Mechanisms and Effects of Isorhamnetin on Imiquimod-Induced Psoriasiform Dermatitis in Mice
Abstract
1. Introduction
2. Material and Methods
2.1. Animal Model
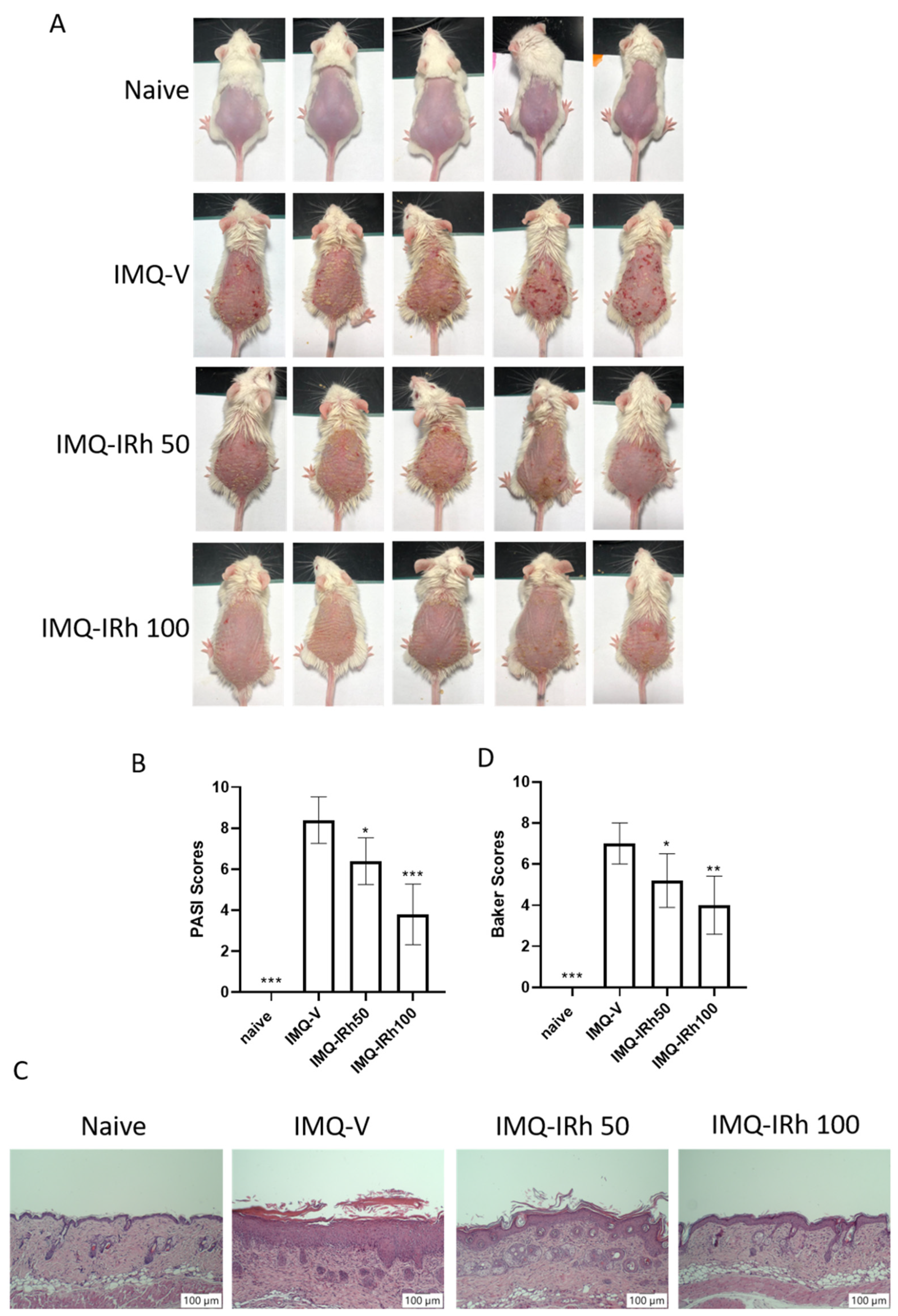
2.2. Psoriasis Area and Severity and Index (PASI)
2.3. Hematoxylin and Eosin (HE) Staining
2.4. Measurement for Oxidative Stress Factors
2.5. Estimation of Inflammatory Cytokines by ELISA
2.6. RT-PCR
2.7. Western Blot
2.8. Flow Cytometry Analysis of Th1 and Th17 Populations
2.9. Flow Cytometry Analysis of Surface Maker Expression by Splenic DCs
2.10. Statistical Analysis
3. Result
3.1. IRh Alleviates Clinical Symptoms in IMQ-Induced Psoriatic Mice
3.2. Effects of IRh on Antioxidative/Oxidative Levels of SOD, CAT, and MDA
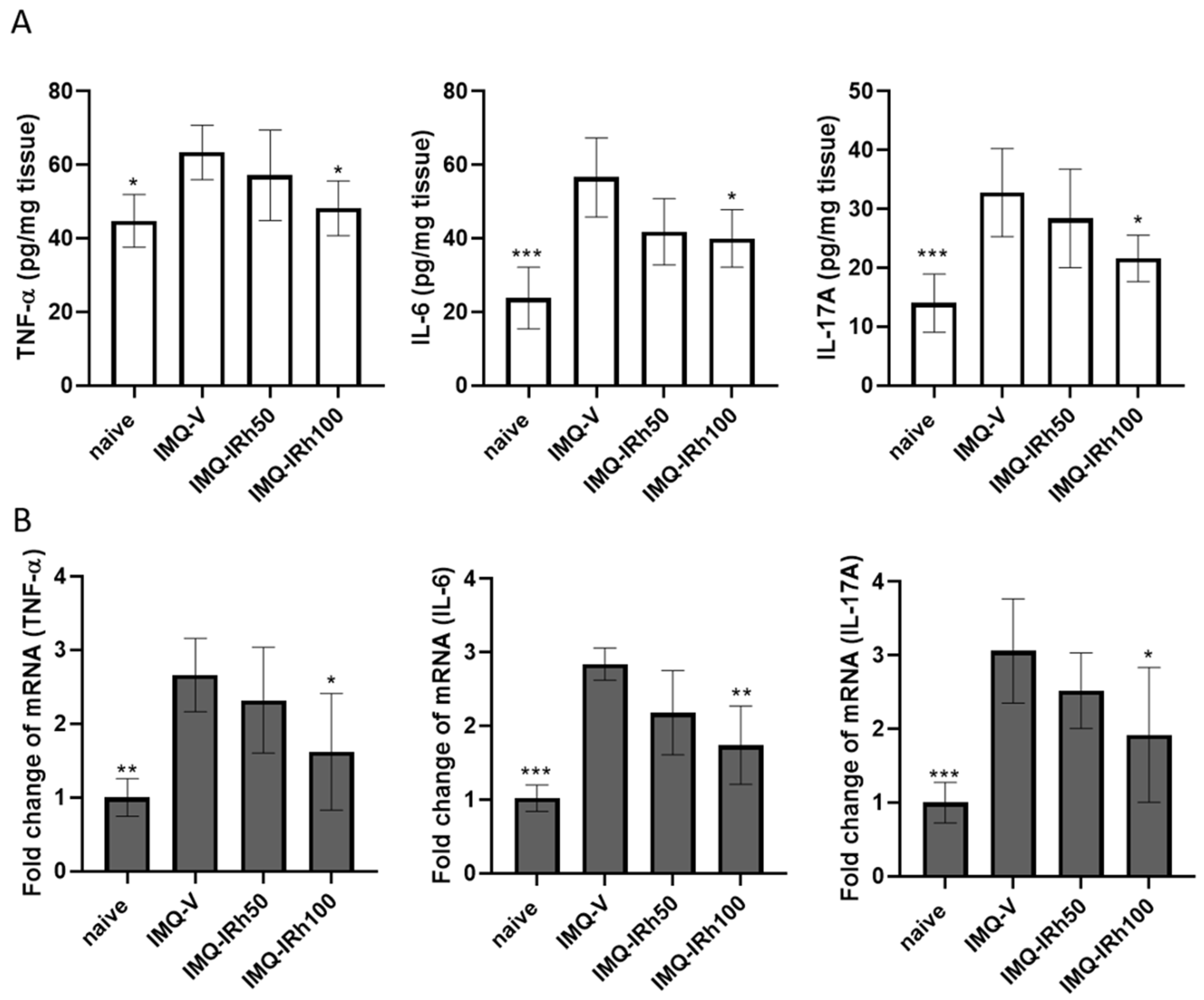
3.3. IRh Suppresses Protein and mRNA Expressions of Proinflammatory Cytokines in IMQ-Induced Psoriatic Mice
3.4. Effect of IRh on Protein Expressions of IκB-α and NF-κB p65 Subunit
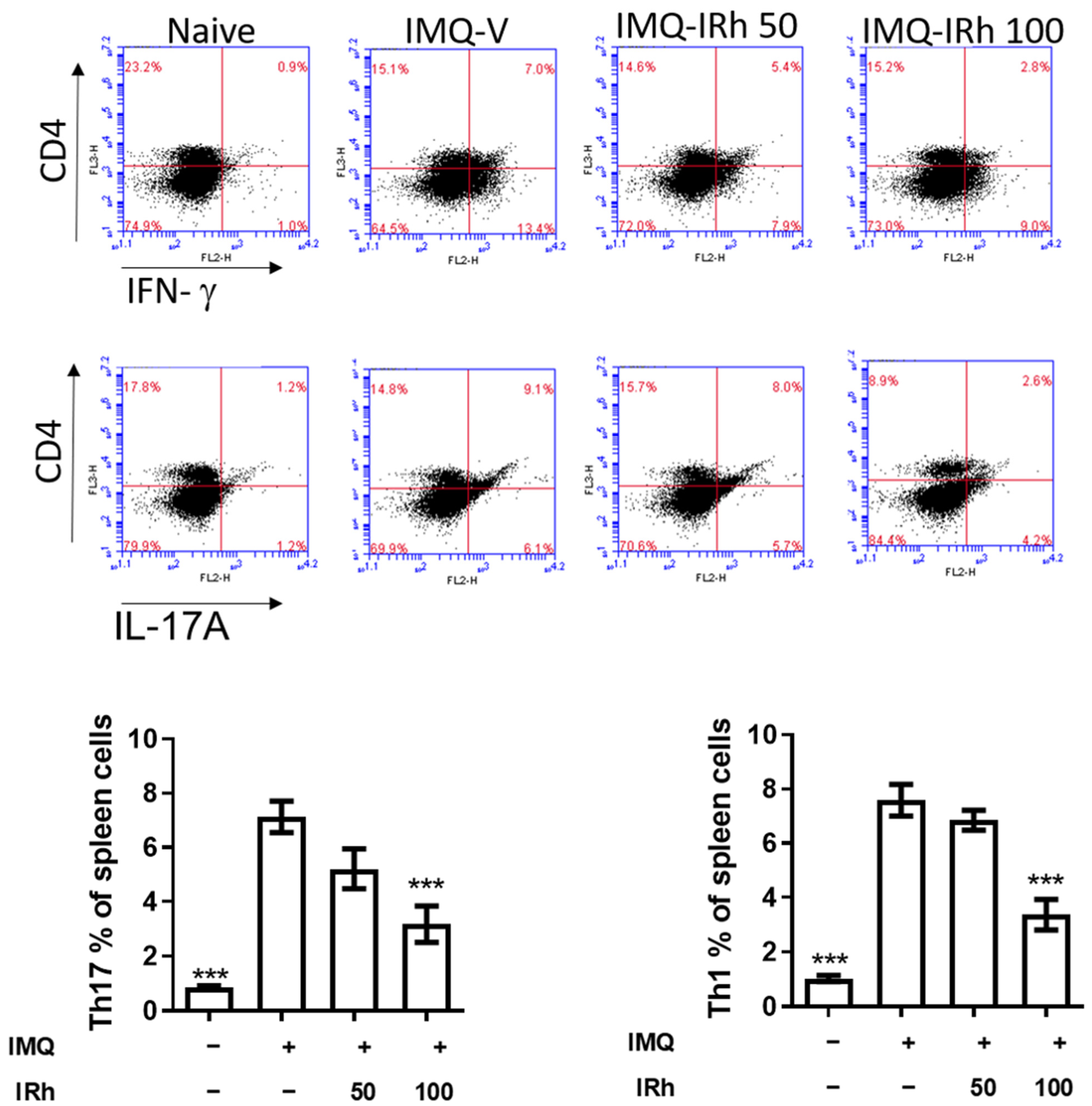
3.5. IRh Reduces the Levels of Splenic Th1 and Th17 Cells in IMQ-Induced Psoriatic Mice
3.6. IRh Suppressed Splenic DC Maturation and Cytokines Production in IMQ-Induced Psoriatic Mice
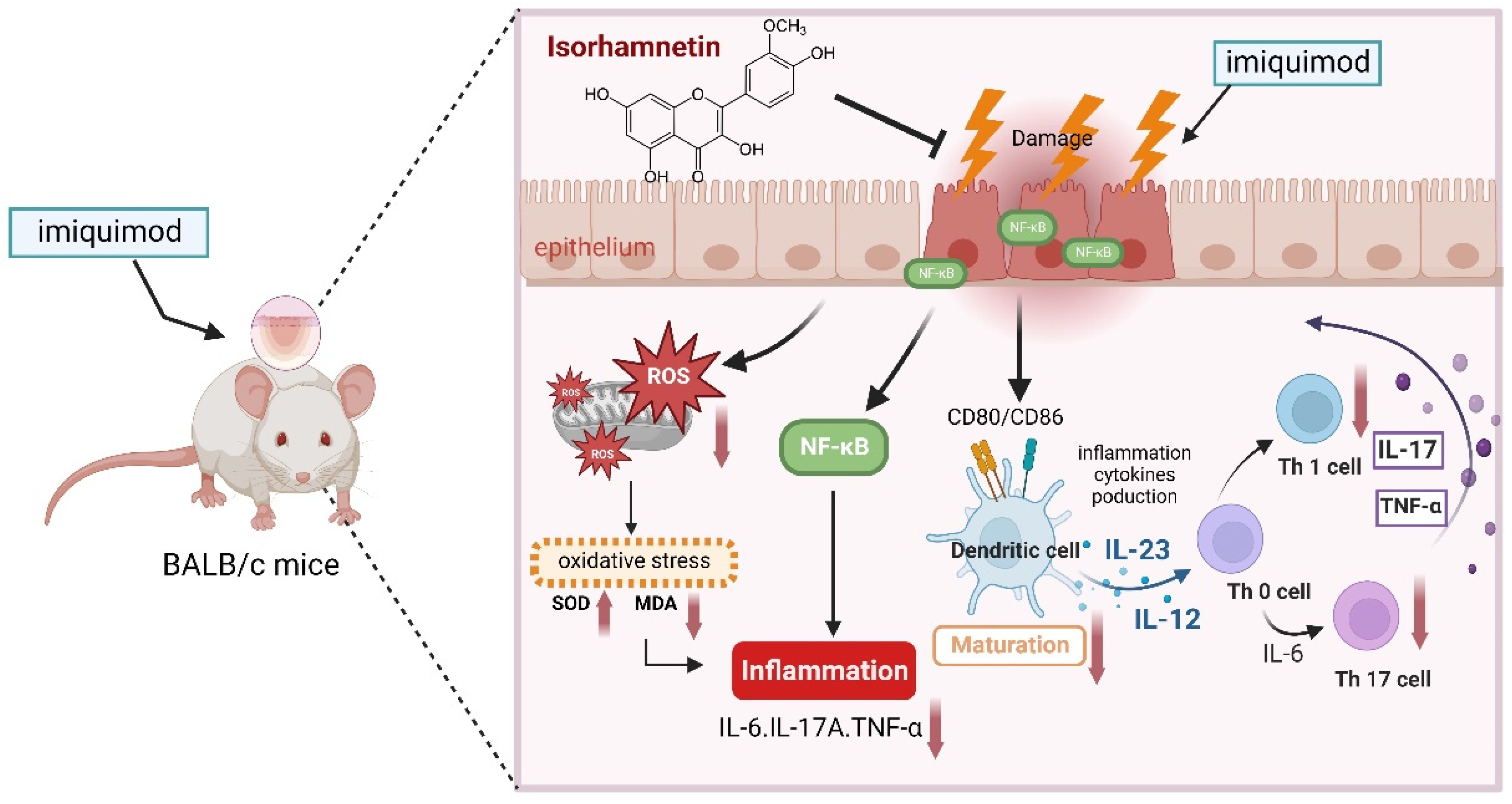
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Griffiths, C.E.M.; Armstrong, A.W.; Gudjonsson, J.E.; Barker, J. Psoriasis. Lancet 2021, 397, 1301–1315. [Google Scholar] [CrossRef] [PubMed]
- Becher, B.; Pantelyushin, S. Hiding under the skin: Interleukin-17-producing gammadelta T cells go under the skin? Nat. Med. 2012, 18, 1748–1750. [Google Scholar] [CrossRef]
- Lowes, M.A.; Russell, C.B.; Martin, D.A.; Towne, J.E.; Krueger, J.G. The IL-23/T17 pathogenic axis in psoriasis is amplified by keratinocyte responses. Trends Immunol. 2013, 34, 174–181. [Google Scholar] [CrossRef] [PubMed]
- Albanesi, C.; Pastore, S. Pathobiology of chronic inflammatory skin diseases: Interplay between keratinocytes and immune cells as a target for anti-inflammatory drugs. Curr. Drug Metab. 2010, 11, 210–227. [Google Scholar] [CrossRef]
- Bastos, K.R.; de Deus Vieira de Moraes, L.; Zago, C.A.; Marinho, C.R.; Russo, M.; Alvarez, J.M.; D’Imperio Lima, M.R. Analysis of the activation profile of dendritic cells derived from the bone marrow of interleukin-12/interleukin-23-deficient mice. Immunology 2005, 114, 499–506. [Google Scholar] [CrossRef]
- Belladonna, M.L.; Renauld, J.C.; Bianchi, R.; Vacca, C.; Fallarino, F.; Orabona, C.; Fioretti, U.G.; Grohmann, U.; Puccetti, P. IL-23 and IL-12 have overlapping, but distinct, effects on murine dendritic cells. J. Immunol. 2002, 168, 5448–5454. [Google Scholar] [CrossRef]
- Lowes, M.A.; Suarez-Farinas, M.; Krueger, J.G. Immunology of psoriasis. Annu. Rev. Immunol. 2014, 32, 227–255. [Google Scholar] [CrossRef]
- Chiricozzi, A.; Guttman-Yassky, E.; Suarez-Farinas, M.; Nograles, K.E.; Tian, S.; Cardinale, I.; Chimenti, S.; Krueger, J.G. Integrative responses to IL-17 and TNF-alpha in human keratinocytes account for key inflammatory pathogenic circuits in psoriasis. J. Investig. Dermatol. 2011, 131, 677–687. [Google Scholar] [CrossRef]
- Jeon, C.; Sekhon, S.; Yan, D.; Afifi, L.; Nakamura, M.; Bhutani, T. Monoclonal antibodies inhibiting IL-12, -23, and -17 for the treatment of psoriasis. Hum. Vaccines Immunother. 2017, 13, 2247–2259. [Google Scholar] [CrossRef] [PubMed]
- Mazloom, S.E.; Yan, D.; Hu, J.Z.; Ya, J.; Husni, M.E.; Warren, C.B.; Fernandez, A.P. TNF-alpha inhibitor-induced psoriasis: A decade of experience at the Cleveland Clinic. J. Am. Acad. Dermatol. 2020, 83, 1590–1598. [Google Scholar] [CrossRef]
- Billowria, K.; Ali, R.; Rangra, N.K.; Kumar, R.; Chawla, P.A. Bioactive Flavonoids: A Comprehensive Review on Pharmacokinetics and Analytical Aspects. Crit. Rev. Anal. Chem. 2022, 1–15. [Google Scholar] [CrossRef]
- Gong, G.; Guan, Y.Y.; Zhang, Z.L.; Rahman, K.; Wang, S.J.; Zhou, S.; Luan, X.; Zhang, H. Isorhamnetin: A review of pharmacological effects. Biomed. Pharmacother. 2020, 128, 110301. [Google Scholar] [CrossRef]
- Li, Y.; Fan, B.; Pu, N.; Ran, X.; Lian, T.; Cai, Y.; Xing, W.; Sun, K. Isorhamnetin Suppresses Human Gastric Cancer Cell Proliferation through Mitochondria-Dependent Apoptosis. Molecules 2022, 27, 5191. [Google Scholar] [CrossRef]
- Dong, X.; Li, J.J.; Ma, N.; Liu, A.Z.; Liu, J.W. Anti-Inflammatory and Antioxidative Effects of Isorhamnetin for Protection Against Lung Injury in a Rat Model of Heatstroke in a Dry-Heat Environment. Med. Sci. Monit. 2022, 28, e935426. [Google Scholar] [CrossRef] [PubMed]
- Li, W.Q.; Xie, X.F.; Peng, C.; Wu, Q.H.; Li, J.; Liu, X.Y.; Luo, S.Y.; Wu, Q.H.; Xie, X.F.; Peng, C. Isorhamnetin: A novel natural product beneficial for cardiovascular disease. Curr. Pharm. Des. 2022, 28, 2569–2582. [Google Scholar] [CrossRef]
- Li, N.; Chen, K.; Bai, J.; Geng, Z.; Tang, Y.; Hou, Y.; Fan, F.; Ai, X.; Hu, T.; Meng, X.; et al. Tibetan medicine Duoxuekang ameliorates hypobaric hypoxia-induced brain injury in mice by restoration of cerebrovascular function. J. Ethnopharmacol. 2021, 270, 113629. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Zhong, W. Isorhamnetin attenuates collagen-induced arthritis via modulating cytokines and oxidative stress in mice. Int. J. Clin. Exp. Med. 2015, 8, 16536–16542. [Google Scholar]
- Li, Y.; Chi, G.; Shen, B.; Tian, Y.; Feng, H. Isorhamnetin ameliorates LPS-induced inflammatory response through downregulation of NF-kappaB signaling. Inflammation 2016, 39, 1291–1301. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Liu, Q.B.; Jing, W. Astragalus membranaceus improves therapeutic efficacy of asthmatic children by regulating the balance of Treg/Th17 cells. Chin. J. Nat. Med. 2019, 17, 252–263. [Google Scholar] [CrossRef]
- Van der Fits, L.; Mourits, S.; Voerman, J.S.; Kant, M.; Boon, L.; Laman, J.D.; Cornelissen, F.; Mus, A.M.; Florencia, E.; Prens, E.P.; et al. Imiquimod-induced psoriasis-like skin inflammation in mice is mediated via the IL-23/IL-17 axis. J. Immunol. 2009, 182, 5836–5845. [Google Scholar] [CrossRef]
- Lowes, M.A.; Kikuchi, T.; Fuentes-Duculan, J.; Cardinale, I.; Zaba, L.C.; Haider, A.S.; Bowman, E.P.; Krueger, J.G. Psoriasis vulgaris lesions contain discrete populations of Th1 and Th17 T cells. J. Investig. Dermatol. 2008, 128, 1207–1211. [Google Scholar] [CrossRef] [PubMed]
- Quaglino, P.; Bergallo, M.; Ponti, R.; Barberio, E.; Cicchelli, S.; Buffa, E.; Comessatti, A.; Costa, C.; Terlizzi, M.E.; Astegiano, S.; et al. Th1, Th2, Th17 and regulatory T cell pattern in psoriatic patients: Modulation of cytokines and gene targets induced by etanercept treatment and correlation with clinical response. Dermatology 2011, 223, 57–67. [Google Scholar] [CrossRef]
- Reagan-Shaw, S.; Nihal, M.; Ahmad, N. Dose translation from animal to human studies revisited. FASEB J. 2008, 22, 659–661. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Wang, S.; Liu, B.; Wang, Y.; Tan, W. (R)-Salbutamol Improves Imiquimod-Induced Psoriasis-like Skin Dermatitis by Regulating the Th17/Tregs Balance and Glycerophospholipid Metabolism. Cells 2020, 9, 511. [Google Scholar] [CrossRef]
- Quintard, B.; Constant, A.; Bouyssou-Gauthier, M.L.; Paul, C.; Truchetet, F.; Thomas, P.; Guiguen, Y.; Taieb, A. Validation of a specific health-related quality of life instrument in a large cohort of patients with psoriasis: The QualiPso Questionnaire. Acta Derm. Venereol. 2011, 91, 660–665. [Google Scholar] [CrossRef]
- Cerutti, P.; Shah, G.; Peskin, A.; Amstad, P. Oxidant carcinogenesis and antioxidant defense. Ann. N. Y. Acad. Sci. 1992, 663, 158–166. [Google Scholar] [CrossRef]
- Pizzino, G.; Irrera, N.; Cucinotta, M.; Pallio, G.; Mannino, F.; Arcoraci, V.; Squadrito, F.; Altavilla, D.; Bitto, A. Oxidative Stress: Harms and Benefits for Human Health. Oxid. Med. Cell. Longev. 2017, 2017, 8416763. [Google Scholar] [CrossRef] [PubMed]
- Draper, H.H.; McGirr, L.G.; Hadley, M. The metabolism of malondialdehyde. Lipids 1986, 21, 305–307. [Google Scholar] [CrossRef]
- Gabr, S.A.; Al-Ghadir, A.H. Role of cellular oxidative stress and cytochrome c in the pathogenesis of psoriasis. Arch. Dermatol. Res. 2012, 304, 451–457. [Google Scholar] [CrossRef] [PubMed]
- Lin, X.; Huang, T. Oxidative stress in psoriasis and potential therapeutic use of antioxidants. Free. Radic. Res. 2016, 50, 585–595. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Zhang, L.; Joo, D.; Sun, S.C. NF-kappaB signaling in inflammation. Signal. Transduct. Target. Ther. 2017, 2, 17023. [Google Scholar] [CrossRef] [PubMed]
- Goldminz, A.M.; Au, S.C.; Kim, N.; Gottlieb, A.B.; Lizzul, P.F. NF-kappaB: An essential transcription factor in psoriasis. J. Dermatol. Sci. 2013, 69, 89–94. [Google Scholar] [CrossRef]
- Ren, X.; Han, L.; Li, Y.; Zhao, H.; Zhang, Z.; Zhuang, Y.; Zhong, M.; Wang, Q.; Ma, W.; Wang, Y. Isorhamnetin attenuates TNF-alpha-induced inflammation, proliferation, and migration in human bronchial epithelial cells via MAPK and NF-kappaB pathways. Anat. Rec. 2021, 304, 901–913. [Google Scholar] [CrossRef] [PubMed]
- Shi, H.; He, J.; Li, X.; Han, J.; Wu, R.; Wang, D.; Yang, F.; Sun, E. Isorhamnetin, the active constituent of a Chinese herb Hippophae rhamnoides L, is a potent suppressor of dendritic-cell maturation and trafficking. Int. Immunopharmacol. 2018, 55, 216–222. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, C.-S.; Lin, C.-C.; Chen, Y.-Y.; Yang, D.-H. Mechanisms and Effects of Isorhamnetin on Imiquimod-Induced Psoriasiform Dermatitis in Mice. Life 2022, 12, 2107. https://doi.org/10.3390/life12122107
Wu C-S, Lin C-C, Chen Y-Y, Yang D-H. Mechanisms and Effects of Isorhamnetin on Imiquimod-Induced Psoriasiform Dermatitis in Mice. Life. 2022; 12(12):2107. https://doi.org/10.3390/life12122107
Chicago/Turabian StyleWu, Chieh-Shan, Chuan-Chao Lin, Yu-Ying Chen, and Deng-Ho Yang. 2022. "Mechanisms and Effects of Isorhamnetin on Imiquimod-Induced Psoriasiform Dermatitis in Mice" Life 12, no. 12: 2107. https://doi.org/10.3390/life12122107
APA StyleWu, C.-S., Lin, C.-C., Chen, Y.-Y., & Yang, D.-H. (2022). Mechanisms and Effects of Isorhamnetin on Imiquimod-Induced Psoriasiform Dermatitis in Mice. Life, 12(12), 2107. https://doi.org/10.3390/life12122107







