1. Introduction
The leather industry promotes China’s economic prosperity but also causes severe environmental pollution [
1]. At present, there are more than 8400 tanning enterprises in China. According to the data, China’s leather output in 2021 was 574 million square meters, with a year-on-year increase of 14.8%. Together with this, a large amount of tanning wastewater is discharged. The tanning industry discharges more than 120–150 million tons of wastewater every year, accounting for about 0.47% of China’s total industrial wastewater. However, tannery wastewater has the characteristics of complex composition, large fluctuations in water quality, many types of organic substances, as well as a high content and difficult degradation of macromolecules [
2].
If the chromium removed from tannery wastewater was directly discharged, it would still cause irreversible pollution to the environment. Therefore, it is imperative to find a process for the advanced treatment of chromium removal from tannery wastewater [
3]. The most significant characteristic of membrane separation involves the driving force, which mainly consists of pressure, with no phase changes during the separation process and no heating. Compared to other separation technologies, membrane separation has significant advantages of energy savings and high efficiency. To make full use of tannery wastewater, improving the reuse rate of wastewater, realizing the comprehensive utilization of wastewater resources, and the combined process of membrane technology and other technologies has become a research hotspot for many scholars [
4,
5,
6].
Li treated integrated wastewater from leather production via the double-membrane technology of UF and reverse osmosis as the core [
7]. The results showed that the UF membrane had strong anti-pollution abilities. In addition, the turbidity of the effluent water was less than 0.5 NTU and the SDI value was less than 3, which fully met the requirements of reverse osmosis equipment (RO). The RO water COD < 30 mg·L
−1 and conductivity < 500 μS/cm met the requirements of leather production process reuse water quality.
For dyeing wastewater, Hang used NF and ED integrated technology for decolorization, which involved the separation of valent salt and salt concentration [
8]. The results showed that after secondary NF treatment, the chroma of the wastewater was reduced to 1/1200 of the raw water, the COD of the raw water was reduced to less than 100 mg·L
−1 from 200 mg·L
−1, and the mass concentration ratio of the Cl
− and SO
42− ions was reduced to 3 from 21. In addition, ED could concentrate the total dissolved salts in the permeate of the NF membrane to more than 10%.
Streit et al. studied the ED process and used it to separate electrolytes from salting or pickling leather to recover water and chemicals reused during production. ED was also used to recover residual tanning fluids from chromate and other neutral salts [
9]. However, the study found that there is a common problem in the process [
10,
11,
12,
13]. Compared with other industrial wastewater, the tannery dechromization wastewater has more suspended solids and macromolecular pollutants in the wastewater, and the membrane fouling is also more serious. When running for a long time, this cause irreversible damages to the membrane and shortens its life span.
In order to overcome this problem, flocculation and ultrafiltration as pretreatment processes are important research directions of tannery wastewater treatment process. Ultrafiltration (UF) is a pressure-driven membrane separation process that separates based upon a screening mechanism of molecular size and morphology [
14]. After a certain pressure is applied to the feed liquid, the polymer and colloidal substances are blocked in the pores due to the primary adsorption of the membrane surface and micropores, and the mechanical screening of the membrane surface will be blocked by the UF membrane, while water and low molecular substances pass through the membrane.
As a pretreatment means of nanofiltration (NF) equipment, UF can provide excellent influent water quality, ensuring the stable operation of subsequent experiments, and prolonging the cleaning cycle of the NF system and the service life of the membrane [
15]. NF intercepts divalent and multivalent ions and various substances with a molecular weight greater than 200 to the NF membrane through a pressure difference.
This offers the advantages of low operating pressure, energy saving, and no pollution production [
16]. The main disadvantage of NF is the volume of highly concentrated salt water that is produced, which can reach 10–20% of the volume of the original wastewater, and the concentrated wastewater contains a large amount of organic and inorganic salts, which need to be further recovered. ED technology has been widely used in brackish water desalination and has started to be applied to the process operation of wastewater desalination [
17,
18,
19].
Therefore, we discussed the UF-NF-ED coupling process, which meets the urgent requirements of reducing and recycling wastewater from the tanning and dechroming wastewater industry. In this experiment, the pretreatment effect of different UF membranes on the removal of chromium from tannery wastewater and the anti-pollution of the membrane were studied, and the effects of different pressures and molecular weights intercepted by the UF membrane were investigated on the membrane flux.
At the same time, the UF water was concentrated by NF, the concentrated wastewater was desalinated by ED, and the operating conditions of the entire process were optimized. Lastly, a set of advanced treatment processes for chromium removal from tanning wastewater was established. It was found that this process can recover 90% of fresh water and 61.9% of inorganic salts. As a new process, it can realize the efficient reuse of tannery dechromization wastewater.
4. Desalting Stage by ED
The wastewater after secondary concentration by NF contained a large number of inorganic salts and organic substances. After analysis and titration, the inorganic salts in the wastewater were mainly Na2SO4 and NaCl, and the Na2SO4 content reached 25 g·L−1 while the NaCl content reached 13.5 g·L−1. The inorganic salts in the wastewater were extracted and utilized as resources, while the wastewater was desalinated by ED technology. The limiting current of the ED system was measured, and the influence of voltage and circulating flow on the recovery rate of inorganic salts was investigated. Two-stage ED was performed on the SO42− that could not be removed.
4.1. Determination of Limiting Current
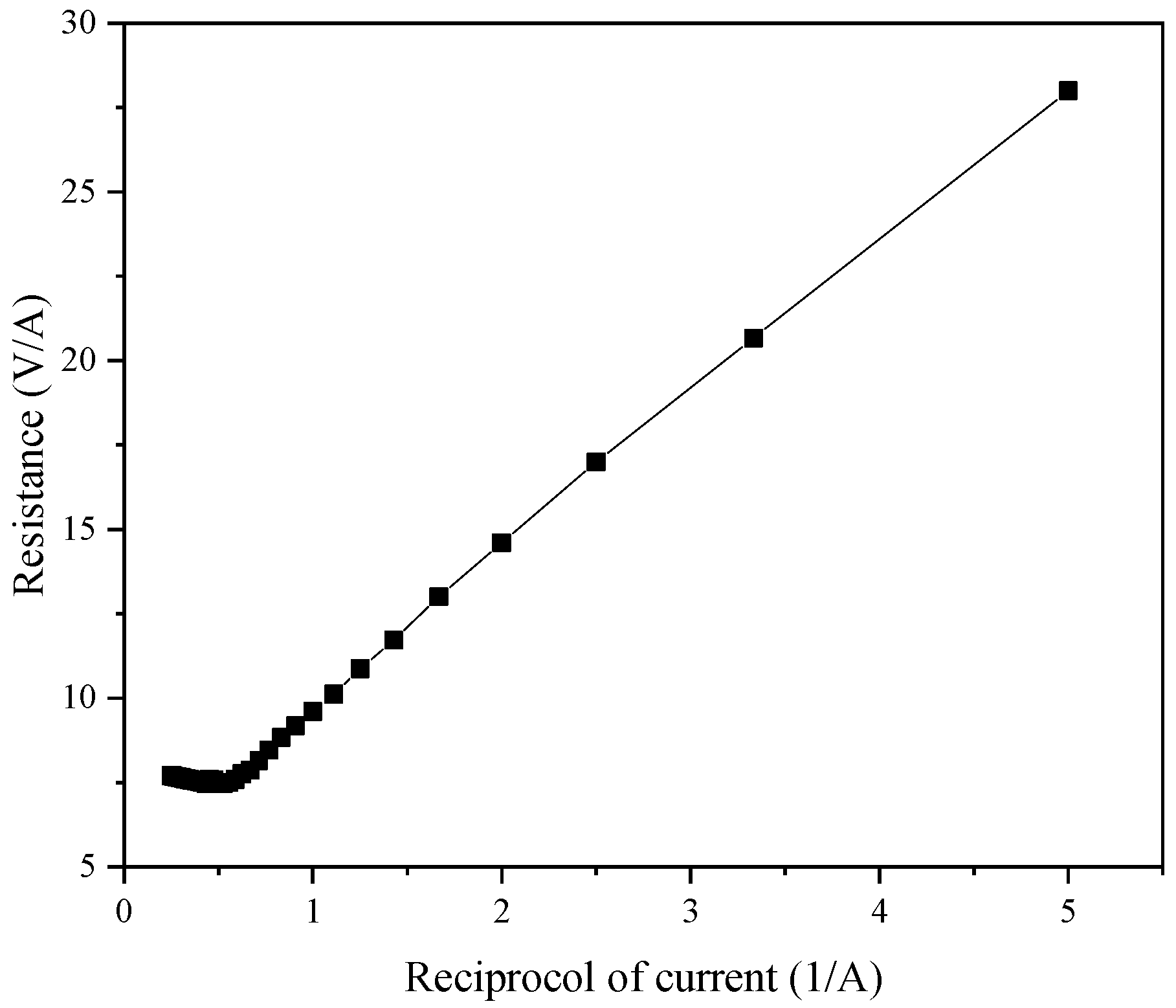
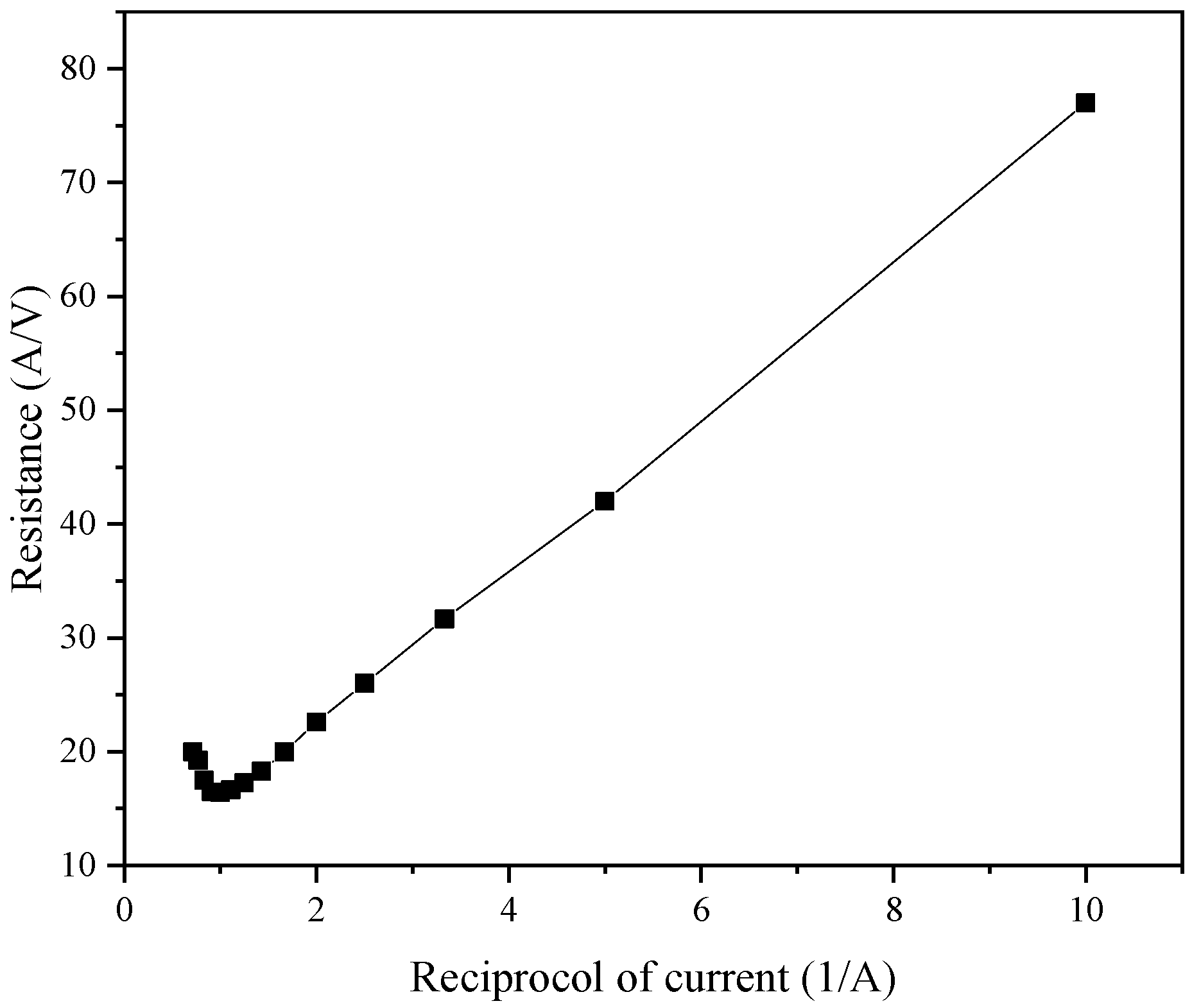
One of the most important parameters in ED applications involves the limiting current. Because the current affects the performance of the ED system, the selection of the limiting voltage and limiting current is very important, and the limiting current will be affected by the plate and membrane specifications. To measure the limiting current, commonly used methods include the voltage–current method, the current-effluent pH value method, and the resistance–current method. After comprehensive consideration, in this experiment, we adopted the resistance–current method to measure the limiting current, and 22 pairs of common anion and cation exchange membranes were used.
The polar liquid consisted of 5% Na
2SO
4 solution, where the concentration chamber contained 15% NaCl solution, and the thin chamber contained NF concentrated water to measure the limit voltage. The actual operation of the experiment finally obtained the current and voltage curve as shown in
Figure 14. The extreme point was the limit current point, and after measurements, the limit voltage and limit current were 22 V and 4.0 A, respectively. However, after consulting the literature, we found that the limit current was not one constant, but varies with the working conditions and working time of the ED process.
4.2. Influence of Operating Conditions on ED
4.2.1. Influence of Voltage on ED
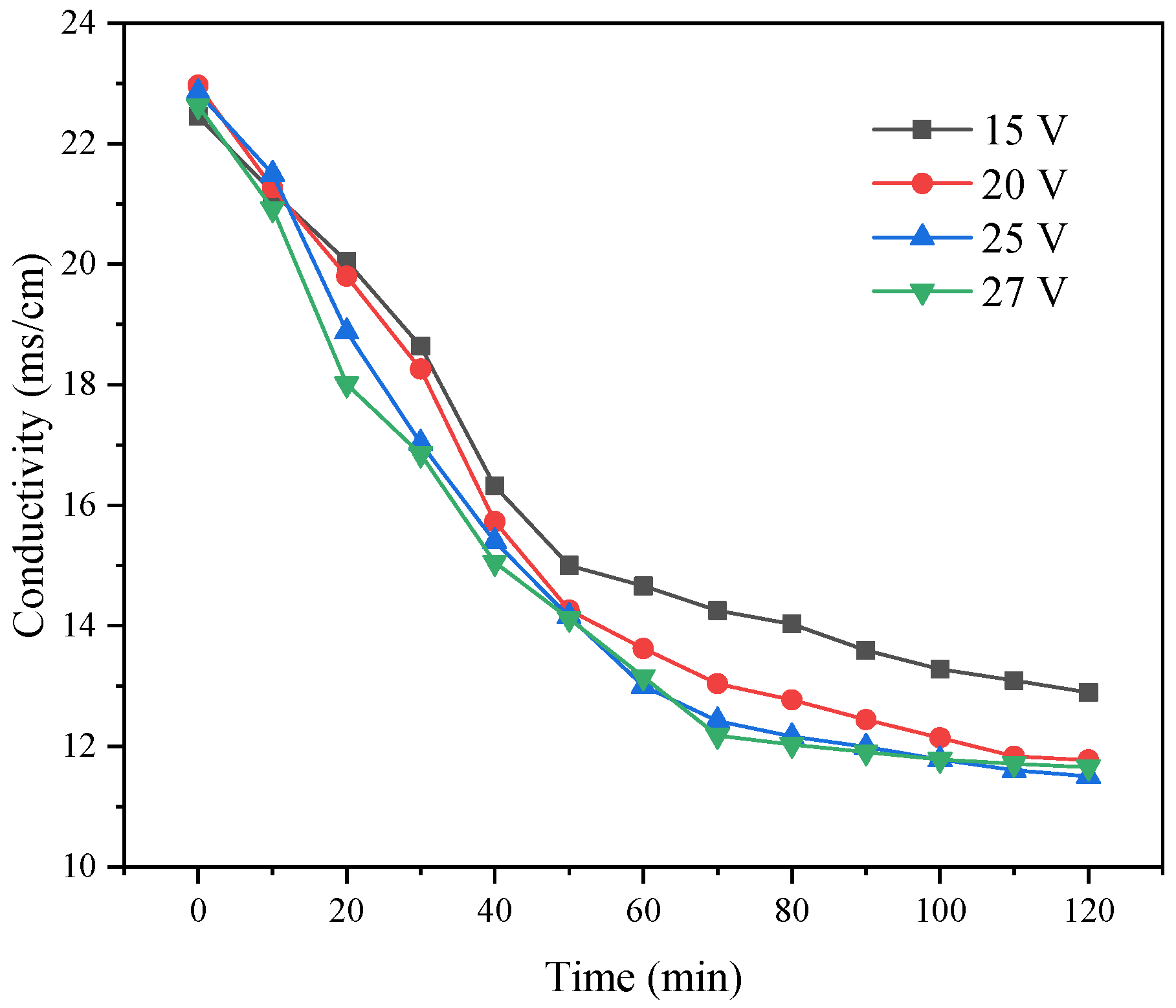
The concentrated NF water was placed into the ED desalination chamber, and the conductivity changed with time under the four constant applied voltages. As shown in
Figure 15, the TDS of the desalination chamber decreased exponentially with time under different voltages. The main reason was that the solution in the desalination chamber was electrolytically separated under the action of voltage. When operating ED under the different applied voltages, we observed certain differences in the conductivity of the desalination chamber, with a high ion migration rate under a voltage of 25 V.
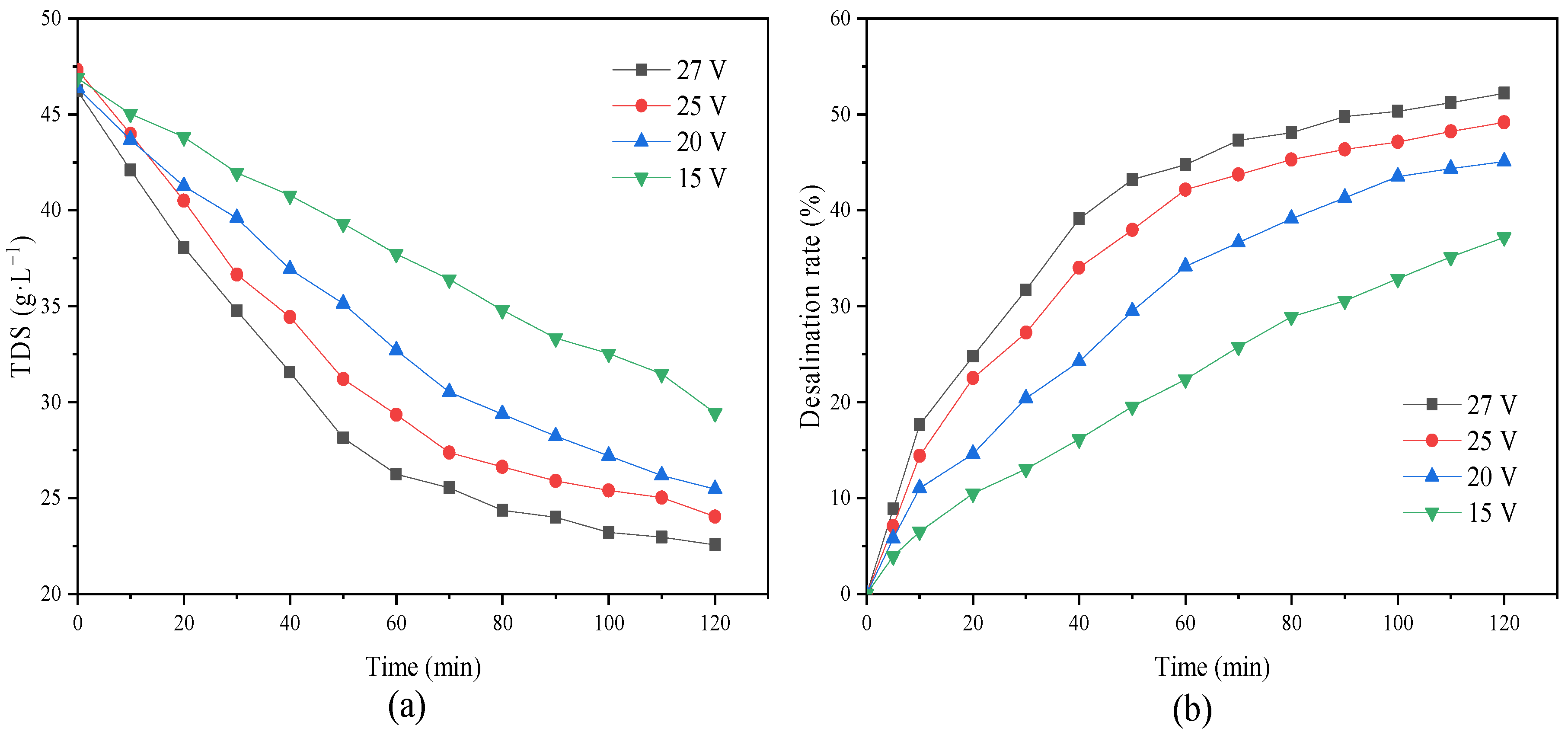
When the voltage continued to increase, the energy consumption increased, resulting in certain energy loss and heat. When the temperature was too high, it also had an adverse impact on the ED membrane, shortening the service life of the membrane. As shown in
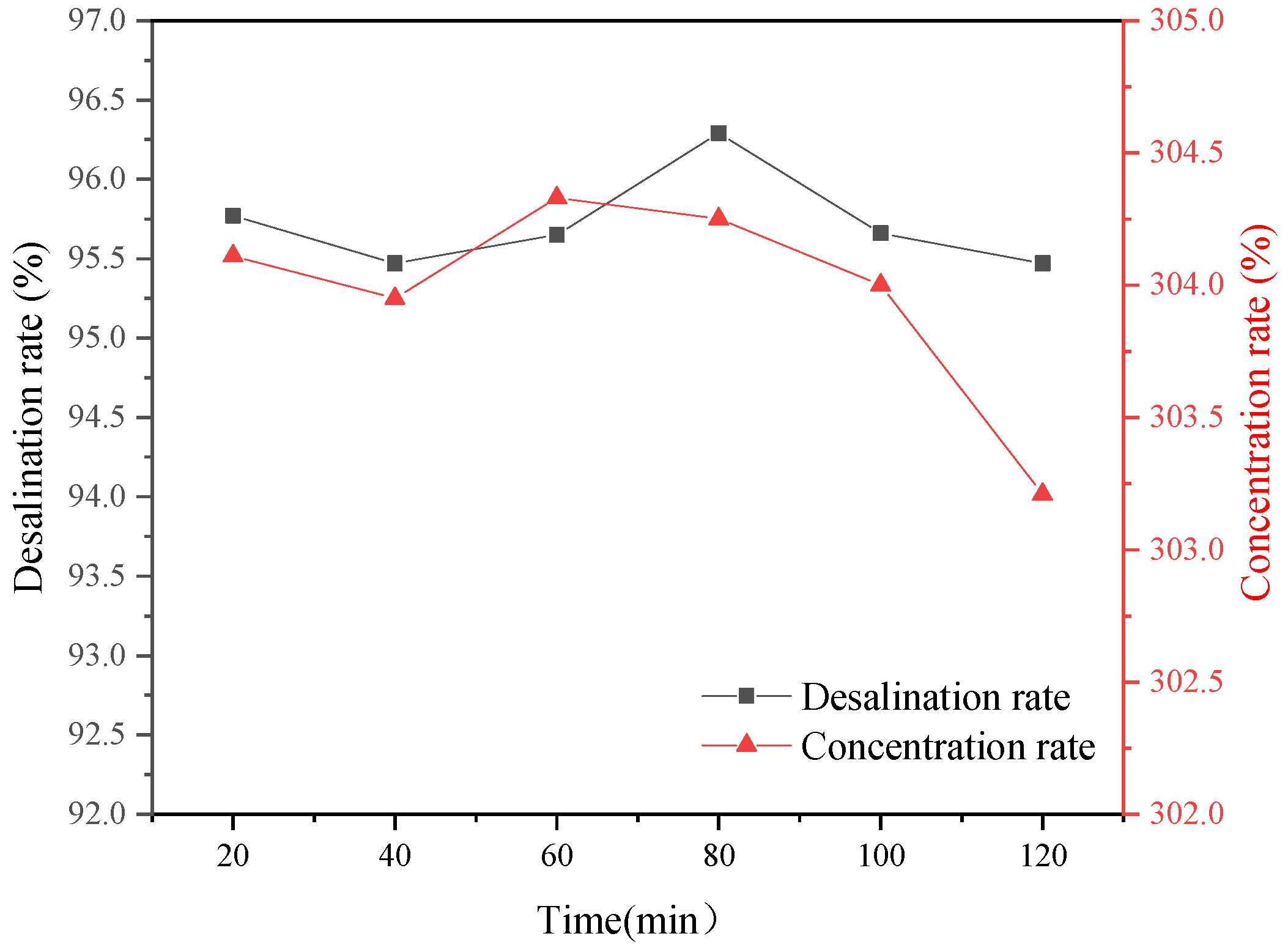
Figure 16, we found that there were also significant differences in the overall desalination rate of the desalination chamber under different voltages. When the voltage was 15 V, the overall desalination rate was only 37.2%; however, when the voltage increased to 27 V, the desalination rate reached 52.2%, and the recovery rate of inorganic salt in the NF concentrated water increased by 15%.
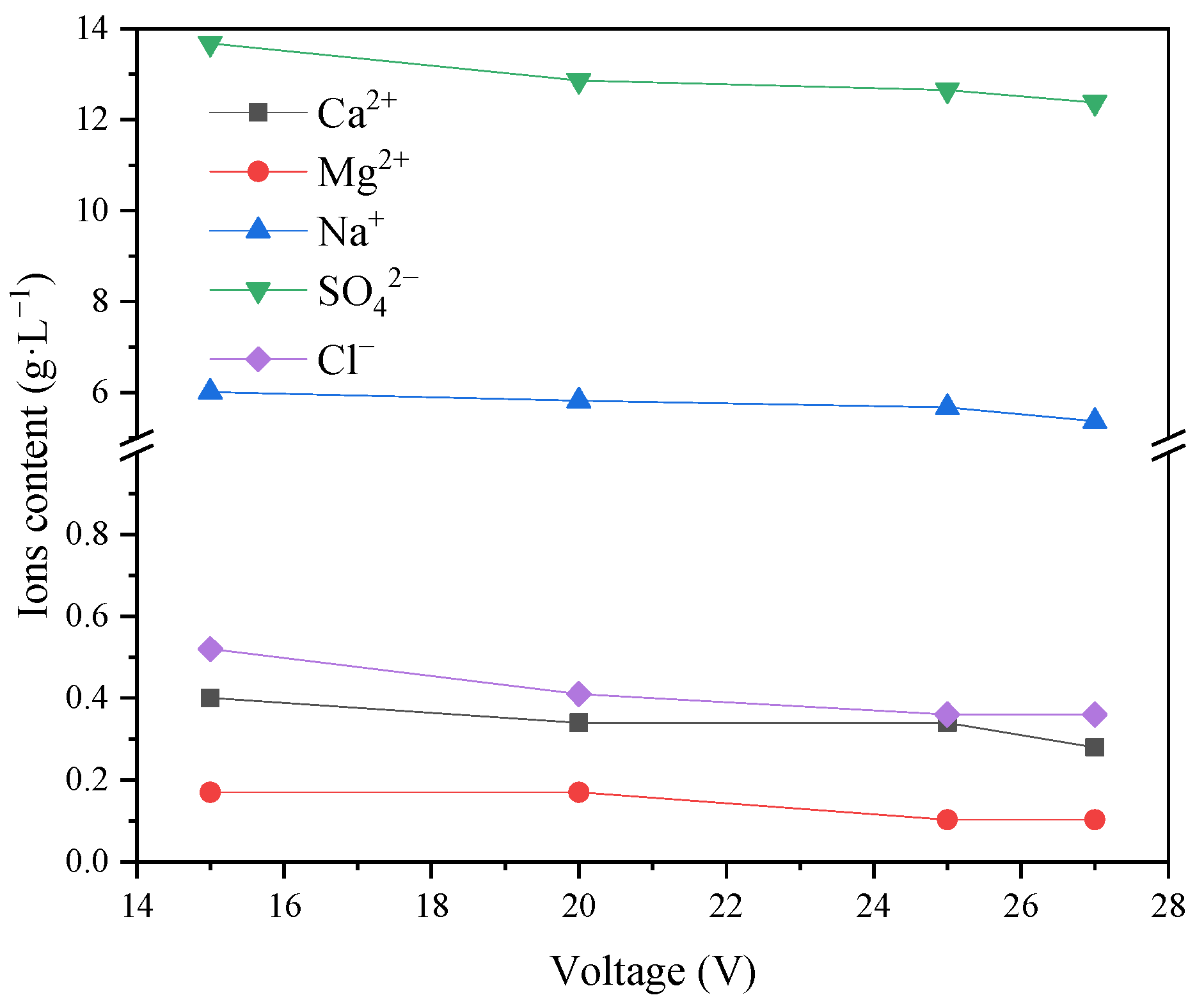
This showed that with an increase in voltage, the current increased. Due to the constant membrane area and current density, the transmembrane migration of ions in the desalination chamber to the concentrated chamber increased. Thus, the recovery rate of inorganic salts improved. At this time, most salts in the concentrated water were recovered. As shown in
Figure 17, the chloride in the concentrated water was essentially removed, and the rest was basically sulfate. The reason for the analysis is that the properties of different ions are different, indicating that under different voltages, the removal rate of Cl
− is faster than that of SO
42−, and Cl
− can be removed more effectively at the same time.
4.2.2. Effect of Feed Flow on ED
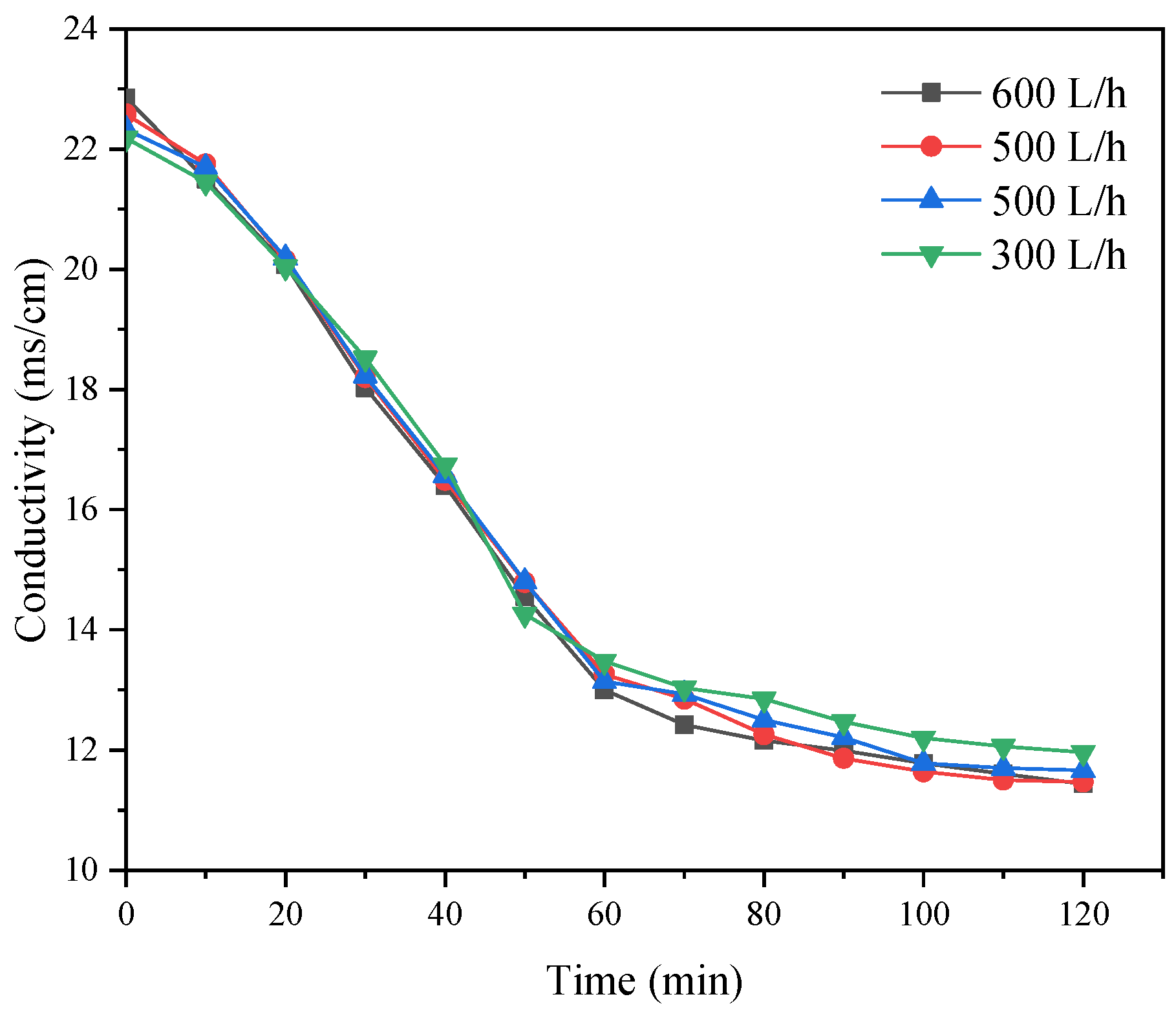
Theoretically, the feed flow can improve desalination efficiency, but this improvement will be limited. Therefore, the operating flow had to be determined and controlled within an appropriate range. In the experiment, the voltage was fixed at 25 V to carry out the research experiments with different feed flow rates. To reduce the influence of differential pressure leakages, the flow rate of the concentration chamber was maintained at 1:1, and in all experiments, the flow rate of the pole chamber remained unchanged. As shown in
Figure 18, we observed that increasing the feed flow had little effect on the desalination effect of the desalination chamber, and the conductivity of the desalination chamber was in the range of 11.44–11.96 ms/cm after 120 min.
At 2 h, the conductivity of the desalination chamber reached the lowest value, and the desalination rate reached a maximum value of 49.93%. When the influent flow was too small, the ion migration rate per unit time accelerated; however, the membrane surface flow rate was too small, and the ion migration amount could not be replenished, resulting in a low desalination rate in the desalination chamber. The mass concentration of the mixed salt and relative concentration rate of the mixed salt was reduced. If the flow velocity of the membrane surface was too large, the ions in the thin chamber would leave the thin chamber because the flow velocity of the membrane surface was too large, and the mass concentration of the mixed salt and relative concentration of the mixed salt rate would cause a reduction.
4.3. Experimental Study on Two-Stage ED
After a period of ED treatment, most sulfates still could not pass ED treatment. After consulting the literature, we found that the temperature had a certain impact on the desalination efficiency of ED. After increasing the temperature to about 31 °C in the desalination chamber, the limiting voltage and current of the water produced in the light chamber after a period of ED treatment were remeasured.
According to
Figure 19, the limit voltage of two-stage ED was 16 V, and at this time, the current did not exceed 1.0 A. Compared to the limit current and voltage of one-stage ED, this was mainly due to the decrease in salt content in the wastewater and the change in wastewater quality. On this basis, the other conditions remained unchanged. Second stage ED was conductedon the wastewater after primary desalination, and the sulfate content in the light chamber was measured. As observed in
Figure 20, SO
42− was further removed after the temperature increased, SO
42− in the overall dilute chamber decreased from 12.28 to 7.13 g·L
−1, and the overall desalination rate also increased to 61.9%.
This is because the ion hydration is inhibited at a higher temperature, the viscosity of the liquid is reduced, and the speed of ion movement is improved. At higher temperature, the conductivity of the ion-exchange membrane increases and the hydration of the counter-ion weakens, which is also beneficial to the electrodialysis. If the temperature is too high, the performance and life of the membrane will be affected.
5. Conclusions
Aiming for the advanced treatment and recycling of tannery wastewater after chromium removal, a new coupling process of flocculation membrane separation technology was proposed. We also investigated the effects of different operating conditions on the overall treatment effect of tannery chromium removal wastewater, and the process conditions were optimized. Our experimental conclusions are as follows.
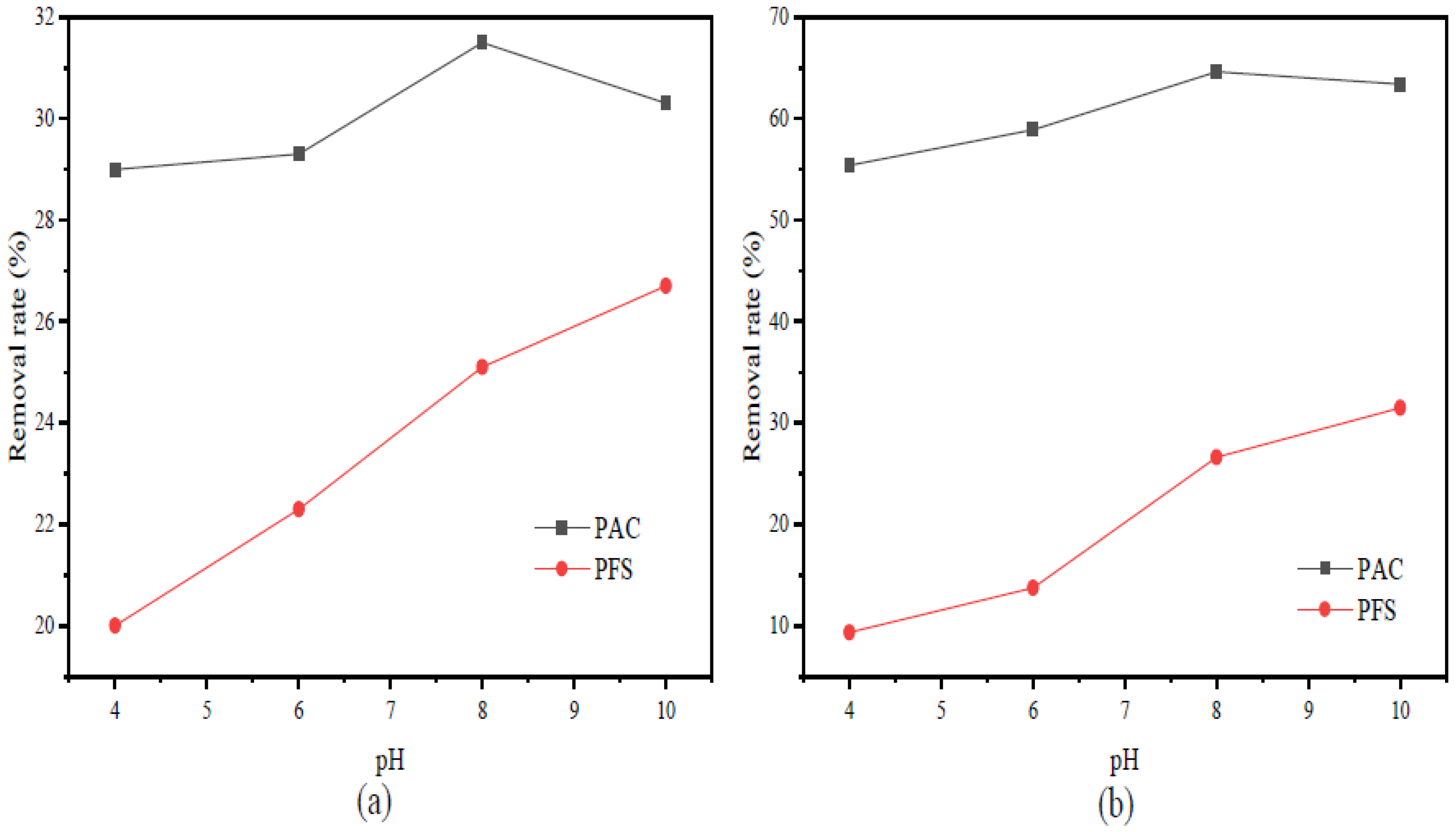
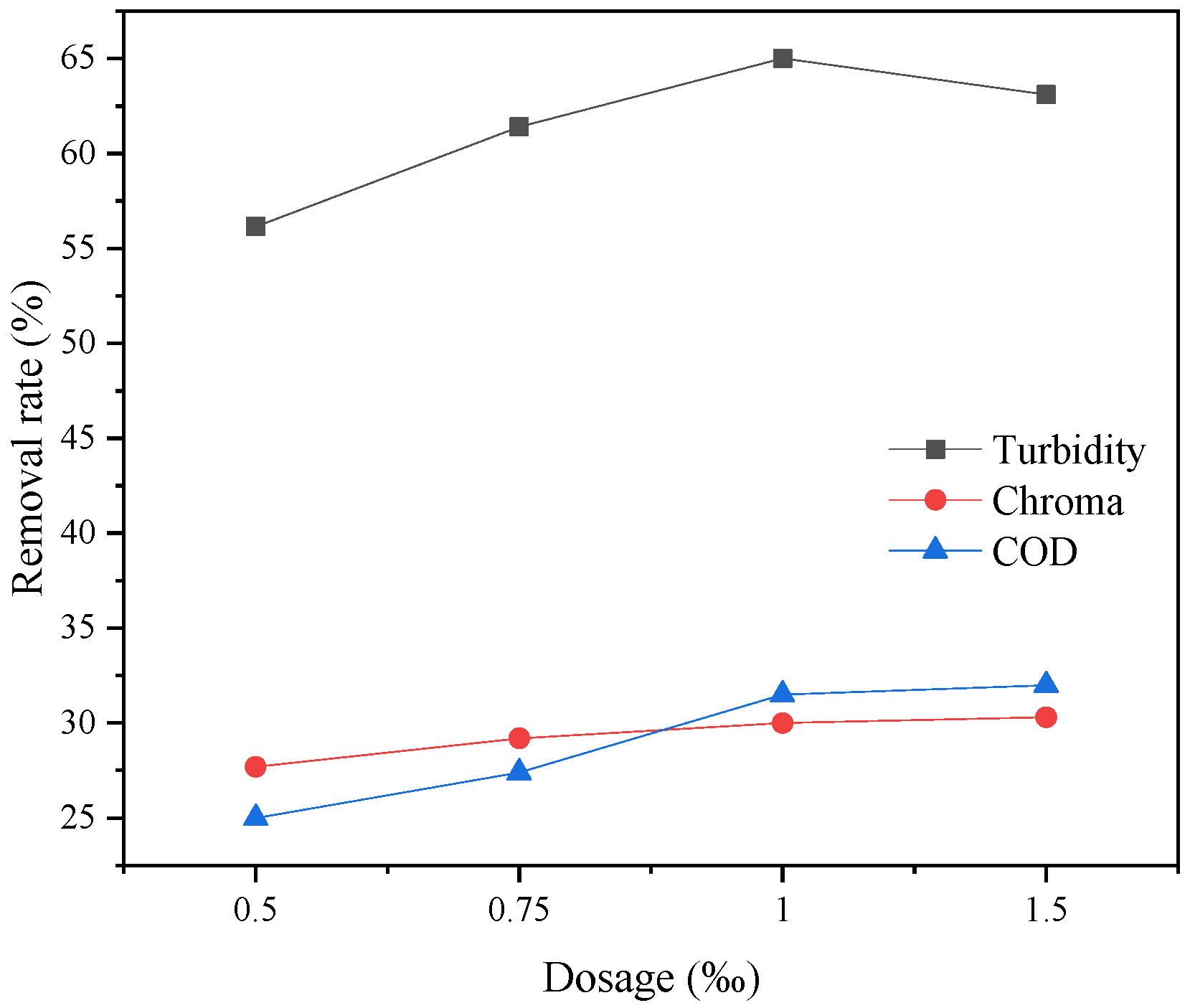
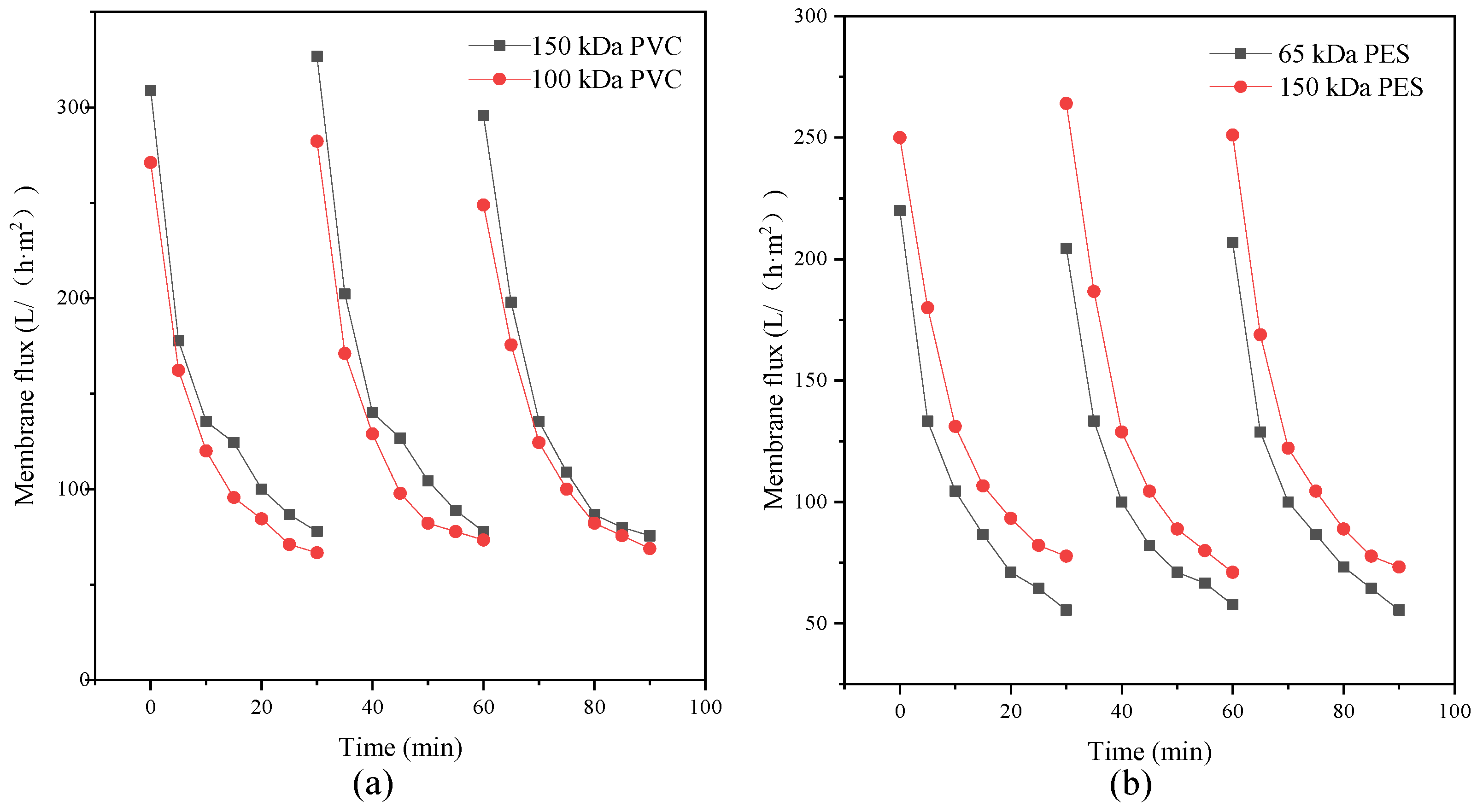
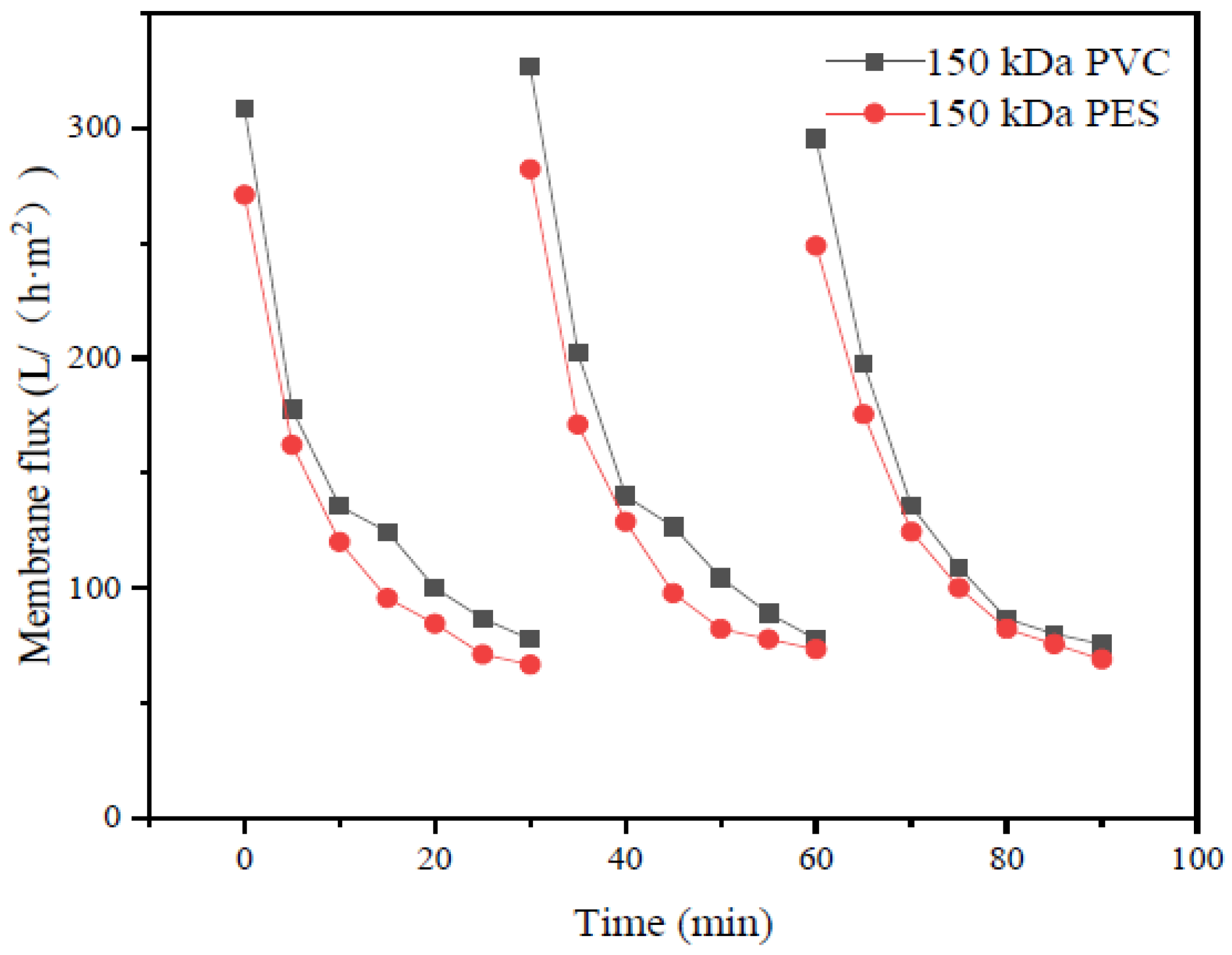
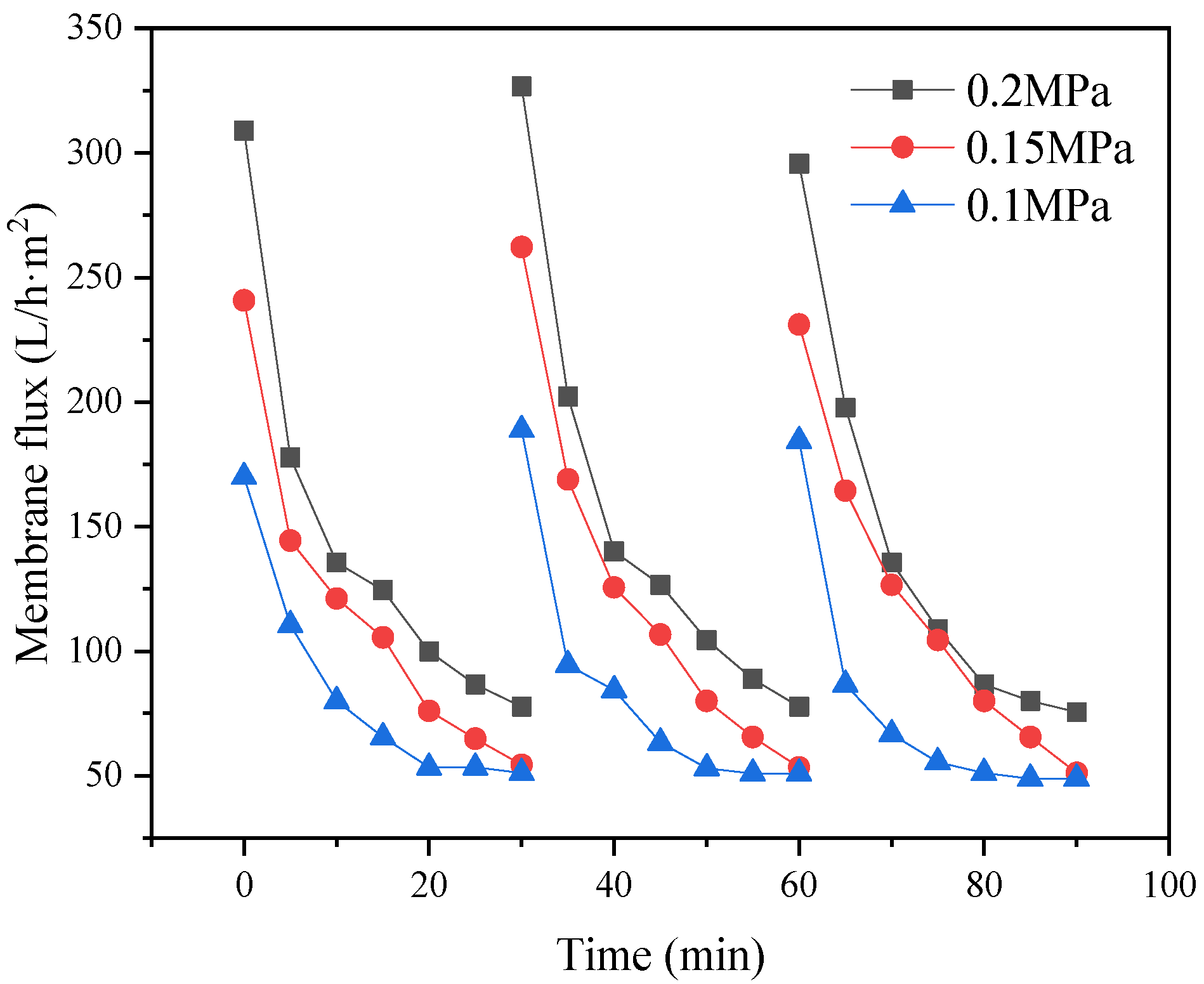
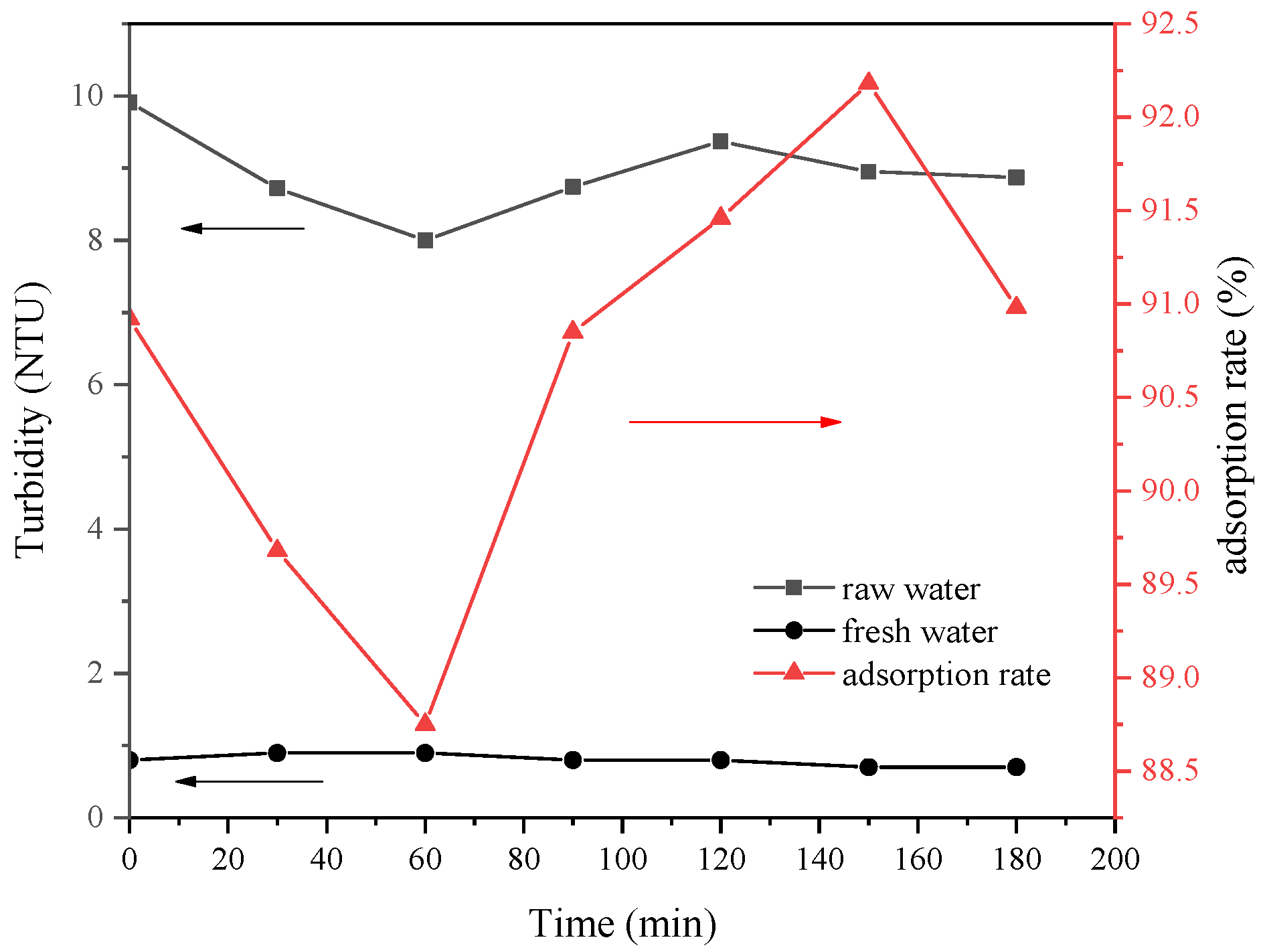
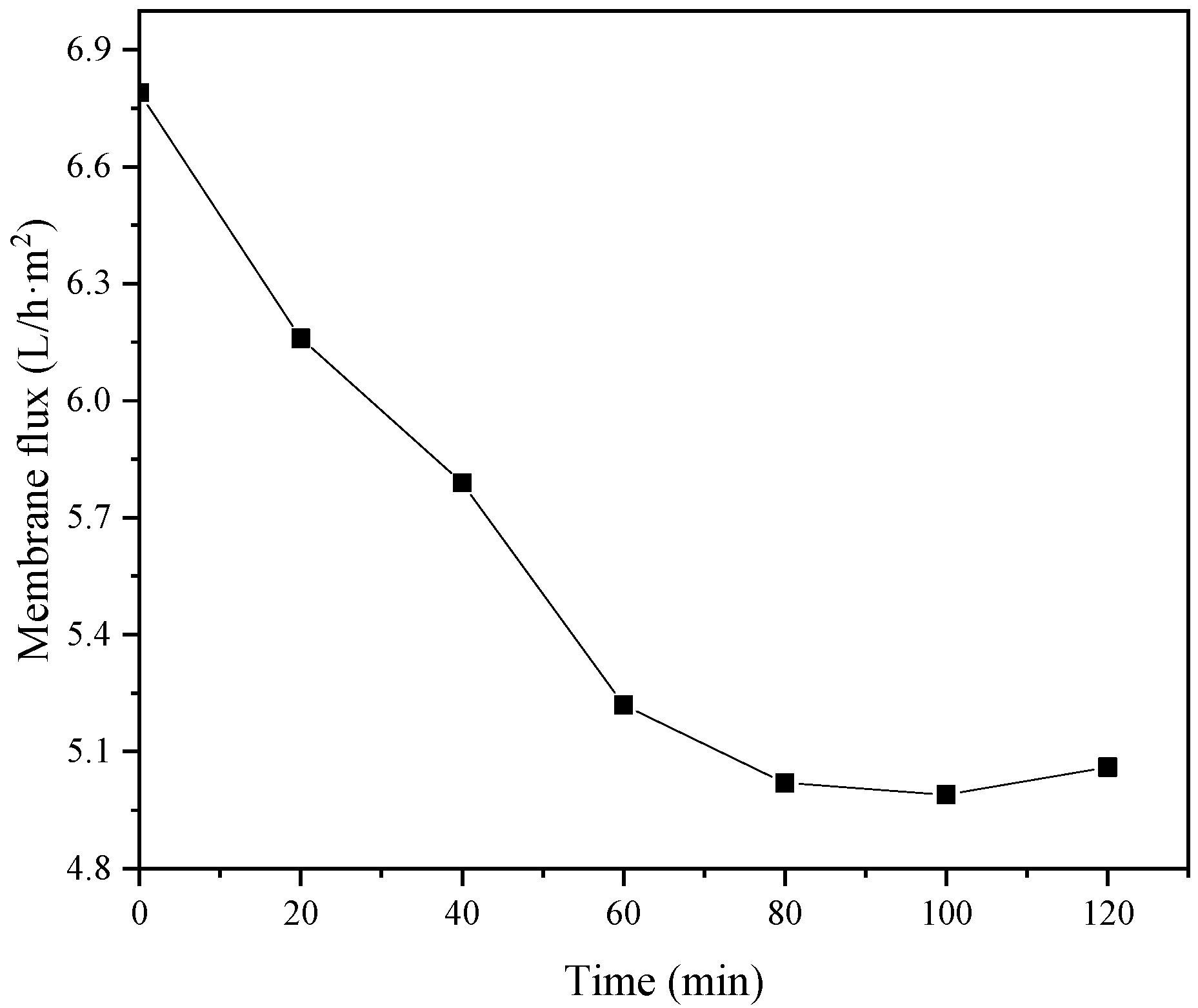
The type of flocculant, the amount of addition and the use of pH all have a certain influence on the flocculation effect. According to this experimental study, the combination of 1‰ PAC + PAM achieved the best effect under the condition of pH = 8. The comparison results of the four UF membrane modules show that the PVC UF membrane with a larger molecular weight cutoff had a greater flux effect. The membrane flux in the UF process increased with the increase of the pressure; however, the excessive pressure also caused the membrane filaments to rupture. The turbidity, chroma and COD removal rates of the pretreated wastewater were 96.5%, 53.7% and 45.8%, respectively.
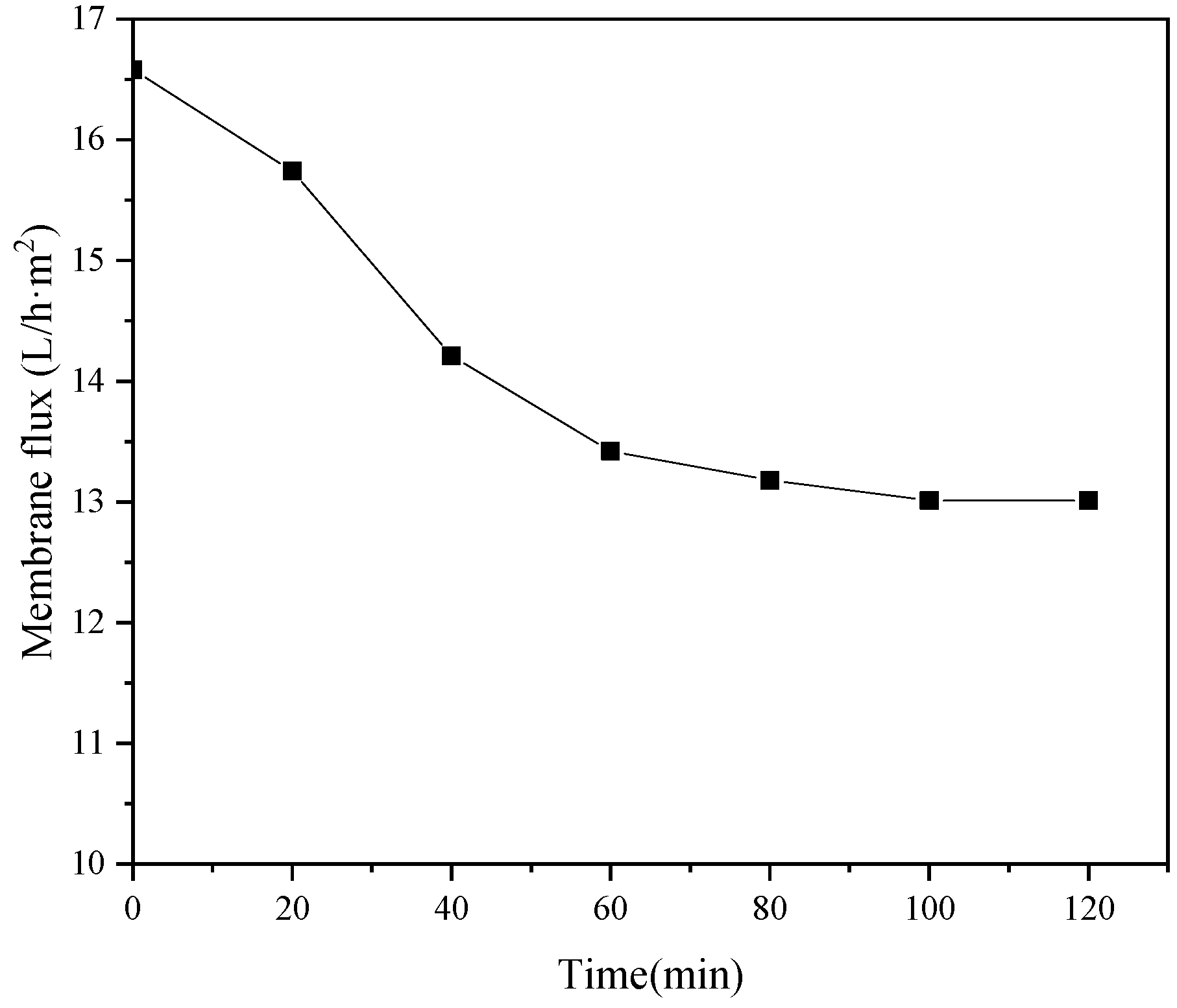
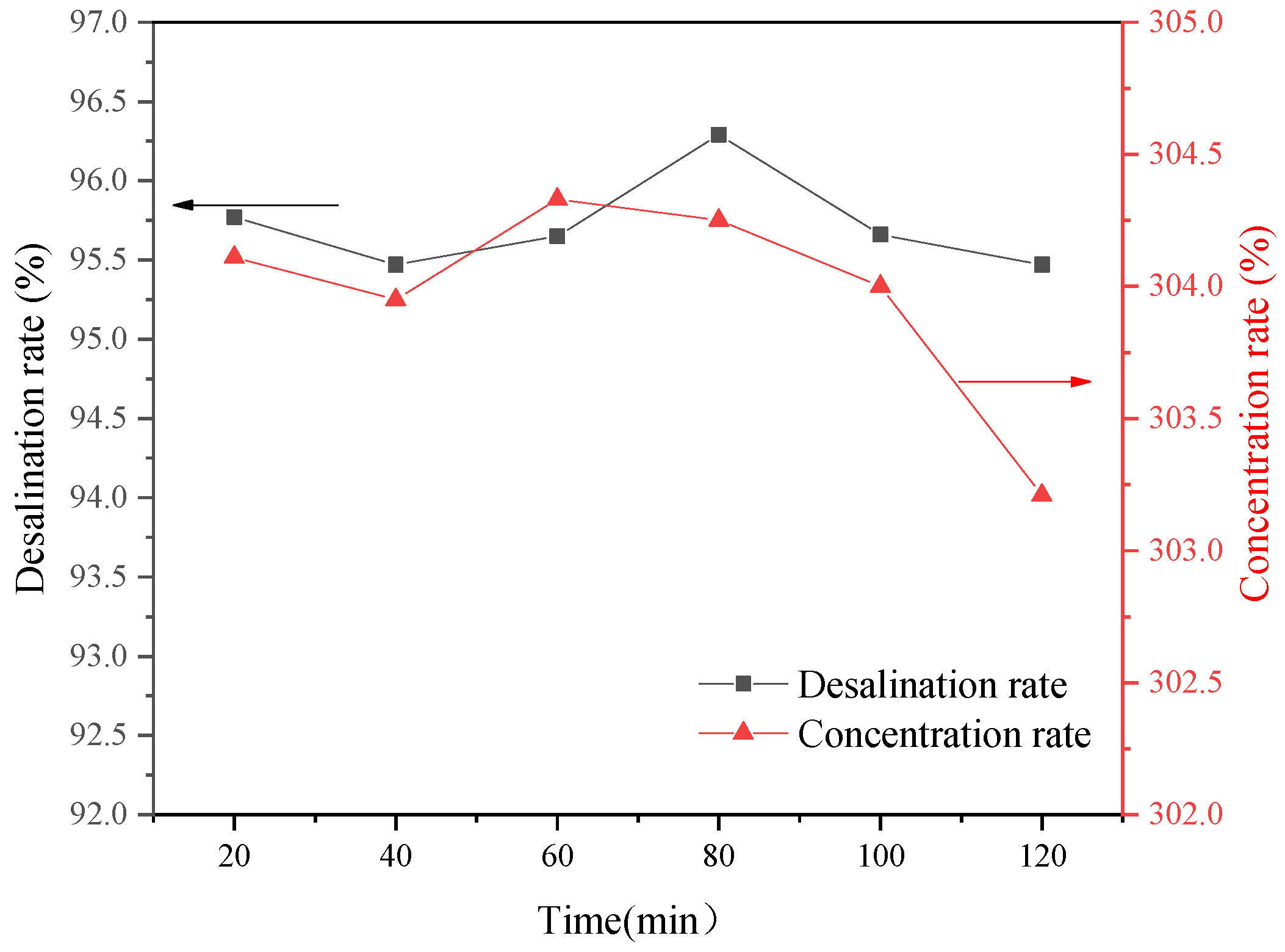
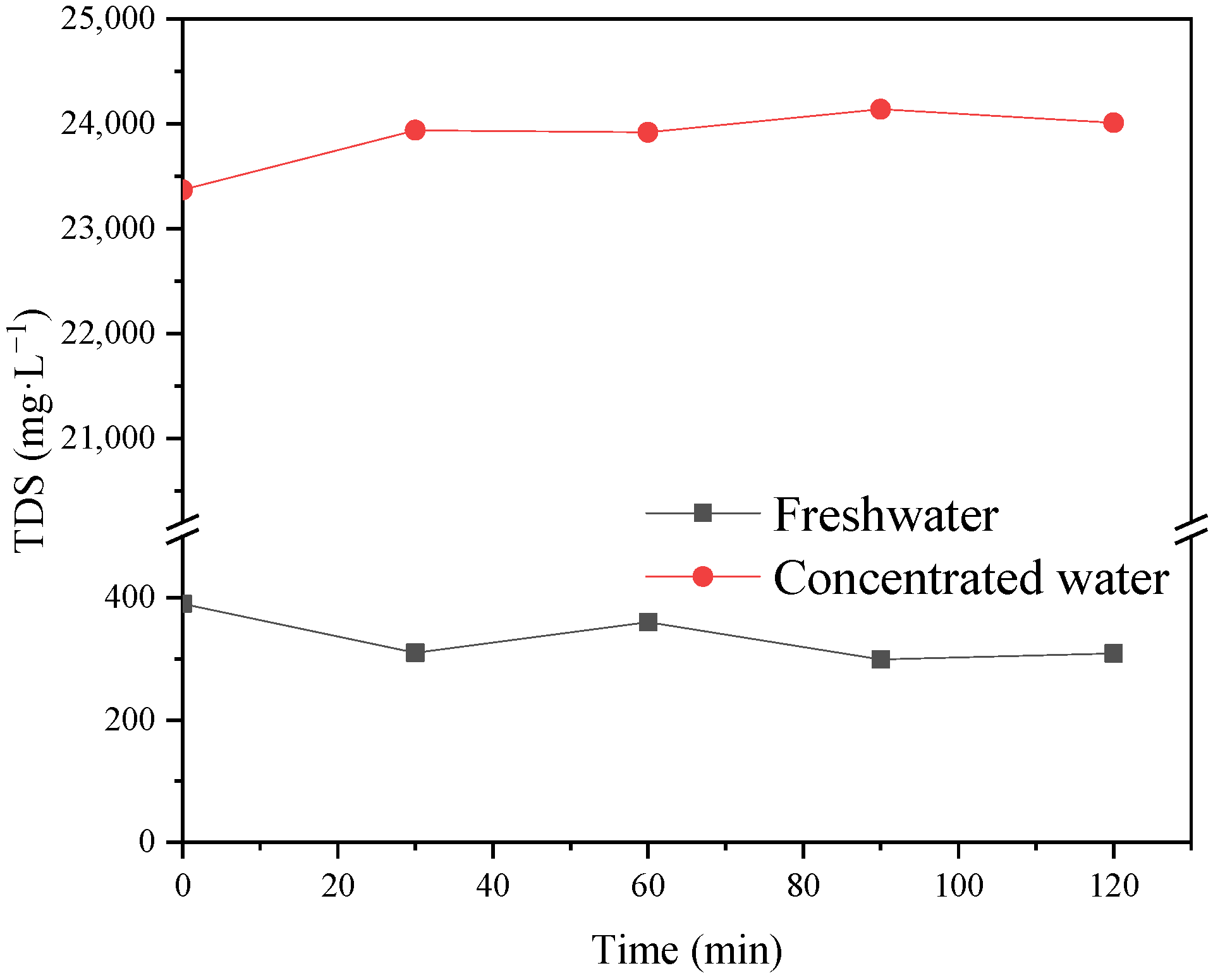
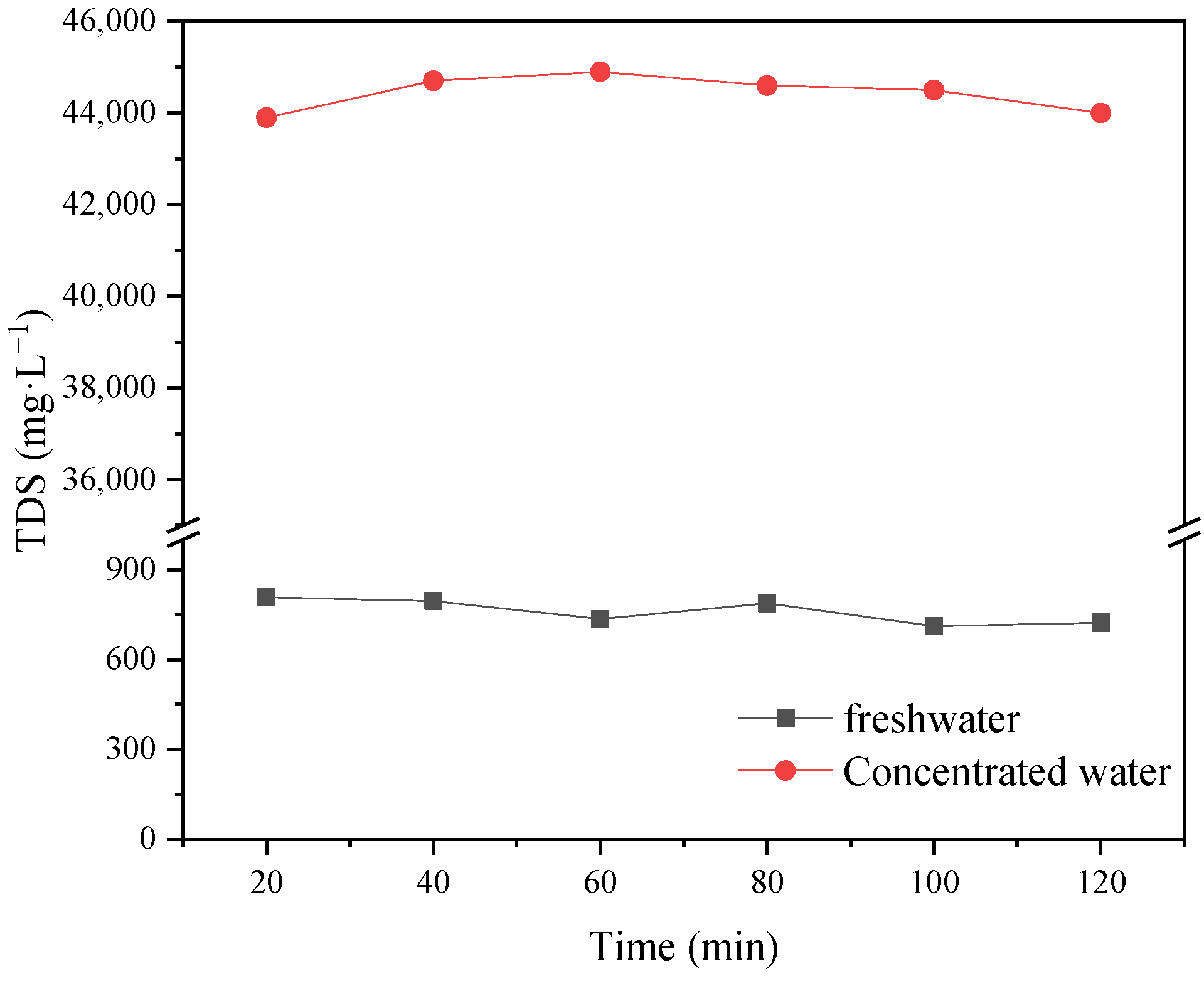
The operating pressure and feed temperature are the key factors affecting the recovery rate and ion interception rate of NF-produced water. The recovery rate of fresh water after two-stage concentration was 90%, and the TDS of concentrated wastewater was more than 36,000 mg·L−1. The operating voltage and circulating flow rate had clear effects on the desalination rate of ED. The desalination rate of two-stage ED reached 61.9%. The results show that this new coupling process can realize the efficient reuse of chromium removal from tannery wastewater.