Influence of Human Milk on Very Preterms’ Gut Microbiota and Alkaline Phosphatase Activity
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design and Participants
2.2. Enteral Nutrition Protocol
2.3. Clinical Data Collection
2.4. Fecal Sample Collection and DNA Extraction
2.5. Quantitative Analysis of Fecal Microbiota by RT-PCR
2.6. Microbial 16S rRNA Sequence Analysis
2.7. Alkaline Phosphatase Activity Assay
2.8. Statistical Analysis
3. Results
3.1. Clinical Characterization of the Preterm Neonates
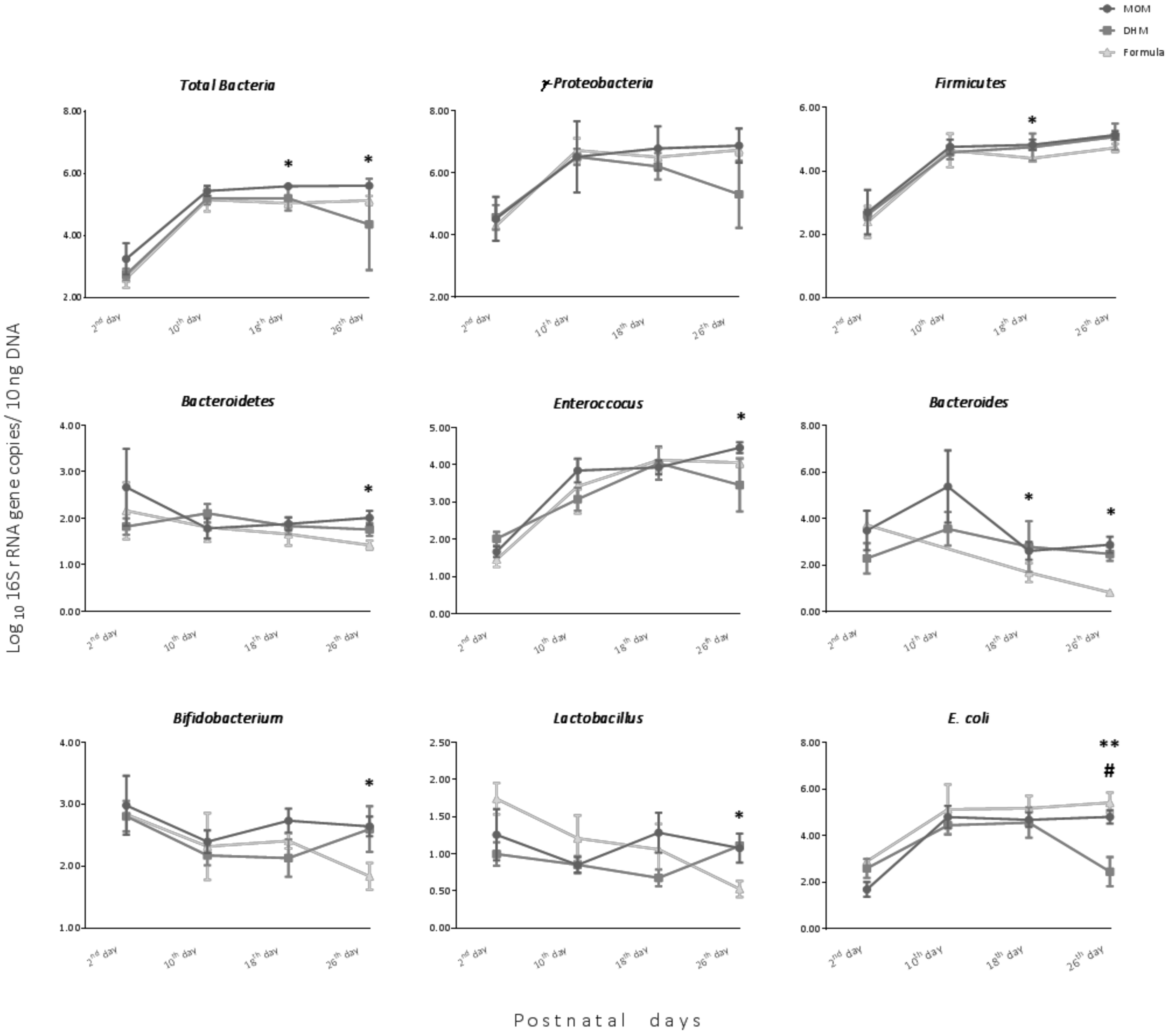
3.2. Impact of Feeding on Intestinal Bacterial Establishment in Premature Neonates
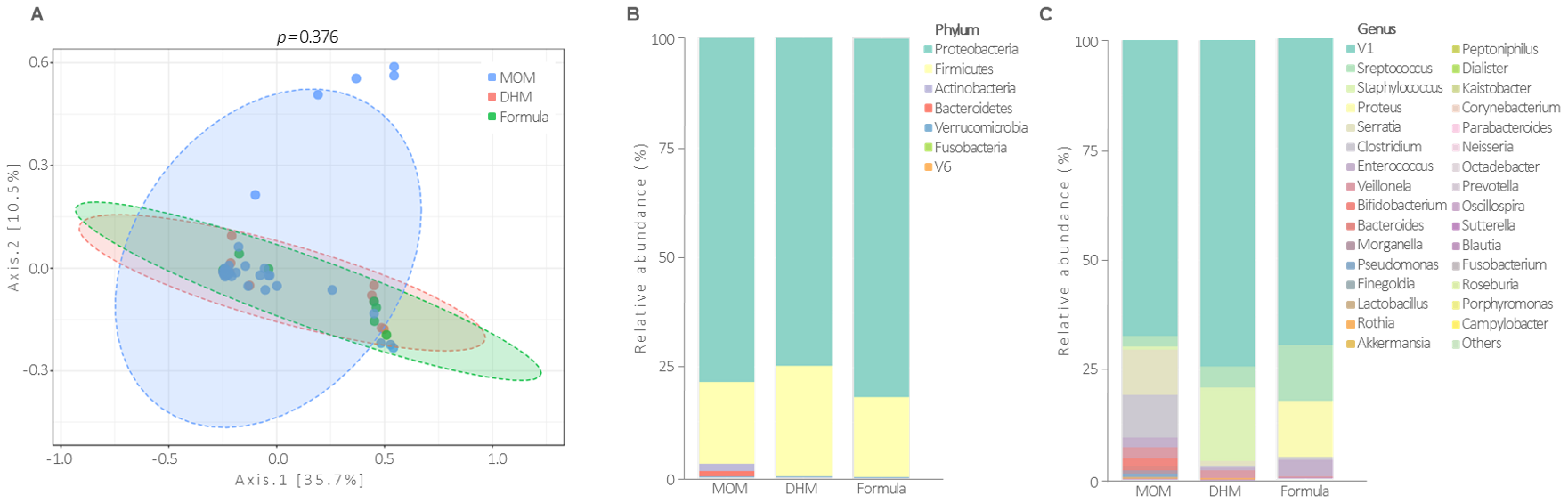
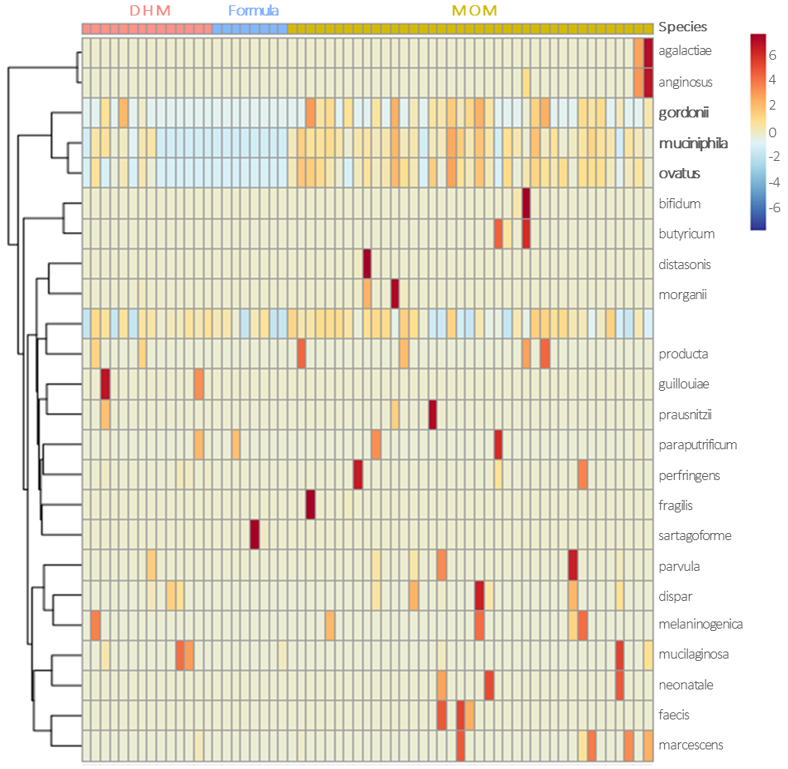
3.3. Gut Microbiota Profile at the 26th Postnatal Day According to Feeding Types
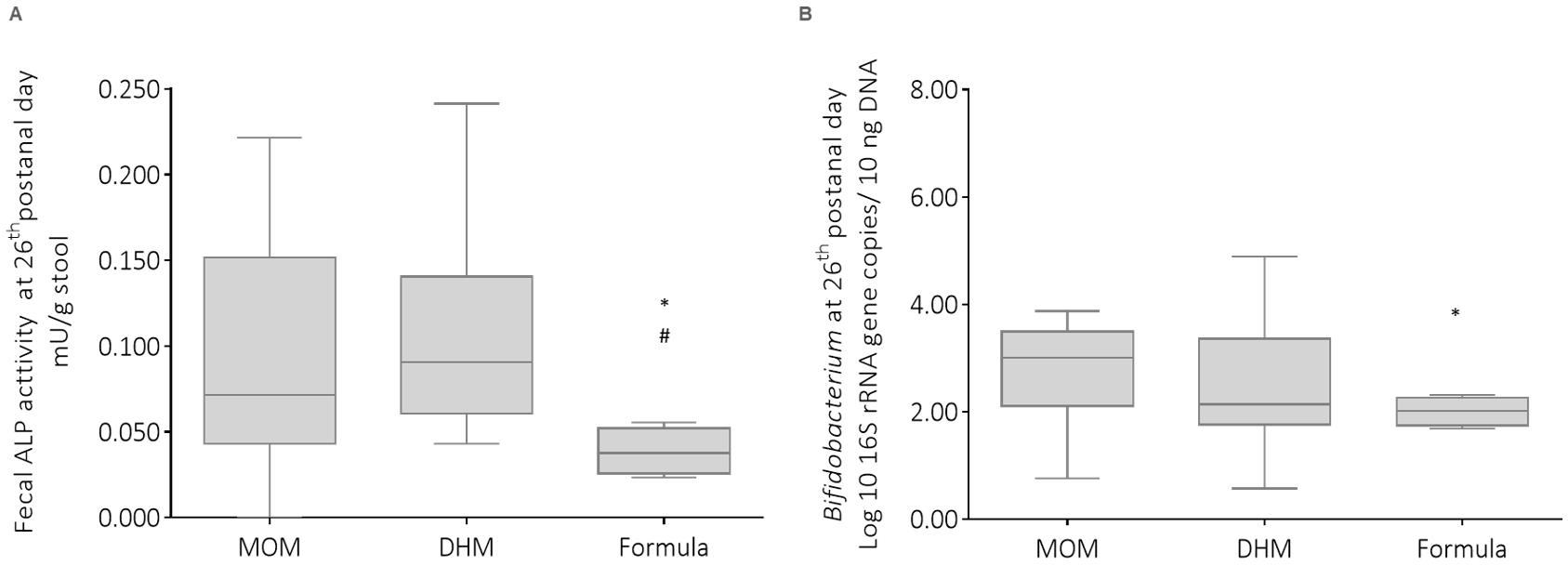
3.4. Influence of Infant Feeding on Fecal Alkaline Phosphatase Activity
3.5. Impact of MOM in Different Proportions
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hill, C.J.; Lynch, D.B.; Murphy, K.; Ulaszewska, M.; Jeffery, I.B.; O’Shea, C.A.; Watkins, C.; Dempsey, E.; Mattivi, F.; Tuohy, K.; et al. Evolution of gut microbiota composition from birth to 24 weeks in the INFANTMET Cohort. Microbiome 2017, 5, 1–18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van Belkum, M.; Alvarez, L.M.; Neu, J. Preterm neonatal immunology at the intestinal interface. Cell. Mol. Life Sci. 2019, 77, 1209–1227. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.; Judge, M.P.; Maas, K.; Hussain, N.; McGrath, J.M.; Henderson, W.A.; Cong, X. Systematic Review of the Effect of Enteral Feeding on Gut Microbiota in Preterm Infants. J. Obstet. Gynecol. Neonatal Nurs. 2018, 47, 451–463. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kumar, R.K.; Singhal, A.; Vaidya, U.; Banerjee, S.; Anwar, F.; Rao, S. Optimizing Nutrition in Preterm Low Birth Weight Infants—Consensus Summary. Front. Nutr. 2017, 4, 20. [Google Scholar] [CrossRef] [Green Version]
- Agostoni, C.; Braegger, C.; Decsi, T.; Kolacek, S.; Koletzko, B.; Michaelsen, K.F.; Mihatsch, W.; A Moreno, L.; Puntis, J.; Shamir, R.; et al. Breast-feeding: A Commentary by the ESPGHAN Committee on Nutrition. J. Pediatr. Gastroenterol. Nutr. 2009, 49, 112–125. [Google Scholar] [CrossRef] [Green Version]
- Korpela, K.; Blakstad, E.W.; Moltu, S.J.; Strømmen, K.; Nakstad, B.; Rønnestad, A.E.; Brække, K.; Iversen, P.O.; Drevon, C.A.; De Vos, W. Intestinal microbiota development and gestational age in preterm neonates. Sci. Rep. 2018, 8, 1–9. [Google Scholar]
- Arslanoglu, S.; Willemijn, C.; Guido, M.; Christian, B.; Cristina, C.; Virginie, C.; Tamas, D.; Magnus, D.; Mary, F.; Iva, H.; et al. Donor human milk for preterm infants: Current evidence and research directions. J. Pediatr. Gastroenterol. Nutr. 2013, 57, 535–542. [Google Scholar] [CrossRef]
- Quigley, M.; Henderson, G.; My, A.; Mcguire, W. Formula milk versus donor breast milk for feeding preterm or low birth weight infants (Review). Cochrane Database Syst. Rev. 2007, CD002971. [Google Scholar] [CrossRef]
- Cheong, J.L.Y.; Burnett, A.C.; Treyvaud, K.; Spittle, A.J. Early environment and long-term outcomes of preterm infants. J. Neural Transm. 2019, 127, 1–8. [Google Scholar] [CrossRef]
- Neu, J. Necrotizing Enterocolitis: A Multi-omic Approach and the Role of the Microbiome. Dig. Dis. Sci. 2020, 65, 789–796. [Google Scholar] [CrossRef] [Green Version]
- Rader, B.A. Alkaline Phosphatase, an Unconventional Immune Protein. Front. Immunol. 2017, 8, 897. [Google Scholar]
- Heath, M.; Buckley, R.; Gerber, Z.; Davis, P.; Linneman, L.; Gong, Q.; Barkemeyer, B.; Fang, Z.; Good, M.; Penn, D.; et al. Association of Intestinal Alkaline Phosphatase With Necrotizing Enterocolitis Among Premature Infants. JAMA Netw. Open 2019, 2, e1914996. [Google Scholar] [CrossRef]
- Yang, Y.; Rader, E.; Peters-Carr, M.; Bent, R.C.; Smilowitz, J.T.; Guillemin, K.; Rader, B. Ontogeny of alkaline phosphatase activity in infant intestines and breast milk. BMC Pediatr. 2019, 19, 1–7. [Google Scholar] [CrossRef]
- Morais, J.; Marques, C.; Teixeira, D.; Durão, C.; Faria, A.; Brito, S.; Cardoso, M.; Macedo, I.; Tomé, T.; Calhau, C. FEEDMI: A Study Protocol to Determine the Influence of Infant-Feeding on Very-Preterm-Infant’s Gut Microbiota. Neonatology 2019, 116, 179–184. [Google Scholar] [CrossRef]
- Fenton, T.R.; Kim, J.H. A systematic review and meta-analysis to revise the Fenton growth chart for preterm infants. BMC Pediatr. 2013, 13, 59. [Google Scholar] [CrossRef] [Green Version]
- Marques, C.; Meireles, M.; Norberto, S.; Leite, J.; Freitas, J.; Pestana, D.; Faria, A.; Calhau, C. High-fat diet-induced obesity Rat model: A comparison between Wistar and Sprague-Dawley Rat. Adipocyte 2015, 5, 11–21. [Google Scholar] [CrossRef] [Green Version]
- Gale, C.; Logan, K.M.; Santhakumaran, S.; Parkinson, J.R.C.; Hyde, M.J.; Modi, N. Effect of Breastfeeding compared with Formula Feeding on Infant Body Composition: A Systematic Review and Meta-Analysis. Am. J. Clin. Nutr. 2012, 95, 656–669. [Google Scholar] [CrossRef] [Green Version]
- Guaraldi, F.; Salvatori, G. Effect of Breast and Formula Feeding on Gut Microbiota Shaping in Newborns. Front. Cell. Infect. Microbiol. 2012, 2, 94. [Google Scholar] [CrossRef] [Green Version]
- Mshvildadze, M.; Neu, J.; Shuster, J.; Theriaque, D.; Li, N.; Mai, V. Intestinal Microbial Ecology in Premature Infants Assessed with Non–Culture-Based Techniques. J. Pediatr. 2010, 156, 20–25. [Google Scholar] [CrossRef] [Green Version]
- Gregory, K.E.; Samuel, B.S.; Houghteling, P.; Shan, G.; Ausubel, F.M.; Sadreyev, R.I.; Walker, W.A. Influence of maternal breast milk ingestion on acquisition of the intestinal microbiome in preterm infants. Microbiome 2016, 4, 1–15. [Google Scholar] [CrossRef] [Green Version]
- Morais, J.; Marques, C.; Teixeira, D.; Durão, C.; Faria, A.; Brito, S.; Cardoso, M.; Macedo, I.; Pereira, E.; Tomé, T.; et al. Extremely preterm neonates have more Lactobacillus in meconium than very preterm neonates – the in utero microbial colonization hypothesis. Gut Microbes 2020, 12, 1–9. [Google Scholar] [CrossRef]
- De Gregoris, T.B.; Aldred, N.; Clare, A.S.; Burgess, J.G. Improvement of phylum- and class-specific primers for real-time PCR quantification of bacterial taxa. J. Microbiol. Methods 2011, 86, 351–356. [Google Scholar] [CrossRef]
- Waitzberg, D.L.; Pereira, C.C.A.; Logullo, L.; Jacintho, T.M.; Almeida, D.; Da Silva, M.L.T.; Torrinhas, R.S.M.D.M. Microbiota benefits after inulin and partially hydrolized guar gum supplementation: A randomized clinical trial in constipated women. Nutr. Hosp. 2012, 27, 123–129. [Google Scholar]
- Walters, W.; Hyde, E.R.; Berg-Lyons, D.; Ackermann, G.; Humphrey, G.; Parada, A.; Gilbert, J.A.; Jansson, J.K.; Caporaso, J.G.; Fuhrman, J.A.; et al. Improved Bacterial 16S rRNA Gene (V4 and V4-5) and Fungal Internal Transcribed Spacer Marker Gene Primers for Microbial Community Surveys. mSystems 2015, 1, e00009-15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Calhau, C.; Martel, F.; Hipólito-Reis, C.; Azevedo, I. Differences between duodenal and jejunal rat alkaline phosphatase. Clin. Biochem. 2000, 33, 571–577. [Google Scholar] [CrossRef]
- Malo, M.S. A High Level of Intestinal Alkaline Phosphatase Is Protective Against Type 2 Diabetes Mellitus Irrespective of Obesity. EBioMedicine 2015, 2, 2016–2023. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chong, J.; Liu, P.; Zhou, G.; Xia, J. Using MicrobiomeAnalyst for comprehensive statistical, functional, and meta-analysis of microbiome data. Nat. Protoc. 2020, 15, 799–821. [Google Scholar] [CrossRef]
- Parra-Llorca, A.; Gormaz, M.; Alcántara, C.; Cernada, M.; Nuñez-Ramiro, A.; Vento, M.; Collado, M.C. Preterm Gut Microbiome Depending on Feeding Type: Significance of Donor Human Milk. Front. Microbiol. 2018, 9, 1376. [Google Scholar] [CrossRef] [PubMed]
- German, J.B.; Freeman, S.L.; Lebrilla, C.B.; Mills, D.A. Human milk oligosaccharides: Evolution, structures and bioselectiivty as substrates for intestinal bacteria. Pers. Nutr. Divers. Needs Infants Child. 2008, 62, 205–222. [Google Scholar]
- Arboleya, S.; Sánchez, B.; Milani, C.; Duranti, S.; Solís, G.; Fernández, N.; Reyes-Gavilán, C.G.D.L.; Ventura, M.; Margolles, A.; Gueimonde, M. Intestinal Microbiota Development in Preterm Neonates and Effect of Perinatal Antibiotics. J. Pediatr. 2015, 166, 538–544. [Google Scholar] [CrossRef] [Green Version]
- Marx, C.; Bridge, R.; Wolf, A.K.; Rich, W.; Kim, J.H.; Bode, L. Human Milk Oligosaccharide Composition Differs between Donor Milk and Mother’s Own Milk in the NICU. J. Hum. Lact. 2013, 30, 54–61. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Mogno, I.; Contijoch, E.J.; Borgerding, J.N.; Aggarwala, V.; Li, Z.; Siu, S.; Grasset, E.K.; Helmus, D.S.; Dubinsky, M.C.; et al. Fecal IgA Levels Are Determined by Strain-Level Differences in Bacteroides ovatus and Are Modifiable by Gut Microbiota Manipulation. Cell Host Microbe 2020, 27, 467–475.e6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Derrien, M.; Belzer, C.; de Vos, W.M. Akkermansia muciniphila and its role in regulating host functions. Microb. Pathog. 2017, 106, 171–181. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Romani, L.; Del Chierico, F.; Chiriaco, M.; Foligno, S.; Reddel, S.; Salvatori, G.; Cifaldi, C.; Faraci, S.; Finocchi, A.; Rossi, P.; et al. Gut Mucosal and Fecal Microbiota Profiling Combined to Intestinal Immune System in Neonates Affected by Intestinal Ischemic Injuries. Front. Cell. Infect. Microbiol. 2020, 10, 59. [Google Scholar] [CrossRef]
- Bavineni, M.; Wassenaar, T.M.; Agnihotri, K.; Ussery, D.W.; Lüscher, T.F.; Mehta, J.L. Mechanisms linking preterm birth to onset of cardiovascular disease later in adulthood. Eur. Heart J. 2019, 40, 1107–1112. [Google Scholar] [CrossRef] [Green Version]
- Ottman, N. Host Immunostimulation and Substrate Utilization of the Gut Symbiont Akkermansia Muciniphila. Ph.D. Thesis, Wageningen University, Wageningen, The Netherlands, 2015. [Google Scholar]
- Neu, J. Mother’s Own Milk: How Does It Differ from Donor Milk for the Baby. Breastfeed. Med. 2019, 14, S3–S4. [Google Scholar] [CrossRef] [Green Version]
- Heinzerling, N.P.; Liedel, J.L.; Welak, S.R.; Fredrich, K.; Biesterveld, B.E.; Pritchard, K.A.; Gourlay, D.M. Intestinal alkaline phosphatase is protective to the preterm rat pup intestine. J. Pediatr. Surg. 2014, 49, 954–960. [Google Scholar] [CrossRef] [Green Version]
- Biesterveld, B.E.; Koehler, S.M.; Heinzerling, N.P.; Rentea, R.M.; Fredrich, K.; Welak, S.R.; Gourlay, D.M. Intestinal alkaline phosphatase to treat necrotizing enterocolitis. J. Surg. Res. 2015, 196, 235–240. [Google Scholar] [CrossRef] [Green Version]
- Rentea, R.; Rentea, M.; Biesterveld, B.; Liedel, J.; Gourlay, D. Factors Known to Influence the Development of Necrotizing Enterocolitis to Modify Expression and Activity of Intestinal Alkaline Phosphatase in a Newborn Neonatal Rat Model. Eur. J. Pediatr. Surg. 2018, 29, 290–297. [Google Scholar] [CrossRef]
- Kaliannan, K.; Wang, B.; Li, X.-Y.; Kim, K.-J.; Kang, J.X. A host-microbiome interaction mediates the opposing effects of omega-6 and omega-3 fatty acids on metabolic endotoxemia. Sci. Rep. 2015, 5, 11276. [Google Scholar] [CrossRef] [Green Version]
- Gonçalves, P.; Catarino, T.A.; Gregório, I.; Martel, F. Inhibition of butyrate uptake by the primary bile salt chenodeoxycholic acid in intestinal epithelial cells. J. Cell. Biochem. 2012, 113, 2937–2947. [Google Scholar] [CrossRef]
- Navis, M.; Muncan, V.; Sangild, P.T.; Willumsen, L.M.; Koelink, P.J.; Wildenberg, M.E.; Abrahamse, E.; Thymann, T.; Van Elburg, R.M.; Renes, I.B. Beneficial Effect of Mildly Pasteurized Whey Protein on Intestinal Integrity and Innate Defense in Preterm and Near-Term Piglets. Nutrients 2020, 12, 1125. [Google Scholar] [CrossRef] [Green Version]
- Underwood, M.A.; Gaerlan, S.C.; De Leoz, M.L.A.; Dimapasoc, L.M.; Kalanetra, K.M.; Lemay, D.G.; German, J.B.; Mills, D.A.; Lebrilla, C.B. Human milk oligosaccharides in premature infants: Absorption, excretion, and influence on the intestinal microbiota. Pediatr. Res. 2015, 78, 670–677. [Google Scholar] [CrossRef] [Green Version]
- Rivière, A.; Selak, M.; Lantin, D.; Leroy, F.; De Vuyst, L. Bifidobacteria and Butyrate-Producing Colon Bacteria: Importance and Strategies for Their Stimulation in the Human Gut. Front. Microbiol. 2016, 7, 979. [Google Scholar] [CrossRef] [Green Version]

| Target Group | Primer Sequence (5′-3′) | Genomic DNA Standard | AT | Ref. |
|---|---|---|---|---|
| Total bacteria | AAA CTC AAA KGA ATT GAC GG CTC ACR RCA CGA GCT GAC | Bacteroides vulgatus ATCC 8482 | 62 °C | [22] |
| Bacteroidetes | CAT GTG GTT TAA TTC GAT GAT AGC TGA CGA CAA CCA TGC AG | Bacteroides vulgatus ATCC 8482 | 60 °C | [16] |
| Firmicutes | ATG TGG TTT AAT TCG AAG CA AGC TGA CGA CAA CCA TGC AC | Lactobacillus gasseri ATCC 33323 | 60 °C | [16] |
| γ-Proteobacteria | TCGTCAGCTCGTGTYGTGA CGTAAGGGCCATGATG | E. coli ATCC 25922 | 61 °C | [22] |
| Lactobacillus | GAG GCA GCA GTA GGG AAT CTT C GGC CAG TTA CTA CCT CTA TCC TTC TTC | Lactobacillus gasseri ATCC 33323 | 60 °C | [16] |
| Bifidobacterium | CGC GTC YGG TGT GAA AG CCC CAC ATC CAG CAT CCA | Bifidobacterium longum ATCC 15697 | 60 °C | [16] |
| Bacteroides | ATA GCC TTT CGA AAG RAA GAT CCA GTA TCA ACT GCA ATT TTA | Bacteroides vulgatus ATCC 33563 | 60 °C | [16] |
| Enterococcus | CCC TTA TTG TTA GTT GCC ATC ATT ACT CGT TGT ACT TCC CT TGT | Enterococcus gilvus ATCC BAA-350 | 61 °C | [16] |
| Escherichia coli | GTA AGT TAC ACT ATA AAA GCA CCG TCG TCT GTG TGG ATG GTA ATA AAT TTT TG | Escherichia coli ATCC 25922 | 60 °C | [23] |
| MOM (n = 75) | DHM (n = 20) | Formula (n = 13) | p-Value (MOM vs. DHM) | p-Value (MOM vs. Formula) | p-Value (DHM vs. Formula) | |
|---|---|---|---|---|---|---|
| Extremely/very preterm n | 28/47 | 5/15 | 0/13 | 0.205 | 0.004 | 0.065 |
| Gestational age (mean ± SD) | 28.3 ± 2.1 | 28.6 ± 1.8 | 29.6 ±1.1 | 0.103 | 0.003 | 0.17 |
| Vaginal delivery/C-section, n | 34/41 | 7/13 | 1/12 | 0.407 | 0.010 | 0.074 |
| Somatometry at birth (mean ± SD) | ||||||
| Weight, g | 1123 ± 345 | 1173 ± 284 | 1416 ± 219 | 0.438 | 0.002 | 0.011 |
| Length, cm | 36.2 ± 3.3 | 36.8 ± 3.2 | 39.1 ± 2.0 | 0.428 | 0.003 | 0.027 |
| Cephalic perimeter, cm | 25.4 ± 2.4 | 25.3 ± 1.8 | 27.2 ± 1.5 | 0.996 | 0.007 | 0.008 |
| Z-score | ||||||
| Weight at birth | −0.27 ± 0.82 | 0.04 ± 1.45 | 0.15 ± 0.70 | 0.977 | 0.057 | 0.789 |
| Length at birth | −0.44 ± 1.09 | −0.25 ± 1.61 | 0.04 ±0.65 | 0.777 | 0.051 | 0.543 |
| Cephalic perimeter at birth | −0.46 ± 0.90 | −0.68 ± 1.79 | −0.77 ± 1.28 | 0.731 | 0.147 | 0.331 |
| Weight at 26th | −0.08 ± 1.85 | 0.85 ± 1.62 | 1.65 ± 1.13 | 0.257 | 0.001 | 0.132 |
| Δ weight until 26th day, g | 200 ± 190 | 200 ± 170 | 420 ± 160 | 0.671 | <0.001 | 0.001 |
| Postnatal day of full enteral nutrition (mean ± SD) | 16.5 ± 8.5 | 16.4 ± 5.6 | 9.5 ± 4.2 | 0.401 | 0.002 | <0.001 |
| Days of antibiotherapy (mean ± SD) | 12 ± 11 | 13 ± 8 | 5 ± 3 | 0.178 | 0.010 | <0.001 |
| Days of hospitalization (mean ± SD) | 58 ± 24 | 59 ± 21 | 42 ± 12 | 0.874 | 0.026 | 0.018 |
| MOM | DHM | Formula | |||
|---|---|---|---|---|---|
| 95% CI | p-Value | 95% CI | p-Value | ||
| Total Bacteria | |||||
| Gestational age-adjusted | 1 (referent) | −0.556 (−1.093 to −0.079) | 0.023 | −0.889 (−1.341 to −0.438) | <0.001 |
| Mode of delivery-adjusted | 1 (referent) | −0.565 (−1.036 to −0.095) | 0.018 | −0.552 (−1.047 to −0.057) | 0.029 |
| Multivariable-adjusted a | 1 (referent) | −0.659 (–1.082 to −0.236) | 0.002 | −0.721 (−1.158 to −0.284) | 0.001 |
| Firmicutes | |||||
| Gestational age-adjusted | 1 (referent) | −0.585 (−1.183 to 0.013) | 0.055 | −0.789 (−1.322 to −0.256) | 0.004 |
| Mode of delivery-adjusted | 1 (referent) | −0.587 (−1.161 to −0.013) | 0.045 | −0.456 (−1.060 to 0.162) | 0.139 |
| Multivariable-adjusted a | 1 (referent) | −0.610 (–1.114 to −0.107) | 0.017 | −0.759 (−1.279 to −0.238) | 0.004 |
| Bifidobacterium | |||||
| Gestational age-adjusted | 1 (referent) | −2.002 (−3.315 to −0.689) | 0.003 | −2.092 (−3.262 to −0.922) | <0.001 |
| Mode of delivery-adjusted | 1 (referent) | −0.291 (−1.016 to 0.434) | 0.431 | −0.684 (−1.447 to 0.079) | 0.079 |
| Multivariable-adjusted a | 1 (referent) | −0.656 (−1.388 to 0.075) | 0.079 | −0.975 (−1.716 to −0.233) | 0.010 |
| * | <90% to ≥80% | <80% to ≥70% | <70% to ≥50% | <50% to ≥35% | <35% | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 95% CI | p | 95% CI | p | 95% CI | p | 95% CI | p | 95% CI | p | ||
| Chao1 index | |||||||||||
| Multivariable -adjusted a | Ref. | 2.559 (–0.707 to 5.824) | 0.125 | −2.384 (–5.749 to 0.980) | 0.165 | 1.603 (–2.467 to 5.673) | 0.440 | −9.451 (−15.660 to−3.242) | 0.003 | −2.820 (–6.273 to 0.634) | 0.110 |
| Total Bacteria | |||||||||||
| Multivariable -adjusted a | Ref. | −0.173 (–0.697 to 0.351) | 0.518 | −0.179 (–0.684 to 0.326) | 0.487 | 0.104 (–0.355 to 0.562) | 0.658 | −0.611 (–1.235 to 0.12) | 0.055 | −0.771 (–1.311 to −0.231) | 0.005 |
| Firmicutes | |||||||||||
| Multivariable -adjusted a | Ref. | −0.551 (–1.165 to 0.063) | 0.079 | −0.502 (–1.094 to 0.090) | 0.097 | −0.401 (–0.939 to 0.137) | 0.144 | −1.297 (–2.028 to −0.566) | 0.001 | −1.115 (–1.749 to −0.482) | 0.001 |
| Bifidobacterium | |||||||||||
| Multivariable -adjusted a | Ref. | 0.605 (−0.495 to 1.704) | 0.281 | −0.039 (−1.018 to 1.096) | 0.943 | −0.237 (−1.196 to 0.723) | 0.628 | 0.207 (−1.078 to 1.492) | 0.753 | −0.424 (−1.543 to 0.696) | 0.458 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Morais, J.; Marques, C.; Faria, A.; Teixeira, D.; Barreiros-Mota, I.; Durão, C.; Araújo, J.; Ismael, S.; Brito, S.; Cardoso, M.; et al. Influence of Human Milk on Very Preterms’ Gut Microbiota and Alkaline Phosphatase Activity. Nutrients 2021, 13, 1564. https://doi.org/10.3390/nu13051564
Morais J, Marques C, Faria A, Teixeira D, Barreiros-Mota I, Durão C, Araújo J, Ismael S, Brito S, Cardoso M, et al. Influence of Human Milk on Very Preterms’ Gut Microbiota and Alkaline Phosphatase Activity. Nutrients. 2021; 13(5):1564. https://doi.org/10.3390/nu13051564
Chicago/Turabian StyleMorais, Juliana, Cláudia Marques, Ana Faria, Diana Teixeira, Inês Barreiros-Mota, Catarina Durão, João Araújo, Shámila Ismael, Sara Brito, Manuela Cardoso, and et al. 2021. "Influence of Human Milk on Very Preterms’ Gut Microbiota and Alkaline Phosphatase Activity" Nutrients 13, no. 5: 1564. https://doi.org/10.3390/nu13051564







