Cutaneous Leishmaniasis Emergence in Southeastern Mexico: The Case of the State of Yucatan
Abstract
:1. Introduction
2. Human LCL in Yucatan State and Mexico
2.1. Historical Records
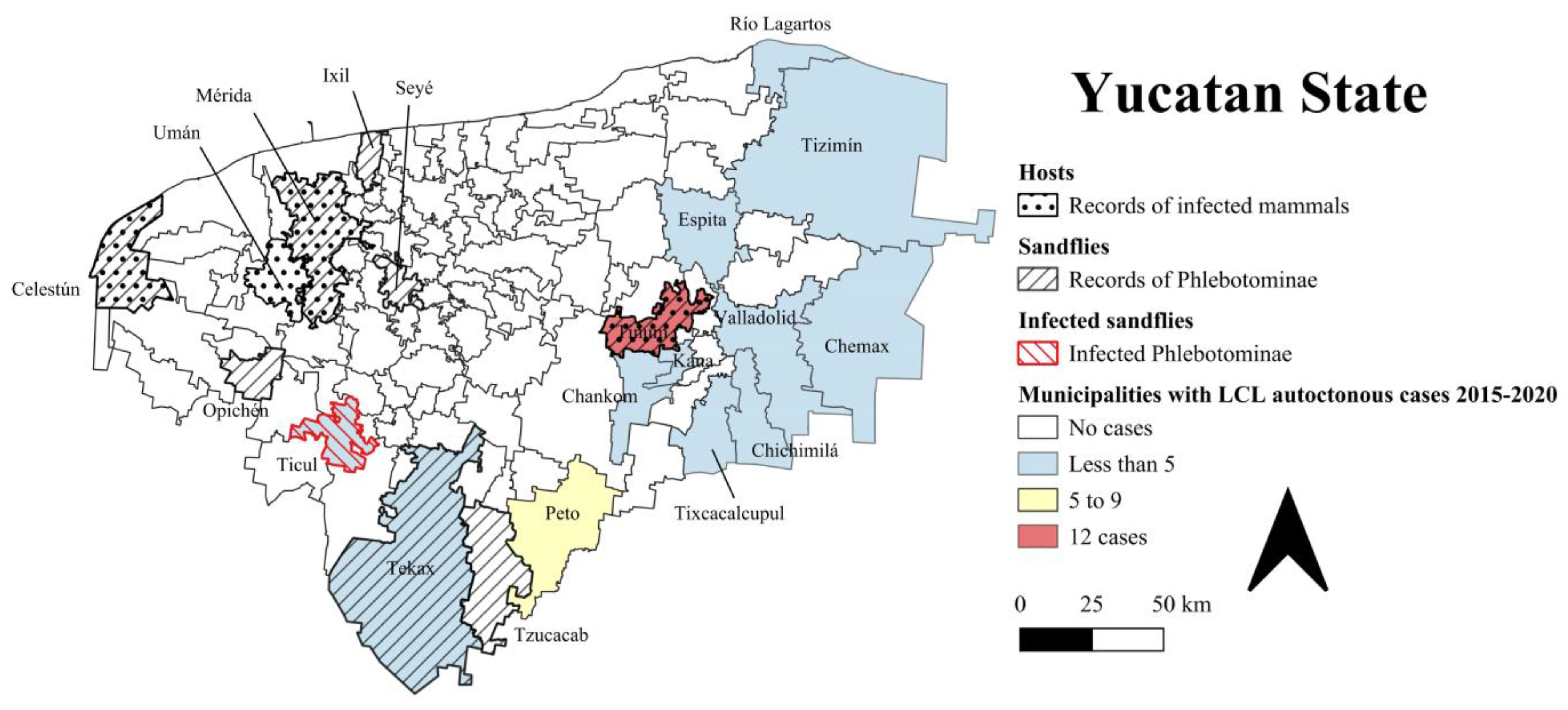
2.2. Current Situation
3. Vector Sandfly Species
4. Vertebrate Hosts
5. Impact of Environmental Factors on the Emergence of Leishmaniasis
5.1. Deforestation and Urbanization
5.2. Climate Change
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- PAHO. Manual of Procedures for Surveillance and Control of Leishmaniasis in the Americas; Pan American Health Organization: Washington, DC, USA, 2019; ISBN 978-92-75-32063-1. [Google Scholar]
- Ruiz-Postigo, J.A.; Jain, S.; Mikhailov, A.; Maia-Elkhoury, A.N.S.; Valadas, S.; Warusavithana, S.; Osman, M.; Lin, Z.; Beshah, A.; Yajima, A.; et al. Global Leishmaniasis Surveillance: 2019–2020 a Baseline for the 2030 Roadmap. Wkly. Epidemiol. Rec. 2021, 35, 401–419. [Google Scholar]
- World Health Organization. Leishmaniasis. Available online: https://www.who.int/health-topics/leishmaniasis#tab=tab_1 (accessed on 26 July 2022).
- Centro Nacional de Vigilancia Epidemiológica y Control de Enfermedades. Dirección General de Programas preventivos. Programa de Enfermedades Transmitidas por Vector. In Manual Para El Diagnóstico, Tratamiento y Control de Las Leishmaniasis; Centro Nacional de Programas Preventivos y Control de Enfermedades, México: Secretaría de Salud: Ciudad de México, México, 2015. [Google Scholar]
- Hernández-Rivera, M.P.; Hernández-Montes, O.; Chiñas-Pérez, A.; Batiza-Avelar, J.M.; Sánchez-Tejeda, G.; Wong-Ramírez, C.; Monroy-Ostria, A. Study of Cutaneous Leishmaniasis in the State of Campeche (Yucatan Peninsula), Mexico, over a Period of Two Years. Salud Publica Mex. 2015, 57, 58–65. [Google Scholar] [CrossRef] [PubMed]
- Pech-May, A.; Escobedo-Ortegón, F.J.; Berzunza-Cruz, M.; Rebollar-Téllez, E.A. Incrimination of Four Sandfly Species Previously Unrecognized as Vectors of Leishmania Parasites in Mexico. Med. Vet. Entomol. 2010, 24, 150–161. [Google Scholar] [CrossRef] [PubMed]
- Beltran, E. Cutaneous Leishmaniasis in Mexico. Sci. Mon. 1944, 59, 108–119. [Google Scholar]
- Zavala-Velazquez, J. Leishmaniasis in Yucatan. Gac. Med. Mex. 1972, 104, 1–7. [Google Scholar] [PubMed]
- Secretaría de Salud Anuarios de Morbilidad 1984 a 2020. Available online: https://www.gob.mx/salud/acciones-y-programas/anuarios-de-morbilidad-1984-a-2020 (accessed on 9 May 2022).
- Moo-Llanes, D.; Ibarra-Cerdeña, C.N.; Rebollar-Téllez, E.A.; Ibáñez-Bernal, S.; González, C.; Ramsey, J.M. Current and Future Niche of North and Central American Sand Flies (Diptera: Psychodidae) in Climate Change Scenarios. PLoS Negl. Trop. Dis. 2013, 7, e2421. [Google Scholar] [CrossRef] [Green Version]
- Moo-Llanes, D.A.; Pech-May, A.; Ibarra-Cerdeña, C.N.; Rebollar-Téllez, E.A.; Ramsey, J.M. Inferring Distributional Shifts of Epidemiologically Important North and Central American Sandflies from Pleistocene to Future Scenarios. Med. Vet. Entomol. 2019, 33, 31–43. [Google Scholar] [CrossRef] [Green Version]
- Albertos-Alpuche, N.E.; Andrade-Narváez, F.J.; Burgos-Patrón, J.P.; Vázquez-Pérez, A. Leishmaniasis Cutánea Localizada: Índice Alérgico En La Comunidad de Becanchén, Tekax, Yucatán, México. Rev. Biomédica 1996, 7, 11–18. [Google Scholar]
- Carstens-Kass, J.; Paulini, K.; Lypaczewski, P.; Matlashewski, G. A Review of the Leishmanin Skin Test: A Neglected Test for a Neglected Disease. PLoS Negl. Trop. Dis. 2021, 15, e0009531. [Google Scholar] [CrossRef]
- Secretaría de Salud México Manual de Procedimientos Estandarizados Para La Vigilancia Epidemiológica de Las Enfermedades Transmitidas Por Vector. Available online: https://epidemiologia.salud.gob.mx/gobmx/salud/documentos/manuales/36_Manual_ETV.pdf (accessed on 9 May 2022).
- Secretaría de Salud México Programa de Acción Específico: Prevención y Control de Las Leishmaniasis 2013–2018. Available online: https://www.gob.mx/salud/documentos/programa-de-accion-especifico-prevencion-y-control-de-las-leishmaniasis-2013-2018 (accessed on 9 May 2022).
- Canché-Pool, E.B.; Canto-Hau, D.M.; Vargas-Meléndez, M.A.; Tello-Martín, R.; Reyes-Novelo, E.; Escobedo-Ortegón, F.J.; Ruiz-Piña, H.A.; Cambranes-Puc, L.H.; Torres-Castro, J.R.; Palacio-Vargas, J.A.; et al. Report of Autochthonous Cases of Localized Cutaneous Leishmaniasis Caused by Leishmania (Leishmania) Mexicana in Vulnerable, Susceptible Areas of Southeastern Mexico. Rev. Inst. Med. Trop. Sao Paulo 2022, 64, e35. [Google Scholar] [CrossRef]
- Loría-Cervera, E.N.; Sosa-Bibiano, E.I.; van Wynsberghe, N.R.; Torres-Castro, J.R.; Andrade-Narváez, F.J. Preliminary Epidemiological Findings of Leishmania Infection in the Municipality of Tinum, Yucatan State, Mexico. Parasite Epidemiol. Control 2019, 4, e00088. [Google Scholar] [CrossRef] [PubMed]
- Longoni, S.S.; Marín, C.; Sauri-Arceo, C.H.; López-Cespedes, A.; Rodríguez-Vivas, R.I.; Villegas, N.; Escobedo-Ortegón, J.; Barrera-Pérez, M.A.; Bolio-Gonzalez, M.E.; Sánchez-Moreno, M. An Iron-Superoxide Dismutase Antigen-Based Serological Screening of Dogs Indicates Their Potential Role in the Transmission of Cutaneous Leishmaniasis and Trypanosomiasis in Yucatan, Mexico. Vector-Borne Zoonotic Dis. 2011, 11, 815–821. [Google Scholar] [CrossRef]
- Arjona-Jiménez, G.; Villegas, N.; López-Céspedes, Á.; Marín, C.; Longoni, S.S.; Bolio-González, M.E.; Rodríguez-Vivas, R.I.; Sauri-Arceo, C.H.; Sánchez-Moreno, M. Prevalence of Antibodies against Three Species of Leishmania (L. mexicana, L. braziliensis, L. infantum) and Possible Associated Factors in Dogs from Mérida, Yucatán, Mexico. Trans. R. Soc. Trop. Med. Hyg. 2012, 106, 252–258. [Google Scholar] [CrossRef]
- Longoni, S.S.; López-Cespedes, A.; Sánchez-Moreno, M.; Bolio-Gonzalez, M.E.; Sauri-Arceo, C.H.; Rodríguez-Vivas, R.I.; Marín, C. Detection of Different Leishmania spp. and Trypanosoma Cruzi Antibodies in Cats from the Yucatan Peninsula (Mexico) Using an Iron Superoxide Dismutase Excreted as Antigen. Comp. Immunol. Microbiol. Infect. Dis. 2012, 35, 469–476. [Google Scholar] [CrossRef]
- Sosa-Bibiano, E.I.; Sánchez -Martínez, L.A.; López-Ávila, K.B.; Chablé-Santos, J.B.; Torres-Castro, J.R.; Fernández-Figueroa, E.A.; Rangel-Escareño, C.; Loría-Cervera, E.N. Leishmania (Leishmania) Mexicana Infection in Wild Rodents from an Emergent Focus of Cutaneous Leishmaniasis in Yucatan, Mexico. J. Trop. Med. 2022, 2022, 1–7. [Google Scholar] [CrossRef]
- de Oca-Aguilar, A.C.M.; Moo-Llanes, D.; Rebollar-Téllez, E.A. Sand Fly Species from a Karstic Cave in the Peninsula of Yucatan, Mexico. Entomol. News 2013, 123, 191–200. [Google Scholar] [CrossRef]
- Ibáñez-Bernal, S.; Durán-Luz, J. An Actualized Catalogue of the Psychodidae (Diptera) of Mexico and Their Known Distribution by State. Zootaxa 2022, 5104, 347–408. [Google Scholar] [CrossRef]
- Navarrete-Carballo, J.; Huerta-Jiménez, H.; Loría-Cervera, E.N.; Manrique-Saide, P.; Sosa-Bibiano, E.I. Phlebotomine Sand Flies (Diptera: Psychodidae) from an Emergent Focus of Localized Cutaneous Leishmaniasis in Yucatan, Southeast Mexico. J. Vector Ecol. 2022, 47, 9–18. [Google Scholar] [CrossRef]
- Pérez-Blas, L.G.; Chiyean-Acosta, A.G.; Canché-Pool, E.B.; Tello-Martín, R.; Torres-Castro, J.R.; Ruiz-Piña, H.A.; Flores-Mejía, R.; Rodríguez-Cortez, O.; Reyes-Novelo, E. Molecular Detection of Leishmania (Leishmania) Mexicana in Sandflies from the State of Yucatan, Mexico. Vector-Borne Zoonotic Dis. 2022, 22, 589–595. [Google Scholar] [CrossRef]
- Bates, P.A.; Depaquit, J.; Galati, E.A.B.; Kamhawi, S.; Maroli, M.; McDowell, M.A.; Picado, A.; Ready, P.D.; Salomón, O.D.; Shaw, J.J.; et al. Recent Advances in Phlebotomine Sand Fly Research Related to Leishmaniasis Control. Parasit Vectors 2015, 8, 131. [Google Scholar] [CrossRef] [Green Version]
- Azevedo, A.C.; Souza, N.A.; Meneses, C.R.; Costa, W.A.; Costa, S.M.; Lima, J.B.; Rangel, E.F. Ecology of Sand Flies (Diptera: Psychodidae: Phlebotominae) in the North of the State of Mato Grosso, Brazil. Mem. Inst. Oswaldo Cruz 2002, 97, 459–464. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dantas-Torres, F. The Role of Dogs as Reservoirs of Leishmania Parasites, with Emphasis on Leishmania (Leishmania) Infantum and Leishmania (Viannia) Braziliensis. Vet. Parasitol. 2007, 149, 139–146. [Google Scholar] [CrossRef] [PubMed]
- Roque, A.L.R.; Jansen, A.M. Wild and Synanthropic Reservoirs of Leishmania Species in the Americas. Int. J. Parasitol. Parasites Wildl. 2014, 3, 251–262. [Google Scholar] [CrossRef]
- Chable-Santos, J.B.; van Wynsberghe, N.; Canto-Lara, S.B.; Andrade-Narvaez, F.J. Isolation of Leishmania (L.) Mexicana from Wild Rodents and Their Possible Role in the Transmission of Localized Cutaneous Leishmaniasis in the State of Campeche, Mexico. Am. J. Trop. Med. Hyg. 1995, 53, 141–145. [Google Scholar] [CrossRef]
- van Wynsberghe, N.R.; Canto-Lara, S.B.; Sosa-Bibiano, E.I.; Rivero-Cárdenas, N.A.; Andrade-Narváez, F.J. Comparison of Small Mammal Prevalence of Leishmania (Leishmania) Mexicana in Five Foci of Cutaneous Leishmaniasis in the State of Campeche, Mexico. Rev. Inst. Med. Trop. Sao Paulo 2009, 51, 87–94. [Google Scholar] [CrossRef] [Green Version]
- Andrade-Narvaez, F.J.; Lara, S.B.C.; van Wynsberghe, N.R.; Rebollar-Tellez, E.A.; Vargas-Gonzalez, A.; Albertos-Alpuche, N.E. Seasonal Transmission of Leishmania (Leishmania) Mexicana in the State of Campeche, Yucatan Peninsula, Mexico. Mem. Inst. Oswaldo Cruz 2003, 98, 995–998. [Google Scholar] [CrossRef] [Green Version]
- Moo-Llanes, D.A.; Baak-Baak, C.M.; Cigarroa-Toledo, N.; Tzuc-Dzul, J.C.; Panti-May, J.A.; García-Rejón, J.E. Impacto Del Cambio Climático En La Distribución de Tres Roedores Endémicos de La Península de Yucatán: Implicaciones Para La Conservación de Otonyctomys Hatti. Rev. Mex. Mastozoología Nueva Época 2021, 11, 1–14. [Google Scholar]
- Hernández-Betancourt, S.F.; López-Willchis, R.; Cimé-Pool, J.A.; Medina-Peralta, S. Área de Actividad, Movimiento y Organización Social de Heteromys Gaumeri Allen y Chapman,1897 (Rodentia: Heteromyidae) En Una Selva Mediana Subcaducifolia de Yucatán, México. Acta Zoológica Mex. Nueva Ser. 2003, 90, 77–91. [Google Scholar] [CrossRef]
- Cimé-Pool, J.A.; Chablé-Santos, J.B.; Sosa-Escalante, J.E.; Hernández-Betancourt, S.F. Quirópteros y Pequeños Roedores de La Reserva de La Biosfera Ría Celestún, Yucatán, México. Acta Zoológica Mex. Nueva Ser. 2006, 22, 127–131. [Google Scholar] [CrossRef]
- Cimé-Pool, J.A.; Hernández-Betancourt, S.F.; Barrientos, R.C.; Castro-Luna, A.A. Diversidad de Pequeños Roedores En Una Selva Baja Caducifolia Del Noreste de Yucatán, México. Therya 2010, 1, 23–39. [Google Scholar] [CrossRef] [Green Version]
- Panti-May, J.A.; Hernández-Betancourt, S.F.; Torres-Castro, M.A.; Parada-López, J.; López-Manzanero, S.G.; Herrera-Meza, M.C. A Population Study of the House Mouse, Mus Musculus (Rodentia: Muridae), in a Rural Community of Mérida, México. Caribb. Nat. 2018, 46, 1–13. [Google Scholar]
- Panti-May, J.A.; Gurubel-González, Y.M.; Palomo-Arjona, E.E.; Cetina-Trejo, R.C.; Machain-Williams, C.; Robles, M.R.; Hernández-Betancourt, S.F. Características Poblacionales de Rattus Rattus y Mus Musculus Presentes En Comunidades Rurales de Yucatán, México. Trop. Subtrop. Agroecosyst. 2018, 21, 345–356. [Google Scholar]
- Berzunza-Cruz, M.; Rodríguez-Moreno, Á.; Gutiérrez-Granados, G.; González-Salazar, C.; Stephens, C.R.; Hidalgo-Mihart, M.; Marina, C.F.; Rebollar-Téllez, E.A.; Bailón-Martínez, D.; Balcells, C.D.; et al. Leishmania (L.) Mexicana Infected Bats in Mexico: Novel Potential Reservoirs. PLoS Negl. Trop. Dis. 2015, 9, 1–15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sosa-Escalante, J.E.; Pech-Canché, J.M.; Cristina MacSwiney, M.; Hernández-Betancourt, S. Mamíferos Terrestres de La Península de Yucatán, México: Riqueza, Endemismo y Riesgo. Rev. Mex. Biodivers. 2013, 84, 949–969. [Google Scholar] [CrossRef] [Green Version]
- Fleming, T.H.; Dávalos, L.M.; Mello, M.A.R. Phyllostomid Bats: A Unique Mammalian Radiation; The University of Chicago Press: Chicago, IL, USA, 2020. [Google Scholar]
- Santiago, M.E.B.; Vasconcelos, R.O.; Fattori, K.R.; Munari, D.P.; de Fátima Michelin, A.; Lima, V.M.F. An Investigation of Leishmania spp. in Didelphis spp. from Urban and Peri-Urban Areas in Bauru (São Paulo, Brazil). Vet. Parasitol. 2007, 150, 283–290. [Google Scholar] [CrossRef]
- Silva, E.M.; Alves, L.C.; Guerra, N.R.; Farias, M.P.O.; Oliveira, E.L.R.; de Souza, R.C.; da Cunha, C.; Ramos, R.A.N.; Porto, W.J.N. Leishmania spp. in Didelphis spp. from Northeastern Bazil. J. Zoo Wildl. Med. 2016, 47, 942–944. [Google Scholar] [CrossRef]
- Araujo Carreira, J.C.; de Avelar Figueiredo Mafra Magalhães, M.; Brazil, R.P.; da Silva, A.V.M. Leishmania in Marsupials—An Overview of Infection Records in the Americas and Australia. Open J. Anim. Sci. 2017, 7, 315–343. [Google Scholar] [CrossRef] [Green Version]
- Ardila, M.M.; Carrillo-Bonilla, L.; Pabón, A.; Robledo, S.M. Surveillance of Phlebotomine Fauna and Didelphis marsupialis (Didelphimorphia: Didelphidae) Infection in an Area Highly Endemic for Visceral Leishmaniasis in Colombia. Biomédica 2019, 39, 252–264. [Google Scholar] [CrossRef] [Green Version]
- Ruiz-Piña, H.A.; Pacheco-Castro, J.; Lugo-Pérez, J.A. El “Zorro” de Yucatán y Su Relación Con La Población Humana. In Estudios Multidisciplinarios de las Enfermedades Zoonóticas y ETVs en Yucatán; Pacheco-Castro, J., Lugo-Perez, J.A., Tzuc-Canché, L., Ruiz-Piña, H.A., Eds.; Universidad Autónoma de Yucatán: Mérida, Mexico, 2013; pp. 216–232. [Google Scholar]
- Bezerra-Santos, M.A.; Ramos, R.A.N.; Campos, A.K.; Dantas-Torres, F.; Otranto, D. Didelphis spp. Opossums and Their Parasites in the Americas: A One Health Perspective. Parasitol. Res. 2021, 120, 4091–4111. [Google Scholar] [CrossRef]
- Harrus, S.; Baneth, G. Drivers for the Emergence and Re-Emergence of Vector-Borne Protozoal and Bacterial Diseases. Int. J. Parasitol. 2005, 35, 1309–1318. [Google Scholar] [CrossRef]
- Afrane, Y.A.; Zhou, G.; Lawson, B.W.; Githeko, A.K.; Yan, G. Effects of Microclimatic Changes Caused by Deforestation on the Survivorship and Reproductive Fitness of Anopheles Gambiae in Western Kenya Highlands. Am. J. Trop. Med. Hyg. 2006, 74, 772–778. [Google Scholar] [CrossRef]
- Githeko, A.K.; Lindsay, S.W.; Confalonieri, U.E.; Patz, J.A. Climate Change and Vector-Borne Diseases: A Regional Analysis. Bull. World Health Organ. 2000, 78, 1136–1147. [Google Scholar]
- Vora, N. Impact of Anthropogenic Environmental Alterations on Vector Borne Diseases. Medscape J. Med. 2008, 10, 238. [Google Scholar]
- Hernández, M.A. Transformación de Los Sistemas Naturales Por Actividades Antropogénicas. In Biodiversidad y Desarrollo Humano en Yucatán; Durán-García, R., Méndez-González, M.E., Eds.; CICY, PPD-FMAM, CONABIO, SEDUMA: Mérida, Mexico, 2010; pp. 316–319. [Google Scholar]
- Pinkus-Rendón, M.A. Dinámica En El Uso de Los Recursos Naturales En El Oriente de Yucatán Durante El Siglo XX. Rev. Pueblos Front. Digit. 2016, 11, 92–113. [Google Scholar] [CrossRef] [Green Version]
- Durán-García, R.; García-Contreras, G. Ditribución Espacial de La Vegetación. In Biodiversidad y Desarrollo Humano en Yucatán; Durán-García, R., Méndez-González, M.E., Eds.; CICY, PPD-FMAM, CONABIO, SEDUMA: Mérida, Mexico, 2010; pp. 131–135. [Google Scholar]
- INEGI Cuéntame de México. Available online: https://cuentame.inegi.org.mx/poblacion/habitantes.aspx?tema=P (accessed on 1 November 2022).
- de Oca-Aguilar, A.C.M.; Rebollar-Téllez, E.A.; Sosa-Bibiano, E.I.; López-Avila, K.B.; Torres-Castro, J.R.; Loría-Cervera, E.N. Effect of Land Use Change on the Phlebotomine Sand Fly Assemblages in an Emergent Focus of Cutaneous Leishmaniasis in Yucatan, Mexico. Acta Trop. 2022, 235, 106628. [Google Scholar] [CrossRef]
- Tidman, R.; Abela-Ridder, B.; de Castañeda, R.R. The Impact of Climate Change on Neglected Tropical Diseases: A Systematic Review. Trans. R. Soc. Trop. Med. Hyg. 2021, 115, 147–168. [Google Scholar] [CrossRef]
- Fouque, F.; Reeder, J.C. Impact of Past and On-Going Changes on Climate and Weather on Vector-Borne Diseases Transmission: A Look at the Evidence. Infect. Dis. Poverty 2019, 8, 51. [Google Scholar] [CrossRef]
- de la Barreda, B.; Metcalfe, S.E.; Boyd, D.S. Precipitation Regionalization, Anomalies and Drought Occurrence in the Yucatan Peninsula, Mexico. Int. J. Climatol. 2020, 40, 4541–4555. [Google Scholar] [CrossRef]
- Murray-Tortarolo, G.N. Seven Decades of Climate Change across Mexico. Atmósfera 2021, 34, 217–226. [Google Scholar] [CrossRef]
- Gobierno del Estado de Yucatán Programa Especial de Acción Ante El Cambio Climático Del Estado de Yucatán. Available online: http://www.ccpy.gob.mx/agenda-yucatan/programa-estatal-cambio-climatico.php (accessed on 30 April 2022).
- World Health Organization; Food and Agriculture Organization of the United Nations; World Organization for Animal Health. Taking a Multisectoral, One Health Approach: A Tripartite Guide to Addressing Zoonotic Diseases in Countries. 2019. Available online: http://www.who.int/about/licensing/copyright_form/en/index.html (accessed on 18 November 2022).
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Canché-Pool, E.B.; Panti-May, J.A.; Ruiz-Piña, H.A.; Torres-Castro, M.; Escobedo-Ortegón, F.J.; Tamay-Segovia, P.; Blum-Domínguez, S.; Torres-Castro, J.R.; Reyes-Novelo, E. Cutaneous Leishmaniasis Emergence in Southeastern Mexico: The Case of the State of Yucatan. Trop. Med. Infect. Dis. 2022, 7, 444. https://doi.org/10.3390/tropicalmed7120444
Canché-Pool EB, Panti-May JA, Ruiz-Piña HA, Torres-Castro M, Escobedo-Ortegón FJ, Tamay-Segovia P, Blum-Domínguez S, Torres-Castro JR, Reyes-Novelo E. Cutaneous Leishmaniasis Emergence in Southeastern Mexico: The Case of the State of Yucatan. Tropical Medicine and Infectious Disease. 2022; 7(12):444. https://doi.org/10.3390/tropicalmed7120444
Chicago/Turabian StyleCanché-Pool, Elsy B., Jesús A. Panti-May, Hugo A. Ruiz-Piña, Marco Torres-Castro, Francisco J. Escobedo-Ortegón, Paulino Tamay-Segovia, Selene Blum-Domínguez, Jimmy R. Torres-Castro, and Enrique Reyes-Novelo. 2022. "Cutaneous Leishmaniasis Emergence in Southeastern Mexico: The Case of the State of Yucatan" Tropical Medicine and Infectious Disease 7, no. 12: 444. https://doi.org/10.3390/tropicalmed7120444
APA StyleCanché-Pool, E. B., Panti-May, J. A., Ruiz-Piña, H. A., Torres-Castro, M., Escobedo-Ortegón, F. J., Tamay-Segovia, P., Blum-Domínguez, S., Torres-Castro, J. R., & Reyes-Novelo, E. (2022). Cutaneous Leishmaniasis Emergence in Southeastern Mexico: The Case of the State of Yucatan. Tropical Medicine and Infectious Disease, 7(12), 444. https://doi.org/10.3390/tropicalmed7120444







