Fully Bio-Based Thermosetting Polyurethanes from Bio-Based Polyols and Isocyanates
Abstract
:1. Introduction
2. Experimental Part
2.1. Materials
2.2. Methods
2.2.1. Nuclear Magnetic Resonance (NMR)
2.2.2. Fourier Transform Infrared (FTIR)
2.2.3. Thermogravimetric Analyses (TGA)
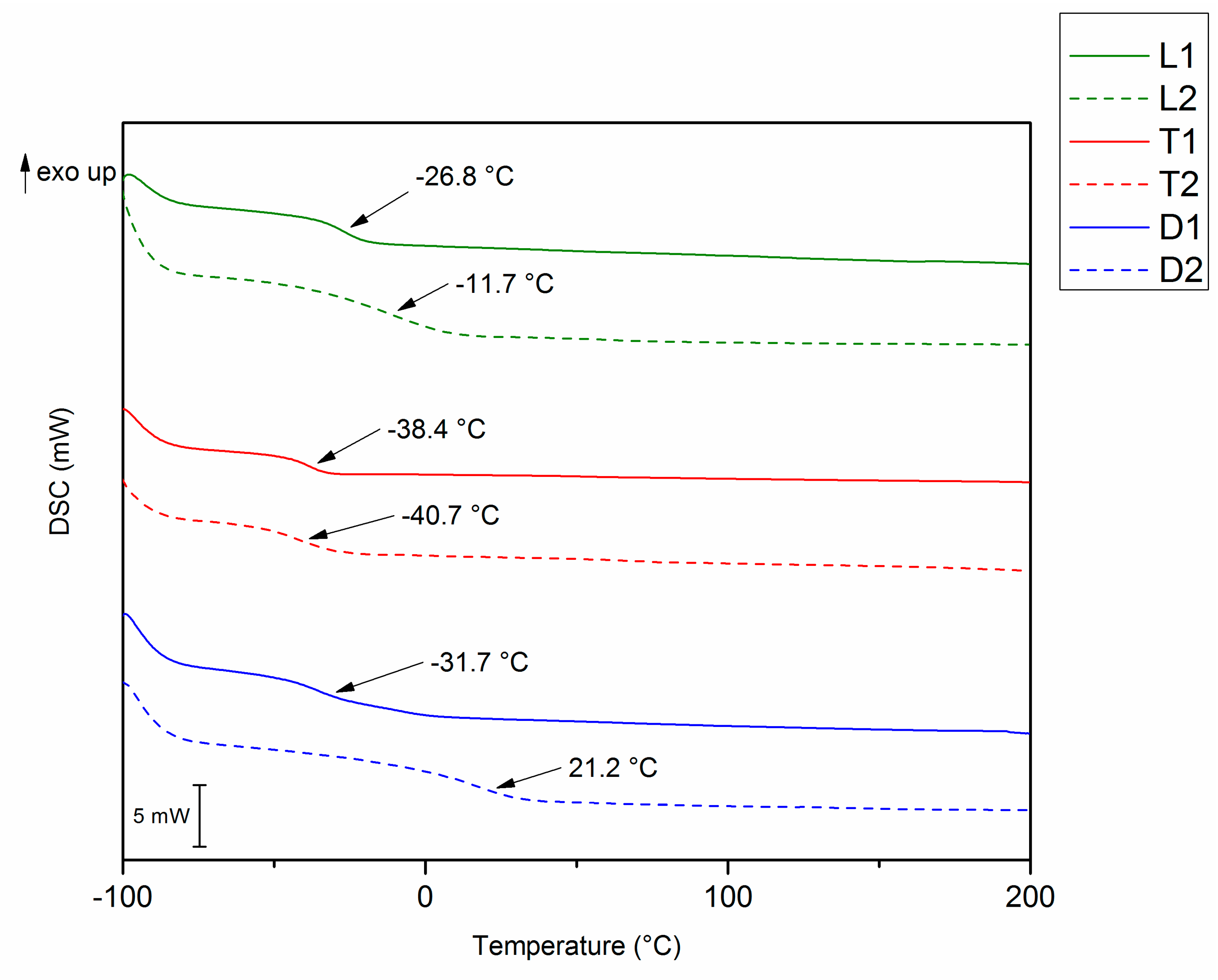
2.2.4. Differential Scanning Calorimetry (DSC)
2.2.5. Dynamic Mechanical Analyses (DMA)
2.2.6. Cross-Linking Density
2.2.7. Gelation Times
2.2.8. Swelling Index
2.2.9. Gel Content
2.2.10. Shore Hardness A
2.2.11. Synthesis of PUs with Castor Oil (One-Pot Method)
2.2.12. Synthesis of PUs with Velvetol® H500 and a Chain Extender (Two-Step Method)
2.2.13. Synthesis of PUs with Velvetol® H500 (One-Pot Method)
3. Results and Discussion
3.1. Synthesis of PUs
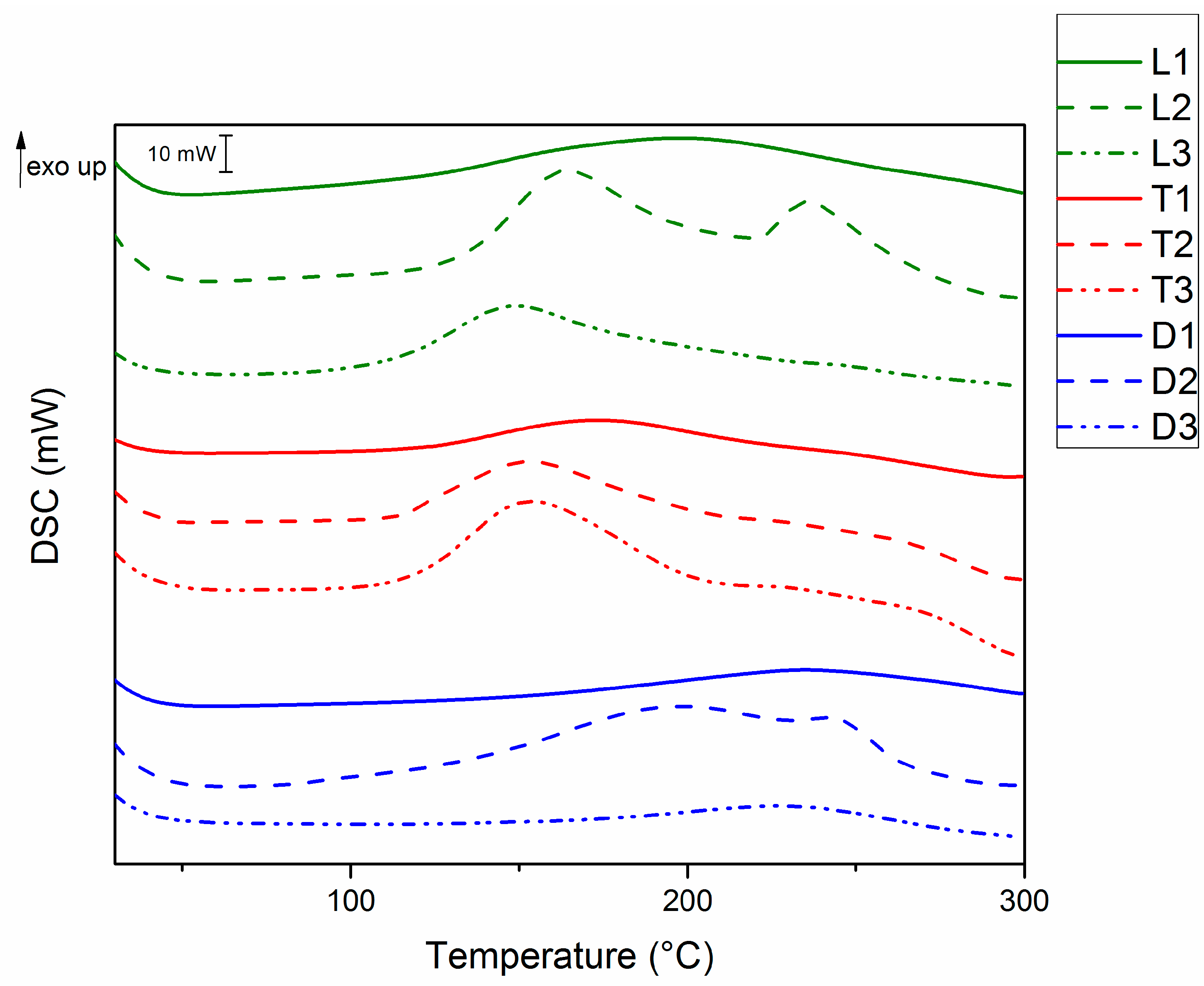
3.2. Curing Behavior
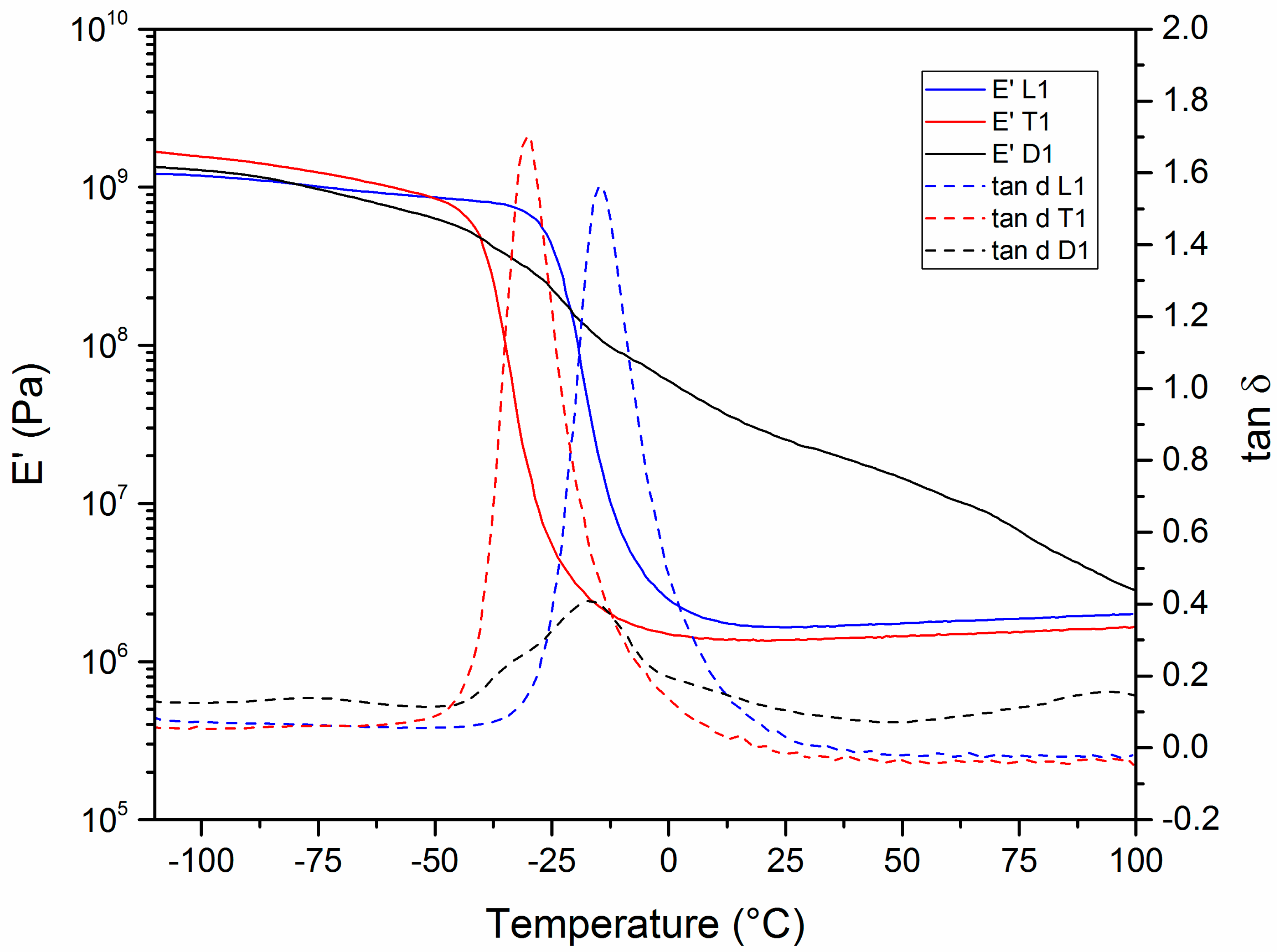
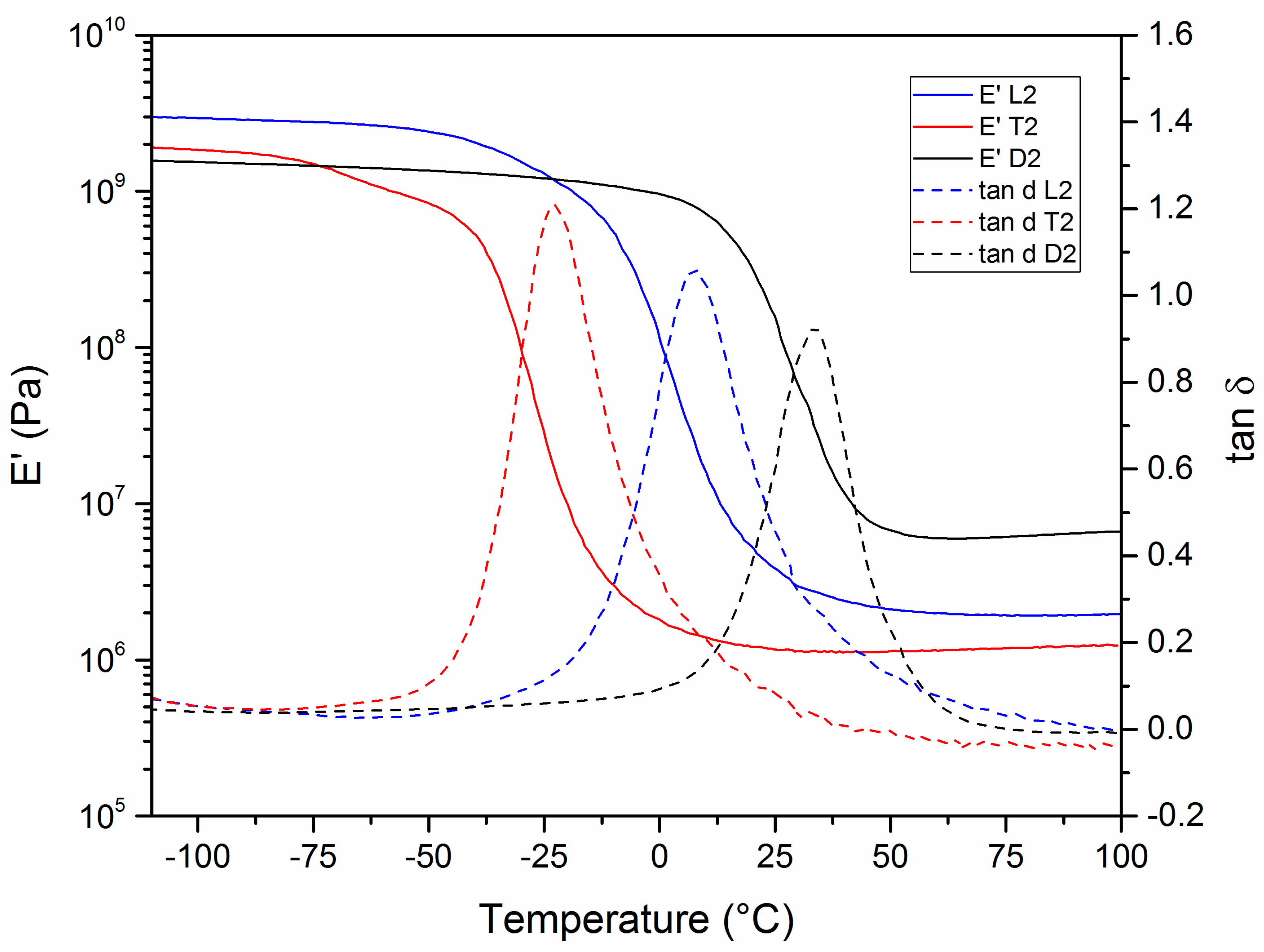
3.3. Thermomechanical Properties
3.4. Swelling Index, Gel Content, and Shore Hardness Values
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Skoczinski, P.; Chinthapalli, R.; Carus, M.; Baltus, W.; Guzman, d.D.; Käb, H.; Raschka, A.; Ravenstijn, J. Bio-Based Building Blocks and Polymers-Global Capacities, Production and Trends 2019–2024; Nova-Institute: Hurth, Germany, 2019. [Google Scholar]
- Álvarez-Chávez, C.R.; Edwards, S.; Moure-Eraso, R.; Geiser, K. Sustainability of bio-based plastics: General comparative analysis and recommendations for improvement. J. Clean. Prod. 2012, 23, 47–56. [Google Scholar] [CrossRef]
- Caillol, S.; Boutevin, B.; Pascault, J.-P. Bio-Sourced Epoxy Monomers and Polymers. In Handbook of Adhesive Technology; CRC Press: Boca Raton, FL, USA, 2017. [Google Scholar]
- Nikafshar, S.; Zabihi, O.; Hamidi, S.; Moradi, Y.; Barzegar, S.; Ahmadi, M.; Naebe, M. A renewable bio-based epoxy resin with improved mechanical performance that can compete with DGEBA. RSC Adv. 2017, 7, 8694–8701. [Google Scholar] [CrossRef] [Green Version]
- Rad, E.R.; Vahabi, H.; de Anda, A.R.; Saeb, M.R.; Thomas, S. Bio-epoxy resins with inherent flame retardancy. Prog. Org. Coat. 2019, 135, 608–612. [Google Scholar] [CrossRef]
- Vahabi, H.; Rohani Rad, E.; Parpaite, T.; Langlois, V.; Saeb, M.R. Biodegradable polyester thin films and coatings in the line of fire: The time of polyhydroxyalkanoate (PHA)? Prog. Org. Coat. 2019, 133, 85–89. [Google Scholar] [CrossRef]
- Winnacker, M.; Rieger, B. Biobased Polyamides: Recent Advances in Basic and Applied Research. Macromol. Rapid Commun. 2016, 37, 1391–1413. [Google Scholar] [CrossRef] [PubMed]
- Bayer, O.; Siefken, W.; Rinke, H.; Orthner, L.; Schild, H. A Process for the Production of Polyurethanes and Polyureas [Verfahren zur Herstellung von Polyurethanen bzw. Polyharnstoffen]; DE728981C; Thieme Gruppe: Stuttgart, Germany, 1937. [Google Scholar]
- Akindoyo, J.O.; Beg, M.D.H.; Ghazali, S.; Islam, M.R.; Jeyaratnam, N.; Yuvaraj, A.R. Polyurethane types, synthesis and applications—A review. RSC Adv. 2016, 6, 114453–114482. [Google Scholar] [CrossRef] [Green Version]
- Rokicki, G.; Parzuchowski, P.G.; Mazurek, M.M. Non-isocyanate polyurethanes: Synthesis, properties, and applications. Polym. Adv. Technol. 2015, 26, 707–761. [Google Scholar] [CrossRef]
- Cornille, A.; Auvergne, R.; Figovsky, O.; Boutevin, B.; Caillol, S. A perspective approach to sustainable routes for non-isocyanate polyurethanes. Eur. Polym. J. 2017, 87, 535–552. [Google Scholar] [CrossRef]
- Delebecq, E.; Pascault, J.-P.; Boutevin, B.; Ganachaud, F. On the Versatility of Urethane/Urea Bonds: Reversibility, Blocked Isocyanate, and Non-isocyanate Polyurethane. Chem. Rev. 2013, 113, 80–118. [Google Scholar] [CrossRef]
- Kathalewar, M.S.; Joshi, P.B.; Sabnis, A.S.; Malshe, V.C. Non-isocyanate polyurethanes: From chemistry to applications. RSC Adv. 2013, 3, 4110–4129. [Google Scholar] [CrossRef]
- Maisonneuve, L.; Lamarzelle, O.; Rix, E.; Grau, E.; Cramail, H. Isocyanate-Free Routes to Polyurethanes and Poly(hydroxy Urethane)s. Chem. Rev. 2015, 115, 12407–12439. [Google Scholar] [CrossRef] [Green Version]
- Błażek, K.; Datta, J. Renewable natural resources as green alternative substrates to obtain bio-based non-isocyanate polyurethanes-review. Crit. Rev. Environ. Sci. Technol. 2019, 49, 173–211. [Google Scholar] [CrossRef]
- Quienne, B.; Poli, R.; Pinaud, J.; Caillol, S. Enhanced aminolysis of cyclic carbonates by β-hydroxylamines for the production of fully biobased polyhydroxyurethanes. Green Chem. 2021, 23, 1678–1690. [Google Scholar] [CrossRef]
- Yebo, L.; Xiaolan, L.; Shengjun, H. Bio-Based Polyols and Polyurethanes; Springer: Cham, Switzerland, 2015. [Google Scholar]
- Hojabri, L.; Kong, X.; Narine, S.S. Novel long chain unsaturated diisocyanate from fatty acid: Synthesis, characterization, and application in bio-based polyurethane. J. Polym. Sci. Part. A Polym. Chem. 2010, 48, 3302–3310. [Google Scholar] [CrossRef]
- Kyriacos, D. Biobased Polyols for Industrial Polymers; John Wiley & Sons: Hoboken, NJ, USA, 2020. [Google Scholar]
- Dworakowska, S.; Bogdal, D.; Prociak, A. Microwave-Assisted Synthesis of Polyols from Rapeseed Oil and Properties of Flexible Polyurethane Foams. Polymers 2012, 4, 1462–1477. [Google Scholar] [CrossRef] [Green Version]
- Lligadas, G.; Ronda, J.C.; Galià, M.; Cádiz, V. Plant Oils as Platform Chemicals for Polyurethane Synthesis: Current State-of-the-Art. Biomacromolecules 2010, 11, 2825–2835. [Google Scholar] [CrossRef]
- Seydibeyoğlu, M.O.; Misra, M.; Mohanty, A.; Blaker, J.J.; Lee, K.-Y.; Bismarck, A.; Kazemizadeh, M. Green polyurethane nanocomposites from soy polyol and bacterial cellulose. J. Mater. Sci. 2012, 48, 2167–2175. [Google Scholar] [CrossRef] [Green Version]
- Tenorio-Alfonso, A.; Sánchez, M.C.; Franco, J.M. A Review of the Sustainable Approaches in the Production of Bio-based Polyurethanes and Their Applications in the Adhesive Field. J. Polym. Environ. 2020, 28, 749–774. [Google Scholar] [CrossRef] [Green Version]
- Furtwengler, P.; Avérous, L. Renewable polyols for advanced polyurethane foams from diverse biomass resources. Polym. Chem. 2018, 9, 4258–4287. [Google Scholar] [CrossRef]
- Trovati, G.; Suman, M.V.N.; Sanches, E.A.; Campelo, P.H.; Neto, R.B.; Neto, S.C.; Trovati, L.R. Production and characterization of polyurethane castor oil (Ricinus communis) foam for nautical fender. Polym. Test. 2019, 73, 87–93. [Google Scholar] [CrossRef]
- Rwahwire, S.; Tomkova, B.; Periyasamy, A.P.; Kale, B.M. Chapter 3—Green thermoset reinforced biocomposites. In Green Composites for Automotive Applications; Koronis, G., Silva, A., Eds.; Woodhead Publishing: Cambridge, UK, 2019; pp. 61–80. [Google Scholar]
- Severino, L.S.; Auld, D.L.; Baldanzi, M.; Cândido, M.J.D.; Chen, G.; Crosby, W.L.; Tan, D.; He, X.; Lakshmamma, P.; Lavanya, C.; et al. A Review on the Challenges for Increased Production of Castor. Agron. J. 2012, 104, 853–880. [Google Scholar] [CrossRef] [Green Version]
- Pfister, D.P.; Xia, Y.; LaRock, R.C. Recent Advances in Vegetable Oil-Based Polyurethanes. ChemSusChem 2011, 4, 703–717. [Google Scholar] [CrossRef] [PubMed]
- Ghasemlou, M.; Daver, F.; Ivanova, E.P.; Adhikari, B. Polyurethanes from seed oil-based polyols: A review of synthesis, mechanical and thermal properties. Ind. Crop. Prod. 2019, 142, 111841. [Google Scholar] [CrossRef]
- Hablot, E.; Zheng, D.; Bouquey, M.; Avérous, L. Polyurethanes Based on Castor Oil: Kinetics, Chemical, Mechanical and Thermal Properties. Macromol. Mater. Eng. 2008, 293, 922–929. [Google Scholar] [CrossRef]
- Desroches, M.; Escouvois, M.; Auvergne, R.; Caillol, S.; Boutevin, B. From Vegetable Oils to Polyurethanes: Synthetic Routes to Polyols and Main Industrial Products. Polym. Rev. 2012, 52, 38–79. [Google Scholar] [CrossRef] [Green Version]
- Ogunniyi, D.S. Castor oil: A vital industrial raw material. Bioresour. Technol. 2006, 97, 1086–1091. [Google Scholar] [CrossRef]
- Mutlu, H.; Meier, M.A.R. Castor oil as a renewable resource for the chemical industry. Eur. J. Lipid Sci. Technol. 2010, 112, 10–30. [Google Scholar] [CrossRef]
- Gebremariam, S.; Marchetti, J. Economics of biodiesel production: Review. Energy Convers. Manag. 2018, 168, 74–84. [Google Scholar] [CrossRef]
- Harmer, M.A.; Confer, D.C.; Hoffman, C.K.; Jackson, S.C.; Liauw, A.Y.; Minter, A.R.; Murphy, E.R.; Spence, R.E.; Sunkara, H.B. Renewably sourced polytrimethylene ether glycol by superacid catalyzed condensation of 1,3-propanediol. Green Chem. 2010, 12, 1410–1416. [Google Scholar] [CrossRef]
- Sonnenschein, M.F. Polyurethane Building Blocks. In Polyurethanes; Wiley: Hoboken, NJ, USA, 2014; pp. 10–104. [Google Scholar]
- Thomas, J.; Singh, V.; Jain, R. Synthesis and characterization of solvent free acrylic copolymer for polyurethane coatings. Prog. Org. Coat. 2020, 145, 105677. [Google Scholar] [CrossRef]
- Kasprzyk, P.; Datta, J. Effect of molar ratio [NCO]/[OH] groups during prepolymer chains extending step on the morphology and selected mechanical properties of final bio-based thermoplastic poly(ether-urethane) materials. Polym. Eng. Sci. 2018, 58, E199–E206. [Google Scholar] [CrossRef]
- Kasprzyk, P.; Sadowska, E.; Datta, J. Investigation of Thermoplastic Polyurethanes Synthesized via Two Different Prepolymers. J. Polym. Environ. 2019, 27, 2588–2599. [Google Scholar] [CrossRef] [Green Version]
- More, A.S.; Lebarbé, T.; Maisonneuve, L.; Gadenne, B.; Alfos, C.; Cramail, H. Novel fatty acid based di-isocyanates towards the synthesis of thermoplastic polyurethanes. Eur. Polym. J. 2013, 49, 823–833. [Google Scholar] [CrossRef]
- Tawade, B.; Shingte, R.; Kuhire, S.; Sadavarte, N.; Garg, K.; Maher, D.; Ichake, A.; More, A.; Wadgaonkar, P. Bio-based di/ polyisocyanates for polyurethanes: An overview. Polyurethanes Today 2017, 11, 1202. [Google Scholar]
- Kamal, M.R.; Kuder, R.C. Diisocyanates. U.S. Patent 3691225A, 12 September 1972. [Google Scholar]
- Chiacchiarelli, L.M. 8-Sustainable, nanostructured, and bio-based polyurethanes for energy-efficient sandwich structures applied to the construction industry. In Biomass, Biopolymer-Based Materials, and Bioenergy, Verma, D.; Fortunati, E., Jain, S., Zhang, X., Eds.; Woodhead Publishing: Cambridge, UK, 2019; pp. 135–160. [Google Scholar]
- Bishop, T.E.; Coady, C.J.; Zimmerman, J.M. Ultraviolet Curable Buffer Coatings for Optical Glass Fiber Based on Long Chain Oxyalkylene Diamines. U.S. Patent 4609718A, 2 September 1986. [Google Scholar]
- Coady, C.J.; Krajewski, J.J.; Bishop, T.E. Polyacrylated Oligomers in Ultraviolet Curable Optical Fiber Coatings. U.S. Patent 4608409A, 26 August 1986. [Google Scholar]
- Charlon, M.; Heinrich, B.; Matter, Y.; Couzigné, E.; Donnio, B.; Avérous, L. Synthesis, structure and properties of fully biobased thermoplastic polyurethanes, obtained from a diisocyanate based on modified dimer fatty acids, and different renewable diols. Eur. Polym. J. 2014, 61, 197–205. [Google Scholar] [CrossRef]
- Das, S.; Pandey, P.; Mohanty, S.; Nayak, S.K. Influence of NCO/OH and transesterified castor oil on the structure and properties of polyurethane: Synthesis and characterization. Mater. Express 2015, 5, 377–389. [Google Scholar] [CrossRef]
- Sahoo, S.; Kalita, H.; Mohanty, S.; Nayak, S. Synthesis and Characterization of Vegetable oil based Polyurethane derived from Biobased isocyanate. J. Polym. Mater. 2017, 34, 601–613. [Google Scholar]
- Wang, C.; Cao, X.; Zhang, Y. A novel bioactive osteogenesis scaffold delivers ascorbic acid, β-glycerophosphate, and dexamethasone in vivo to promote bone regeneration. Oncotarget 2017, 8, 31612–31625. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hao, H.; Shao, J.; Deng, Y.; He, S.; Luo, F.; Wu, Y.; Li, J.; Tan, H.; Li, J.; Fu, Q. Synthesis and characterization of biodegradable lysine-based waterborne polyurethane for soft tissue engineering applications. Biomater. Sci. 2016, 4, 1682–1690. [Google Scholar] [CrossRef]
- Guelcher, S.A.; Srinivasan, A.; Dumas, J.E.; Didier, J.E.; McBride, S.; Hollinger, J.O. Synthesis, mechanical properties, biocompatibility, and biodegradation of polyurethane networks from lysine polyisocyanates. Biomaterials 2008, 29, 1762–1775. [Google Scholar] [CrossRef]
- Storey, R.F.; Wiggins, J.S.; Puckett, A.D. Hydrolyzable poly(ester-urethane) networks from L-lysine diisocyanate and D,L-lactide/ε-caprolactone homo- and copolyester triols. J. Polym. Sci. Part. A Polym. Chem. 1994, 32, 2345–2363. [Google Scholar] [CrossRef]
- Lee, S.-H.; Wang, S. Biodegradable polymers/bamboo fiber biocomposite with bio-based coupling agent. Compos. Part. A Appl. Sci. Manuf. 2006, 37, 80–91. [Google Scholar] [CrossRef]
- Liu, H.; Chen, N.; Shan, P.; Song, P.; Liu, X.; Chen, J. Toward Fully Bio-based and Supertough PLA Blends via in Situ Formation of Cross-Linked Biopolyamide Continuity Network. Macromolecules 2019, 52, 8415–8429. [Google Scholar] [CrossRef]
- Acik, G.; Karabulut, H.R.F.; Altinkok, C.; Karatavuk, A.O. Synthesis and characterization of biodegradable polyurethanes made from cholic acid and l-lysine diisocyanate ethyl ester. Polym. Degrad. Stab. 2019, 165, 43–48. [Google Scholar] [CrossRef]
- Acik, G.; Kamaci, M.; Altinkok, C.; Karabulut, H.F.; Tasdelen, M.A. Synthesis and properties of soybean oil-based biodegradable polyurethane films. Prog. Org. Coat. 2018, 123, 261–266. [Google Scholar] [CrossRef]
- Cifarelli, A.; Boggioni, L.; Vignali, A.; Tritto, I.; Bertini, F.; Losio, S. Flexible Polyurethane Foams from Epoxidized Vegetable Oils and a Bio-Based Diisocyanate. Polymers 2021, 13, 612. [Google Scholar] [CrossRef] [PubMed]
- Sahoo, S.; Kalita, H.; Mohanty, S.; Nayak, S.K. Meticulous study on curing kinetics of green polyurethane-clay nanocomposite adhesive derived from plant oil: Evaluation of decomposition activation energy using TGA analysis. J. Macromol. Sci. Part. A 2017, 54, 819–826. [Google Scholar] [CrossRef]
- Gurunathan, T.; Chung, J.S. Physicochemical Properties of Amino–Silane-Terminated Vegetable Oil-Based Waterborne Polyurethane Nanocomposites. ACS Sustain. Chem. Eng. 2016, 4, 4645–4653. [Google Scholar] [CrossRef]
- Hu, J.; Chen, Z.; He, Y.; Huang, H.; Zhang, X. Synthesis and structure investigation of hexamethylene diisocyanate (HDI)-based polyisocyanates. Res. Chem. Intermed. 2017, 43, 2799–2816. [Google Scholar] [CrossRef]
- Widemann, M.; Driest, P.J.; Orecchia, P.; Naline, F.; Golling, F.E.; Hecking, A.; Eggert, C.; Pires, R.; Danielmeier, K.; Richter, F.U. Structure–Property Relations in Oligomers of Linear Aliphatic Diisocyanates. ACS Sustain. Chem. Eng. 2018, 6, 9753–9759. [Google Scholar] [CrossRef]
- Kurańska, M.; Cabulis, U.; Auguścik, M.; Prociak, A.; Ryszkowska, J.; Kirpluks, M. Bio-based polyurethane-polyisocyanurate composites with an intumescent flame retardant. Polym. Degrad. Stab. 2016, 127, 11–19. [Google Scholar] [CrossRef]
- Sasaki, N.; Yokoyama, T.; Tanaka, T. Properties of isocyanurate-type crosslinked polyurethanes. J. Polym. Sci. Polym. Chem. Ed. 1973, 11, 1765–1779. [Google Scholar] [CrossRef]
- Levita, G.; De Petris, S.; Marchetti, A.; Lazzeri, A. Crosslink density and fracture toughness of epoxy resins. J. Mater. Sci. 1991, 26, 2348–2352. [Google Scholar] [CrossRef]
- Schroeder, J.A.; Madsen, P.A.; Foister, R.T. Structure/property relationships for a series of crosslinked aromatic/aliphatic epoxy mixtures. Polymers 1987, 28, 929–940. [Google Scholar] [CrossRef]
- Lapprand, A.; Boisson, F.; Delolme, F.; Méchin, F.; Pascault, J.-P. Reactivity of isocyanates with urethanes: Conditions for allophanate formation. Polym. Degrad. Stab. 2005, 90, 363–373. [Google Scholar] [CrossRef]
- Olejnik, A.; Gosz, K.; Piszczyk, L. Kinetics of cross-linking processes of fast-curing polyurethane system. Thermochim. Acta 2020, 683, 178435. [Google Scholar] [CrossRef]
- Vyazovkin, S.; Burnham, A.K.; Favergeon, L.; Koga, N.; Moukhina, E.; Pérez-Maqueda, L.A.; Sbirrazzuoli, N. ICTAC Kinetics Committee recommendations for analysis of multi-step kinetics. Thermochim. Acta 2020, 689, 178597. [Google Scholar] [CrossRef]
- Rochery, M.; Lam, T.M. Chemorheology of polyurethane. I. Vitrification and gelation studies. J. Polym. Sci. Part. B Polym. Phys. 2000, 38, 544–551. [Google Scholar] [CrossRef]
- Kasprzyk, P.; Benes, H.; Donato, R.K.; Datta, J. The role of hydrogen bonding on tuning hard-soft segments in bio-based thermoplastic poly(ether-urethane)s. J. Clean. Prod. 2020, 274, 122678. [Google Scholar] [CrossRef]
- Hassan, M.K.; Mauritz, K.A.; Storey, R.F.; Wiggins, J.S. Biodegradable aliphatic thermoplastic polyurethane based on poly(ε-caprolactone) and L-lysine diisocyanate. J. Polym. Sci. A Polym. Chem. 2006, 44, 2990–3000. [Google Scholar] [CrossRef]
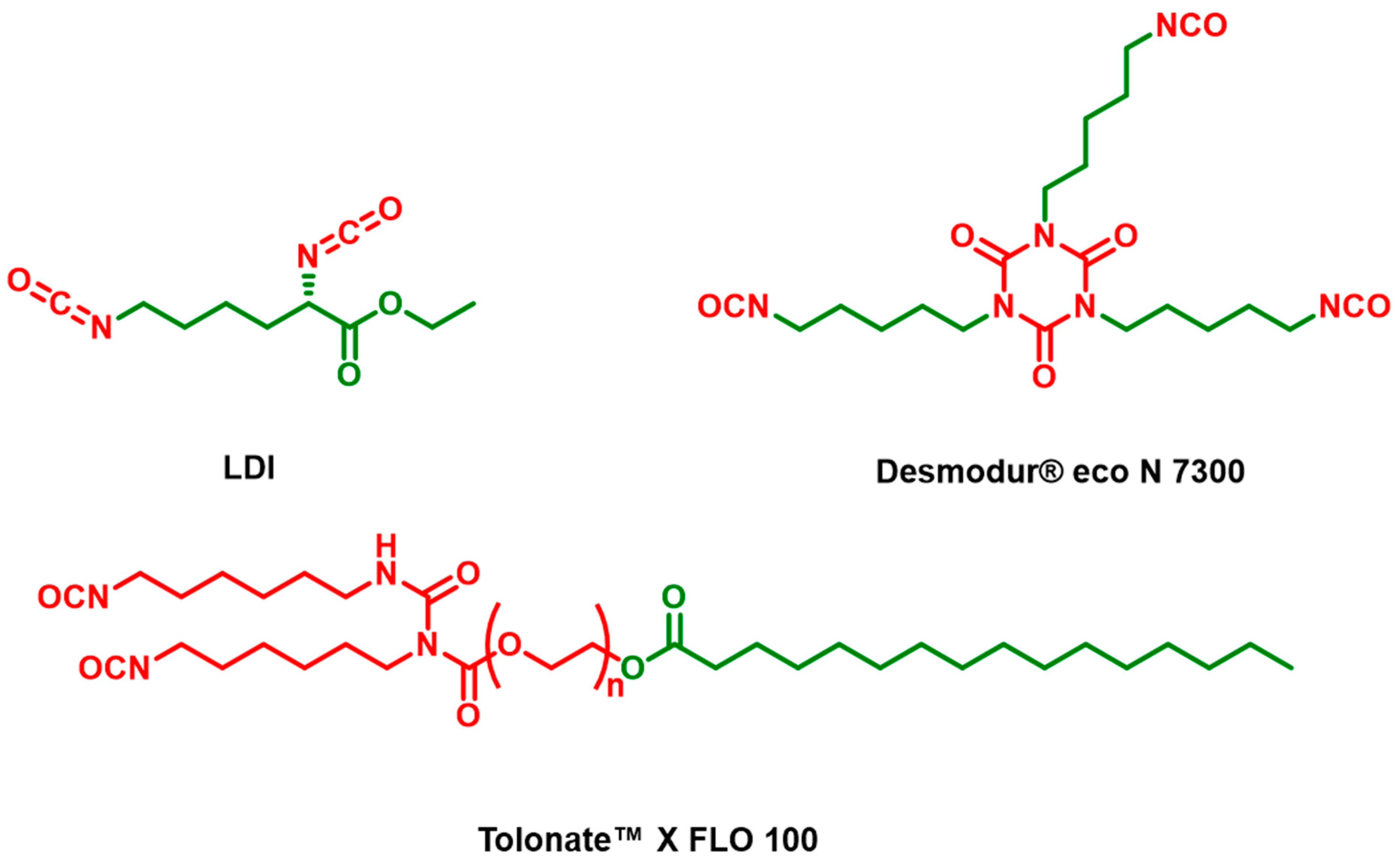



| Entry | Polyisocyanate | Polyol | Method (Chain Extender) | Bio-Based Carbon Content (%) |
|---|---|---|---|---|
| L1 | LDI | Castor oil | One-pot | 95 |
| L2 | LDI | Velvetol® H500 | Two-step (glycerol) | 90 |
| L3 | LDI | Velvetol® H500 + 5 wt% Glycerol | One-pot | 94 |
| T1 | Tolonate™ X FLO 100 | Castor oil | One-pot | 66 |
| T2 | Tolonate™ X FLO 100 | Velvetol® H500 | Two-step (glycerol) | 50 |
| T3 | Tolonate™ X FLO 100 | Velvetol® H500 + 5 wt% Glycerol | One-pot | 61 |
| D1 | Desmodur® eco N 7300 | Castor oil | One-pot | 89 |
| D2 | Desmodur® eco N 7300 | Velvetol® H500 | Two-step (1,3-propanediol) | 79 |
| D3 | Desmodur® eco N 7300 | Velvetol® H500 | One-pot | 87 |
| Frequency | Gelation Times (min) | |||||
|---|---|---|---|---|---|---|
| L1 | L2 | T1 | T2 | D1 | D2 | |
| 1 Hz | 140 | 328 | 327 | 80 | 107 | 32 |
| 2.6 Hz | 142 | 435 | 332 | 83 | 107 | 35 |
| 7 Hz | 143 | 513 | 340 | 88 | 108 | 38 |
| Average | 142 ± 2 | 425 ± 93 | 333 ± 7 | 84 ± 4 | 107 ± 1 | 35 ± 3 |
| Sample | Tg (°C) a | Tα (°C) b | E’ (Pa) (Glassy) | E’ (Pa) (Rubbery) | ν (mol·m−3) | Td5% (°C) | Char Yield (800 °C, %) | Td5% (°C) | Char Yield (800 °C, %) |
|---|---|---|---|---|---|---|---|---|---|
| (Tα − 50 °C) | (Tα + 50 °C) | Argon | Air | ||||||
| L1 | −27 | −14 | 9.3·108 | 1.7·106 | 221 | 316 | 1 | 317 | 0 |
| L2 | −12 | 7 | 2.2·109 | 2.0·106 | 243 | 299 | 2 | 288 | 0 |
| T1 | −38 | −30 | 1.3·109 | 1.4·106 | 192 | 303 | 0 | 297 | 0 |
| T2 | −41 | −23 | 1.4·109 | 1.2·106 | 160 | 292 | 0 | 290 | 0 |
| D1 | −32 | −17 | 8.6·108 | 2.1·107 | n.d. | 326 | 0 | 332 | 0 |
| D2 | 21 | 33 | 1.1·109 | 6.3·106 | 710 | 315 | 4 | 319 | 1 |
| Entry | SI (%) | GC (%) | Shore A Hardness (ShA) |
|---|---|---|---|
| L1 | 353 | 94 | 38.0 ± 0.2 |
| L2 | 266 | 96 | 57.1 ± 3.0 |
| T1 | 403 | 92 | 49.4 ± 0.4 |
| T2 | 420 | 94 | 41.2 ± 1.0 |
| D1 | 275 | 75 | 57.3 ± 1.0 |
| D2 | 107 | 98 | 85.2 ± 1.2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Morales-Cerrada, R.; Tavernier, R.; Caillol, S. Fully Bio-Based Thermosetting Polyurethanes from Bio-Based Polyols and Isocyanates. Polymers 2021, 13, 1255. https://doi.org/10.3390/polym13081255
Morales-Cerrada R, Tavernier R, Caillol S. Fully Bio-Based Thermosetting Polyurethanes from Bio-Based Polyols and Isocyanates. Polymers. 2021; 13(8):1255. https://doi.org/10.3390/polym13081255
Chicago/Turabian StyleMorales-Cerrada, Roberto, Romain Tavernier, and Sylvain Caillol. 2021. "Fully Bio-Based Thermosetting Polyurethanes from Bio-Based Polyols and Isocyanates" Polymers 13, no. 8: 1255. https://doi.org/10.3390/polym13081255







