mediated Fenton-like processes under sunlight and in the dark. Photochem. Photobiol. Sci. 2013, 12, 1596–1605. [Google Scholar] [CrossRef]
- Gutierrez, L.; Li, X.; Wang, J.; Nangmenyi, G.; Economy, J.; Kuhlenschmidt, T.B.; Kuhlenschmidt, M.S.; Nguyen, T.H. Adsorption of rotavirus and bacteriophage MS2 using glass fiber coated with hematite nanoparticles. Water Res. 2009, 43, 5198–5208. [Google Scholar] [CrossRef]
- Bradley, I.; Straub, A.; Maraccini, P.; Markazi, S.; Nguyen, T.H. Iron oxide amended biosand filters for virus removal. Water Res. 2011, 45, 4501–4510. [Google Scholar] [CrossRef] [Green Version]
- De Cortalezzi, M.M.F.; Gallardo, M.V.; Yrazu, F.; Gentile, G.J.; Opezzo, O.; Pizarro, R.; Poma, H.R.; Rajal, V.B. Virus removal by iron oxide ceramic membranes. J. Environ. Chem. Eng. 2014, 2, 1831–1840. [Google Scholar] [CrossRef]
- You, Y.; Han, J.; Chiu, P.C.; Jin, Y. Removal and inactivation of waterborne viruses using zerovalent iron. Environ. Sci. Technol. 2005, 39, 9263–9269. [Google Scholar] [CrossRef]
- Briggiler Marcó, M.; del Quiberoni, A.L.; Negro, A.C.; Reinheimer, J.A.; Alfano, O.M. Evaluation of the photocatalytic inactivation efficiency of dairy bacteriophages. Chem. Eng. J. 2011, 172, 987–993. [Google Scholar] [CrossRef]
- Nieto-Juarez, J.I.; Pierzchla, K.; Sienkiewicz, A.; Kohn, T. Inactivation of MS2 coliphage in Fenton and Fenton-like systems: Role of transition metals, hydrogen peroxide and sunlight. Environ. Sci. Technol. 2010, 44, 3351–3356. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.Y.; Lee, C.; Sedlak, D.L.; Yoon, J.; Nelson, K.L. Inactivation of MS2 coliphage by Fenton’s reagent. Water Res. 2010, 44, 2647–2653. [Google Scholar] [CrossRef] [PubMed]
- Hu, L.; Page, M.A.; Sigstam, T.; Kohn, T.; Mariñas, B.J.; Strathmann, T.J. Inactivation of bacteriophage MS2 with potassium ferrate(VI). Environ. Sci. Technol. 2012, 46, 12079–12087. [Google Scholar] [CrossRef]
- Cheng, R.; Li, G.; Shi, L.; Xue, X.; Kang, M.; Zheng, X. The mechanism for bacteriophage f2 removal by nanoscale zero-valent iron. Water Res. 2016, 105, 429–435. [Google Scholar] [CrossRef] [PubMed]
- Heffron, J.; McDermid, B.; Maher, E.; McNamara, P.J.; Mayer, B.K. Mechanisms of virus mitigation and suitability of bacteriophages as surrogates in drinking water treatment by iron electrocoagulation. Water Res. 2019, 163, 114877. [Google Scholar] [CrossRef]
- Kim, J.Y.; Lee, C.; Love, D.C.; Sedlak, D.L.; Yoon, J.; Nelson, K.L. Inactivation of MS2 coliphage by ferrous ion and zero-valent iron nanoparticles. Environ. Sci. Technol. 2011, 45, 6978–6984. [Google Scholar] [CrossRef]
- Cheng, R.; Li, G.; Cheng, C.; Liu, P.; Shi, L.; Ma, Z.; Zheng, X. Removal of bacteriophage f2 in water by nanoscale zero-valent iron and parameters optimization using response surface methodology. Chem. Eng. J. 2014, 252, 150–158. [Google Scholar] [CrossRef]
- Shi, C.; Wei, J.; Jin, Y.; Kniel, K.E.; Chiu, P.C. Removal of viruses and bacteriophages from drinking water using zero-valent iron. Sep. Purif. Technol. 2012, 84, 72–78. [Google Scholar] [CrossRef]
- Yuan, D.; Zhai, L.; Zhang, X.; Cui, Y.; Wang, X.; Zhao, Y.; Xu, H.; He, L.; Yan, C.; Cheng, R.; et al. Study on the characteristics and mechanism of bacteriophage MS2 inactivated by bacterial cellulose supported nanoscale zero-valent iron. J. Clean. Prod. 2020, 270, 122527. [Google Scholar] [CrossRef]
- Shearer, A.E.H.; Kniel, K.E. Enhanced removal of norovirus surrogates, murine norovirus and tulane virus, from aqueous systems by zero-valent iron. J. Food Prot. 2018, 81, 1432–1438. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Yang, Z.; Liu, H.; Lv, X.; Tu, Q.; Ren, Q.; Xia, X.; Jing, C. Common oxidants activate the reactivity of zero-valent iron (ZVI) and hence remarkably enhance nitrate reduction from water. Sep. Purif. Technol. 2015, 146, 227–234. [Google Scholar] [CrossRef]
- Mokete, R.; Eljamal, O.; Sugihara, Y. Exploration of the reactivity of nanoscale zero-valent iron (NZVI) associated nanoparticles in diverse experimental conditions. Chem. Eng. Processing-Process Intensif. 2020, 150, 107879. [Google Scholar] [CrossRef]
- Foltynowicz, Z.; Bardenshtein, A.; Sängerlaub, S.; Antvorskov, H.; Kozak, W. Nanoscale, zero valent iron particles for application as oxygen scavenger in food packaging. Food Packag. Shelf Life 2017, 11, 74–83. [Google Scholar] [CrossRef]
- Foltynowicz, Z.W.; Kozak, J.; Stoińska, M.; Urbańska, K.; Muc, A.; Forysiak, K.K. Nanoiron-based oxygen scavengers. Patent EPO EP2658666A1, 2018. [Google Scholar]
- Richter, Ł.; Księżarczyk, K.; Paszkowska, K.; Janczuk-Richter, M.; Niedziółka-Jönsson, J.; Gapiński, J.; Łoś, M.; Hołyst, R.; Paczesny, J.; Richter, Ł.; et al. Adsorption of bacteriophages on polypropylene labware affects reproducibility of phage research. Sci. Rep. 2021, 11, 7387. [Google Scholar] [CrossRef]
- Ligaj, M.; Tichoniuk, M.; Cierpiszewski, R.; Foltynowicz, Z. Efficiency of novel antimicrobial coating based on iron nanoparticles for dairy products’ packaging. Coatings 2020, 10, 156. [Google Scholar] [CrossRef] [Green Version]
- Lawrence, D.; Baldridge, M.T.; Handley, S.A. Phages and human health: More than idle hitchhikers. Viruses 2019, 11, 587. [Google Scholar] [CrossRef] [Green Version]
- Dawson, D.J.; Paish, A.; Staffell, L.M.; Seymour, I.J.; Appleton, H. Survival of viruses on fresh produce, using MS2 as a surrogate for norovirus. J. Appl. Microbiol. 2005, 98, 203–209. [Google Scholar] [CrossRef]
- Jacquet, N.; Wurtzer, S.; Darracq, G.; Wyart, Y.; Moulin, L.; Moulin, P. Effect of concentration on virus removal for ultrafiltration membrane in drinking water production. J. Membr. Sci. 2021, 634, 119417. [Google Scholar] [CrossRef]
- String, G.M.; White, M.R.; Gute, D.M.; Mühlberger, E.; Lantagne, D.S. Selection of a SARS-CoV-2 Surrogate for Use in Surface Disinfection Efficacy Studies with Chlorine and Antimicrobial Surfaces. Environ. Sci. Technol. Lett. 2021, 8, 995–1001. [Google Scholar] [CrossRef]
- Peng, H.; Chen, I.A. Phage engineering and the evolutionary arms race. Curr. Opin. Biotechnol. 2021, 68, 23–29. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.Y.; Chang, Y.C.; Hu, C.W.; Kao, C.Y.; Yu, Y.A.; Lim, S.K.; Mou, K.Y. Development of a Novel Cytokine Vehicle Using Filamentous Phage Display for Colorectal Cancer Treatment. ACS Synth. Biol. 2021, 10, 2087–2095. [Google Scholar] [CrossRef] [PubMed]
- Richter, Ł.; Janczuk-Richter, M.; Niedziółka-Jönsson, J.; Paczesny, J.; Hołyst, R. Recent advances in bacteriophage-based methods for bacteria detection. Drug Discov. Today 2018, 23, 448–455. [Google Scholar] [CrossRef]
- Peng, H.; Pearce, C.I.; Huang, W.; Zhu, Z.; N’Diaye, A.T.; Rosso, K.M.; Liu, J. Reversible Fe(II) uptake/release by magnetite nanoparticles. Environ. Sci. Nano 2018, 5, 1545–1555. [Google Scholar] [CrossRef]
- Hem, J.D.; Cropper, W.H. Survey of Ferrous-Ferric Chemical Equilibria and Redox Potentials. Chem. Iron Nat. Water 1962, 268. [Google Scholar]
- Kuzmanovic, D.A.; Elashvili, I.; Wick, C.; Connell, C.O.; Krueger, S. Bacteriophage MS2: Molecular Weight and Spatial Distribution of the Protein and RNA Components by Small-Angle Neutron Scattering and Virus Counting. Structure 2003, 11, 1339–1348. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moghimian, P.; Srot, V.; Pichon, B.P.; Facey, S.J.; Aken, P.A. van Stability of M13 Phage in Organic Solvents. J. Biomater. Nanobiotechnol. 2016, 07, 72–77. [Google Scholar] [CrossRef] [Green Version]
- Yap, M.L.; Rossmann, M.G. Structure and function of bacteriophage T4. Future Microbiol. 2014, 9, 1319–1337. [Google Scholar] [CrossRef] [Green Version]
- Leiman, P.G.; Arisaka, F.; Van Raaij, M.J.; Kostyuchenko, V.A.; Aksyuk, A.A.; Kanamaru, S.; Rossmann, M.G. Morphogenesis of the T4 tail and tail fibers. Virol. J. 2010, 7, 355. [Google Scholar] [CrossRef] [Green Version]
- Deng, X.; Wang, L.; You, X.; Dai, P.; Zeng, Y. Advances in the T7 phage display system (Review). Mol. Med. Rep. 2018, 17, 714–720. [Google Scholar] [CrossRef] [Green Version]
- Hu, B.; Margolin, W.; Molineux, I.J.; Liu, J. The Bacteriophage T7 Virion Undergoes Extensive Structural Remodeling During Infection. Science 2013, 339, 576–579. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Armanious, A.; Aeppli, M.; Jacak, R.; Refardt, D.; Sigstam, T.; Kohn, T.; Sander, M. Viruses at Solid-Water Interfaces: A Systematic Assessment of Interactions Driving Adsorption. Environ. Sci. Technol. 2016, 50, 732–743. [Google Scholar] [CrossRef] [PubMed]
- Putra, B.R.; Szot-Karpińska, K.; Kudła, P.; Yin, H.; Boswell, J.A.; Squires, A.M.; Da Silva, M.A.; Edler, K.J.; Fletcher, P.J.; Parker, S.C.; et al. Bacteriophage M13 Aggregation on a Microhole Poly(ethylene terephthalate) Substrate Produces an Anionic Current Rectifier: Sensitivity toward Anionic versus Cationic Guests. ACS Appl. Bio Mater. 2019, 3, 512–521. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Han, J.H.; Wang, M.S.; Das, J.; Sudheendra, L.; Vonasek, E.; Nitin, N.; Kennedy, I.M. Capture and detection of T7 bacteriophages on a nanostructured interface. ACS Appl. Mater. Interfaces 2014, 6, 4758–4765. [Google Scholar] [CrossRef]
- Nobrega, F.L.; Costa, A.R.; Santos, J.F.; Siliakus, M.F.; Van Lent, J.W.M.; Kengen, S.W.M.; Azeredo, J.; Kluskens, L.D. Genetically manipulated phages with improved pH resistance for oral administration in veterinary medicine. Sci. Rep. 2016, 6, 39235. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Greve, J.; Blok, J. Transient electric birefringence of T-even bacteriophages. II. T4B in the Presence of tryptophan and T4D. Biopolymers 1975, 14, 139–154. [Google Scholar] [CrossRef] [PubMed]
- Richter, Ł.; Matuła, K.; Leśniewski, A.; Kwaśnicka, K.; Łoś, J.; Łoś, M.; Paczesny, J.; Hołyst, R. Ordering of bacteriophages in the electric field: Application for bacteria detection. Sens. Actuators B Chem. 2016, 224, 233–240. [Google Scholar] [CrossRef]
- De Groot, G.; Greve, J.; Block, J. Transient electric birefringence of the bacteriophages T3 and T7. Biopolymers 1977, 16, 639–654. [Google Scholar] [CrossRef]
- Matuła, K.; Richter, Ł.; Adamkiewicz, W.; Åkerström, B.; Paczesny, J.; Hołyst, R.; Yamamoto, O.; Pan, Y.; Neuss, S.; Leifert, A.; et al. Influence of nanomechanical stress induced by ZnO nanoparticles of different shapes on the viability of cells. Soft Matter 2016, 12, 4162–4169. [Google Scholar] [CrossRef]
- Taylor, N.M.I.; Prokhorov, N.S.; Guerrero-Ferreira, R.C.; Shneider, M.M.; Browning, C.; Goldie, K.N.; Stahlberg, H.; Leiman, P.G. Structure of the T4 baseplate and its function in triggering sheath contraction. Nature 2016, 533, 346–352. [Google Scholar] [CrossRef]
- Zhou, Z.; Alhadidi, Q.; Quiñones Deliz, K.; Yamaguchi Greenslet, H.; Bonzongo, J.C. Removal of Oxyanion Forming Elements from Contaminated Soils through Combined Sorption onto Zero-Valent Iron (ZVI) and Magnetic Separation: Arsenic and Chromium as Case Studies. Soil Sediment Contam. 2020, 29, 180–191. [Google Scholar] [CrossRef]
- Richter, Ł.; Paszkowska, K.; Cendrowska, U.; Olgiati, F.; Silva, P.J.; Gasbarri, M.; Guven, Z.P.; Paczesny, J.; Stellacci, F. Broad-spectrum nanoparticles against bacteriophage infections. Nanoscale 2021, 13, 18684–18694. [Google Scholar] [CrossRef] [PubMed]
- Cheng, R.; Zhang, Y.; Zhang, T.; Hou, F.; Cao, X.; Shi, L.; Jiang, P. The inactivation of bacteriophages MS2 and PhiX174 by nanoscale zero-valent iron: Resistance difference and mechanisms. Front. Environ. Sci. Eng. 2022, 16, 108. [Google Scholar] [CrossRef]
| MS2 | M13 | T4 | T7 | |
|---|---|---|---|---|
| shape | icosahedral | filamentous | myophage (long contractile tail) | podophage (short tail) |
| size | 23 to 28 nm [58] | 880 × 5.5 nm [59] | 115 × 85 nm capsid, 92 × 24 nm tail, 145 nm motile fibers [60,61] | 60 nm capsid [62], 23 nm tail [63], fibers usually bound to capsid, with 90° kink separating two parts around 30 nm and around 20 nm long [63] |
| genetic material | ssRNA | ssDNA | dsDNA | dsDNA |
| genome size | 3569 nucleotides | 6407 nucleotides | 168 903 bp | 39 936 bp |
| zeta potential at pH around 7 | around −40 mV [64] | −18 mV [65] | −26 mV [47] | −21.1mV [66] −5.49 mV [67] |
| dipole moment | NA | NA | 24 kD (200 kD when the fibers are extended) [68,69] | 5096 D [70]. The charges are unequal, with a larger negative charge on the head and a smaller charge on fibers/little tail [66]. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Raza, S.; Folga, M.; Łoś, M.; Foltynowicz, Z.; Paczesny, J. The Effect of Zero-Valent Iron Nanoparticles (nZVI) on Bacteriophages. Viruses 2022, 14, 867. https://doi.org/10.3390/v14050867
Raza S, Folga M, Łoś M, Foltynowicz Z, Paczesny J. The Effect of Zero-Valent Iron Nanoparticles (nZVI) on Bacteriophages. Viruses. 2022; 14(5):867. https://doi.org/10.3390/v14050867
Chicago/Turabian StyleRaza, Sada, Michał Folga, Marcin Łoś, Zenon Foltynowicz, and Jan Paczesny. 2022. "The Effect of Zero-Valent Iron Nanoparticles (nZVI) on Bacteriophages" Viruses 14, no. 5: 867. https://doi.org/10.3390/v14050867
APA StyleRaza, S., Folga, M., Łoś, M., Foltynowicz, Z., & Paczesny, J. (2022). The Effect of Zero-Valent Iron Nanoparticles (nZVI) on Bacteriophages. Viruses, 14(5), 867. https://doi.org/10.3390/v14050867







