Polytriphenylamine and Poly(styrene-co-hydroxystyrene) Blends as High-Performance Anticorrosion Coating for Iron
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials Synthesis
2.2. Sample Preparation
2.2.1. Sample Preparation for the Optical Microscope Measurement
2.2.2. Corrosion Test
3. Results and Discussion
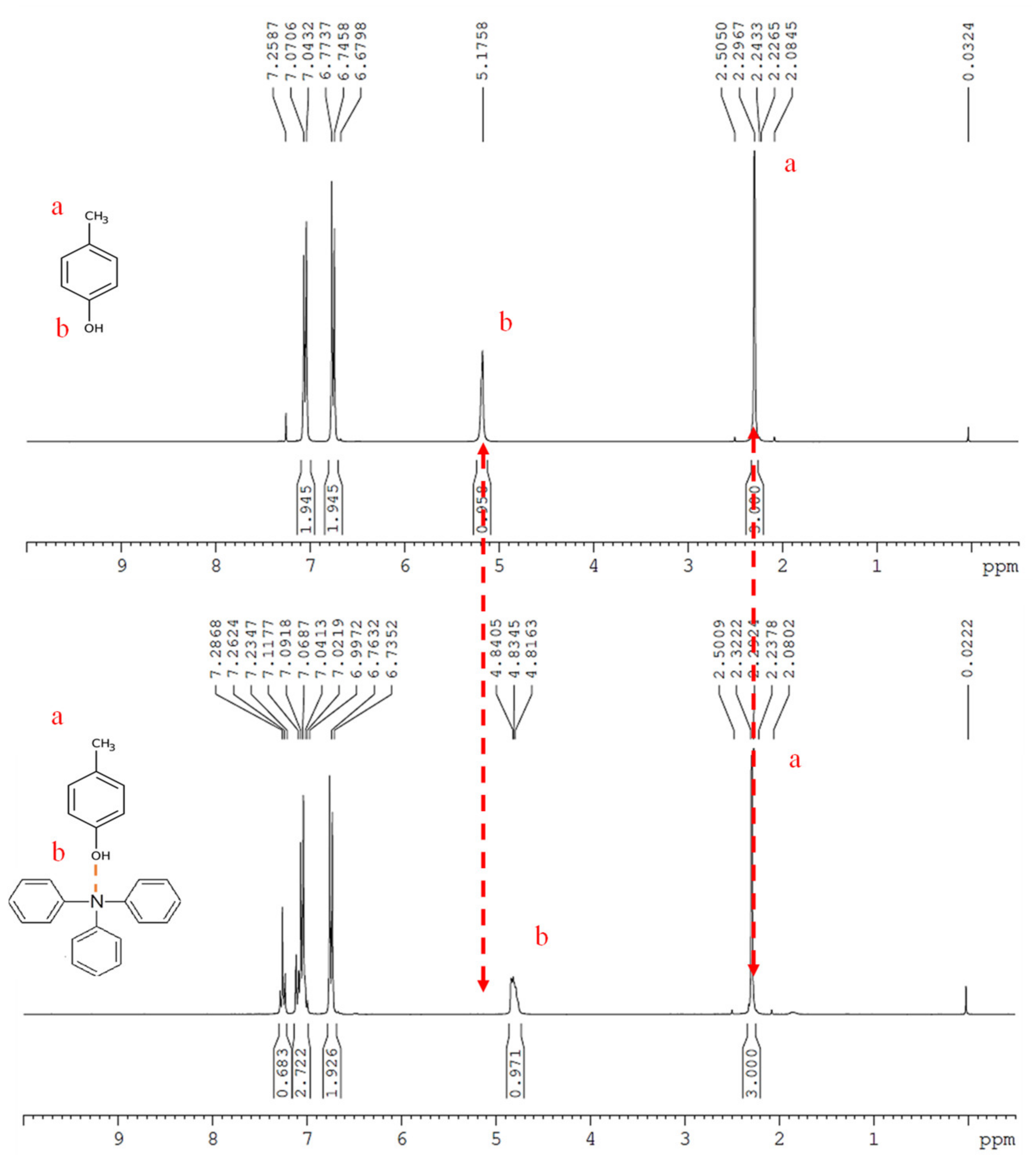
3.1. Hydrogen Bonding Study
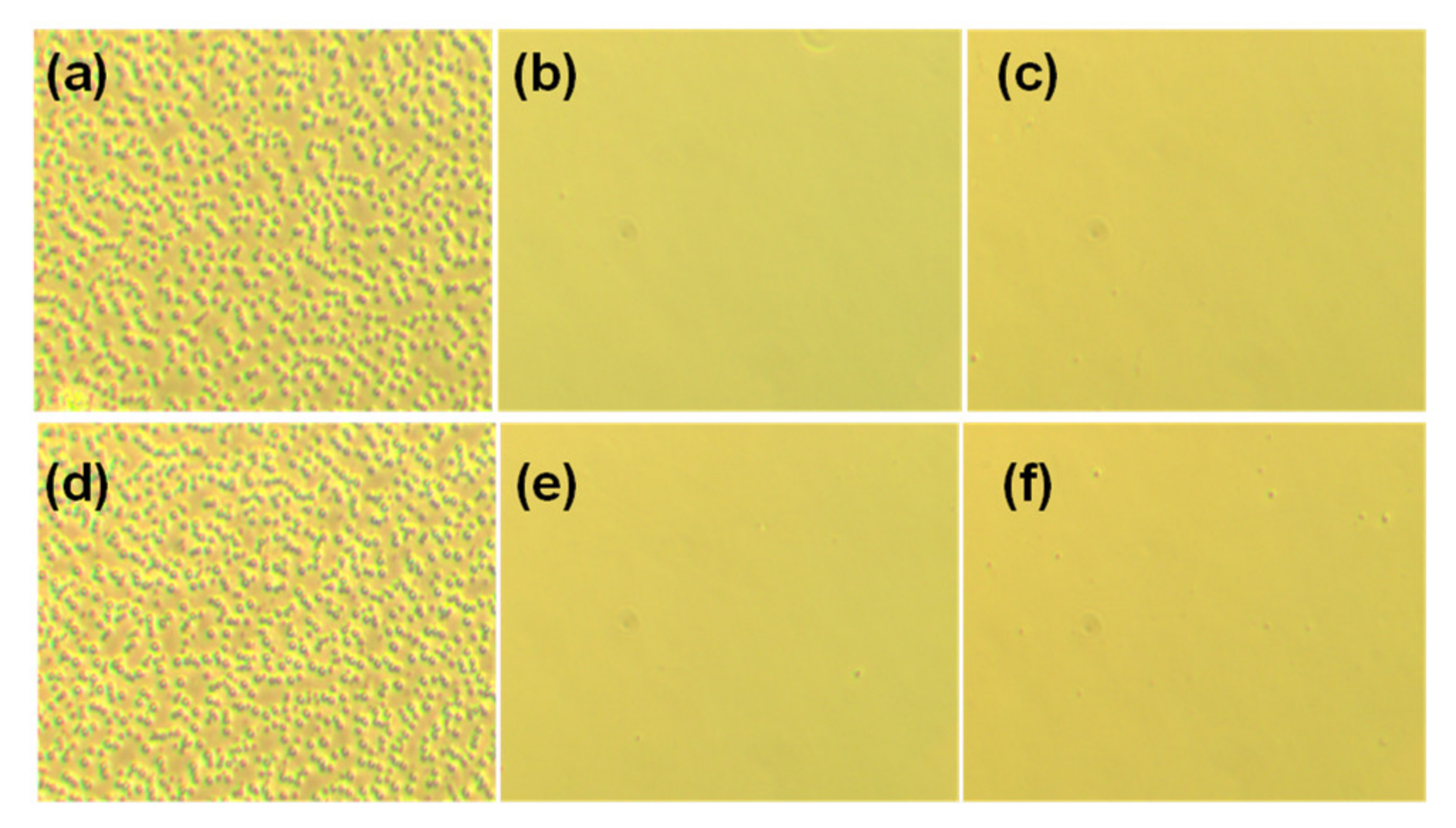
3.2. Morphology Study
3.3. Adhesion Test and Contact Angle Study
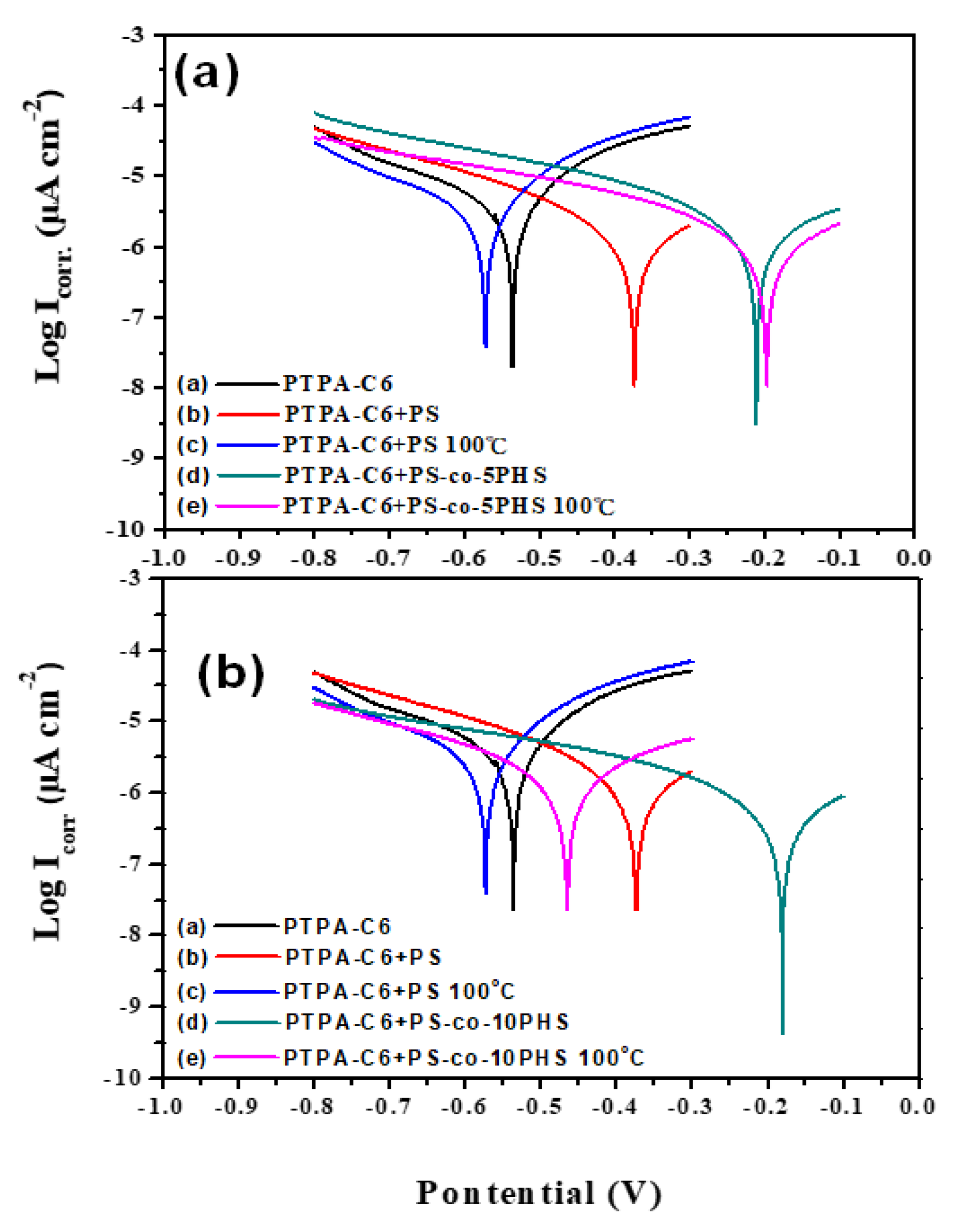
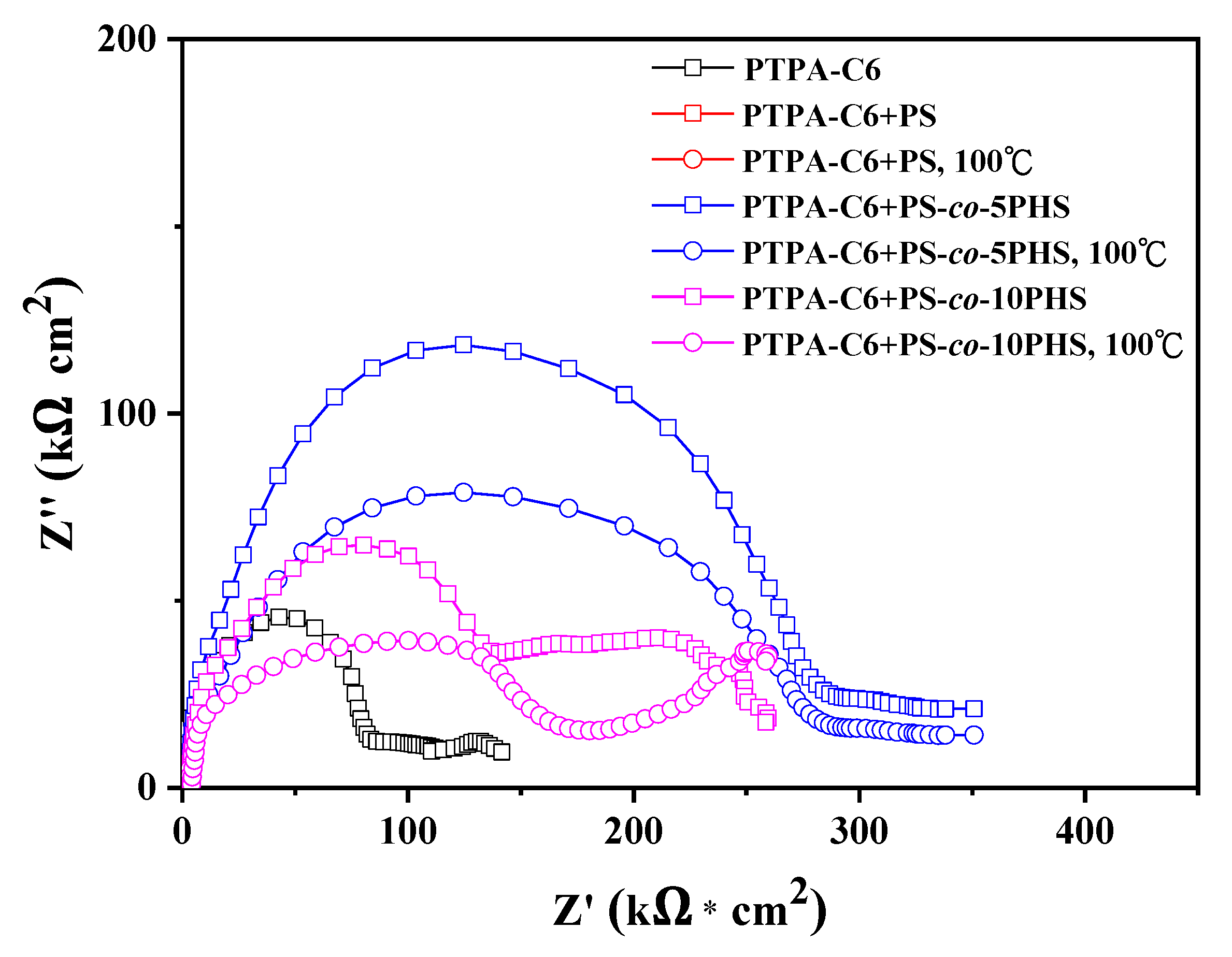
3.4. Corrosion Test
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Javaherdashti, R. How corrosion affects industry and life. Anti-Corros. Methods Mater. 2000, 47, 30–34. [Google Scholar] [CrossRef]
- Angst, U.M. Challenges and opportunities in corrosion of steel in concrete. Mater. Struct. 2018, 51, 4. [Google Scholar] [CrossRef]
- Zhu, Y.; Free, M.L.; Woollam, R.; Durnie, W. A review of surfactants as corrosion inhibitors and associated modeling. Prog. Mater. Sci. 2017, 90, 159–223. [Google Scholar] [CrossRef]
- Rohwerder, M.; Michalik, A. Conducting polymers for corrosion protection: What makes the difference between failure and success? Electrochim. Acta 2007, 53, 1300–1313. [Google Scholar] [CrossRef]
- Olajire, A.A. Recent advances on organic coating system technologies for corrosion protection of offshore metallic structures. J. Mol. Liquids 2018, 269, 572–606. [Google Scholar] [CrossRef]
- Lyon, S.B.; Bingham, R.; Mills, D.J. Advances in corrosion protection by organic coatings: What we know and what we would like to know. Prog. Org. Coat. 2017, 102, 2–7. [Google Scholar] [CrossRef]
- Zhang, Y.; Deng, J.; Shao, Y.; Shi, Q.; Meng, G.; Ping, L. Effect of polyaniline/organophilic montmorillonite composites on properties of epoxy coating. Corros. Rev. 2014, 32, 227–236. [Google Scholar] [CrossRef]
- Choi, D.; Park, C.; Yang, S.; Kim, H.; Kim, S. Effect of Solvent-Assisted Dispersions of Clay/Epoxy Nanocomposites on Steel Passivation. J. Nanosci. Nanotechnol. 2016, 16, 981–986. [Google Scholar] [CrossRef] [PubMed]
- Zabihi, O.; Ahmadi, M.; Nikafshar, S.; Chandrakumar Preyeswary, K.; Naebe, M. A technical review on epoxy-clay nanocomposites: Structure, properties, and their applications in fiber reinforced composites. Compos. Part B Eng. 2018, 135, 1–24. [Google Scholar] [CrossRef]
- Kalaivasan, N.; Syed Shafi, S. Enhancement of corrosion protection effect in mechanochemically synthesized Polyaniline/MMT clay nanocomposites. Arab. J. Chem. 2017, 10, S127–S133. [Google Scholar] [CrossRef]
- Viswanathan, A.; Shetty, A.N. Facile in-situ single step chemical synthesis of reduced graphene oxide-copper oxide-polyaniline nanocomposite and its electrochemical performance for supercapacitor application. Electrochim. Acta 2017, 257, 483–493. [Google Scholar] [CrossRef]
- Zhao, Y.; Ma, J.; Chen, K.; Zhang, C.; Yao, C.; Zuo, S.; Kong, Y. One-Pot Preparation of Graphene-Based Polyaniline Conductive Nanocomposites for Anticorrosion Coatings. Nano 2017, 12, 1750056. [Google Scholar] [CrossRef]
- Liu, Z.; Tian, S.; Li, Q.; Wang, J.; Pu, J.; Wang, G.; Zhao, W.; Feng, F.; Qin, J.; Ren, L. Integrated Dual-Functional ORMOSIL Coatings with AgNPs@rGO Nanocomposite for Corrosion Resistance and Antifouling Applications. ACS Sustain. Chem. Eng. 2020, 8, 6786–6797. [Google Scholar] [CrossRef]
- Zhu, A.; Shi, P.; Sun, S.; Rui, M. Construction of rGO/Fe3O4/PANI nanocomposites and its corrosion resistance mechanism in waterborne acrylate-amino coating. Prog. Org. Coat. 2019, 133, 117–124. [Google Scholar] [CrossRef]
- Wang, M.-H.; Li, Q.; Li, X.; Liu, Y.; Fan, L.-Z. Effect of oxygen-containing functional groups in epoxy/reduced graphene oxide composite coatings on corrosion protection and antimicrobial properties. Appl. Surf. Sci. 2018, 448, 351–361. [Google Scholar] [CrossRef]
- Ruben, B.; Zhang, G.; Xin, T.; Giorgio, S.; Victor, M.; Gloria, G.; Michele, F.; Filippo, P.; Shuhui, S.; Nadhira, L.; et al. Graphene oxide/reduced graphene oxide films as protective barriers on lead against differential aeration corrosion induced by water drops. Nanoscale Adv. 2020, 2, 5412–5420. [Google Scholar] [CrossRef]
- Tallman, D.E.; Spinks, G.; Dominis, A.; Wallace, G.G. Electroactive conducting polymers for corrosion control. J. Solid State Electrochem. 2002, 6, 73–84. [Google Scholar] [CrossRef]
- Cortés, M.T.; Sierra, E.V. Effect of synthesis parameters in polyaniline: Influence on yield and thermal behavior. Polym. Bull. 2006, 56, 37–45. [Google Scholar] [CrossRef]
- Arabzadeh, H.; Shahidi, M.; Foroughi, M.M. Electrodeposited polypyrrole coatings on mild steel: Modeling the EIS data with a new equivalent circuit and the influence of scan rate and cycle number on the corrosion protection. J. Electroanal. Chem. 2017, 807, 162–173. [Google Scholar] [CrossRef]
- Jadhav, N.; Kasisomayajula, S.; Gelling, V. Polypyrrole/Metal Oxides-Based Composites/Nanocomposites for Corrosion Protection. Front. Mater. 2020, 7, 95. [Google Scholar] [CrossRef]
- Qiu, S.; Li, W.; Zheng, W.; Zhao, H.; Wang, L. Synergistic Effect of Polypyrrole-Intercalated Graphene for Enhanced Corrosion Protection of Aqueous Coating in 3.5% NaCl Solution. ACS Appl. Mater. Interfaces 2017, 9, 34294–34304. [Google Scholar] [CrossRef] [PubMed]
- Williams, G.; Gabriel, A.; Cook, A.; McMurray, H.N. Dopant Effects in Polyaniline Inhibition of Corrosion-Driven Organic Coating Cathodic Delamination on Iron. J. Electrochem. Soc. 2006, 153, B425. [Google Scholar] [CrossRef]
- Fahlman, M.; Jasty, S.; Epstein, A.J. Corrosion protection of iron/steel by emeraldine base polyaniline: An X-ray photoelectron spectroscopy study. Synth. Met. 1997, 85, 1323–1326. [Google Scholar] [CrossRef]
- MacDiarmid, A.G. Polyaniline and polypyrrole: Where are we headed? Synth. Met. 1997, 84, 27–34. [Google Scholar] [CrossRef]
- Bernard, M.C.; Hugot-LeGoff, A.; Joiret, S.; Dinh, N.N.; Toan, N.N. Polyaniline layer for iron protection in sulfate medium. Synth. Met. 1999, 102, 1383–1384. [Google Scholar] [CrossRef]
- Ahmad, N.; MacDiarmid, A.G. Inhibition of corrosion of steels with the exploitation of conducting polymers. Synth. Met. 1996, 78, 103–110. [Google Scholar] [CrossRef]
- Alam, M.A.; Samad, U.A.; Khan, R.; Alam, M.; Al-Zahrani, S.M. Anti-corrosive performance of epoxy coatings containing various nano-particles for splash zone applications. Korean J. Chem. Eng. 2017, 34, 2301–2310. [Google Scholar] [CrossRef]
- Mostafaei, A.; Nasirpouri, F. Epoxy/polyaniline–ZnO nanorods hybrid nanocomposite coatings: Synthesis, characterization and corrosion protection performance of conducting paints. Prog. Org. Coat. 2014, 77, 146–159. [Google Scholar] [CrossRef]
- Sathiyanarayanan, S.; Azim, S.S.; Venkatachari, G. A new corrosion protection coating with polyaniline–TiO2 composite for steel. Electrochim. Acta 2007, 52, 2068–2074. [Google Scholar] [CrossRef]
- Zandi-zand, R.; Ershad-langroudi, A.; Rahimi, A. Silica based organic–inorganic hybrid nanocomposite coatings for corrosion protection. Prog. Org. Coat. 2005, 53, 286–291. [Google Scholar] [CrossRef]
- Lamaka, S.V.; Xue, H.B.; Meis, N.N.A.H.; Esteves, A.C.C.; Ferreira, M.G.S. Fault-tolerant hybrid epoxy-silane coating for corrosion protection of magnesium alloy AZ31. Prog. Org. Coat. 2015, 80, 98–105. [Google Scholar] [CrossRef]
- Chang, C.-M.; Weng, C.-J.; Chien, C.-M.; Chuang, T.-L.; Lee, T.-Y.; Yeh, J.-M.; Wei, Y. Polyaniline/carbon nanotube nanocomposite electrodes with biomimetic hierarchical structure for supercapacitors. J. Mater. Chem. A 2013, 1, 14719–14728. [Google Scholar] [CrossRef]
- Liu, Q.; Jin, J.; Zhang, J. NiCo2S4@graphene as a Bifunctional Electrocatalyst for Oxygen Reduction and Evolution Reactions. ACS Appl. Mater. Interfaces 2013, 5, 5002–5008. [Google Scholar] [CrossRef] [PubMed]
- Deshpande, P.P.; Jadhav, N.G.; Gelling, V.J.; Sazou, D. Conducting polymers for corrosion protection: A review. J. Coat. Technol. Res. 2014, 11, 473–494. [Google Scholar] [CrossRef]
- Yu, Y.-H.; Lin, Y.-Y.; Lin, C.-H.; Chan, C.-C.; Huang, Y.-C. High-performance polystyrene/graphene-based nanocomposites with excellent anti-corrosion properties. Polym. Chem. 2014, 5, 535–550. [Google Scholar] [CrossRef]
- Hernández-Martínez, D.; León-Silva, U.; Nicho Maria, E. Corrosion protection of steel by poly(3-hexylthiophene) polymer blends. Anti-Corros. Methods Mater. 2015, 62, 229–240. [Google Scholar] [CrossRef]
- Tsai, M.-C.; Yang, C.-R.; Tsai, J.-H.; Yu, Y.-H.; Huang, P.-T. Enhanced Corrosion Protection of Iron by Poly(3-hexylthiophene)/Poly(styrene-co-hydroxystyrene) Blends. Coatings 2018, 8, 383. [Google Scholar] [CrossRef]
- Tsai, J.-H.; Tsai, M.-C.; Lee, T.-H.; Huang, P.-T. Corrosion-resistant coating of iron: A synergistic effect of electroactive poly(triphenylamine) coating with posttreatment for high-corrosion-protection efficiency. J. Chin. Chem. Soc. 2020, 67, 1174–1182. [Google Scholar] [CrossRef]
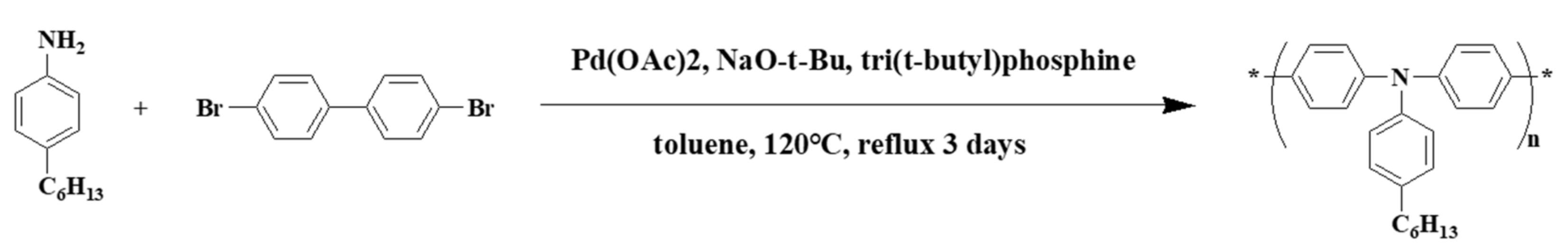

| Coating Layer | 100 Grid Test | Contact Angle (°) |
|---|---|---|
| PTPA-C6, 25 °C |  | 96.1  |
| PTPA-C6, 100 °C |  | 98.8  |
| PTPA-C6 + PS, 25 °C |  | 93.9  |
| PTPA-C6 + PS, 100 °C |  | 99.2  |
| PTPA-C6 + PS-co-5PHS, 25 °C |  | 99.5  |
| PTPA-C6 + PS-co-5PHS, 100 °C |  | 102.3  |
| PTPA-C6 + PS-co-10PHS, 25 °C |  | 99.7  |
| PTPA-C6 + PS-co-10PHS, 100 °C |  | 101.4  |
| Coating Layer a | Rp (kΩ cm2) | Icorr (μA/cm2) | Rcorr (MPY c) | PE b (%) | Thickness (μm) |
|---|---|---|---|---|---|
| Bare-Fe | 4.20 | 15.38 | 7.02 | - | - |
| PTPA-C6, 25 °C | 160.25 | 2.84 | 1.30 | 81.52 | 14 |
| PTPA-C6 + PS, 25 °C | 32.80 | 3.09 | 1.41 | 79.88 | 12.8 |
| PTPA-C6 + PS, 100 °C | 10.57 | 3.60 | 1.64 | 76.67 | 13.5 |
| PTPA-C6 + PS-co-5PHS, 25 °C | 417.00 | 0.15 | 0.07 | 98.97 | 18 |
| PTPA-C6 + PS-co-5PHS, 100 °C | 372.41 | 0.86 | 0.39 | 94.40 | 10.7 |
| PTPA-C6 + PS-co-10PHS, 25 °C | 284.93 | 0.12 | 0.06 | 99.19 | 12.7 |
| PTPA-C6 + PS-co-10PHS, 100 °C | 246.04 | 0.30 | 0.14 | 97.99 | 12.6 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, T.-H.; Tsai, J.-H.; Chen, H.-Y.; Huang, P.-T. Polytriphenylamine and Poly(styrene-co-hydroxystyrene) Blends as High-Performance Anticorrosion Coating for Iron. Polymers 2021, 13, 1629. https://doi.org/10.3390/polym13101629
Lee T-H, Tsai J-H, Chen H-Y, Huang P-T. Polytriphenylamine and Poly(styrene-co-hydroxystyrene) Blends as High-Performance Anticorrosion Coating for Iron. Polymers. 2021; 13(10):1629. https://doi.org/10.3390/polym13101629
Chicago/Turabian StyleLee, Ting-Hsuan, Jen-Hao Tsai, Hong-Yu Chen, and Ping-Tsung Huang. 2021. "Polytriphenylamine and Poly(styrene-co-hydroxystyrene) Blends as High-Performance Anticorrosion Coating for Iron" Polymers 13, no. 10: 1629. https://doi.org/10.3390/polym13101629
APA StyleLee, T.-H., Tsai, J.-H., Chen, H.-Y., & Huang, P.-T. (2021). Polytriphenylamine and Poly(styrene-co-hydroxystyrene) Blends as High-Performance Anticorrosion Coating for Iron. Polymers, 13(10), 1629. https://doi.org/10.3390/polym13101629





