Gold Nanospikes Formation on Screen-Printed Carbon Electrode through Electrodeposition Method for Non-Enzymatic Electrochemical Sensor
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
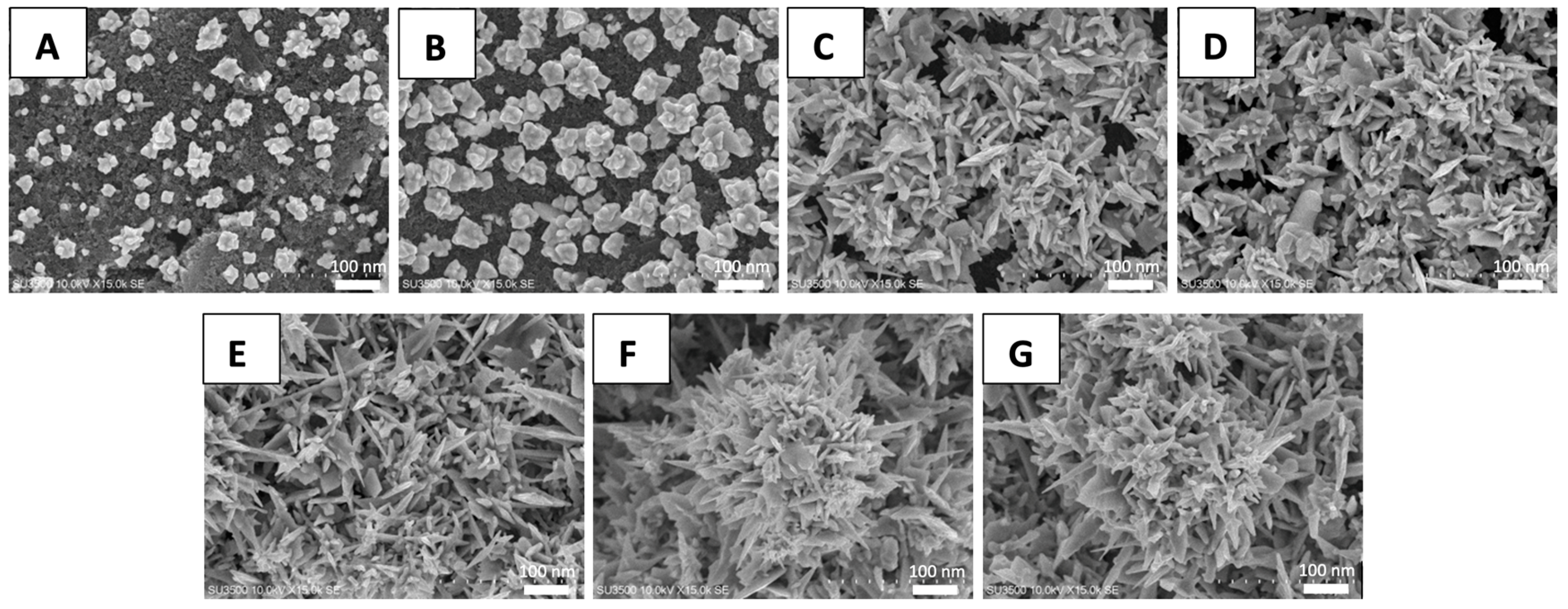
3.1. Characterization of the AuNS Morphology
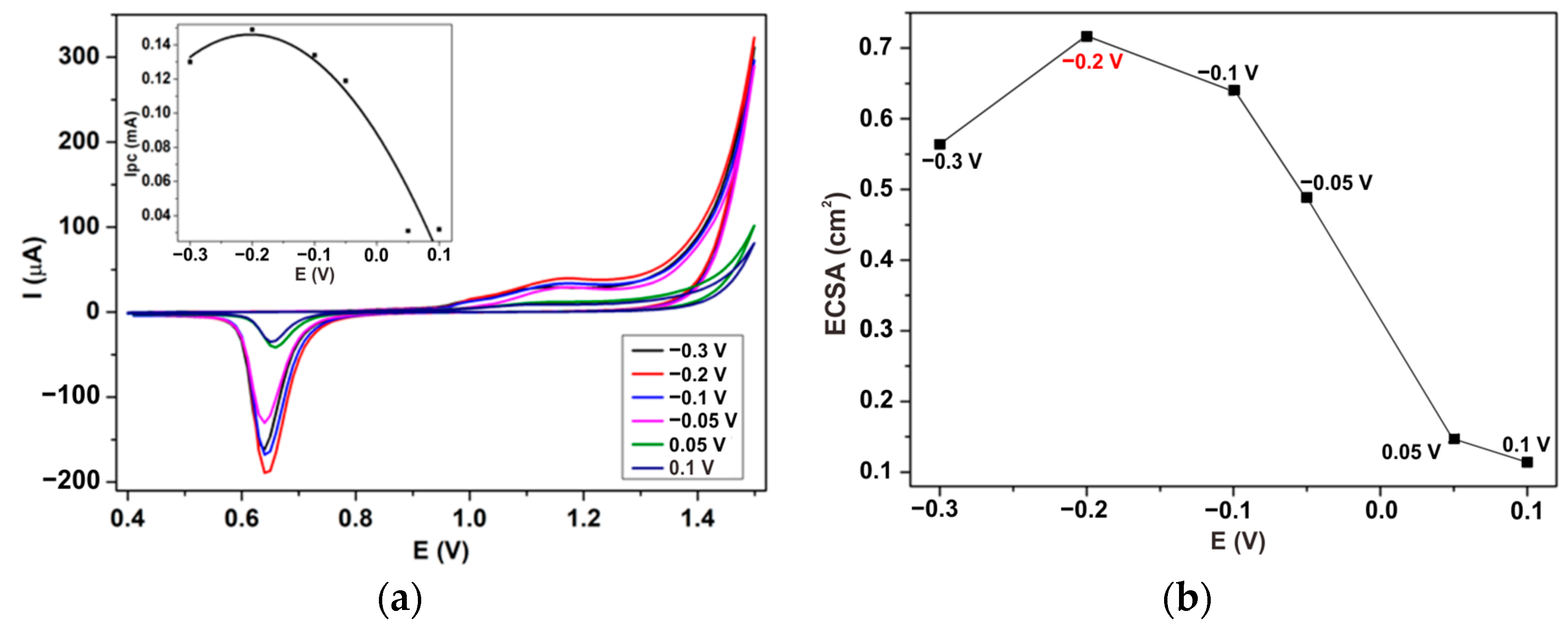
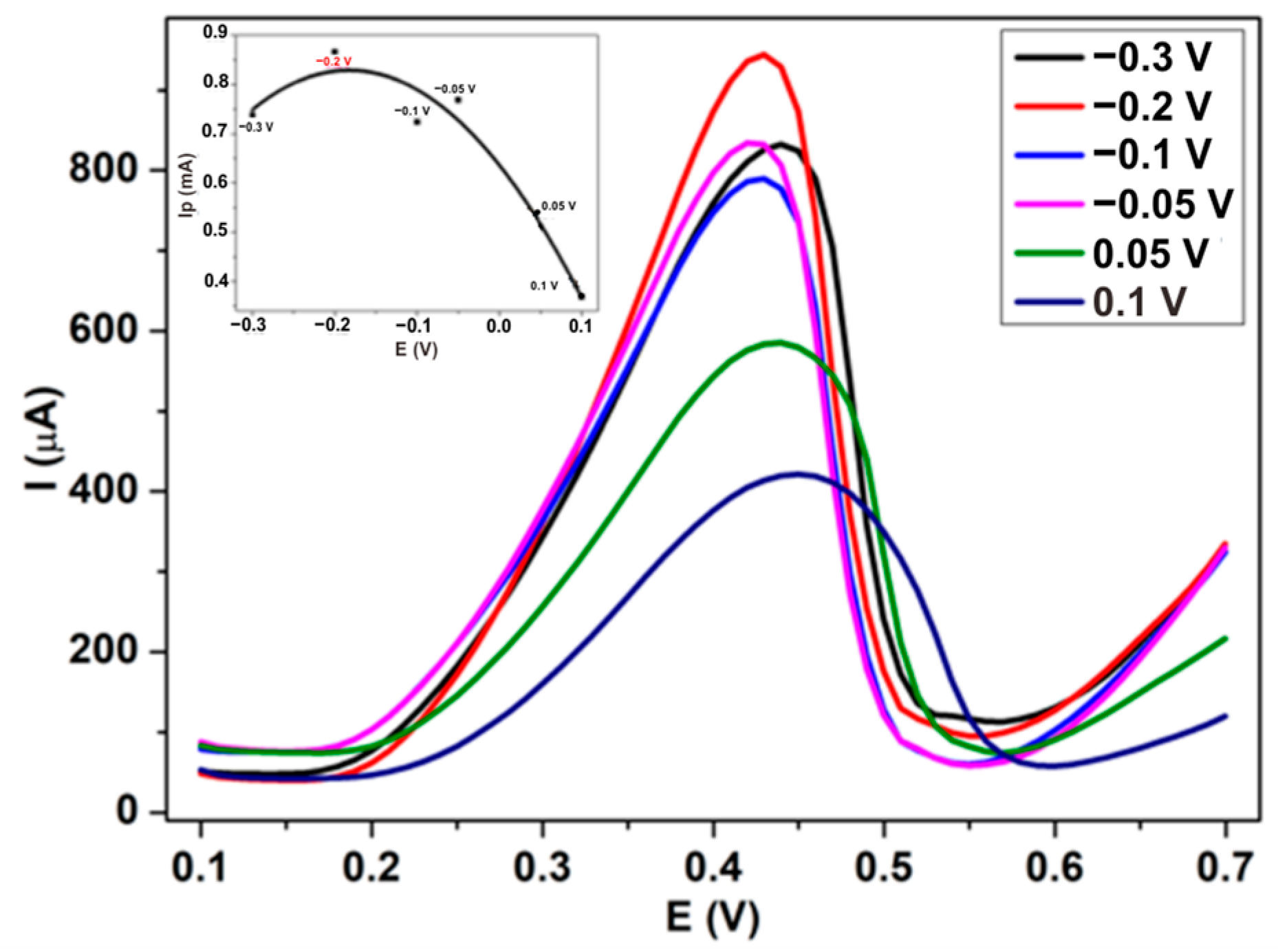
3.1.1. The Effect of the Electrodeposition Potential
3.1.2. The Optimization of Deposition Time and Precursor Concentration on the AuNS-Modified SPCE
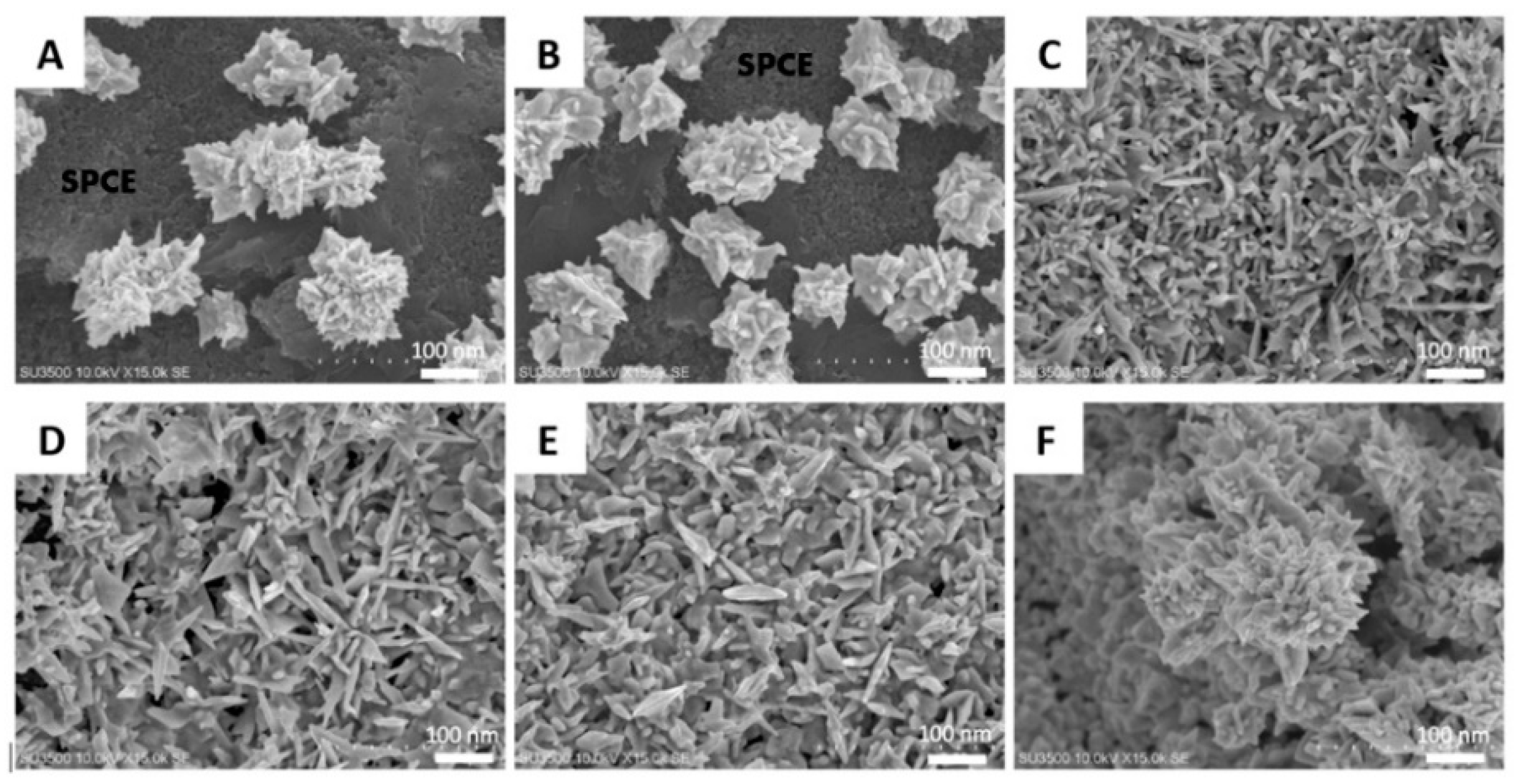
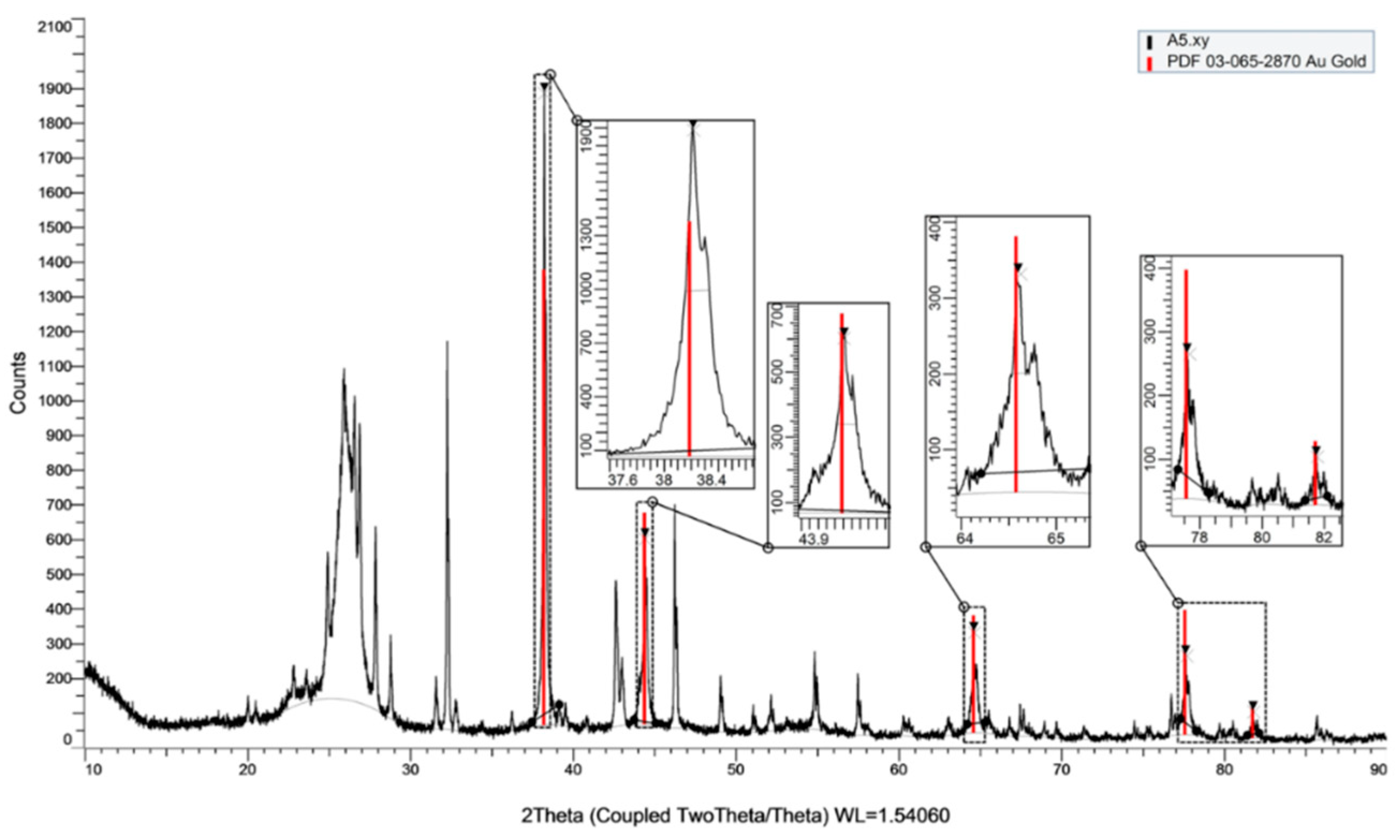
3.1.3. FE-SEM and XRD Characterization on the AuNS-Modified SPCE
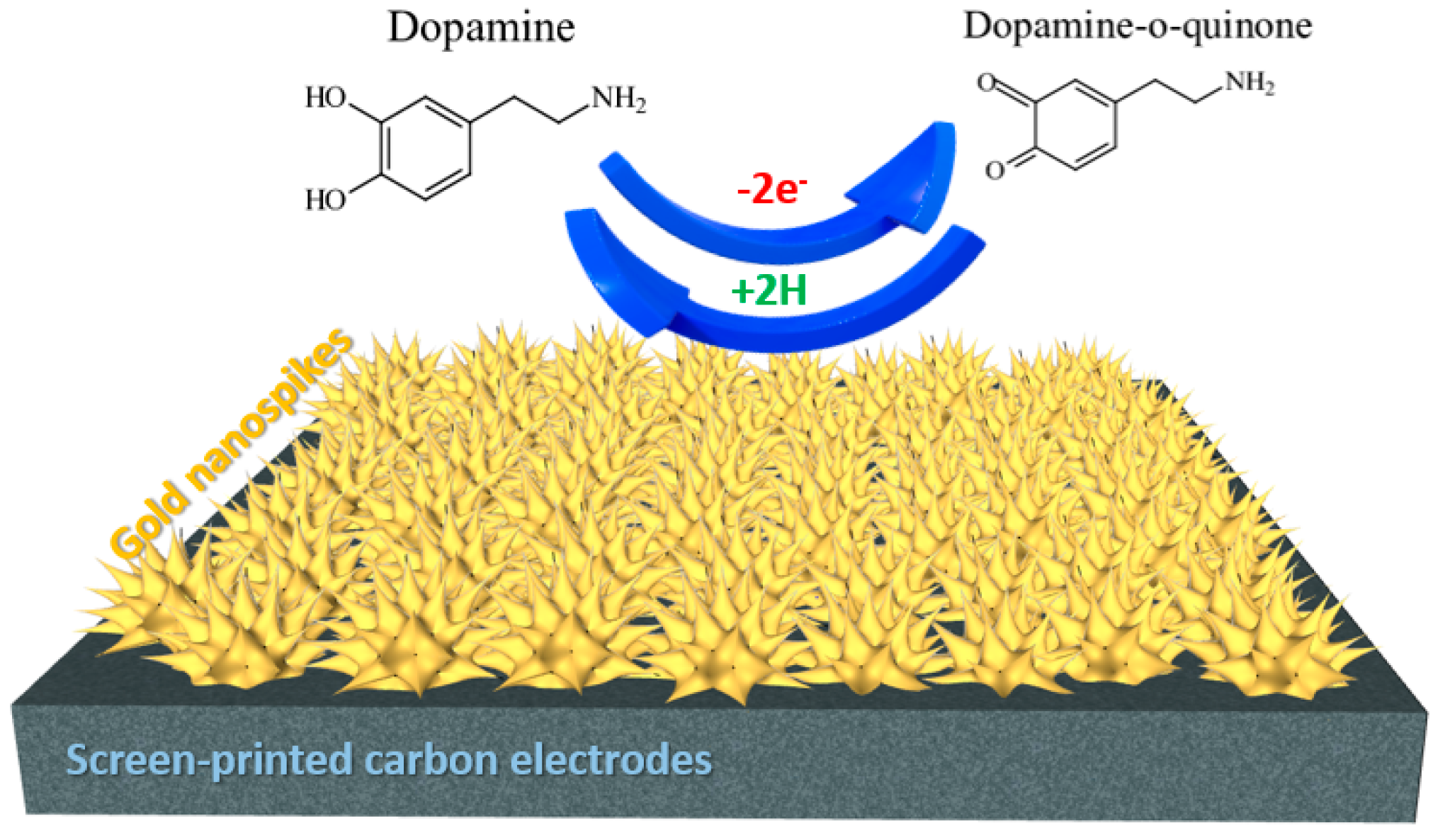
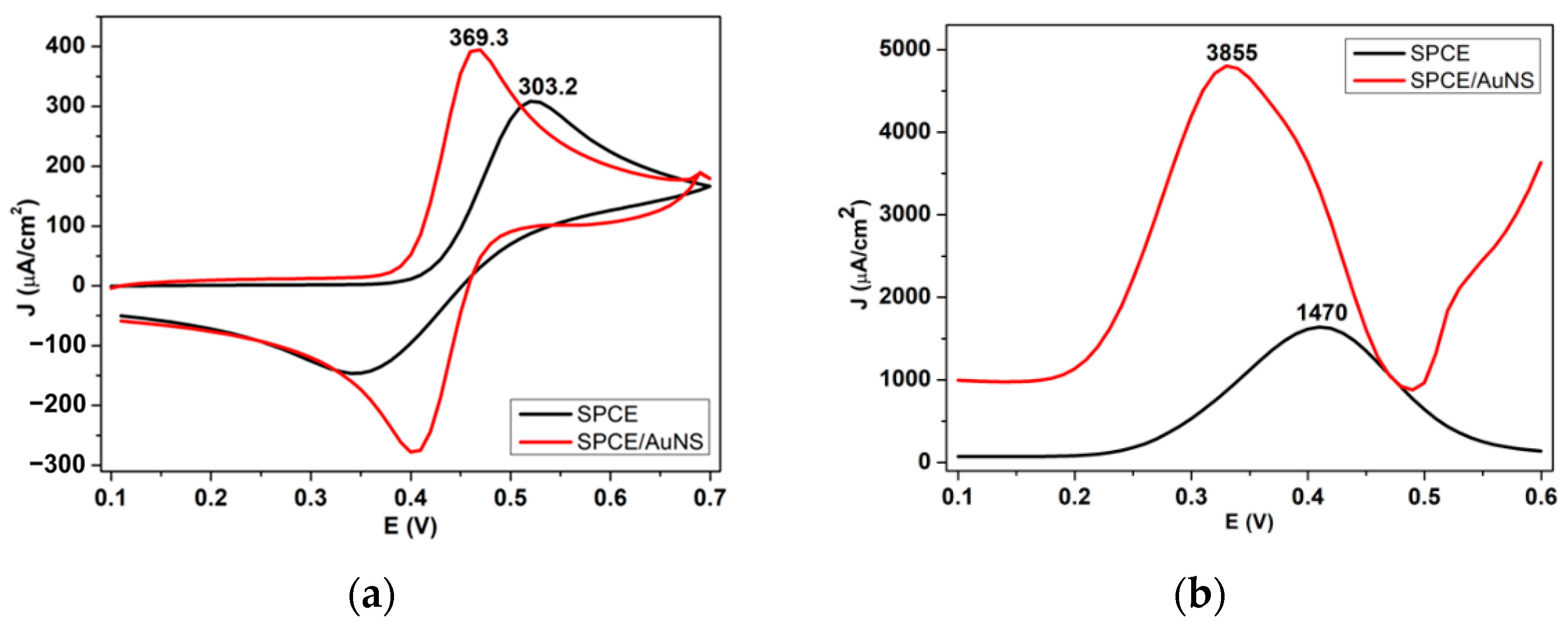
3.2. Electrocatalytic Activity of the AuNS-Modified SPCE towards Dopamine Detection
3.2.1. Electrochemical Measurement towards Dopamine
3.2.2. Effect of the Potential Scan Rate Variation
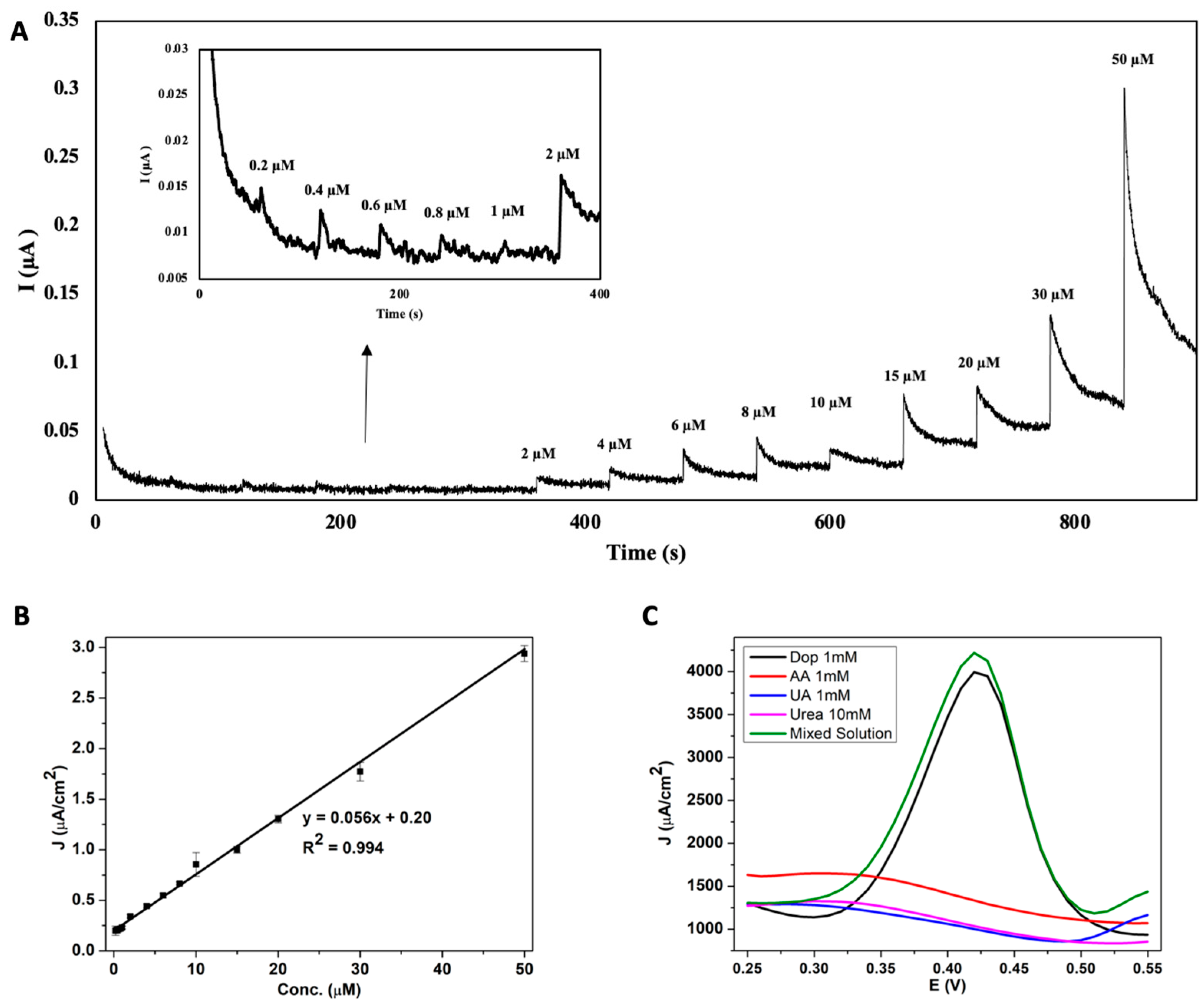
3.2.3. Sensitivity and Linearity of the AuNS-Modified SPCE for Dopamine Concentration
3.2.4. Selectivity of the AuNS-Modified SPCE Dopamine Sensor
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lakard, S.; Pavel, I.A.; Lakard, B. Electrochemical Biosensing of Dopamine Neurotransmitter: A Review. Biosensors 2021, 11, 179. [Google Scholar] [CrossRef] [PubMed]
- Plowman, B.J.; Mahajan, M.; O’Mullane, A.P.; Bhargava, S.K. Electrochemical Detection of Dopamine and Cytochrome c at a Nanostructured Gold Electrode. Electrochim. Acta 2010, 55, 8953–8959. [Google Scholar] [CrossRef]
- Huang, J.; Liu, Y.; Hou, H.; You, T. Simultaneous Electrochemical Determination of Dopamine, Uric Acid and Ascorbic Acid Using Palladium Nanoparticle-Loaded Carbon Nanofibers Modified Electrode. Biosens. Bioelectron. 2008, 24, 632–637. [Google Scholar] [CrossRef] [PubMed]
- Choe, J.E.; Ahmed, M.S.; Jeon, S. Trouble Free Dopamine Sensing by Palladium Nanoparticles Fabricated Poly(3,4-Ethylenedioxythiophene) Functionalized Graphene. J. Electrochem. Soc. 2016, 163, B113–B118. [Google Scholar] [CrossRef]
- Wei, Y.; Liu, Y.; Xu, Z.; Wang, S.; Chen, B.; Zhang, D.; Fang, Y. Simultaneous Detection of Ascorbic Acid, Dopamine, and Uric Acid Using a Novel Electrochemical Sensor Based on Palladium Nanoparticles/Reduced Graphene Oxide Nanocomposite. Int. J. Anal. Chem. 2020, 2020, 8812443. [Google Scholar] [CrossRef]
- Anshori, I.; Nuraviana Rizalputri, L.; Rona Althof, R.; Sean Surjadi, S.; Harimurti, S.; Gumilar, G.; Yuliarto, B.; Handayani, M. Functionalized Multi-Walled Carbon Nanotube/Silver Nanoparticle (f-MWCNT/AgNP) Nanocomposites as Non-Enzymatic Electrochemical Biosensors for Dopamine Detection. Nanocomposites 2021, 7, 97–108. [Google Scholar] [CrossRef]
- Adhikari, A.; De, S.; Rana, D.; Nath, J.; Ghosh, D.; Dutta, K.; Chakraborty, S.; Chattopadhyay, S.; Chakraborty, M.; Chattopadhyay, D. Selective Sensing of Dopamine by Sodium Cholate Tailored Polypyrrole-Silver Nanocomposite. Synth. Met. 2020, 260, 116296. [Google Scholar] [CrossRef]
- Yang, Z.; Liu, X.; Zheng, X.; Zheng, J. Synthesis of Au@Pt Nanoflowers Supported on Graphene Oxide for Enhanced Electrochemical Sensing of Dopamine. J. Electroanal. Chem. 2018, 817, 48–54. [Google Scholar] [CrossRef] [Green Version]
- Zhao, D.; Yu, G.; Tian, K.; Xu, C. A Highly Sensitive and Stable Electrochemical Sensor for Simultaneous Detection towards Ascorbic Acid, Dopamine, and Uric Acid Based on the Hierarchical Nanoporous PtTi Alloy. Biosens. Bioelectron. 2016, 82, 119–126. [Google Scholar] [CrossRef]
- Chen, D.; Tian, C.; Li, X.; Li, Z.; Han, Z.; Zhai, C.; Quan, Y.; Cui, R.; Zhang, G. Electrochemical Determination of Dopamine Using a Glassy Carbon Electrode Modified with a Nanocomposite Consisting of Nanoporous Platinum-Yttrium and Graphene. Microchimica. Acta 2018, 185, 13–15. [Google Scholar] [CrossRef]
- Elewi, A.S.; Al-Shammaree, S.A.W.; AL Sammarraie, A.K.M.A. Hydrogen Peroxide Biosensor Based on Hemoglobin-Modified Gold Nanoparticles–Screen Printed Carbon Electrode. Sens. Biosensing Res. 2020, 28, 100340. [Google Scholar] [CrossRef]
- Ramanaviciene, A.; German, N.; Kausaite-minkstimiene, A. Glucose Biosensor Based on Dendritic Gold Nanostructures Electrodeposited on Graphite Electrode by Different Electrochemical Methods. Chemosensors 2021, 9, 188. [Google Scholar] [CrossRef]
- Xiao, T.; Huang, J.; Wang, D.; Meng, T.; Yang, X. Au and Au-Based Nanomaterials: Synthesis and Recent Progress in Electrochemical Sensor Applications. Talanta 2020, 206, 120210. [Google Scholar] [CrossRef] [PubMed]
- Alonso-Lomillo, M.A.; Domínguez-Renedo, O.; Saldaña-Botín, A.; Arcos-Martínez, M.J. Determination of Ascorbic Acid in Serum Samples by Screen-Printed Carbon Electrodes Modified with Gold Nanoparticles. Talanta 2017, 174, 733–737. [Google Scholar] [CrossRef]
- Pingarrón, J.M.; Yáñez-Sedeño, P.; González-Cortés, A. Gold Nanoparticle-Based Electrochemical Biosensors. Electrochim. Acta 2008, 53, 5848–5866. [Google Scholar] [CrossRef]
- Liu, X.Y.; Wang, J.Q.; Ashby, C.R.; Zeng, L.; Fan, Y.F.; Chen, Z.S. Gold Nanoparticles: Synthesis, Physiochemical Properties and Therapeutic Applications in Cancer. Drug Discov. Today 2021, 26, 1284–1292. [Google Scholar] [CrossRef]
- Malvano, F.; Albanese, D.; Crescitelli, A.; Pilloton, R.; Esposito, E. Impedimetric Label-Free Immunosensor on Disposable Modified Screen-Printed Electrodes for Ochratoxin A. Biosensors 2016, 6, 33. [Google Scholar] [CrossRef] [Green Version]
- Graule, T.; Michler, J. Electrodeposition of Gold Thin Films with Controlled Morphologies and Their Applications in Electrocatalysis and SERS. Nanotechnology 2012, 23, 255705. [Google Scholar] [CrossRef]
- Purwidyantri, A.; Karina, M.; Hsu, C.-H.; Srikandace, Y.; Prabowo, B.A.; Lai, C.-S. Facile Bacterial Cellulose Nanofibrillation for the Development of Plasmonic Paper Sensor. ACS Biomater. Sci. Eng. 2020, 6, 3122–3131. [Google Scholar] [CrossRef]
- Purwidyantri, A.; Tian, Y.; Muhammad, G.; Saputra, A.; Prabowo, B.A. Gold Nanoframe Array Electrode for Straightforward Detection of Hydrogen Peroxide. Chemosensors 2021, 9, 37. [Google Scholar] [CrossRef]
- Hsu, C.H.; Gupta, A.K.; Purwidyantri, A.; Prabowo, B.A.; Chen, C.H.; Chuang, C.C.; Tian, Y.C.; Lu, Y.J.; Lai, C.S. Sensing Alzheimer’s Disease Utilizing Au Electrode by Controlling Nanorestructuring. Chemosensors 2022, 10, 94. [Google Scholar] [CrossRef]
- Ren, B.; Jones, L.A.; Chen, M.; Oppedisano, D.K.; Qiu, D.; Ippolito, S.J.; Bhargava, S.K. The Effect of Electrodeposition Parameters and Morphology on the Performance of Au Nanostructures for the Detection of As (III). J. Electrochem. Soc. 2017, 164, H1121. [Google Scholar] [CrossRef] [Green Version]
- Yao, S.; Lv, Y.; Wang, Q.; Yang, J.; Li, H.; Gao, N.; Zhong, F.; Fu, J.; Tang, J.; Wang, T.; et al. Facile Preparation of Highly Sensitive SERS Substrates Based on Gold Nanoparticles Modified Graphdiyne/Carbon Cloth. Appl. Surf. Sci. 2023, 609, 155098. [Google Scholar] [CrossRef]
- Madhu, S.; Han, J.H.; Jeong, C.W.; Choi, J. Sensitive Electrochemical Sensing Platform Based on Au Nanoflower-Integrated Carbon Fiber for Detecting Interleukin-6 in Human Serum. Anal. Chim. Acta 2023, 1238, 340644. [Google Scholar] [CrossRef]
- Sun, C.; Shen, Y.; Zhang, Y.; Du, Y.; Peng, Y.; Xie, Q. Sandwich Photoelectrochemical Biosensing of Concanavalin A Based on CdS/AuNPs/NiO Z-Scheme Heterojunction and Lectin-Sugar Binding. Talanta 2023, 253, 123882. [Google Scholar] [CrossRef] [PubMed]
- Mohan Mundotiya, B.; Ullah, W. Morphology Controlled Synthesis of the Nanostructured Gold by Electrodeposition Techniques. In Novel Metal Electrodeposition and the Recent Application; IntechOpen: London, UK, 2018; pp. 1–17. [Google Scholar] [CrossRef] [Green Version]
- Tian, Y.; Liu, H.; Zhao, G.; Tatsuma, T. Shape-Controlled Electrodeposition of Gold Nanostructures. J. Phys. Chem. B 2006, 110, 23478–23481. [Google Scholar] [CrossRef]
- Stine, K.J. Biosensor Applications of Electrodeposited Nanostructures. Appl. Sci. 2019, 9, 797. [Google Scholar] [CrossRef] [Green Version]
- Plowman, B.; Ippolito, S.J.; Bansal, V.; Sabri, Y.M.; O’Mullane, A.P.; Bhargava, S.K. Gold Nanospikes Formed through a Simple Electrochemical Route with High Electrocatalytic and Surface Enhanced Raman Scattering Activity. Chem. Commun. 2009, 33, 5039–5041. [Google Scholar] [CrossRef] [Green Version]
- Weinstein, J.J.; Chohan, M.O.; Slifstein, M.; Kegeles, L.S.; Moore, H.; Abi-dargham, A. Review Pathway-Speci Fi c Dopamine Abnormalities in Schizophrenia. Biol. Psychiatry 2017, 81, 31–42. [Google Scholar] [CrossRef]
- Prieto, G.A. Abnormalities of Dopamine D 3 Receptor Signaling in the Diseased Brain. J. Cent. Nerv. Syst. Dis. 2017, 9, 1179573517726335. [Google Scholar] [CrossRef] [Green Version]
- Lin, C.; Chen, T.; Cheng, P.; Liu, Y. Early Life Social Experience affects Adulthood Fear Extinction deficit and Associated Dopamine profile Abnormalities in a Rat Model of PTSD. Prog. Neuropsychopharmacol. Biol. Psychiatry 2020, 101, 109914. [Google Scholar] [CrossRef] [PubMed]
- Cernat, A.; Geanina, S.; Tertis, M.; Cristea, C.; Simon, I. An Overview of the Detection of Serotonin and Dopamine with Graphene-Based Sensors. Bioelectrochemistry 2020, 136, 107620. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Liu, J. Biosensors and Sensors for Dopamine Detection. View 2021, 2, 20200102. [Google Scholar] [CrossRef]
- Cho, Y.W.; Park, J.H.; Lee, K.H.; Lee, T.; Luo, Z.; Kim, T.H. Recent Advances in Nanomaterial-Modified Electrical Platforms for the Detection of Dopamine in Living Cells. Nano Converg. 2020, 7. [Google Scholar] [CrossRef] [PubMed]
- Tangkuaram, T.; Ponchio, C.; Kangkasomboon, T.; Katikawong, P.; Veerasai, W. Design and Development of a Highly Stable Hydrogen Peroxide Biosensor on Screen Printed Carbon Electrode Based on Horseradish Peroxidase Bound with Gold Nanoparticles in the Matrix of Chitosan. Biosens. Bioelectron. 2007, 22, 2071–2078. [Google Scholar] [CrossRef]
- Shu, H.; Cao, L.; Chang, G.; He, H.; Zhang, Y.; He, Y. Electrochimica Acta Direct Electrodeposition of Gold Nanostructures onto Glassy Carbon Electrodes for Non-Enzymatic Detection of Glucose. Electrochim. Acta 2014, 132, 524–532. [Google Scholar] [CrossRef]
- Zhang, K.; Wei, J.; Zhu, H.; Ma, F.; Wang, S. Electrodeposition of Gold Nanoparticle Arrays on ITO Glass as Electrode with High Electrocatalytic Activity. Mater. Res. Bull. 2013, 48, 1338–1341. [Google Scholar] [CrossRef]
- Gowthaman, N.S.K.; Arul, P.; Lim, H.N.; John, S.A. Negative Potential-Induced Growth of Surfactant-Free CuO Nanostructures on an Al−C Substrate: A Dual In-Line Sensor for Biomarkers of Diabetes and Oxidative Stress. ACS Sustainable Chem. Eng. 2020, 8, 2640–2651. [Google Scholar] [CrossRef]
- Zakaria, N.D.; Omar, M.H.; Ahmad Kamal, N.N.; Abdul Razak, K.; Sönmez, T.; Balakrishnan, V.; Hamzah, H.H. Effect of Supporting Background Electrolytes on the Nanostructure Morphologies and Electrochemical Behaviors of Electrodeposited Gold Nanoparticles on Glassy Carbon Electrode Surfaces. ACS Omega. 2021, 6, 24419–24431. [Google Scholar] [CrossRef]
- Shen, Y. Determination of the Stability of Dopamine in Aqueous Solutions by High Performance Liquid Chromatography. J. Liq. Chromatogr. Relat. Technol. 2006, 17, 1557–1565. [Google Scholar] [CrossRef]
- Aoki, K.J.; Chen, J.; Liu, Y.; Jia, B. Peak Potential Shift of Fast Cyclic Voltammograms Owing to Capacitance of Redox Reactions. J. Electroanal. Chem. 2020, 856, 113609. [Google Scholar] [CrossRef]
- Speiser, B. Linear Sweep and Cyclic Voltammetry. In Comprehensive Chemical Kinetics; Elsevier: Amsterdam, The Netherlands, 2007; Volume 26, pp. 145–202. [Google Scholar] [CrossRef]
- Zheng, Y.; Huang, Z.; Zhao, C.; Weng, S.; Zheng, W.; Lin, X. A Gold Electrode with a Flower-like Gold Nanostructure for Simultaneous Determination of Dopamine and Ascorbic Acid. Microchimica Acta 2013, 180, 537–544. [Google Scholar] [CrossRef]
- Hsu, M.S.; Chen, Y.L.; Lee, C.Y.; Chiu, H.T. Gold Nanostructures on Flexible Substrates as Electrochemical Dopamine Sensors. ACS Appl. Mater Interfaces 2012, 4, 5570–5575. [Google Scholar] [CrossRef] [PubMed]
- Ramirez, S.; Silva, N.; Oyarzun, M.P.; Pavez, J.; Silva, J.F. Gold Nanostructures on Self-Assembled Monolayers Activity for Epinephrine, Noradrenaline and Dopamine. J. Electroanal. Chem. 2017, 799, 349–357. [Google Scholar] [CrossRef]
- Kumar, K.K.; Devendiran, M.; Kalaivani, R.A.; Narayanan, S.S. Enhanced Electrochemical Sensing of Dopamine in the Presence of AA and UA Using a Curcumin Functionalized Gold Nanoparticle Modified Electrode. New J. Chem. 2019, 43, 19003–19013. [Google Scholar] [CrossRef]
- Kim, D.S.; Kang, E.S.; Baek, S.; Choo, S.S.; Chung, Y.H.; Lee, D.; Min, J.; Kim, T.H. Electrochemical Detection of Dopamine Using Periodic Cylindrical Gold Nanoelectrode Arrays. Sci. Rep. 2018, 8, 14049. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, C.; Du, J.; Wang, H.; Zou, C.; Jiang, F.; Yang, P.; Du, Y. A Facile Electrochemical Sensor Based on Reduced Graphene Oxide and Au Nanoplates Modified Glassy Carbon Electrode for Simultaneous Detection of Ascorbic Acid, Dopamine and Uric Acid. Sens. Actuators B Chem. 2014, 204, 302–309. [Google Scholar] [CrossRef]
- Immanuel, S.; Aparna, T.K.; Sivasubramanian, R. A Facile Preparation of Au—SiO2 Nanocomposite for Simultaneous Electrochemical Detection of Dopamine and Uric Acid. Surf. Interfaces 2019, 14, 82–91. [Google Scholar] [CrossRef]
- Venkata, S.J.A.; Narayanasamy, A.; Srinivasan, V.; Iyer, G.; Sivaramakrishnan, R.; Subramanian, M.; Mahadevan, R. Tear Ascorbic Acid Levels and the Total Antioxidant Status in Contact Lens Wearers: A Pilot Study. Indian J. Ophthalmol. 2009, 57, 289–292. [Google Scholar] [CrossRef]
- Zhao, J.; Huang, Y. Salivary Uric Acid as a Noninvasive Biomarker for Monitoring the Efficacy of Urate-Lowering Therapy in a Patient with Chronic Gouty Arthropathy. Clinica Chimica Acta 2015, 450, 115–120. [Google Scholar] [CrossRef]
- Roy, D.; Singh, P.; Halder, S.; Chanda, N.; Mandal, S. 3-D Printed Electrode Integrated Sensing Chip and a PoC Device for Enzyme Free Electrochemical Detection of Blood Urea. Bioelectrochemistry 2021, 142, 107893. [Google Scholar] [CrossRef] [PubMed]



| Material | Linear Range (µM) | Limit of Detection (µM) | Detection Technique | Ref. |
|---|---|---|---|---|
| Gold electrode/GNF | 1.0–150 | 0.2 | DPV | [44] |
| PET/Au thin film/GNC | 0.2–500 | 0.06 | CPA | [45] |
| Au/SAMs/AuNRs | 10–60 | 7.76 | SWV | [46] |
| PIG/CR-GNP | 0.4–56 | 0.042 | CPA | [47] |
| ITO/CAuNE | 1–100 | 5.83 | CV | [48] |
| GCE/Au/rGO | 6.8–41 | 1.4 | DPV | [49] |
| GCE/Au-SiO2 | 10–100 | 1.98 | DPV | [50] |
| SPCE/AuNS | 0.2–50 | 0.33 | CPA | This work |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Anshori, I.; Althof, R.R.; Rizalputri, L.N.; Ariasena, E.; Handayani, M.; Pradana, A.; Akbar, M.R.; Syamsunarno, M.R.A.A.; Purwidyantri, A.; Prabowo, B.A.; et al. Gold Nanospikes Formation on Screen-Printed Carbon Electrode through Electrodeposition Method for Non-Enzymatic Electrochemical Sensor. Metals 2022, 12, 2116. https://doi.org/10.3390/met12122116
Anshori I, Althof RR, Rizalputri LN, Ariasena E, Handayani M, Pradana A, Akbar MR, Syamsunarno MRAA, Purwidyantri A, Prabowo BA, et al. Gold Nanospikes Formation on Screen-Printed Carbon Electrode through Electrodeposition Method for Non-Enzymatic Electrochemical Sensor. Metals. 2022; 12(12):2116. https://doi.org/10.3390/met12122116
Chicago/Turabian StyleAnshori, Isa, Raih Rona Althof, Lavita Nuraviana Rizalputri, Eduardus Ariasena, Murni Handayani, Arfat Pradana, Mohammad Rizki Akbar, Mas Rizky Anggun Adipurna Syamsunarno, Agnes Purwidyantri, Briliant Adhi Prabowo, and et al. 2022. "Gold Nanospikes Formation on Screen-Printed Carbon Electrode through Electrodeposition Method for Non-Enzymatic Electrochemical Sensor" Metals 12, no. 12: 2116. https://doi.org/10.3390/met12122116







