Synthesis and Evaluation of Some Uracil Nucleosides as Promising Anti-Herpes Simplex Virus 1 Agents
Abstract
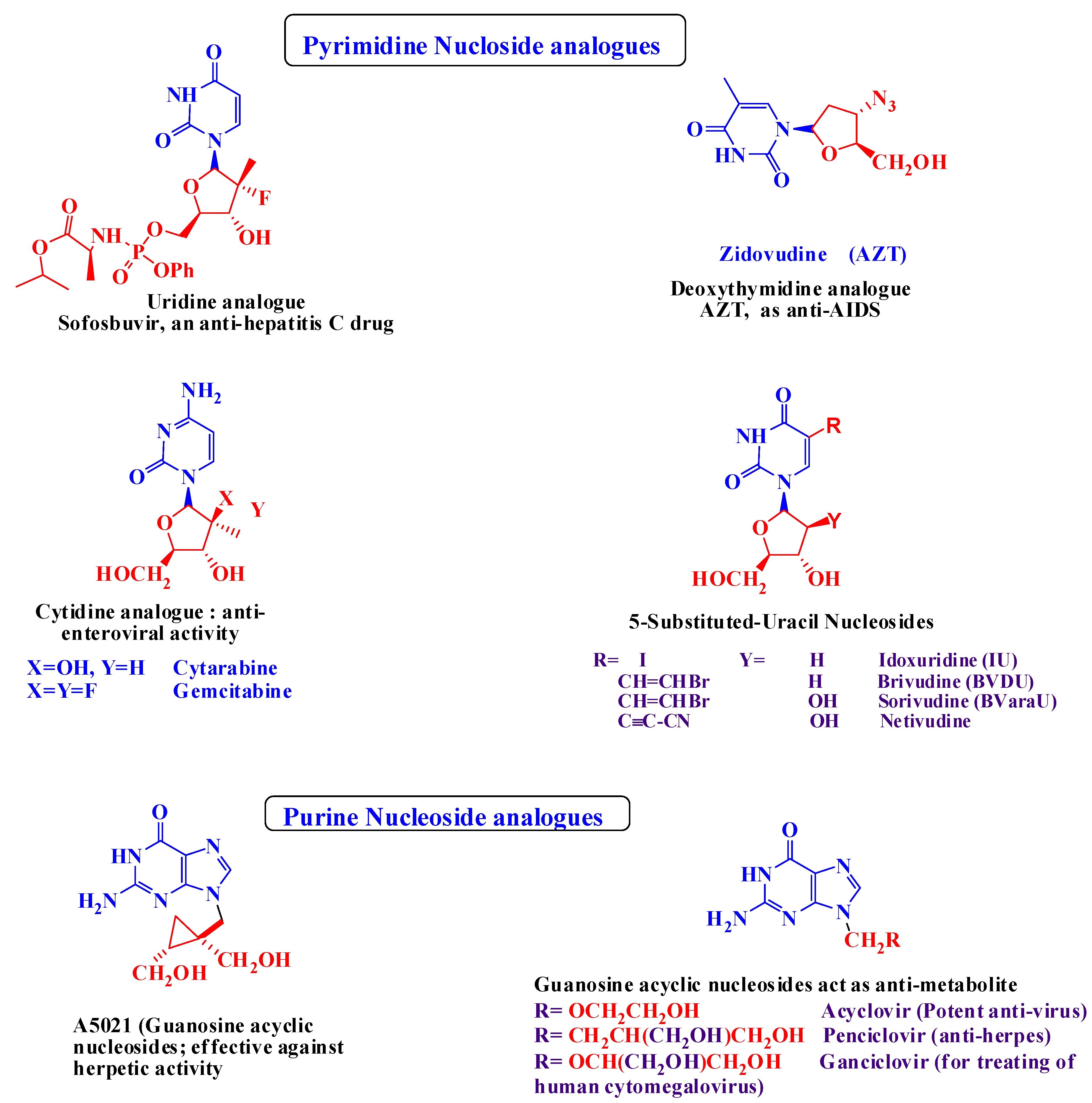
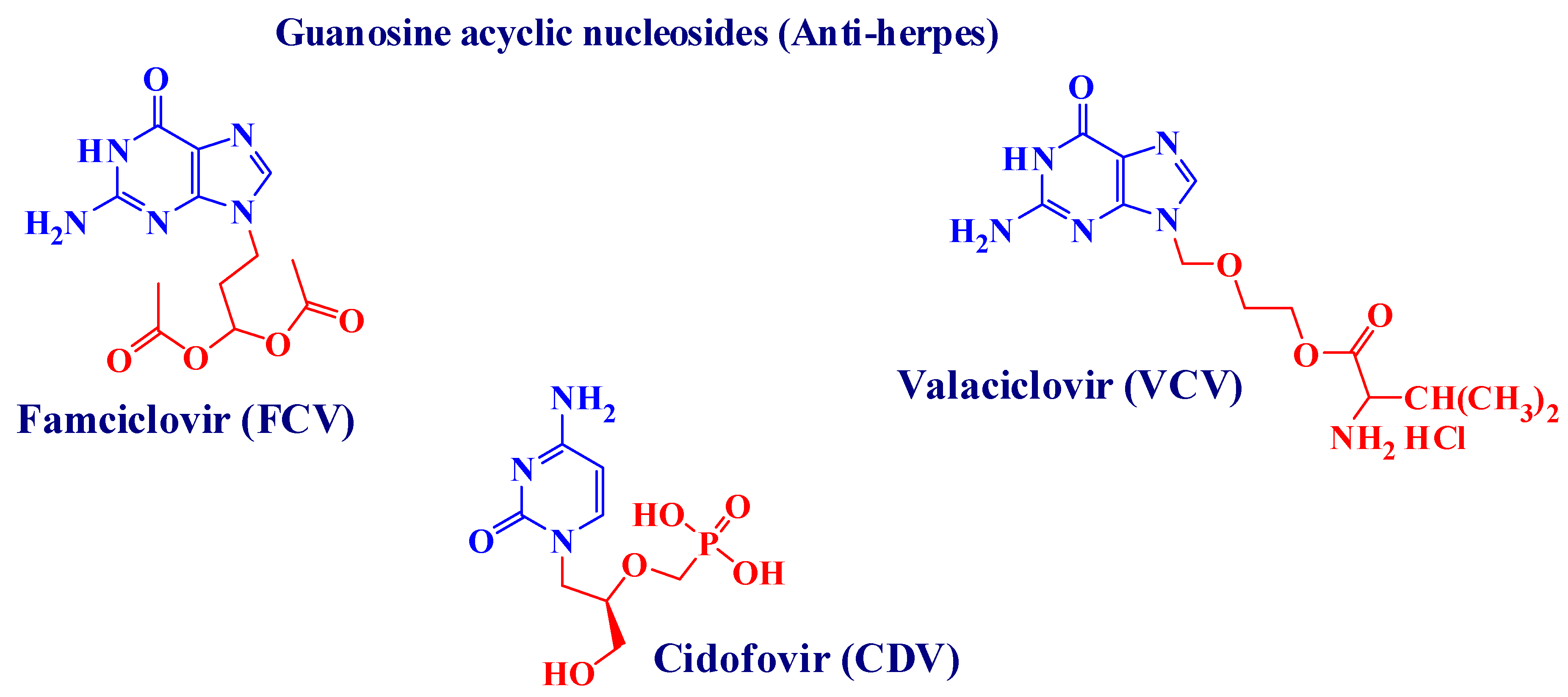
:1. Introduction
2. Results
2.1. Chemical Results
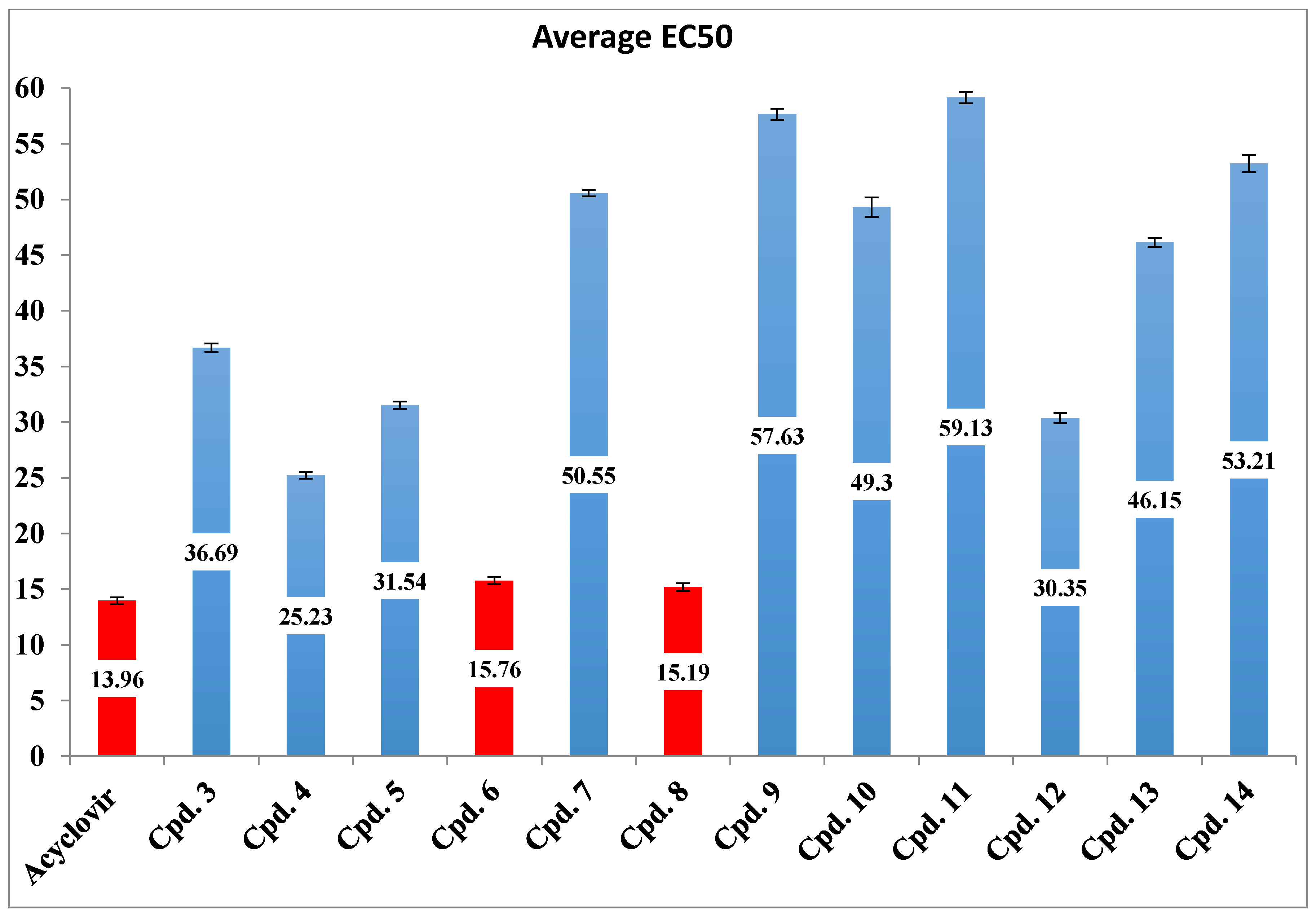
2.2. Biological Results
Antiherpetic Activity of the Synthesized Compounds
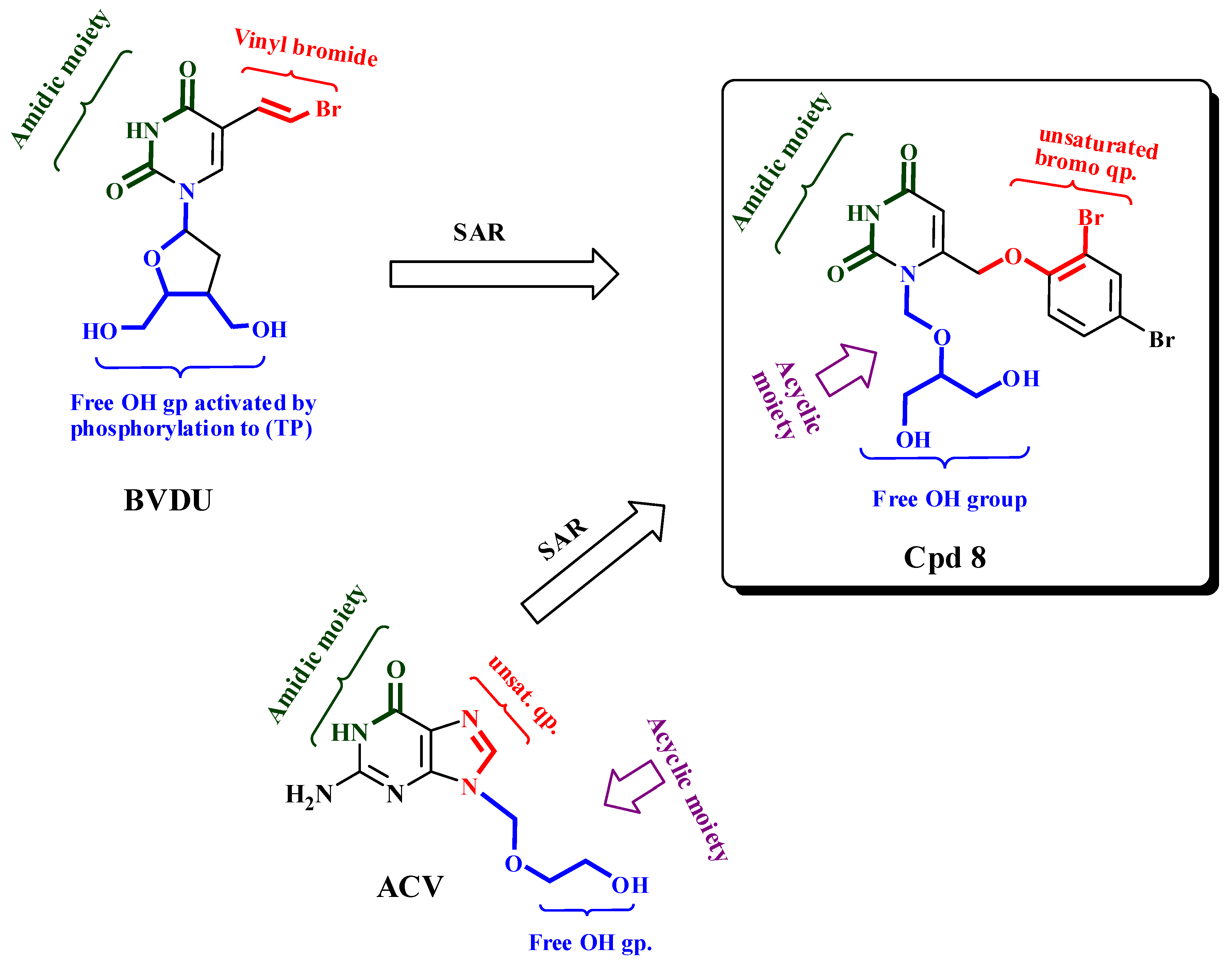
3. Discussion
4. Materials and Methods
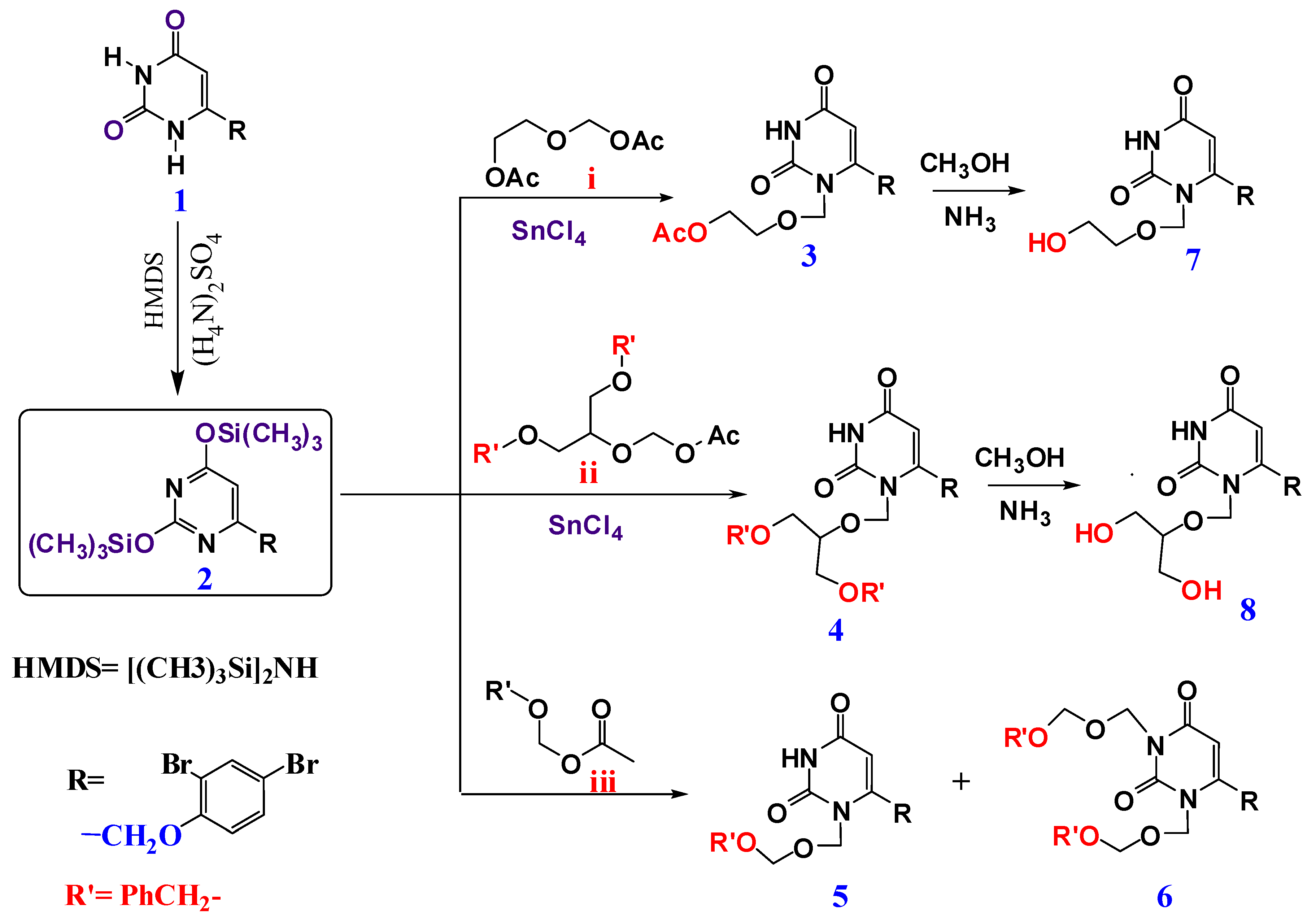
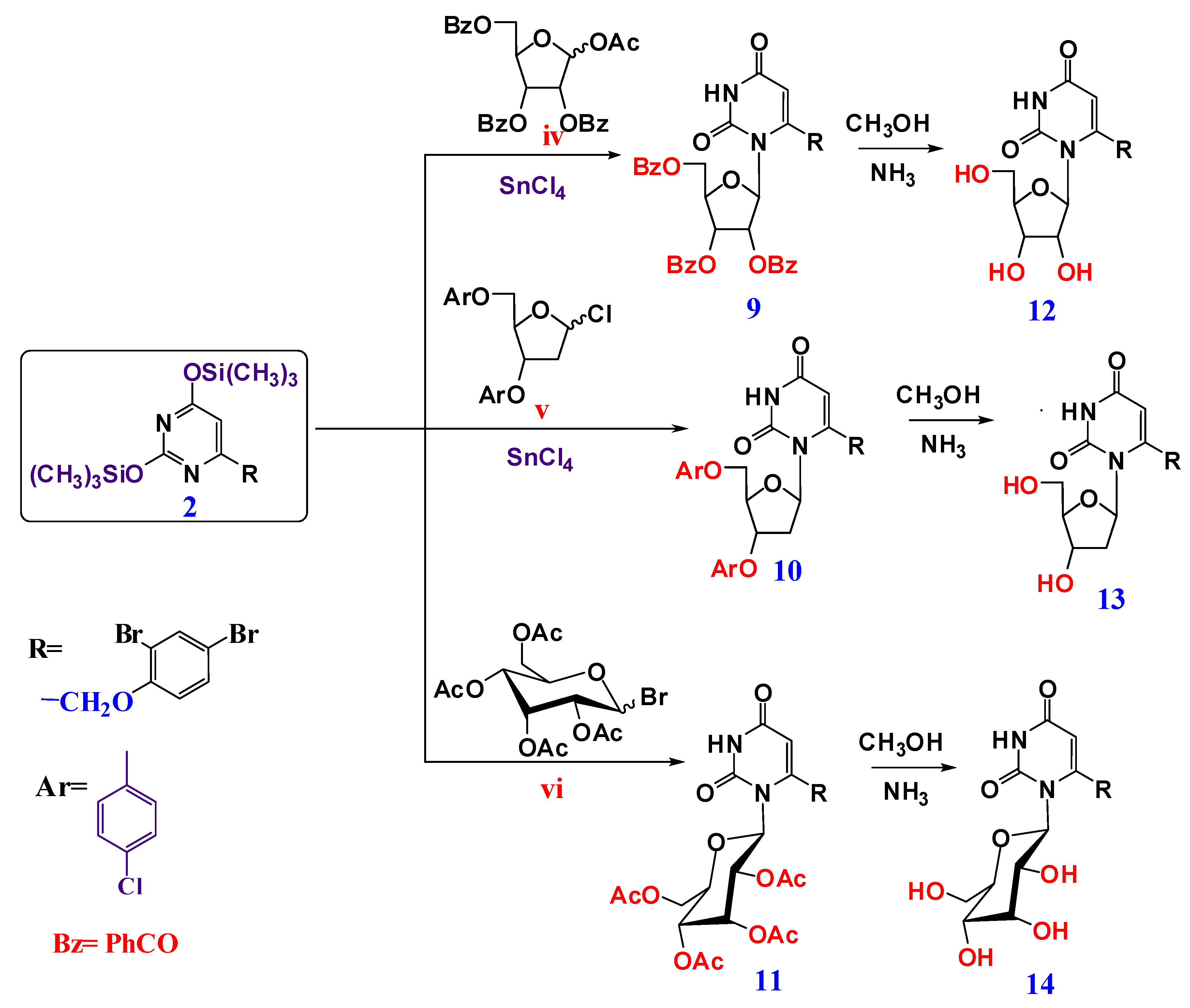
4.1. Chemistry
4.1.1. Preparation of 4-((2,4-Dibromophenoxy)methyl)-2,6-bis (trimethylsilyloxy) pyrimidine 2
4.1.2. General Procedure for Preparation of Acyclic and Cyclic Nucleosides
1-[(2-Acetoxyethoxy)methyl]-6-(2,4-dibromophenoxymethyl)uracil (3)
2-[(6-(2,4-dibromophenoxymethyl)-2,4-dioxo-1-pyrimidinyl)methoxy]-1,3-propanediyl dibenzoate (4)
1-(Benzyloxymethyl)-6-(2,4-dibromophenoxy methyl) uracil (5)
1,3-di(Benzyloxymethyl)-6-(2,4-dibromophenoxy methyl) uracil (6)
1-(2,3,5-Tri-O-benzoyl-ß-D-ribofuranosyl)-6-(2,4-dibromophenoxy methyl) uracil (9)
((2R,3S,5R)-3-(4-chlorobenzoloxy)-5-(6-((2,4-dibromophenoxy)methyl)-2,4-dioxo-3,4-dihydropyrimidin-1-(2H)-yl)tetrahydrofuran-2-yl)methyl-4-chlorobenzoate (10)
1-(2,3,4,6-Tetra-O-acetyl-ß-D-glucopyranosyl)-6-(2,4-dibromophenoxymethyl) uracil (11)
4.1.3. General Procedure for De-Protection of Nucleosides to Prepare (7, 8, 12–14)
1-(2-Hydroxyethoxy methyl)-6-(2,4-dibromophenoxy methyl) uracil (7)
1-[2-Hydroxy-1-(hydroxyl methyl) ethoxymethyl]-6-(2,4-dibromo phenoxymethyl) uracil (8)
1-(ß-D-Ribofuranosyl)-6-(2,4-dibromophenoxy methyl) uracil (12)
6-(2,4-Dibromophenoxy)methyl)-1-((2R,4S,5R)-4-hydroxy-5-(hydroxylmethyl)tetra-hydrofuran-2-yl)pyrimidine-2,4-(1H,3H)-dione (13)
1-(ß-D-Glucopyranosyl)-6-(2,4-dibromophenoxy methyl) uracil (14)
4.2. Biology
4.2.1. Cell and Virus
4.2.2. Preparation of Tested Compounds and the Standard
4.2.3. Antiherpetic Activity Assay
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jiang, Y.C.; Feng, H.; Lin, Y.C.; Guo, X.R. New strategies against drug resistance to herpes simplex virus. Int. J. Oral Sci. 2016, 8, 1–6. [Google Scholar] [CrossRef]
- Coen, N.; Singh, U.; Vuyyuru, V.; Van den Oord, J.J.; Balzarini, J.; Duraffour, S.; Snoeck, R.; Cheng, Y.C.; Chu, C.K.; Andrei, G. Activity and Mechanism of Action of HDVD, a Novel Pyrimidine Nucleoside Derivative with High Levels of Selectivity and Potency against Gammaherpesviruses. J. Virol. 2013, 87, 3839–3851. [Google Scholar] [CrossRef] [Green Version]
- Wilson, S.S.; Fakioglu, E.; Herold, B.C. Novel approaches in fighting herpes simplex virus infections. Expert Rev. Anti Infect. Ther. 2009, 7, 559–568. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aichelburg, M.C.; Weseslindtner, L.; Mandorfer, M.; Strassl, R.; Rieger, A.; Reiberger, T.; Puchhammer-Stöckl, E.; Grabmeier-Pfistershammer, K. Association of CMV-specific T cell-mediated immunity with CMV DNAemia and development of CMV disease in HIV-1-infected individuals. PLoS ONE 2015, 10, e0137096. [Google Scholar] [CrossRef] [Green Version]
- Hill, J.A.; Boeckh, M.J.; Sedlak, R.H.; Jerome, K.R.; Zerr, D.M. Human herpesvirus 6 can be detected in cerebrospinal fluid without associated symptoms after allogeneic hematopoietic cell transplantation. J. Clin. Virol. 2014, 61, 289–292. [Google Scholar] [CrossRef] [Green Version]
- Lei, X.; Bai, Z.; Ye, F.; Huang, Y.; Gao, S.J. Regulation of herpesvirus lifecycle by viral microRNAs. Virulence 2010, 1, 433–435. [Google Scholar] [CrossRef]
- Gilbert, C.; Bestman-Smith, J.; Boivin, G. Resistance of herpesviruses to antiviral drugs: Clinical impacts and molecular mechanisms. Drug Resist. Updates 2002, 5, 88–114. [Google Scholar] [CrossRef]
- De Clercq, E. Selective anti-herpesvirus agents. Antivir. Chem. Chemother. 2013, 23, 93–101. [Google Scholar] [CrossRef]
- De Clercq, E. Nucleoside Analogues Exerting Antiviral Activity through a Non-nucleoside Mechanism. Nucleosides Nucleotides Nucleic Acids 2004, 23, 457–470. [Google Scholar] [CrossRef]
- El-Din Abuo-Rahma, G.A.; Mohamed, M.F.A.; Ibrahim, T.S.; Shoman, M.E.; Samir, E.; Abd El-Baky, R.M. Potential repurposed SARS-CoV-2 (COVID-19) infection drugs. RSC Adv. 2020, 10, 26895–26916. [Google Scholar] [CrossRef]
- Andrei, G.; Snoeck, R. Emerging drugs for varicella-zoster virus infections. Expert Opin. Emerg. Drugs 2011, 16, 507–535. [Google Scholar] [CrossRef]
- Ramesh, D.; Vijayakumar, B.G.; Kannan, T. Therapeutic potential of uracil and its derivatives in countering pathogenic and physiological disorders. Eur. J. Med. Chem. 2020, 207, 112801. [Google Scholar] [CrossRef] [PubMed]
- De Clercq, E.; Holý, A. Case history: Acyclic nucleoside phosphonates: A key class of antiviral drugs. Nat. Rev. Drug Discov. 2005, 4, 928–940. [Google Scholar] [CrossRef] [PubMed]
- Onishi, T.; Mukai, C.; Nakagawa, R.; Sekiyama, T.; Aoki, M.; Suzuki, K.; Nakazawa, H.; Ono, N.; Ohmura, Y.; Iwayama, S.; et al. Synthesis and antiviral activity of novel anti-VZV 5-substituted uracil nucleosides with a cyclopropane sugar moiety. J. Med. Chem. 2000, 43, 278–282. [Google Scholar] [CrossRef] [PubMed]
- Shigeta, S.; Mori, S.; Kira, T.; Takahashi, K.; Kodama, E.; Konno, K.; Nagata, T.; Kato, H.; Wakayama, T.; Koike, N.; et al. Anti-herpesvirus activities and cytotoxicities of 2-thiopyrimidine nucleoside analogues in vitro. Antivir. Chem. Chemother. 1999, 10, 195–209. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.; Li, L.; Grill, S.; Gullen, E.; Lee, C.S.; Gumina, G.; Tsujii, E.; Cheng, Y.C.; Chu, C.K. Structure-activity relationships of (E)-5-(2-bromovinyl)uracil and related pyrimidine nucleosides as antiviral agents for herpes viruses. J. Med. Chem. 2000, 43, 2538–2546. [Google Scholar] [CrossRef] [PubMed]
- De Clercq, E. Guanosine analogues as anti-herpesvirus agents. Nucleosides Nucleotides Nucleic Acids 2000, 19, 1531–1541. [Google Scholar] [CrossRef]
- Machida, H.; Sakata, S.; Ashida, N.; Takenuki, K.; Matsuda, A. In vitro anti-herpesvirus activities of 5-substituted 2′-deoxy-2′-methylidene pyrimidine nucleosides. Antivir. Chem. Chemother. 1993, 4, 11–17. [Google Scholar] [CrossRef] [Green Version]
- Pałasz, A.; Ciez, D. In search of uracil derivatives as bioactive agents. Uracils and fused uracils: Synthesis, biological activity and applications. Eur. J. Med. Chem. 2015, 97, 582–611. [Google Scholar] [CrossRef]
- Katsuyama, A.; Ichikawa, S. Medicinal and Bioorganic Chemistry of Nucleosides and Nucleotides Synthesis and Medicinal Chemistry of Muraymycins, Nucleoside Antibiotics. Chem. Pharm. Bull. 2018, 66, 139–146. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Clercq, E. Antivirals and antiviral strategies. Nat. Rev. Microbiol. 2004, 2, 704–720. [Google Scholar] [CrossRef] [PubMed]
- Mahmoud, S.; Hasabelnaby, S.; Hammad, S.; Sakr, T. Antiviral Nucleoside and Nucleotide Analogs: A Review. J. Adv. Pharm. Res. 2018, 2, 73–88. [Google Scholar] [CrossRef] [Green Version]
- Azzam, R.A.; Osman, R.R.; Elgemeie, G.H. Efficient Synthesis and Docking Studies of Novel Benzothiazole-Based Pyrimidinesulfonamide Scaffolds as New Antiviral Agents and Hsp90α Inhibitors. ACS Omega 2020, 5, 1640–1655. [Google Scholar] [CrossRef]
- Pona, A.; Jiwani, R.A.; Afriyie, F.; Labbe, J.; Cook, P.P.; Mao, Y. Herpes zoster as a potential complication of coronavirus disease 2019. Dermatol. Ther. 2020, 18–19. [Google Scholar] [CrossRef] [PubMed]
- Altayeb, H.; Bouslama, L.; Abdulhakimc, J.A.; Chaieb, K.; Baothman, O.; Zamzami, M. Potential activity of a selected natural compounds on SARS-CoV-2 RNA-dependent-RNA polymerase, and binding affinity of the receptor-binding domain (RBD). Res. Sq. 2020, 1–39. [Google Scholar] [CrossRef]
- Wang, Y.; Anirudhan, V.; Du, R.; Cui, Q.; Rong, L. RNA-dependent RNA polymerase of SARS-CoV-2 as a therapeutic target. J. Med. Virol. 2020. [Google Scholar] [CrossRef] [PubMed]
- Machitani, M.; Yasukawa, M.; Nakashima, J.; Furuichi, Y.; Masutomi, K. RNA-dependent RNA polymerase, RdRP, a promising therapeutic target for cancer and potentially COVID-19. Cancer Sci. 2020, 111, 3976–3984. [Google Scholar] [CrossRef]
- Mancilla-Galindo, J.; Óscar García-Méndez, J.; Márquez-Sánchez, J.; Reyes-Casarrubias, R.E.; Aguirre-Aguilar, E.; Rocha-González, H.I.; Kammar-García, A. Use of antivirals and antibiotics for COVID-19 in Mexico City: A Real-World Multicenter Cohort Study. MedRxiv 2020. [Google Scholar] [CrossRef]
- Gao, Y.; Yan, L.; Huang, Y.; Liu, F.; Zhao, Y.; Cao, L.; Wang, T.; Sun, Q.; Ming, Z.; Zhang, L.; et al. Structure of the RNA-dependent RNA polymerase from COVID-19 virus. Science 2020, 368, 779–782. [Google Scholar] [CrossRef] [Green Version]
- Bocci, G.; Bradfute, S.B.; Ye, C.; Garcia, M.J.; Parvathareddy, J.; Reichard, W.; Surendranathan, S.; Bansal, S.; Bologa, C.G.; Perkins, D.J.; et al. Virtual and in Vitro Antiviral Screening Revive Therapeutic Drugs for COVID-19. ACS Pharmacol. Transl. Sci. 2020, 3, 1278–1292. [Google Scholar] [CrossRef]
- Yin, W.; Mao, C.; Luan, X.; Shen, D.D.; Shen, Q.; Su, H.; Wang, X.; Zhou, F.; Zhao, W.; Gao, M.; et al. Structural basis for inhibition of the RNA-dependent RNA polymerase from SARS-CoV-2 by remdesivir. Science 2020, 368, 1499–1504. [Google Scholar] [CrossRef] [PubMed]
- Jockusch, S.; Tao, C.; Li, X.; Anderson, T.K.; Chien, M.; Kumar, S.; Russo, J.J.; Kirchdoerfer, R.N.; Ju, J. A library of nucleotide analogues terminate RNA synthesis catalyzed by polymerases of coronaviruses that cause SARS and COVID-19. Antivir. Res. 2020, 180, 104857. [Google Scholar] [CrossRef] [PubMed]
- Mocarski, E.S., Jr. Comparative analysis of herpesvirus-common proteins. In Human Herpesviruses: Biology, Therapy, and Immunoprophylaxis; Cambridge University Press: Cambridge, UK, 2007; Chapter 4; pp. 1–17. [Google Scholar]
- Zhao, J.; Qin, C.; Liu, Y.; Rao, Y.; Feng, P. Herpes Simplex Virus and Pattern Recognition Receptors: An Arms Race. Front. Immunol. 2021, 11, 1–10. [Google Scholar] [CrossRef] [PubMed]
- De Clercq, E.; Li, G. Approved antiviral drugs over the past 50 years. Clin. Microbiol. Rev. 2016, 29, 695–747. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Coen, N.; Duraffour, S.; Snoeck, R.; Andrei, G. KSHV targeted therapy: An update on inhibitors of viral lytic replication. Viruses 2014, 6, 4731–4759. [Google Scholar] [CrossRef] [Green Version]
- Madavaraju, K.; Koganti, R.; Volety, I.; Yadavalli, T.; Shukla, D. Herpes Simplex Virus Cell Entry Mechanisms: An Update. Front. Cell. Infect. Microbiol. 2021, 10, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Stebbing, J.; Phelan, A.; Griffin, I.; Tucker, C.; Oechsle, O.; Smith, D.; Richardson, P. COVID-19: Combining antiviral and anti-inflammatory treatments. Lancet Infect. Dis. 2020, 20, 400–402. [Google Scholar] [CrossRef]
- Heidary, F.; Gharebaghi, R. Ivermectin: A systematic review from antiviral effects to COVID-19 complementary regimen. J. Antibiot. 2020, 73, 593–602. [Google Scholar] [CrossRef]
- Niedballa, U.; Vorbrüggen, H. A General Synthesis of N-Glycosides. I. Synthesis of Pyrimidine Nucleosides. J. Org. Chem. 1974, 39, 3654–3660. [Google Scholar] [CrossRef]
- Vorbrüggen, H.; Krolikiewicz, K.; Bennua, B. Nucleoside syntheses, XXII1) Nucleoside synthesis with trimethylsilyl triflate and perchlorate as catalysts. Chem. Ber. 1981, 114, 1234–1255. [Google Scholar] [CrossRef]
- Niedballa, U.; Vorbrüggen, H. A General Synthesis of N-Glycosides. 6. On the Mechanism of the Stannic Chloride Catalyzed Silyl Hilbert–Johnson Reaction. J. Org. Chem. 1976, 41, 2084–2086. [Google Scholar] [CrossRef]
- Andersen, G.W.; Halverstadt, I.F.; Miller, W.H.; Roblin, R.O. Studies in Chemotherapy. X. Antithyroid Compounds. Synthesis of 5- and 6- Substituted 2-Thiouracils from β-Oxoesters and Thiourea. J. Am. Chem. Soc. 1945, 67, 2197–2200. [Google Scholar] [CrossRef]
- El-Telbani, E.M.; El Shehry, M.F.; Nawwar, G.A.M. Meldrum’s acid in heterocyclic synthesis: Azoles incorporating a 2,4-dichlorophenoxy moiety with anticipated molluscicidal activity. Monatshefte fur Chemie 2008, 139, 685–689. [Google Scholar] [CrossRef]
- Elshehry, M.F.; Balzarini, J.; Meier, C. Synthesis of new cyclic and acyclic 5-halouridine derivatives as potential antiviral agents. Synthesis 2009, 841–847. [Google Scholar] [CrossRef]
- Meier, L.; Monteiro, G.C.; Baldissera, R.A.M.; Sá, M.M. Simple method for fast deprotection of nucleosides by triethylamine- catalyzed methanolysis of acetates in aqueous medium. J. Braz. Chem. Soc. 2010, 21, 859–866. [Google Scholar] [CrossRef] [Green Version]
- McCabe Dunn, J.M.; Reibarkh, M.; Sherer, E.C.; Orr, R.K.; Ruck, R.T.; Simmons, B.; Bellomo, A. The protecting-group free selective 3′-functionalization of nucleosides. Chem. Sci. 2017, 8, 2804–2810. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sauer, R.; El-Tayeb, A.; Kaulich, M.; Müller, C.E. Synthesis of uracil nucleotide analogs with a modified, acyclic ribose moiety as P2Y2 receptor antagonists. Bioorg. Med. Chem. 2009, 17, 5071–5079. [Google Scholar] [CrossRef] [PubMed]
- Boháčová, S.; Ludvíková, L.; Poštová Slavětínská, L.; Vaníková, Z.; Klán, P.; Hocek, M. Protected 5-(hydroxymethyl)uracil nucleotides bearing visible-light photocleavable groups as building blocks for polymerase synthesis of photocaged DNA. Org. Biomol. Chem. 2018, 16, 1527–1535. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vorbrüggen, H.; Bennua, B. Nucleoside syntheses, XXV1) A new simplified nucleoside synthesis. Chem. Ber. 1981, 114, 1279–1286. [Google Scholar] [CrossRef]
- Reefschläger, J.; Herrmann, G.; Bärwolff, D.; Schwarz, B.; Cech, D.; Langen, P. Antiherpes activity of (E)-5-(2-bromovinyl)- and 5-vinyl-1-β-D-arabinofuranosyluracil and some other 5-substituted uracil arabinosyl nucleosides in two different cell lines. Antivir. Res. 1983, 3, 175–187. [Google Scholar] [CrossRef]
- Mattelaer, H.P.; Van Hool, A.S.; de Jong, F.; Van der Auweraer, M.; Van Meervelt, L.; Dehaen, W.; Herdewijn, P. New Metal-Free Route towards Imidazole-Substituted Uridine. Eur. J. Org. Chem. 2020, 2020, 4022–4025. [Google Scholar] [CrossRef] [PubMed]
- Crute, J.J.; Lehman, I.R. Herpes simplex-1 DNA polymerase. Identification of an intrinsic 5′→3′ exonuclease with ribonuclease H activity. J. Biol. Chem. 1989, 264, 19266–19270. [Google Scholar] [CrossRef]
- Weller, S.K.; Coen, D.M. Herpes simplex viruses: Mechanisms of DNA replication. Cold Spring Harb. Perspect. Biol. 2012, 4, 1–14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bednarski, K.; Dixit, D.M.; Wang, W.; Evans, C.A.; Jin, H.; Yuen, L.; Mansour, T.S. Inhibitory activities of herpes simplex viruses type 1 and 2 and human cytomegalovirus by stereoisomers of 2′-deoxy-3′-oxa-5(E)-(2-bromovinyl)uridines and their 4′-thio analogues. Bioorg. Med. Chem. Lett. 1994, 4, 2667–2672. [Google Scholar] [CrossRef]
- Piret, J.; Boivin, G. Resistance of herpes simplex viruses to nucleoside analogues: Mechanisms, prevalence, and management. Antimicrob. Agents Chemother. 2011, 55, 459–472. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schnitzler, P.; Koch, C.; Reichling, J. Susceptibility of drug-resistant clinical herpes simplex virus type 1 strains to essential oils of ginger, thyme, hyssop, and sandalwood. Antimicrob. Agents Chemother. 2007, 51, 1859–1862. [Google Scholar] [CrossRef] [Green Version]
- Czartoski, T.; Liu, C.; Koelle, D.M.; Schmechel, S.; Kalus, A.; Wald, A. Fulminant, acyclovir-resistant, herpes simplex virus type 2 hepatitis in an immunocompetent woman. J. Clin. Microbiol. 2006, 44, 1584–1586. [Google Scholar] [CrossRef] [Green Version]
- Razonable, R.R. Antiviral drugs for viruses other than human immunodeficiency virus. Mayo Clin. Proc. 2011, 86, 1009–1026. [Google Scholar] [CrossRef] [Green Version]
- Chrisp, P.; Clissold, S.P. Foscarnet: A Review of its Antiviral Activity, Pharmacokinetic Properties and Therapeutic Use in Immunocompromised Patients with Cytomegalovirus Retinitis. Drugs 1991, 41, 104–129. [Google Scholar] [CrossRef]
- Kotton, C.N.; Kumar, D.; Caliendo, A.M.; Åsberg, A.; Chou, S.; Snydman, D.R.; Allen, U.; Humar, A.; Emery, V.; Lautenschlager, I.; et al. International consensus guidelines on the management of cytomegalovirus in solid organ transplantation. Transplantation 2010, 89, 779–795. [Google Scholar] [CrossRef]
- Humar, A.; Snydman, D. Cytomegalovirus in solid organ transplant recipients. Am. J. Transplant. 2009, 9, 78–86. [Google Scholar] [CrossRef] [PubMed]
- Garvey, L.; Thomson, E.C.; Taylor, G.P. Progressive multifocal leukoencephalopathy: Prolonged survival in patients treated with protease inhibitors and cidofovir: A case series. AIDS 2006, 20, 791–793. [Google Scholar] [CrossRef] [PubMed]
- LoPresti, A.E.; Levine, J.F.; Munk, G.B.; Tai, C.Y.; Mendel, D.B. Successful treatment of an acyclovir- and foscarnet- resistant herpes virus simplex virus type 1 lesion with intravenous cidofovir. Clin. Infect. Dis. 1998, 26, 512–513. [Google Scholar] [CrossRef] [Green Version]
- Bestman-Smith, J.; Boivin, G. Drug Resistance Patterns of Recombinant Herpes Simplex Virus DNA Polymerase Mutants Generated with a Set of Overlapping Cosmids and Plasmids. J. Virol. 2003, 77, 7820–7829. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zandi, K.; Ramedani, E.; Khosro, M.; Tajbakhsh, S.; Dailami, I.; Rastian, Z.; Fouladvand, M.; Yousefi, F.; Farshadpour, F. Natural Product Communications Evaluation of Antiviral Activities of Curcumin Derivatives. Nat. Prod. Commun. 2010, 5, 8–11. [Google Scholar]
- Aguilar, J.S.; Roy, D.; Ghazal, P.; Wagner, E.K. Dimethyl sulfoxide blocks herpes simplex virus-I productive infection in vitro acting at different stages with positive cooperativity. Application of micro-array analysis. BMC Infect. Dis. 2002, 2, 1–10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| Concentration (μg/mL) | CPE Inhibition (%) | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ACV * | Cpd. 3 | Cpd. 4 * | Cpd. 5 | Cpd. 6 * | Cpd. 7 | Cpd. 8 * | Cpd. 9 | Cpd. 10 | Cpd. 11 | Cpd. 12 * | Cpd. 13 | Cpd. 14 | |
| 6 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 12 | 29 | 0 | 14 | 0 | 30 | 0 | 32 | 0 | 0 | 0 | 0 | 0 | 0 |
| 18 | 50 | 12 | 22 | 15 | 45 | 0 | 42 | 0 | 0 | 0 | 0 | 0 | 0 |
| 24 | 70 | 28 | 42 | 28 | 65 | 10 | 72 | 0 | 10 | 0 | 32 | 0 | 0 |
| 30 | 77 | 34 | 62 | 40 | 80 | 16 | 80 | 22 | 20 | 18 | 44 | 22 | 18 |
| 36 | 92 | 38 | 70 | 65 | 100 | 20 | 100 | 28 | 32 | 22 | 75 | 34 | 28 |
| 42 | 100 | 55 | 100 | 82 | 26 | 34 | 50 | 26 | 86 | 45 | 36 | ||
| 48 | 77 | 100 | 66 | 38 | 82 | 34 | 100 | 52 | 46 | ||||
| 54 | 80 | 74 | 47 | 100 | 46 | 66 | 58 | ||||||
| 60 | 100 | 88 | 65 | 48 | 72 | 76 | |||||||
| 66 | 100 | 88 | 74 | 77 | 82 | ||||||||
| 72 | 100 | 78 | 82 | 100 | |||||||||
| 78 | 85 | 100 | |||||||||||
| 84 | 100 | ||||||||||||
| Concentration (μg/mL) | Compound 4 | Compound 6 | Compound 8 | ACV |
|---|---|---|---|---|
| CPE Inhibition (%) Mean ± SE | ||||
| 6 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 |
| 12 | 14.25 ± 3.75 | 30.30 ± 3.40 | 31.95 ± 2.75 | 29.00 ± 3.00 |
| 18 | 22.35 ± 2.25 | 45.90 ± 3.40 | 41.25 ± 4.05 | 49.80 ± 3.40 |
| 24 | 42.35 ± 1.85 | 65.15 ± 1.75 | 72.25 ± 2.05 | 70.45 ± 2.75 |
| 32 | 62.00 ± 5.00 | 80.40 ± 10.00 | 80.00 ± 10.00 | 77.65 ± 1.75 |
| 36 | 75.75 ± 9.85 | 99.00 ± 1.00 | 99.00 ± 1.00 | 92.50 ± 4.50 |
| 42 | 99.50 ± 0.50 | 99.50 ± 0.50 | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Awad, S.M.; Ali, S.M.; Mansour, Y.E.; Fatahala, S.S. Synthesis and Evaluation of Some Uracil Nucleosides as Promising Anti-Herpes Simplex Virus 1 Agents. Molecules 2021, 26, 2988. https://doi.org/10.3390/molecules26102988
Awad SM, Ali SM, Mansour YE, Fatahala SS. Synthesis and Evaluation of Some Uracil Nucleosides as Promising Anti-Herpes Simplex Virus 1 Agents. Molecules. 2021; 26(10):2988. https://doi.org/10.3390/molecules26102988
Chicago/Turabian StyleAwad, Samir Mohamed, Shima Mahmoud Ali, Yara Essam Mansour, and Samar Said Fatahala. 2021. "Synthesis and Evaluation of Some Uracil Nucleosides as Promising Anti-Herpes Simplex Virus 1 Agents" Molecules 26, no. 10: 2988. https://doi.org/10.3390/molecules26102988





