Abstract
Cyclic voltammograms and optical absorption spectra of PEDOT/WO3 composite films were recorded in order to identify possible interactions and modes of improved performance of the composite as compared to the single materials. Changes in the shape of redox peaks related to the W(VI)/W(V) couple in the CVs of WO3 and the composite PEDOT/WO3 films indicate electrostatic interactions between the negatively charged tungsten oxide species and the positively charged conducting polymer. Smaller peak separation suggests a more reversible redox process due to the presence of the conducting polymer matrix, accelerating electron transfer between tungsten ions. Electronic absorption spectra of the materials were analyzed with respect to changes of the shapes of the spectra and characteristic band positions. There are no noticeable changes in the position of the electronic absorption bands of the main chromophores in the electronic spectra of the composite film. Obviously, the interactions accelerating the redox performance do not show up in the optical spectra. This suggests that the existing electrostatic interactions in the composite do not significantly change the opto-electronic properties of components of the composite but resulted in the redistribution of fractions of polaron and bipolaron forms in the polymer.
1. Introduction
In recent years growing attention has been paid to new hybrid materials combining organic and inorganic compounds. In particular, conducting polymers as a conductive matrix in combination with transition metal oxides have been widely investigated as novel functional materials for different applications such as electrochromic materials [1], energy storage materials [2,3,4] and photocatalysts [5].
Among the inorganic materials, tungsten oxide has been frequently studied as an electrochromic negative electrode [6,7,8] and energy storage material [8,9,10] with good chemical stability and strong adherence to the substrate.
Tungsten oxide WO3 can be obtained in different crystallographic structures, that are composed of three-dimensional networks of WO6 octahedra in corner-sharing or edge-sharing arrangements. This provides abundant channels or chains of interstitial sites for insertion of small ions [11], which are associated with the origin of electrochromism.
The kinetics of redox processes and the degree of reduction of tungsten oxides strongly depend on their structure and, in turn, on the synthesis procedures. In many investigated cases, the kinetics of the redox response of WO3 remain relatively sluggish due to slow diffusion in compact structures of tungsten oxides. Therefore, intrinsically conducting polymers have been employed recently as a matrix for incorporated WO3 particles to facilitate charge transport to the oxide.
In particular, the incorporation of WO3 nanostructures into polyaniline (PANI) [12,13,14,15,16,17], polypyrrole (PPY) [18] and poly(3,4-ethylenedioxythiophene) (PEDOT) [3,19,20,21] yielding composites has been reported; for an overview see [4]. The main achievements noticed with such combination is the acceleration of redox processes in the WO3 fraction due to faster charge transport across the polymer layer to the metal oxide and higher surface-to-volume ratio in the obtained composite structures further facilitating ion transport. The synthesis of nanostructured WO3/conducting polymer composites leads to synergistic enhancement of their electrochromic and pseudo-capacitive properties, combining the benefits from both components. For example, both components of PEDOT/WO3 composite (PEDOT and WO3) undergo blue coloration during the reduction process, thus ensuring the complementarity of the electrochromic responses and higher coloration efficiency.
An important question hardly addressed in most studies of composite materials aims at conceivable interactions between the constituents of the composite materials [4]: Are there any? Are they pronounced? What are their effects? It may also be of interest whether they will lead to new properties of the material. On the other hand, it is also possible that the composite simply inherits the properties of the original components (additive behavior), suggesting that the electronic structure and properties of the components is maintained in the composite.
In this report, we focus on a comparative study of cyclic voltammetry and electronic absorption spectra of single component films (PEDOT, WO3) and their composite material (PEDOT/WO3) at different electrode potentials in non-aqueous electrolyte solutions, in order to identify the contributions of redox processes and optoelectronic absorption and to conclude on the possible interaction between the components conceivably affecting their performance as supercapacitor electrode material. To our knowledge, such combined electrochemical and spectroelectrochemical investigations of PEDOT/WO3 composite films prepared by electrochemical deposition have not been reported.
However, an in-depth investigation of the electrochromic properties of film-coated electrodes and a full characterization of their electrooptical response was beyond the scope of our study. Instead, we used a spectroelectrochemical approach as a tool of independent evaluation of possible interactions between components of the formed composite and their impact on optical properties. Nevertheless, such full characterization, including further experiments and taking into account electrolyte solution effects as addressed elsewhere ) [22], are planned to be performed in the future, aiming at proper optimization of the composite.
2. Materials and Methods
3,4-ethylenedioxythiophene (EDOT, 98%) and lithium perchlorate (LiClO4, 99.9%) were purchased from Sigma-Aldrich (Darmstadt, Germany). Acetonitrile (HPLC grade, water content below 0.05%) was obtained from abcr GmbH (Karslruhe, Germany), propylenecarbonate (PC, 99%) from Alfa Aesar (Kandel, Germany). Sodium tungstate dihydrate (Na2WO4∙2H2O) and 18 M solution of H2SO4 were from Neva Reactive Co., Russia. All aqueous solutions were prepared using deionized water of resistivity > 18 MΩ·cm−1 (Merck Millipore, Darmstadt, Germany).
The electropolymerization and all electrochemical studies were conducted in a three-electrode cell at room temperature (20 ± 2 °C) using a PGSTAT302N potentiostat/galvanostat (Metrohm Autolab, Utrecht, The Netherlands) controlled by GPES software.
To produce optically transparent working electrodes (FTO-electrodes) SnO2/F-coated glass slides from Sigma-Aldrich (transmittance 82–84.5% (in the visible range), surface resistivity ~ 13 Ω cm−2) were cut to pieces with sizes of about 50 mm × 8 mm. Before film deposition, the FTO-electrodes were cleaned ultrasonically in ethanol for 20 min, washed with deionized water, and dried.
PEDOT was electropolymerized galvanostatically on FTO-electrodes at j = 1 mA·cm−2 from solutions of 0.05 M 3,4-ethylenedioxythiophene (EDOT) and 0.5 M of lithium perchlorate (LiClO4) in acetonitrile. To obtain relatively transparent films, the time of electrodeposition was limited to 100 s. Deposited amounts of both constituents were the same for the single constituent and the composite electrodes, in order to enable quantitative evaluation.
Tungsten oxide was electrodeposited either on pristine FTO-electrodes or on the pre-formed FTO/PEDOT film electrodes from a metastable acidic solution of isopolytungstate containing 0.005 M Na2WO4 and 0.5 M H2SO4 following a procedure described in [20,21], i.e., under potentiodynamic conditions within the potential range −0.3 to 0.7 V at a scan rate of 50 mV·s−1; the number of cycles was usually 60.
After film deposition, the WO3 and PEDOT/WO3 electrodes were carefully rinsed with PC, dried in air first at ambient temperature, then at an increased temperature (80 °C). The redox cycling of electrodes was performed in an aprotic solution 0.5 M LiClO4/PC in the potential range −0.6 to +0.8 V (vs. Ag/AgCl electrode) at scan rates 10–100 mV/s.
The spectroelectrochemical studies were performed in situ using a Shimadzu UV-1700 spectrophotometer (Japan) coupled to the PGSTAT302N potentiostat/galvanostat. The electronic absorption spectra were acquired at selected constant potentials of film electrodes in 0.5 M LiClO4/PC supporting electrolyte in the UV-vis-NIR range 300–1100 nm.
The spectroelectrochemical studies were performed using a custom-made three-electrode cell, comprising a 10 mm quartz cuvette (Hellma Müllheim, Germany) with a PTFE insert, supporting the vertically inserted FTO working electrode, a Pt foil counter electrode and an Ag/Ag+ reference electrode, the latter two arranged so that they did not block the light beam. For more convenient data presentation, the potential values of the Ag/Ag+ non-aqueous reference electrode were recalculated to the aqueous Ag/AgCl electrode.
The morphology of prepared composites was characterized by scanning electron microscopy (SEM, SUPRA 40VP Carl Zeiss, Oberkochen, Germany). EDX analysis was performed with an energy-dispersive X-ray spectrometer X-act (Oxford Instruments, Oxon, UK).
3. Results
3.1. Morphology
The morphology of tungsten oxide and the PEDOT/WO3 composite deposited on FTO and the localization of tungsten oxide deposits was studied with scanning electron microscopy and local EDX analysis.
With pristine PEDOT films, a rather typical globular structure of the polymer globules of about 50–200 nm sizes were observed (Figure 1a). During the electrodeposition of tungsten oxide, these globular structures were covered with a thin deposit of nanometer sized lamellar WO3 formations (100–500 nm), resulting in cauliflower-like agglomerates up to 2–3 μm size (Figure 1b).
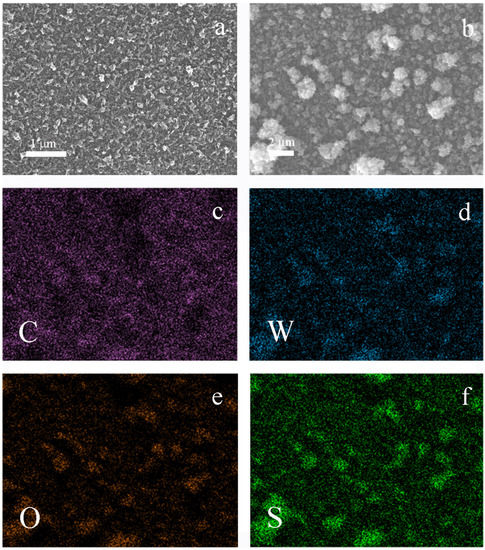
Figure 1.
(a) SEM of PEDOT film; (b) SEM of PEDOT/WO3 composite film; (c–f) EDX element mapping of PEDOT/WO3 composite film as shown in Figure 1b.
EDX element distribution maps (C, W, O, S) across the surface of the scanned areas of the PEDOT/WO3 composite electrode were obtained (Figure 1c–f). The coincidence of the relative distribution density of sulfur from PEDOT and tungsten indicates the predominant deposition of tungsten oxide on dense globular supramolecular structures of the polymer.
3.2. Cyclic Voltammetry
Typical CV responses of WO3, PEDOT and composite PEDOT/WO3 film electrodes in LiClO4/PC aprotic electrolyte solution are shown in Figure 2. The stability of PEDOT, WO3 and composite PEDOT/WO3 films was tested by long-term voltammetric cycling of film electrodes in the potential range from −0.1 to + 0.9 V. The peak currents were rather stable, showing a small gradual decrease with an increasing number of cycles. For example, continuous potential cycling of PEDOT and PEDOT/WO3 electrodes over 30 cycles lead to gradual decrease in the currents related to tungsten oxide less than 10%, whereas for WO3 electrodes, the stability was much better (decrease within 1–2%). The obtained result suggests a moderate stability of the composite electrodes.
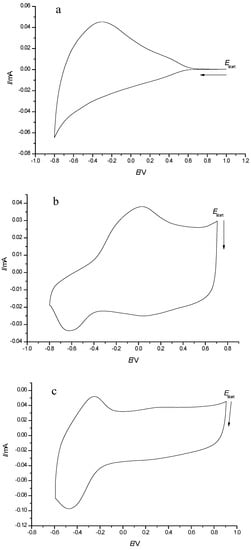
Figure 2.
Cyclic voltammograms of: (a) FTO/WO3, (b) FTO/PEDOT, and (c) FTO/PEDOT/WO3 composite film electrodes in 0.5 M LiClO4/PC, v = 50 mV·s−1.
By comparison of CV shapes, it can be confirmed that both components, WO3 and PEDOT, are present in the PEDOT/WO3 composite film; however, in the composite film, their particular responses have changed already suggesting specific interactions.
The CV of the tungsten oxide film (Figure 2a) shows a rather broad redox response without a distinct cathodic peak and with a pronounced anodic peak at about −0.3 V. This type of CV response is characteristic for tungsten oxide deposited by different methods on FTO- or ITO-substrates. It is related to the reversible redox process W(VI)/W(V) in tungsten oxide, accompanied by the intercalation/deintercalation of Li+ ions into/out of the WO3 films with the formation of nonstoichiometric intercalated tungsten oxides according to:
WO3 + xLi+ + xe− = Lix [W(6+)]1−x[W(5+)]xO3
Figure 2b shows the characteristic CV of PEDOT in an organic electrolyte solution, with a broad anodic peak and two cathodic peaks.
The cyclic voltammogram of the PEDOT/WO3 composite film (Figure 2c) shows a pair of peaks (around E0 = −0.3 V) due to WO3 redox processes and a pseudo-capacitive response without any distinct peaks in the more positive potential range −0.1 to + 0.9 V, characteristic for PEDOT charging/discharging processes. As seen in Figure 2c, more pronounced and symmetric redox peaks of WO3 with smaller peak-to-peak separation are observed in the case of the PEDOT/WO3 composite film. The pronounced in the shape of CV response related to the W(VI)/W(V) redox couple indicates interactions between the constituents of the composite. The improved reversibility of the redox process of WO3 is due to the presence of the conducting polymer matrix, which accelerates electron transfer between tungsten ions. Apparently, electrostatic interactions between the negatively charged tungsten oxide species and the positively charged oxidized fragments of conducting polymer take place.
Thus, it can be stated that currents are not simply additive in the overall electrochemical response of the composite film.
3.3. Spectroelectrochemistry
The spectra of PEDOT/WO3 composite films, as well as their components (PEDOT and tungsten oxide films) were recorded in solutions of 0.5 M LiClO4 in propylene carbonate in the range 300–1100 nm at fixed electrode potentials in the range from −0.8 V to +1.0 V. All spectra were collected at stationary conditions (after the registered current remained almost unchanged).
Figure 3 shows a series of steady-state electronic absorption spectra of the PEDOT film. The arrows indicate the direction of the optical density variations in the case of gradual film oxidation (shift to positive potentials).
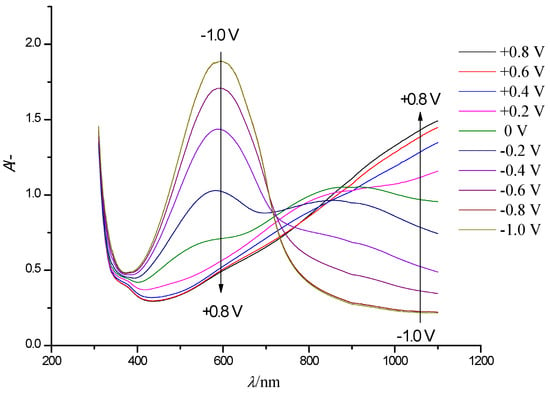
Figure 3.
Electronic absorption spectra of a PEDOT film on an FTO electrode with a controlled change in the electrode potential in the range of −1.0 V to + 0.8 V (step 0.2 V, arrows show the direction of the absorption band change with increasing potential) in solution of 0.5 M LiClO4/PC.
In the neutral state of PEDOT (E = −0.8 V), the main absorption band at 600 nm, related to π-π* interband electronic transitions in neutral film fragments [23,24] is dominant. During the oxidation of PEDOT, the absorbance at this wavelength decreases, and at potentials higher than +0.4 V it completely disappears. Instead, a new band with a maximum at 840 nm appears, which is assigned to a similar electronic transition in the first oxidation state of the polymer units–polaron species. When approaching the highest potential, the absorption growth at 840 nm slows down, and an edge of a new absorption band appears in the near IR NIR spectral range with a maximum located beyond the registered wavelength range (approx. λmax > 1100 nm). It is noteworthy that this type of spectral evolution is typical for several thiophene derivatives at various degrees of oxidation [25,26,27,28,29], this suggesting that similar processes occur in the polymers even with chemically different substituents. Two isosbestic points are observed in the set of spectra: the first isosbestic point near λ = 720 nm occurs in the potential range from −0.8 to 0.2 V, while the further positive potential shift (E > 0.3 V) leads to the appearance of the second, poorly expressed isosbestic point (isosbestic range) around λmax = 860 nm (Figure 3). The presence of isosbestic points is typical for mixtures of absorbing components/species with a constant overall concentration.
The presented spectroelectrochemical data suggest the presence of three types of absorbing species in the PEDOT film. This is in agreement with the well-known polaron-bipolaron model of redox processes in ICPs [30,31,32]. The oxidation of neutral PEDOT fragments (λmax = 600 nm) leads to consecutive formation of oxidized species: polarons (λmax = 860 nm) and consecutively bipolarons (λmax > 1100 nm).
Figure 4 shows a similar series of steady-state electronic absorption spectra of a WO3 film. In the spectra recorded at potentials ranging from 1 V to −0.8 V, we can see the appearance of a broad absorption band in the vis-NIR range of the spectrum (850–1100 nm), which grows with a negative shift of electrode potential.
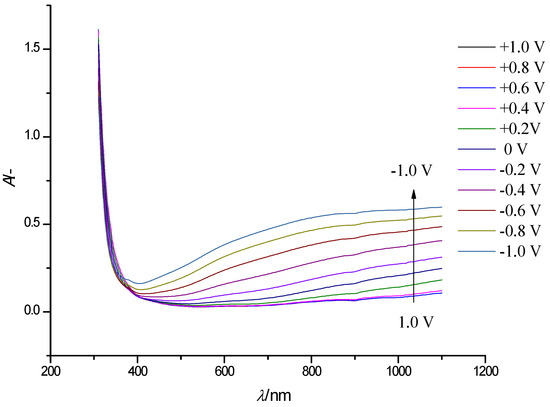
Figure 4.
Electronic absorption spectra of a WO3 film on an FTO electrode at electrode potentials in the range −1.0 to +1.0 V (step width 0.2 V, arrows show the direction of the absorption band change with decreasing potential) in solution of 0.5 M LiClO4/PC.
Following gradual WO3 reduction, at potentials lower than +0.4 V, an additional broad absorption band was observed in the range of wavelengths 500–800 nm. Because of the considerable width of both absorption bands, a clear maximum of the second band can hardly be located; this prevents separation of the bands. Maximum absorption of WO3 in the visible and NIR range, and blue coloration was achieved at the most negative electrode potential −1.0 V (Figure 4). This is associated with the intercalation of the Li+-ions and electron flow into the WO3 film. The intense blue color is caused by intervalence charge transfer between adjacent W5+ and W6+ sites in tungsten oxide [33,34].
There are three major absorption ranges in the spectra of PEDOT/WO3 composite film (see Figure 5): Range I: 400–720 nm, range II: 720–970 nm and range III: 970–1100 nm. Detailed analysis and comparison of absorption spectra of a PEDOT film and a PEDOT/WO3 composite film reveals the main features and nature of spectral changes.
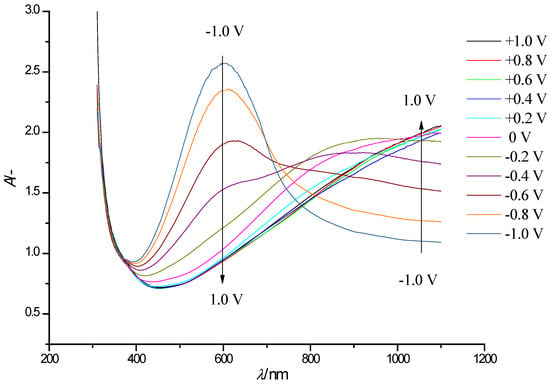
Figure 5.
Electronic absorption spectra of a PEDOT/WO3 film on an FTO electrode at electrode potentials in the range −1.0 to + 1.0 V (step width 0.2 V, arrows show the direction of the absorption band change with increasing potential) in solution of 0.5 M LiClO4/PC.
In the spectra of the composite, in range I, the absorption of the PEDOT film dominates with an additional contribution from tungsten oxide to the overall absorption at potentials from 0.2 to −0.5 V. Range II (720–970 nm) undergoes the strongest change in the shape of the overall spectrum of the composite film compared to the plain PEDOT polymer film, associated with a noticeable absorption of tungsten oxide in addition to the contribution of PEDOT both in the visible part of the spectrum (at λ = 500–800 nm, with a maximum around λ = 600 nm), and in the NIR range. At negative potentials, both PEDOT and WO3 components absorb in the visible range, synergistically providing blue color of the PEDOT/WO3 film. This observation is consistent with the data on the enhancement of electrochromic properties due to complementary change of both constituents in the composite film in the color (blue) at negative potential values available in the literature [35].
There are also three characteristic bands in the in situ absorption spectra of the composite, showing intensity changes with the applied electrode potential (Figure 5). These bands dominate at λmax about 600 nm, λmax about 900 nm and λmax > 1100 nm. At the first glance, there are no obvious changes of the wavelengths of the maxima of observed bands in the PEDOT/WO3 nanocomposite in comparison to the pristine PEDOT polymer film at relatively low electrode potentials. At negative potentials both, the absorption band of PEDOT and bands of WO3, contribute, but due to the flat bands of WO3, band shapes and peak locations of maxima from PEDOT are preserved.
This suggests that the inclusion of WO3 into the polymer matrix did not significantly change its electronic structure at a moderate level of polymer oxidation, and the electrostatic interactions noticed in CVs accelerating the kinetics of redox performance (reversibility of W(VI)/W(V) redox couple) do not show up in the optical spectra.
The comparison of electronic absorption spectra of PEDOT and PEDOT/WO3 films at two selected potentials (Figure 6) clearly shows the differences between the spectra of PEDOT and PEDOT/WO3.
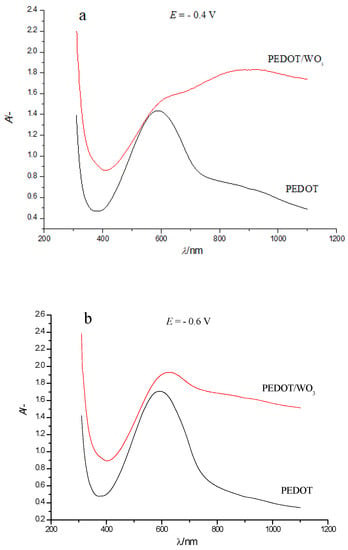
Figure 6.
Comparison of electronic absorption spectra of PEDOT and PEDOT/WO3 films on an FTO electrode in solution of 0.5 M LiClO4/PC at selected electrode potentials: (a) −0.4 V, (b) −0.6 V.
When comparing Figure 3 and Figure 5 more closely, we can also see that at the transition from neutral to oxidized state there is no expressed isosbestic point, like the one observed in the case of PEDOT only. In addition, the strongest changes in the shape of the spectrum of PEDOT/WO3 composite compared to the pristine PEDOT film are associated with a noticeable absorption of tungsten oxide both in the visible and NIR parts of the spectrum. In particular, at high positive potentials, where contributions from WO3 absorption is quite low (see Figure 4), the absorption in the NIR-range (λ ~ 1090 nm) from bipolaronic species of PEDOT in PEDOT/WO3 composite is much lower compared to pristine PEDOT film.
The reason for this decrease in absorption (so-called quenching) of bipolaronic fragments of PEDOT in the composite is probably the redistribution of fractions of polaron and bipolaron forms in the polymer, which resulted in the change of intensities of corresponding bands. Probably, at high positive electrode potentials of the composite film, the electrostatic interaction between the anionic form of tungsten oxide and the positively charged polarons leads to the stabilization of the polaronic species and a decrease in the fraction of bipolaronic forms in the polymer, which in turn results in lower intensity of the corresponding absorption band of the PEDOT/WO3 composite.
4. Conclusions
As noted above, the CVs and electronic absorption spectra of PEDOT/WO3 composite film are not the simple sum of individual components PEDOT and WO3 electrodeposited on FTO electrodes, as might have been expected for the case of a simple additive mixture. The shape of CV peaks attributed to the WO3 component in of PEDOT/WO3 composite film is significantly different from that observed with simple electrodeposited WO3. This is most likely caused by different mechanisms of electrodeposition of tungsten oxide on an FTO electrode and a PEDOT-covered FTO electrode. The difference was clearly observed before in a systematic investigation of electrodeposition conditions and their effects on the electrochemical response of tungsten oxide-covered FTO electrode and reported recently [36]. As shown, the potentiodynamic method of electrodeposition may result in trapping of polyoxotungstate anion species into the polymer during p-doping of the polymer and may thus facilitate preliminary formation of precursors of tungsten oxide nanostructures. The resulting precipitate of WO3 displays more symmetrical redox peaks with smaller peak-to-peak potential separation, this may be enhanced by the conductive matrix. These conditions and arguments do not apply to the case of electrodeposition on an FTO-surface from the same electrolyte solution.
Detailed analysis of electronic absorption spectra of PEDOT, WO3 films and PEDOT/WO3 composite films also suggests a situation more complex than simple additivity of the spectral components of the composite constituents. Although we do not see pronounced shifts of main electronic bands, which would indicate specific chemical interactions between the components, we nevertheless observe a redistribution of the fractions of different oxidized forms (polarons and bipolarons) of PEDOT. We propose that electrostatic interactions between positively charged polymer fragments and negatively charged polymer oxoanions of tungsten oxide lead to a stabilization of polaron species of the polymer and to a decrease in the degree of charge delocalization in the composite film. Therefore, at high positive electrode potentials of the composite film, the redistribution of fractions of polaron and bipolaron forms in the polymer was observed, which resulted in the change of intensities of corresponding bands. These observations shed light on performance improvements of these functional composite materials for both supercapacitor and optoelectronic applications.
Author Contributions
Conceptualization, V.V.K.; methodology, V.V.K. and E.G.T.; investigation, A.O.E.; writing–original draft preparation, V.V.K. and R.H.; writing–review and editing, R.H. and E.G.T.; visualization, A.O.E. and E.G.T.; funding acquisition, V.V.K. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Russian Foundation for Basic Research, grant number 19-03-00593.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Acknowledgments
The authors are thankful to the colleagues from Interdisciplinary Resource Centre for Nanotechnology of St. Petersburg State University.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Deb, S.K. Opportunities and challenges in science and technology of WO3 for electrochromic and related applications. Sol. Energy Mater. Sol. Cells 2008, 92, 245–258. [Google Scholar] [CrossRef]
- Holze, R. Metal oxide/conducting polymers based hybrid materials designed for application in supercapacitors. In Metal Oxides in Supercapacitors; Dubal, D.P., Gomez-Romero, P., Eds.; Elsevier: Amsterdam, The Netherlands, 2017; pp. 219–245. [Google Scholar]
- Meng, Q.; Cai, K.; Chen, Y.; Chen, L. Research progress on conducting polymer based supercapacitor electrode materials. Nano Energy 2017, 36, 268–285. [Google Scholar] [CrossRef]
- Fu, L.; Qu, Q.; Holze, R.; Kondratiev, V.V.; Wu, Y. Composites of metal oxides and intrinsically conducting polymers as supercapacitor electrode materials: The best of both worlds? J. Mater. Chem. A 2019, 7, 14937–14970. [Google Scholar] [CrossRef]
- Sadakane, M.; Sasaki, K.; Kunioku, H.; Ohtani, B.; Ueda, W.; Abe, R. Preparation of nano-structured crystalline tungsten(VI) oxide and enhanced photocatalytic activity for decomposition of organic compounds under visible light irradiation. Chem. Commun. 2008, 6552–6554. [Google Scholar] [CrossRef] [PubMed]
- Granqvist, C.G. Electrochromic tungsten oxide films: Review of progress 1993–1998. Sol. Energy Mater. Sol. Cells 2000, 60, 201–262. [Google Scholar] [CrossRef]
- Granqvist, C.G.; Avendaño, E.; Azens, A. Electrochromic coatings and devices: Survey of some recent advances. Thin Solid Films 2003, 442, 201–211. [Google Scholar] [CrossRef]
- Yang, P.; Sun, P.; Du, L.; Liang, Z.; Xie, W.; Cai, X.; Huang, L.; Tan, S.; Mai, W. Quantitative analysis of charge storage process of tungsten oxide that combines pseudocapacitive and electrochromic properties. J. Phys. Chem. C 2015, 119, 16483–16489. [Google Scholar] [CrossRef]
- Li, W.J.; Fu, Z.W. Nanostructured WO3 thin film as a new anode material for lithium-ion batteries. Appl. Surf. Sci. 2010, 256, 2447–2452. [Google Scholar] [CrossRef]
- Lokhande, V.; Lokhande, A.; Namkoong, G.; Kim, J.H.; Ji, T. Charge storage in WO3 polymorphs and their application as supercapacitor electrode material. Results Phys. 2019, 12, 2012–2020. [Google Scholar] [CrossRef]
- Kalagi, S.S.; Dalavi, D.S.; Mali, S.S.; Inamdar, A.I.; Patil, R.S.; Patil, P.S. Study of novel WO3-PEDOT:PSS bilayered thin film for electrochromic applications. Nanosci. Nanotechnol. Lett. 2012, 4, 1146–1154. [Google Scholar] [CrossRef]
- Zou, B.X.; Liang, Y.; Liu, X.X.; Diamond, D.; Lau, K.T. Electrodeposition and pseudocapacitive properties of tungsten oxide/polyaniline composite. J. Power Sources 2011, 196, 4842–4848. [Google Scholar] [CrossRef]
- Amaechi, I.C.; Nwanya, A.C.; Ekwealor, A.B.C.; Asogwa, P.U.; Osuji, R.U.; Maaza, M.; Ezema, F.I. Electronic thermal conductivity, thermoelectric properties and supercapacitive behaviour of conjugated polymer nanocomposite (polyaniline-WO3) thin film. Eur. Phys. J. Appl. Phys. 2015, 69, 30901. [Google Scholar] [CrossRef]
- Wei, H.; Yan, X.; Wu, S.; Luo, Z.; Wei, S.; Guo, Z. Electropolymerized polyaniline stabilized tungsten oxide nanocomposite films: Electrochromic behavior and electrochemical energy storage. J. Phys. Chem. C 2012, 116, 25052–25064. [Google Scholar] [CrossRef]
- Zou, B.; Gong, S.; Wang, Y.; Liu, X. Tungsten oxide and polyaniline composite fabricated by surfactant-templated electrodeposition and its use in supercapacitors. J. Nanomater. 2014, 2014, 813120. [Google Scholar] [CrossRef]
- Yuksel, R.; Durucan, C.; Unalan, H.E. Ternary nanocomposite SWNT/WO3/PANI thin film electrodes for supercapacitors. J. Alloys Compd. 2016, 658, 183–189. [Google Scholar] [CrossRef]
- Kadam, A.V.; Patil, S.B. Polyaniline globules as a catalyst for WO3 nanoparticles for supercapacitor application. Mater. Res. Expr. 2018, 5, 085036. [Google Scholar] [CrossRef]
- Wang, F.; Zhan, X.; Cheng, Z.; Wang, Z.; Wang, Q.; Xu, K.; Safdar, M.; He, J. Tungsten oxide@polypyrrole core-shell nanowire arrays as novel negative electrodes for asymmetric supercapacitors. Small 2015, 11, 749–755. [Google Scholar] [CrossRef]
- Lokhande, V.C.; Lokhande, A.C.; Lokhande, C.D.; Kim, J.H.; Ji, T. Supercapacitive composite metal oxide electrodes formed with carbon, metal oxides and conducting polymers. J. Alloys Compd. 2016, 685, 381–403. [Google Scholar] [CrossRef]
- Zhuzhelskii, D.V.; Tolstopjatova, E.G.; Eliseeva, S.N.; Ivanov, A.V.; Miao, S.; Kondratiev, V.V. Electrochemical properties of PEDOT/WO3 composite films for high performance supercapacitor application. Electrochim. Acta 2019, 299, 182–190. [Google Scholar] [CrossRef]
- Szymanska, D.; Rutkowska, I.A.; Adamczyk, L.; Zoladek, S.; Kulesza, P.J. Effective charge propagation and storage in hybrid films of tungsten oxide and poly(3,4-ethylenedioxythiophene). J. Solid State Electrochem. 2010, 14, 2049–2056. [Google Scholar] [CrossRef]
- Zhu, Y.; Otley, M.T.; Alamer, F.A.; Kumar, A.; Zhang, X.; Mamangun, D.M.D.; Li, M.; Arden, B.G.; Sotzing, G.A. Electrochromic properties as a function of electrolyte on the performance of electrochromic devices consisting of a single-layer polymer. Organ. Electron. 2014, 15, 1378–1386. [Google Scholar] [CrossRef]
- Bredas, J.L.; Scott, J.C.; Yakushi, K.; Street, G.B. Polarons and bipolarons in polypyrrole: Evolution of the band structure and optical spectrum upon doping. Phys. Rev. B Condens. Matter. 1984, 30, 1023–1025. [Google Scholar] [CrossRef]
- Bredas, J.L.; Street, G.B. Polarons, bipolarons and solitons in conducting polymers. Acc. Chem. Res. 1985, 18, 309–315. [Google Scholar] [CrossRef]
- Hoier, S.N.; Park, S.M. Electrochemistry of conductive polymers. 11. Spectroelectrochemical studies of poly(3-methylthiophene) oxidation. J. Phys. Chem. 1992, 96, 5188–5193. [Google Scholar] [CrossRef]
- Trznadel, M.; Zagórska, M.; Lapkowski, M.; Louarn, G.; Lefrant, S.; Pron, A. UV–VIS–NIR and Raman spectroelectrochemistry of regioregular poly(3-octylthiophene): Comparison with its non-regioregular analogue. J. Chem. Soc. Faraday Trans. 1996, 92, 1387–1393. [Google Scholar] [CrossRef]
- Neugebauer, H.; Blomquist, S.; Ahonen, H.J.; Kankare, J.; Ivaska, A. In situ spectroelectrochemical characterization of poly(3,4-ethylenedioxythiophene). Electrochim. Acta 1999, 44, 2739–2750. [Google Scholar] [CrossRef]
- Łapkowski, M.; Proń, A. Electrochemical oxidation of poly(3,4-ethylenedioxythiophene)—“In situ” conductivity and spectroscopic investigations. Synth. Met. 2000, 110, 79–83. [Google Scholar] [CrossRef]
- Tolstopyatova, E.G.; Pogulaichenko, N.A.; Eliseeva, S.N.; Kondratiev, V.V. Spectroelectrochemical study of poly-3,4-ethylenedioxythiophene films in the presence of different supporting electrolytes. Russ. J. Electrochem. 2009, 45, 252–262. [Google Scholar] [CrossRef]
- Chen, X.; Inganaes, O. Three-step redox in polythiophenes: Evidence from electrochemistry at an ultramicroelectrode. J. Phys. Chem. 1996, 100, 15202–15206. [Google Scholar] [CrossRef]
- Carlberg, C.; Chen, X.; Inganaes, O. Ionic transport and electronic structure in poly(3,4-ethylenedioxythiophene). Solid State Ionics 1996, 85, 73–78. [Google Scholar] [CrossRef]
- Tourillon, G. Polythiophene and its derivatives. In Handbook of Conducting Polymers; Skotheim, T.A., Ed.; Marcel Dekker: New York, NY, USA, 1986; pp. 293–350. [Google Scholar]
- Darmawi, S.; Burkhardt, S.; Leichtweiss, T.; Weber, D.A.; Wenzel, S.; Janek, J.; Elm, M.T.; Klar, P.J. Correlation of electrochromic properties and oxidation states in nanocrystalline tungsten trioxide. Phys. Chem. Chem. Phys. 2015, 17, 15903–15911. [Google Scholar] [CrossRef] [PubMed]
- Deepa, M.; Joshi, A.G.; Srivastava, A.K.; Shivaprasad, S.M.; Agnihotry, S.A. Electrochromic nanostructured tungsten oxide films by sol-gel: Structure and intercalation properties. J. Electrochem. Soc. 2006, 153, C365–C376. [Google Scholar] [CrossRef]
- Shi, Y.; Zhang, Y.; Tang, K.; Cui, J.; Shu, X.; Wang, Y.; Liu, J.; Jiang, Y.; Tan, H.H.; Wu, Y. Designed growth of WO3/PEDOT core/shell hybrid nanorod arrays with modulated electrochromic properties. Chem. Eng. J. 2019, 355, 942–951. [Google Scholar] [CrossRef]
- Zhuzhelskii, D.V.; Tolstopjatova, E.G.; Volkov, A.I.; Eliseeva, S.N.; Láng, G.G.; Kondratiev, V.V. Insights on the electrodeposition mechanism of tungsten oxide into conducting polymers: Potentiostatic vs. potentiodynamic deposition. Synth. Met. 2020, 267, 116469. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).