Correlations between Endoscopic and Histopathological Assessment of Helicobacter pylori-Induced Gastric Pathology—A Cross-Sectional Retrospective Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Patient Characteristics
2.2. Endoscopy
2.3. Rapid Urease Test (RUT) for the Diagnosis of H. pylori
2.4. Histopathology
2.5. Statistical Analysis
3. Results
3.1. Patient Characteristics
3.2. Endoscopic Diagnostics
Gastric Pathology
3.3. Histopathology
Correlation of Endoscopy and Histopathology Results in H. pylori Positive Patients
- A.
- Atrophic gastritis
- B.
- Gastric metaplasia
- C.
- Correlation of corpus atrophy endoscopy with patients’ age
- D.
- Gender correlation with endoscopic atrophy and metaplasia
- E.
- Gastric polyps
- F.
- Gastric tumors
- G.
- Sensitivity and specificity
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ahn, H.J.; Lee, D.S. Helicobacter Pylori in Gastric Carcinogenesis. World J. Gastrointest. Oncol. 2015, 7, 455–465. [Google Scholar] [CrossRef]
- Stolte, M.; Bayerdörffer, E.; Morgner, A.; Alpen, B.; Wündisch, T.; Thiede, C.; Neubauer, A. Helicobacter and Gastric MALT Lymphoma. Gut 2002, 50 (Suppl. S3), 19–24. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sjomina, O.; Pavlova, J.; Niv, Y.; Leja, M. Epidemiology of Helicobacter Pylori Infection. Helicobacter 2018, 23, e12514. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zamani, M.; Ebrahimtabar, F.; Zamani, V.; Miller, W.H.; Alizadeh-Navaei, R.; Shokri-Shirvani, J.; Derakhshan, M.H. Systematic Review with Meta-Analysis: The Worldwide Prevalence of Helicobacter Pylori Infection. Aliment. Pharmacol. Ther. 2018, 47, 868–876. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cardos, I.A.; Zaha, D.C.; Sindhu, R.K.; Cavalu, S. Revisiting Therapeutic Strategies for H. pylori Treatment in the Context of Antibiotic Resistance: Focus on Alternative and Complementary Therapies. Molecules 2021, 26, 6078. [Google Scholar] [CrossRef]
- Adadi, S.; Bennani, B.; Elabkari, M.; Ibrahimi, A.; Alaoui, S.; Elkhadir, M.; Harmouch, T.; Mahmoud, M.; Nejjari, C.; Benajah, D. Gastric Atrophy, Intestinal Metaplasia in Helicobacter Pylori Gastritis: Prevalence and Predictors Factors. J. Biosci. Med. 2016, 4, 43–49. [Google Scholar] [CrossRef] [Green Version]
- Leung, W.K.; Lin, S.-R.; Ching, J.Y.L.; To, K.-F.; Ng, E.K.W.; Chan, F.K.L.; Lau, J.Y.W.; Sung, J.J.Y. Factors Predicting Progression of Gastric Intestinal Metaplasia: Results of a Randomised Trial on Helicobacter Pylori Eradication. Gut 2004, 53, 1244–1249. [Google Scholar] [CrossRef] [Green Version]
- Hassan, T.M.M.; Al-Najjar, S.; Al-Zahrani, I.; Alanazi, F.I.B.; Alotibi, M. Helicobacter Pylori Chronic Gastritis Updated Sydney Grading in Relation to Endoscopic Findings and H. Pylori IgG Antibody: Diagnostic Methods. J. Microsc. Ultrastruct. 2016, 4, 167. [Google Scholar] [CrossRef] [Green Version]
- Zhou, J.; Wang, W.; Xie, Y.; Zhao, Y.; Chen, X.; Xu, W.; Wang, Y.; Guan, Z. Proteomics-Based Identification and Analysis of Proteins Associated with Helicobacter Pylori in Gastric Cancer. PLoS ONE 2016, 11, e0146521. [Google Scholar] [CrossRef] [Green Version]
- Kim, S.S.; Ruiz, V.E.; Carroll, J.D.; Moss, S.F. Helicobacter Pylori in the Pathogenesis of Gastric Cancer and Gastric Lymphoma. Cancer Lett. 2011, 305, 228–238. [Google Scholar] [CrossRef]
- Sasazuki, S.; Inoue, M.; Iwasaki, M.; Otani, T.; Yamamoto, S.; Ikeda, S.; Hanaoka, T.; Tsugane, S.; Japan Public Health Center Study Group. Effect of Helicobacter Pylori Infection Combined with CagA and Pepsinogen Status on Gastric Cancer Development among Japanese Men and Women: A Nested Case-Control Study. Cancer Epidemiol. Biomark. Prev. Publ. Am. Assoc. Cancer Res. Cospons. Am. Soc. Prev. Oncol. 2006, 15, 1341–1347. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, B.R.; Shun, C.T.; Wang, T.H.; Lin, J.T. Endoscopic Diagnosis of Intestinal Metaplasia of Stomach—Accuracy Judged by Histology. Hepatogastroenterology 1999, 46, 162–166. [Google Scholar] [PubMed]
- Ouyang, Y.; Zhang, W.; Huang, Y.; Wang, Y.; Shao, Q.; Wu, X.; Lu, N.; Xie, C. Effect of Helicobacter Pylori Eradication on Hyperplastic Gastric Polyps: A Systematic Review and Meta-Analysis. Helicobacter 2021, 26, e12838. [Google Scholar] [CrossRef] [PubMed]
- Nam, S.Y.; Park, B.J.; Ryu, K.H.; Nam, J.H. Effect of Helicobacter Pylori Eradication on the Regression of Gastric Polyps in National Cancer Screening Program. Korean J. Intern. Med. 2018, 33, 506–511. [Google Scholar] [CrossRef] [Green Version]
- Uotani, T.; Graham, D.Y. Diagnosis of Helicobacter Pylori Using the Rapid Urease Test. Ann. Transl. Med. 2015, 3, 9. [Google Scholar] [CrossRef]
- Dore, M.P.; Pes, G.M. What Is New in Helicobacter Pylori Diagnosis. An Overview. J. Clin. Med. 2021, 10, 2091. [Google Scholar] [CrossRef]
- Godbole, G.; Mégraud, F.; Bessède, E. Review: Diagnosis of Helicobacter Pylori Infection. Helicobacter 2020, 25 (Suppl. S1), e12735. [Google Scholar] [CrossRef]
- Chey, W.D.; Leontiadis, G.I.; Howden, C.W.; Moss, S.F. ACG Clinical Guideline: Treatment of Helicobacter Pylori Infection. Am. J. Gastroenterol. 2017, 112, 212–239. [Google Scholar] [CrossRef]
- Cardos, A.I.; Maghiar, A.; Zaha, D.C.; Pop, O.; Fritea, L.; Miere Groza, F.; Cavalu, S. Evolution of Diagnostic Methods for Helicobacter Pylori Infections: From Traditional Tests to High Technology, Advanced Sensitivity and Discrimination Tools. Diagnostics 2022, 12, 508. [Google Scholar] [CrossRef]
- Dixon, M.F.; Genta, R.M.; Yardley, J.H.; Correa, P. Histological Classification of Gastritis and Helicobacter Pylori Infection: An Agreement at Last? The International Workshop on the Histopathology of Gastritis. Helicobacter 1997, 2 (Suppl. S1), S17–S24. [Google Scholar] [CrossRef]
- Dixon, M.F.; Genta, R.M.; Yardley, J.H.; Correa, P. Classification and Grading of Gastritis. The Updated Sydney System. International Workshop on the Histopathology of Gastritis, Houston 1994. Am. J. Surg. Pathol. 1996, 20, 1161–1181. [Google Scholar] [CrossRef] [PubMed]
- Schoonjans, F. MedCalc’s Comparison of Proportions Calculator. Available online: https://www.medcalc.org/calc/comparison_of_proportions.php (accessed on 3 December 2022).
- Correa, P. Gastric Cancer: Overview. Gastroenterol. Clin. North Am. 2013, 42, 211–217. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rugge, M.; Savarino, E.; Sbaraglia, M.; Bricca, L.; Malfertheiner, P. Gastritis: The Clinico-Pathological Spectrum. Dig. Liver Dis. Off. J. Ital. Soc. Gastroenterol. Ital. Assoc. Study Liver 2021, 53, 1237–1246. [Google Scholar] [CrossRef] [PubMed]
- Correa, P.; Piazuelo, M.B. The Gastric Precancerous Cascade. J. Dig. Dis. 2012, 13, 2–9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ferro, A.; Morais, S.; Pelucchi, C.; Dierssen-Sotos, T.; Martín, V.; López-Carrillo, L.; Malekzadeh, R.; Tsugane, S.; Hamada, G.S.; Hidaka, A.; et al. Sex Differences in the Prevalence of Helicobacter Pylori Infection: An Individual Participant Data Pooled Analysis (StoP Project). Eur. J. Gastroenterol. Hepatol. 2019, 31, 593–598. [Google Scholar] [CrossRef] [PubMed]
- Windsor, H.M.; Abioye-Kuteyi, E.A.; Leber, J.M.; Morrow, S.D.; Bulsara, M.K.; Marshall, B.J. Prevalence of Helicobacter Pylori in Indigenous Western Australians: Comparison between Urban and Remote Rural Populations. Med. J. Aust. 2005, 182, 210–213. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Prunduș, C.; Ciobanu, L.; Bolboacă, S.; Tanțău, M.; Matei, D.; Cruciat, C.; Pojoga, C.; Andreica, V. The Evolution over Time of the Prevalence of Helicobacter Pylori Infection among Patients with Dyspeptic Syndrome—The Experience of a Tertiary Center in Romania. Med. Connect. 2018, 13, 23–27. [Google Scholar]
- Cavalu, S.; Damian, G.; Dansoreanu, M. EPR study of non-covalent spin labeled serum albumin and hemoglobin. Biophys. Chem. 2002, 99, 181–188. [Google Scholar] [CrossRef]
- Delić, D.; Ellinger-Ziegelbauer, H.; Vohr, H.W.; Dkhil, M.; Al-Quraishy, S.; Wunderlich, F. Testosterone Response of Hepatic Gene Expression in Female Mice Having Acquired Testosterone-Unresponsive Immunity to Plasmodium chabaudi Malaria. Steroids 2011, 76, 1204–1212. [Google Scholar] [CrossRef]
- Cerqueira, L.; Fernandes, R.M.; Ferreira, R.M.; Oleastro, M.; Carneiro, F.; Brandão, C.; Pimentel-Nunes, P.; Dinis-Ribeiro, M.; Figueiredo, C.; Keevil, C.W.; et al. Validation of a Fluorescence In Situ Hybridization Method Using Peptide Nucleic Acid Probes for Detection of Helicobacter Pylori Clarithromycin Resistance in Gastric Biopsy Specimens. J. Clin. Microbiol. 2013, 51, 1887–1893. [Google Scholar] [CrossRef] [Green Version]
- Akbari, M.; Tabrizi, R.; Kardeh, S.; Lankarani, K.B. Gastric Cancer in Patients with Gastric Atrophy and Intestinal Metaplasia: A Systematic Review and Meta-Analysis. PLoS ONE 2019, 14, e0219865. [Google Scholar] [CrossRef] [PubMed]
- Domșa, A.-M.T.; Lupușoru, R.; Gheban, D.; Șerban, R.; Borzan, C.M. Helicobacter Pylori Gastritis in Children—The Link between Endoscopy and Histology. J. Clin. Med. 2020, 9, 784. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mărginean, C.O.; Cotoi, O.S.; Pitea, A.M.; Mocanu, S.; Mărginean, C. Assessment of the Relationship between Helicobacter Pylori Infection, Endoscopic Appearance and Histological Changes of the Gastric Mucosa in Children with Gastritis (a Single Center Experience). Rom. J. Morphol. Embryol. 2013, 54, 709–715. [Google Scholar] [PubMed]
- Kawai, S.; Wang, C.; Lin, Y.; Sasakabe, T.; Okuda, M.; Kikuchi, S. Lifetime Incidence Risk for Gastric Cancer in the Helicobacter Pylori-Infected and Uninfected Population in Japan: A Monte Carlo Simulation Study. Int. J. Cancer 2022, 150, 18–27. [Google Scholar] [CrossRef]
- Kumar, S.; Metz, D.C.; Ellenberg, S.; Kaplan, D.E.; Goldberg, D.S. Risk Factors and Incidence of Gastric Cancer After Detection of Helicobacter Pylori Infection: A Large Cohort Study. Gastroenterology 2020, 158, 527–536. [Google Scholar] [CrossRef]

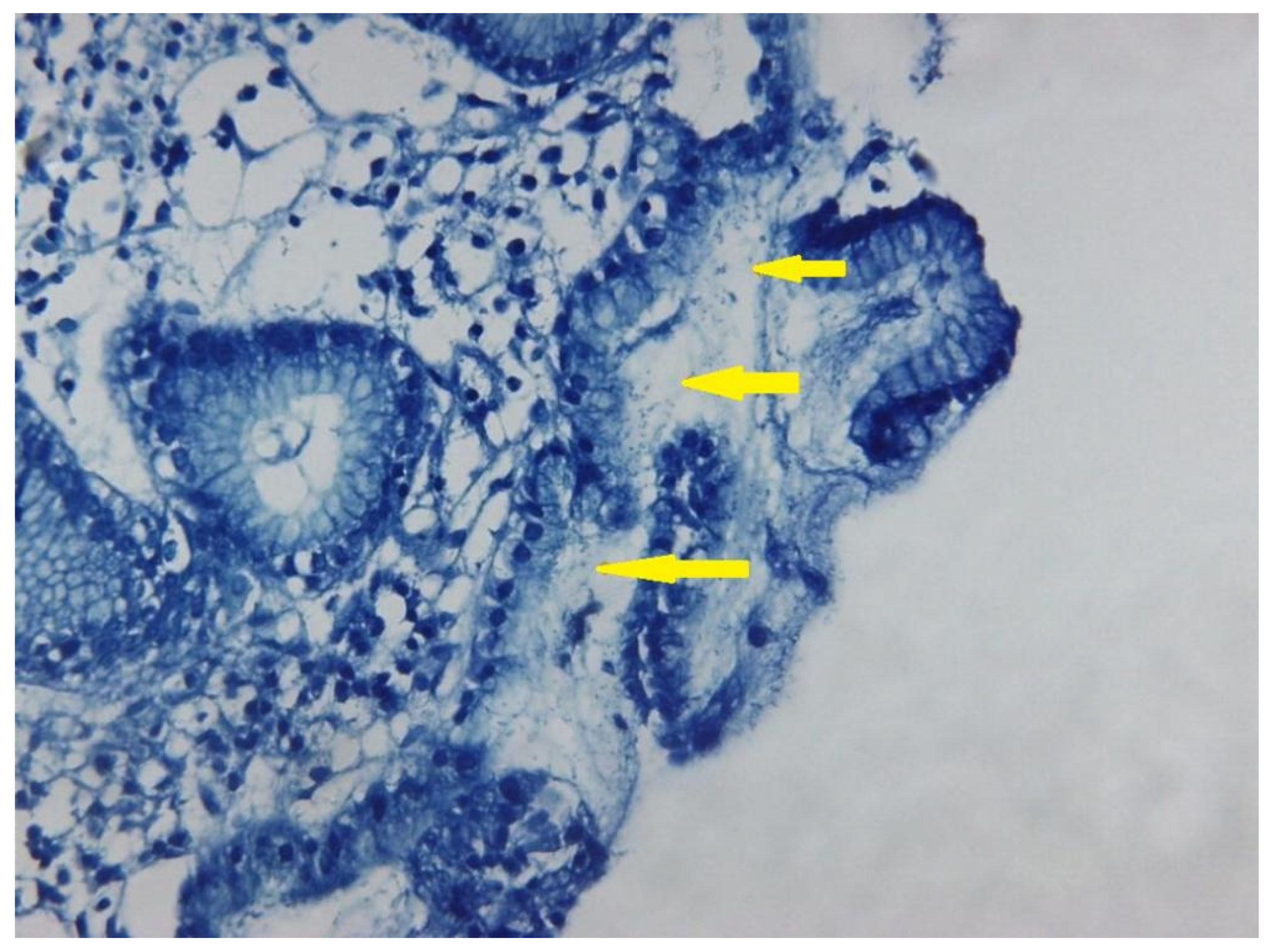
| Demographic Data | Number of Cases/Total/Percentage | |
|---|---|---|
| Female | 63/133 (47.4%) | |
| Male | 70/133 (52.6%) | |
| Rural | 59/133 (44.4%) | |
| Urban | 74/133 (55.6%) | |
| Age < 20 | 0/133 (0%) | |
| Age 20–29 | 2/133 (1.5%) | |
| Age 30–39 | 5/133 (3.8%) | |
| Age 40–49 | 16/133 (12%) | |
| Age 50–59 | 24/133 (18%) | |
| Age 60–69 | 44/133 (33.1%) | |
| Age 70–79 | 36/133 (27.1%) | |
| Age > 79 | 5/133 (4.5%) | |
| Gastric Pathologies (endoscopy) | Number of cases/total/percentage | |
| Corpus | Antrum | |
| Atrophic Gastritis | 19/133 (14.3%) | 14/133 (10.5%) |
| Acute Gastritis | 2/133 (1.5%) | 6/133 (4.5%) |
| Chronic Gastritis | 55/133 (41.4%) | 106/133 (79.7%) |
| Hypertrophic Gastritis | 13/133 (9.8%) | 19/133 (14.3%) |
| Metaplasia | 7/133 (5.3%) | 23/133 (17.3%) |
| Polyps | 13/132 (9.8%) | 11/133 (8.3%) |
| Ulcer | 10/132 (7.6%) | 18/133 (13.5%) |
| Tumor | 4/132 (3.5%) | Not available |
| Gastric Pathologies | ||||||
|---|---|---|---|---|---|---|
| Corpus | Antrum | |||||
| Polymorphonuclear cell | 15/55 (27.3%) | 16/65 (24.6%) | ||||
| Lymphoplasmocitar cell | 47/55 (85.5%) | 57/65 (87.7%) | ||||
| Atrophy | 19/55 (34.5%) | 16/65 (24.6%) | ||||
| Complete Metaplasia | 4/55 (7.3%) | All Metaplasia | 6/55 (10.9%) | 19/65 (29.2%) | All Metaplasia | 25/65 (38.5%) |
| Incomplete Metaplasia | 2/55 (3.6%) | 6/65 (9.2%) | ||||
| Reduced Dysplasia | 7/55 (12.7%) | 13/65 (20%) | ||||
| High Dysplasia | 1/55 (1.8%) | 2/65 (3.1%) | ||||
| Hyperplasic Polyps | 8/55 (14.5%) | All Polyps | 12/55 (21.8%) | 10/66 (15.2%) | All Polyps | 15/66 (22.7%) |
| Adenomatous Polyps | 0/55 (0%) | 1/66 (1.5%) | ||||
| Dysplasic Polyps | 0/55 (0%) | 1/66 (1.5%) | ||||
| Inflammatory Polyps | 4/55 (7.3%) | 5/66 (7.6%) | ||||
| Tumor | 3/55 (5.5%) | 2/66 (3%) | ||||
| Giemsa | 22/105 RUT positive (21%) | |||||
| Antrum Atrophic Gastritis | Antrum Metaplasia | All Metaplasia | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Correlation between endoscopy and histopathology in antrum atrophic gastritis | Fisher exact test for the correlation of the endoscopic and histopathology results for metaplasia in antrum | Fisher exact test for the correlation of the endoscopic and histopathology results for all metaplasia (both corpus and antrum). | ||||||||||
| Histopathology | Total | Histopathology | Total | Histopathology | Total | |||||||
| 0 | 1 | 0 | 1 | 0 | 1 | |||||||
| Endoscopy | 0 | 46 | 9 | 55 | 0 | 31 | 12 | 43 | 0 | 76 | 16 | 92 |
| 1 | 3 | 7 | 10 | 1 | 9 | 13 | 22 | 1 | 13 | 15 | 28 | |
| Total | 49 | 16 | 65 | 40 | 25 | 65 | 89 | 31 | 129 | |||
| p = 0.001 * | p = 0.018 * | p << 0.001 * | ||||||||||
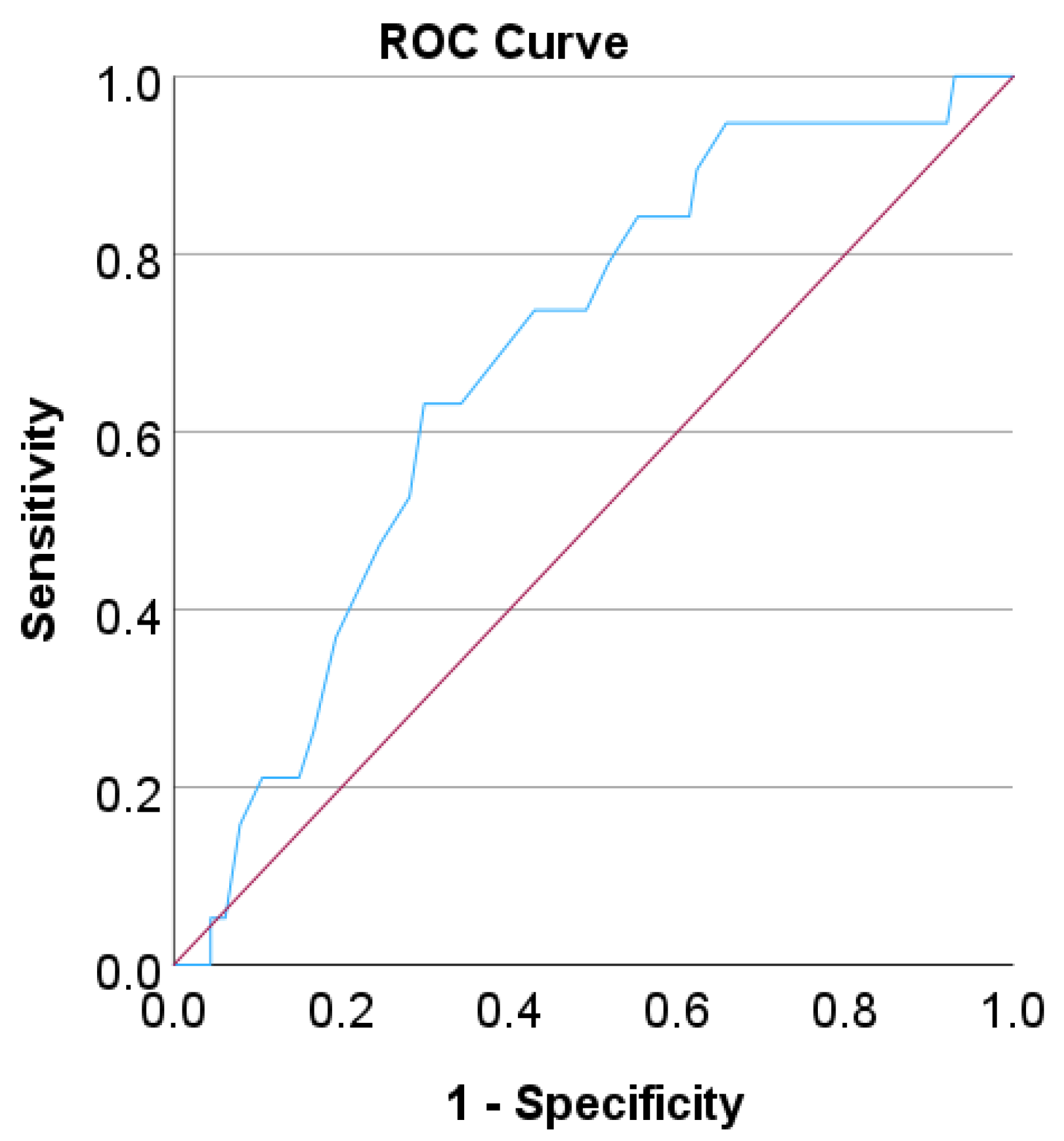
| Sensitivity (CI) | Specificity (CI) | PPV | NPV | p (Fisher Exact Test) | |
|---|---|---|---|---|---|
| Antrum Metaplasia | 52% (31–72%) | 77.5% (62–89%) | 59% | 72% | 0.018 * |
| All Metaplasia | 48% (30–67%) | 85% (76–92%) | 54% | 83% | <<0.001 * |
| Corpus Polyps | 83% (52–98%) | 93% (81–99%) | 77% | 95% | <0.001 * |
| Antrum Polyps | 53% (27–79%) | 94% (84–99%) | 73% | 87% | <0.001 * |
| Corpus Tumors | 67% (9–99%) | 96% (87–100%) | 50% | 98% | 0.012 * |
| Antrum Atrophy | 44% (20–70%) | 94% (83–99%) | 70% | 84% | 0.001 * |
| Overall | 55% (43–66%) | 92% (88–95%) | 64% | 88% | <<0.001 * |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dănilă, C.; Cardos, I.A.; Pop-Crisan, A.; Marc, F.; Hoza, A.; Chirla, R.; Pascalău, A.; Magheru, C.; Cavalu, S. Correlations between Endoscopic and Histopathological Assessment of Helicobacter pylori-Induced Gastric Pathology—A Cross-Sectional Retrospective Study. Life 2022, 12, 2096. https://doi.org/10.3390/life12122096
Dănilă C, Cardos IA, Pop-Crisan A, Marc F, Hoza A, Chirla R, Pascalău A, Magheru C, Cavalu S. Correlations between Endoscopic and Histopathological Assessment of Helicobacter pylori-Induced Gastric Pathology—A Cross-Sectional Retrospective Study. Life. 2022; 12(12):2096. https://doi.org/10.3390/life12122096
Chicago/Turabian StyleDănilă, Cătălina, Ioana Alexandra Cardos, Andrea Pop-Crisan, Felicia Marc, Anica Hoza, Razvan Chirla, Andrei Pascalău, Calin Magheru, and Simona Cavalu. 2022. "Correlations between Endoscopic and Histopathological Assessment of Helicobacter pylori-Induced Gastric Pathology—A Cross-Sectional Retrospective Study" Life 12, no. 12: 2096. https://doi.org/10.3390/life12122096
APA StyleDănilă, C., Cardos, I. A., Pop-Crisan, A., Marc, F., Hoza, A., Chirla, R., Pascalău, A., Magheru, C., & Cavalu, S. (2022). Correlations between Endoscopic and Histopathological Assessment of Helicobacter pylori-Induced Gastric Pathology—A Cross-Sectional Retrospective Study. Life, 12(12), 2096. https://doi.org/10.3390/life12122096







