Abstract
Diabetes is the most common chronic disease in the world, and it brings a heavy burden to people’s health. Against this background, diabetic research, including islet functionalization has become a hot topic in medical institutions all over the world. Especially with the rapid development of microencapsulation and three-dimensional (3D) bioprinting technologies, organ engineering and manufacturing have become the main trends for disease modeling and drug screening. Especially the advanced 3D models of pancreatic islets have shown better physiological functions than monolayer cultures, suggesting their potential in elucidating the behaviors of cells under different growth environments. This review mainly summarizes the latest progress of islet capsules and 3D printed pancreatic organs and introduces the activities of islet cells in the constructs with different encapsulation technologies and polymeric materials, as well as the vascularization and blood glucose control capabilities of these constructs after implantation. The challenges and perspectives of the pancreatic organ engineering/manufacturing technologies have also been demonstrated.
1. Introduction
Diabetes is caused by a fault in the insulin production of the body and has different types. Type 1 diabetes mellitus is a common chronic disease in which the human immune system constantly attacks and destroys β cells, leading to insufficient insulin supply or insulin resistance, further causing the rise of blood glucose levels [1]. The main feature of type 2 diabetes is insulin resistance. The pancreas can produce insulin, but human organs no longer fully respond to insulin. As a result, with the continuous increase in insulin production, the overall β cell function and quality decline distinctively [2]. Currently, patients with type 1 diabetes can be controlled by injecting insulin and taking drugs to alleviate the disease (Figure 1). Type 2 diabetes is mainly controlled by diet and movement. However, diabetes can lead to irreversible tissue and organ damage with a variety of life-threatening secondary metabolic syndromes (MS), including neuropathy, retinopathy, nephropathy, stroke, and heart failure [3].
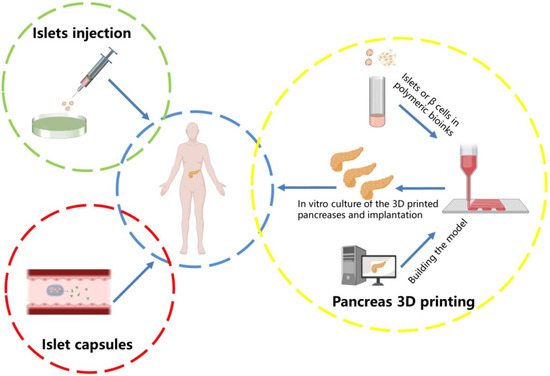
Figure 1.
Diabetes treatment strategies. The traditional treatment for Type 1 diabetes is to inject insulin or take islet capsules. With the development of science and technologies, 3D printing implantable pancreatic organs is becoming more and more popular.
Clinical trials over the past few decades have demonstrated that pancreatic islet transplantation is an effective treatment [4,5]. Nevertheless, this therapeutic approach has been greatly limited by the shortage of islet donors and the low survival rate of the transplanted islets. Meanwhile, the allogeneic immune response of the transplanted islets can cause tissue rejection and further death of the transplanted cells. The need for lifelong immunosuppressants has also significantly restricted the widespread of this therapeutic approach [6,7].
It is recognized that the use of immunosuppressive drugs can cause a variety of serious adverse effects, such as nephrotoxicity, liver toxicity, and other abnormalities [8]. Over the last two decades, many researchers turned to wrapping the islets with biocompatible polymers as an immunoprotective barrier [9]. However, the traditional encapsulation methods have numerous shortcomings, such as hypoxia, lack of blood supply networks, and difficulty in the degradation of the polymers [10]. Without proper vascular networks, most of the transplanted cells disappear in the body or die quickly inside the capsules [11].
Parallelly, the traditional tissue engineering approaches for bioartificial tissue and organ engineering/manufacturing have been totally substituted by three-dimensional (3D) bioprinting technologies. Typical 3D bioprinting technology is characterized by printing cells, growth factors, hydrogels, and other biomaterials as ‘bioinks’ to produce bioartificial tissues and organs through automatic layer-by-layer deposition processes under the guidance of computer-aided design (CAD) models. Different types of cells can be encapsulated in different polymeric ‘bioinks’ and deposited simultaneously through multi-nozzle 3D printers. The hydrogels can absorb and retain large amounts of water, which is beneficial for cell growth, proliferation, differentiation, and tissue/organ formation [12]. The advanced 3D bioprinting technologies represent a high potential for pancreas constructions and type 1 diabetes therapies.
2. Pancreatic Islets and β-Cells
In the human body, the adult pancreas is a heterogeneous gland consisting of an exocrine chamber and an endocrine chamber. The exocrine part of the pancreas consists of secretory cells that produce digestive enzymes and release them into pancreatic ducts. The endocrine part consists of islets, which produce hormones and regulate glucose homeostasis (Figure 2). There are about 1.5 million islets in the pancreas. In each islet, 50–60% of the cells are β-cells, which secrete insulin, 30–45% are α-cells secreting glucagon, less than 10% are δ-cells secreting somatostatin, about 1% are pancreatic polypeptide cells (PP-cells) secreting pancreatic polypeptide, and less than 1% are ε-cells secreting ghrelin [13,14].
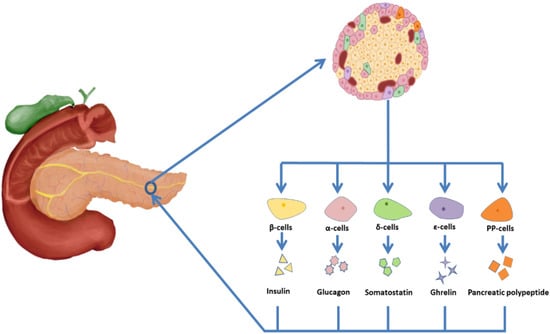
Figure 2.
Islet cell composition and secretions. Islet cells are generally divided into β cells (50–60%), which secrete insulin, α cells (30–45%), which secrete glucagon, δ (less than 10%) cells, which secrete somatostatin, PP cells (about 1%), which secrete pancreatic polypeptide, and ε cells (less than 1%), which secrete growth hormone.
β cells are so special that half of the protein they create can be converted into insulin. The newly translated insulins can package into small particles. The insulin particle is an organelle in which many regulatory pathways intersect, which acts as the origin of several signals to regulate the activities of β cells. The changes of β cell activities in the plasma membrane directly result in the changes of glucose concentrations in blood with the stimulation or inhibition of secretions of insulins [15,16].
3. Islet Encapsulation
For nearly 20 years, cell encapsulation techniques have been explored to protect the transplanted heterospecific cells from the host immune system. The principle of islet encapsulation is to engraft cells into compartments separated by a semipermeable polymer membrane of capsules. The capsules can protect the islets from damage caused by the immune response. In addition to the protective mechanism provided by the capsules, the islets in the capsules can regulate blood sugar levels by releasing insulin, while small molecules (e.g., glucose and nutrients) and metabolic wastes can pass through the semipermeable polymeric membrane. Therefore, the encapsulation system can be considered as a ‘mini-bioartificial pancreas’, ‘micro-organ’, or ‘organoid’ [17,18].
However, the progress of cell encapsulation is very slow. At present, only a few cell encapsulation techniques have been applied in clinical trials with little therapeutic effects. Due to the complexity of cell encapsulation techniques, the immune response generated by the components (e.g., polymer materials, embedded cells, foreign genes, and genetically engineered DNA vectors) has not been effectively exempted. Although the use of high-purity polymers can reduce the immune response to some extent, more and more researchers believe that reducing the immunogenicity of the cells inside the microcapsules is the key to preventing the immune rejection of the transplants.
It is generally believed that the ideal polymers for islet cell encapsulation should be completely inert (no immune rejection), non-degradable (exist in the body for a long time), highly compatible with the encapsulated cells (maintaining cell survival and function), and have a smooth surface and strong hydrophilicity (reduce protein and cell attachment) [19]. To improve the properties of the encapsulation polymers, many methods, such as changing the chemical composition of the encapsulating materials, and co-encapsulating immunomodulators, have been exploited [20]. It is expected that after the microcapsules are transplanted into the recipients, new blood vessels should be established immediately to maintain the survival of the islets [21]. In past studies, nearly all the islet encapsulation techniques employ natural polymer, such as alginate, to encapsulate islets, since alginate has a certain degree of biocompatibility and can be cross-linked by divalent cations [22,23]. Nowadays, some synthetic hydrogels and their derivatives have been employed in islet encapsulation, since these synthetic hydrogels outperform natural hydrogels with respect to tunable properties, such as porosity, stability, mechanical strength, and biocompatibility [24].
As the goal of islet xenotransplantation is to restore insulin secretion in the recipients, a large number of islets is a prerequisite to ensure that the transplantation has enough living cells. When sufficient insulin is secreted by the transplanted islets, the sugar level of the blood can be controlled efficaciously. At the same time, oxygen and nutrient supplies for the grafts should be kept up with [25,26]. In order to ensure the stability of the transplants, rapid vascularization is desired, to maintain the insulin secretion function under the stimulation of glucose.
A typical islet encapsulation technique was described in 2009 by Zhang et al. [27]. In this study, a non-adhesive islet encapsulation layer based on synthetic polyethylene glycol diacrylate (PEGDA) was used as the first layer. To increase the vascularization effects, thiogelatin, thioheparin, and thiohyaluronic acid were used as the second layer to provide endothelial cell adhesion points and act as a growth factor release matrix [28]. The PEGDA coatings can be covalently applied on the surface of islets, and the islets can be subsequently embedded in hydrogels containing thioglycosaminoglycans. Experiments have shown that this method can effectively control the release of growth factors, promote the growth of blood vessels to the embedded islets, and maintain the shape and function of the islets after implantation [29].
Another typical example of islet encapsulation was described in 2015 by Wertz and colleagues [30]. In this study, the ubiquitin-editing protein A20, encoded by TNFAIP3, is a negative regulator of immunostimulatory factors. Polyethylene glycol (PEG) hydrogel capsules loaded with A20-expressing islets are used as a drug release system to release immunosuppressants and growth factors to improve the state of transplanted islets. Once injected, the hydrogel can gel and provide support for the A20-expressing islets. The hydrogel shell of the capsules can promote the vascularization processes and prevents the immune system from attacking the pancreatic islets [31]. In order to protect the cells encapsulated in the hydrogel from being damaged by cytokines diffused into the capsules and to accelerate the vascularization processes, the researchers further added IL-1β, TNF-α, INF-γ, and other cytokines to modify the hydrogel. As a result, the cytokines can effectively protect the encapsulated cells against β cell-specific T lymphocytes and maintain glucose-stimulated insulin release from the islet cells [32].
Likewise, mesenchymal stem cells (MSCs) can release soluble cytokines and growth factors to neighboring cells to suppress the immune response, resulting in no local immunosuppression for MS [33]. In this context, MSCs and islet cells were simultaneously encapsulated into alginate hydrogel to improve the survival rate of the transplanted islet cells, promoting insulin secretion and new blood vessel formation [34,35].
Besides the pancreatic cells, the changes in the components of the encapsulation hydrogels can also change the destiny of the capsules. Studies have shown that the incorporation of tripeptide sequence Arg-Gly-Asp (RGD) in the islet-encapsulated PEG hydrogels could improve insulin response to glucose stimulation. Furthermore, the degradable hydrogel layer could enhance the vascular density at the graft site of the greater omentum, thereby improving the viability of encapsulated islets in a syngeneic diabetic rat model [36].
To facilitate nutrient diffusion, researchers employed a microfluidic encapsulation system to enhance the insulin responsiveness of the encapsulated islets and allow the islets to engraft within the vascularized tissue space [37] (Figure 3). Pham et al. used surface modification technology, 3,4-dihydroxyphenethylamine (DOPA)-conjugated polylactide–polyethylene glycol nanoparticles carrying immune-suppressant FK506 (FK506/DOPA-NPs) (DOPA-NPs), and functionalized DOPA-NPs to form a multifunctional coating for antigen camouflage without interfering with islet viability and function. The coating effectively preserved the morphology and viability of the islets when co-cultured with xenogeneic lymphocytes for 7 days. The mean survival time of the islets coated with FK506/DOPA-NP was higher. This study suggests that the combination of surface camouflages and local low-dose immunosuppressive agents may prolong the survival time of the transplanted islets [38].
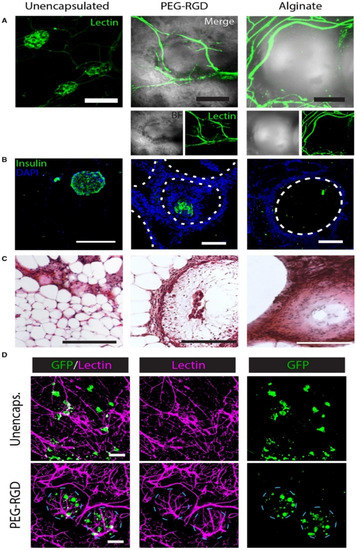
Figure 3.
Histological evaluation of EFP-transplanted unencapsulated and encapsulated islets. (A) Lectin staining shows the vascular system of the graft; (B) IHC staining of the samples; (C) H & E staining of the samples; (D) Imaging of the PEG-RGD-encapsulated islets shows dense blood vessel formation on the surface of the microgels and living islets within the microgels. Reprinted with permission from Ref. [37] Copyright 2019, John Wiley and Sons.
During the past few years, decellularized extracellular matrix (dECM), as a kind of natural polymer, contains various proteins, as well as growth factors, required for cell growth and differentiation, regulating biological balance with low toxicity and immunogenicity, has attracted much attention in biomedical fields [39,40]. Compared with polysaccharide- or protein/peptide-based materials, dECM-based materials can better mimic the ecological niche of natural tissues or organs [41]. In some cases, dECM plays a crucial role in particular tissue homeostasis, growth, and maturation, which makes it a special candidate for islet encapsulation with improved microenvironments [42].
Analogously, composite polymers for the formation of an interpenetrating network by complexing extracellular matrix (ECM) components of human-derived liposuction fluid with ionized gels of alginate matrices and heat-induced gels of pepsin-solubilized ECM pregels can achieve the in situ encapsulation of pancreatic islet cells (MIN6 β cells) [43,44]. Islets encapsulated in the microcapsules (≈640 µm), proliferated rapidly in vitro and displayed glucose-stimulated insulin responses due to the enhanced cell-matrix interactions [45]. When alginate was combined with the ECM-derived peptides, such as RGD, LRE, YIGSR, PDGEA, and PDSGR, islet dysfunctions due to the disruption of ECM interactions during the earlier islet isolation and side effects associated with immunosuppression can be overcome [46]. Porcine islets encapsulated in peptide-functionalized alginate microcapsules exhibit enhanced viability and glucose-stimulated insulin release. This study suggests that the ECM-derived peptides help to maintain the health of the encapsulated islets and may contribute to prolonging the lifespan of the encapsulated islet grafts [47].
In general, islet cell transplantation is one of the most promising treatments for type 1 diabetes, but the recipient’s immune response to the encapsulation polymers and cells is a major obstacle to the clinical application of islet cell transplantation. The control of the polymer component, thickness, and pore size around the islets is related to the level of mass exchange between the islets and the external small molecules and immunosuppression. Until the present, the traditional cell encapsulation techniques still have many limitations in clinical trials, with regard to islet cell protection effects, transplantation sites, and graft stabilities. The in-depth study of islet cell encapsulation materials, encapsulation strategies, and stem cell technologies is expected to improve the success rate of cell-based remedies.
4. Pancreas 3D Printing
3D bioprinting is a fully automatic layer-by-layer additive manufacturing process, which can deposit cells, growth factors, and other biomaterials through rapid prototyping (RP) technologies to fabricate bioartificial tissues and organs with multicellular components, hierarchical structures (especially branching vascular networks), and complex functions [48,49]. Currently, 3D bioprinting technologies have been successfully used to print many living tissues and organs [12], including blood vessels [50], skins [51], bones [52], cartilages [53], hearts [54], and livers [55]. Most of the 3D bioprinting technologies used for producing bioartificial pancreases belong to inkjet 3D printing, fused deposition modeling (FDM), extrusion-based 3D printing, and UV curing-based 3D printing [56,57,58,59,60,61]. The raw biomaterials for cell/growth factor-loading include natural polymeric solutions or hydrogels, synthetic polymeric solutions, and ECMs [62,63,64]. With these 3D bioprinting technologies and ‘bioinks’, all of the bottleneck problems which have perplexed tissue engineers, biomaterial researchers, pharmaceutists, and other scientists for several decades have been overcome sensibly [65]. These can be reflected in the following sections.
4.1. Natural Polymers for Pancreas 3D Printing
Natural polymers are macromolecular compounds that exist in nature, including proteins, polysaccharides, and their combinations, such as glycoproteins and proteoglycans [66,67]. Most of the natural polymers, such as gelatin, alginate, fibrinogen, and hyaluronic acid, are water-soluble, dissolving in inorganic solvents such as cell culture medium. The polymer solutions usually have good fluidities, excellent cytocompatibility, and can form water-rich hydrogels through the physical, chemical, and enzymatic cross-linking of the polymer molecules [68,69,70]. The water-rich hydrogels can not only embed living cells, growth factors, and other bioactive agents, transporting nutrients/oxygen to cells, but also discharge metabolic wastes produced by cells through the interpenetrating networks [71,72].
As the main component of 3D printable ‘bioinks’, natural polymers have been widely used in pancreas 3D printing. The 3D printed islets embedded in natural hydrogels can maintain excellent biological activities with glucose regulation functions [73]. The first pancreas 3D printing technology was reported by Prof. Wang early in 2009 using gelatin/alginate/fibrin hydrogels [74] in which, adipose stem cells (ASCs), embedded in gelatin/alginate/fibrinogen solutions, and islets were printed into large-scale living organs with similar biological and physiological functions of their natural counterparts. When the pancreatic islets were deposited at designated locations with the ASC-laden gelatin/alginate/fibrin hydrogel, the ASCs can be induced to differentiate into vascular endothelial cells (ECs) and adipocytes dividually (or separately). The differentiation and self-organization of ASCs can be totally controlled by the growth factor combinations and the incorporated islets. This is a huge milestone in complex organ engineering/manufacturing areas, which has shown great potential in the establishment of physiological models of MS. When different drugs are applied to this model, the physiological responses are consistent with the in vivo experiments, suggesting that this model has strong advantages in high-throughput drug screening, pathological model establishment, as well as contributing to a better understanding of the multiple sclerosis pathogenesis of cells and drug development strategies [75] (Figure 4).
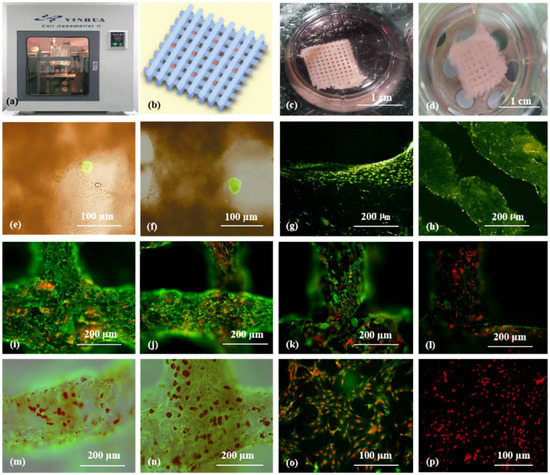
Figure 4.
Cell-laden hydrogel constructs printed by Professor Wang: (a–d) grid gelatin/alginate/fibrin constructs containing ASCs and islets; (e,f) immunofluorescence staining of islet cells in the constructs; (g,h) immunofluorescence staining of ASCs differentiated into endothelial cells with EGF; (i–l) endothelial cell (green) immunostaining, nuclear (red) propidium iodide (PI) staining; (m,n) immunostained endothelial cells (green), and adipocytes (red) stained with oil red O; (o,p) immunostaining of two-dimensional (2D) cultured endothelial cells (green), differentiated from ASCs with pyridine iodide staining nucleus (red). Reprinted from Ref. [75].
Later, in 2019, Duin et al. encapsulated islets into an alginate/methylcellulose hydrogel and constructed macroporous hydrogel structures via a simple 3D bioprinting technique. It was shown that the islets within the hydrogel had good viability and morphology and could continuously produce insulin and glucagon throughout the observation stage in responding to glucose stimulation [76] (Figure 5).
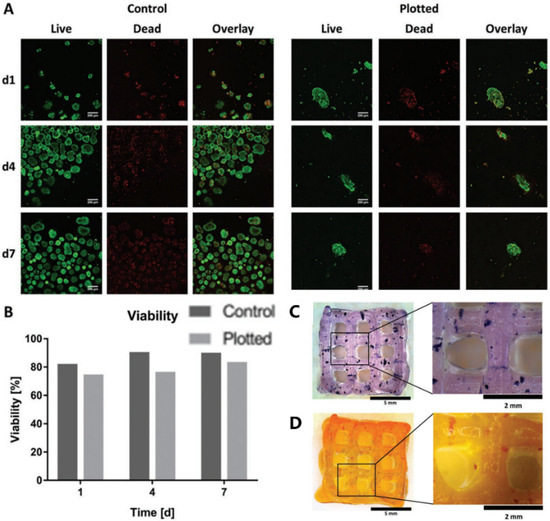
Figure 5.
Islet viability assay and staining. (A) Live/dead staining of islets in Alg/MC gel (right) and free control islets (left). Live and dead cells are shown in green and red, respectively. (B) Semi-quantitative assessment of islet viability based on live/dead staining as shown in (A), n > 60 islets. (C) Islet-containing scaffolds were stained with thiazolyl blue tetrazolium bromide (MTT). (D) Islet-containing scaffolds were stained with dithizone (DTZ). Reprinted with permission from Ref. [76], Copyright 2019, John Wiley and Sons.
In 2021, Hu et al. developed a new ‘bioink’ based on natural alginate molecules. They added polymer Pluronic F127 to the alginate solution, which greatly improved the printability of the alginate-based hydrogel and the flexibility of the cross-linked structure. Meanwhile, hypomethylated pectin was added to reduce inflammation. The experimental results showed that the cellular constructs printed with pectin-alginate-pluronic ‘bioink’ could reduce tissue rejections by inhibiting TLR2/1 and ensure the survival of the insulin-producing β cells under inflammatory stress. It provides an improved strategy for the long-term survival of the transplanted islets in the treatment of type 1 diabetes [77].
To overcome the fundamental problems for islet or pancreatic cell transplantation, such as lacking adequate blood vessels in the constructs and allogeneic immune attack after implantation, the development of custom-designed bioartificial pancreases is urgently needed. This problem is expected to be solved using multi-nozzle 3D bioprinting technologies [78]. With the multiple nozzles, the distribution of many different cell types, including multicellular islets, can be controlled simultaneously to mimic the natural pancreas with the desired physiological functions.
4.2. Synthetic Polymers for Pancreas 3D Printing
Synthetic polymers are artificially manufactured macromolecular compounds that cannot be obtained from nature. They are often obtained through a certain polymerization reaction, using small molecules called monomers, with known structures and relatively low molecular weights as raw materials [78]. Synthetic polymers are widely used in various fields such as electronics, automobiles, and transportation due to their excellent chemical and physical properties. Synthetic polymers, such as polylactic acid (PLA), polylactic-co-glycolic acid (PLGA), polyurethane (PU), and polycaprolactone (PCL) with good mechanical properties, in vivo histocompatibility, and structural stability, have been 3D printed widely as tissue engineering scaffolds for cell attachment and vascular/neural network building templates for organ implantation [75,79,80,81,82] (Figure 6).
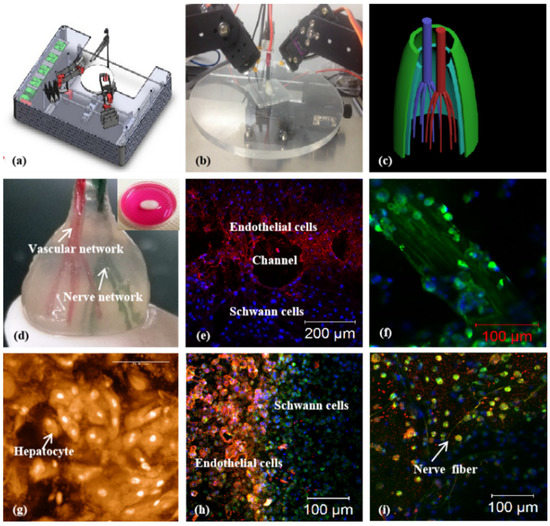
Figure 6.
Vascularized and neuralized liver tissue models constructed by Professor Wang: (a,b) combined four nozzle printer; (c) a CAD model of the vascularized and neuralized liver tissue; (d) the 3D printed constructs containing vascularized and neuralized liver tissues; (e) immunofluorescence staining of endothelial cells and Schwann cells around the branching channels of the constructs; (f) nerve fibers formed in the 3D constructs; (g) hepatocytes in the 3D constructs, some of the cells in proliferation stage with two nucleus; (h) the interface between the endothelial cells and Schwann cells; (i) nerve fibers formed in the constructs. Reprinted from Ref. [75].
Compared with natural polymers, most synthetic polymers have super mechanical properties, and 3D printed structures can be maintained in vivo for a long time [83]. For example, Song et al. printed a PLA structure by tuning the parameters of a low-cost 3D printer that could be accommodated by clusters of SC-β cells in a degradable fibrin gel. A finite element model of cellular oxygen diffusion consumption was used to determine the diameter of cell clusters to avoid severe hypoxia before vascularization. After the constructs were transplanted into mice, insulin was secreted in response to glucose injection, and the transplanted constructs maintained their structural integrity for 12 weeks. Unlike the pure cell encapsulation techniques, this approach could serve as a platform for advanced diabetes therapies using 3D printed cell replacements [84].
In another study, Farina presented a novel 3D printing and functionalized encapsulation system for the subcutaneous transplantation of pancreatic islets or islet-like cells. When the surface of the 3D printed PLA structure underwent some treatments, the hydrophilicity of the synthetic polymers was increased, which could facilitate cell attachment and proliferation. The implantation of a growth factor-rich platelet gel in a surface-treated encapsulation system could help to create a vascularized environment prior to loading human islets. Islets encased in this device could be protected from acute hypoxia and retain their function [85].
Similarly, Marchioli developed a PCL scaffold that could actively promote vascularization in extrahepatic islet transplantation. The PCL scaffold with a heparinized surface could electrostatically bind vascular endothelial growth factor (VEGF) to the alginate-encapsulated islets. Compared with the untreated PCL scaffold, heparin immobilization could increase the retention of the VEGF in the scaffold up to 3.6-fold. In a chicken chorioallantoic membrane model, the VEGF immobilized on the surface of the PCL scaffold could promote angiogenesis. After 7 days of implantation, the alginate-encapsulated islets exhibited functional responses to the glucose stimulation similar to the free-floating islets. The model has the potential to support rapid vascularization and islet endocrine function [86] (Figure 7).
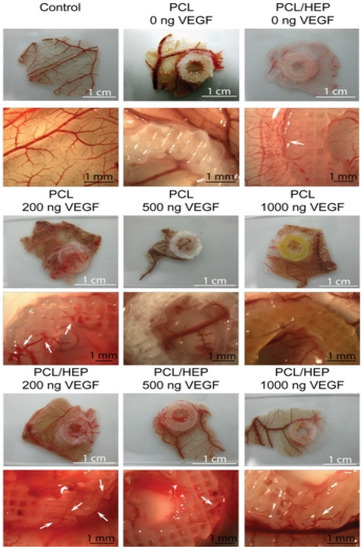
Figure 7.
CAM assays were performed on PCL and heparin-coated 3D-printed PCL scaffolds with three different concentrations of VEGF. The 200 ng loading of VEGF induced stent formation with normal morphological vessels. Reprinted with permission from Ref. [86], Copyright 2016, Elsevier.
4.3. ECM and dECM for Pancreas 3D Printing
As stated above, ECM is a macromolecular substance secreted by cells with a complex network, supporting, connecting, and regulating cell behaviors with the occurrence of tissue and organ formation [87,88]. An acellular matrix is a process of the decellularization of allogeneic tissue to remove antigenic components that can cause immune rejection, while completely retaining the 3D structure of the ECM with some growth factors, such as the fibroblast growth factor, VEGF, that play a significant role in stem cell differentiation [89,90]. Some of the ECMs have relatively good mechanical properties compared with the single natural polymeric hydrogels. Some of the ECMs demonstrate good histocompatibility and low immune rejection when they are implanted in the body [91,92].
Similarly, an acellular extracellular matrix or dECM is a biological material derived from living organisms, and its 3D printed pancreas model is closer to the living environment of real islets, which is more conducive to the maintenance of islet function and the release of insulin [93]. In 2019, Kim et al. printed pancreatic-derived ECM (pdECM) for the creation of a native microenvironment for transplantable 3D pancreatic tissues. The results showed that the insulin secretion of human pluripotent stem cells and the maturity of insulin-producing cells were highly enhanced when they were cultured in the pdECM ‘bioinks’ and that the co-culture with human umbilical vein-derived endothelial cells could reduce the central islet necrosis under 3D culture conditions. The possibility of fabricating 3D islet structures with therapeutic graft dimensions was validated by the fusion with 3D bioprinting technology [93]. Hwang et al. developed a hybrid packaging system using 3D bioprinting technology, which consists of macroporous polymer capsules and nanoporous dECM hydrogels with islet-like aggregates. The exterior of the construct is designed as a go-through porous structure, β-cells can be encapsulated inside, and can maintain their activities with insulin secretion functions. The islet-like aggregates are formed through 3D bioprinting technology to improve cell vitalities and functions. The experimental results show that the hybrid packaging system has good biocompatibility, and the cells in the construct can connect through the go-through pores. These approaches are expected to solve the donor shortage problems to some degree and realize the clinical application of 3D printed pancreatic organs [94]. Wang et al. fabricated a novel ’bioink’ by combining pancreatic extracellular matrix (pECM) and hyaluronic acid methacrylate (HAMA) and used 3D printing technology to construct islet organoids. The islet cells maintained the biological functions in the structure through the Rac1/ROCK/MLCK signal pathway with improved bioactivities. When the pancreas structure was implanted into the diabetic model mice, the insulin level in the mice was significantly increased, and the blood glucose level in the mice remained at the normal level for 90 days. Compared with HAMA hydrogel, the HAMA/pECM hydrogel is more conducive to angiogenesis, and the blood vessel density is significantly increased, which brings hope for the construction of vascularized 3D pancreatic organs [95] (Figure 8).
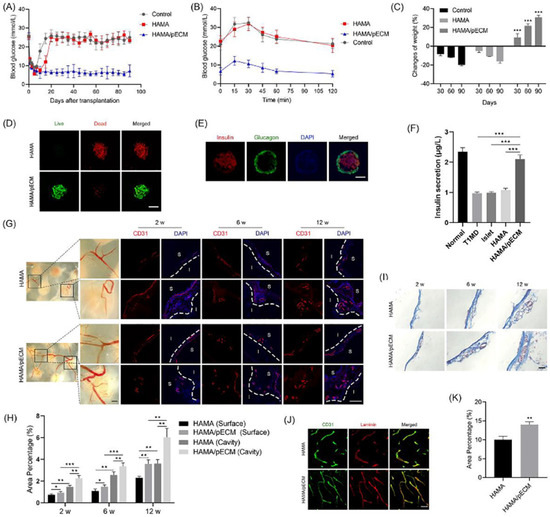
Figure 8.
In vivo transplantation of the 3D printed pancreas-like organ. (A) Blood glucose level in the diabetic mice. (B) Intraperitoneal glucose tolerance test (IPGTT). (C) Weight changes of mice after the 3D printed pancreas-like organ transplantation. (D) Living and dead cell staining. (E) Insulin/glucagon/DAPI immunofluorescence images. (F) Comparison of serum insulin levels in different groups of mice. (G,H) CD31 immunostaining image and intensity comparison. (I) Masson trichrome staining images of the 3D printed pancreas-like organ. (J) Typical CD31 and layer adhesion immunostaining images of the 3D printed pancreas-like organ. (K) Percentage of angiogenesis. * p ≤ 0.05, ** p ≤ 0.01, *** p ≤ 0.001. Reprinted with permission from Ref. [95], Copyright 2022, Elsevier.
Concisely, the most outstanding 3D bioprinting technologies and ‘bioinks’ for bioartificial ogan engineering/manufacturing are summarized in Table 1

Table 1.
Outstanding 3D bioprinting technologies and polymers for bioartificial organ engineering/manufacturing.
5. Discussion
At present, great progress has been achieved in the field of cell encapsulation and 3D bioprinting. It has been proven that islet cells can maintain high activity and secrete insulin in most of the constructed models. Like other bioartificial organ engineering/manufacturing, there are still some unsolved issues to be explored in order to obtain an implantable bioartificial pancreatic organ [110,111].
Firstly, the bioartificial pancreases constructed from pure natural polymers and ECMs can hardly maintain their original shapes before the cells grow into mature pancreatic tissues [112], while the bioartificial pancreases constructed from pure synthetic polymers are difficult to load cells and bioactive agents. Therefore, material scientists need to develop new biomaterials or use a variety of composite materials to build a functional bioartificial pancreas with proper mechanical strengths and biological activities [113,114].
Secondly, it is better to use the patients’ own pancreatic cells or stem cell-derived pancreatic cells to build the implantable bioartificial pancreases for custom or personalized pancreas engineering/manufacturing and restoration. With the patients’ own pancreatic cells, most of the immunological rejection of the implantable bioartificial pancreases can be surmounted. A large amount of living cells with no immunogenicity is the guarantee for the organ-level replacement and respondence.
Thirdly, it is hard to construct precise structures that fully conform to the distribution of different cells in a natural pancreas. The pancreas contains a variety of adult cells, so mechanical engineers are required to design new 3D printers with multiple nozzles and high precision to deposit different cells in a predefined construct, mimicking their respective locations in natural pancreatic organs [115,116,117].
Fourthly, there is no such powerful equipment at present that can build a complex hierarchical vascular network, containing large arteries, branched arterioles, elaborate capillaries, and venous vessels (veins) integrally in a construct to maintain the nutrient transport and waste metabolism required inside the bioartificial pancreas [104,105]. Consequently, scientists need to design an induction strategy that can generate complex vascular networks in bioartificial pancreatic organs [118,119].
It is expected the 3D bioprinting pancreas will become the mainstream for future diabetes treatment. Problems such as the transportation of nutrients in the complex bioartificial organs, the formation of the branched hierarchical vascular networks, the biocompatibility of the 3D printed ‘bioinks’ in hosts, and the anti-suture/stress capabilities of the implanted bioartificial organs can be solved by more powerful updated 3D printers [120,121,122,123,124]. 3D bioprinting, as the most effective technology in the field of complex organ engineering/manufacturing, can fundamentally solve all of the problems faced by donor organ shortage and various MSs.
6. Conclusions
The construction of a clinically implantable bioartificial pancreatic organ requires the joint efforts of researchers in different fields, such as medicine, biology, materials, computers, engineering, chemistry, etc. Currently, 3D bioprinting, along with cell encapsulation technologies, has solved nearly all the bottleneck problems for bioartificial pancreas engineering/manufacturing. There are still some particular features, including an anti-sutural hierarchical vascular network with a full spectrum of blood vessels, that should be incorporated. With the rapid development of stem cells, biomaterials, and 3D printers, we can foresee that the 3D printing of bioartificial pancreases will save numerous patients in the future.
Author Contributions
Y.X. and D.S. wrote the main content, Y.X. contributed some detailed techniques; X.W. supervised and revised the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
The work was supported by grants from the National Natural Science Foundation of China (NSFC) (Nos. 81571832) and the Key Research and Development Project of Liaoning Province (No. 2018225082).
Institutional Review Board Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Abbreviations
| 3D | three-dimensional |
| CAD | computer-aided design |
| PEGDA | PEG-diacrylate |
| PEG | polyethylene glycol |
| MSCs | mesenchymal stem cells |
| PLGA | polylactic-co-glycolic acid |
| dECM | decellularized extracellular matrix |
| ASCs | adipose stem cells |
| MS | metabolic syndrome |
| ALG | alginate |
| Gel | gelatin |
| PLA | polylactic acid |
| PU | polyurethane |
| ECs | endothelial cells |
| EGF | epidermal growth factor |
| PI | propidium iodide |
| IBMX | isobutylmethylxanthine |
| 2D | two-dimensional |
| DTZ | DTZ |
| PCL | polycaprolactone |
| VEGF | vascular endothelial growth factor |
| ECM | extracellular matrix |
| HAMA | hyaluronic acid methacrylate |
| pECM | pancreatic extracellular matrix |
| IPGTT | intraperitoneal glucose tolerance test |
| GelMA | gelatin-methacryloyl |
References
- Barnett, R. Type 1 diabetes. Lancet 2018, 391, 195. [Google Scholar] [CrossRef] [PubMed]
- Gloyn, A.L.; Drucker, D.J. Precision medicine in the management of type 2 diabetes. Lancet Diabetes Endocrinol. 2018, 11, 891–900. [Google Scholar] [CrossRef]
- Bjerg, L.; Gudbjörnsdottir, S.; Franzén, S.; Carstensen, B.; Witte, D.R.; Jørgensen, M.E.; Svensson, A.M. Duration of diabetes-related complications and mortality in type 1 diabetes: A national cohort study. Int. J. Epidemiol. 2021, 4, 1250–1259. [Google Scholar] [CrossRef] [PubMed]
- Ahearn, A.J.; Parekh, J.R.; Posselt, A.M. Islet transplantation for Type 1 diabetes: Where are we now? Expert Rev. Clin. Immunol. 2015, 1, 59–68. [Google Scholar] [CrossRef]
- Menger, M.M.; Nalbach, L.; Roma, L.P.; Körbel, C.; Wrublewsky, S.; Glanemann, M.; Laschke, M.W.; Menger, M.D.; Ampofo, E. Erythropoietin accelerates the revascularization of transplanted pancreatic islets. Br. J. Pharmacol. 2020, 7, 1651–1665. [Google Scholar] [CrossRef]
- Troppmann, C. Complications after pancreas transplantation. Curr. Opin. Organ Transplant. 2010, 1, 112–118. [Google Scholar] [CrossRef]
- Gibly, R.F.; Graham, J.G.; Luo, X.; Lowe, W.L., Jr.; Hering, B.J.; Shea, L.D. Advancing islet transplantation: From engraftment to the immune response. Diabetologia 2011, 10, 2494–2505. [Google Scholar] [CrossRef] [PubMed]
- Hyder, A.; Laue, C.; Schrezenmeir, J. Effect of the immunosuppressive regime of Edmonton protocol on the long-term in vitro insulin secretion from islets of two different species and age categories. Toxicol. Vitr. 2005, 4, 541–546. [Google Scholar] [CrossRef] [PubMed]
- Sakai, S.; Inagaki, H.; Inamoto, K.; Taya, M. Wrapping tissues with a pre-established cage-like layer composed of living cells. Biomaterials 2012, 28, 6721–6727. [Google Scholar] [CrossRef]
- Desai, T.; Shea, L.D. Advances in islet encapsulation technologies. Nat. Rev. Drug Discov. 2017, 5, 338–350. [Google Scholar] [CrossRef] [PubMed]
- Strand, B.L.; Coron, A.E.; Skjak-Braek, G. Current and future perspectives on alginate encapsulated pancreatic islet. Stem Cells Transl. Med. 2017, 4, 1053–1058. [Google Scholar] [CrossRef] [PubMed]
- Song, D.; Xu, Y.; Liu, S.; Wen, L.; Wang, X. Progress of 3D bioprinting in organ manufacturing. Polymers 2021, 18, 3178. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Q.; Melton, D.A. Pancreas regeneration. Nature 2018, 7705, 351–358. [Google Scholar] [CrossRef] [PubMed]
- van der Meulen, T.; Mawla, A.M.; DiGruccio, M.R.; Adams, M.W.; Nies, V.; Dólleman, S.; Liu, S.; Ackermann, A.M.; Cáceres, E.; Hunter, A.E.; et al. Virgin beta cells persist throughout life at a neogenic niche within pancreatic islets. Cell Metab. 2017, 4, 911–926.e6. [Google Scholar] [CrossRef]
- Lemaire, K.; Thorrez, L.; Schuit, F. Disallowed and allowed gene expression: Two faces of mature islet beta cells. Annu. Rev. Nutr. 2016, 36, 45–71. [Google Scholar] [CrossRef]
- Rutter, G.A.; Pullen, T.J.; Hodson, D.J.; Martinez-Sanchez, A. Pancreatic β-cell identity, glucose sensing and the control of insulin secretion. Biochem. J. 2015, 2, 203–218. [Google Scholar] [CrossRef]
- Xu, Y.; Li, D.; Wang, X. The construction of vascularized pancreas based on 3D printing techniques. In Organ Manufacturing; Wang, X., Ed.; Nova Science Publishers Inc.: Hauppauge, NY, USA, 2015; pp. 245–268. [Google Scholar]
- Espona-Noguera, A.; Ciriza, J.; Cañibano-Hernández, A.; Villa, R.; Saenz Del Burgo, L.; Alvarez, M.; Pedraz, J.L. 3D printed polyamide macroencapsulation devices combined with alginate hydrogels for insulin-producing cell-based therapies. Int. J. Pharm. 2019, 566, 604–614. [Google Scholar] [CrossRef]
- Borg, D.J.; Bonifacio, E. The use of biomaterials in islet transplantation. Curr. Diabetes Rep. 2011, 5, 434–444. [Google Scholar] [CrossRef]
- Kothale, D.; Verma, U.; Dewangan, N.; Jana, P.; Jain, A.; Jain, D. Alginate as promising natural polymer for pharmaceutical, food, and biomedical applications. Curr. Drug Deliv. 2020, 9, 755–775. [Google Scholar] [CrossRef]
- Hogan, M.F.; Hull, R.L. The islet endothelial cell: A novel contributor to beta cell secretory dysfunction in diabetes. Diabetologia 2017, 6, 952–959. [Google Scholar] [CrossRef]
- Vegas, A.J.; Veiseh, O.; Gürtler, M.; Millman, J.R.; Pagliuca, F.W.; Bader, A.R.; Doloff, J.C.; Li, J.; Chen, M.; Olejnik, K.; et al. Long-term glycemic control using polymer-encapsulated human stem cell-derived beta cells in immune-competent mice. Nat. Med. 2016, 3, 306–311. [Google Scholar] [CrossRef] [PubMed]
- Cao, H.; Lee, M.K.H.; Yang, H.; Sze, S.K.; Tan, N.S.; Tay, C.Y. Mechanoregulation of cancer-associated fibroblast phenotype in three-dimensional interpenetrating hydrogel networks. Langmuir 2019, 23, 7487–7495. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Yan, Y.; Zhang, R. Gelatin-based hydrogels for controlled cell assembly. In Biomedical Applications of Hydrogels Handbook; Ottenbrite, R.M., Ed.; Springer: New York, NY, USA, 2010; pp. 269–284. [Google Scholar]
- Dufrane, D.; Gianello, P. Pig islet for xenotransplantation in human: Structural and physiological compatibility for human clinical application. Transplant. Rev. 2012, 3, 183–188. [Google Scholar] [CrossRef] [PubMed]
- Thompson, P.; Cardona, K.; Russell, M.; Badell, I.R.; Shaffer, V.; Korbutt, G.; Rayat, G.R.; Cano, J.; Song, M.; Jiang, W.; et al. CD40-specific costimulation blockade enhances neonatal porcine islet survival in nonhuman primates. Am. J. Transplant. 2011, 5, 947–957. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Aung, A.; Liao, L.; Varghese, S. A novel single precursor-based biodegradable hydrogel with enhanced mechanical properties. Soft Matter 2009, 5, 3831–3834. [Google Scholar] [CrossRef]
- Lin, S.; Sangaj, N.; Razafiarison, T.; Zhang, C.; Varghese, S. Influence of physical properties of biomaterials on cellular behavior. Pharm. Res. 2011, 6, 1422–1430. [Google Scholar] [CrossRef] [PubMed]
- Marchioli, G.; Zellner, L.; Oliveira, C.; Engelse, M.; Koning, E.; Mano, J.; Karperien; Apeldoorn, A.V.; Moroni, L. Layered PEGDA hydrogel for islet of Langerhans encapsulation and improvement of vascularization. J. Mater. Sci. Mater. Med. 2017, 12, 195. [Google Scholar] [CrossRef]
- Wertz, I.E.; Newton, K.; Seshasayee, D.; Kusam, S.; Lam, C.; Zhang, J.; Popovych, N.; Helgason, E.; Schoeffler, A.; Jeet, S.; et al. Phosphorylation and linear ubiquitin direct A20 inhibition of inflammation. Nature 2015, 7582, 370–375. [Google Scholar] [CrossRef]
- Bai, X.; Pei, Q.; Pu, C.; Chen, Y.; He, S.; Wang, B. Multifunctional islet transplantation hydrogel encapsulating A20 high-expressing islets. Drug Des. Dev. Ther. 2020, 14, 4021–4027. [Google Scholar] [CrossRef] [PubMed]
- Su, J.; Hu, B.H.; Lowe, W.L., Jr.; Kaufman, D.B.; Messersmith, P.B. Anti-inflammatory peptide-functionalized hydrogels for insulin-secreting cell encapsulation. Biomaterials 2010, 2, 308–314. [Google Scholar] [CrossRef] [PubMed]
- Soleymaninejadian, E.; Pramanik, K.; Samadian, E. Immunomodulatory properties of mesenchymal stem cells: Cytokines and factors. Am. J. Reprod. Immunol. 2012, 1, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Vaithilingam, V.; Evans, M.D.M.; Lewy, D.M.; Bean, P.A.; Bal, S.; Tuch, B.E. Co-encapsulation and co-transplantation of mesenchymal stem cells reduces pericapsular fibrosis and improves encapsulated islet survival and function when allografted. Sci. Rep. 2017, 1, 10059. [Google Scholar] [CrossRef] [PubMed]
- Mochizuki, Y.; Kogawa, R.; Takegami, R.; Nakamura, K.; Wakabayashi, A.; Ito, T.; Yoshioka, Y. Co-microencapsulation of islets and MSC CellSaics, Mosaic-like aggregates of MSCs and recombinant peptide pieces, and therapeutic effects of their subcutaneous transplantation on diabetes. Biomedicines 2020, 9, 318. [Google Scholar] [CrossRef]
- Weaver, J.D.; Headen, D.M.; Hunckler, M.D.; Coronel, M.M.; Stabler, C.L.; García, A.J. Design of a vascularized synthetic poly(ethylene glycol) macroencapsulation device for islet transplantation. Biomaterials 2018, 172, 54–65. [Google Scholar] [CrossRef]
- Weaver, J.D.; Headen, D.M.; Coronel, M.M.; Hunckler, M.D.; Shirwan, H.; García, A.J. Synthetic poly(ethylene glycol)-based microfluidic islet encapsulation reduces graft volume for delivery to highly vascularized and retrievable transplant site. Am. J. Transplant. 2019, 5, 1315–1327. [Google Scholar] [CrossRef]
- Pham, T.T.; Nguyen, T.T.; Pathak, S.; Regmi, S.; Nguyen, H.T.; Tran, T.H.; Yong, C.S.; Kim, J.O.; Park, P.H.; Park, M.H.; et al. Tissue adhesive FK506-loaded polymeric nanoparticles for multi-layered nano-shielding of pancreatic islets to enhance xenograft survival in a diabetic mouse model. Biomaterials 2018, 154, 182–196. [Google Scholar] [CrossRef] [PubMed]
- Ullah, I.; Busch, J.F.; Rabien, A.; Ergün, B.; Stamm, C.; Knosalla, C. Adult tissue extracellular matrix determines tissue specification of human iPSC-derived embryonic stage mesodermal precursor cells. Adv. Sci. 2020, 7, 1901198. [Google Scholar] [CrossRef] [PubMed]
- Raza, F.; Zafar, H.; Zhu, Y.; Ren, Y.; Ullah, A.; Khan, A.U.; He, X.; Han, H.; Aquib, M.; Boakye-Yiadom, K.O.; et al. A Review on recent advances in stabilizing peptides/proteins upon fabrication in hydrogels from biodegradable polymers. Pharmaceutics 2018, 1, 16. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Kim, S.W.; Lee, S.; Kim, S.C.; Cho, D.W.; Jang, J. 3D cell printing of islet-laden pancreatic tissue-derived extracellular matrix bioink constructs for enhancing pancreatic functions. J. Mater. Chem. B 2019, 10, 1773–1781. [Google Scholar] [CrossRef]
- Czerwinski, M.; Spence, J.R. Hacking the Matrix. Cell Stem Cell 2017, 1, 9–10. [Google Scholar] [CrossRef] [PubMed]
- Freytes, D.O.; Martin, J.; Velankar, S.S.; Lee, A.S.; Badylak, S.F. Preparation and rheological characterization of a gel form of the porcine urinary bladder matrix. Biomaterials 2008, 11, 1630–1637. [Google Scholar] [CrossRef]
- Giobbe, G.G.; Crowley, C.; Luni, C.; Campinoti, S.; Khedr, M.; Kretzschmar, K.; De Santis, M.M.; Zambaiti, E.; Michielin, F.; Meran, L.; et al. Extracellular matrix hydrogel derived from decellularized tissues enables endodermal organoid culture. Nat. Commun. 2019, 1, 5658. [Google Scholar] [CrossRef]
- Wang, J.K.; Cheam, N.M.J.; Irvine, S.A.; Tan, N.S.; Venkatraman, S.; Tay, C.Y. Interpenetrating network of alginate-human adipose extracellular matrix hydrogel for islet cells encapsulation. Macromol. Rapid Commun. 2020, 21, e2000275. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Williams, C.G.; Wang, D.A.; Lee, H.; Manson, P.N.; Elisseeff, J. The effect of incorporating RGD adhesive peptide in polyethylene glycol diacrylate hydrogel on osteogenesis of bone marrow stromal cells. Biomaterials 2005, 30, 5991–5998. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; He, K.; Zhang, W. Optimizing the fabrication processes for manufacturing a hybrid hierarchical polyurethane-cell/hydrogel construct. J. Bioact. Compat. Polym. 2013, 28, 303–319. [Google Scholar] [CrossRef]
- Wang, X.; Liu, C. 3D Bioprinting of adipose-derived stem cells for organ manufacturing. Adv. Exp. Med. Biol. 2018, 1078, 3–14. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Ao, Q.; Tian, X.; Fan, J.; Tong, H.; Hou, W.; Bai, S. Gelatin-based hydrogels for organ 3D bioprinting. Polymers 2017, 9, 401. [Google Scholar] [CrossRef] [PubMed]
- Zhu, W.; Qu, X.; Zhu, J.; Ma, X.; Patel, S.; Liu, J.; Wang, P.; Lai, C.S.; Gou, M.; Xu, Y.; et al. Direct 3D bioprinting of prevascularized tissue constructs with complex microarchitecture. Biomaterials 2017, 124, 106–115. [Google Scholar] [CrossRef]
- Baltazar, T.; Merola, J.; Catarino, C.; Xie, C.B.; Kirkiles-Smith, N.C.; Lee, V.; Hotta, S.; Dai, G.; Xu, X.; Ferreira, F.C.; et al. Three dimensional bioprinting of a vascularized and perfusable skin graft using human keratinocytes, fibroblasts, pericytes, and endothelial cells. Tissue Eng. Part A 2020, 5–6, 227–238. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Tian, X.; Fan, J.; Tong, H.; Ao, Q.; Wang, X. Chitosans for tissue repair and organ three-dimensional (3D) bioprinting. Micromachines 2019, 10, 765. [Google Scholar] [CrossRef] [PubMed]
- Lam, T.; Dehne, T.; Krüger, J.P.; Hondke, S.; Endres, M.; Thomas, A.; Lauster, R.; Sittinger, M.; Kloke, L. Photopolymerizable gelatin and hyaluronic acid for stereolithographic 3D bioprinting of tissue-engineered cartilage. J. Biomed. Mater. Res. B Appl. Biomater. 2019, 8, 2649–2657. [Google Scholar] [CrossRef] [PubMed]
- Lee, A.; Hudson, A.R.; Shiwarski, D.J.; Tashman, J.W.; Hinton, T.J.; Yerneni, S.; Bliley, J.M.; Campbell, P.G.; Feinberg, A.W. 3D bioprinting of collagen to rebuild components of the human heart. Science 2019, 6452, 482–487. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Yan, Y.; Pan, Y.; Xiong, Z.; Liu, H.; Cheng, J.; Liu, F.; Lin, F.; Wu, R.; Zhang, R.; et al. Generation of three-dimensional hepatocyte/gelatin structures with rapid prototyping system. Tissue Eng. 2006, 1, 83–90. [Google Scholar] [CrossRef] [PubMed]
- Gao, G.; Hubbell, K.; Schilling, A.F.; Dai, G.; Cui, X. Bioprinting cartilage tissue from mesenchymal stem cells and PEG hydrogel. Methods Mol. Biol. 2017, 1612, 391–398. [Google Scholar] [CrossRef] [PubMed]
- Wickramasinghe, S.; Do, T.; Tran, P. FDM-based 3D printing of polymer and associated composite: A review on mechanical properties, defects and treatments. Polymers 2020, 12, 1529. [Google Scholar] [CrossRef]
- Ding, L.; Lu, W.; Zhang, J.; Yang, C.; Wu, G. Preparation and performance evaluation of duotone 3D-printed polyetheretherketone as oral prosthetic materials: A proof-of-concept study. Polymers 2021, 13, 1949. [Google Scholar] [CrossRef]
- Rutz, A.L.; Gargus, E.S.; Hyland, K.E.; Lewis, P.L.; Setty, A.; Burghardt, W.R.; Shah, R.N. Employing PEG crosslinkers to optimize cell viability in gel phase bioinks and tailor post printing mechanical properties. Acta Biomater. 2019, 99, 121–132. [Google Scholar] [CrossRef]
- Gu, Y.; Zhang, L.; Du, X.; Fan, Z.; Wang, L.; Sun, W.; Cheng, Y.; Zhu, Y.; Chen, C. Reversible physical crosslinking strategy with optimal temperature for 3D bioprinting of human chondrocyte-laden gelatin methacryloyl bioink. J. Biomater. Appl. 2018, 33, 609–618. [Google Scholar] [CrossRef]
- Quan, H.; Zhang, T.; Xu, H.; Luo, S.; Nie, J.; Zhu, X. Photo-curing 3D printing technique and its challenges. Bioact. Mater. 2020, 22, 110–115. [Google Scholar] [CrossRef]
- Taghipour, Y.D.; Hokmabad, V.R.; Del Bakhshayesh, A.R.; Asadi, N.; Salehi, R.; Nasrabadi, H.T. The application of hydrogels based on natural polymers for tissue engineering. Curr. Med. Chem. 2020, 27, 2658–2680. [Google Scholar] [CrossRef]
- Hung, B.P.; Naved, B.A.; Nyberg, E.L.; Dias, M.; Holmes, C.; Elisseeff, J.H.; Dorafshar, A.; Grayson, W.L. Three-dimensional printing of bone extracellular matrix for craniofacial regeneration. ACS Biomater. Sci. Eng. 2016, 2, 1806–1816. [Google Scholar] [CrossRef] [PubMed]
- Mott, E.J.; Busso, M.; Luo, X.; Dolder, C.; Wang, M.O.; Fisher, J.P.; Dean, D. Digital micromirror device (DMD)-based 3D printing of poly(propylene fumarate) scaffolds. Mater. Sci. Eng. C Mater. Biol. Appl. 2016, 61, 301–311. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Yan, Y.; Zhang, R. Rapid prototyping as a tool for manufacturing bioartificial livers. Trends Biotechnol. 2007, 25, 505–513. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Yan, Y.; Xiong, Z.; Weng, C.; Zhang, R.; Wang, X. Gradient hydrogel construct based on an improved cell assembling system. J. Bioact. Compat. Polym. 2009, 24, 84–99. [Google Scholar] [CrossRef]
- Mahendiran, B.; Muthusamy, S.; Sampath, S.; Jaisankar, S.N.; Popat, K.C.; Selvakumar, R.; Krishnakumar, G.S. Recent trends in natural polysaccharide based bioinks for multiscale 3D printing in tissue regeneration: A review. Int. J. Biol. Macromol. 2021, 183, 564–588. [Google Scholar] [CrossRef]
- Hong, H.; Seo, Y.B.; Kim, D.Y.; Lee, J.S.; Lee, Y.J.; Lee, H.; Ajiteru, O.; Sultan, M.T.; Lee, O.J.; Kim, S.H.; et al. Digital light processing 3D printed silk fibroin hydrogel for cartilage tissue engineering. Biomaterials 2020, 232, 119679. [Google Scholar] [CrossRef]
- Wang, X. Spatial effects of stem cell engagement in 3D printing constructs. J. Stem Cells Res. Rev. Rep. 2014, 1, 5–9. [Google Scholar]
- Wang, X. 3D printing of tissue/organ analogues for regenerative medicine. In Handbook of Intelligent Scaffolds for Regenerative Medicine, 2nd ed.; Pan Stanford Publishing: Palo Alto, CA, USA, 2016; pp. 557–570. [Google Scholar]
- Erkoc, P.; Uvak, I.; Nazeer, M.A.; Batool, S.R.; Odeh, Y.N.; Akdogan, O.; Kizilel, S. 3D Printing of cytocompatible gelatin-cellulose-alginate blend hydrogels. Macromol. Biosci. 2020, 10, e2000106. [Google Scholar] [CrossRef]
- Jose, G.; Shalumon, K.T.; Chen, J.P. Natural polymers based hydrogels for cell culture applications. Curr. Med. Chem. 2020, 16, 2734–2776. [Google Scholar] [CrossRef]
- Luetchford, K.A.; Chaudhuri, J.B.; De Bank, P.A. Silk fibroin/gelatin microcarriers as scaffolds for bone tissue engineering. Mater. Sci. Eng. C 2020, 106, 110116. [Google Scholar] [CrossRef]
- Xu, M.; Yan, Y.; Liu, H.; Yao, Y.; Wang, X. Control adipose-derived stromal cells differentiation into adipose and endothelial cells in a 3D structure established by cell-assembly technique. J. Bioact. Compat. Polym. 2009, 24, 31–47. [Google Scholar] [CrossRef]
- Wang, X. Advanced polymers for three-dimensional (3D) organ bioprinting. Micromachines 2019, 12, 814. [Google Scholar] [CrossRef]
- Duin, S.; Schütz, K.; Ahlfeld, T.; Lehmann, S.; Lode, A.; Ludwig, B.; Gelinsky, M. 3D bioprinting of functional islets of langerhans in an alginate/methylcellulose hydrogel blend. Adv. Healthc. Mater. 2019, 7, e1801631. [Google Scholar] [CrossRef]
- Hu, S.; Martinez-Garcia, F.D.; Moeun, B.N.; Burgess, J.K.; Harmsen, M.C.; Hoesli, C.; de Vos, P. An immune regulatory 3D-printed alginate-pectin construct for immunoisolation of insulin producing β-cells. Mater. Sci. Eng. C Mater. Biol. Appl. 2021, 123, 112009. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; He, K.; Wang, X. Rapid Prototyping of a hybrid hierarchical polyurethane-cell/hydrogel construct for regenerative medicine. Mater. Sci. Eng. C 2013, 33, 3220–3229. [Google Scholar] [CrossRef]
- Xu, W.; Wang, X.; Yan, Y.; Zhang, R. Rapid prototyping of polyurethane for the creation of vascular systems. J. Bioact. Compat. Polym. 2008, 23, 103–114. [Google Scholar] [CrossRef]
- Ashwin, B.; Abinaya, B.; Prasith, T.P.; Chandran, S.V.; Yadav, L.R.; Vairamani, M.; Patil, S.; Selvamurugan, N. 3D-poly (lactic acid) scaffolds coated with gelatin and mucic acid for bone tissue engineering. Int. J. Biol. Macromol. 2020, 162, 523–532. [Google Scholar] [CrossRef]
- Babilotte, J.; Martin, B.; Guduric, V.; Bareille, R.; Agniel, R.; Roques, S.; Héroguez, V.; Dussauze, M.; Gaudon, M.; Le Nihouannen, D.; et al. Development and characterization of a PLGA-HA composite material to fabricate 3D-printed scaffolds for bone tissue engineering. Mater. Sci. Eng. C Mater. Biol. Appl. 2021, 118, 111334. [Google Scholar] [CrossRef]
- Hung, K.C.; Tseng, C.S.; Dai, L.G.; Hsu, S.H. Water-based polyurethane 3D printed scaffolds with controlled release function for customized cartilage tissue engineering. Biomaterials 2016, 83, 156–168. [Google Scholar] [CrossRef]
- Liu, F.; Chen, Q.; Liu, C.; Ao, Q.; Tian, X.; Fan, J.; Tong, H.; Wang, X. Natural polymers for organ 3D bioprinting. Polymers 2018, 11, 1278. [Google Scholar] [CrossRef]
- Lei, M.; Wang, X. Biodegradable polymers and stem cells for bioprinting. Molecules 2016, 21, 539. [Google Scholar] [CrossRef] [PubMed]
- Farina, M.; Ballerini, A.; Fraga, D.W.; Nicolov, E.; Hogan, M.; Demarchi, D.; Scaglione, F.; Sabek, O.M.; Horner, P.; Thekkedath, U.; et al. 3D printed vascularized device for subcutaneous transplantation of human islets. Biotechnol. J. 2017, 9, 1700169. [Google Scholar] [CrossRef]
- Marchioli, G.; Luca, A.D.; de Koning, E.; Engelse, M.; Van Blitterswijk, C.A.; Karperien, M.; Van Apeldoorn, A.A.; Moroni, L. Hybrid polycaprolactone/alginate scaffolds functionalized with VEGF to promote de Novo vessel formation for the transplantation of islets of Langerhans. Adv. Healthc. Mater. 2016, 13, 1606–1616. [Google Scholar] [CrossRef] [PubMed]
- Foyt, D.A.; Norman, M.D.A.; Yu, T.T.L.; Gentleman, E. Exploiting Advanced Hydrogel Technologies to Address Key Challenges in Regenerative Medicine. Adv. Healthc. Mater. 2018, 8, e1700939. [Google Scholar] [CrossRef] [PubMed]
- Ge, F.; Lu, Y.; Li, Q.; Zhang, X. Decellularized extracellular matrices for tissue engineering and regeneration. Adv. Exp. Med. Biol. 2020, 1250, 15–31. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Liu, L.; Wang, J.; Xu, Y.F.; Zhang, W.M.; Khang, G.; Wang, X. In vitro vascularization of a combined system based on a 3D bioprinting technique. J. Tissue Eng. Regen. Med. 2016, 10, 833–842. [Google Scholar] [CrossRef]
- Yao, Q.; Zheng, Y.W.; Lan, Q.H.; Kou, L.; Xu, H.L.; Zhao, Y.Z. Recent development and biomedical applications of decellularized extracellular matrix biomaterials. Mater. Sci. Eng. C Mater. Biol. Appl. 2019, 104, 109942. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.S.; Kwon, Y.W.; Kong, J.S.; Park, G.T.; Gao, G.; Han, W.; Kim, M.B.; Lee, H.; Kim, J.H.; Cho, D.W. 3D cell printing of in vitro stabilized skin model and in vivo pre-vascularized skin patch using tissue-specific extracellular matrix bioink: A step towards advanced skin tissue engineering. Biomaterials 2018, 168, 38–53. [Google Scholar] [CrossRef]
- Taylor, D.A.; Sampaio, L.C.; Ferdous, Z.; Gobin, A.S.; Taite, L.J. Decellularized matrices in regenerative medicine. Acta Biomater. 2018, 74, 74–89. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Kim, M.; Hwang, D.G.; Shim, I.K.; Kim, S.C.; Jang, J. Pancreatic tissue-derived extracellular matrix bioink for printing 3D cell-laden pancreatic tissue constructs. J. Vis. Exp. 2019, 154, e60434. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Tuomi, J.; Mäkitie, A.A.; Poloheimo, K.-S.; Partanen, J.; Yliperttula, M. The integrations of biomaterials and rapid prototyping techniques for intelligent manufacturing of complex organs. In Advances in Biomaterials Science and Applications in Biomedicine; Lazinica, R., Ed.; InTech: Rijeka, Croatia, 2013; pp. 437–463. [Google Scholar]
- Wang, D.; Guo, Y.; Zhu, J.; Liu, F.; Xue, Y.; Huang, Y.; Zhu, B.; Wu, D.; Pan, H.; Gong, T.; et al. Hyaluronic acid methacrylate/pancreatic extracellular matrix as a potential 3D printing bioink for constructing islet organoids. Acta Biomater. 2022, 22, 00375. [Google Scholar] [CrossRef] [PubMed]
- Yan, Y.; Wang, X.; Pan, Y.; Liu, H.; Cheng, J.; Xiong, Z.; Lin, F.; Wu, R.; Zhang, R.; Lu, Q. Fabrication of viable tissue-engineered constructs with 3D cell-assembly technique. Biomaterials 2005, 26, 5864–5871. [Google Scholar] [CrossRef] [PubMed]
- Yan, Y.; Wang, X.; Xiong, Z.; Liu, H.; Liu, F.; Lin, F.; Wu, R.; Zhang, R.; Lu, Q. Direct construction of a three-dimensional structure with cells and hydrogel. J. Bioact. Compat. Polym. 2005, 20, 259–269. [Google Scholar] [CrossRef]
- Xu, W.; Wang, X.; Yan, Y.; Zheng, W.; Xiong, Z.; Lin, F.; Wu, R.; Zhang, R. Rapid prototyping three-dimensional cell/gelatin/fibrinogen constructs for medical regeneration. J. Bioact. Compat. Polym. 2007, 22, 363–377. [Google Scholar] [CrossRef]
- Zhang, T.; Yan, Y.; Wang, X.; Xiong, Z.; Lin, F.; Wu, R.; Zhang, R. Three-dimensional gelatin and gelatin/hyaluronan hydrogel structures for traumatic brain injury. J. Bioact. Compat. Polym. 2007, 22, 19–29. [Google Scholar] [CrossRef]
- Xu, W.; Wang, X.; Yan, Y.; Zhang, R. A polyurethane-gelatin hybrid construct for the manufacturing of implantable bioartificial livers. J. Bioact. Compat. Polym. 2008, 23, 409–422. [Google Scholar] [CrossRef]
- Li, S.; Xiong, Z.; Wang, X.; Yan, Y.; Liu, H.; Zhang, R. Direct fabrication of a hybrid cell/hydrogel construct by a double-nozzle assembling technology. J. Bioact. Compat. Polym. 2009, 24, 249–265. [Google Scholar]
- Xu, M.; Wang, X.; Yan, Y.; Yao, R.; Ge, Y. A cell-assembly derived physiological 3D model of the metabolic syndrome, based on adipose-derived stromal cells and a gelatin/alginate/fibrinogen matrix. Biomaterials 2010, 31, 3868–3877. [Google Scholar] [CrossRef] [PubMed]
- Sui, S.; Wang, X.; Liu, P.; Yan, Y.; Zhang, R. Cryopreservation of cells in 3D constructs based on controlled cell assembly processes. J. Bioact. Compat. Polym. 2009, 24, 473–487. [Google Scholar] [CrossRef]
- Wang, X.; Xu, H. Incorporation of DMSO and dextran-40 into a gelatin/alginate hydrogel for controlled assembled cell cryopreservation. Cryobiology 2010, 61, 345–351. [Google Scholar] [CrossRef]
- Cui, T.; Yan, Y.; Zhang, R.; Liu, L.; Xu, W.; Wang, X. Rapid prototyping of a double layer polyurethane-collagen conduit for peripheral nerve regeneration. Tissue Eng. C 2009, 15, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Cui, T.; Yan, Y.; Zhang, R. Peroneal nerve regeneration along a new polyurethane-collagen guide conduit. J. Bioact. Compat. Polym. 2009, 24, 109–127. [Google Scholar] [CrossRef]
- He, K.; Wang, X. Rapid prototyping of tubular polyurethane and cell/hydrogel construct. J. Bioact. Compat. Polym. 2011, 26, 363–374. [Google Scholar]
- Zhao, X.; Du, S.; Chai, L.; Xu, Y.; Liu, L.; Zhou, X.; Wang, J.; Zhang, W.; Liu, C.-H.; Wang, X. Anti-cancer drug screening based on an adipose-derived stem cell/hepatocyte 3D printing technique. J. Stem Cell Res. Ther. 2015, 5, 273. [Google Scholar]
- Zhou, X.; Liu, C.; Zhao, X.; Wang, X. A 3D bioprinting liver tumor model for drug screening. World J. Pharm. Pharm. Sci. 2016, 5, 196–213. [Google Scholar]
- Wang, X. Bioartificial organ manufacturing technologies. Cell Transplant. 2019, 1, 5–17. [Google Scholar] [CrossRef]
- Pati, F.; Gantelius, J.; Svahn, H.A. 3D bioprinting of tissue/organ models. Angew. Chem. Int. Ed. Engl. 2016, 15, 4650–4665. [Google Scholar] [CrossRef]
- Wang, X. Overview on biocompatibilities of implantable biomaterials. In Advances in Biomaterials Science and Biomedical Applications in Biomedicine; Lazinica, R., Ed.; InTech: Rijeka, Croatia, 2013; pp. 111–115. [Google Scholar]
- Dey, M.; Ozbolat, I.T. 3D bioprinting of cells, tissues and organs. Sci. Rep. 2020, 1, 14023. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Wang, X. Synthetic polymers for organ 3D printing. Polymers 2020, 8, 1765. [Google Scholar] [CrossRef]
- Hann, S.Y.; Cui, H.; Esworthy, T.; Miao, S.; Zhou, X.; Lee, S.J.; Fisher, J.P.; Zhang, L.G. Recent advances in 3D printing: Vascular network for tissue and organ regeneration. Transl. Res. 2019, 211, 46–63. [Google Scholar] [CrossRef]
- Chen, E.P.; Toksoy, Z.; Davis, B.A.; Geibel, J.P. 3D Bioprinting of vascularized tissues for in vitro and in vivo applications. Front. Bioeng. Biotechnol. 2021, 9, 664188. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Wang, L.; Li, T.; Liu, S.; Guo, B.; Huang, W.; Wu, Y. 3D bioprinting in cardiac tissue engineering. Theranostics 2021, 16, 7948–7969. [Google Scholar] [CrossRef] [PubMed]
- Mandrycky, C.; Wang, Z.; Kim, K.; Kim, D.H. 3D bioprinting for engineering complex tissues. Biotech. Adv. 2016, 4, 422–434. [Google Scholar] [CrossRef] [PubMed]
- Matai, I.; Kaur, G.; Seyedsalehi, A.; McClinton, A.; Laurencin, C.T. Progress in 3D bioprinting technology for tissue/organ regenerative engineering. Biomaterials 2020, 226, 119536. [Google Scholar] [CrossRef]
- Berg, J.; Kurreck, J. Clean bioprinting-fabrication of 3D organ models devoid of animal components. ALTEX 2021, 2, 269–288. [Google Scholar] [CrossRef] [PubMed]
- Deo, K.A.; Singh, K.A.; Peak, C.W.; Alge, D.L.; Gaharwar, A.K. Bioprinting 101: Design, fabrication, and evaluation of cell-laden 3D bioprinted scaffolds. Tissue Eng. Part A 2020, 5–6, 318–338. [Google Scholar] [CrossRef]
- Sorkio, A.; Koch, L.; Koivusalo, L.; Deiwick, A.; Miettinen, S.; Chichkov, B.; Skottman, H. Human stem cell based corneal tissue mimicking structures using laser-assisted 3D bioprinting and functional bioinks. Biomaterials 2018, 171, 57–71. [Google Scholar] [CrossRef] [PubMed]
- Huh, D.; Hamilton, G.A.; Ingber, D.E. From 3D cell culture to organs-on-chips. Trends Cell Biol. 2011, 12, 745–754. [Google Scholar] [CrossRef]
- Zhu, J.; Wang, Y.; Zhong, L.; Pan, F.; Wang, J. Advances in tissue engineering of vasculature through three-dimensional bioprinting. Dev. Dyn. Off. Publ. Am. Assoc. Anat. 2021, 12, 1717–1738. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).