Gypenoside XVII, an Active Ingredient from Gynostemma Pentaphyllum, Inhibits C3aR-Associated Synaptic Pruning in Stressed Mice
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Chemicals and Reagents
2.3. Drug Administration
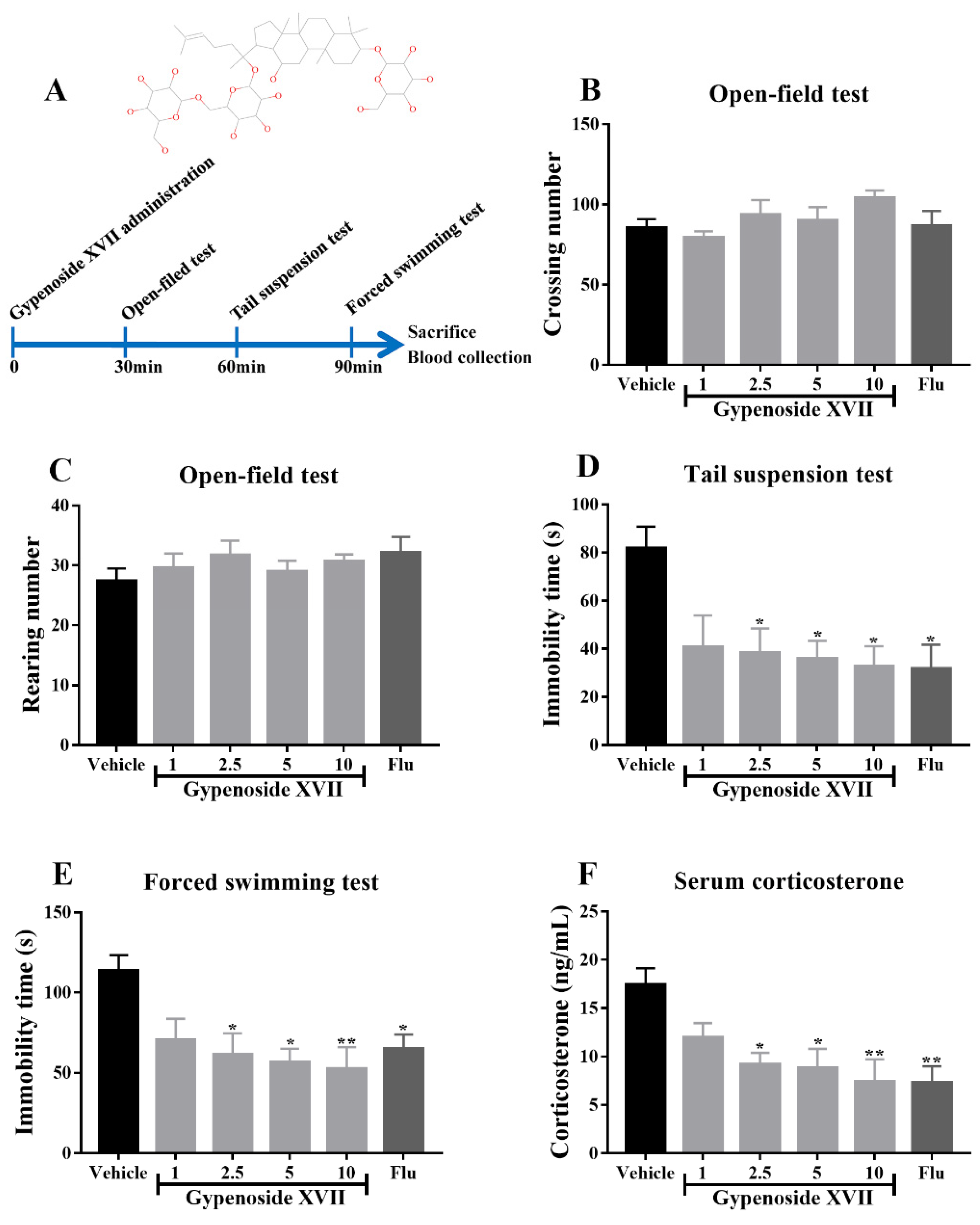
2.4. Open-Field Test
2.5. Tail Suspension Test
2.6. Forced Swimming Test
2.7. Corticosterone Measurement
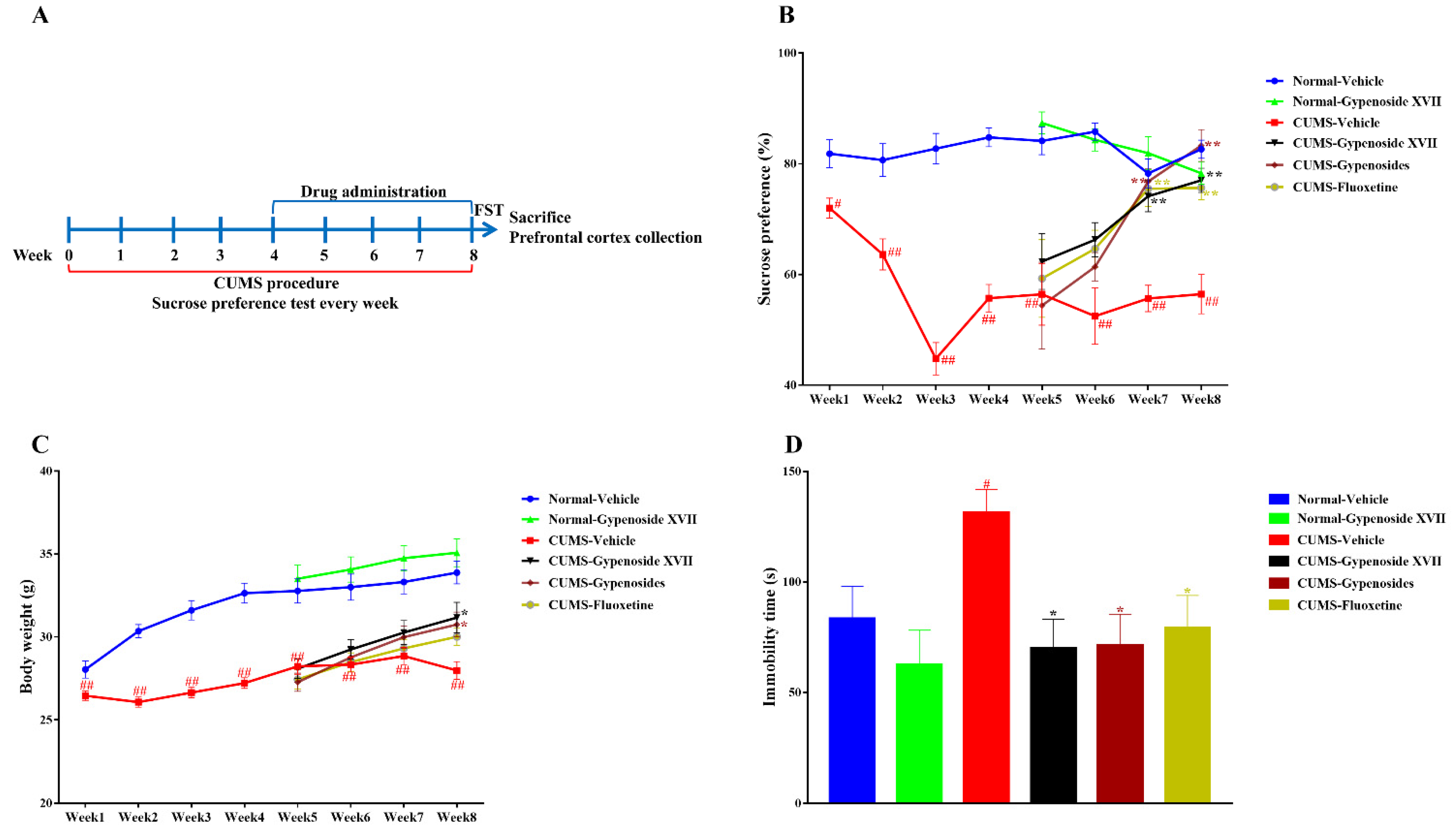
2.8. Chronic Unpredictable Mild Stress (CUMS)
2.9. Sucrose Preference Test
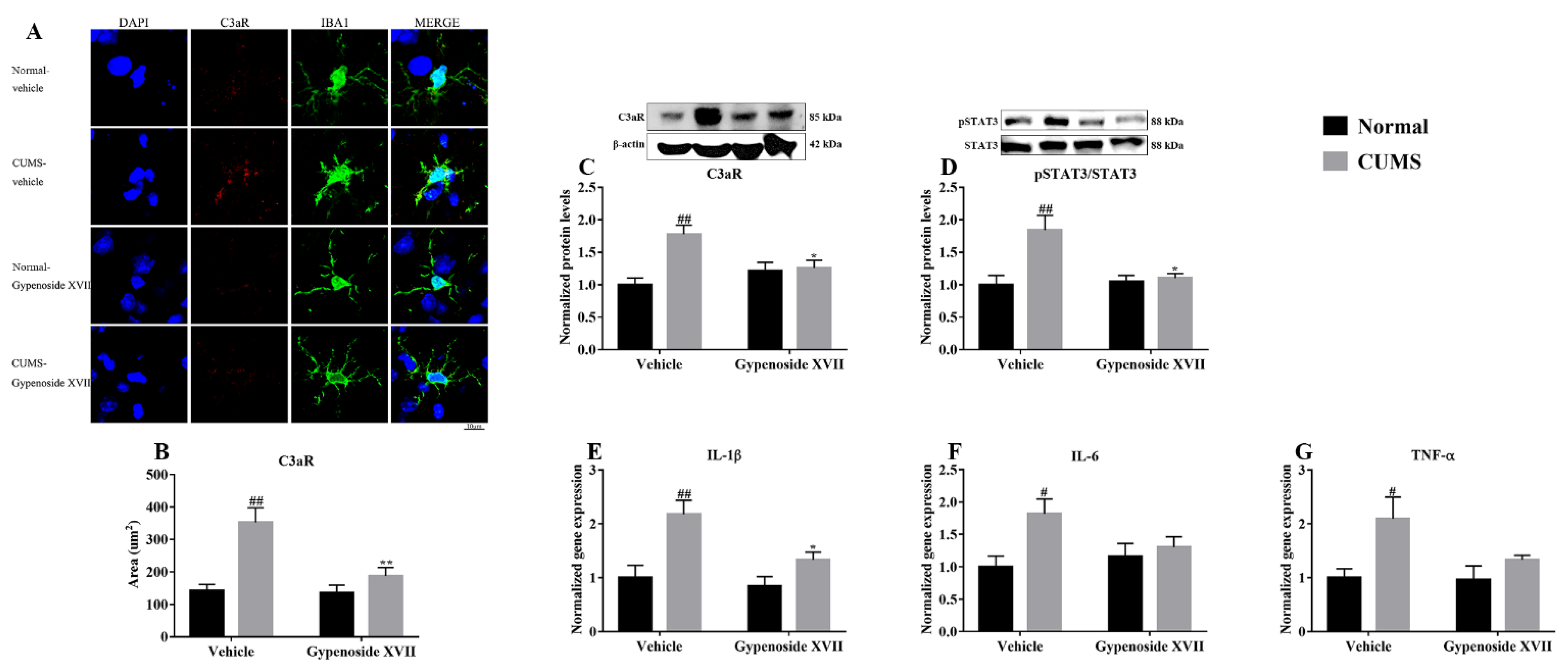
2.10. Western Blotting
2.11. Real-Time PCR
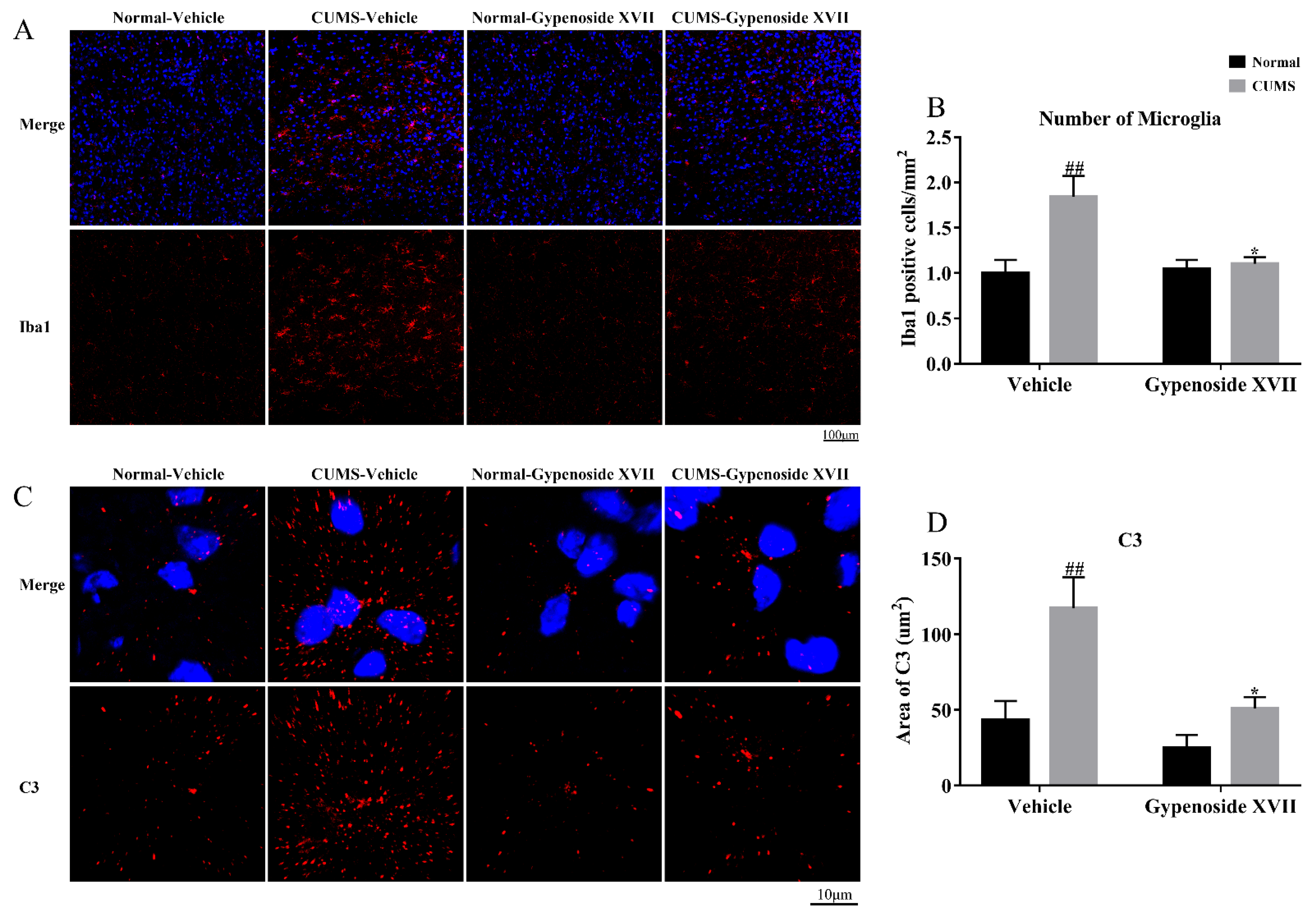
2.12. Brain Extraction and Immunofluorescence
2.13. Statistical Analyses
3. Results
3.1. Gypenoside XVII Exhibited the Antidepressant-Like Effects in Mice
3.2. Gypenoside XVII Attenuated the Depressive-Like Symptoms in CUMS Mice
3.3. Gypenoside XVII Inhibited Microglia Proliferation and Decreased C3 Expression in the Prefrontal Cortex
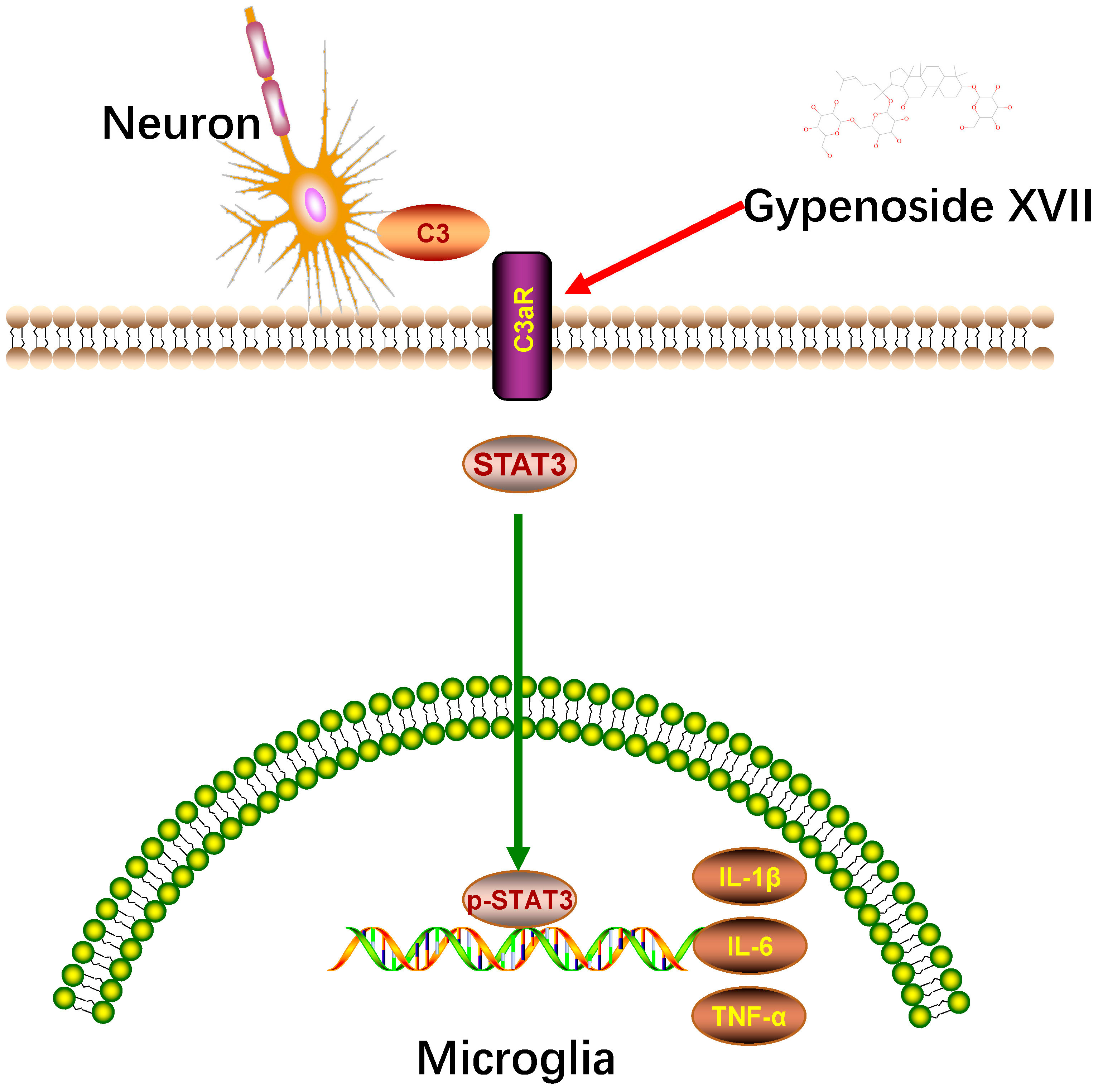
3.4. Gypenoside XVII Inhibited C3aR/STAT3/Cytokine Signaling Pathway in CUMS Mice
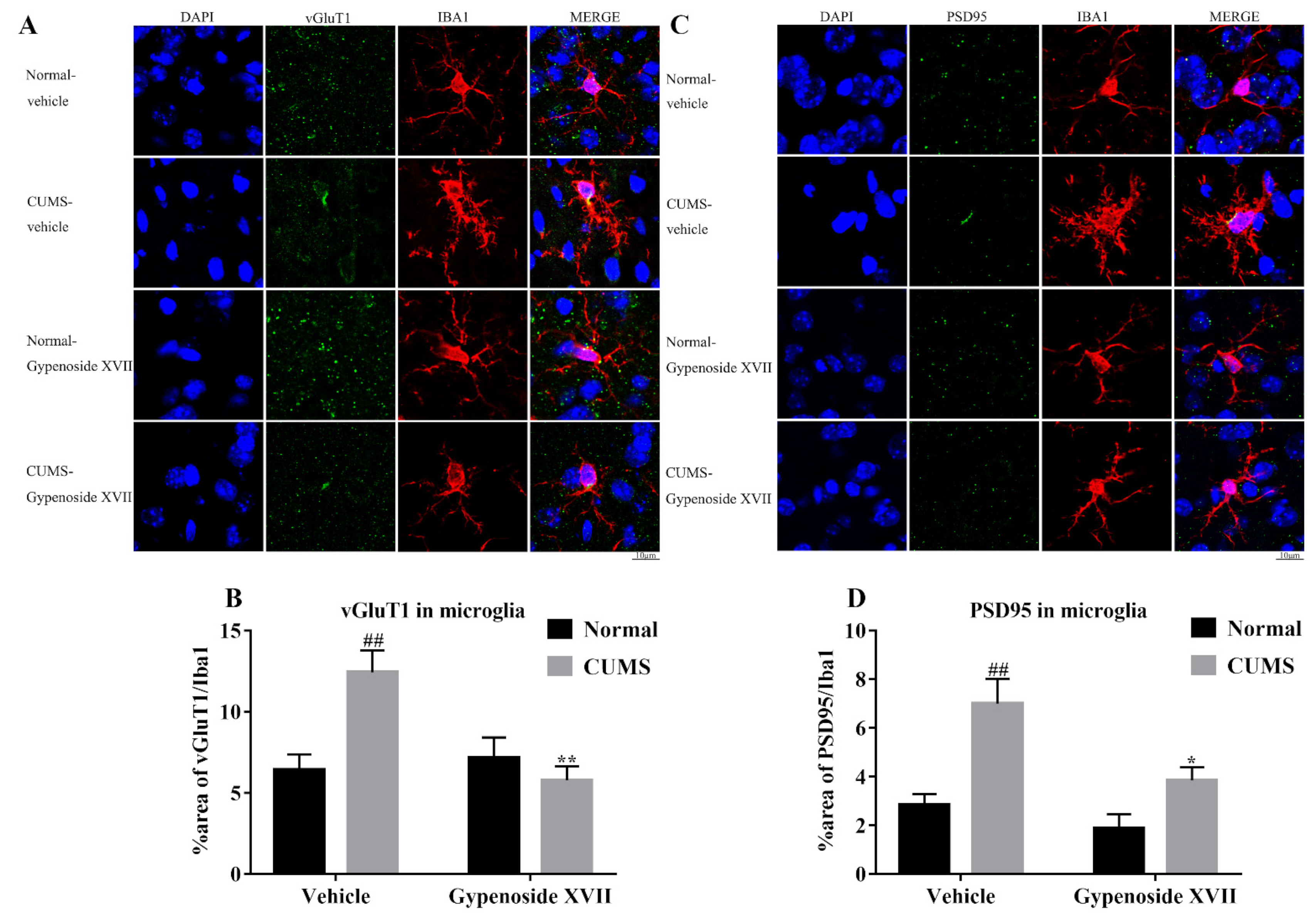
3.5. Gypenoside XVII Inhibited Microglia-Mediated Synaptic Pruning in CUMS Mice
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Iob, E.; Lacey, R.; Giunchiglia, V.; Steptoe, A. Adverse childhood experiences and severity levels of inflammation and depression from childhood to young adulthood: A longitudinal cohort study. Mol. Psychiatry 2022, 27, 2255–2263. [Google Scholar] [CrossRef] [PubMed]
- Katrinli, S.; Smith, A.K. Immune system regulation and role of the human leukocyte antigen in posttraumatic stress disorder. Neurobiol. Stress 2021, 15, 100366. [Google Scholar] [CrossRef] [PubMed]
- Nookabkaew, S.; Rangkadilok, N.; Prachoom, N.; Satayavivad, J. Concentrations of Trace Elements in Organic Fertilizers and Animal Manures and Feeds and Cadmium Contamination in Herbal Tea (Gynostemma pentaphyllum Makino). J. Agric. Food Chem. 2016, 64, 3119–3126. [Google Scholar] [CrossRef] [PubMed]
- Su, C.; Li, N.; Ren, R.; Wang, Y.; Su, X.; Lu, F.; Zong, R.; Yang, L.; Ma, X. Progress in the Medicinal Value, Bioactive Compounds, and Pharmacological Activities of Gynostemma pentaphyllum. Molecules 2021, 26, 6249. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Ye, W.; Mo, Z.; Yu, B.; Zhao, S.; Wu, H.; Che, C.; Jiang, R.; Mak, T.C.; Hsiao, W.L. Five new Ocotillone-type saponins from Gynostemma pentaphyllum. J. Nat. Prod. 2004, 67, 1147–1151. [Google Scholar] [CrossRef]
- Yin, F.; Zhang, Y.N.; Yang, Z.Y.; Hu, L.H. Nine new dammarane saponins from Gynostemma pentaphyllum. Chem. Biodivers. 2006, 3, 771–782. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Ye, W.; Mo, Z.; Yu, B.; Wu, H.; Zhao, S.; Che, C.; Hsiao, W.L. Three dammarane-type saponins from Gynostemma pentaphyllum. Planta Med. 2005, 71, 880–884. [Google Scholar] [CrossRef]
- Liu, J.; Li, Y.; Yang, P.; Wan, J.; Chang, Q.; Wang, T.T.Y.; Lu, W.; Zhang, Y.; Wang, Q.; Yu, L.L. Gypenosides Reduced the Risk of Overweight and Insulin Resistance in C57BL/6J Mice through Modulating Adipose Thermogenesis and Gut Microbiota. J. Agric. Food Chem. 2017, 65, 9237–9246. [Google Scholar] [CrossRef] [Green Version]
- Yi, L.T.; Mu, R.H.; Dong, S.Q.; Wang, S.S.; Li, C.F.; Geng, D.; Liu, Q. miR-124 antagonizes the antidepressant-like effects of standardized gypenosides in mice. J. Psychopharmacol. 2018, 32, 458–468. [Google Scholar] [CrossRef]
- Mu, R.H.; Fang, X.Y.; Wang, S.S.; Li, C.F.; Chen, S.M.; Chen, X.M.; Liu, Q.; Li, Y.C.; Yi, L.T. Antidepressant-like effects of standardized gypenosides: Involvement of brain-derived neurotrophic factor signaling in hippocampus. Psychopharmacology 2016, 233, 3211–3221. [Google Scholar] [CrossRef]
- Lee, B.; Shim, I.; Lee, H.; Hahm, D.H. Gypenosides attenuate lipopolysaccharide-induced neuroinflammation and anxiety-like behaviors in rats. Anim. Cells Syst. 2018, 22, 305–316. [Google Scholar] [CrossRef] [Green Version]
- Lee, B.; Shim, I.; Lee, H. Gypenosides Attenuate Lipopolysaccharide-Induced Neuroinflammation and Memory Impairment in Rats. Evid.-Based Complementary Altern. Med. 2018, 2018, 1–10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dong, S.Q.; Zhang, Q.P.; Zhu, J.X.; Chen, M.; Li, C.F.; Liu, Q.; Geng, D.; Yi, L.T. Gypenosides reverses depressive behavior via inhibiting hippocampal neuroinflammation. Biomed. Pharmacother. 2018, 106, 1153–1160. [Google Scholar] [CrossRef] [PubMed]
- Woodburn, S.C.; Bollinger, J.L.; Wohleb, E.S. Synaptic and behavioral effects of chronic stress are linked to dynamic and sex-specific changes in microglia function and astrocyte dystrophy. Neurobiol. Stress 2021, 14, 100312. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Lu, J.; Lukasiewicz, K.; Pan, B.; Zuo, Y. Stress induces microglia-associated synaptic circuit alterations in the dorsomedial prefrontal cortex. Neurobiol. Stress 2021, 15, 100342. [Google Scholar] [CrossRef]
- Bourel, J.; Planche, V.; Dubourdieu, N.; Oliveira, A.; Sere, A.; Ducourneau, E.G.; Tible, M.; Maitre, M.; Leste-Lasserre, T.; Nadjar, A.; et al. Complement C3 mediates early hippocampal neurodegeneration and memory impairment in experimental multiple sclerosis. Neurobiol. Dis. 2021, 160, 105533. [Google Scholar] [CrossRef]
- Pekna, M.; Stokowska, A.; Pekny, M. Targeting Complement C3a Receptor to Improve Outcome After Ischemic Brain Injury. Neurochem. Res. 2021, 46, 2626–2637. [Google Scholar] [CrossRef]
- Petrisko, T.J.; Gomez-Arboledas, A.; Tenner, A.J. Complement as a powerful "influencer" in the brain during development, adulthood and neurological disorders. Adv. Immunol. 2021, 152, 157–222. [Google Scholar] [CrossRef]
- Farley, S.; Apazoglou, K.; Witkin, J.M.; Giros, B.; Tzavara, E.T. Antidepressant-like effects of an AMPA receptor potentiator under a chronic mild stress paradigm. Int. J. Neuropsychopharmacol. 2010, 13, 1207–1218. [Google Scholar] [CrossRef] [Green Version]
- Paxinos, G.; Franklin, K.B. Paxinos and Franklin’s the Mouse Brain in Stereotaxic Coordinates; Academic Press: Cambridge, MA, USA, 2019. [Google Scholar]
- Alvarez, S.A.; Rocha-Guzman, N.E.; Gonzalez-Laredo, R.F.; Gallegos-Infante, J.A.; Moreno-Jimenez, M.R.; Bravo-Munoz, M. Ancestral Food Sources Rich in Polyphenols, Their Metabolism, and the Potential Influence of Gut Microbiota in the Management of Depression and Anxiety. J. Agric. Food Chem. 2022, 70, 944–956. [Google Scholar] [CrossRef]
- Wang, Q.; Jia, M.; Zhao, Y.; Hui, Y.; Pan, J.; Yu, H.; Yan, S.; Dai, X.; Liu, X.; Liu, Z. Supplementation of Sesamin Alleviates Stress-Induced Behavioral and Psychological Disorders via Reshaping the Gut Microbiota Structure. J. Agric. Food Chem. 2019, 67, 12441–12451. [Google Scholar] [CrossRef] [PubMed]
- Elias, E.; Zhang, A.Y.; Manners, M.T. Novel Pharmacological Approaches to the Treatment of Depression. Life 2022, 12, 196. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Qin, C.; Hu, Z.W.; Zhou, L.Q.; Yu, H.H.; Chen, M.; Bosco, D.B.; Wang, W.; Wu, L.J.; Tian, D.S. Microglia reprogram metabolic profiles for phenotype and function changes in central nervous system. Neurobiol. Dis. 2021, 152, 105290. [Google Scholar] [CrossRef] [PubMed]
- Kam, T.I.; Hinkle, J.T.; Dawson, T.M.; Dawson, V.L. Microglia and astrocyte dysfunction in parkinson’s disease. Neurobiol. Dis. 2020, 144, 105028. [Google Scholar] [CrossRef]
- Jun, G.R.; You, Y.; Zhu, C.; Meng, G.; Chung, J.; Panitch, R.; Hu, J.; Xia, W.; Alzheimer’s Disease Genetics, C.; Bennett, D.A.; et al. Protein phosphatase 2A and complement component 4 are linked to the protective effect of APOE varepsilon2 for Alzheimer’s disease. Alzheimer’s Dement 2022, 1–13. [Google Scholar] [CrossRef]
- Rupprecht, R.; Rupprecht, C.; Di Benedetto, B.; Rammes, G. Neuroinflammation and psychiatric disorders: Relevance of C1q, translocator protein (18 kDa) (TSPO), and neurosteroids. World J. Biol. Psychiatry 2021, 1–7. [Google Scholar] [CrossRef]
- Li, J.; Tian, S.; Wang, H.; Wang, Y.; Du, C.; Fang, J.; Wang, X.; Wang, Y.; Gong, Z.; Yan, B.; et al. Protection of hUC-MSCs against neuronal complement C3a receptor-mediated NLRP3 activation in CUMS-induced mice. Neurosci. Lett. 2021, 741, 135485. [Google Scholar] [CrossRef]
- Tang, J.; Jila, S.; Luo, T.; Zhang, B.; Miao, H.; Feng, H.; Chen, Z.; Zhu, G. C3/C3aR inhibition alleviates GMH-IVH-induced hydrocephalus by preventing microglia-astrocyte interactions in neonatal rats. Neuropharmacology 2022, 205, 108927. [Google Scholar] [CrossRef]
- Luchini, L.S.G.; Pidde, G.; Squaiella-Baptistao, C.C.; Tambourgi, D.V. Complement System Inhibition Modulates the Pro-Inflammatory Effects of a Snake Venom Metalloproteinase. Front. Immunol. 2019, 10, 1137. [Google Scholar] [CrossRef]
- Kwon, S.H.; Han, J.K.; Choi, M.; Kwon, Y.J.; Kim, S.J.; Yi, E.H.; Shin, J.C.; Cho, I.H.; Kim, B.H.; Jeong Kim, S.; et al. Dysfunction of Microglial STAT3 Alleviates Depressive Behavior via Neuron-Microglia Interactions. Neuropsychopharmacology 2017, 42, 2072–2086. [Google Scholar] [CrossRef]
- Al-Samhari, M.M.; Al-Rasheed, N.M.; Al-Rejaie, S.; Al-Rasheed, N.M.; Hasan, I.H.; Mahmoud, A.M.; Dzimiri, N. Possible involvement of the JAK/STAT signaling pathway in N-acetylcysteine-mediated antidepressant-like effects. Exp. Biol. Med. 2016, 241, 509–518. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jarlestedt, K.; Rousset, C.I.; Stahlberg, A.; Sourkova, H.; Atkins, A.L.; Thornton, C.; Barnum, S.R.; Wetsel, R.A.; Dragunow, M.; Pekny, M.; et al. Receptor for complement peptide C3a: A therapeutic target for neonatal hypoxic-ischemic brain injury. FASEB J. 2013, 27, 3797–3804. [Google Scholar] [CrossRef] [PubMed]
- Gomez-Arboledas, A.; Acharya, M.M.; Tenner, A.J. The Role of Complement in Synaptic Pruning and Neurodegeneration. Immunotargets Ther. 2021, 10, 373–386. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, M.-M.; Huo, G.-M.; Cheng, J.; Zhang, Q.-P.; Li, N.-Z.; Guo, M.-X.; Liu, Q.; Xu, G.-H.; Zhu, J.-X.; Li, C.-F.; et al. Gypenoside XVII, an Active Ingredient from Gynostemma Pentaphyllum, Inhibits C3aR-Associated Synaptic Pruning in Stressed Mice. Nutrients 2022, 14, 2418. https://doi.org/10.3390/nu14122418
Zhang M-M, Huo G-M, Cheng J, Zhang Q-P, Li N-Z, Guo M-X, Liu Q, Xu G-H, Zhu J-X, Li C-F, et al. Gypenoside XVII, an Active Ingredient from Gynostemma Pentaphyllum, Inhibits C3aR-Associated Synaptic Pruning in Stressed Mice. Nutrients. 2022; 14(12):2418. https://doi.org/10.3390/nu14122418
Chicago/Turabian StyleZhang, Man-Man, Guo-Ming Huo, Jie Cheng, Qiu-Ping Zhang, Na-Zhi Li, Min-Xia Guo, Qing Liu, Guang-Hui Xu, Ji-Xiao Zhu, Cheng-Fu Li, and et al. 2022. "Gypenoside XVII, an Active Ingredient from Gynostemma Pentaphyllum, Inhibits C3aR-Associated Synaptic Pruning in Stressed Mice" Nutrients 14, no. 12: 2418. https://doi.org/10.3390/nu14122418
APA StyleZhang, M.-M., Huo, G.-M., Cheng, J., Zhang, Q.-P., Li, N.-Z., Guo, M.-X., Liu, Q., Xu, G.-H., Zhu, J.-X., Li, C.-F., Zhou, F., & Yi, L.-T. (2022). Gypenoside XVII, an Active Ingredient from Gynostemma Pentaphyllum, Inhibits C3aR-Associated Synaptic Pruning in Stressed Mice. Nutrients, 14(12), 2418. https://doi.org/10.3390/nu14122418






