Counter-Acting Candida albicans-Staphylococcus aureus Mixed Biofilm on Titanium Implants Using Microbial Biosurfactants
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Microbial Strains and Growth Conditions
2.3. Biosurfactant Production
2.4. Medical-Grade Titanium Discs Preparation
2.5. Surface Coating Process
2.6. Biofilm Growth on Titanium Surface
2.7. Quantitative Tests for Biofilm Formation
2.8. Scanning Electron Microscopy of Multi-Species Biofilms
2.9. Eukaryotic Cell Viability Tests
2.10. Data Analysis and Statistics
3. Results
3.1. Formation of C. Albicans and S. Aureus Single- and Dual-Species Biofilm on Titanium Discs
3.2. Anti-Biofilm Activity of R89BS-Coated Titanium Discs against Multi-Species Biofilm
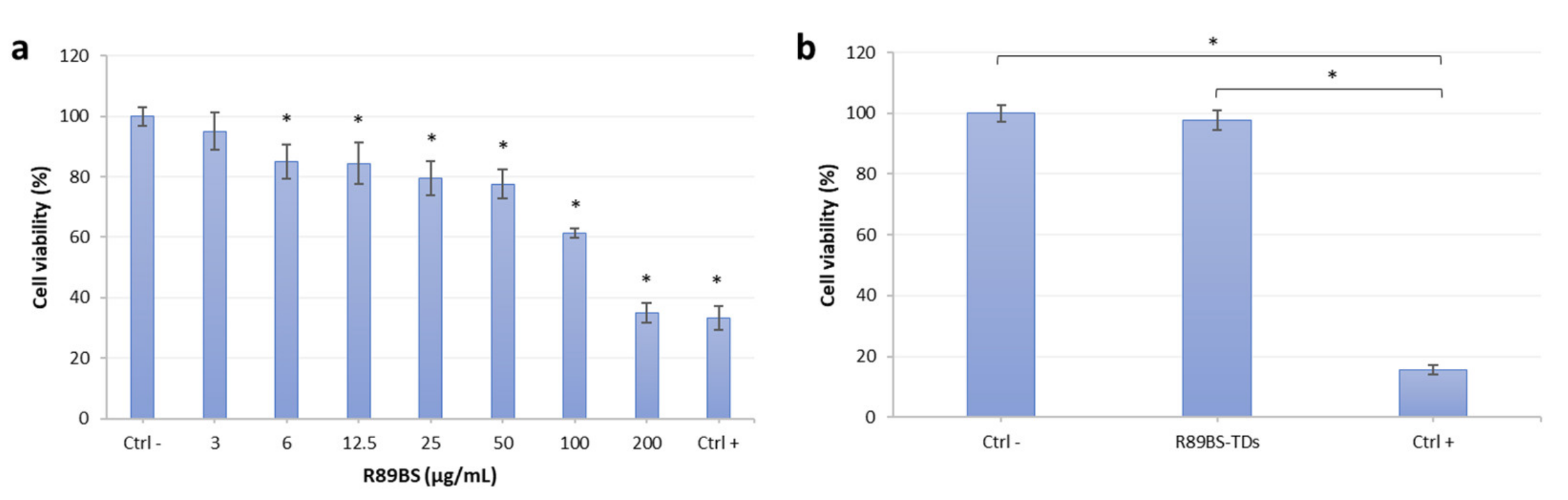
3.3. R89BS Effect on Eucaryotic Cell Viability
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dewhirst, F.E.; Chen, T.; Izard, J.; Paster, B.J.; Tanner, A.C.R.; Yu, W.-H.; Lakshmanan, A.; Wade, W.G. The Human Oral Microbiome. J. Bacteriol. 2010, 192, 5002–5017. [Google Scholar] [CrossRef] [Green Version]
- Khurshid, Z.; Naseem, M.; Sheikh, Z.; Najeeb, S.; Shahab, S.; Zafar, M.S. Oral antimicrobial peptides: Types and role in the oral cavity. Saudi Pharm. J. 2016, 24, 515–524. [Google Scholar] [CrossRef] [Green Version]
- Marsh, P.D.; Zaura, E. Dental biofilm: Ecological interactions in health and disease. J. Clin. Periodontol. 2017, 44, S12–S22. [Google Scholar] [CrossRef]
- Montelongo-Jauregui, D.; Lopez-Ribot, J.L. Candida Interactions with the Oral Bacterial Microbiota. J. Fungi 2018, 4, 122. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Negrini, T.D.C.; Koo, H.; Arthur, R.A. Candida–Bacterial Biofilms and Host–Microbe Interactions in Oral Diseases. Adv. Exp. Med. Biol. 2019, 1197, 119–141. [Google Scholar] [CrossRef] [PubMed]
- Smeets, R.; Henningsen, A.; Jung, O.; Heiland, M.; Hammächer, C.; Stein, J.M. Definition, etiology, prevention and treatment of peri-implantitis—A review. Head Face Med. 2014, 10, 34. [Google Scholar] [CrossRef] [Green Version]
- Lindhe, J.; Meyle, J.; Group D of the European Workshop on Periodontology. Peri-implant diseases: Consensus Report of the Sixth European Workshop on Periodontology. J. Clin. Periodontol. 2008, 35, 282–285. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pye, A.; Lockhart, D.; Dawson, M.; Murray, C.; Smith, A. A review of dental implants and infection. J. Hosp. Infect. 2009, 72, 104–110. [Google Scholar] [CrossRef]
- Ata-Ali, J.; Candel-Marti, M.; Flichy-Fernandez, A.; Penarrocha-Oltra, D.; Balaguer-Martinez, J.; Penarrocha, M. Peri-implantitis: Associated microbiota and treatment. Med. Oral Patol. Oral Cir. Bucal 2011, 16, e937–e943. [Google Scholar] [CrossRef] [Green Version]
- Ghensi, P.; Manghi, P.; Zolfo, M.; Armanini, F.; Pasolli, E.; Bolzan, M.; Bertelle, A.; Dell’Acqua, F.; Dellasega, E.; Waldner, R.; et al. Strong oral plaque microbiome signatures for dental implant diseases identified by strain-resolution metagenomics. NPJ Biofilms Microbiomes 2020, 6, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Najeeb, S.; Mali, M.; Syed, A.U.Y.; Zafar, M.S.; Khurshid, Z.; Alwadaani, A.; Matinlinna, J.P. Dental implants materials and surface treatments. In Advanced Dental Biomaterials; Elsevier BV: Amsterdam, The Netherlands, 2019; pp. 581–598. [Google Scholar]
- Berbel, L.O.; Banczek, E.D.P.; Karousis, I.K.; Kotsakis, G.A.; Costa, I. Determinants of corrosion resistance of Ti-6Al-4V alloy dental implants in an In Vitro model of peri-implant inflammation. PLoS ONE 2019, 14, e0210530. [Google Scholar] [CrossRef] [Green Version]
- Elias, C.N.; Lima, J.H.C.; Valiev, R.; Meyers, M. Biomedical applications of titanium and its alloys. JOM 2008, 60, 46–49. [Google Scholar] [CrossRef]
- Li, Y.; Yang, C.; Zhao, H.; Qu, S.; Li, X.; Li, Y. New Developments of Ti-Based Alloys for Biomedical Applications. Materials 2014, 7, 1709–1800. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Belibasakis, G.N.; Charalampakis, G.; Bostanci, N.; Stadlinger, B. Peri-Implant Infections of Oral Biofilm Etiology. Adv. Exp. Med. Biol. 2015, 830, 69–84. [Google Scholar] [CrossRef] [PubMed]
- Harriott, M.M.; Noverr, M.C. Importance of Candida–bacterial polymicrobial biofilms in disease. Trends Microbiol. 2011, 19, 557–563. [Google Scholar] [CrossRef] [Green Version]
- Elias, S.; Banin, E. Multi-species biofilms: Living with friendly neighbors. FEMS Microbiol. Rev. 2012, 36, 990–1004. [Google Scholar] [CrossRef]
- Burmølle, M.; Ren, D.; Bjarnsholt, T.; Sørensen, S. Interactions in multispecies biofilms: Do they actually matter? Trends Microbiol. 2014, 22, 84–91. [Google Scholar] [CrossRef]
- Shirtliff, M.E.; Peters, B.M.; Jabra-Rizk, M.A. Cross-kingdom interactions: Candida albicansand bacteria. FEMS Microbiol. Lett. 2009, 299, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Diaz, P.I.; Strausbaugh, L.D.; Edongari-Bagtzoglou, A. Fungal-bacterial interactions and their relevance to oral health: Linking the clinic and the bench. Front. Cell. Infect. Microbiol. 2014, 4, 101. [Google Scholar] [CrossRef] [Green Version]
- Rodrigues, M.E.; Gomes, F.; Rodrigues, C.F. Candida spp./Bacteria Mixed Biofilms. J. Fungi 2019, 6, 5. [Google Scholar] [CrossRef] [Green Version]
- Ghannoum, M.A.; Jurevic, R.J.; Mukherjee, P.K.; Cui, F.; Sikaroodi, M.; Naqvi, A.; Gillevet, P.M. Characterization of the Oral Fungal Microbiome (Mycobiome) in Healthy Individuals. PLoS Pathog. 2010, 6, e1000713. [Google Scholar] [CrossRef] [Green Version]
- Samaranayake, L.P.; Leung, W.K.; Jin, L. Oral mucosal fungal infections. Periodontology 2000 2009, 49, 39–59. [Google Scholar] [CrossRef] [PubMed]
- Mayer, F.L.; Wilson, D.; Hube, B. Candida albicans pathogenicity mechanisms. Virulence 2013, 4, 119–128. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bertolini, M.; Dongari-Bagtzoglou, A. The Dysbiosis and Inter-Kingdom Synergy Model in Oropharyngeal Candidiasis, a New Perspective in Pathogenesis. J. Fungi 2019, 5, 87. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mombelli, A.; Décaillet, F. The characteristics of biofilms in peri-implant disease. J. Clin. Periodontol. 2011, 38, 203–213. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Canullo, L.; Penarrocha-Oltra, D.; Covani, U.; Rossetti, P. Microbiologic and Clinical Findings of Implants in Healthy Condition and with Peri-Implantitis. Int. J. Oral Maxillofac. Implant. 2015, 30, 834–842. [Google Scholar] [CrossRef]
- Morales, D.; Hogan, D.A. Candida albicans Interactions with Bacteria in the Context of Human Health and Disease. PLoS Pathog. 2010, 6, e1000886. [Google Scholar] [CrossRef]
- Tamai, R.; Sugamata, M.; Kiyoura, Y. Candida albicans enhances invasion of human gingival epithelial cells and gingival fibroblasts by Porphyromonas gingivalis. Microb. Pathog. 2011, 51, 250–254. [Google Scholar] [CrossRef]
- Baena-Monroy, T.; Moreno-Maldonado, V.; Franco-Martínez, F.; Aldape-Barrios, B.; Quindós, G.; Sánchez-Vargas, L.O. Candida albicans, Staphylococcus aureus and Streptococcus mutans colonization in patients wearing dental prosthesis. Med. Oral Patol. Oral Cir. Bucal 2005, 10 (Suppl. S1), E27–E39. [Google Scholar]
- Pereira, C.A.; Toledo, B.C.; Santos, C.T.; Costa, A.C.B.P.; Back-Brito, G.N.; Kaminagakura, E.; Jorge, A.O.C. Opportunistic microorganisms in individuals with lesions of denture stomatitis. Diagn. Microbiol. Infect. Dis. 2013, 76, 419–424. [Google Scholar] [CrossRef] [Green Version]
- Otto, M. Staphylococcal Biofilms. Gram Posit. Pathog. 2019, 6, 699–711. [Google Scholar] [CrossRef]
- Fürst, M.M.; Salvi, G.E.; Lang, N.P.; Persson, G.R. Bacterial colonization immediately after installation on oral titanium implants. Clin. Oral Implant. Res. 2007, 18, 501–508. [Google Scholar] [CrossRef] [PubMed]
- Salvi, G.E.; Fürst, M.M.; Lang, N.P.; Persson, G.R. One-year bacterial colonization patterns of Staphylococcus aureus and other bacteria at implants and adjacent teeth. Clin. Oral Implant. Res. 2008, 19, 242–248. [Google Scholar] [CrossRef] [PubMed]
- Persson, G.R.; Renvert, S. Cluster of Bacteria Associated with Peri-Implantitis. Clin. Implant. Dent. Relat. Res. 2014, 16, 783–793. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, L.-F.; Watt, R.M.; Mattheos, N.; Si, M.-S.; Lai, H.-C.; Lang, N.P. Periodontal and peri-implant microbiota in patients with healthy and inflamed periodontal and peri-implant tissues. Clin. Oral Implant. Res. 2014, 27, 13–21. [Google Scholar] [CrossRef]
- Qin, S.; Xu, K.; Nie, B.; Ji, F.; Zhang, H. Approaches based on passive and active antibacterial coating on titanium to achieve antibacterial activity. J. Biomed. Mater. Res. Part A 2018, 106, 2531–2539. [Google Scholar] [CrossRef]
- Chouirfa, H.; Bouloussa, H.; Migonney, V.; Falentin-Daudré, C. Review of titanium surface modification techniques and coatings for antibacterial applications. Acta Biomater. 2019, 83, 37–54. [Google Scholar] [CrossRef]
- Ghensi, P.; Bettio, E.; Maniglio, D.; Bonomi, E.; Piccoli, F.; Gross, S.; Caciagli, P.; Segata, N.; Nollo, G.; Tessarolo, F. Dental Implants with Anti-Biofilm Properties: A Pilot Study for Developing a New Sericin-Based Coating. Materials 2019, 12, 2429. [Google Scholar] [CrossRef] [Green Version]
- De Avila, E.D.; Van Oirschot, B.A.; Van Den Beucken, J.J. Biomaterial-based possibilities for managing peri-implantitis. J. Periodontal Res. 2019, 55, 165–173. [Google Scholar] [CrossRef]
- Bumgardner, J.D.; Adatrow, P.; O Haggard, W.; Norowski, P.A. Emerging antibacterial biomaterial strategies for the prevention of peri-implant inflammatory diseases. Int. J. Oral Maxillofac. Implant. 2011, 26, 553–560. [Google Scholar]
- Santos, D.K.F.; Rufino, R.D.; Luna, J.M.; Santos, V.A.; Sarubbo, L.A. Biosurfactants: Multifunctional Biomolecules of the 21st Century. Int. J. Mol. Sci. 2016, 17, 401. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Skoula, M.; Harborne, J.B.; Harborne, J.B. The Taxonomy and Chemistry of Origanum. Available online: https://www.taylorfrancis.com/ (accessed on 9 May 2019).
- Naughton, P.; Marchant, R.; Naughton, V.; Banat, I. Microbial biosurfactants: Current trends and applications in agricultural and biomedical industries. J. Appl. Microbiol. 2019, 127, 12–28. [Google Scholar] [CrossRef] [Green Version]
- Ceresa, C.; Fracchia, L.; Fedeli, E.; Porta, C.; Banat, I. Recent Advances in Biomedical, Therapeutic and Pharmaceutical Applications of Microbial Surfactants. Pharmaceutics 2021, 13, 466. [Google Scholar] [CrossRef]
- Rodrigues, L.; Banat, I.; Teixeira, J.; Oliveira, R. Biosurfactants: Potential applications in medicine. J. Antimicrob. Chemother. 2006, 57, 609–618. [Google Scholar] [CrossRef]
- Banat, I.M.; Franzetti, A.; Gandolfi, I.; Bestetti, G.; Martinotti, M.G.; Fracchia, L.; Smyth, T.; Marchant, R. Microbial biosurfactants production, applications and future potential. Appl. Microbiol. Biotechnol. 2010, 87, 427–444. [Google Scholar] [CrossRef]
- Gudiña, E.; Rangarajan, V.; Sen, R.; Rodrigues, L.R. Potential therapeutic applications of biosurfactants. Trends Pharmacol. Sci. 2013, 34, 667–675. [Google Scholar] [CrossRef] [Green Version]
- Varvaresou, A.; Iakovou, K. Biosurfactants in cosmetics and biopharmaceuticals. Lett. Appl. Microbiol. 2015, 61, 214–223. [Google Scholar] [CrossRef]
- Chen, J.; Wu, Q.; Hua, Y.; Chen, J.; Zhang, H.; Wang, H. Potential applications of biosurfactant rhamnolipids in agriculture and biomedicine. Appl. Microbiol. Biotechnol. 2017, 101, 8309–8319. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R.; Das, A.J. Application of Rhamnolipids in Medical Sciences. In Rhamnolipid Biosurfactant; Springer Science and Business Media LLC: Berlin/Heidelberg, Germany, 2018; pp. 79–87. [Google Scholar]
- Elshikh, M.; Marchant, R.; Banat, I.M. Biosurfactants: Promising bioactive molecules for oral-related health applications. FEMS Microbiol. Lett. 2016, 363, fnw213. [Google Scholar] [CrossRef] [Green Version]
- Dusane, D.H.; Dam, S.; Nancharaiah, Y.V.; Kumar, A.R.; Venugopalan, V.P.; Zinjarde, S.S. Disruption of Yarrowia lipolytica biofilms by rhamnolipid biosurfactant. Aquat. Biosyst. 2012, 8, 17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Singh, N.; Pemmaraju, S.C.; Pruthi, P.A.; Cameotra, S.S.; Pruthi, V. Candida Biofilm Disrupting Ability of Di-rhamnolipid (RL-2) Produced from Pseudomonas aeruginosa DSVP20. Appl. Biochem. Biotechnol. 2013, 169, 2374–2391. [Google Scholar] [CrossRef]
- Edas, P.; Eyang, X.-P.; Ma, L.Z. Analysis of biosurfactants from industrially viable Pseudomonas strain isolated from crude oil suggests how rhamnolipids congeners affect emulsification property and antimicrobial activity. Front. Microbiol. 2014, 5, 696. [Google Scholar] [CrossRef]
- Hajfarajollah, H.; Mehvari, S.; Habibian, M.; Mokhtarani, B.; Noghabi, K.A. Rhamnolipid biosurfactant adsorption on a plasma-treated polypropylene surface to induce antimicrobial and antiadhesive properties. RSC Adv. 2015, 5, 33089–33097. [Google Scholar] [CrossRef]
- De Araujo, L.V.; Guimarães, C.R.; Marquita, R.L.D.S.; Santiago, V.M.; De Souza, M.P.; Nitschke, M.; Freire, D.M.G. Rhamnolipid and surfactin: Anti-adhesion/antibiofilm and antimicrobial effects. Food Control. 2016, 63, 171–178. [Google Scholar] [CrossRef]
- Elshikh, M.; Funston, S.; Chebbi, A.; Ahmed, S.; Marchant, R.; Banat, I.M. Rhamnolipids from non-pathogenic Burkholderia thailandensis E264: Physicochemical characterization, antimicrobial and antibiofilm efficacy against oral hygiene related pathogens. New Biotechnol. 2017, 36, 26–36. [Google Scholar] [CrossRef]
- Ceresa, C.; Tessarolo, F.; Maniglio, D.; Tambone, E.; Carmagnola, I.; Fedeli, E.; Caola, I.; Nollo, G.; Chiono, V.; Allegrone, G.; et al. Medical-Grade Silicone Coated with Rhamnolipid R89 Is Effective against Staphylococcus spp. Biofilms. Molecules 2019, 24, 3843. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tambone, E.; Bonomi, E.; Ghensi, P.; Maniglio, D.; Ceresa, C.; Agostinacchio, F.; Caciagli, P.; Nollo, G.; Piccoli, F.; Caola, I.; et al. Rhamnolipid coating reduces microbial biofilm formation on titanium implants: An in vitro study. BMC Oral Health 2021, 21, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Foster, K.R.; Bell, T. Competition, Not Cooperation, Dominates Interactions among Culturable Microbial Species. Curr. Biol. 2012, 22, 1845–1850. [Google Scholar] [CrossRef] [Green Version]
- Ceresa, C.; Tessarolo, F.; Caola, I.; Nollo, G.; Cavallo, M.; Rinaldi, M.; Fracchia, L. Inhibition of Candida albicans adhesion on medical-grade silicone by a Lactobacillus -derived biosurfactant. J. Appl. Microbiol. 2015, 118, 1116–1125. [Google Scholar] [CrossRef] [PubMed]
- Little, B.; Wagner, P.; Ray, R.; Pope, R.; Scheetz, R. Biofilms: An ESEM evaluation of artifacts introduced during SEM preparation. J. Ind. Microbiol. Biotechnol. 1991, 8, 213–221. [Google Scholar] [CrossRef]
- Hrubanova, K.; Krzyzanek, V.; Nebesarova, J.; Ruzicka, F.; Pilat, Z.; Samek, O. Monitoring Candida parapsilosis and Staphylococcus epidermidis Biofilms by a Combination of Scanning Electron Microscopy and Raman Spectroscopy. Sensors 2018, 18, 4089. [Google Scholar] [CrossRef] [Green Version]
- Tessarolo, F.; Caola, I.; Fedel, M.; Stacchiotti, A.; Caciagli, P.; Guarrera, G.; Motta, A.; Nollo, G. Different experimental protocols for decontamination affect the cleaning of medical devices. A preliminary electron microscopy analysis. J. Hosp. Infect. 2007, 65, 326–333. [Google Scholar] [CrossRef] [PubMed]
- Signoretto, C.; Marchi, A.; Bertoncelli, A.; Burlacchini, G.; Milli, A.; Tessarolo, F.; Caola, I.; Papetti, A.; Pruzzo, C.; Zaura, E.; et al. Effects of mushroom and chicory extracts on the shape, physiology and proteome of the cariogenic bacterium Streptococcus mutans. BMC Complement. Altern. Med. 2013, 13, 117. [Google Scholar] [CrossRef] [Green Version]
- Signoretto, C.; Marchi, A.; Bertoncelli, A.; Burlacchini, G.; Tessarolo, F.; Caola, I.; Pezzati, E.; Zaura, E.; Papetti, A.; Lingström, P.; et al. Effects of Mushroom and Chicory Extracts on the Physiology and Shape of Prevotella intermedia, a Periodontopathogenic Bacterium. J. Biomed. Biotechnol. 2011, 2011, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Tessarolo, F.; Piccoli, F.; Caola, I.; Tomasi, C.; Bressan, E.; Nollo, G.; Caciagli, P. Optimizing Protocols for Preparation and Imaging of Natural Teeth, Dental Implant and Peri-Implant Tissues in High Vacuum, Low Vacuum, and Environmental SEM. J. Appl. Biomater. Biomech. (JABB) 2009, 7, 73. [Google Scholar]
- Bressan, E.; Tessarolo, F.; Sbricoli, L.; Caola, I.; Nollo, G.; Di Fiore, A. Effect of Chlorhexidine in Preventing Plaque Biofilm on Healing Abutment. Implant. Dent. 2014, 23, 64–68. [Google Scholar] [CrossRef]
- Bosetti, M.; Fusaro, L.; Nicoli, E.; Borrone, A.; Aprile, S.; Cannas, M. Poly-L-lactide acid-modified scaffolds for osteoinduction and osteoconduction. J. Biomed. Mater. Res. Part A 2013, 102, 3531–3539. [Google Scholar] [CrossRef]
- Bosetti, M.; Santin, M.; Lloyd, A.W.; Denyer, S.P.; Sabbatini, M.; Cannas, M. Cell behaviour on phospholipids-coated surfaces. J. Mater. Sci. Mater. Electron. 2007, 18, 611–617. [Google Scholar] [CrossRef] [PubMed]
- Peters, B.M.; Jabra-Rizk, M.A.; O’May, G.A.; Costerton, J.W.; Shirtliff, M.E. Polymicrobial Interactions: Impact on Pathogenesis and Human Disease. Clin. Microbiol. Rev. 2012, 25, 193–213. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gomes, M.Z.D.V.; Nitschke, M. Evaluation of rhamnolipid and surfactin to reduce the adhesion and remove biofilms of individual and mixed cultures of food pathogenic bacteria. Food Control. 2012, 25, 441–447. [Google Scholar] [CrossRef]
- De Rienzo, M.A.D.; Banat, I.; Dolman, B.; Winterburn, J.; Martin, P. Sophorolipid biosurfactants: Possible uses as antibacterial and antibiofilm agent. New Biotechnol. 2015, 32, 720–726. [Google Scholar] [CrossRef]
- Ceresa, C.; Rinaldi, M.; Tessarolo, F.; Maniglio, D.; Fedeli, E.; Tambone, E.; Caciagli, P.; Banat, I.M.; De Rienzo, M.A.D.; Fracchia, L. Inhibitory Effects of Lipopeptides and Glycolipids on C. albicans–Staphylococcus spp. Dual-Species Biofilms. Front. Microbiol. 2021, 11. [Google Scholar] [CrossRef]
- Cuesta, A.I.; Jewtuchowicz, V.; I Brusca, M.; Nastri, M.L.; Rosa, A.C. Prevalence of Staphylococcus spp. and Candida spp. in the oral cavity and periodontal pockets of periodontal disease patients. Acta Odontol. Latinoam. 2010, 23, 20–26. [Google Scholar]
- Listgarten, M.A.; Lai, C.-H. Comparative Microbiological Characteristics of Failing Implants and Periodontally Diseased Teeth. J. Periodontol. 1999, 70, 431–437. [Google Scholar] [CrossRef]
- Albertini, M.; López-Cerero, L.; O’Sullivan, M.G.; Chereguini, C.F.; Ballesta, S.; Ríos, V.; Herrero-Climent, M.; Bullón, P. Assessment of periodontal and opportunistic flora in patients with peri-implantitis. Clin. Oral Implant. Res. 2015, 26, 937–941. [Google Scholar] [CrossRef]
- Carolus, H.; Van Dyck, K.; Van Dijck, P. Candida albicans and Staphylococcus Species: A Threatening Twosome. Front. Microbiol. 2019, 10, 2162. [Google Scholar] [CrossRef] [PubMed]
- Russel, J.; Røder, H.L.; Madsen, J.S.; Burmølle, M.; Sørensen, S.J. Antagonism correlates with metabolic similarity in diverse bacteria. Proc. Natl. Acad. Sci. USA 2017, 114, 10684–10688. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rendueles, O.; Ghigo, J.-M. Multi-species biofilms: How to avoid unfriendly neighbors. FEMS Microbiol. Rev. 2012, 36, 972–989. [Google Scholar] [CrossRef]
- Jabra-Rizk, M.A.; Meiller, T.F.; James, C.; Shirtliff, M.E. Effect of Farnesol on Staphylococcus aureus Biofilm Formation and Antimicrobial Susceptibility. Antimicrob. Agents Chemother. 2006, 50, 1463–1469. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zago, C.E.; Silva, S.; Sanitá, P.V.; Barbugli, P.; Dias, C.M.I.; Lordello, V.B.; Vergani, C.E. Dynamics of Biofilm Formation and the Interaction between Candida albicans and Methicillin-Susceptible (MSSA) and -Resistant Staphylococcus aureus (MRSA). PLoS ONE 2015, 10, e0123206. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kean, R.; Rajendran, R.; Haggarty, J.; Townsend, E.; Short, B.; Burgess, K.; Lang, S.; Millington, O.; Mackay, W.; Williams, C.; et al. Candida albicans Mycofilms Support Staphylococcus aureus Colonization and Enhances Miconazole Resistance in Dual-Species Interactions. Front. Microbiol. 2017, 8, 258. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gabrilska, R.A.; Rumbaugh, K.P. Biofilm models of polymicrobial infection. Futur. Microbiol. 2015, 10, 1997–2015. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Neu, T.R. Significance of Bacterial Surface-Active Compounds in Interaction of Bacteria with Interfaces. Microbiol. Rev. 1996, 60, 151. [Google Scholar] [CrossRef]
- Walencka, E.; Różalska, S.; Sadowska, B. The influence of Lactobacillus acidophilus-derived surfactants on staphylococcal adhesion and biofilm formation. Folia Microbiol. 2008, 53, 61–66. [Google Scholar] [CrossRef] [PubMed]
- Monteiro, A.S.; Miranda, T.T.; Lula, I.; Denadai, Â.M.; Sinisterra, R.; Santoro, M.M.; Santos, V.L. Inhibition of Candida albicans CC biofilms formation in polystyrene plate surfaces by biosurfactant produced by Trichosporon montevideense CLOA72. Colloids Surf. B Biointerfaces 2011, 84, 467–476. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, J.D.F.; Vieira, E.A.; Nitschke, M. The antibacterial activity of rhamnolipid biosurfactant is pH dependent. Food Res. Int. 2019, 116, 737–744. [Google Scholar] [CrossRef]
- Harriott, M.M.; Noverr, M.C. Candida albicans and Staphylococcus aureus Form Polymicrobial Biofilms: Effects on Antimicrobial Resistance. Antimicrob. Agents Chemother. 2009, 53, 3914–3922. [Google Scholar] [CrossRef] [Green Version]
- Peters, B.M.; Ovchinnikova, E.S.; Krom, B.; Schlecht, L.M.; Zhou, H.; Hoyer, L.; Busscher, H.J.; Van Der Mei, H.C.; Jabra-Rizk, M.A.; Shirtliff, M.E. Staphylococcus aureus adherence to Candida albicans hyphae is mediated by the hyphal adhesin Als3p. Microbiology 2012, 158, 2975–2986. [Google Scholar] [CrossRef] [Green Version]
- Dalle, F.; Wãchtler, B.; L’Ollivier, C.; Holland, G.; Bannert, N.; Wilson, D.; Labruère, C.; Bonnin, A.; Hube, B. Cellular interactions of Candida albicans with human oral epithelial cells and enterocytes. Cell. Microbiol. 2010, 12, 248–271. [Google Scholar] [CrossRef]
- Schlecht, L.M.; Peters, B.M.; Krom, B.; Freiberg, J.A.; Hänsch, G.M.; Filler, S.G.; Jabra-Rizk, M.A.; Shirtliff, M.E. Systemic Staphylococcus aureus infection mediated by Candida albicans hyphal invasion of mucosal tissue. Microbiology 2015, 161, 168–181. [Google Scholar] [CrossRef] [Green Version]
- Socransky, S.S.; Haffajee, A.D.; Cugini, M.A.; Smith, C.; Kent, R.L., Jr. Microbial complexes in subgingival plaque. J. Clin. Periodontol. 1998, 25, 134–144. [Google Scholar] [CrossRef] [PubMed]
- Teles, F.R.F. The Microbiome of Peri-Implantitis: Is It Unique? Compend. Contin. Educ. Dent. 2017, 38, 22–25. [Google Scholar] [PubMed]
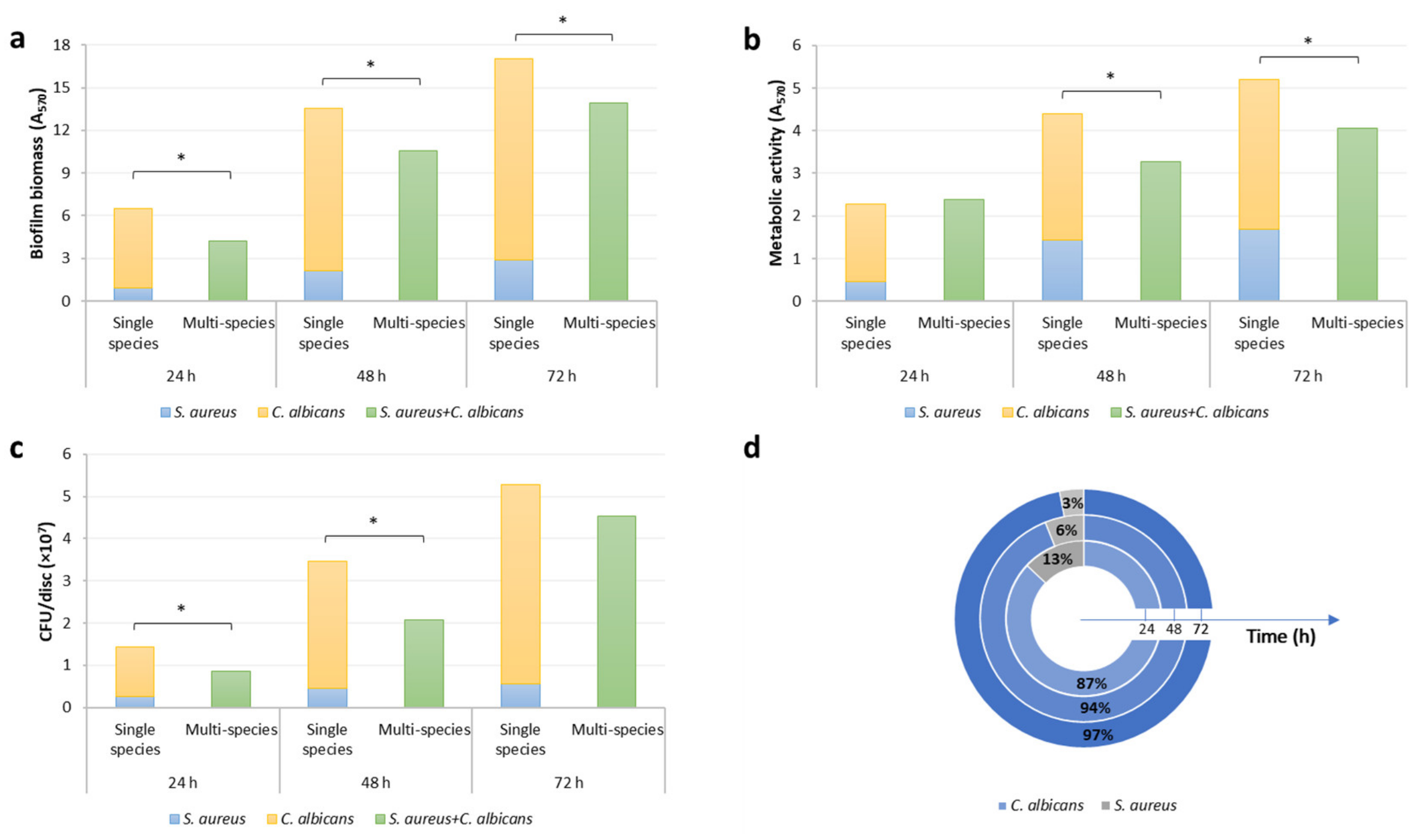
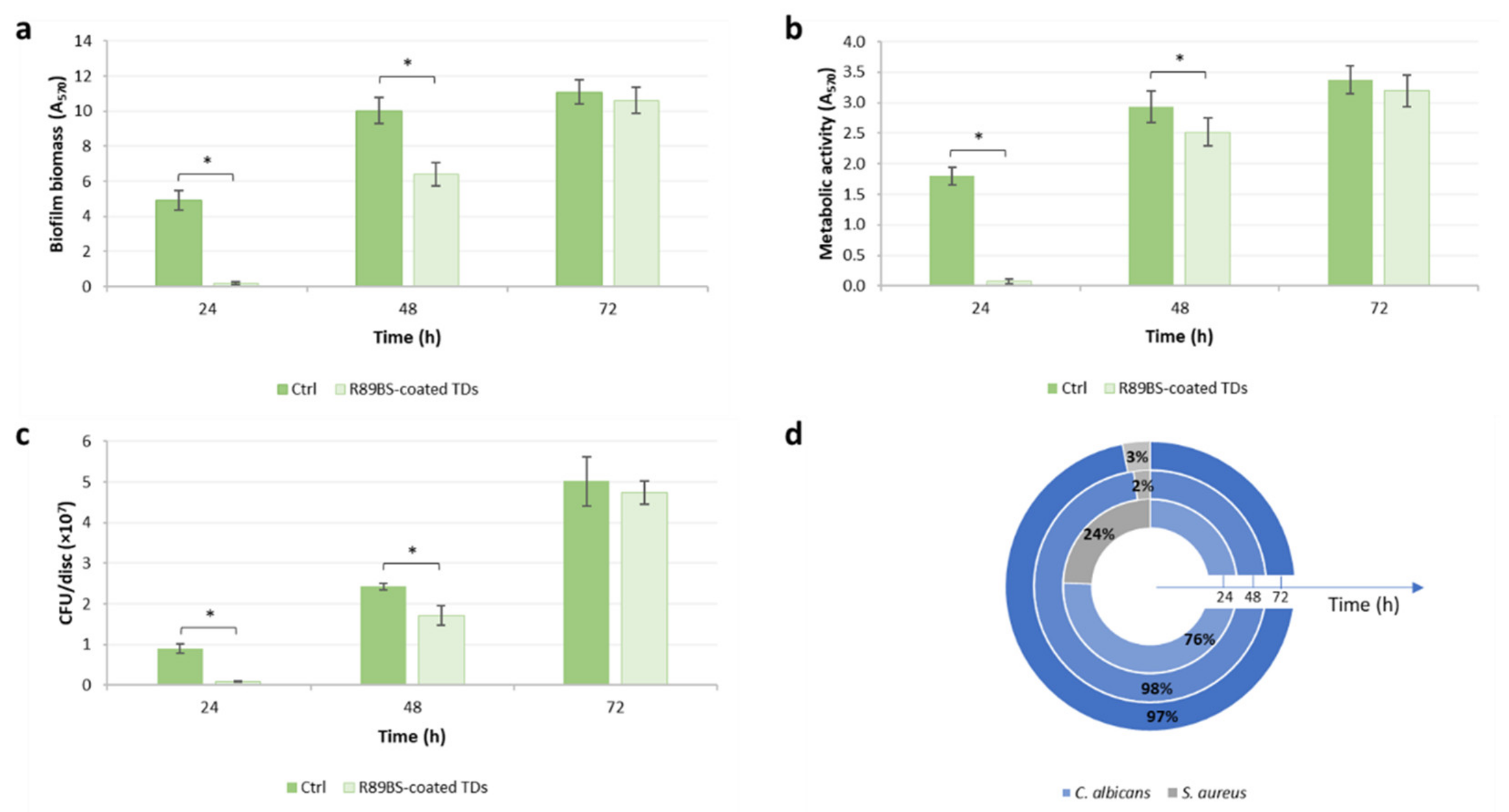

| Incubation Time (h) | Inhibition (%) | ||
|---|---|---|---|
| Biomass | Metabolic Activity | Cell Viability | |
| 24 | 96.3 (1.2) * | 95.9 (1.8) * | 90.6 (1.1) * |
| 48 | 36.1 (6.7) * | 14.0 (7.7) * | 29.1 (9.8) * |
| 72 | 4.4 (6.7) | 5.3 (7.8) | 5.4 (4.5) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tambone, E.; Marchetti, A.; Ceresa, C.; Piccoli, F.; Anesi, A.; Nollo, G.; Caola, I.; Bosetti, M.; Fracchia, L.; Ghensi, P.; et al. Counter-Acting Candida albicans-Staphylococcus aureus Mixed Biofilm on Titanium Implants Using Microbial Biosurfactants. Polymers 2021, 13, 2420. https://doi.org/10.3390/polym13152420
Tambone E, Marchetti A, Ceresa C, Piccoli F, Anesi A, Nollo G, Caola I, Bosetti M, Fracchia L, Ghensi P, et al. Counter-Acting Candida albicans-Staphylococcus aureus Mixed Biofilm on Titanium Implants Using Microbial Biosurfactants. Polymers. 2021; 13(15):2420. https://doi.org/10.3390/polym13152420
Chicago/Turabian StyleTambone, Erica, Alice Marchetti, Chiara Ceresa, Federico Piccoli, Adriano Anesi, Giandomenico Nollo, Iole Caola, Michela Bosetti, Letizia Fracchia, Paolo Ghensi, and et al. 2021. "Counter-Acting Candida albicans-Staphylococcus aureus Mixed Biofilm on Titanium Implants Using Microbial Biosurfactants" Polymers 13, no. 15: 2420. https://doi.org/10.3390/polym13152420






