Electrolyte-Gated Graphene Field Effect Transistor-Based Ca2+ Detection Aided by Machine Learning
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents and Chemicals
2.2. Prepare Graphene Ink
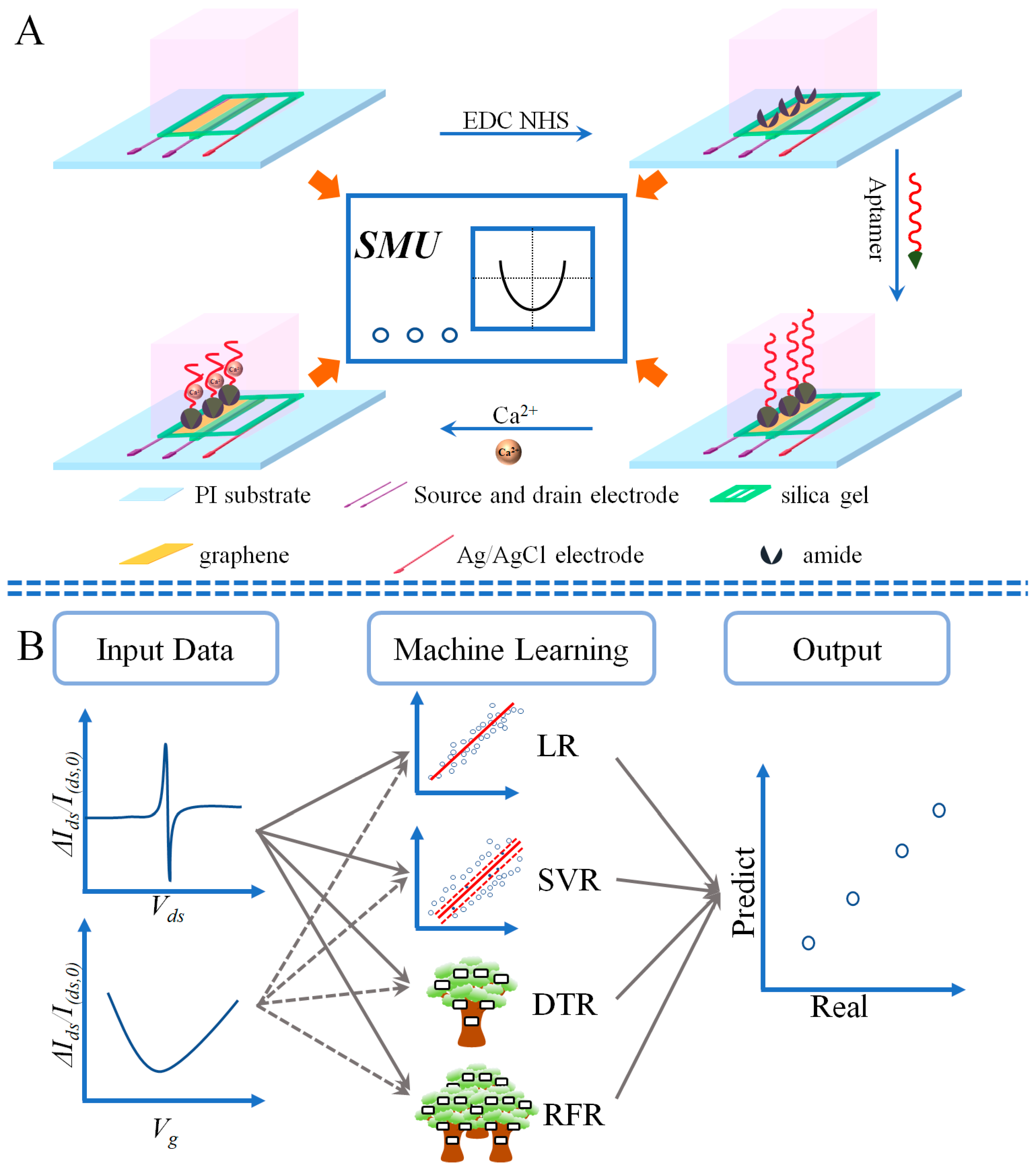
2.3. Fabricate the Eg-GFET
2.4. Ions Detection
2.5. Regression Modeling
3. Results
3.1. X-ray Photoelectron Spectroscopy (XPS) Characteristic
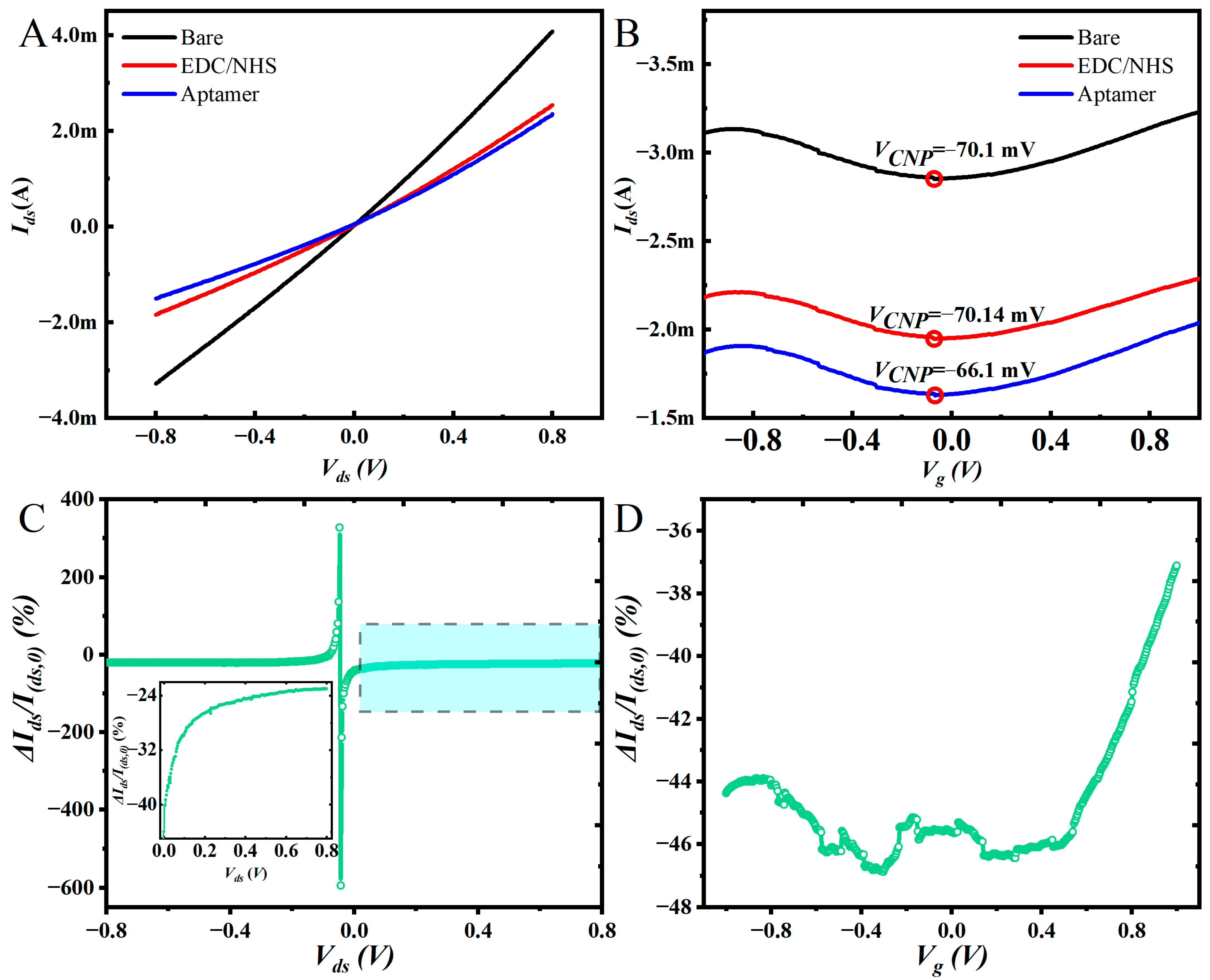
3.2. Electrical Properties of Eg-GFETs
3.3. Ca2+ Ion Test
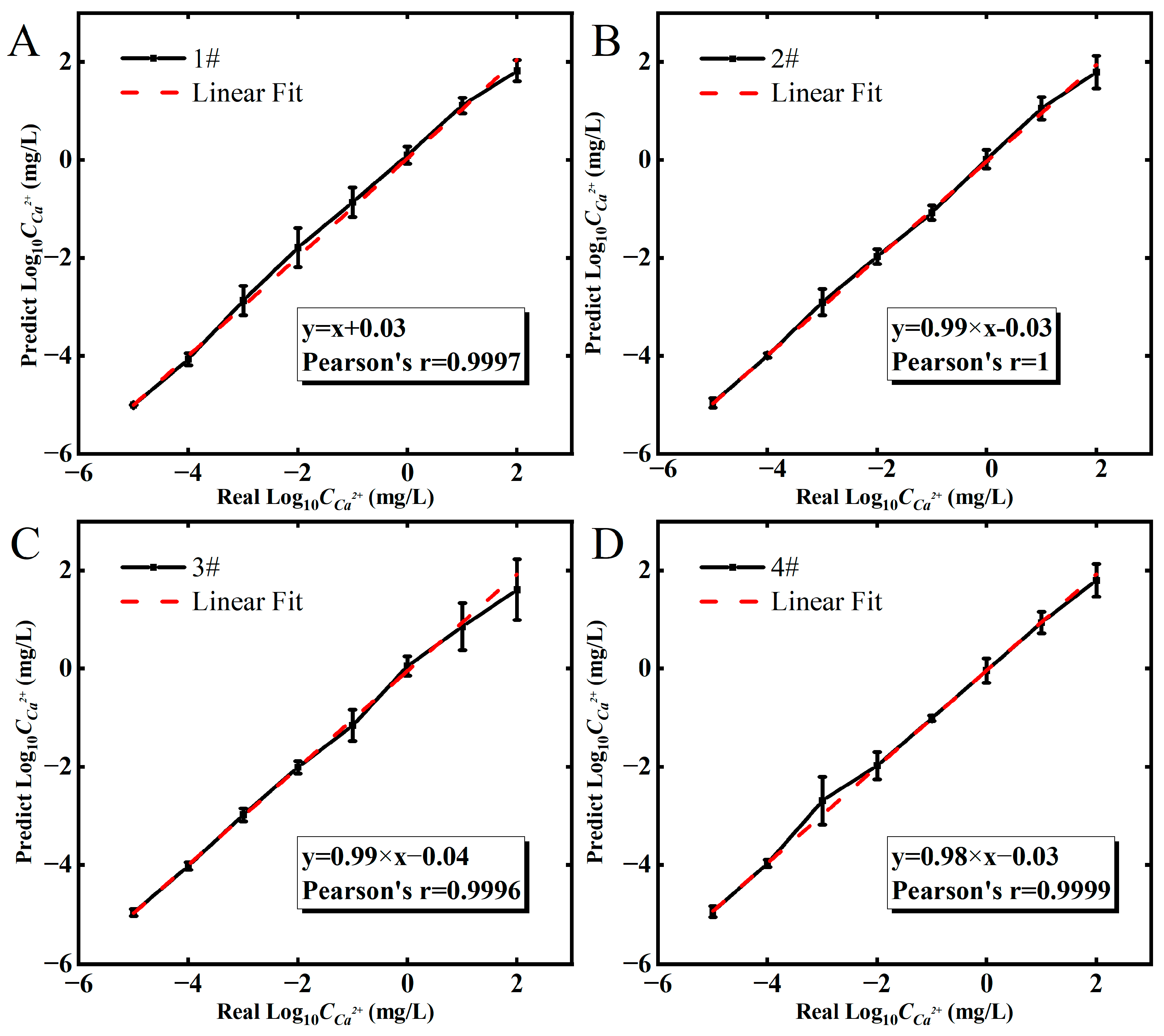
3.4. Regression Algorithm Analyzes Output Curves and Transfer Curves
3.5. Prediction of
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Welch, J.M.; Weaver, C.M. Calcium and exercise affect the growing skeleton. Nutr. Rev. 2005, 63, 361–373. [Google Scholar] [CrossRef] [PubMed]
- Ermak, G.; Davies, K.J.A. Calcium and oxidative stress: From cell signaling to cell death. Mol. Immunol. 2002, 38, 713–721. [Google Scholar] [CrossRef] [PubMed]
- Nanou, E.; Catterall, W.A. Calcium Channels, Synaptic Plasticity, and Neuropsychiatric Disease. Neuron 2018, 98, 466–481. [Google Scholar] [CrossRef]
- Reid, I.R.; Bolland, M.J.; Grey, A. Effects of vitamin D supplements on bone mineral density: A systematic review and meta-analysis. Lancet 2014, 383, 146–155. [Google Scholar] [CrossRef] [PubMed]
- Sorokin, I.; Mamoulakis, C.; Miyazawa, K.; Rodgers, A.; Talati, J.; Lotan, Y. Epidemiology of stone disease across the world. World J. Urol. 2017, 35, 1301–1320. [Google Scholar] [CrossRef] [PubMed]
- Hossienifar, F.; Entezari, M.; Hosseini, S. Water hardness zoning of Isfahan Province, Iran, and its relationship with cardiovascular mortality, 2013–2015. ARYA Atheroscler. 2019, 15, 275–280. [Google Scholar] [CrossRef]
- Thanikachalam, K.; Khan, G. Colorectal Cancer and Nutrition. Nutrients 2019, 11, 164. [Google Scholar] [CrossRef]
- Kim, M.-H.; Bu, S.Y.; Choi, M.-K. Daily calcium intake and its relation to blood pressure, blood lipids, and oxidative stress biomarkers in hypertensive and normotensive subjects. Nutr. Res. Pract. 2012, 6, 421–428. [Google Scholar] [CrossRef]
- Scancar, J.; Milacic, R.; Benedik, M.; Bukovec, P. Determination of trace elements and calcium in bone of the human iliac crest by atomic absorption spectrometry. Clin. Chim. Acta 2000, 293, 187–197. [Google Scholar] [CrossRef]
- Roopa, R.; Kumar, N.; Kumar, M.; Bhalla, V. Design and Applications of Small Molecular Probes for Calcium Detection. Chem. Asian. J. 2019, 14, 4493–4505. [Google Scholar] [CrossRef]
- Deng, B.; Zhu, P.; Wang, Y.; Feng, J.; Li, X.; Xu, X.; Lu, H.; Xu, Q. Determination of free calcium and calcium-containing species in human plasma by capillary electrophoresis-inductively coupled plasma optical emission spectrometry. Anal. Chem. 2008, 80, 5721–5726. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.-X.; He, Y.-B.; Lai, L.-N.; Li, J.-B.; Song, X.-L. Electrochemical sensors using gold submicron particles modified electrodes based on calcium complexes formed with alizarin red S for determination of Ca2+ in isolated rat heart mitochondria. Biosens. Bioelectron. 2015, 66, 417–422. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Z. An overview of carbon nanotubes and graphene for biosensing applications. Nanomicro. Lett. 2017, 9, 1–24. [Google Scholar] [CrossRef] [PubMed]
- Novoselov, K.S.; Geim, A.K.; Morozov, S.V.; Jiang, D.; Zhang, Y.; Dubonos, S.V.; Grigorieva, I.V.; Firsov, A.A. Electric field effect in atomically thin carbon films. Science 2004, 306, 666–669. [Google Scholar] [CrossRef] [PubMed]
- Ganguli, A.; Faramarzi, V.; Mostafa, A.; Hwang, M.T.; You, S.; Bashir, R. High Sensitivity Graphene Field Effect Transistor-Based Detection of DNA Amplification. Adv. Funct. Mater. 2020, 30, 2001031. [Google Scholar] [CrossRef]
- Kim, H.E.; Schuck, A.; Lee, J.H.; Kim, Y.-S. Solution-gated graphene field effect transistor for TP53 DNA sensor with coplanar electrode array. Sens. Actuators B 2019, 291, 96–101. [Google Scholar] [CrossRef]
- Huang, Y.; Dong, X.; Liu, Y.; Li, L.-J.; Chen, P. Graphene-based biosensors for detection of bacteria and their metabolic activities. J. Mater. Chem. 2011, 21, 12358–12362. [Google Scholar] [CrossRef]
- Park, S.J.; Seo, S.E.; Kim, K.H.; Lee, S.H.; Kim, J.; Ha, S.; Song, H.S.; Lee, S.H.; Kwon, O.S. Real-time monitoring of geosmin based on an aptamer-conjugated graphene field-effect transistor. Biosens. Bioelectron. 2021, 174, 112804. [Google Scholar] [CrossRef]
- Cui, F.; Yue, Y.; Zhang, Y.; Zhang, Z.; Zhou, H.S. Advancing Biosensors with Machine Learning. ACS Sens. 2020, 5, 3346–3364. [Google Scholar] [CrossRef]
- Bian, L.; Wang, Z.; White, D.L.; Star, A. Machine learning-assisted calibration of Hg2+ sensors based on carbon nanotube field-effect transistors. Biosens. Bioelectron. 2021, 180, 113085. [Google Scholar] [CrossRef]
- Wang, H.; Luo, Q.; Zhao, Y.; Nan, X.; Zhang, F.; Wang, Y.; Wang, Y.; Hua, D.; Zheng, S.; Jiang, L.; et al. Electrochemical device based on nonspecific DNAzyme for the high-accuracy determination of Ca2+ with Pb2+ interference. Bioelectrochemistry 2021, 140, 107732. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Jia, Y. A disposable printed liquid gate graphene field effect transistor for a salivary cortisol test. ACS Sens. 2021, 6, 3024–3031. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Jia, Y. Total solution processable and low-cost DNA methylation sensor. J. Instrum. 2021, 16, P09021. [Google Scholar] [CrossRef]
- Wang, Q.; Hu, W.; Huang, Y. Nitrogen doped graphene anchored cobalt oxides efficiently bi-functionally catalyze both oxygen reduction reaction and oxygen revolution reaction. Int. J. Hydrogen Energy 2017, 42, 5899–5907. [Google Scholar] [CrossRef]
- Tahir, M.; Cao, C.; Butt, F.K.; Butt, S.; Idrees, F.; Ali, Z.; Aslam, I.; Tanveer, M.; Mahmood, A.; Mahmood, N. Large scale production of novel g-C3N4 micro strings with high surface area and versatile photodegradation ability. CrystEngComm 2014, 16, 1825–1830. [Google Scholar] [CrossRef]
- Kawata, T.; Ono, T.; Kanai, Y.; Ohno, Y.; Maehashi, K.; Inoue, K.; Matsumoto, K. Improved sensitivity of a graphene FET biosensor using porphyrin linkers. Jpn. J. Appl. Phys. 2018, 57, 065103. [Google Scholar] [CrossRef]
- Beraud, A.; Sauvage, M.; Bazan, C.M.; Tie, M.; Bencherif, A.; Bouilly, D. Graphene field-effect transistors as bioanalytical sensors: Design, operation and performance. Analyst 2021, 146, 403–428. [Google Scholar] [CrossRef]
- Yuan, Q.; Wu, S.; Ye, C.; Liu, X.; Gao, J.; Cui, N.; Guo, P.; Lai, G.; Wei, Q.; Yang, M.; et al. Sensitivity enhancement of potassium ion (K+) detection based on graphene field-effect transistors with surface plasma pretreatment. Sens. Actuators B 2019, 285, 333–340. [Google Scholar] [CrossRef]



| Algorithms | LR | SVR | DTR | RFR | |||||
|---|---|---|---|---|---|---|---|---|---|
| Metrics | Output | Transfer | Output | Transfer | Output | Transfer | Output | Transfer | |
| MSE | 6.62 | 1.68 | 2.41 | 1.53 | 1.06 | 0.54 | 0.84 | 0.24 | |
| RMSE | 2.57 | 1.29 | 1.55 | 1.24 | 1.03 | 0.73 | 0.91 | 0.49 | |
| MAE | 2.23 | 1.03 | 1.19 | 0.97 | 0.56 | 0.39 | 0.43 | 0.21 | |
| R2 | 0.03 | 0.75 | 0.65 | 0.77 | 0.84 | 0.92 | 0.88 | 0.96 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, R.; Hao, T.; Hu, S.; Wang, K.; Ren, S.; Tian, Z.; Jia, Y. Electrolyte-Gated Graphene Field Effect Transistor-Based Ca2+ Detection Aided by Machine Learning. Sensors 2023, 23, 353. https://doi.org/10.3390/s23010353
Zhang R, Hao T, Hu S, Wang K, Ren S, Tian Z, Jia Y. Electrolyte-Gated Graphene Field Effect Transistor-Based Ca2+ Detection Aided by Machine Learning. Sensors. 2023; 23(1):353. https://doi.org/10.3390/s23010353
Chicago/Turabian StyleZhang, Rong, Tiantian Hao, Shihui Hu, Kaiyang Wang, Shuhui Ren, Ziwei Tian, and Yunfang Jia. 2023. "Electrolyte-Gated Graphene Field Effect Transistor-Based Ca2+ Detection Aided by Machine Learning" Sensors 23, no. 1: 353. https://doi.org/10.3390/s23010353
APA StyleZhang, R., Hao, T., Hu, S., Wang, K., Ren, S., Tian, Z., & Jia, Y. (2023). Electrolyte-Gated Graphene Field Effect Transistor-Based Ca2+ Detection Aided by Machine Learning. Sensors, 23(1), 353. https://doi.org/10.3390/s23010353











