Biodegradable Molybdenum (Mo) and Tungsten (W) Devices: One Step Closer towards Fully-Transient Biomedical Implants
Abstract
:1. Introduction
2. Transient Electronic Devices
2.1. The Arrival of Transient Technology
2.2. Transient Bioelectronic Devices
Design and Fabrication Requirements
3. Bioresorbable Metals Mo and W
3.1. Biocompatibility and Bioresorbability
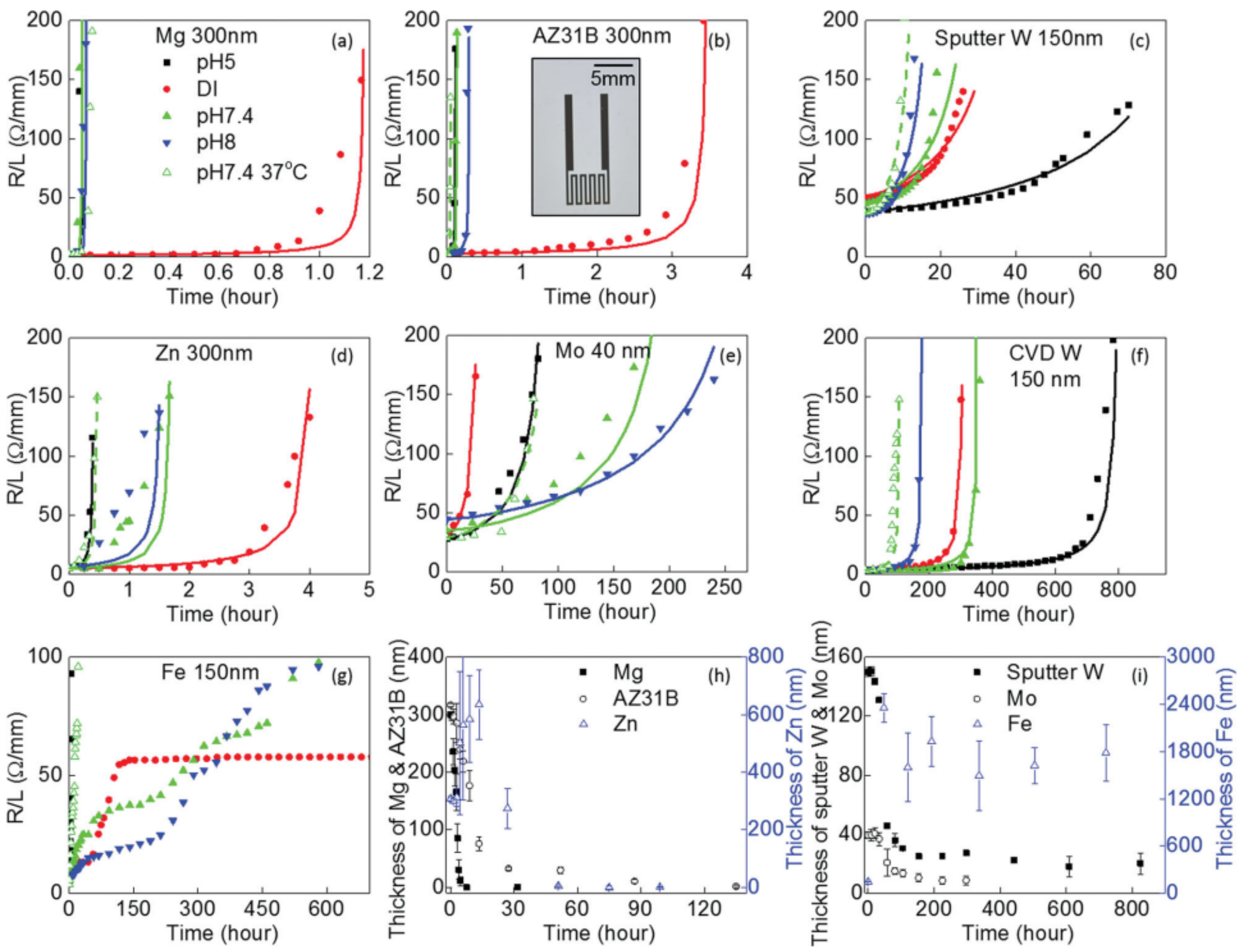
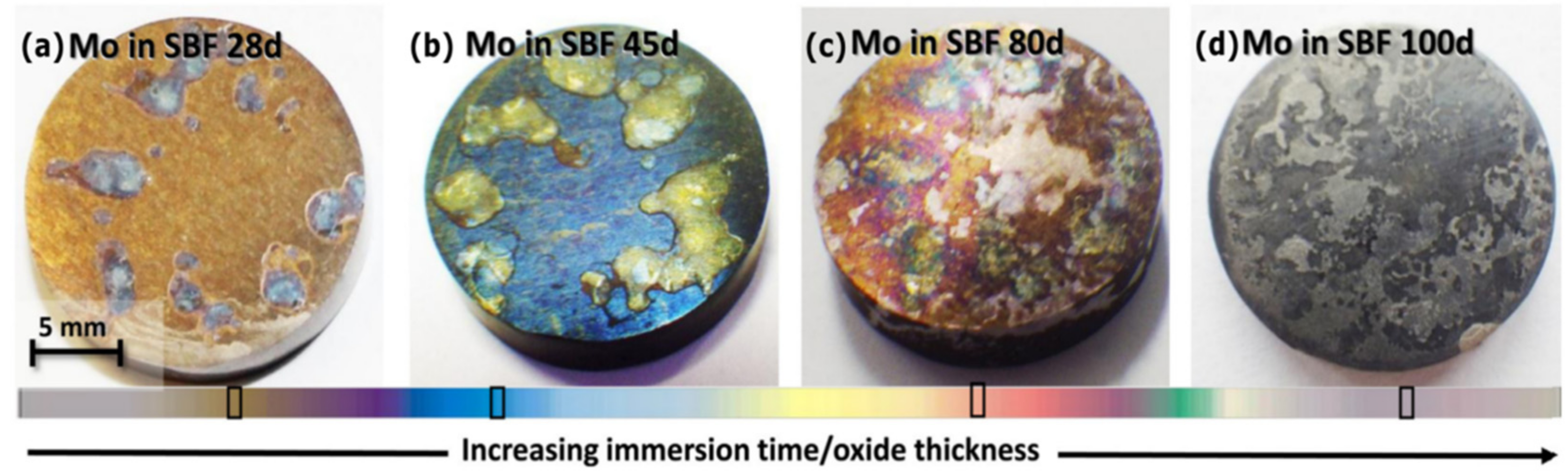
3.2. General Dissolution Mechanisms
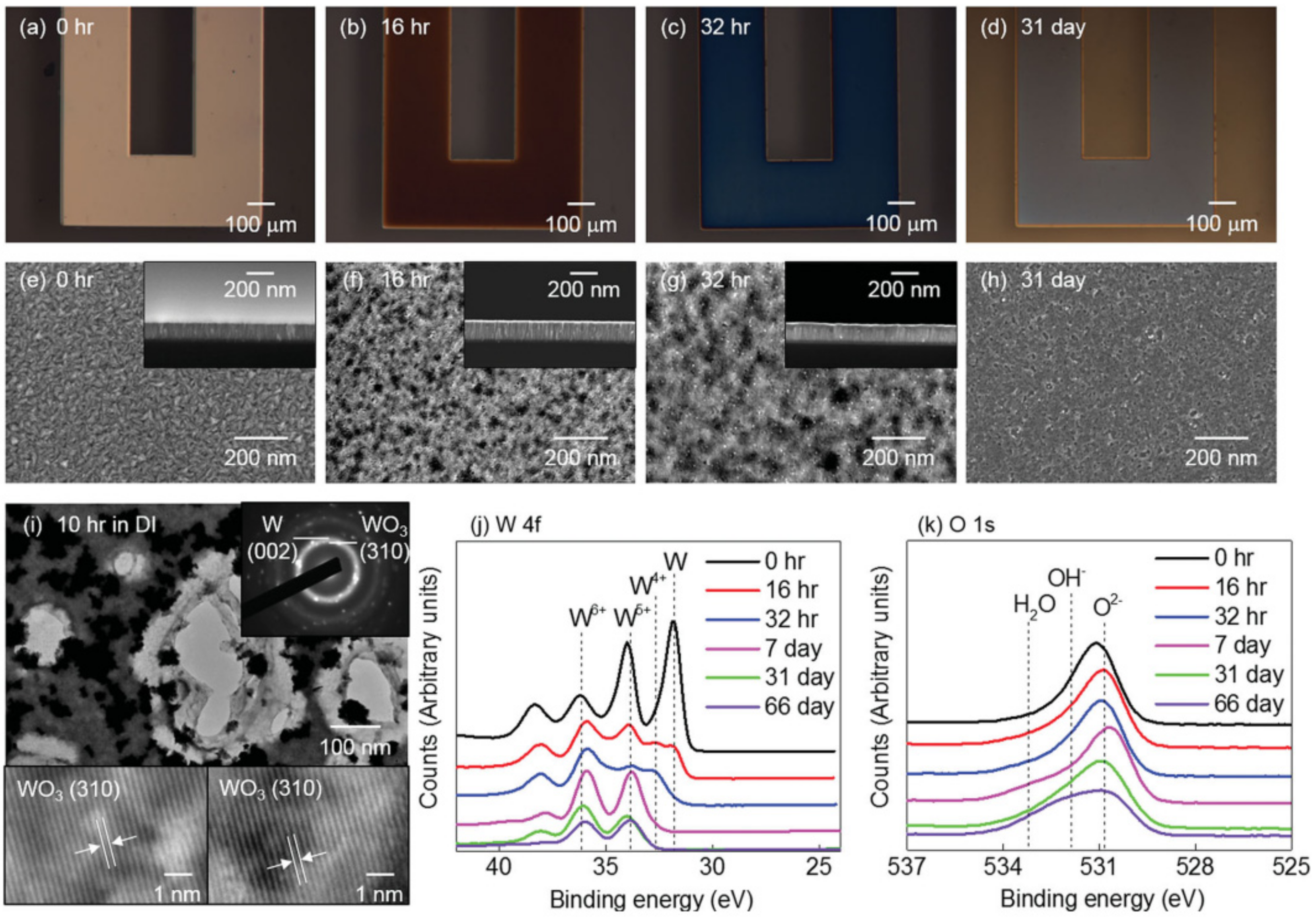
3.3. Surface Chemistry
4. Mo and W-Based Electrochemical Transient Devices
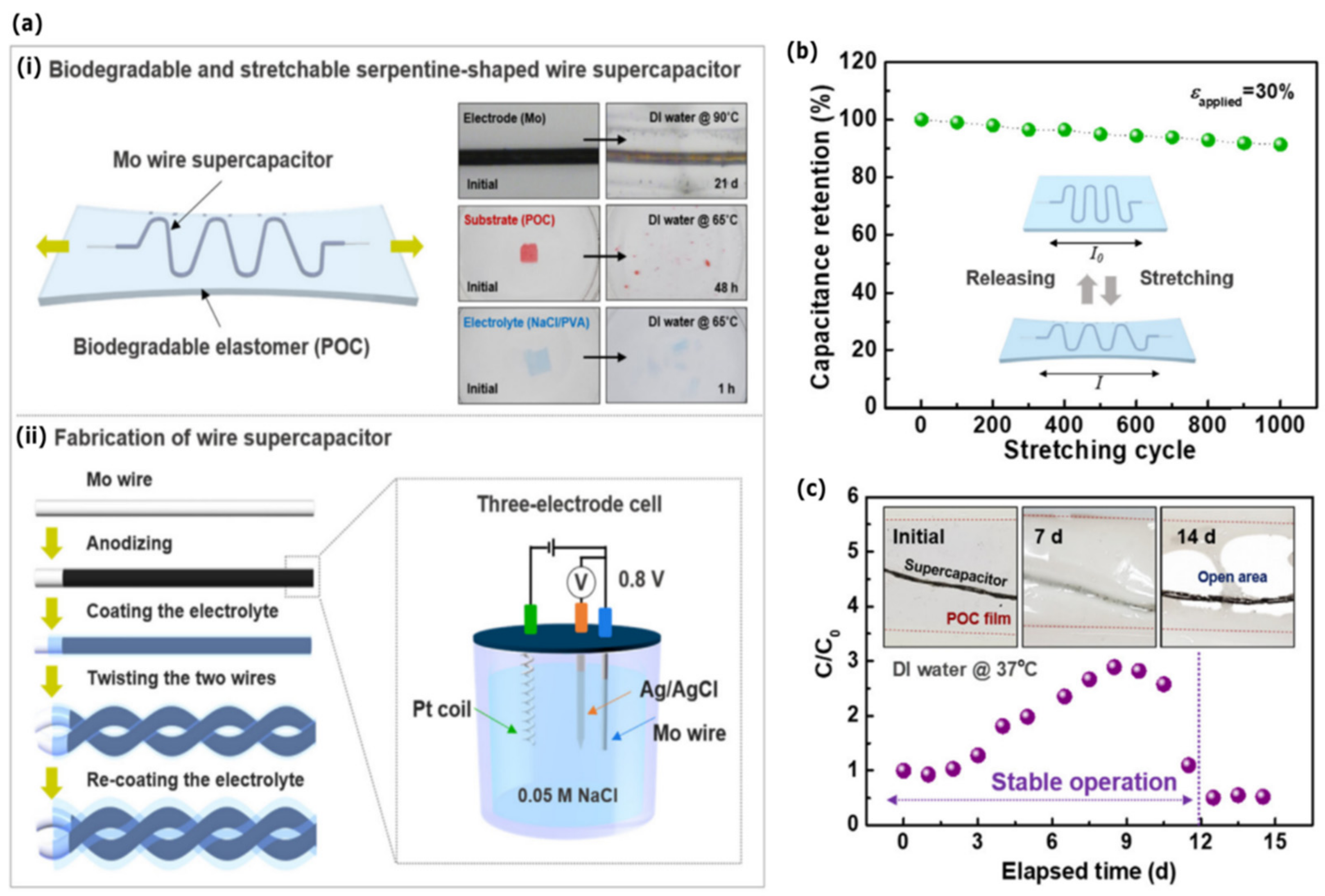
4.1. Bioresorbable Energy Devices
4.2. Bioresorbable Electrical Connectors
4.3. Bioresorbable Electrochemical Sensors
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Li, R.; Wang, L.; Kong, D.; Yin, L. Recent Progress on Biodegradable Materials and Transient Electronics. Bioact. Mater. 2018, 3, 322–333. [Google Scholar] [CrossRef] [PubMed]
- Fu, K.K.; Wang, Z.; Dai, J.; Carter, M.; Hu, L. Transient Electronics: Materials and Devices. Chem. Mater. 2016, 28, 3527–3539. [Google Scholar] [CrossRef]
- Hwang, S.-W.; Tao, H.; Kim, D.; Cheng, H.; Song, J.; Rill, E.; Brenckle, M.A.; Panilaitis, B.; Won, S.M.; Kim, Y.-S.; et al. A Physically Transient Form of Silicon Electronics. Science 2012, 337, 1640–1644. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yin, L.; Cheng, H.; Mao, S.; Haasch, R.; Liu, Y.; Xie, X.; Hwang, S.W.; Jain, H.; Kang, S.K.; Su, Y.; et al. Dissolvable Metals for Transient Electronics. Adv. Funct. Mater. 2014, 24, 645–658. [Google Scholar] [CrossRef]
- Redlich, C.; Schauer, A.; Scheibler, J.; Poehle, G.; Barthel, P.; Maennel, A.; Adams, V.; Weissgaerber, T.; Linke, A.; Quadbeck, P. In Vitro Degradation Behavior and Biocompatibility of Bioresorbable Molybdenum. Metals 2021, 11, 761. [Google Scholar] [CrossRef]
- Chatterjee, S.; Saxena, M.; Padmanabhan, D.; Jayachandra, M.; Pandya, H.J. Futuristic Medical Implants Using Bioresorbable Materials and Devices. Biosens. Bioelectron. 2019, 142, 111489. [Google Scholar] [CrossRef]
- Li, J.; Liu, J.; Lu, W.; Wu, Z.; Yu, J.; Wang, B.; Ma, Z.; Huo, W.; Huang, X. Water-Sintered Transient Nanocomposites Used as Electrical Interconnects for Dissolvable Consumer Electronics. ACS Appl. Mater. Interfaces 2021, 13, 32136–32148. [Google Scholar] [CrossRef]
- Kiddee, P.; Naidu, R.; Wong, M.H. Electronic Waste Management Approaches: An Overview. Waste Manag. 2013, 33, 1237–1250. [Google Scholar] [CrossRef]
- Shinkuma, T. Nguyen Thi Minh Huong the Flow of E-Waste Material in the Asian Region and a Reconsideration of International Trade Policies on E-Waste. Environ. Impact Assess. Rev. 2009, 29, 25–31. [Google Scholar] [CrossRef]
- Leung, A.; Cai, Z.W.; Wong, M.H. Environmental Contamination from Electronic Waste Recycling at Guiyu, Southeast China. J. Mater. Cycles Waste Manag. 2006, 8, 21–33. [Google Scholar] [CrossRef]
- Luo, J.; Qi, S.; Xie, X.; Gu, X.W.S.; Wang, J. The Assessment of Source Attribution of Soil Pollution in a Typical E-Waste Recycling Town and Its Surrounding Regions Using the Combined Organic and Inorganic Dataset. Environ. Sci. Pollut. Res. 2017, 24, 3131–3141. [Google Scholar] [CrossRef]
- Liu, J.; He, X.X.; Lin, X.R.; Chen, W.C.; Zhou, Q.X.; Shu, W.S.; Huang, L.N. Ecological Effects of Combined Pollution Associated with E-Waste Recycling on the Composition and Diversity of Soil Microbial Communities. Environ. Sci. Technol. 2015, 49, 6438–6447. [Google Scholar] [CrossRef]
- Jamshidi, R.; Taghavimehr, M.; Chen, Y.; Hashemi, N.; Montazami, R. Transient Electronics as Sustainable Systems: From Fundamentals to Applications. Adv. Sustain. Syst. 2021, 6, 2100057. [Google Scholar] [CrossRef]
- Han, W.B.; Lee, J.H.; Shin, J.W.; Hwang, S.W. Advanced Materials and Systems for Biodegradable, Transient Electronics. Adv. Mater. 2020, 32, 2002211. [Google Scholar] [CrossRef]
- Baldo, T.A.; de Lima, L.F.; Mendes, L.F.; de Araujo, W.R.; Paixão, T.R.L.C.; Coltro, W.K.T. Wearable and Biodegradable Sensors for Clinical and Environmental Applications. ACS Appl. Electron. Mater. 2021, 3, 68–100. [Google Scholar] [CrossRef]
- Ko, G.J.; Han, S.D.; Kim, J.K.; Zhu, J.; Han, W.B.; Chung, J.; Yang, S.M.; Cheng, H.; Kim, D.H.; Kang, C.Y.; et al. Biodegradable, Flexible Silicon Nanomembrane-Based NOx Gas Sensor System with Record-High Performance for Transient Environmental Monitors and Medical Implants. NPG Asia Mater. 2020, 12, 71. [Google Scholar] [CrossRef]
- She, D.; Tsang, M.; Allen, M. Biodegradable Batteries with Immobilized Electrolyte for Transient MEMS. Biomed. Microdevices 2019, 21, 17. [Google Scholar] [CrossRef]
- Huang, X.; Wang, D.; Yuan, Z.; Xie, W.; Wu, Y.; Li, R.; Zhao, Y.; Luo, D.; Cen, L.; Chen, B.; et al. A Fully Biodegradable Battery for Self-Powered Transient Implants. Small 2018, 14, 1800994. [Google Scholar] [CrossRef]
- Joung, Y.H. Development of Implantable Medical Devices: From an Engineering Perspective. Int. Neurourol. J. 2013, 17, 98–106. [Google Scholar] [CrossRef]
- Cha, G.D.; Kang, D.; Lee, J.; Kim, D.H. Bioresorbable Electronic Implants: History, Materials, Fabrication, Devices, and Clinical Applications. Adv. Healthc. Mater. 2019, 8, e1801660. [Google Scholar] [CrossRef]
- Tayal, M.; Mukherjee, A.; Chauhan, U.; Uniyal, M.; Garg, S.; Singh, A.; Bhadoria, A.S.; Kant, R. Evaluation of Remote Monitoring Device for Monitoring Vital Parameters against Reference Standard: A Diagnostic Validation Study for COVID-19 Preparedness. Indian J. Community Med. 2020, 45, 235–239. [Google Scholar] [CrossRef] [PubMed]
- De Santis, M.; Cacciotti, I. Wireless Implantable and Biodegradable Sensors for Postsurgery Monitoring: Current Status and Future Perspectives. Nanotechnology 2020, 31, 252001. [Google Scholar] [CrossRef] [PubMed]
- Coleman, T.; Hung, C.; Hsiai, K.T.; Khademhosseini, A. Interfacing Bioelectronics and Biomedical Sensing; Springer: Berlin/Heidelberg, Germany, 2020. [Google Scholar]
- La Mattina, A.A.; Mariani, S.; Barillaro, G. Bioresorbable Materials on the Rise: From Electronic Components and Physical Sensors to In Vivo Monitoring Systems. Adv. Sci. 2020, 7, 1902872. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, R.; Wang, L.; Yin, L. Materials and Devices for Biodegradable and Soft Biomedical Electronics. Materials 2018, 11, 2108. [Google Scholar] [CrossRef] [Green Version]
- Taurino, I.; Sanzó, G.; Mazzei, F.; Favero, G.; de Micheli, G.; Carrara, S. Fast Synthesis of Platinum Nanopetals and Nanospheres for Highly-Sensitive Non-Enzymatic Detection of Glucose and Selective Sensing of Ions. Sci. Rep. 2015, 5, 15277. [Google Scholar] [CrossRef]
- Taurino, I.; Magrez, A.; Matteini, F.; Cavallini, A.; Forró, L.; de Micheli, G.; Carrara, S. High-Performance Multipanel Biosensors Based on a Selective Integration of Nanographite Petals. Nano Lett. 2014, 14, 3180–3184. [Google Scholar] [CrossRef]
- Kieninger, J. Electrochemical Methods for the Micro- and Nanoscale: Theoretical Essentials, Instrumentation and Methods for Applications in MEMS and Nanotechnology, 1st ed.; Walter de Gruyter GmbH: Berlin, Germany; Boston, MA, USA, 2022. [Google Scholar]
- Boutry, C.M.; Nguyen, A.; Lawal, Q.O.; Chortos, A.; Rondeau-Gagné, S.; Bao, Z. A Sensitive and Biodegradable Pressure Sensor Array for Cardiovascular Monitoring. Adv. Mater. 2015, 27, 6954–6961. [Google Scholar] [CrossRef]
- Li, R.; Qi, H.; Ma, Y.; Deng, Y.; Liu, S.; Jie, Y.; Jing, J.; He, J.; Zhang, X.; Wheatley, L.; et al. A Flexible and Physically Transient Electrochemical Sensor for Real-Time Wireless Nitric Oxide Monitoring. Nat. Commun. 2020, 11, 11. [Google Scholar] [CrossRef]
- Kang, S.K.; Murphy, R.K.J.; Hwang, S.W.; Lee, S.M.; Harburg, D.V.; Krueger, N.A.; Shin, J.; Gamble, P.; Cheng, H.; Yu, S.; et al. Bioresorbable Silicon Electronic Sensors for the Brain. Nature 2016, 530, 71–76. [Google Scholar] [CrossRef]
- Marland, J.R.K.; Gray, M.E.; Dunare, C.; Blair, E.O.; Tsiamis, A.; Sullivan, P.; González-Fernández, E.; Greenhalgh, S.N.; Gregson, R.; Clutton, R.E.; et al. Real-Time Measurement of Tumour Hypoxia Using an Implantable Microfabricated Oxygen Sensor. Sens. Biosensing Res. 2020, 30, 100375. [Google Scholar] [CrossRef]
- Ribeiro, A.M.; Flores-Sahagun, T.H.S.; Paredes, R.C. A Perspective on Molybdenum Biocompatibility and Antimicrobial Activity for Applications in Implants. J. Mater. Sci. 2016, 51, 2806–2816. [Google Scholar] [CrossRef]
- Ryu, H.; Seo, M.H.; Rogers, J.A. Bioresorbable Metals for Biomedical Applications: From Mechanical Components to Electronic Devices. Adv. Healthc. Mater. 2021, 10, 2002236. [Google Scholar] [CrossRef]
- Phan, H.P. Implanted Flexible Electronics: Set Device Lifetime with Smart Nanomaterials. Micromachines 2021, 12, 157. [Google Scholar] [CrossRef]
- Lu, L.; Garcia, C.A.; Mikos, A.G. In Vitro Degradation of Thin Poly(DL-Lactic-Co-Glycolic Acid) Films. J. Biomed. Mater. Res. 1999, 46, 236–244. [Google Scholar] [CrossRef]
- Silva, A.T.C.R.; Cardoso, B.C.O.; Ribeiro e Silva, M.E.S.; Freitas, R.F.S.; Sousa, R.G. Synthesis, Characterization, and Study of PLGA Copolymer in Vitro Degradation. J. Biomater. Nanobiotechnol. 2015, 06, 8–19. [Google Scholar] [CrossRef] [Green Version]
- Hwang, S.W.; Song, J.K.; Huang, X.; Cheng, H.; Kang, S.K.; Kim, B.H.; Kim, J.H.; Yu, S.; Huang, Y.; Rogers, J.A. High-Performance Biodegradable/Transient Electronics on Biodegradable Polymers. Adv. Mater. 2014, 26, 3905–3911. [Google Scholar] [CrossRef]
- Kim, D.H.; Kim, Y.S.; Amsden, J.; Panilaitis, B.; Kaplan, D.L.; Omenetto, F.G.; Zakin, M.R.; Rogers, J.A. Silicon Electronics on Silk as a Path to Bioresorbable, Implantable Devices. Appl. Phys. Lett. 2009, 95, 133701. [Google Scholar] [CrossRef] [Green Version]
- Gesheva, K.; Szekeres, A.; Ivanova, T. Optical Properties of Chemical Vapor Deposited Thin Films of Molybdenum and Tungsten Based Metal Oxides. Sol. Energy Mater. Sol. Cells 2003, 76, 563–576. [Google Scholar] [CrossRef]
- Jörg, T.; Cordill, M.J.; Franz, R.; Glushko, O.; Winkler, J.; Mitterer, C. The Electro-Mechanical Behavior of Sputter-Deposited Mo Thin Films on Flexible Substrates. Thin Solid Film. 2016, 606, 45–50. [Google Scholar] [CrossRef]
- Chen, R.; Lan, L. Solution-Processed Metal-Oxide Thin-Film Transistors: A Review of Recent Developments. Nanotechnology 2019, 30, 312001. [Google Scholar] [CrossRef]
- Zhang, X.; Ma, G.; Wang, J. Hydrothermal Synthesis of Two-Dimensional MoS2 and Its Applications. Tungsten 2019, 1, 59–79. [Google Scholar] [CrossRef] [Green Version]
- Feig, V.R.; Tran, H.; Bao, Z. Biodegradable Polymeric Materials in Degradable Electronic Devices. ACS Cent. Sci. 2018, 4, 337–348. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, C.; Guo, C.; Fitzpatrick, V.; Ibrahim, A.; Zwierstra, M.J.; Hanna, P.; Lechtig, A.; Nazarian, A.; Lin, S.J.; Kaplan, D.L. Design of Biodegradable, Implantable Devices towards Clinical Translation. Nat. Rev. Mater. 2020, 5, 61–81. [Google Scholar] [CrossRef]
- Ben Amar, A.; Kouki, A.B.; Cao, H. Power Approaches for Implantable Medical Devices. Sensors 2015, 15, 28889–28914. [Google Scholar] [CrossRef]
- Zheng, Y.F.; Gu, X.N.; Witte, F. Biodegradable Metals. Mater. Sci. Eng. R Rep. 2014, 77, 1–34. [Google Scholar] [CrossRef]
- Manivasagam, G.; Dhinasekaran, D.; Rajamanickam, A. Biomedical Implants: Corrosion and Its Prevention—A Review. Recent Pat. Corros. Sci. 2010, 2, 40–54. [Google Scholar] [CrossRef] [Green Version]
- Yamamoto, A.; Honma, R.; Sumita, M. Cytotoxicity Evaluation of 43 Metal Salts Using Murine Fibroblasts and Osteoblastic Cells. J. Biomed. Mater. Res. 1998, 39, 331–340. [Google Scholar] [CrossRef]
- Li, H.; Zheng, Y.; Qin, L. Progress of Biodegradable Metals. Prog. Nat. Sci. Mater. Int. 2014, 24, 414–422. [Google Scholar] [CrossRef] [Green Version]
- Redlich, C.; Quadbeck, P.; Thieme, M.; Kieback, B. Molybdenum—A Biodegradable Implant Material for Structural Applications? Acta Biomater. 2020, 104, 241–251. [Google Scholar] [CrossRef]
- Hagen, W. Cellular uptake of molybdenum and tungsten. Coord. Chem. Rev. 2011, 255, 1117–1128. [Google Scholar] [CrossRef]
- Hille, R. Molybdenum and Tungsten in Biology. Trends Biochem. Sci. 2002, 27, 361–367. [Google Scholar] [CrossRef]
- Mendel, R.R.; Bittner, F. Cell Biology of Molybdenum. Biochim. Biophys. Acta Mol. Cell Res. 2006, 1763, 621–635. [Google Scholar] [CrossRef] [Green Version]
- Bolt, A.M.; Mann, K.K. Tungsten: An Emerging Toxicant, Alone or in Combination. Curr. Environ Health Rep. 2016, 3, 405–415. [Google Scholar] [CrossRef]
- Mendel, R.R. Molybdenum: Biological Activity and Metabolism. Dalton Trans. 2005, 21, 3404–3409. [Google Scholar] [CrossRef]
- Peuster, M.; Fink, C.; von Schnakenburg, C. Biocompatibility of Corroding Tungsten Coils: In Vitro Assessment of Degradation Kinetics and Cytotoxicity on Human Cells. Biomaterials 2003, 24, 4057–4061. [Google Scholar] [CrossRef]
- Yu, X.; Shou, W.; Mahajan, B.K.; Huang, X.; Pan, H. Materials, Processes, and Facile Manufacturing for Bioresorbable Electronics: A Review. Adv. Mater. 2018, 30, 1707624. [Google Scholar] [CrossRef]
- Kim, A.; Ochoa, M.; Rahimi, R.; Ziaie, B. New and Emerging Energy Sources for Implantable Wireless Microdevices. IEEE Access 2015, 3, 89–98. [Google Scholar] [CrossRef]
- Ronkainen, N.J.; Halsall, H.B.; Heineman, W.R. Electrochemical Biosensors. Chem. Soc. Rev. 2010, 39, 1747–1763. [Google Scholar] [CrossRef]
- Pandolfo, A.G.; Hollenkamp, A.F. Carbon Properties and Their Role in Supercapacitors. J. Power Sources 2006, 157, 11–27. [Google Scholar] [CrossRef]
- González, A.; Goikolea, E.; Barrena, J.A.; Mysyk, R. Review on Supercapacitors: Technologies and Materials. Renew. Sustain. Energy Rev. 2016, 58, 1189–1206. [Google Scholar] [CrossRef]
- Lee, H.; Lee, G.; Yun, J.; Keum, K.; Hong, S.Y.; Song, C.; Kim, J.W.; Lee, J.H.; Oh, S.Y.; Kim, D.S.; et al. Facile Fabrication of a Fully Biodegradable and Stretchable Serpentine-Shaped Wire Supercapacitor. Chem. Eng. J. 2019, 366, 62–71. [Google Scholar] [CrossRef]
- Tang, W.; Liu, L.; Tian, S.; Li, L.; Yue, Y.; Wu, Y.; Zhu, K. Aqueous Supercapacitors of High Energy Density Based on MoO3 Nanoplates as Anode Material. Chem. Commun. 2011, 47, 10058–10060. [Google Scholar] [CrossRef] [PubMed]
- Yin, L.; Huang, X.; Xu, H.; Zhang, Y.; Lam, J.; Cheng, J.; Rogers, J.A. Materials, Designs, and Operational Characteristics for Fully Biodegradable Primary Batteries. Adv. Mater. 2014, 26, 3879–3884. [Google Scholar] [CrossRef] [PubMed]
- Ali, A. Ensafi Electrochemical Biosensors; Elsevier: Amsterdam, The Netherlands, 2019. [Google Scholar]
- Clark, L.C.; Lyons, C. Electrode Systems for Continuous Monitoring in Cardiovascular Surgery. Autom. Semi-Autom. Syst. Clin. Chem. 1962, 102, 29–45. [Google Scholar] [CrossRef]
- Grieshaber, D.; Mackenzie, R.; Vörös, J.; Reimhult, E. Electrochemical Biosensors-Sensor Principles and Architectures. Sensors 2008, 8, 1400–1458. [Google Scholar] [CrossRef]
- Cordeiro, C.A.; Sias, A.; Koster, T.; Westerink, B.H.C.; Cremers, T.I.F.H. In Vivo “Real-Time” Monitoring of Glucose in the Brain with an Amperometric Enzyme-Based Biosensor Based on Gold Coated Tungsten (W-Au) Microelectrodes. Sens. Actuators B Chem. 2018, 263, 605–613. [Google Scholar] [CrossRef]


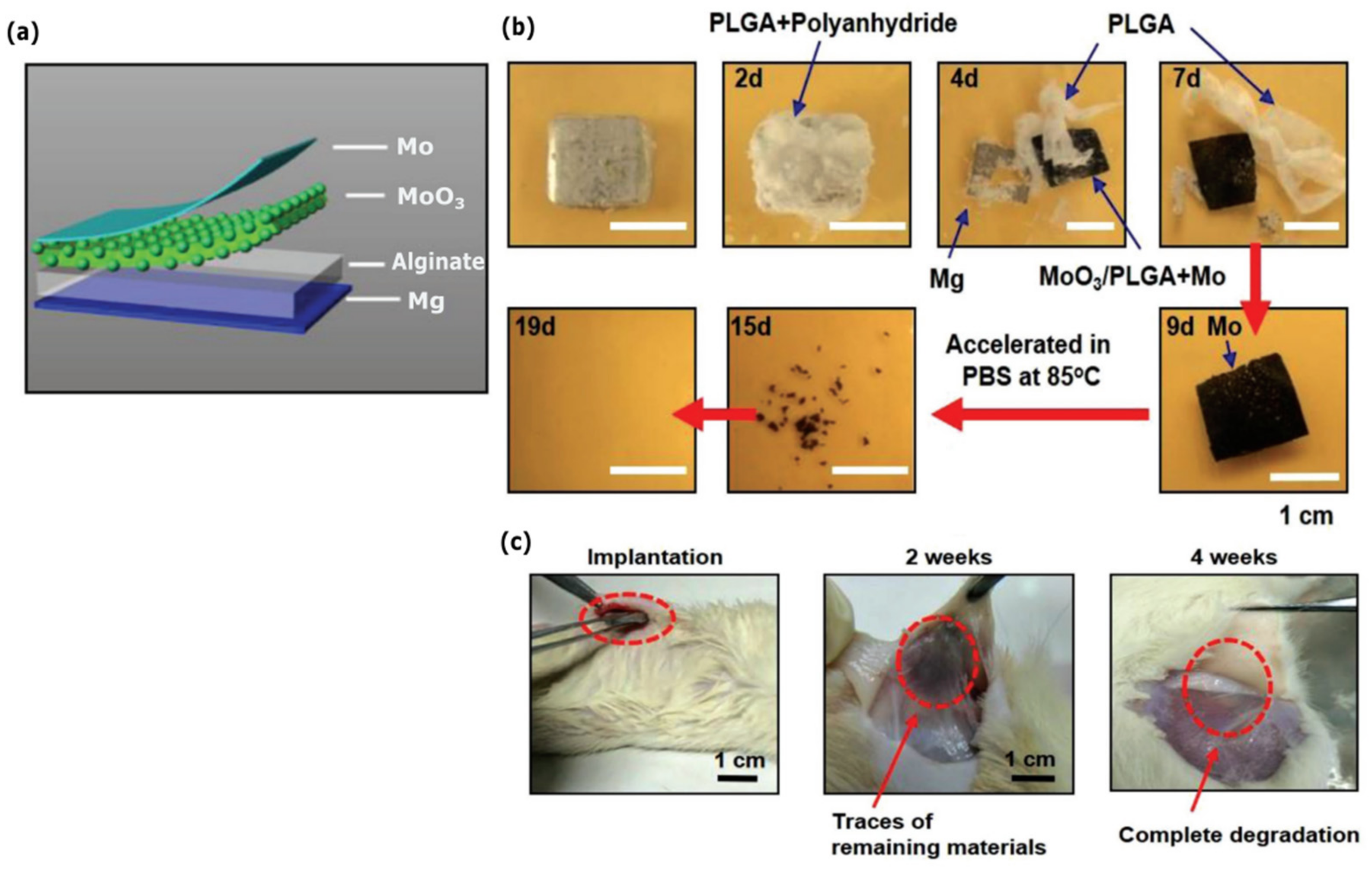

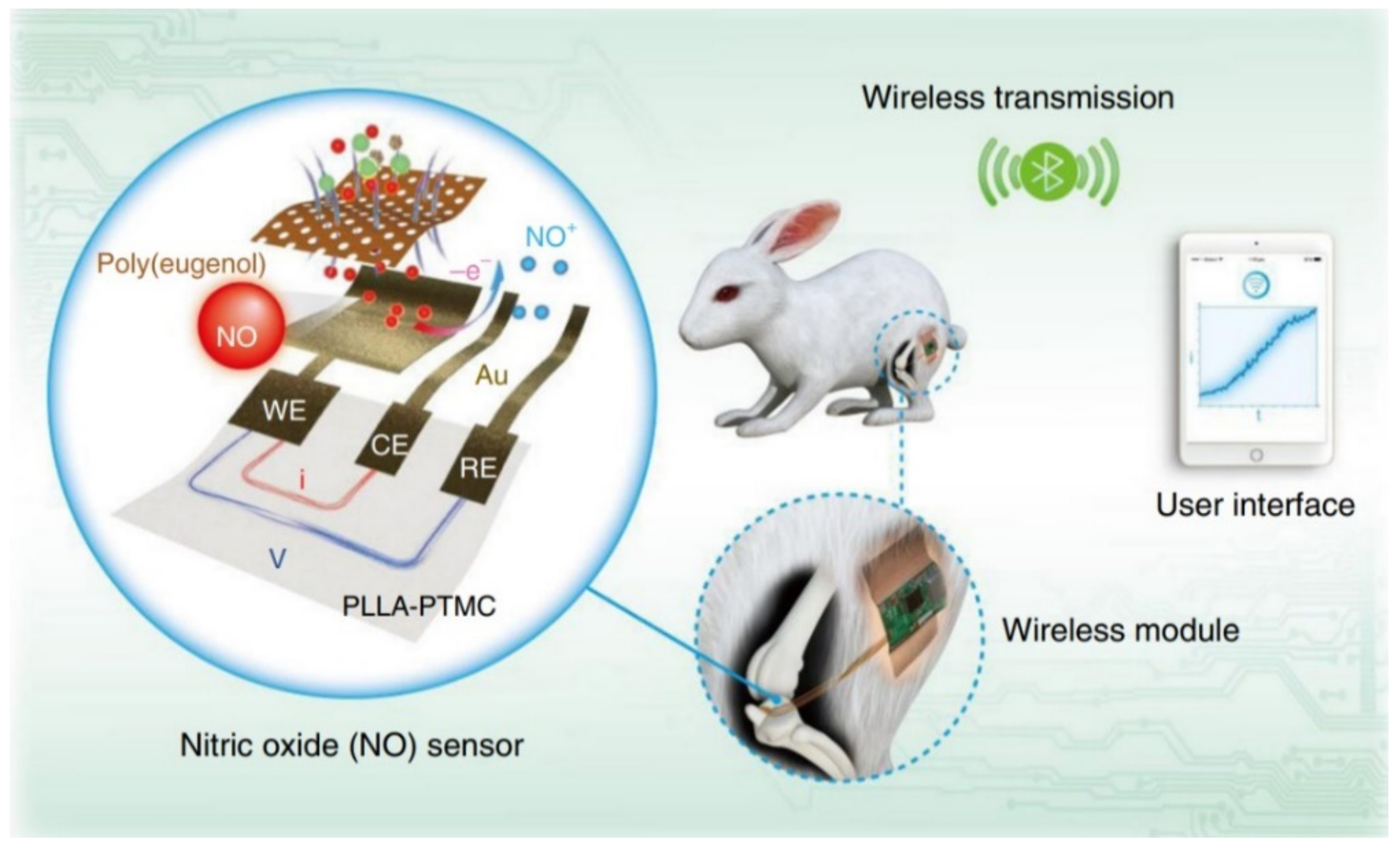
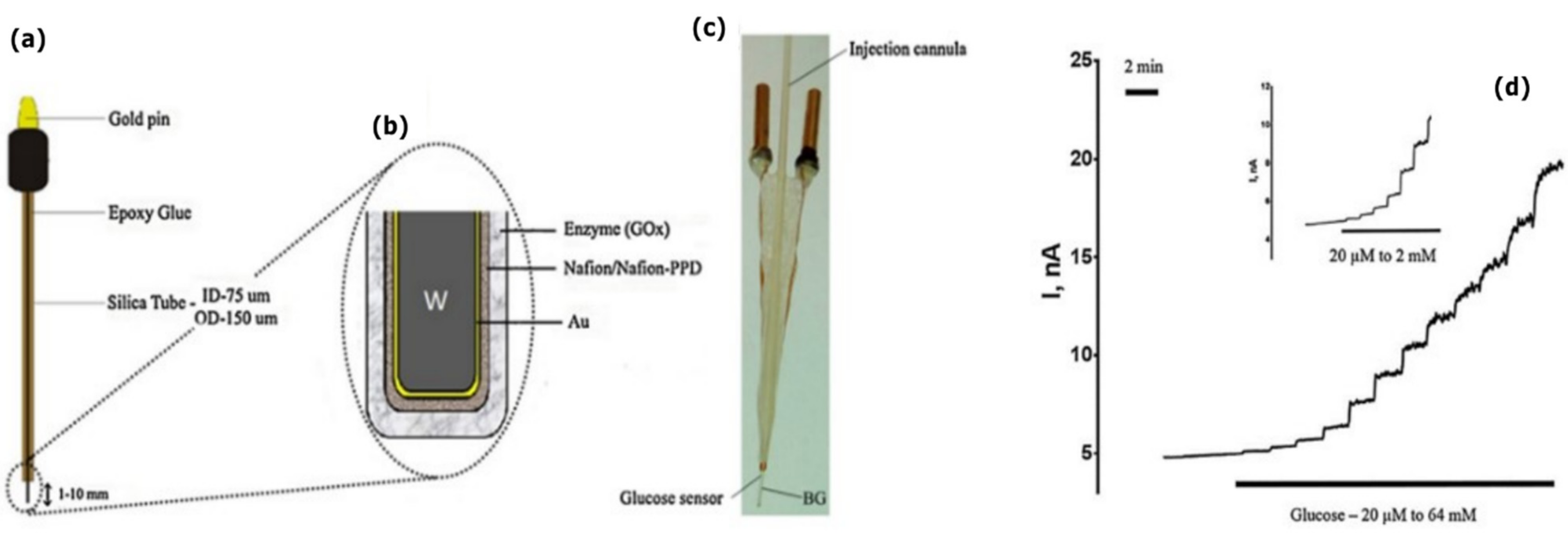
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fernandes, C.; Taurino, I. Biodegradable Molybdenum (Mo) and Tungsten (W) Devices: One Step Closer towards Fully-Transient Biomedical Implants. Sensors 2022, 22, 3062. https://doi.org/10.3390/s22083062
Fernandes C, Taurino I. Biodegradable Molybdenum (Mo) and Tungsten (W) Devices: One Step Closer towards Fully-Transient Biomedical Implants. Sensors. 2022; 22(8):3062. https://doi.org/10.3390/s22083062
Chicago/Turabian StyleFernandes, Catarina, and Irene Taurino. 2022. "Biodegradable Molybdenum (Mo) and Tungsten (W) Devices: One Step Closer towards Fully-Transient Biomedical Implants" Sensors 22, no. 8: 3062. https://doi.org/10.3390/s22083062
APA StyleFernandes, C., & Taurino, I. (2022). Biodegradable Molybdenum (Mo) and Tungsten (W) Devices: One Step Closer towards Fully-Transient Biomedical Implants. Sensors, 22(8), 3062. https://doi.org/10.3390/s22083062






