Full Exploitation of Peach Palm (Bactris gasipaes Kunth): State of the Art and Perspectives
Abstract
1. Introduction
2. History of Peach Palm (Bactris gasipaes Kunth)
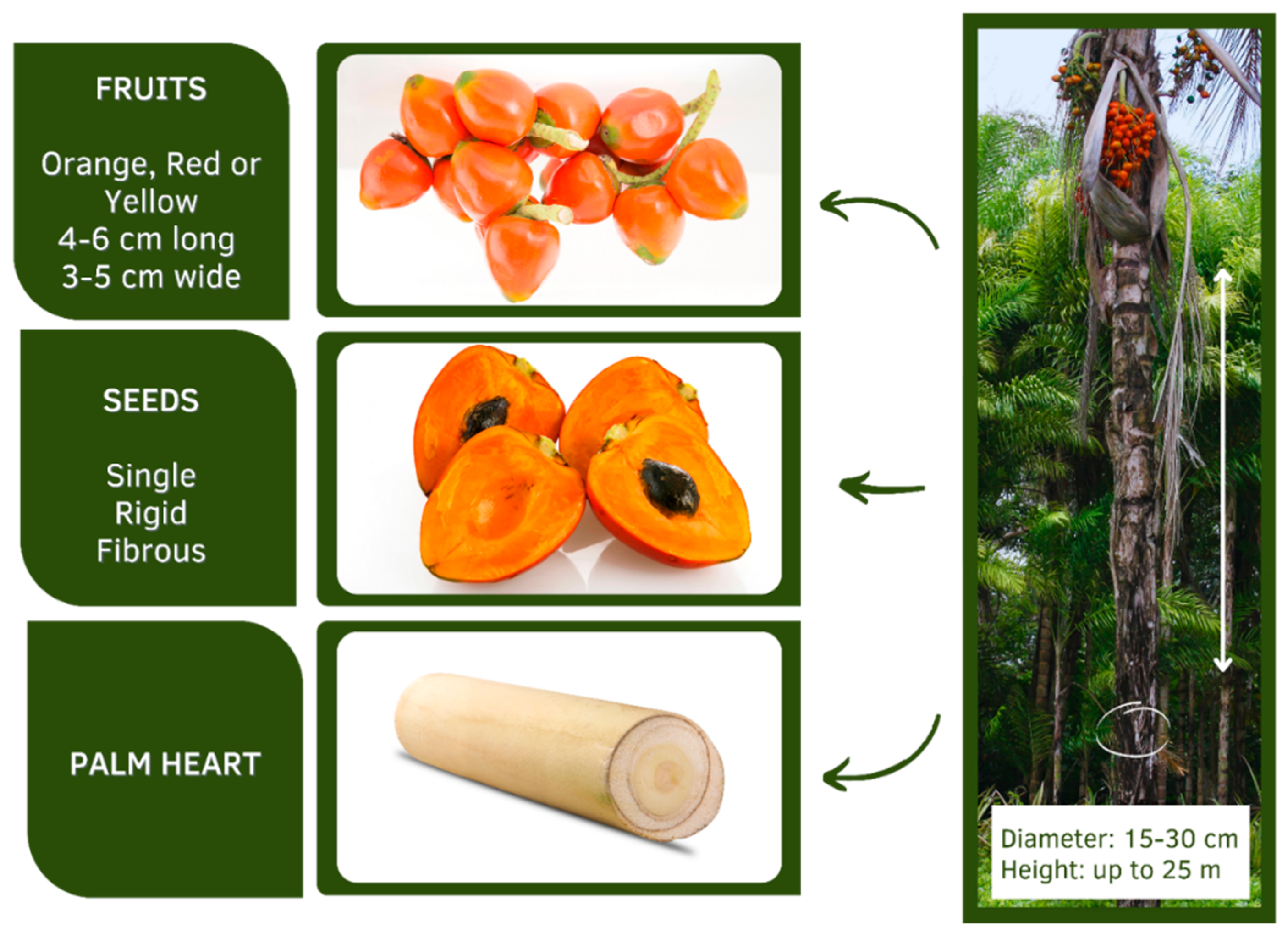
3. Botanical and Morphological Aspects of Bactris gasipaes Kunth
4. Edible Parts of the Peach Palm: Palm Heart and Fruits
4.1. Peach Palm Fruit
4.2. Peach Heart
| Component | Pupunha Palm Heart (100 g) | Juçara Palm Heart (100 g) |
|---|---|---|
| Moisture | 89.4% | 91.4% |
| Energetic value | 29.0 kcal = 124 kJ | 23.0 kcal = 97 kJ |
| Carbohydrates | 5.5 g | 4.3 g |
| Proteins | 2.5 g | 1.8 g |
| Lipids | 0.5 mg | 0.4 g |
| Dietary fibers | 2.6 g | 3.2 g |
| Ashes | 2.1 g | 2.1 g |
| Calcium, Ca | 32.4 mg | 58.0 mg |
| Vitamin C | 8.7 mg | 2.0 mg |
| Phosphorus, P | 55 mg | 40 mg |
| Manganese, Mn | 0.14 mg | - |
| Magnesium, Mg | 25.5 mg | - |
| Iron, Fe | 0.2 mg | - |
| Potassium, K | 206.4 mg | - |
| Cooper, Cu | 0.1 µg | - |
| Zinc, Zn | 0.4 mg | - |
| Sodium, Na | 562.7 mg | - |
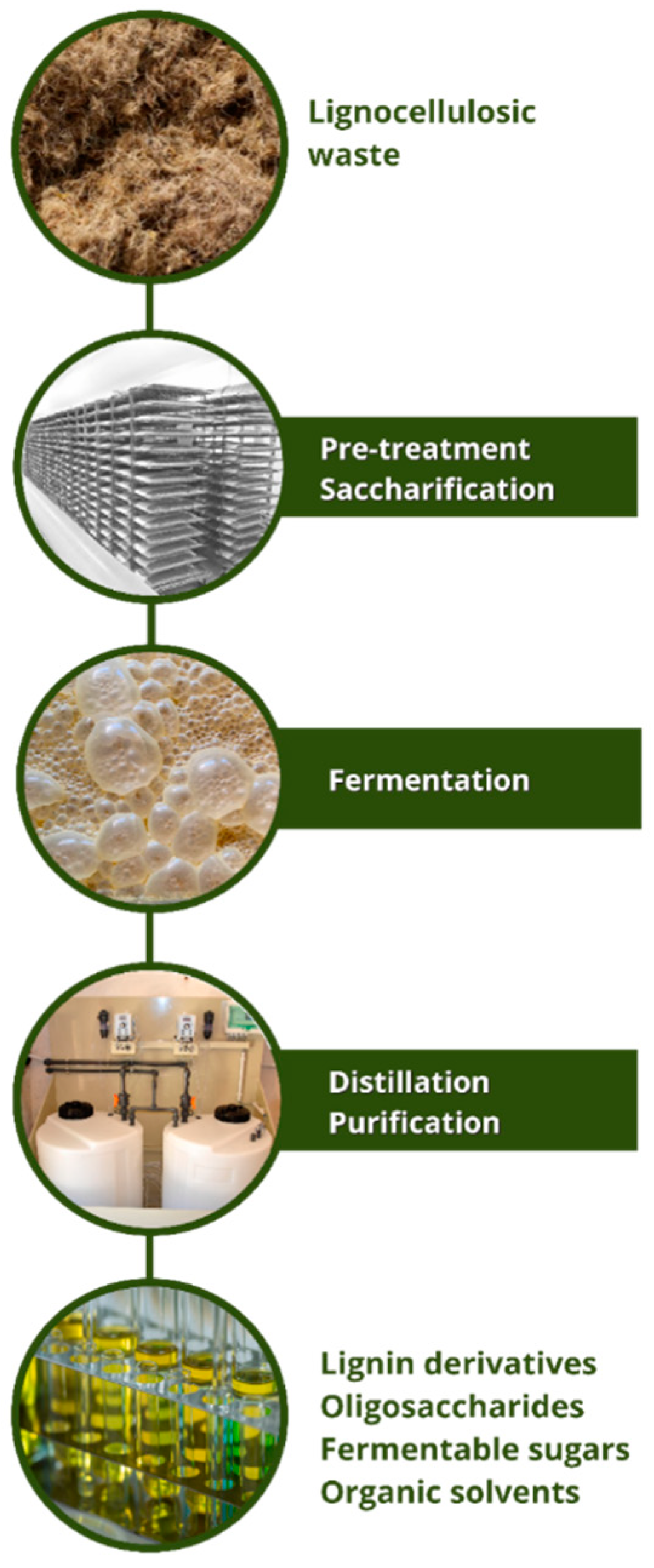
5. Lignocellulosic Residues of Peach Palm
5.1. Obtaining High Fiber Flours through B. gasipaes Lignocellulosic Wastes
5.2. Obtaining Prebiotics (Xylooligosaccharides) B. gasipaes Lignocellulosic Wastes
5.3. Obtaining Cellulose Nanofibrils from Lignocellulosic Residues of Peach Palm
5.4. Application of Peach Palm Lignocellulosic Residues as Substrate for Cultivation of Microorganisms and Obtainment of Useful Molecules
6. Patents Based on Bactris gasipaes Products
7. Concluding Remarks
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Bezerra, C.V.; Silva, L.H.M.D. Pupunha (Bactris gasipaes): General and consumption aspects. In Traditional Foods; Kristbergsson, K., Oliveira, J., Eds.; Springer: Boston, MA, USA, 2016; pp. 399–405. [Google Scholar] [CrossRef]
- Pires, M.B.; Amante, E.R.; Lopes, A.S.; Rodrigues, A.M.C.; Silva, L.H.M. Peach palm flour (Bactris gasipaes Kunth): Potential application in the food industry. Food Sci. Technol. 2019, 39, 613–619. [Google Scholar] [CrossRef]
- Felisberto, M.H.F.; Costa, M.S.; Boas, F.V.; Leiva, C.L.; Franco, C.M.L.; Souza, S.M.; Clerici, M.T.P.S.; Cordeiro, L.M.C. Characterization and technological properties of peach palm (Bactris gasipaes var. gasipaes) fruit starch. Food Res. Int. 2020, 136, 109569. [Google Scholar] [CrossRef] [PubMed]
- Mora-Urpí, J.; Weber, J.; Clement, C. Peach Palm Bactris gasipaes Kunth; Promoting the Conservation and Use of Underutilized and Neglected Crops; International Plant Genetics Resources Institute: Rome, Italy, 1997; p. 83. [Google Scholar]
- da Costa, R.D.S.D.; Rodrigues, A.M.D.C.; Silva, L.H.M.D. The fruit of peach palm (Bactris gasipaes) and its technological potential: An overview. Food Sci. Technol. 2022, 42, e82721. [Google Scholar] [CrossRef]
- Arkcoll, D.B.; Aguiar, J.P.L. Peach palm (Bactris gasipaes H.B.K.), a new source of vegetable oil from the wet tropics. J. Sci. Food Agric. 1984, 35, 520–526. [Google Scholar] [CrossRef]
- Santos, O.V.; Soares, S.D.; Dias, P.C.S.; Duarte, S.P.A.; Santos, M.P.L.; Nascimento, F.C.A. Chromatographic profile and bioactive compounds found in the composition of pupunha oil (Bactris gasipaes Kunth): Implications for human health. Rev. de Nutr. 2020, 33, e190146. [Google Scholar] [CrossRef]
- Spacki, K.C.; Vieira, T.F.; Helm, C.V.; de Lima, E.A.; Bracht, A.; Peralta, R.M. Pupunha (Bactris gasipaes Kunth):uma revisão. In Agricultura E Agroindústria No Contexto Do Desenvolvimento Rural Sustentável, 1st ed.; Lima, F.S., Melo Neto, B.A., Melo, G.J.A., Cavalcante, D.K., Santos, T.R., Eds.; Editora Científica Digital: Guarujá, Brazil, 2021; pp. 332–350. [Google Scholar] [CrossRef]
- Anefalos, L.C.; Modolo, V.A.; Tucci, M.L.S. Expansão do cultivo da pupunheira no Vale do Ribeira, Estado de São Paulo, 2002–2006. Inf. Econômicas 2007, 37, 37–43. [Google Scholar]
- Seben, L.; de Paula, I.C.; Viana, S.G. Análise do processo de beneficiamento da Palmeira Real da Austrália (palmito em conserva) para determinação das variáveis que influenciam as operações de valorização de seus resíduos. Prod. Produção 2012, 13, 75–92. [Google Scholar] [CrossRef]
- Oxford Dictionary. Upcycle, Oxford University Press. Available online: https://en.oxforddictionaries.com/definition/upcycle (accessed on 7 July 2022).
- Coppola, C.; Vollero, A.; Siano, A. Consumer upcycling as emancipated self-production: Understanding motivations and identifying upcycler types. J. Clean. Prod. 2021, 285, 124812. [Google Scholar] [CrossRef]
- Chisté, R.C.; Fernandes, E. Bioactive compounds from Amazonian fruits and their antioxidant properties. In Natural Bioactive Compounds from Fruits and Vegetables as Health Promoters—Part I; Silva, L.R., Silva, B.M., Eds.; Bentham Science Publishers: Sharjah, United Arab Emirates, 2016; pp. 244–264. [Google Scholar]
- Baker, W.J.; Couvreur, T.L.P. Global biogeography and diversification of palms sheds light on the evolution of tropical lineages. II. Diversification history and origin of regional assemblages. J. Biogeogr. 2013, 40, 286–298. [Google Scholar] [CrossRef]
- Couvreur, T.L.P.; Forest, F.; Baker, W.J. Origin and global diversification patterns of tropical rain forests: Inferences from a complete genus-level phylogeny of palms. BMC Biol. 2011, 9, 44–56. [Google Scholar] [CrossRef]
- Alves, W.F.; Ribeiro, G.S.; de Souza, M.C.; Souza, R.L.; Oliveira, F.N.L.; Mesquita, F.R. Análise físico-química do óleo essencial de pupunha (Bactris gasipaes Kunth-Arecaceae), do município de Cruzeiro do Sul, Acre, Brasil. Ciênc. Florest. 2021, 31, 533–549. [Google Scholar] [CrossRef]
- Cymerys, M.; Clement, C.R. Pupunha, Bactris gasipaes Kunth. In Frutíferas E Plantas úteis Na Vida Amazônica; Sanley, P., Medina, G., Eds.; CIFOR & Imazon: Belém, Brazil, 2005; pp. 203–208. [Google Scholar]
- Clement, C.R.; Weber, J.C.; van Leeuwen, J.; Astorga Domian, C.; Cole, D.M.; Arévalo Lopez, L.A.; Argüello, H. Why extensive research and development did not promote use of peach palm fruit in Latin America. Agrofor. Syst. 2004, 61, 195–206. [Google Scholar]
- Clement, C.R.; Urpí, J.E.M. Pejibaye palm (Bactris gasipaes, Arecaceae): Multi-use potential for the lowland humid tropics. Econ. Bot. 1987, 41, 302–311. [Google Scholar] [CrossRef]
- Clement, C.R. Domestication of the pejibaye palm (Bactris gasipaes): Past and present in the palm—tree of life: Biology, utilization and conservation. Adv. Econ. Bot. 1988, 6, 155–174. [Google Scholar]
- Ferreira, C.D.; Pena, R.S. Hygroscopic behavior of the pupunha flour (Bactris gasipaes). Food Sci. Technol. 2003, 23, 251–255. [Google Scholar] [CrossRef]
- Neri-Numa, I.A.; Sancho, R.A.S.; Pereira, A.P.A.; Pastore, G.M. Small Brazilian wild fruits: Nutrients, bioactive compounds, health-promotion properties, and commercial interest. Food Res. Int. 2018, 103, 345–360. [Google Scholar] [CrossRef]
- Villachica, H.; Chávez, E.; Sanchez, J. Informe Técnico. 30: Manejo Postcosecha e Industrialización Del Pijuayo (Bactris gasipaes Kunth.); Sector Agrario, Instituto Nacional de Investigación Agraria (INIA): Lima, Peru, 1994; p. 55. [Google Scholar]
- Matos, K.A.N.; Lima, D.P.; Barbosa, A.P.P.; Mercadante, A.Z.; Chisté, R.S. Peels of tucumã (Astrocaryum vulgare) and peach palm (Bactris gasipaes) are by-products classified as very high carotenoid sources. Food Chem. 2019, 272, 216–221. [Google Scholar] [CrossRef]
- Carvalho, A.N.; Beckman, J.C.; Maciel, R.A.; Farias Neto, J.T. Características físicas e químicas de frutos de pupunheira no Estado do Pará. Rev. Bras. Frutic. 2013, 15, 763–768. [Google Scholar] [CrossRef]
- de Souza Mesquisa, L.M.; Murador, D.C.; Neves, B.V.; Braga, A.R.C.; Pisani, L.P.; de Rosso, V.V. Bioaccessibility and cellular uptake of carotenoids extracted from Bactris gasipaes fruit: Differences between conventional and ionic liquid-mediated extraction. Molecules 2021, 30, 3989–4005. [Google Scholar] [CrossRef]
- Rojas-Garbanzo, C.; Pérez, A.M.; Castro, M.L.P.; Vaillant, F. Major physicochemical and antioxidant changes during peach-palm (Bactris gasipaes H.B.K.) flour processing. Fruits 2012, 67, 6415–6427. [Google Scholar] [CrossRef]
- Melo, C.M.T.; Costa, L.L.; Pereira, F.C.; Castro, L.M.; Nepumoceno, S. Physical and chemical analysis of fruit “in natura” of pupunha. Innov. Sci. Technol. J. Revista Inova Ciência e Tecnologia 2017, 3, 13–17. [Google Scholar]
- da Silva, R.F.; Furtado, M.T.; Rodrigues, D.P. Qualidade nutricional de frutos da pupunheira vermelha integral desidratados a diferentes temperaturas. Agrotec 2020, 41, 101–108. [Google Scholar] [CrossRef]
- Cantu-Jungles, T.M.; Cipriani, T.R.; Iacomini, M.; Hamaker, B.R.; Cordeiro, L.M.C. A pectic polysaccharide from peach palm fruits (Bactris gasipaes) and its fermentation profile by the human gut microbiota In Vitro. Bioact. Carbohydr. Diet. Fibre 2017, 9, 1–6. [Google Scholar] [CrossRef]
- Pereira, T.C.J.; Ribeiro, L.S.O.; Pires, A.J.V.; Pereira, M.L.A.; Santos, A.B.; Silva, H.G.O.; Carvalho, G.G.P. Growth performance and apparent digestibility by goats fed diets with peach palm meal replacing maize. Appl. Anim. Sci. 2019, 35, 563–569. [Google Scholar] [CrossRef]
- Rojas-Garbanzo, C.; Pérez, A.M.; Bustos-Carmona, J.; Vaillant, F. Identification and quantification of carotenoids by HPLC-DAD during the process of peach palm (Bactris gasipaes H.B.K.) flour. Food Res. Int. 2011, 44, 2377–2384. [Google Scholar] [CrossRef]
- Quesada, S.; Azofeifa, G.; Jatunov, S.; Jiménez, G.; Gó, G. Carotenoids composition, antioxidant activity and glycemic index of two varieties of Bactris gasipaes. Emir. J. Food Agric. 2011, 23, 482–489. [Google Scholar]
- dos Santos, M.F.G.; Mamede, R.V.S.; Rufino, M.S.M.; de Brito, E.S.; Alves, R.E. Amazonian native palm fruits as sources of antioxidant bioactive compounds. Antioxidants 2015, 4, 591–602. [Google Scholar] [CrossRef]
- Jaramillo-Vivanco, T.; Balslev, H.; Montúfar, R.; Cámara, R.M.; Giampieri, F.; Battino, M.; Cámara, M.; Alvarez-Suarez, J.M. Three Amazonian palms as underestimated and little-known sources of nutrients, bioactive compounds, and edible insects. Food Chem. 2022, 372, 131273. [Google Scholar] [CrossRef]
- Araujo, N.M.P.; Arruda, H.S.; Marques, D.R.; Oliveira, W.Q.; Pereira, G.A.; Pastore, G.M. Functional and nutritional properties of selected Amazon fruits: A review. Food Res. Int. 2021, 147, 110520. [Google Scholar] [CrossRef]
- Monteiro, S.F.; Costa, E.L.N.; Ferreira, R.S.B.; Chiste, R.C. Simultaneous extraction of carotenoids and phenolic compounds from pulps of orange and yellow peach palm fruits (Bactris gasipaes) by ultrasound-assisted extraction. Food. Sci. Technol. 2022, 42, e34021. [Google Scholar] [CrossRef]
- de Souza-Mesquita, L.M.; Ventura, S.P.M.; Braga, A.R.C.; Pisani, L.P.; Dias, A.C.R.V.; Rosso, V.V. Ionic liquid-high performance extractive approach to recover carotenoids from Bactris gasipaes fruits. Green Chem. 2019, 21, 2380–2391. [Google Scholar] [CrossRef]
- de Souza Mesquita, L.M.; Martins, M.; Pisani, L.P.; Ventura, S.P.M.; Rosso, V.V. Insights on the use of alternative solvents and technologies to recover biobased food pigments. Compr. Rev. Food Sci. Food Saf. 2021, 20, 787–818. [Google Scholar] [CrossRef] [PubMed]
- Santamarina, A.B.; de Souza Mesquita, L.M.; Casagrande, P.; Sertorio, M.N.; de Souza, D.V.; Mennitti, L.V.; Ribeiro, D.A.; Estadella, D.; Ventura, S.P.M.; de Rosso, V.V.; et al. Supplementation of carotenoids from peach palm waste (Bactris gasipaes) obtained with an ionic liquid mediated process displays kidney anti-inflammatory and antioxidant outcomes. Food Chem. X 2022, 13, 100245. [Google Scholar] [CrossRef] [PubMed]
- Ordóñez-Santos, L.E.; Pinzón-Zarate, L.X.; González-Salcedo, L.O. Optimization of ultrasonic-assisted extraction of total carotenoids from peach palm fruit (Bactris gasipaes) by-products with sunflower oil using response surface methodology. Ultrason. Sonochem. 2015, 27, 560–566. [Google Scholar] [CrossRef]
- de Souza Mesquita, L.M.; Casagrande, B.P.; Santamarina, A.B.; Sertorio, M.N.; de Souza, D.V.; Mennitti, L.V.; Jucá, A.; Jamar, G.; Estadella, D.; Ribeiro, D.A.; et al. Carotenoids obtained from an ionic liquid-mediated process display anti-inflammatory response in the adipose tissue-liver axis. Food Funct. 2021, 12, 8478–8491. [Google Scholar] [CrossRef]
- Espinosa-Pardo, F.A.; Martinez, J.; Martinez-Correa, H.A. Extraction of bioactive compounds from peach palm pulp (Bactris gasipaes) using supercritical CO2. J. Supercrit. Fluid. 2014, 93, 2–6. [Google Scholar] [CrossRef]
- Martinez-Girón, J.; Osorio, C.; Onrdoñez-Santos, L.E. Effect of temperature and particle size on physicochemical and techno-functional properties of peach palm peel flour (Bactris gasipaes, red and yellow ecotypes). Food Sci. Technol. Int. 2022, 28, 535–544. [Google Scholar] [CrossRef]
- Ribeiro, G.S.; Monteiro, K.C.; Carmo, J.R.; Pena, R.S.; Chisté, R.C. Peach palm flour: Production, hygroscopic behaviour and application in cookies. Heliyon 2021, 7, e07062. [Google Scholar] [CrossRef]
- Santos, Y.J.S.; Facchinatto, W.M.; Rochetti, A.L.; Carvalho, R.A.; Le Feunteun, S.; Fukumasu, H.; Morzel, M.; Colnago, L.A.; Vanin, F.M. Systemic characterization of pupunha (Bactris gasipaes) flour with views of polyphenol content on cytotoxicity and protein In Vitro digestion. Food Chem. 2023, 405, 134888. [Google Scholar] [CrossRef]
- Ibiapina, A.; Gualberto, L.S.; Dias, B.B.; Freitas, B.C.B.; Martins, G.A.S.; Melo Filho, A.A. Essential and fixed oils from Amazonian fruits: Properties and applications. Crit. Rev. Food Sci. Nutr. 2021, 17, 8842–8854. [Google Scholar] [CrossRef]
- Basto, G.J.; Carvalho, W.P.; Soares, A.G.; Costa, H.T.G.B.; Chávez, D.W.H.; Godoy, R.L.O.; Pacheco, S. Physicochemical properties and carotenoid content of extruded and non-extruded corn and peach palm (Bactris gasipaes, Kunth). LWT–Food Sci. Technol. 2016, 69, 312–318. [Google Scholar] [CrossRef]
- Maranhão, R. O palmito pupunha, a gastronomia e o meio ambiente. Rev. Rosa Dos Ventos Dossiê Tur. E Gastron. 2012, 4, 352–368. [Google Scholar]
- Galdino, N.O.; Clemente, E. Palmito de pupunha (Bactris gasipaes Kunth.) composição mineral e cinética de enzimas oxidativas. Ciência Tecnol. Aliment. 2008, 28, 540–544. [Google Scholar] [CrossRef]
- Santos, A.F.; Corrêa Júnior, C.; Neves, E.J.M. Palmeiras Para Produção De Palmito: Juçara, Pupunheira E Palmeira Real; Embrapa Florestas: Colombo, Brazil, 2008. [Google Scholar]
- dos Santos, A.F.; Santarosa, E.; Penteado Júnior, J.F.; Neves, E.J.M.; Tessmann, D.; Bellettini, S. Panorama da produção da pupunheira no Sul do Brasil. In Seminário Sobre Sistemas de Produção de Pupunheira e Palmeira-Real-Australiana No SUL Do Brasil 2019; Embrapa Florestas: Colombo, Brazil, 2021; pp. 17–25. [Google Scholar]
- Oliveira-Junior, O.L.; das Neves, Y.T.R.; Junqueira, P.S. Caiçara communities, atlantic forest and actual situation of the palmito juçara (Euterpe edulis mart.) around River Una da Aldeia (Iguape, SP) surrounding Estação Ecologica Jureia-Itatins. Rev. Arvore 2010, 34, 1065–1073. [Google Scholar] [CrossRef][Green Version]
- Guimarães, L.A.O.P.; Souza, R.G. Palmeira Juçara: Patrimônio Natural Da Mata Atlântica No Espírito Santo; INCAPER: Vitória, ES, Brasil, 2017; p. 68. [Google Scholar]
- TACO—Tabela Brasileira de Composição de Alimentos/NEPA—UNICAMP, 4th ed.; rev. e ampl.; NEPA—UNICAMP: Campinas, Brazil, 2011; p. 161. Available online: https://www.cfn.org.br/wp-content/uploads/2017/03/taco_4_edicao ampliada_e_ revisada.pdf (accessed on 10 July 2022).
- Vieira, T.F.; Corrêa, R.C.G.; Moreira, R.F.P.M.; Peralta, R.A.; de Lima, E.A.; Helm, C.V.; Garcia, J.A.A.; Bracht, A.; Peralta, R.M. Valorization of peach palm (Bactris gasipaes Kunth) waste: Production of antioxidant xylooligosaccharides. Waste Biomass Valori. 2021, 12, 727–6740. [Google Scholar] [CrossRef]
- Franco, T.S.; Potulski, D.C.; Viana, L.C.; Forville, E.; Andrade, A.S.; Muniz, G.I.B. Nanocellulose obtained from residues of peach palm extraction (Bactris gasipaes). Carbohydr. Polym. 2019, 218, 8–19. [Google Scholar] [CrossRef]
- Giombelli, C.; Iwassa, I.J.; Silva, C.; Barros, B.C.B. Valorization of peach palm by-product through subcritical water extraction of soluble sugars and phenolic compounds. J. Supercrit. Fluids 2020, 165, 2–9. [Google Scholar] [CrossRef]
- Sun, Z.; Bottari, G.; Afanasenko, A.; Stuart, M.C.A.; Deuss, P.J.; Fridrich, B.; Barta, K. Complete lignocellulose conversion with integrated catalyst recycling yielding valuable aromatics and fuels. Nat. Catal. 2018, 1, 82–92. [Google Scholar] [CrossRef]
- Corrado, I.; Varriale, S.; Pezzella, C. Microbial processes for upcycling food wastes into sustainable bioplastics. In Reference Module in Food Science; Elsevier: Amsterdam, The Netherlands, 2023. [Google Scholar] [CrossRef]
- Shrestha, S.; Kognou, A.L.M.; Zhang, J.; Qin, W. Different facets of lignocellulosic biomass including pectin and its perspectives. Waste Biomass Valori. 2020, 12, 4805–4823. [Google Scholar] [CrossRef]
- Braghiroli, F.L.; Passarini, L. Valorization of biomass residues from forest operations and wood manufacturing presents a wide range of sustainable and innovative possibilities. Curr. For. Rep. 2020, 6, 172–183. [Google Scholar] [CrossRef]
- Peralta, R.M.; Silva, B.P.; Correa, R.C.G.; Kato, C.G.; Seixas, F.A.V.; Bracht, A. Enzymes from Basidiomycetes-peculiar and efficient tools for biotechnology. In Biotechnology of Microbial Enzymes, 1st ed.; Brahmachari, G., Demain, A.L., Adrio, J.L., Eds.; Academic Press: London, UK, 2016; pp. 119–214. [Google Scholar]
- Muktham, R.; Bhargava, S.K.; Bankupalli, S.; Ball, A.S. A review on 1st and 2nd generation bioethanol production-recent progress. J. Sustain. Bioenergy Syst. 2016, 6, 72–92. [Google Scholar] [CrossRef]
- Kumar, A.; Gautam, A.; Dutt, D. Biotechnological transformation of lignocellulosic biomass into industrial products: An overview. Adv. Biosci. Biotechnol. 2016, 7, 149–168. [Google Scholar] [CrossRef]
- Zeng, F.; Wang, S.; Liang, J.; Cao, L.; Liu, X.; Qin, C.; Liang, C.; Si, C.; Yu, Z.; Yao, S. High-efficiency separation of hemicellulose from bamboo by one-step freeze–thaw-assisted alkali treatment. Bioresour. Technol. 2022, 361, 127735. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Li, F.; Zhou, X.; Hu, J.; Liu, P. Biomass, lignocellulolytic enzyme production and lignocellulose degradation patterns by Auricularia auricula during solid state fermentation of corn stalk residues under different pretreatments. Food Chem. 2022, 384, 132622. [Google Scholar] [CrossRef]
- Valdebenito, F.; Pereira, M.; Ciudad, G.; Azocar, L.; Briones, R.; Chinga-Carrasco, G. On the nanofibrillation of corn husks and oat hulls fibres. Ind. Crops Prod. 2017, 95, 528–534. [Google Scholar] [CrossRef]
- Rodriguez-Sanz, A.; Fuciños, C.; Torrado, A.M.; Rúa, M.L. Extraction of the wheat straw hemicellulose fraction assisted by commercial endo-xylanases. Role of the accessory enzyme activities. Ind. Crops Prod. 2022, 179, 114655. [Google Scholar] [CrossRef]
- Ponce, J.; Andrade, J.G.S.; Santos, L.N.; Bulla, M.K.; Barros, B.C.B.; Favaro, S.L.; Hioka, N.; Caetano, W.; Batistela, V.R. Alkali pretreated sugarcane bagasse, rice husk and corn husk wastes as lignocellulosic biosorbents for dyes. Carbohydr. Polym. Technol. Appl. 2021, 2, 100061. [Google Scholar] [CrossRef]
- Castoldi, R.; Bracht, A.; Morais, G.R.; Baesso, M.L.; Correa, R.C.G.; Peralta, R.A.; Peralta-Muniz-Moreira, R.F.; Polizeli, M.L.T.M.; Souza, C.G.M.; Peralta, R.M. Biological pretreatment of Eucalyptus grandis sawdust with white-rot fungi: Study of degradation patterns and saccharification kinetics. Chem. Eng. J. 2014, 258, 240–246. [Google Scholar] [CrossRef]
- Castoldi, R.; Correa, V.G.; Morais, G.R.; Souza, C.G.M.; Bracht, A.; Peralta, R.A.; Peralta-Muniz-Moreira, R.F.; Peralta, R.M. Liquid nitrogen pretreatment of eucalyptus sawdust and rice hull for enhanced enzymatic saccharification. Bioresour Technol. 2017, 224, 648–655. [Google Scholar] [CrossRef]
- Karunarathna, M.S.; Smith, R.C. Valorization of lignin as a sustainable component of structural materials and composites: Advances from 2011 to 2019. Sustainability 2020, 12, 734–749. [Google Scholar] [CrossRef]
- Isikgor, F.H.; Remzi Becer, C. Lignocellulosic biomass: A sustainable platform for the production of bio-based chemicals and polymers. Polym. Chem. 2015, 6, 4497–4559. [Google Scholar] [CrossRef]
- Fatma, S.; Hameed, A.; Noman, M.; Ahmed, T.; Shahid, M.; Tariq, M.; Sohail, I.; Tabassum, R. Lignocellulosic biomass: A sustainable bioenergy source for the future. Protein Pept. Lett. 2018, 25, 148–163. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.; Bajar, S.; Devi, A.; Pant, D. An overview on the recent developments in fungal cellulase production and their industrial applications. Bioresour. Technol. Rep. 2021, 14, 100652. [Google Scholar] [CrossRef]
- Kumla, J.; Suwannarach, N.; Sujarit, K.; Penkhrue, W.; Kakumyan, P.; Jatuwong, K.; Vadthanarat, S.; Lumyong, S. Cultivation of mushrooms and their lignocellulolytic enzyme production through the utilization of agro-industrial waste. Molecules 2020, 18, 2811–2852. [Google Scholar] [CrossRef]
- Ye, S.; Shah, B.R.; Li, J.; Liang, H.; Zhan, F.; Geng, F.; Li, B. A critical review on interplay between dietary fibers and gut microbiota. Trends Food Sci. Technol. 2022, 124, 237–249. [Google Scholar] [CrossRef]
- Bolanho, B.C.; Danesi, E.D.G.; Beleia, A.D.P. Peach palm (Bactris gasipaes Kunth.) characterization and the potential the of by-products flour processing. Food Sci. Technol. Res. 2013, 19, 1061–1069. [Google Scholar] [CrossRef][Green Version]
- Bolanho, B.C.; Danesi, E.D.G.; Beléia, A.D.P. Carbohydrate composition of peach palm (Bactris gasipaes Kunth) by-products flours. Carbohydr. Polym. 2015, 124, 196–200. [Google Scholar] [CrossRef]
- Helm, C.V.; Raup, D.S.; dos Santos, A.F. Development of peach palm fibrous flour from the waste generated by the heart of palm agribusiness. Acta Sci. Technol. 2014, 36, 171–177. [Google Scholar] [CrossRef]
- Meyer, T.S.M.; Miguel, A.S.M.; Fernández, D.E.R.; Ortiz, G.M.D. Biotechnological production of oligosaccharides—Applications in the food industry. In Food Production and Industry; Eissa, A.H.A., Ed.; InTech: London, UK, 2015; pp. 25–78. [Google Scholar] [CrossRef]
- Mano, M.C.R.; Neri-Numa, I.A.; da Silva, J.B.; Paulino, B.N.; Pessoa, M.G.; Pastore, G.M. Oligosaccharide biotechnology: An approach of prebiotic revolution on the industry. Appl. Microbiol. Biotechnol. 2018, 102, 17–37. [Google Scholar] [CrossRef]
- Santibánez, L.; Henrique, C.; Corro-Tejeda, R.; Bernal, S.; Armijo, B.; Salazar, O. Xylooligosaccharides from lignocellulosic biomass: A comprehensive review. Carbohydr. Polym. 2021, 251, 117118. [Google Scholar] [CrossRef]
- Huang, C.; Wang, X.; Laing, X.; Liang, C.; Jiang, X.; Yang, G.; Xu, J.; Yong, Q. A sustainable process for procuring biologically active fractions of high purity xylooligosaccharides and water-soluble lignin from Moso bamboo prehydrolyzate. Biotechnol. Biofuels 2019, 12, 189–202. [Google Scholar] [CrossRef] [PubMed]
- de Campos, A.; Correa, A.C.; Cannella, D.; Teixeira, E.M.; Marconcini, J.M.; Dufresne, A.; Mattoso, L.H.C.; Cassland, P.; Sanadi, A.R. Obtaining nanofibers from curauá and sugarcane bagasse fibers using enzymatic hydrolysis followed by sonication. Cellulose 2013, 20, 1491–1500. [Google Scholar] [CrossRef]
- Chandra, C.S.J.; George, N.; Narayanankutty, S.K. Isolation and characterization of cellulose nanofibrils from arecanut husk fibre. Carbohydr. Polym. 2016, 142, 158–166. [Google Scholar] [CrossRef]
- Trache, D.; Tarchoun, A.F.; Derradji, M.; Hamidon, T.S.; Masruchin, N.; Brosse, N.; Hazwan Hussin, M. Nanocellulose: From fundamentals to advanced applications. Front. Chem. 2020, 8, 392. [Google Scholar] [CrossRef]
- Martins, M.P.; Dagostin, J.L.A.; Franco, T.S.; Muniz, G.I.B.; Masson, M.L. Application of cellulose nanofibrils isolated from an agroindustrial residue of peach palm in cassava starch films. Food Biophys. 2020, 15, 323–334. [Google Scholar] [CrossRef]
- Webb, C.; Manan, M.A. Design aspects of solid state fermentation as applied to microbial bioprocessing. J. Appl. Biotechnol. Bioeng. 2017, 4, 511–532. [Google Scholar] [CrossRef]
- Chilakamarry, C.R.; Sakinah, A.M.M.; Zularisam, A.W.; Sirohi, R.; Khilji, I.A.; Ahmad, N.; Pandey, A. Advances in solid-state fermentation for bioconversion of agricultural wastes to value-added products: Opportunities and challenges. Biorresour. Technol. 2022, 343, 126065. [Google Scholar] [CrossRef]
- Yafetto, L. Application of solid-state fermentation by microbial biotechnology for bioprocessing of agro-industrial wastes from 1970 to 2020: A review and bibliometric analysis. Heliyon 2022, 8, e09173. [Google Scholar] [CrossRef]
- Roasa, J.; de Villa, R.; Mine, Y.; Tsao, R. Phenolics of cereal, pulse and oilseed processing by-products and potential effects of solid-state fermentation on their bioaccessibility, bioavailability and health benefits: A review. Trends Food Sci. Technol. 2021, 116, 954–974. [Google Scholar] [CrossRef]
- dos Santos Zenni, R.; Helm, C.V.; Tavares, L.B.B. Cascas do processamento de palmito para uso na alimentação humana: Uma abordagem socioambiental. Rev. Gest. Ambient. Sustentabilidade 2018, 7, 276–299. [Google Scholar] [CrossRef]
- de Lima, G.G.; Schoenherr, Z.C.P.; Magalhães, W.L.E.; Tavares, L.B.B.; Helm, C.V. Enzymatic activities and analysis of a mycelium-based composite formation using peach palm (Bactris gasipaes) residues on Lentinula edodes. Biorresour. Bioprocess. 2020, 7, 58. [Google Scholar] [CrossRef]
- Pasko, R.Z.; Timm, T.G.; de Lima, G.G.; Helm, C.V.; de Lima, E.A.; Henriques, G.S.; Tavares, L.B.B. In Vivo evaluation and nutritional quality of by-products Subjected to Solid-State Fermentation Using Shiitake Culinary-Medicinal Mushroom, Lentinula edodes (Agaricomycetes). Int. J. Med. Mushrooms 2022, 24, 53–66. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, E.A.; Nunes, L.V.; Goes, L.M.S.; Silva, E.G.P.; Franco, M.; Gross, E.; Uetanabaro, A.P.; Costa, A.M. Peach-palm (Bactris gasipaes Kunth.) waste as substrate for xylanase production by Trichoderma stromaticum AM7. Chem. Eng. Commun. 2018, 205, 975–985. [Google Scholar] [CrossRef]
- Chicatto, J.A.; Rainert, K.T.; Gonçalves, M.J.; Helm, C.V.; Altmajer-Vaz, D.; Tavares, L.B.B. Decolorization of textile industry wastewater in solid state fermentation with peach-palm (Bactris gasipaes) residue. Braz. J. Biol. 2018, 78, 718–727. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, V.C. Patent Number WO2006130939A2, 2006. Available online: https://patents.google.com/patent/WO2006130939A2/en?oq=WO+2006%2f130939+A2+ (accessed on 15 July 2022).
- Marinho, H.A.; Borrás, M.R.L.; Da Costa, P.R.C.; Dos Santos, S.M.P.B.; Galaxe, B.O.; De Sá, J.W.A. Patent WO2009082790A1, 2007. Available online: https://patents.google.com/patent/WO2009082790A1/en?oq=WO+2009%2f082790+A1 (accessed on 15 July 2022).
- de Pinheiro, B.P.; da Silva Santos, B.; Pelais, A.C.A. Patent Number BR102017026631A2, 2017. Available online: https://patents.google.com/patent/BR102017026631A2/en?oq=BR102017026631A2 (accessed on 15 July 2022).
- de Melo Neto, B.A.; Pontes, K.V.; de Almeida, P.F. Patent Number BR1020150243871, 2021. Available online: https://ifbaiano.edu.br/portal/wp-content/uploads/2021/03/Carta-Patente.pdf (accessed on 15 July 2022).
- Herbert, C.G.; Polkowski, R.D.O.; Gonçalves, C.C.; Aguiar, A.N.; de Souza, J.G.F.; dos Santos, H.L.; dos Santos Almeida, O.; Kiziltas, A.; Oliveira, P.R.T.; Rezende, F.M. Patent Number US20210284831A1, 2021. Available online: https://patents.google.com/patent/US20210284831A1/en?oq=US+2021%2f0284831+A1 (accessed on 15 July 2022).


| Aims and Procedures | Main Results and Implications | Reference |
|---|---|---|
| The ultrasound-assisted extraction (UAE) technique with various solvents [ethanol, ethyl acetate and ethanol/water (1:1, v/v)] was used to determine the best conditions for the simultaneous extraction of carotenoids and phenolic compounds from orange pulps and yellow peach palm fruits to produce extracts with high bioactive compound contents. | Regardless of the solvent, the UAE proved to be an efficient technique to carry out simultaneous extraction of high contents of carotenoids and phenolic compounds from pulps of peach palm fruits. | [37] |
| A new form of extraction mediated by ionic liquids (ILs) was used for the extraction of carotenoids of B. gasipaes. Four ILs were examined, as well as the solid-liquid ratio R(S/L), the number of extractions, the time of extraction, the co-solvent-ratio R(IL/E) and the homogenization method employed. | After selecting the best solvent ([C4mim][BF4]) and process conditions (extraction yield of 172 ± 18 μg carotenoids g dried biomass−1), the IL-ethanolic solution recyclability was tested by freezing/precipitating the IL (maximum of 94% of IL recovered), proving its success for at least 10 cycles while decreasing the process carbon footprint by 50% compared with the conventional method using acetone. | [38] |
| The extracts of B. gasipaes rich in pro-vitamin A carotenoids were emulsified and subjected to an in vitro digestion model followed by the Caco-2 cell absorption assay. | The cellular uptake of the carotenoids extracted with ionic liquid was 1.4-fold higher than that of those ones extracted using conventional organic solvents. | [39] |
| Carotenoids from B. gasipaes waste obtained by an ionic liquid (IL)-based process were investigated in terms of safety, anti-inflammatory and antioxidant activity on the kidney of high-fat-diet (HFD) animals. Wistar rats were supplemented or not by carotenoids extracted with IL or deep eutectic solvents (VOS). | The animals supplemented with carotenoids had lower weights than the controls and the high-fat diet group. In the animals supplemented with carotenoids, the IL group had improved anti-inflammatory and antioxidant activities compared with the group supplemented with carotenoids obtained by VOS. Also, the HFD-VOS group showed moderate-severe injuries in the kidney. ILs could represent a novel tool for natural pigments safely applied to the food industry. | [40] |
| Response surface methodology was used to investigate the effect of process variables on the ultrasound-assisted extraction (UAE) of total carotenoids from peach palm fruit with sunflower oil. | The optimal UAE condition was obtained with an ultrasonic intensity of 1528 W/m2, extraction temperature of 35 °C and extraction time of 30 min. Under these conditions, the maximum extraction of total carotenoids was 163.47 mg/100 g dried peel. | [41] |
| This work proposed to investigate the potential of carotenoids extracted from Bactris gasipaes feedstocks, mediated by an ethanolic solution of an imidazolium-based ionic liquid (IL). Male Wistar rats were randomized into six different groups, supplemented or not by carotenoids extracted by IL or deep eutectic solvents (VOS) and fed by control- and/or high-fat diets (HFD). The adipose tissue-liver axis was studied as a model to investigate the influence of carotenoids on the levels of inflammation and oxidative stress markers. | The main results showed that animals supplemented with carotenoids extracted with IL displayed improvements in serum parameters, besides lower metabolic efficiency, and improved antioxidant response in the liver, even when fed with HFD. However, animals supplemented with carotenoids extracted by VOS showed higher levels of pro-inflammatory markers and pronounced oxidative stress in the liver. | [42] |
| Extracts obtained from peach palm fruit using supercritical carbon dioxide were compared in terms of yield, total phenolic content, total flavonoids, total carotenoids, and antioxidant activity by means of the β-carotene bleaching method. | The recommended operating condition for supercritical extraction was 300 bar–40 °C because this allows for obtaining the highest carotenoid concentration. | [43] |
| Aims and Main Results | Implications | Reference |
|---|---|---|
| Water-extracted starch from the mesocarp of peach palm fruit presented small granules with a smooth surface and oval or conical shapes and larger spherical granules with holes and cracks on the surface. The amylose content was less than 20%, and the amylopectin revealed a crystalline structure with a high proportion of medium chains (13–24 residues). X-ray diffraction suggested a low digestible starch. The crystals were apparently homogeneous, and a weak gel was formed after 24 h of storage. | The peach palm fruit starch can be used in products where slow and smooth retrogradation is desired, such as in bread, soups, chowder, and porridges, without the use of emulsifiers or fat. | [3] |
| Techno-functional properties of the flours from two ecotypes of peach palm fruit peels were evaluated. Temperature and particle size had a pronounced influence on most of these properties except the swelling capacity. The flour from the red ecotype revealed superior nutritional properties in terms of total dietary fiber and protein contents and was also superior in terms of its water retention capacity, oil retention capacity, emulsifier activity and emulsifier stability. | These results suggest that by considering its protein contents and the fact that it can be considered as a source of dietary fibers or emulsifiers, the flour from the peach palm fruit peels could be used as a promising natural additive by the food industry. | [44] |
| The possibility of producing cookies using flour from peach palm fruits was investigated. Analysis ranged from composition, physicochemical properties and hygroscopic behavior. Cookies produced with two types of peach palm flour, whole fruit (pulp + peel) and solely pulp, presented good sensory acceptance (>70%), but the purchase intention favored the cookies prepared with whole fruit flour (85%). Both types of cookies (whole fruit-pulp) presented low moisture (4.9–6.2%), high lipid content (25.56–26.37%) and total carbohydrates (59.10–61.84%), resulting in products with high total energetic value (501.8–502.8 kcal/100 g). | The authors concluded that peach palm peels represent an excellent alternative for the use of by-products in the development of new food products | [45] |
| Pupunha flours (PF) from fruits harvested at different locations were characterized concerning their phenolic contents, cytotoxic effects, and inhibition of protein digestion in vitro. PF has high contents of phenolic compounds and antioxidant potential. | Potential negative effects such as cytotoxicity cells and inhibition of protein digestion in vitro were described for the first time and related to the high contents of phenolic compounds in the flours. | [46] |
| Lignocellulosic Biomass | Dry Weight (%) | Reference | ||
|---|---|---|---|---|
| Cellulose | Hemicellulose | Lignin | ||
| Bamboo | 45.8 | 26.6 | 23.4 | [66] |
| Corn stalk | 27.8 | 10.1 | 34.1 | [67] |
| Corn husk | 48.6 | 16.1 | 6.5 | [68] |
| Oat hull | 38.7 | 35.3 | 10.1 | [68] |
| Wheat straw | 35.5 | 25.2 | 25.1 | [69] |
| Sugar cane bagasse | 58.8 | 17.7 | 12.7 | [70] |
| Eucalyptus sawdust | 42.0 | 19.0 | 23.0 | [71] |
| Rice husk | 49.6 | 10.4 | 21.8 | [70] |
| Rice hull | 35.1 | 26.3 | 20.0 | [72] |
| Peach palm | 34.2 | 21.3 | 19.5 | [57] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
de Cássia Spacki, K.; Corrêa, R.C.G.; Uber, T.M.; Barros, L.; Ferreira, I.C.F.R.; Peralta, R.A.; de Fátima Peralta Muniz Moreira, R.; Helm, C.V.; de Lima, E.A.; Bracht, A.; et al. Full Exploitation of Peach Palm (Bactris gasipaes Kunth): State of the Art and Perspectives. Plants 2022, 11, 3175. https://doi.org/10.3390/plants11223175
de Cássia Spacki K, Corrêa RCG, Uber TM, Barros L, Ferreira ICFR, Peralta RA, de Fátima Peralta Muniz Moreira R, Helm CV, de Lima EA, Bracht A, et al. Full Exploitation of Peach Palm (Bactris gasipaes Kunth): State of the Art and Perspectives. Plants. 2022; 11(22):3175. https://doi.org/10.3390/plants11223175
Chicago/Turabian Stylede Cássia Spacki, Kamila, Rúbia Carvalho Gomes Corrêa, Thaís Marques Uber, Lillian Barros, Isabel C. F. R. Ferreira, Rosely Aparecida Peralta, Regina de Fátima Peralta Muniz Moreira, Cristiane Vieira Helm, Edson Alves de Lima, Adelar Bracht, and et al. 2022. "Full Exploitation of Peach Palm (Bactris gasipaes Kunth): State of the Art and Perspectives" Plants 11, no. 22: 3175. https://doi.org/10.3390/plants11223175
APA Stylede Cássia Spacki, K., Corrêa, R. C. G., Uber, T. M., Barros, L., Ferreira, I. C. F. R., Peralta, R. A., de Fátima Peralta Muniz Moreira, R., Helm, C. V., de Lima, E. A., Bracht, A., & Peralta, R. M. (2022). Full Exploitation of Peach Palm (Bactris gasipaes Kunth): State of the Art and Perspectives. Plants, 11(22), 3175. https://doi.org/10.3390/plants11223175










