Facile Synthesis of Formaldehyde-Free Bio-Based Thermoset Resins for Fabrication of Highly Efficient Foams
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
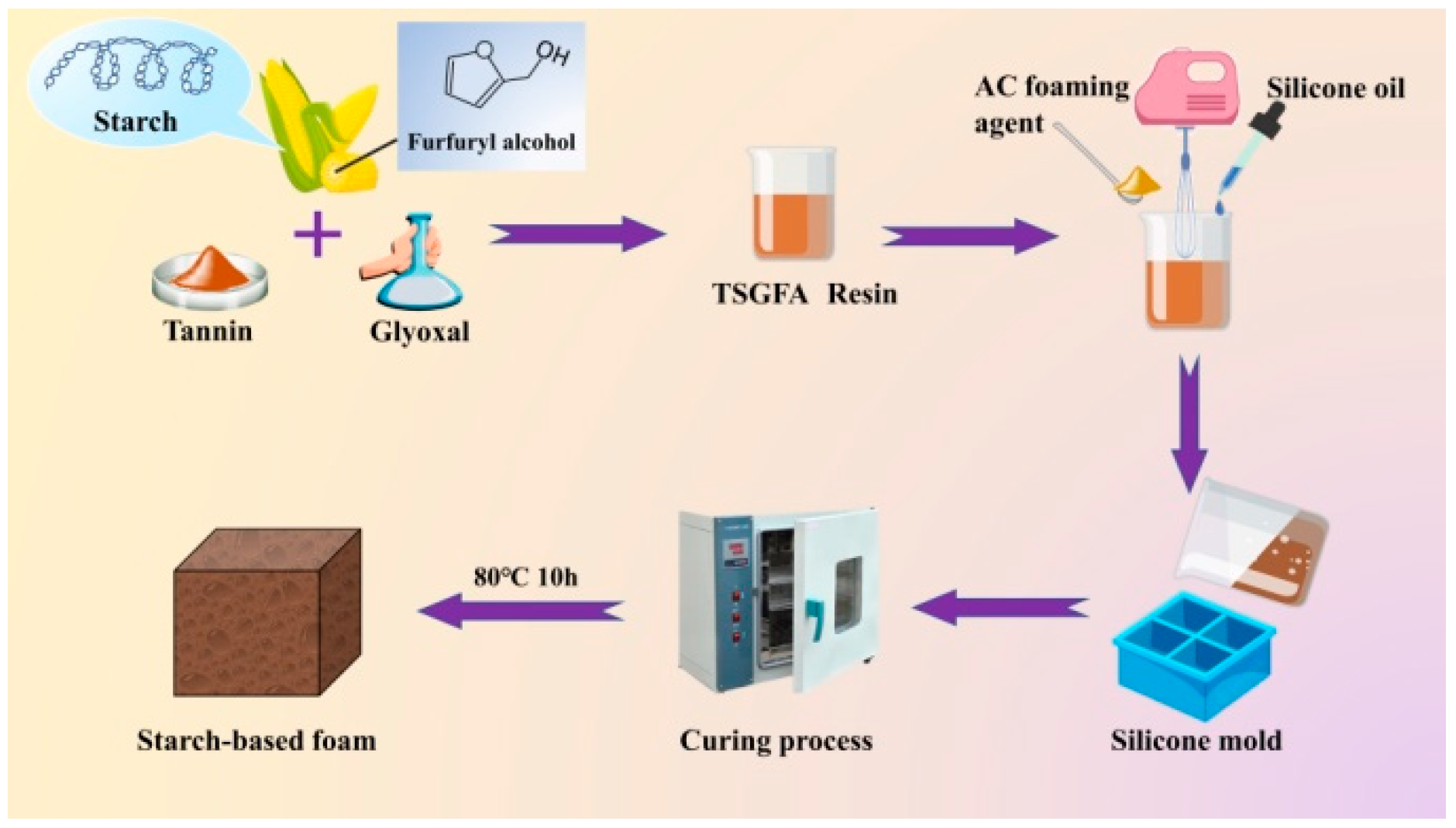
2.2. Preparation of Tannin–Starch–Glyoxal–Furfuryl Alcohol (TSGFA) Resin-Based Foam
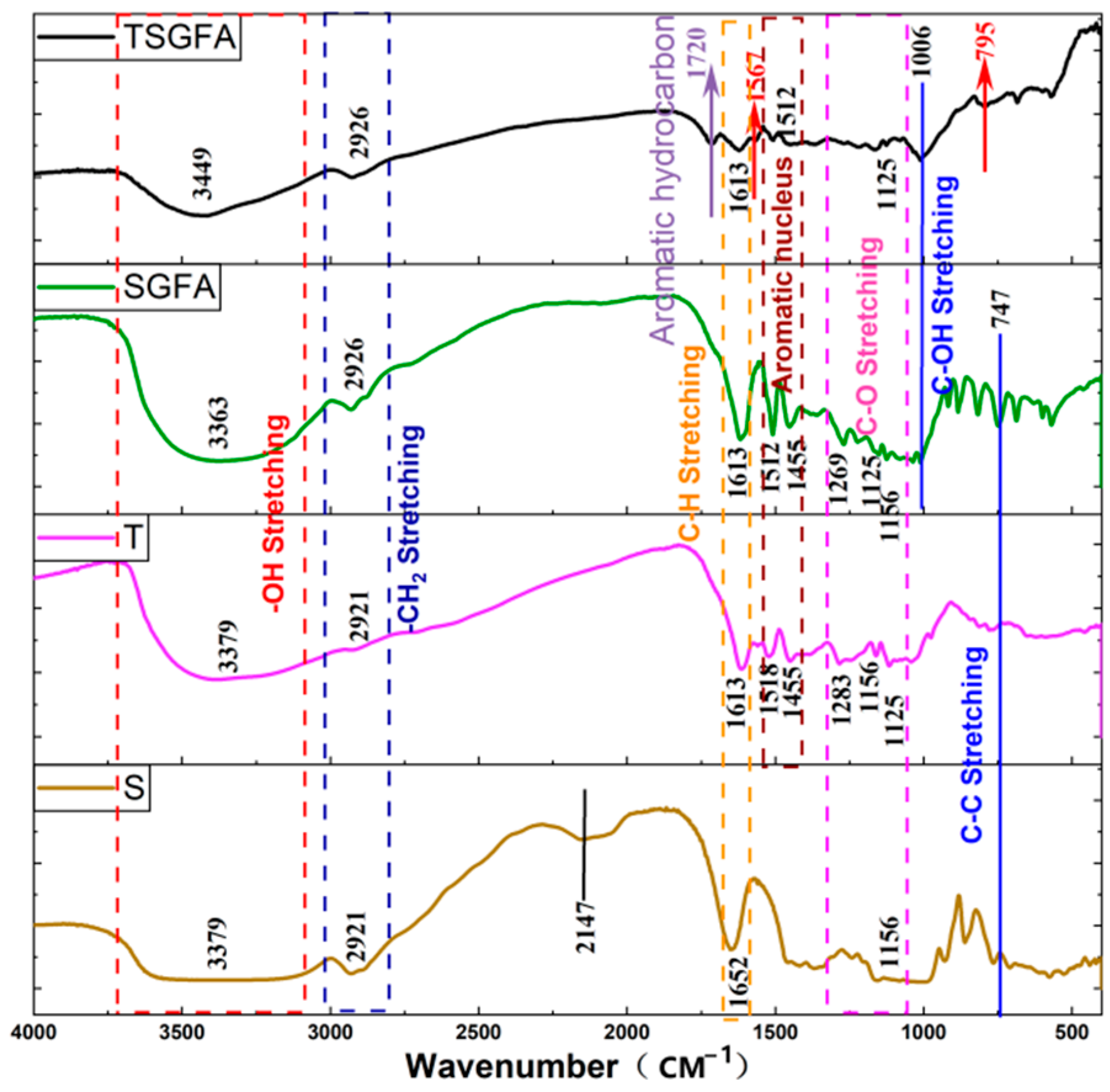
2.3. Characterizations
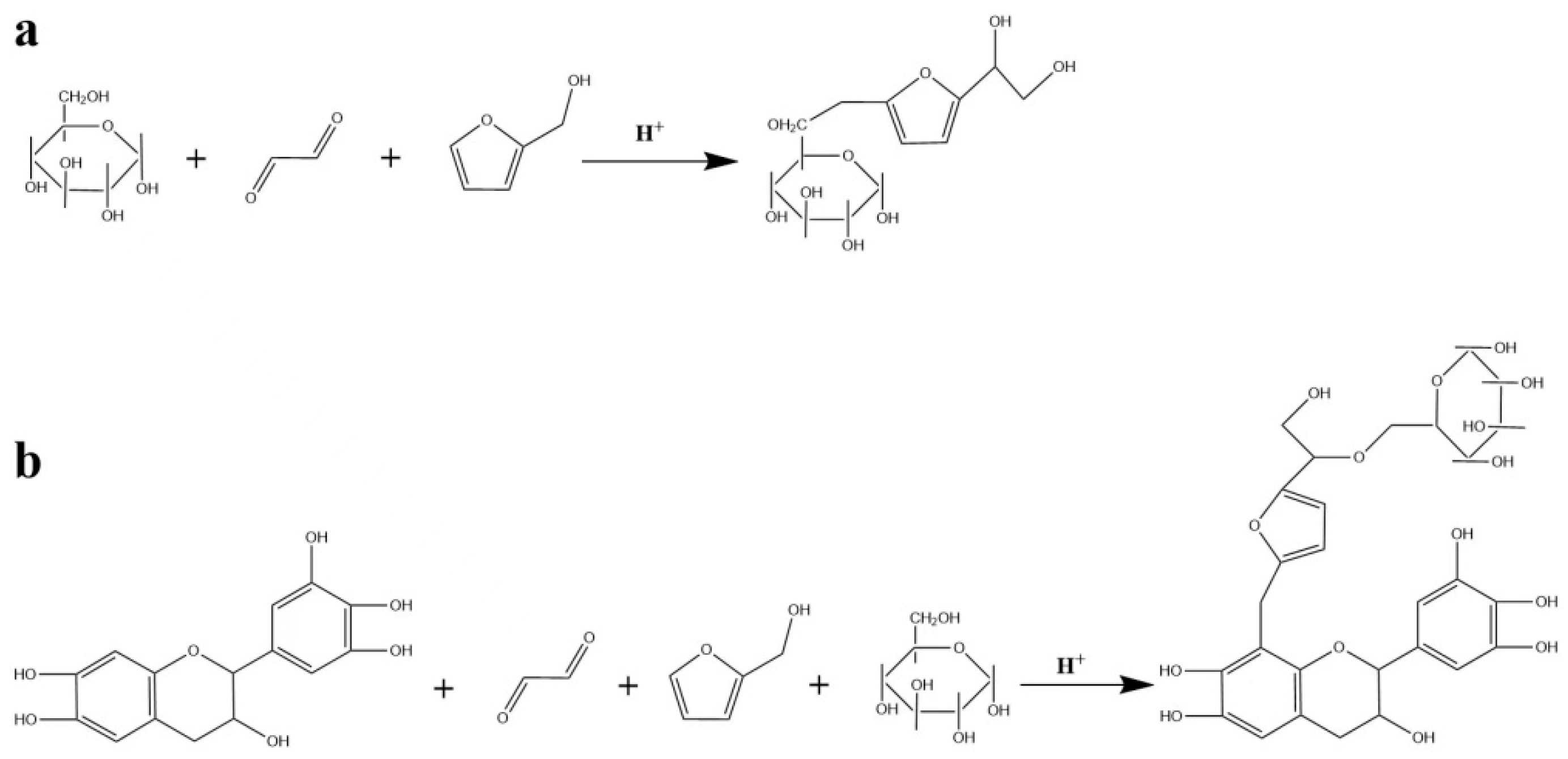
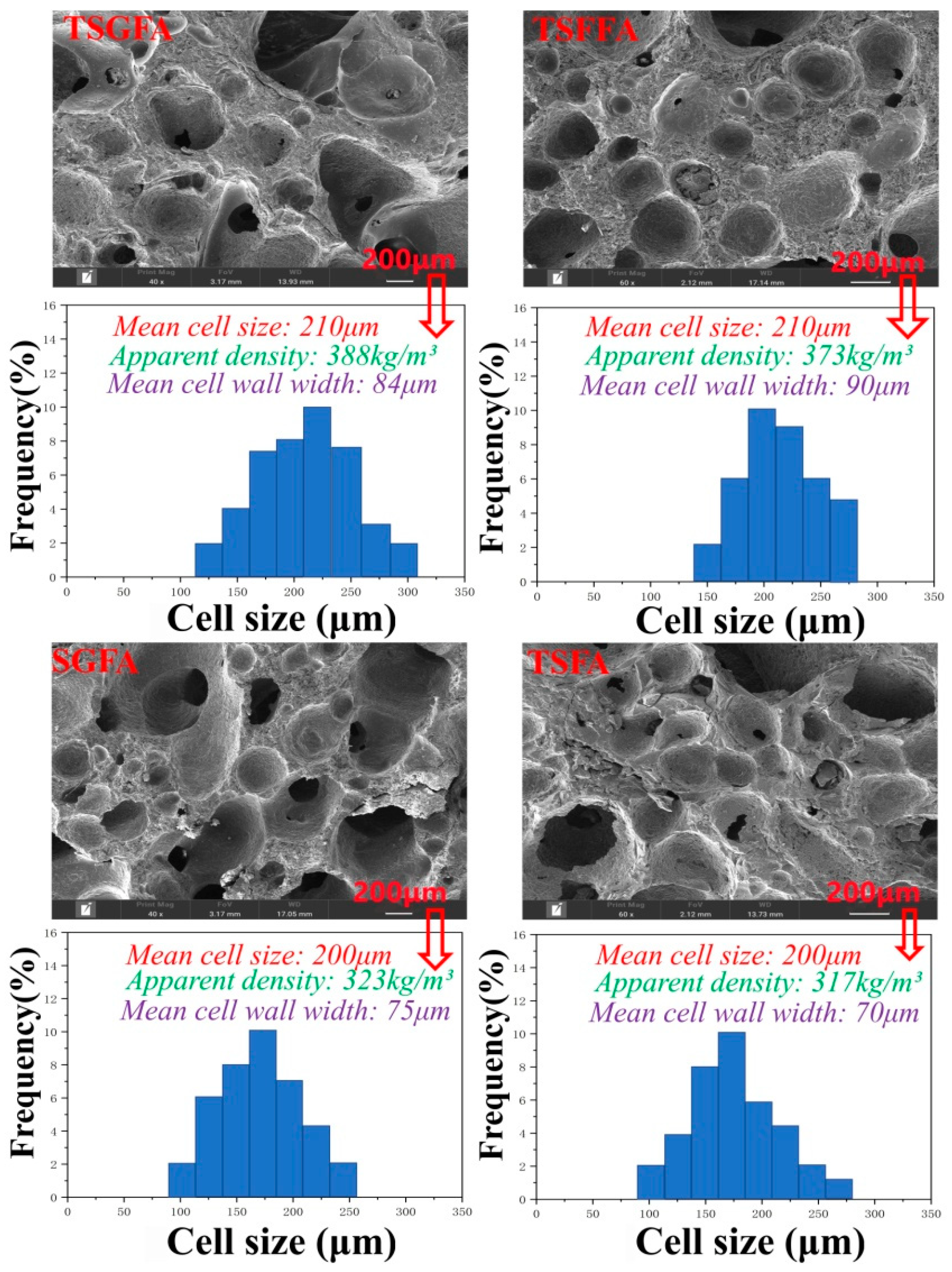
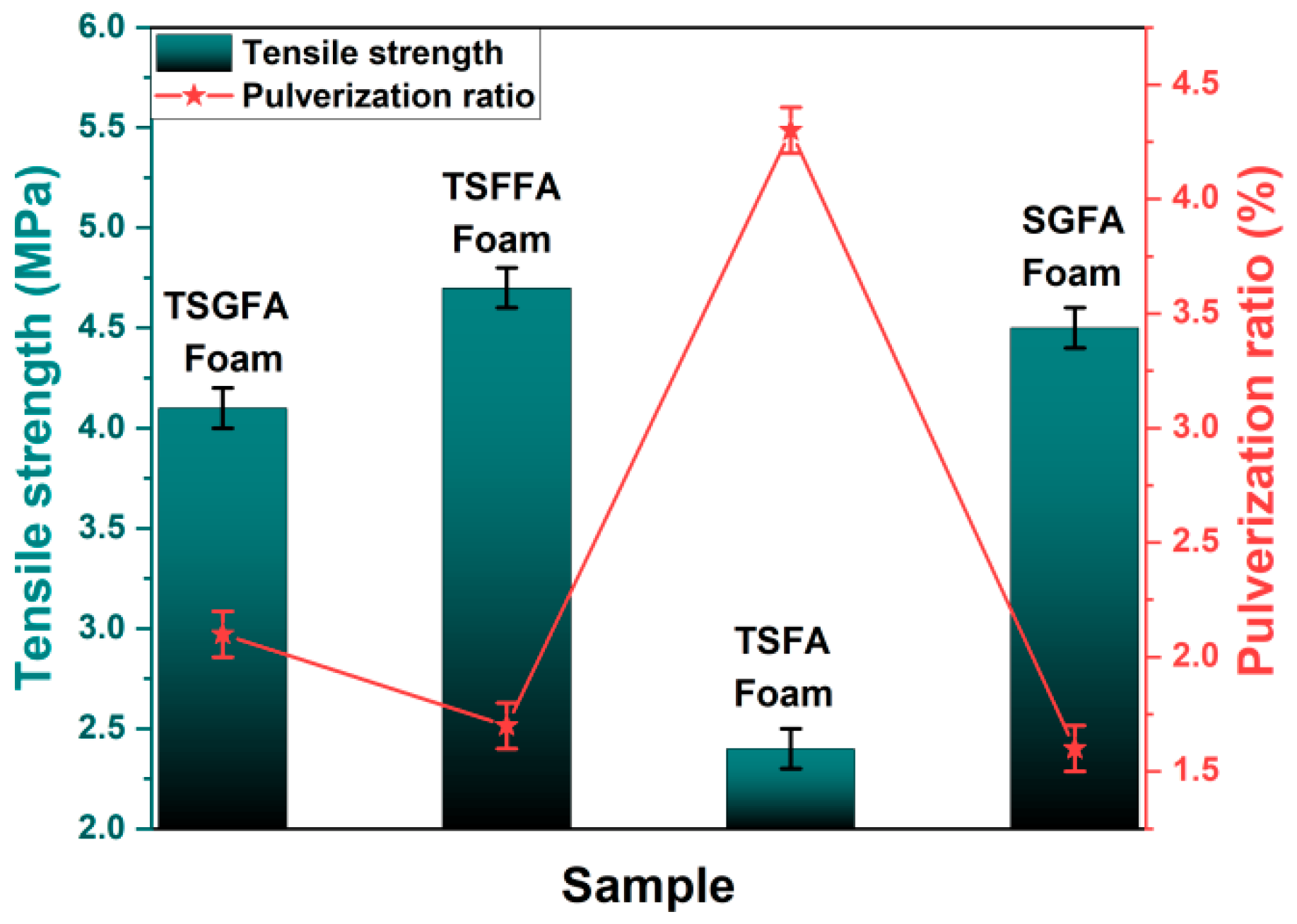
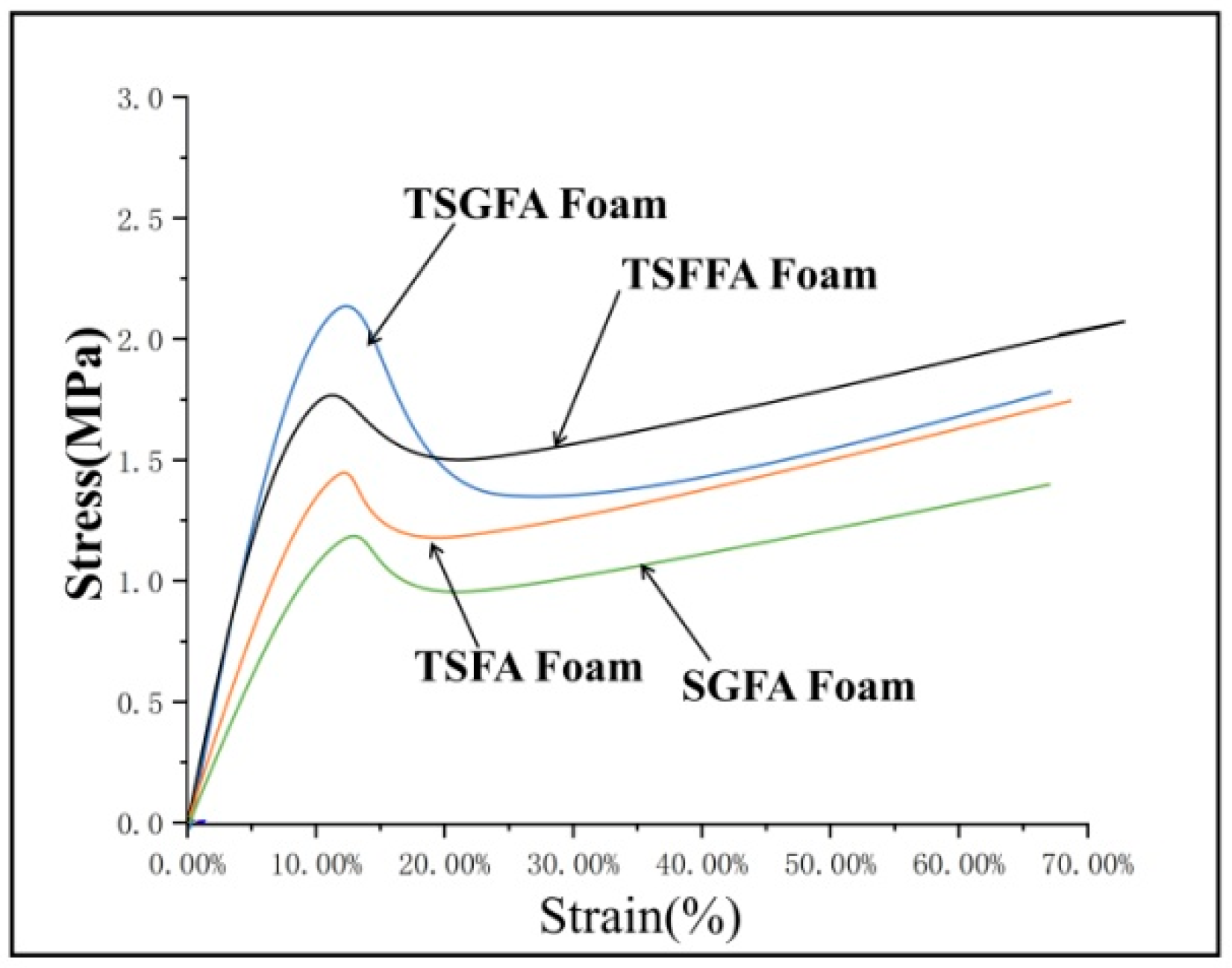
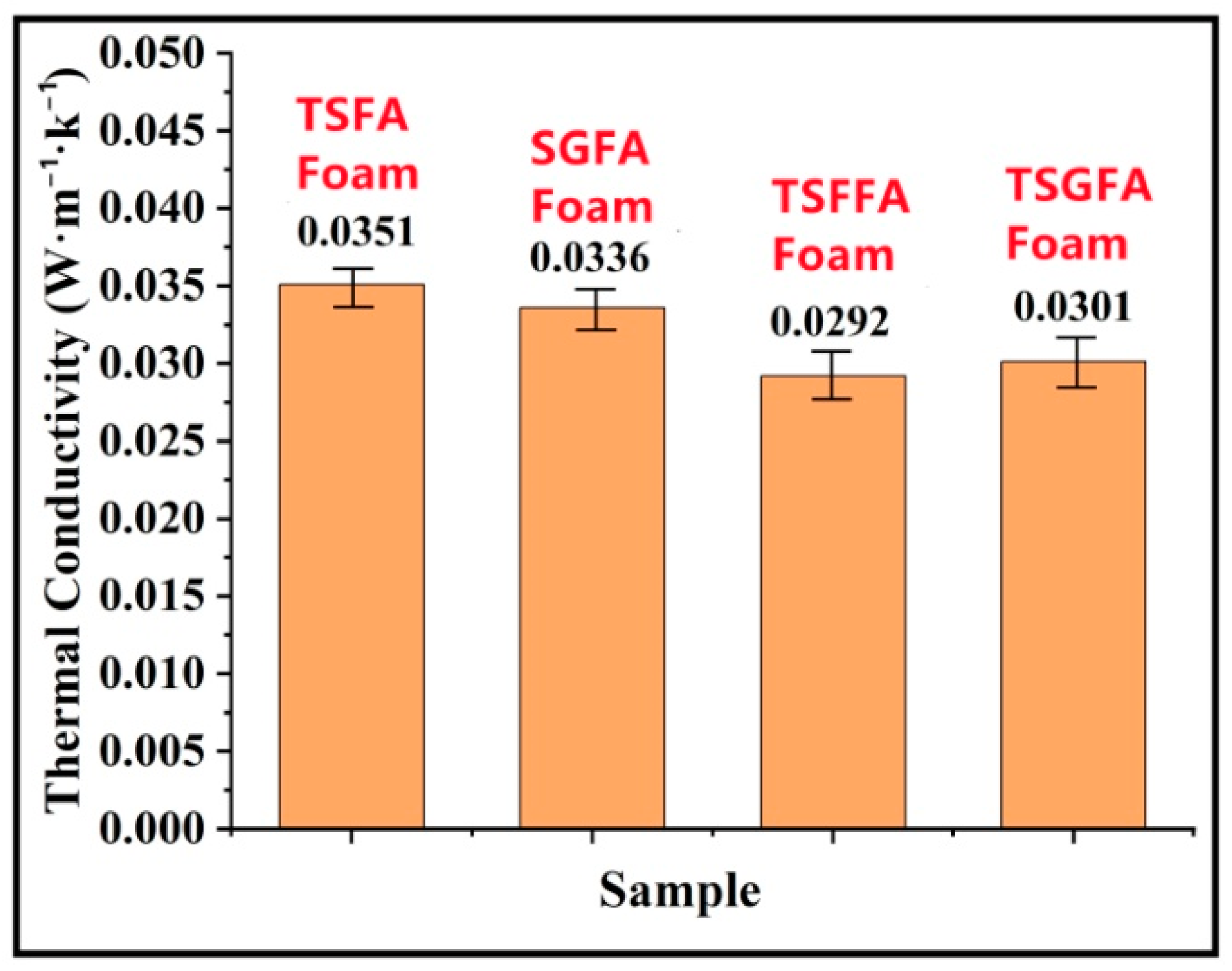
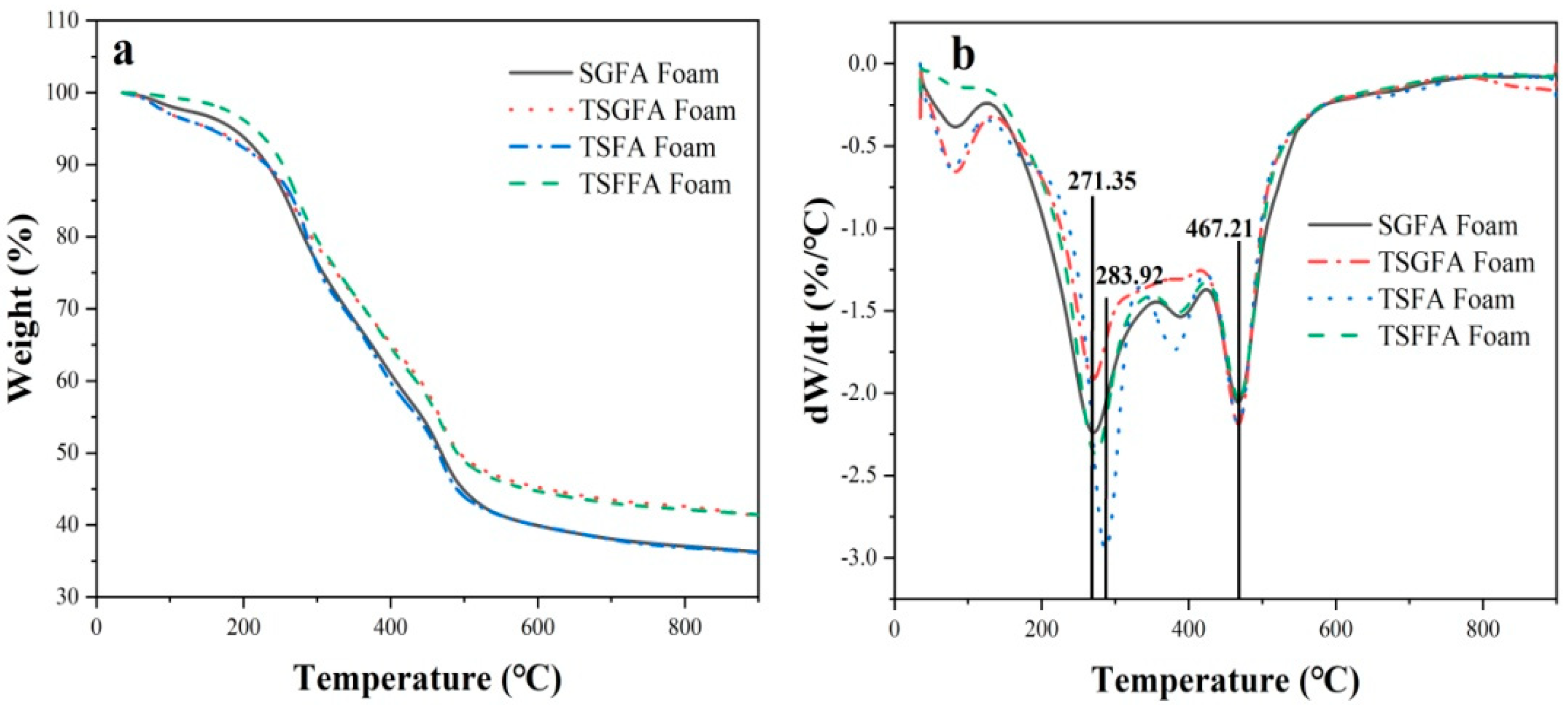
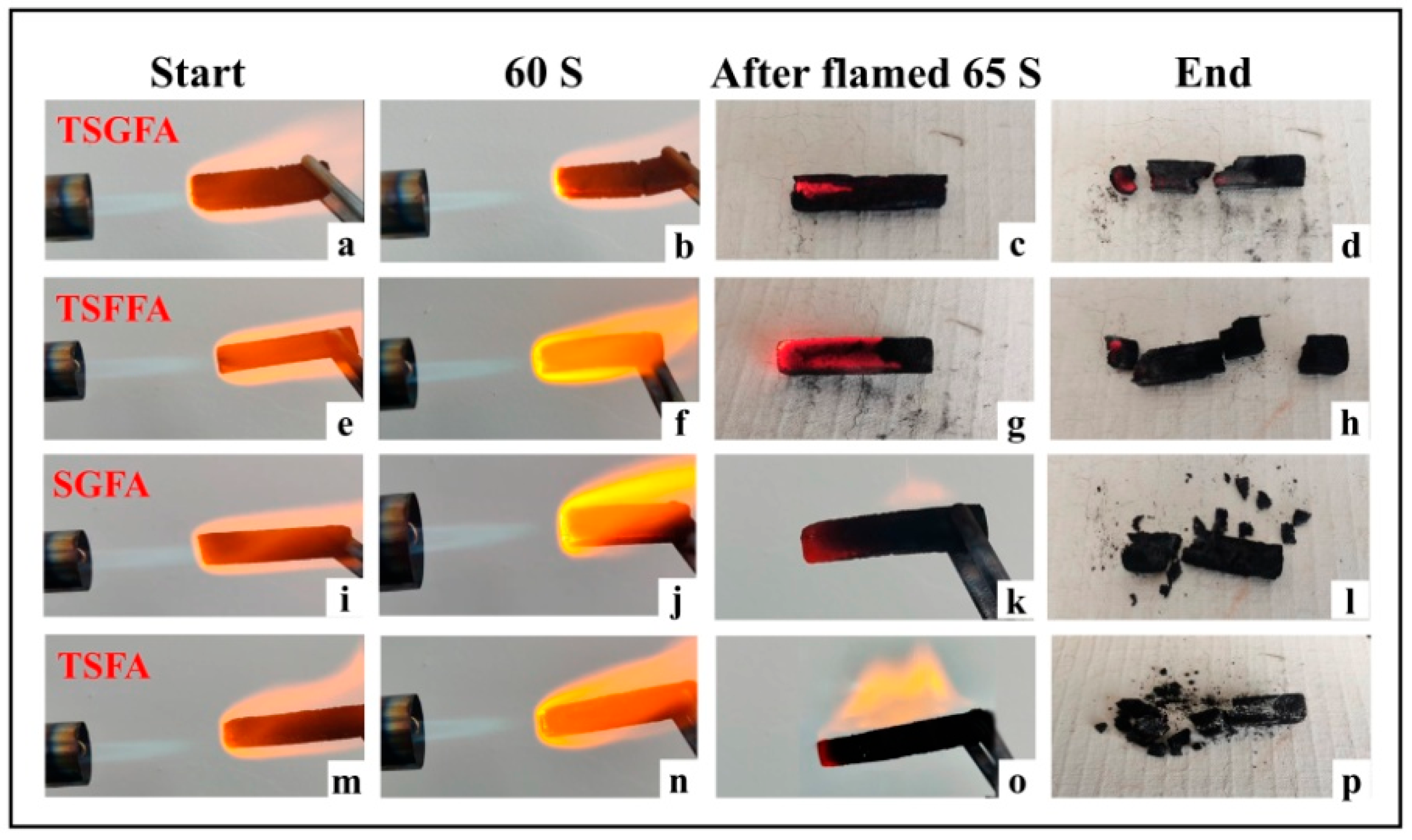
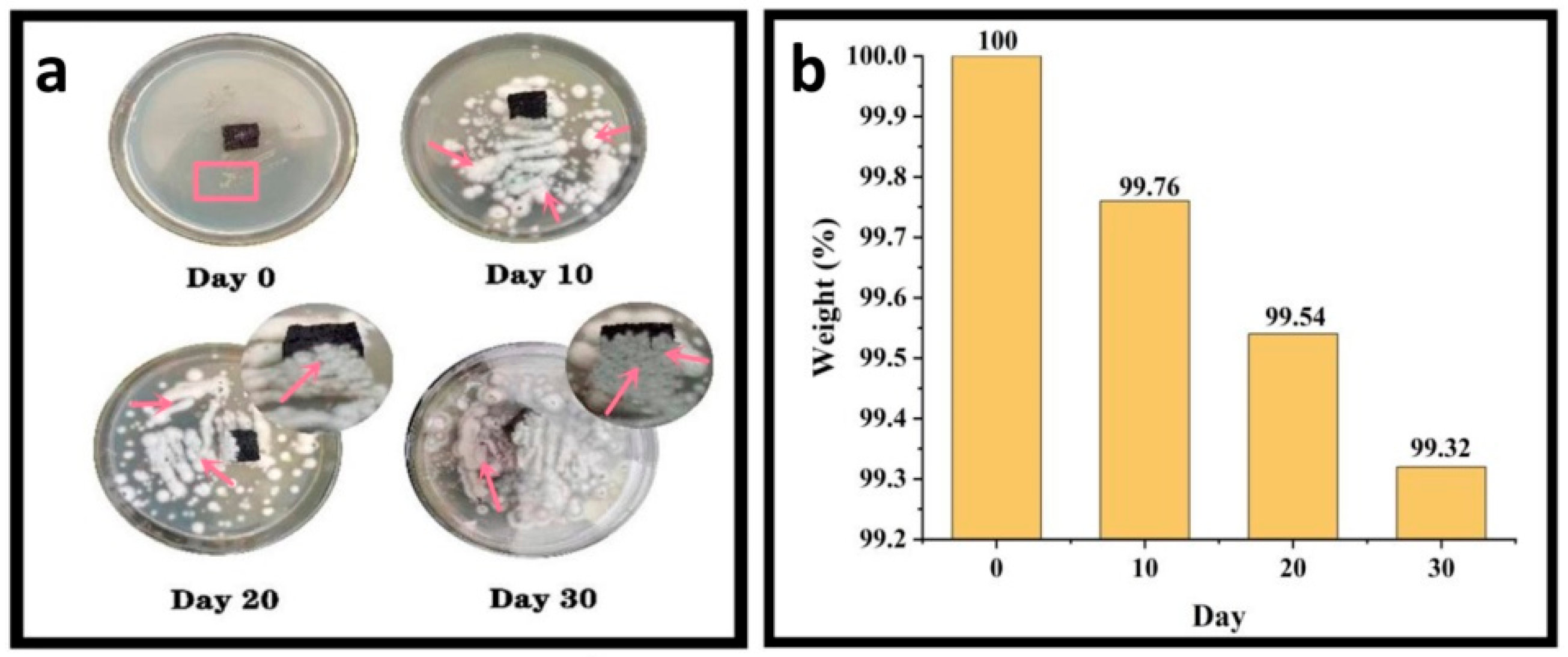
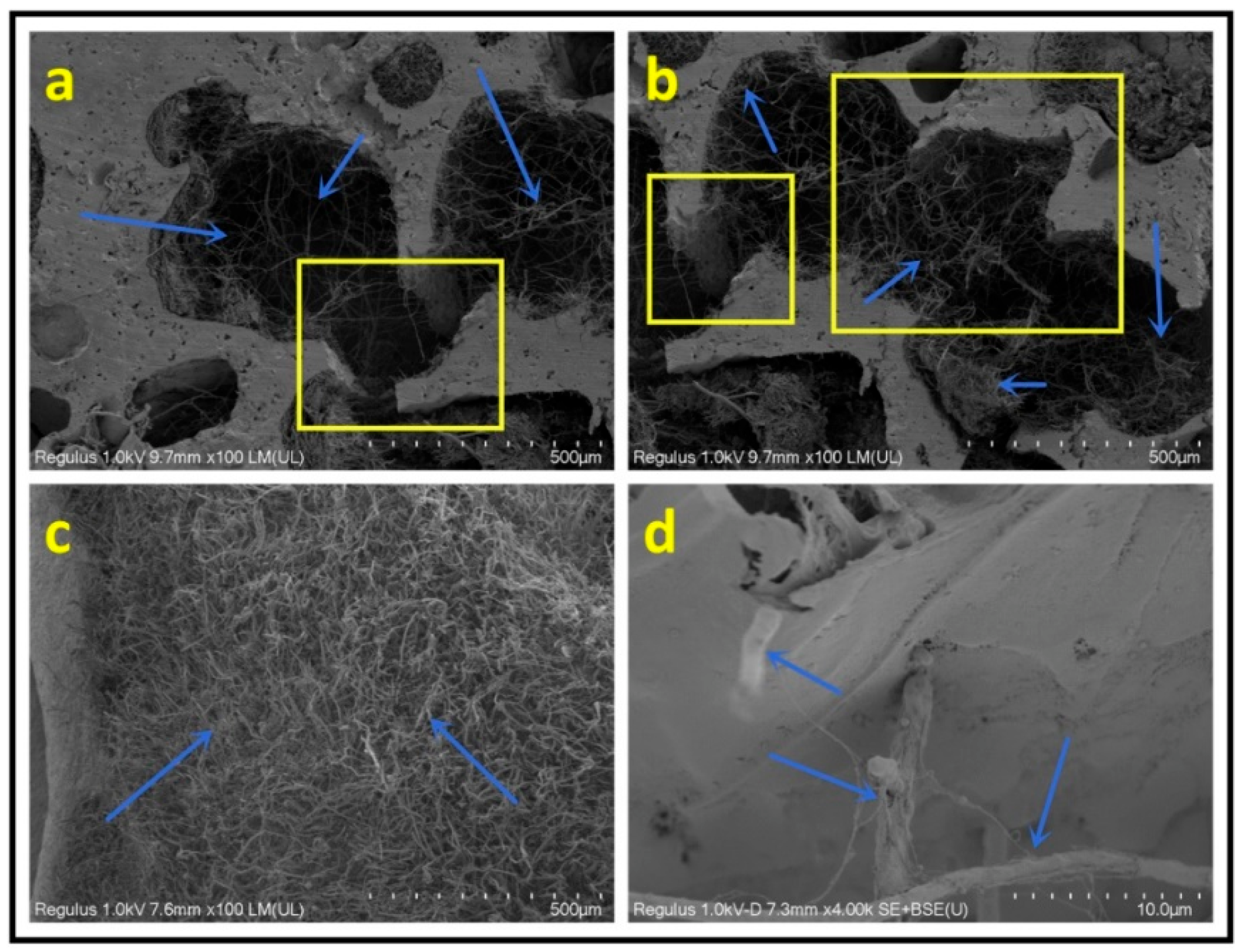
3. Results and Discussion
4. Conclusions
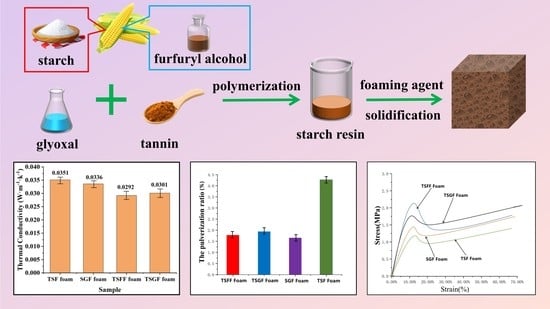
- Different bio-based foam structures in crosslinked form can be prepared from a polycondensation reaction incorporating starch, furfuryl alcohol, glyoxal, and condensed tannin under mild acidic conditions and an appropriate foaming agent. The selection of catalytic system and foaming agent determines, to a large extent, the cell formation characteristics of the foam structure, while the mechanical strength is a parameter that is dependent on the condensing agents’ formulation.
- It can be concluded that the addition of tannin into the formulation contributed significantly to the high compressive strength and low pulverization ratio.
- The crosslinking between tannin, starch, glyoxal, and furfuryl alcohol under the employed reaction conditions provoked formation of closed cells with uniform cell distribution and appropriate apparent density, which contributed to the good thermal insulation and flame retardancy of the foam.
- The induced biodegradability of the prepared foams using Penicillium sp. is ascribed to the bio-based nature of the structural units involved in building the chemical skeleton of the foam.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kumar, D.; Alam, M.; Zou, P.X.W.; Sanjayan, J.G.; Memon, R.A. Comparative analysis of building insulation material properties and performance. Renew. Sustain. Energy Rev. 2020, 131, 110038. [Google Scholar] [CrossRef]
- Aditya, L.; Mahlia, T.; Rismanchi, B.; Ng, H.; Hasan, M.; Metselaar, H.; Muraza, O.; Aditiya, H.A. review on insulation materials for energy conservation in buildings. Renew. Sustain. Energy Rev. 2017, 73, 1352–1365. [Google Scholar] [CrossRef]
- Ramlee, N.A.; Jawaid, M.; Yamani, S.A.K.; Zainudin, E.S.; Alamery, S. Effect of surface treatment on mechanical, physical and morphological properties of oil palm/bagasse fiber reinforced phenolic hybrid composites for wall thermal insulation application. Constr. Build. Mater. 2021, 276, 122239. [Google Scholar] [CrossRef]
- Ahmadzadeh, S.; Nasirpour, A.; Keramat, J.; Hamdami, N.; Behzad, T.; Desobry, S. Nanoporous cellulose nanocomposite foams as high insulated food packaging materials. Colloids Surf. A Physicochem. Eng. Asp. 2015, 468, 201–210. [Google Scholar] [CrossRef]
- Zhu, G.; Wang, J.; Yuan, X.; Yuan, B. Hydrophobic and fire safe polyurethane foam coated with chitosan and nano-montmorillonite via layer-by-layer assembly for emergency absorption of oil spill. Mater. Lett. 2022, 316, 132009. [Google Scholar] [CrossRef]
- Razali, N.; Ali, F.; Azmi, A.S.; Ismail, T.; Mirghani, M.; Omar, M.F. Microwave-assisted synthesis of polylactic acid-diol for polyurethane as biodegradable packaging material. IOP Conf. Ser. Mater. Sci. Eng. 2021, 1192, 012015. [Google Scholar] [CrossRef]
- Amirabadi, S.; Kakroodi, A.R.; Dias, O.; Sain, M.; Park, C.B. Tailoring nano-fibrillated polystyrene composite with enhanced fire retarding properties for foam applications. Mater. Des. 2022, 214, 110419. [Google Scholar] [CrossRef]
- Liu, C.; Xie, Y.; Gao, D.; Shi, X.; Rao, Z. Fabrication of fire-retardant building materials via a hyper-crosslinking chemical conversion process from waste polystyrenes. Energy Built Environ. 2021, 3, 226–232. [Google Scholar] [CrossRef]
- Zou, S.i.; Li, H.; Wang, S.; Jiang, R.; Zou, J.; Zhang, X.; Liu, L.; Zhang, G. Experimental research on an innovative sawdust biomass-based insulation material for buildings. J. Clean. Prod. 2020, 260, 121029. [Google Scholar] [CrossRef]
- Lee, J.; Kim, J.; Song, D.; Kim, J.; Jang, C. Impact of external insulation and internal thermal density upon energy consumption of buildings in a temperate climate with four distinct seasons. Renew. Sustain. Energy Rev. 2017, 75, 1081–1088. [Google Scholar] [CrossRef]
- Oh, T.H.; Chua, S.C. Energy efficiency and carbon trading potential in Malaysia. Renew. Sustain. Energy Rev. 2010, 14, 2095–2103. [Google Scholar] [CrossRef]
- Huang, Y.; Chen, C.; Huang, H. Analyzing life-cycle water footprint for advanced bio-liquid fuel: Crop residues and non-grain biofuels in China. J. Clean. Prod. 2021, 293, 126151. [Google Scholar] [CrossRef]
- Zhang, Y.N.; Ghaly, A.E.; Li, B.X. Physical properties of corn residues. Am. J. Biochem. Biotechnol. 2012, 8, 44–53. [Google Scholar] [CrossRef]
- Wang, Z.P.; Zhao, L.; Jin, B.C.; Jia, S.L.; Han, L.J.; Pan, H.W.; Zhang, H.L. Effect of maleic anhydride and titanate coupling agent as additives on the properties of poly(butylene adipate-co-terephthalate)/thermoplastic starch films. Polym. Bull. 2021, 11, 7193–7213. [Google Scholar] [CrossRef]
- Zhang, J.; Liu, B.W.; Zhou, Y.X.; Essawy, H.; Chen, Q.; Zhou, X.J.; Du, G.B. Preparation of a starch-based adhesive cross-linked with furfural, furfuryl alcohol and epoxy resin. Int. J. Adhes. Adhes. 2021, 110, 102958. [Google Scholar] [CrossRef]
- Zhang, Y.H.; Ding, L.L.; Gu, J.Y.; Tan, H.Y.; Zhu, L.B. Preparation and properties of a starch-based wood adhesive with high bonding strength and water resistance. Carbohydr. Polym. 2015, 115, 32–37. [Google Scholar] [CrossRef]
- Zheng, X.; Cheng, L.; Gu, Z.; Hong, Y.; Li, Z. Effects of heat pretreatment of starch on graft copolymerization reaction and performance of resulting starch-based wood adhesive. Int. J. Biol. Macromol. 2017, 96, 11–18. [Google Scholar] [CrossRef]
- Mitrus, M.; Moscicki, L. Extrusion-cooking of starch protective loose-fill foams. Chem. Eng. Res. Des. 2014, 92, 778–783. [Google Scholar] [CrossRef]
- Glenn, G.M.; Orts, W.J.; Nobes, G.A.R.; Gray, G.M. In situ laminating process for baked starch-based foams. Ind. Crops Prod. 2001, 14, 125–134. [Google Scholar] [CrossRef]
- Liu, H.; Xie, F.; Yu, L.; Chen, L.; Li, L. Thermal processing of starch-based polymers. Prog. Polym. Sci. 2009, 34, 1348–1368. [Google Scholar] [CrossRef]
- Akrami, M.; Ghasemi, I.; Azizi, H.; Karrabi, M.; Seyedabadi, M. A new approach in compatibilization of the poly(lactic acid)/thermoplastic starch (PLA/TPS) blends. Carbohydr. Polym. 2016, 144, 254–262. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Liu, B.W.; Zhou, Y.X.; Essawy, H.; Liang, J.K.; Zhou, X.J.; Du, G.B. Preparation and characterization of a bio-based rigid plastic based on gelatinized starch cross-linked with furfural and formaldehyde. Ind. Crops Prod. 2022, 186, 115246. [Google Scholar] [CrossRef]
- Bénézet, J.C.; Stanojlovic-Davidovic, A.; Bergeret, A.; Ferry, L.; Crespy, A. Mechanical and physical properties of expanded starch, reinforced by natural fibres. Ind. Crops Prod. 2012, 37, 435–440. [Google Scholar] [CrossRef]
- Ghanbari, A.; Tabarsa, T.; Ashori, A.; Shakeri, A.; Mashkour, M. Thermoplastic starch foamed composites reinforced with cellulose nanofibers: Thermal and mechanical properties. Carbohydr Polym. 2018, 197, 305–311. [Google Scholar] [CrossRef]
- Hendrawati, N.; Dewi, E.N.; Santosa, S. Karakterisasi biodegradable foam dari pati sagu termodifikasi dengan kitosan sebagai aditif. J. Tek. Kim. Dan Lingkunga 2019, 3, 47. [Google Scholar] [CrossRef]
- Zhang, X.; Teng, Z.; Huang, R. Biodegradable starch/chitosan foam via microwave assisted preparation: Morphology and performance properties. Polymers 2020, 12, 2612. [Google Scholar] [CrossRef]
- Yu, Y.X.; Wang, Y.F.; Xu, P.P.; Chang, J.M. Preparation and Characterization of Phenolic Foam Modified with Bio-Oil. Materials 2018, 11, 2228. [Google Scholar] [CrossRef]
- Cao, Z.; He, Y.H.; Sun, L.X.; Cao, X.Q. Preparation and Characterization of Lignin-Phenolic Foam. Adv. Mater. Res. 2011, 236–238, 1014–1018. [Google Scholar] [CrossRef]
- Li, X.J.; Nicollin, A.; Pizzi, A.; Zhou, X.J.; Saugeta, A. Natural tannin–furanic thermosetting moulding plastics. RSC Adv. 2013, 3, 17732–17740. [Google Scholar] [CrossRef]
- Basso, M.C.; Giovando, S.; Pizzi, A.; Celzard, A.; Fierro, V. Tannin/furanic foams without blowing agents and formaldehyde. Ind. Crops Prod. 2013, 49, 17–22. [Google Scholar] [CrossRef]
- Celzard, A.; Fierro, V.; Amaral-Labat, G.; Pizzi, A.; Torero, J. Flammability assessment of tannin-based cellular materials. Polym. Degrad. Stab. 2011, 96, 477–482. [Google Scholar] [CrossRef]
- Lacoste, C.; Basso, M.C.; Pizzi, A.; Celzard, A.; Ebang, E.E.; Gallon, N.; Charrier, B. Pine (P. pinaster) and quebracho (S. lorentzii) tannin-based foams as green acoustic absorbers. Ind. Crops Prod. 2015, 67, 70–73. [Google Scholar] [CrossRef]
- Pizzi, A. Tannin-based biofoams—A review. J. Renew. Mater. 2019, 7, 16. [Google Scholar] [CrossRef]
- Zhang, J.; Liang, J.; Du, G.; Zhou, X.; Wang, H.; Lei, H. Development and characterization of a Bayberry tannin-based adhesive for particleboard. BioResources 2017, 12, 6082–6093. [Google Scholar] [CrossRef][Green Version]
- Zhang, J.; Xi, X.D.; Liang, J.K.; Pizzi, A.; Du, G.B.; Deng, S.D. Tannin-based adhesive cross-linked by furfuryl alcohol-glyoxal and epoxy resins. Int. J. Adhes. Adhes. 2019, 94, 47–52. [Google Scholar] [CrossRef]
- Liu, B.W.; Zhou, Y.X.; Essawy, H.; Chen, Q.; Liang, J.K.; Zhou, X.J.; Zhang, J.; Du, G.B. Formaldehyde free tannin-based adhesive with epoxy as hardener for plywood. Maderas Cienc. Tecnol. 2022, 24, 33. [Google Scholar] [CrossRef]
- Lagel, M.C.; Zhang, J.; Pizzi, A. Cutting and grinding wheels for angle grinders with a bioresin matrix. Ind. Crops Prod. 2015, 67, 264–269. [Google Scholar] [CrossRef]
- Zhang, J.; Liu, B.W.; Zhou, Y.X.; Essawy, H.; Li, J.X.; Chen, Q.; Zhou, X.J.; Du, G.B. Preparation and performance of tannin-glyoxal-urea resin-bonded grinding wheel loaded with SiO2 reinforcing particles. Maderas Cienc. Tecnol. 2021, 23, 48. [Google Scholar] [CrossRef]
- Li, J.; Zhang, J.; Zhou, Y.; Zhou, Z.; Essawy, H.; Zhou, X.; Du, G.B. Preparation of an Abrasive Grinding Wheel Based on Tannin Resin Cross-Linked by Furfuryl Alcohol, Urea and Glyoxal. J. Renew. Mater. 2020, 8, 1019–1032. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, W.L.; Zhou, X.J.; Liang, J.K.; Du, G.B.; Wu, Z.G. Lignin-based adhesive crosslinked by furfuryl alcohol–glyoxal and epoxy resins. Nord. Pulp Pap. Res. J. 2019, 34, 228–238. [Google Scholar] [CrossRef]
- Liu, B.W.; Zhou, Y.X.; Essawy, H.; Feng, S.; Li, X.H.; Liao, J.J.; Zhou, X.J.; Zhang, J.; Xie, S.D. Formaldehyde Free Renewable Thermosetting Foam Based on Biomass Tannin with a Lignin Additive. J. Renew. Mater. 2022, 10, 3009–3024. [Google Scholar] [CrossRef]
- Sánchez-Martín, J.; Beltrán-Heredia, J.; Delgado-Regaña, A.; Rodríguez-González, M.A.; Rubio-Alonso, F. Optimization of Tannin Rigid Foam as Adsorbents in Wastewater Treatment. Ind. Crops Prod. 2013, 49, 507–514. [Google Scholar] [CrossRef]
- Wang, H.; Wang, S.; Liu, H.; Wang, S.; Wang, D.; Shen, M.; Xu, X.; Shang, S. Biobased phosphorus siloxane-containing polyurethane foam with flame-retardant and smoke-suppressant performances. ACS Sustain. Chem. Eng. 2021, 9, 8623–8634. [Google Scholar] [CrossRef]
- Sawangpet, K.; Walong, A.; Thongnuanchan, B.; Kaesaman, A.; Lopattananon, N. Foaming and Physical Properties, Flame Retardancy, and Combustibility of Polyethylene Octene Foams Modified by Natural Rubber and Expandable Graphite. J. Vinyl Addit. Technol. 2020, 26, 423–433. [Google Scholar] [CrossRef]
- Fu, Z.Q.; Wang, L.J.; Li, D.; Zhou, Y.G.; Adhikari, B. The effect of partial gelatinization of corn starch on its retrogradation. Carbohydr. Polym. 2013, 97, 512–517. [Google Scholar] [CrossRef]
- Sánchez, C. Lignocellulosic residues: Biodegradation and bioconversion by fungi. Biotechnol. Adv. 2009, 27, 185–194. [Google Scholar] [CrossRef]
- Chen, X.Y.; Li, J.X.; Essawy, H.; Pizzi, A.; Fredon, E.; Gerardin, C.; Du, G.B.; Zhou, X.J. Flame-retardant and thermally-insulating tannin and soybean protein isolate (SPI) based foams for potential applications in building materials. Constr. Build. Mater. 2021, 315, 125711. [Google Scholar] [CrossRef]
- Li, J.; Liao, J.; Essawy, H.; Zhang, J.; Li, T.; Wu, Z.; Du, G.; Zhou, X. Preparation and characterization of novel cellular/nonporous foam structures derived from tannin furanic resin. Ind. Crops. Prod. 2021, 162, 113264. [Google Scholar] [CrossRef]
- Muntoni, A.P.; Braunstein, A.; Pagnani, A.; Martino, D.D.; Martino, A.D. Relationship between fitness and heterogeneity in exponentially growing microbial populations. Biophys. J. 2021, 121, 1919–1930. [Google Scholar] [CrossRef]
- Abou-Zeid, D.M.; Müller, R.J.; Deckwer, W.D. Degradation of natural and synthetic polyesters under anaerobic conditions. J. Biotechnol. 2001, 86, 113–126. [Google Scholar] [CrossRef]
| TSGFA | TSFFA | SGFA | TSFA | |
|---|---|---|---|---|
| Starch/g | 31 | 31 | 44 | 31 |
| Tannin/g | 13 | 13 | -- | 13 |
| Furfuryl alcohol/mL | 35 | 35 | 35 | 35 |
| Glyoxal/mL | 20 | -- | 20 | -- |
| Formaldehyde/mL | -- | 20 | -- | -- |
| AC/g | 16 | 16 | 16 | 16 |
| pTSA/mL | 20 | 20 | 20 | 20 |
| Silicone oil/mL | 10 | 10 | 10 | 0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, X.; Liu, B.; Zheng, L.; Essawy, H.; Liu, Z.; Liu, C.; Zhou, X.; Zhang, J. Facile Synthesis of Formaldehyde-Free Bio-Based Thermoset Resins for Fabrication of Highly Efficient Foams. Polymers 2022, 14, 5140. https://doi.org/10.3390/polym14235140
Li X, Liu B, Zheng L, Essawy H, Liu Z, Liu C, Zhou X, Zhang J. Facile Synthesis of Formaldehyde-Free Bio-Based Thermoset Resins for Fabrication of Highly Efficient Foams. Polymers. 2022; 14(23):5140. https://doi.org/10.3390/polym14235140
Chicago/Turabian StyleLi, Xuehui, Bowen Liu, Lulu Zheng, Hisham Essawy, Zhiyan Liu, Can Liu, Xiaojian Zhou, and Jun Zhang. 2022. "Facile Synthesis of Formaldehyde-Free Bio-Based Thermoset Resins for Fabrication of Highly Efficient Foams" Polymers 14, no. 23: 5140. https://doi.org/10.3390/polym14235140
APA StyleLi, X., Liu, B., Zheng, L., Essawy, H., Liu, Z., Liu, C., Zhou, X., & Zhang, J. (2022). Facile Synthesis of Formaldehyde-Free Bio-Based Thermoset Resins for Fabrication of Highly Efficient Foams. Polymers, 14(23), 5140. https://doi.org/10.3390/polym14235140