Thermal Liquid Biopsy (TLB) of Blood Plasma as a Potential Tool to Help in the Early Diagnosis of Multiple Sclerosis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Subjects
2.2. Blood Sample Processing
2.3. Sample Preparation and DSC Measurements
2.4. Thermogram Analysis and Deconvolution
2.5. Multiparametric Data Analysis
2.6. Statistical Model
3. Results
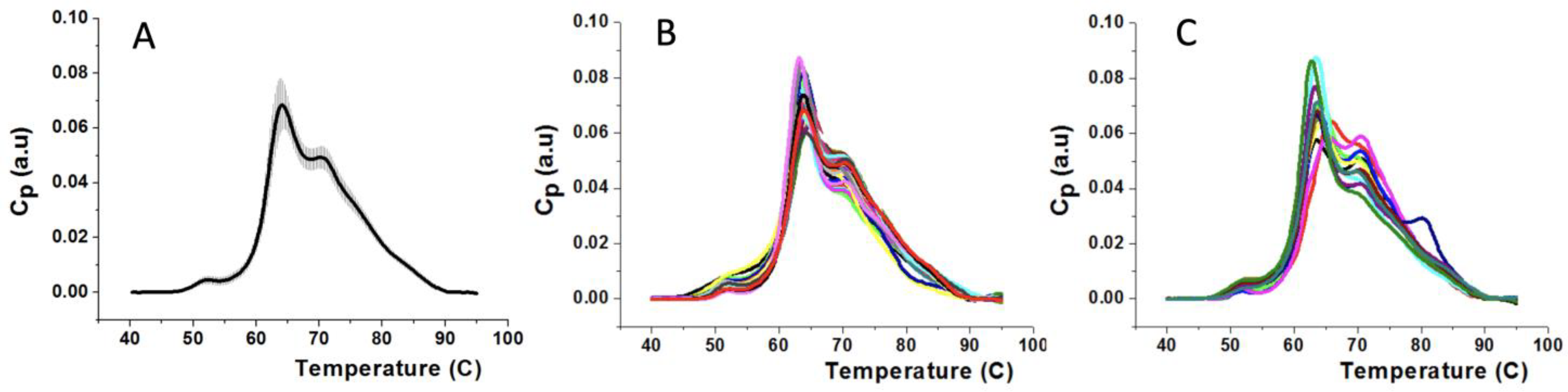
3.1. Thermograms of Plasma Samples for Subjects from HC and MS Groups
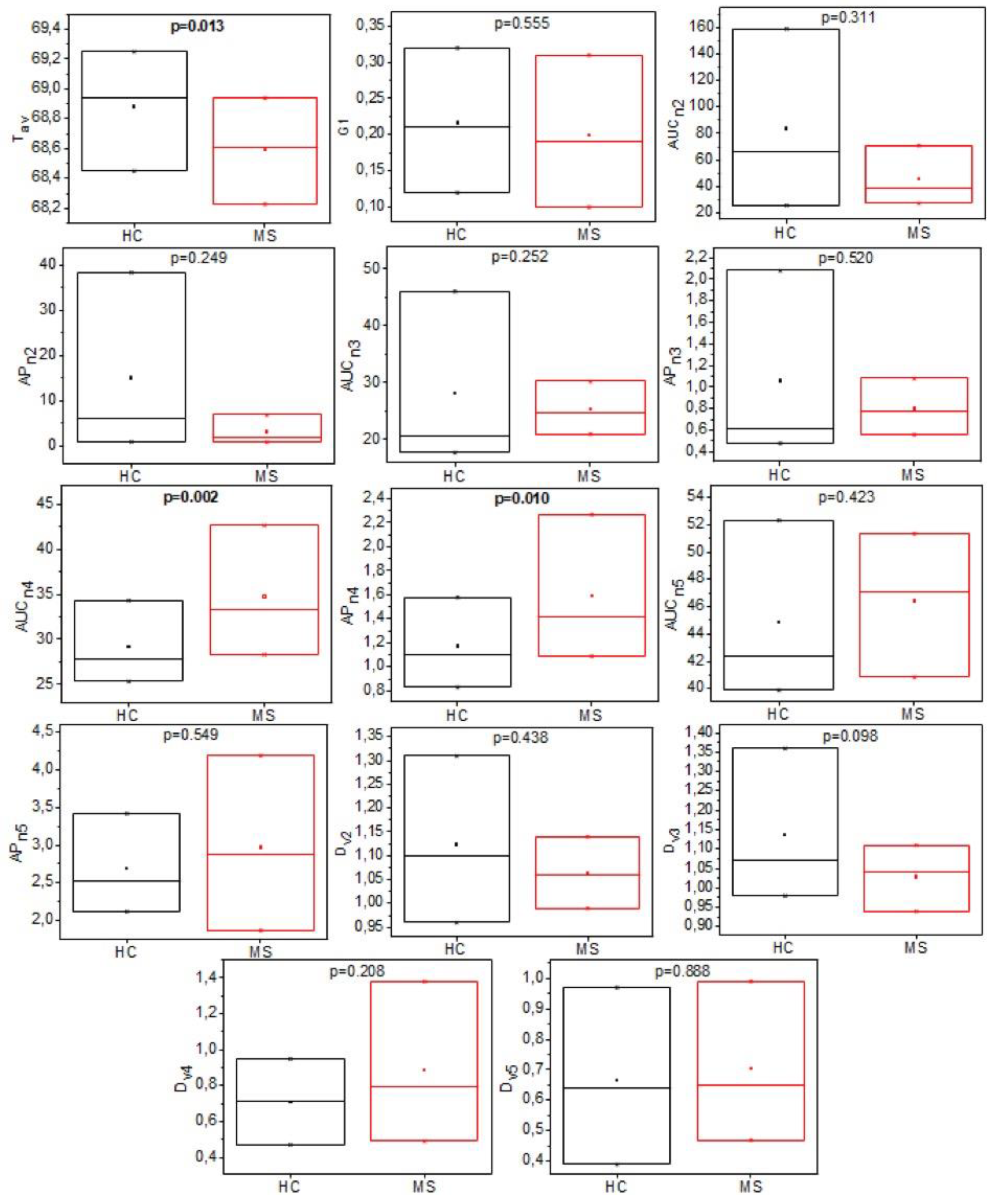
3.2. Analysis of Individual TLB-Derived Parameters
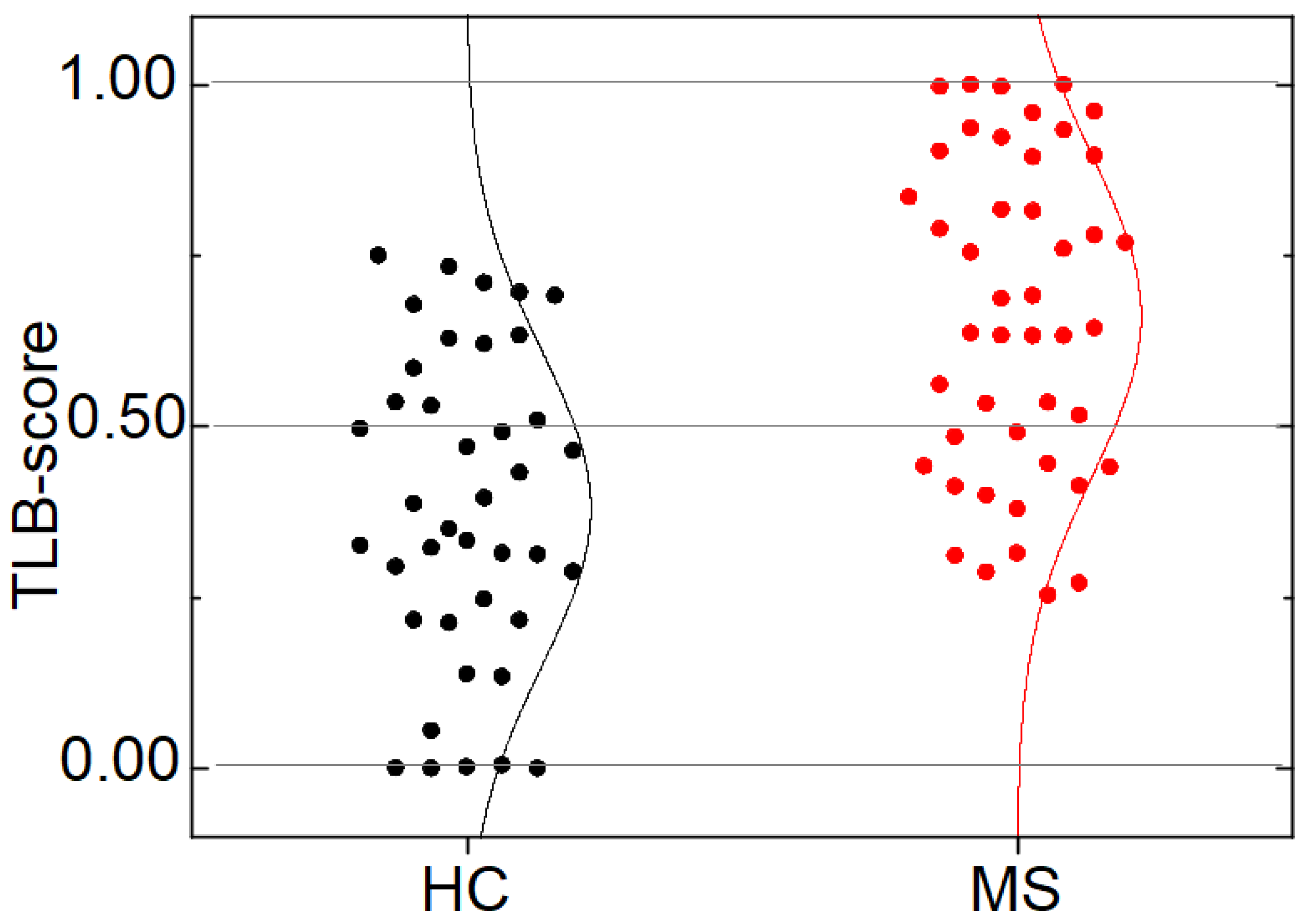
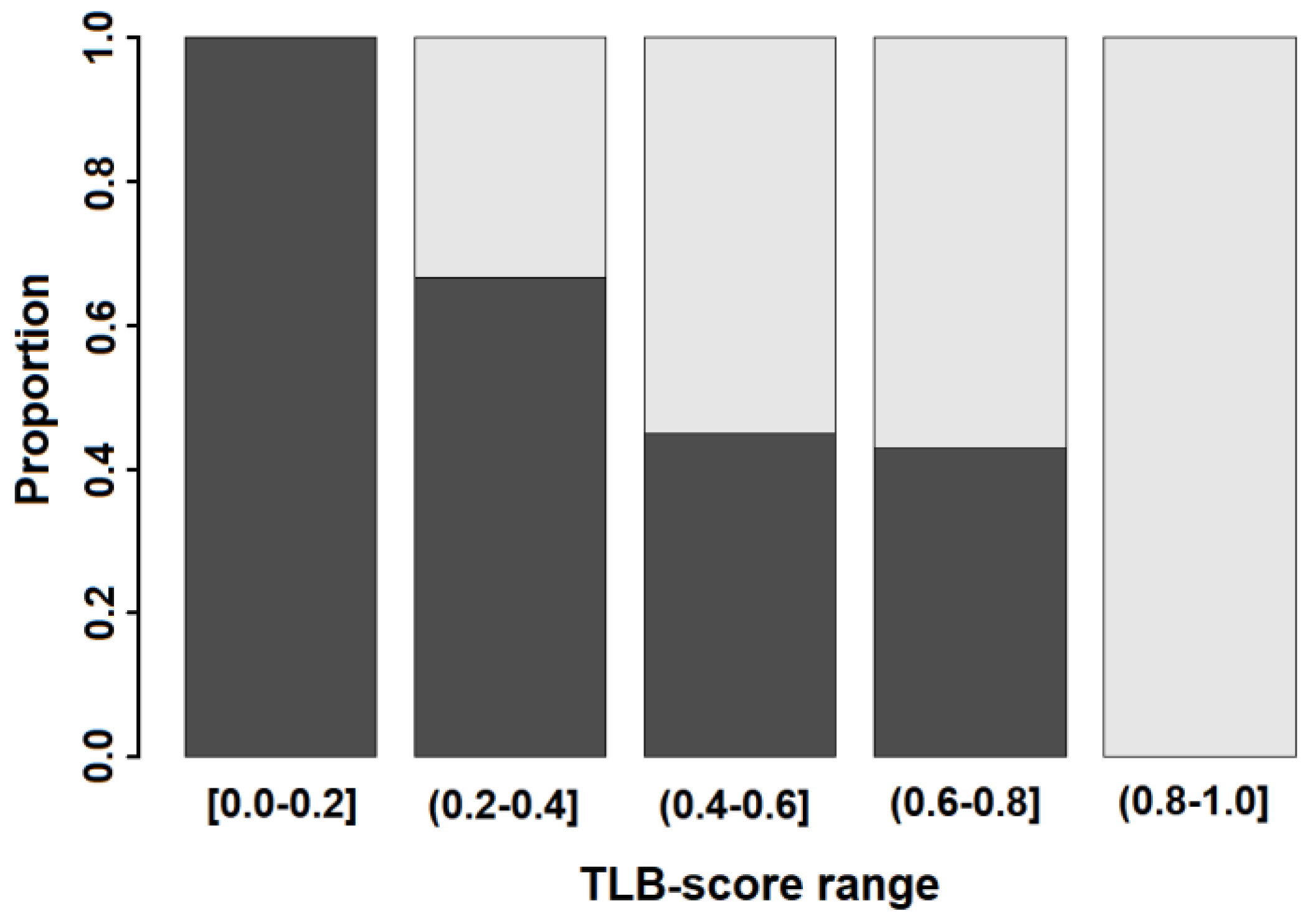
3.3. TLB Score: A Classifying Predictor for MS
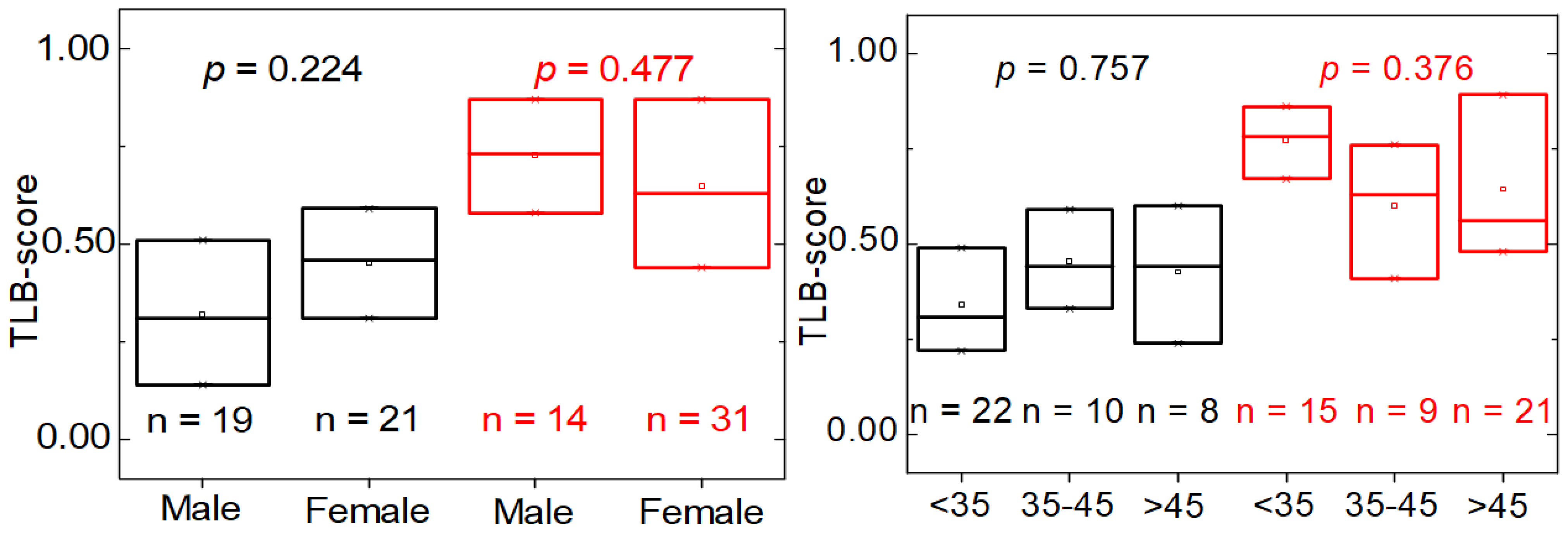
3.4. Distinctive Features of MS Patients versus HC Individuals according to TLB Values
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Reich, D.S.; Lucchinetti, C.F.; Calabresi, P.A. Multiple Sclerosis. N. Engl. J. Med. 2018, 378, 169–180. [Google Scholar] [CrossRef] [PubMed]
- Walton, C.; King, R.; Rechtman, L.; Kaye, W.; Leray, E.; Marrie, R.A.; Robertson, N.; La Rocca, N.; Uitdehaag, B.; van der Mei, I.; et al. Rising prevalence of multiple sclerosis worldwide: Insights from the Atlas of MS, third edition. Mult. Scler. 2020, 26, 1816–1821. [Google Scholar] [CrossRef] [PubMed]
- Collaborators, G.M.S. Global, regional, and national burden of multiple sclerosis 1990–2016: A systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol. 2019, 18, 269–285. [Google Scholar]
- Milo, R. Effectiveness of multiple sclerosis treatment with current immunomodulatory drugs. Expert Opin. Pharmacother. 2015, 16, 659–673. [Google Scholar] [CrossRef] [PubMed]
- Brownlee, W.J.; Hardy, T.A.; Fazekas, F.; Miller, D.H. Diagnosis of multiple sclerosis: Progress and challenges. Lancet 2017, 389, 1336–1346. [Google Scholar] [CrossRef]
- Thompson, A.J.; Banwell, B.L.; Barkhof, F.; Carroll, W.M.; Coetzee, T.; Comi, G.; Correale, J.; Fazekas, F.; Filippi, M.; Freedman, M.S.; et al. Diagnosis of multiple sclerosis: 2017 revisions of the McDonald criteria. Lancet Neurol. 2018, 17, 162–173. [Google Scholar] [CrossRef]
- Burman, J.; Zetterberg, H.; Fransson, M.; Loskog, A.S.; Raininko, R.; Fagius, J. Assessing tissue damage in multiple sclerosis: A biomarker approach. Acta Neurol. Scand. 2014, 130, 81–89. [Google Scholar] [CrossRef] [PubMed]
- Harris, V.K.; Tuddenham, J.F.; Sadiq, S.A. Biomarkers of multiple sclerosis: Current findings. Degener. Neurol. Neuromuscul. Dis. 2017, 7, 19–29. [Google Scholar] [CrossRef] [Green Version]
- Kivisakk, P.; Healy, B.C.; Francois, K.; Gandhi, R.; Gholipour, T.; Egorova, S.; Sevdalinova, V.; Quintana, F.; Chitnis, T.; Weiner, H.L.; et al. Evaluation of circulating osteopontin levels in an unselected cohort of patients with multiple sclerosis: Relevance for biomarker development. Mult. Scler. 2014, 20, 438–444. [Google Scholar] [CrossRef]
- Liguori, M.; Qualtieri, A.; Tortorella, C.; Direnzo, V.; Bagala, A.; Mastrapasqua, M.; Spadafora, P.; Trojano, M. Proteomic profiling in multiple sclerosis clinical courses reveals potential biomarkers of neurodegeneration. PLoS ONE 2014, 9, e103984. [Google Scholar] [CrossRef]
- Hye, A.; Lynham, S.; Thambisetty, M.; Causevic, M.; Campbell, J.; Byers, H.L.; Hooper, C.; Rijsdijk, F.; Tabrizi, S.J.; Banner, S.; et al. Proteome-based plasma biomarkers for Alzheimer’s disease. Brain 2006, 129, 3042–3050. [Google Scholar] [CrossRef] [Green Version]
- Mordechai, S.; Shufan, E.; Porat Katz, B.S.; Salman, A. Early diagnosis of Alzheimer’s disease using infrared spectroscopy of isolated blood samples followed by multivariate analyses. Analyst 2017, 142, 1276–1284. [Google Scholar] [CrossRef]
- Elshemey, W.M.; Ismail, A.M.; Elbialy, N.S. Molecular-Level Characterization of Normal, Benign, and Malignant Breast Tissues Using FTIR Spectroscopy. J. Med. Biol. Eng. 2016, 36, 369–378. [Google Scholar] [CrossRef]
- Garbett, N.C.; Miller, J.J.; Jenson, A.B.; Chaires, J.B. Calorimetry Outside the Box: A New Window into the Plasma Proteome. Biophys. J. 2008, 94, 1377–1383. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Crutchfield, C.A.; Thomas, S.N.; Sokoll, L.J.; Chan, D.W. Advances in mass spectrometry-based clinical biomarker discovery. Clin. Proteomics 2016, 13, 1–12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cooper, A. Differential scanning microcalorimetry. In Protein-Ligand Interactions: Hydrodynamics and Calorimetry; Oxford University Press: Oxford, UK, 2000. [Google Scholar]
- Privalov, G.; Kavina, V.; Freire, E.; Privalov, P.L. Precise scanning calorimeter for studying thermal properties of biological macromolecules in dilute solution. Anal. Biochem. 1995, 232, 79–85. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guglielmelli, A.; Rizzuti, B.; Guzzi, R. Stereoselective and domain-specific effects of ibuprofen on the thermal stability of human serum albumin. Eur. J. Pharm. Sci. 2018, 112, 122–131. [Google Scholar] [CrossRef] [PubMed]
- Rizzuti, B.; Bartucci, R.; Pey, A.L.; Guzzi, R. Warfarin increases thermal resistance of albumin through stabilization of the protein lobe that includes its binding site. Arch. Biochem. Biophys. 2019, 676, 108123. [Google Scholar] [CrossRef]
- Guzzi, R.; Sportelli, L.; Yanagisawa, S.; Li, C.; Kostrz, D.; Dennison, C. The influence of active site loop mutations on the thermal stability of azurin from Pseudomonas aeruginosa. Arch. Biochem. Biophys. 2012, 521, 18–23. [Google Scholar] [CrossRef]
- Chagovetz, A.A.; Jensen, R.L.; Recht, L.; Glantz, M.; Chagovetz, A.M. Preliminary use of differential scanning calorimetry of cerebrospinal fluid for the diagnosis of glioblastoma multiforme. J. Neurooncol. 2011, 105, 499–506. [Google Scholar] [CrossRef]
- Garbett, N.C.; Mekmaysy, C.S.; DeLeeuw, L.; Chaires, J.B. Clinical application of plasma thermograms. Utility, practical approaches and considerations. Methods 2015, 76, 41–50. [Google Scholar] [CrossRef] [Green Version]
- Krumova, S.; Rukova, B.; Todinova, S.; Gartcheva, L.; Milanova, V.; Toncheva, D.; Taneva, S.G. Calorimetric monitoring of the serum proteome in schizophrenia patients. Thermochim. Acta 2013, 572, 59–64. [Google Scholar] [CrossRef]
- Vega, S.; Garcia-Gonzalez, M.A.; Lanas, A.; Velazquez-Campoy, A.; Abian, O. Deconvolution analysis for classifying gastric adenocarcinoma patients based on differential scanning calorimetry serum thermograms. Sci. Rep. 2015, 5, 7988. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tsvetkov, P.O.; Tabouret, E.; Roman, A.Y.; Romain, S.; Bequet, C.; Ishimbaeva, O.; Honore, S.; Figarella-Branger, D.; Chinot, O.; Devred, F. Differential scanning calorimetry of plasma in glioblastoma: Toward a new prognostic/monitoring tool. Oncotarget 2018, 9, 9391–9399. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Garbett, N.C.; Brock, G.N.; Chaires, J.B.; Mekmaysy, C.S.; DeLeeuw, L.; Sivils, K.L.; Harley, J.B.; Rovin, B.H.; Kulasekera, K.B.; Jarjour, W.N. Characterization and classification of lupus patients based on plasma thermograms. PLoS ONE 2017, 12, e0186398. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rodrigo, A.; Ojeda, J.L.; Vega, S.; Sanchez-Gracia, O.; Lanas, A.; Isla, D.; Velazquez-Campoy, A.; Abian, O. Thermal Liquid Biopsy (TLB): A Predictive Score Derived from Serum Thermograms as a Clinical Tool for Screening Lung Cancer Patients. Cancers 2019, 11, 1012. [Google Scholar] [CrossRef] [Green Version]
- Velazquez-Campoy, A.; Vega, S.; Sanchez-Gracia, O.; Lanas, A.; Rodrigo, A.; Kaliappan, A.; Hall, M.B.; Nguyen, T.Q.; Brock, G.N.; Chesney, J.A.; et al. Thermal liquid biopsy for monitoring melanoma patients under surveillance during treatment: A pilot study. Biochim. Biophys. Acta Gen. Subj. 2018, 1862, 1701–1710. [Google Scholar] [CrossRef] [PubMed]
- Polman, C.H.; Reingold, S.C.; Banwell, B.; Clanet, M.; Cohen, J.A.; Filippi, M.; Fujihara, K.; Havrdova, E.; Hutchinson, M.; Kappos, L.; et al. Diagnostic criteria for multiple sclerosis: 2010 revisions to the McDonald criteria. Ann. Neurol. 2011, 69, 292–302. [Google Scholar] [CrossRef] [Green Version]
- Gornall, A.G.; Bardawill, C.J.; David, M.M. Determination of serum proteins by means of the biuret reaction. J. Biol. Chem. 1949, 177, 751–766. [Google Scholar] [CrossRef]
- Fluss, R.; Faraggi, D.; Reiser, B. Estimation of the Youden Index and its associated cutoff point. Biom. J. 2005, 47, 458–472. [Google Scholar] [CrossRef] [Green Version]
- Huang, J.; Khademi, M.; Fugger, L.; Lindhe, Ö.; Novakova, L.; Axelsson, M.; Malmeström, C.; Constantinescu, C.; Lycke, J.; Piehl, F.; et al. Inflammation-related plasma and CSF biomarkers for multiple sclerosis. Proc. Natl. Acad. Sci. USA 2020, 117, 12952–12960. [Google Scholar] [CrossRef] [PubMed]
- Malekzadeh, A.; Leurs, C.; van Wieringen, W.; Steenwijk, M.D.; Schoonheim, M.M.; Amann, M.; Naegelin, Y.; Kuhle, J.; Killestein, J.; Teunissen, C.E. Plasma proteome in multiple sclerosis disease progression. Ann. Clin. Transl. Neurol. 2019, 6, 1582–1594. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bhargava, P.; Anthony, D.C. Metabolomics in multiple sclerosis disease course and progression. Mult. Scler. J. 2020, 26, 591–598. [Google Scholar] [CrossRef]
- Porter, L.; Shoushtarizadeh, A.; Jelinek, G.A.; Brown, C.R.; Lim, C.K.; de Livera, A.M.; Jacobs, K.R.; Weiland, T.J. Metabolomic Biomarkers of Multiple Sclerosis: A Systematic Review. Front. Mol. Biosci. 2020, 7, 591–598. [Google Scholar] [CrossRef]
- Andersen, S.L.; Briggsb, F.B.S.; Winnikec, J.H.; Natanzonb, Y.; Maichled, S.; Knaggec, K.J.; Newbye, L.K.; Gregorya, S.G. Metabolome-based signature of disease pathology in MS. Mult. Scler. Relat. Disord. 2019, 31, 12–21. [Google Scholar] [CrossRef] [PubMed]


| Groups (1) | Gender | N (%) | Minimum | Q1 | Q2 | Mean | Q3 | Maximum |
|---|---|---|---|---|---|---|---|---|
| HC | Male | 19 (47.50%) | 24.00 | 26.00 | 32.00 | 36.58 | 40.50 | 71.00 |
| Female | 21 (52.50%) | 27.00 | 30.00 | 38.00 | 37.95 | 43.00 | 52.00 | |
| Total | 40 | 24.00 | 29.00 | 35.00 | 37.30 | 42.25 | 71.00 | |
| MS | Male | 14 (31.11%) | 24.00 | 31.25 | 39.00 | 41.21 | 49.50 | 63.00 |
| Female | 31 (68.89%) | 22.00 | 34.50 | 45.00 | 43.32 | 50.50 | 69.00 | |
| Total | 45 | 22.00 | 33.00 | 45.00 | 42.67 | 50.00 | 69.00 | |
| Diagnosis (2) | Gender | N (%) | Minimum | Q1 | Q2 | Mean | Q3 | Maximum |
| RRMS | Male | 12 (31.58%) | 24.00 | 31.00 | 33.50 | 38.42 | 47.25 | 58.00 |
| Female | 26 (68.42%) | 22.00 | 33.75 | 45.00 | 43.23 | 50.75 | 69.00 | |
| Total | 38 | 22.00 | 32.25 | 43.50 | 41.71 | 50.00 | 69.00 | |
| SPMS | Male | 2 (28.57%) | 53.00 | 55.50 | 58.00 | 58.00 | 60.50 | 63.00 |
| Female | 5 (71.43%) | 30.00 | 43.00 | 46.00 | 43.80 | 48.00 | 52.00 | |
| Total | 7 | 30.00 | 44.50 | 48.00 | 47.86 | 52.50 | 63.00 | |
| EDSS (3) | Gender | N (%) | Minimum | Q1 | Q2 | Mean | Q3 | Maximum |
| 0.5–3.0 | Male | 10 (31.25%) | 24.00 | 31.00 | 32.50 | 35.90 | 43.75 | 50.00 |
| Female | 22 (68.75%) | 22.00 | 33.00 | 42.00 | 41.50 | 50.00 | 69.00 | |
| Total | 32 | 22.00 | 31.00 | 38.00 | 39.75 | 49.25 | 69.00 | |
| 3.5–7.0 | Male | 4 (30.77%) | 44.00 | 50.75 | 55.50 | 54.50 | 59.25 | 63.00 |
| Female | 9 (69.23%) | 30.00 | 45.00 | 48.00 | 47.78 | 52.00 | 59.00 | |
| Total | 13 | 30.00 | 45.00 | 52.00 | 49.85 | 55.00 | 63.00 | |
| Disease onset (4) | Gender | N (%) | Minimum | Q1 | Q2 | Mean | Q3 | Maximum |
| ≤10 years | Male | 10 (37.04%) | 29.00 | 31.25 | 33.50 | 39.30 | 47.75 | 58.00 |
| Female | 17 (62.96%) | 22.00 | 30.00 | 39.00 | 40.59 | 52.00 | 59.00 | |
| Total | 27 | 22.00 | 30.50 | 37.00 | 40.11 | 50.50 | 59.00 | |
| >10 years | Male | 4 (22.22%) | 24.00 | 39.00 | 48.50 | 46.00 | 55.50 | 63.00 |
| Female | 14 (77.78%) | 33.00 | 43.50 | 46.00 | 46.64 | 49.75 | 69.00 | |
| Total | 18 | 24.00 | 43.25 | 46.00 | 46.50 | 50.00 | 69.00 | |
| Therapy (5) | Gender | N (%) | Minimum | Q1 | Q2 | Mean | Q3 | Maximum |
| No | Male | 2 (28.57%) | 32.00 | 39.75 | 47.50 | 47.50 | 55.25 | 63.00 |
| Female | 5 (71.43%) | 30.00 | 36.00 | 39.00 | 43.80 | 45.00 | 69.00 | |
| Total | 7 | 30.00 | 34.00 | 39.00 | 44.86 | 54.00 | 69.00 | |
| Yes | Male | 12 (31.58%) | 24.00 | 31.00 | 39.00 | 40.17 | 48.50 | 58.00 |
| Female | 26 (68.42%) | 22.00 | 34.00 | 46.00 | 43.23 | 50.75 | 59.00 | |
| Total | 38 | 22.00 | 33.00 | 45.50 | 42.26 | 50.00 | 59.00 |
| Model | Success Rate | Sensitivity | Specificity | PPV | NPV |
|---|---|---|---|---|---|
| 1 | 63.53% | 64.44% | 62.50% | 65.91% | 60.98% |
| 2 | 56.47% | 64.44% | 47.50% | 58.00% | 54.29% |
| 3 | 68.24% | 68.89% | 67.50% | 70.45% | 65.85% |
| Group | Gender | TLB Score < 0.5 | TLB Score > 0.5 | p-Value |
|---|---|---|---|---|
| HC | Male (n = 19) | 14 (73.68%) | 5 (26.32%) | 0.511 |
| Female (n = 21) | 13 (61.90%) | 8 (38.10%) | ||
| MS | Male (n = 14) | 2 (14.29%) | 12 (85.71%) | 0.165 |
| Female (n = 31) | 12 (38.71%) | 19 (61.29%) | ||
| Age | TLB Score < 0.5 | TLB Score > 0.5 | p-Value | |
| HC | <35 (n = 22) | 17 (77.27%) | 5 (22.73%) | 0.315 |
| 35–45 (n = 10) | 6 (60.00%) | 4 (40.00%) | ||
| >45 (n = 8) | 4 (50.00%) | 4 (50.00%) | ||
| MS | <35 (n = 15) | 3 (20.00%) | 12 (80.00%) | 0.444 |
| 35–45 (n = 9) | 4 (44.44%) | 5 (55.56%) | ||
| >45 (n = 21) | 7 (33.33%) | 14 (66.67%) |
| EDSS | TLB Score < 0.5 | TLB Score > 0.5 | p-Value |
|---|---|---|---|
| 0.5–3.0 (n = 32) | 7 (21.87%) | 25 (78.13%) | 0.072 |
| 3.5–7.0 (n = 13) | 7 (53.85%) | 6 (46.15%) | |
| Diagnosis | TLB Score < 0.5 | TLB Score > 0.5 | p-Value |
| RRMS (n = 38) | 9 (23.68%) | 29 (76.32%) | 0.023 |
| SPMS (n = 7) | 5 (71.43%) | 2 (28.57%) | |
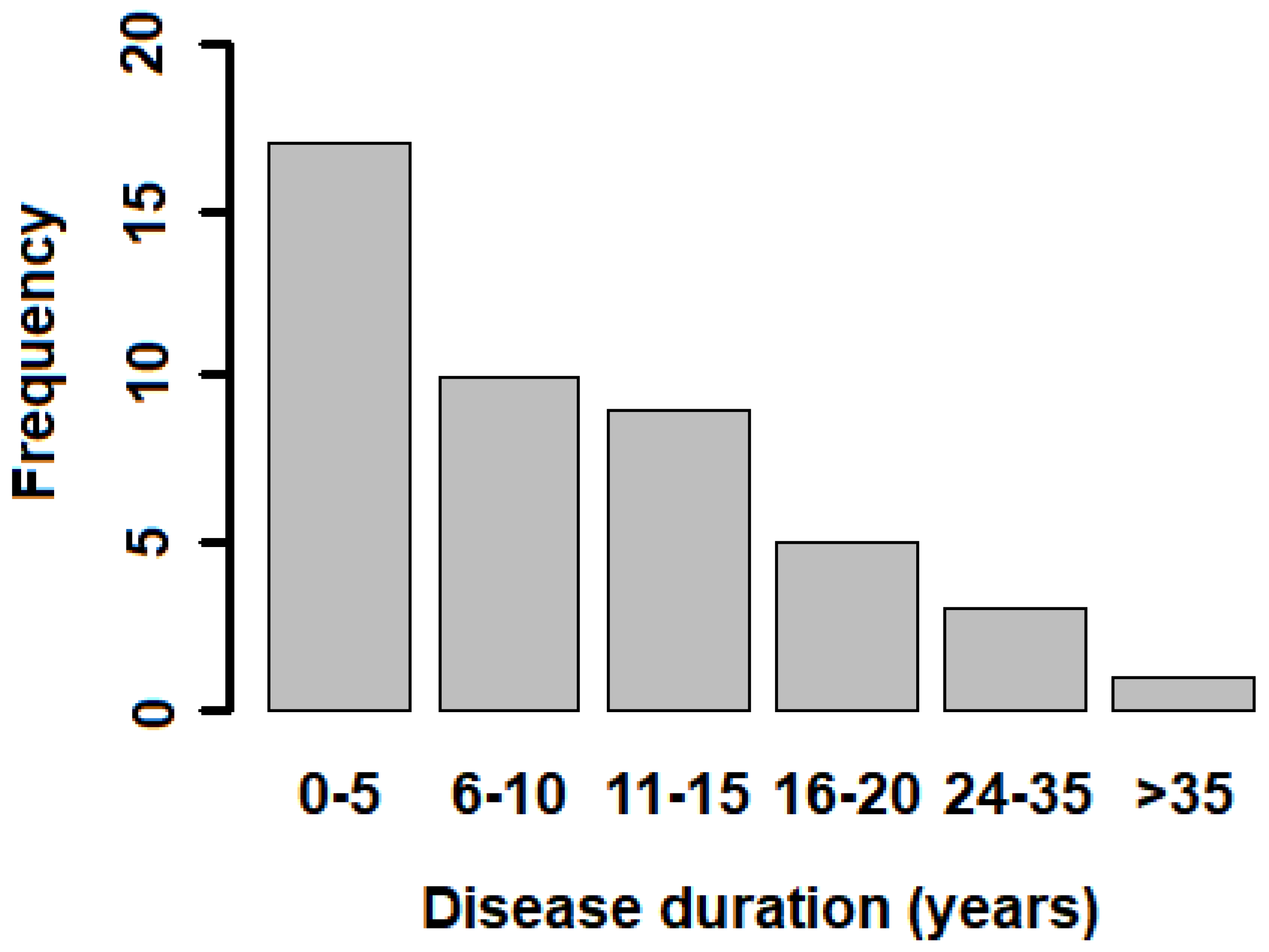
| Disease duration | TLB Score < 0.5 | TLB Score > 0.5 | p-Value |
| <10 years (n = 27) | 9 (33.33%) | 18 (66.67%) | 0.753 |
| >10 years (n = 18) | 5 (27.78%) | 13 (72.22%) | |
| Therapy | TLB Score < 0.5 | TLB Score > 0.5 | p-Value |
| No (n = 7) | 0 (0.00%) | 7 (100.00%) | 0.081 |
| Yes (n = 38) | 14 (36.84%) | 24 (63.16%) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Annesi, F.; Hermoso-Durán, S.; Rizzuti, B.; Bruno, R.; Pirritano, D.; Petrone, A.; Del Giudice, F.; Ojeda, J.; Vega, S.; Sanchez-Gracia, O.; et al. Thermal Liquid Biopsy (TLB) of Blood Plasma as a Potential Tool to Help in the Early Diagnosis of Multiple Sclerosis. J. Pers. Med. 2021, 11, 295. https://doi.org/10.3390/jpm11040295
Annesi F, Hermoso-Durán S, Rizzuti B, Bruno R, Pirritano D, Petrone A, Del Giudice F, Ojeda J, Vega S, Sanchez-Gracia O, et al. Thermal Liquid Biopsy (TLB) of Blood Plasma as a Potential Tool to Help in the Early Diagnosis of Multiple Sclerosis. Journal of Personalized Medicine. 2021; 11(4):295. https://doi.org/10.3390/jpm11040295
Chicago/Turabian StyleAnnesi, Ferdinanda, Sonia Hermoso-Durán, Bruno Rizzuti, Rosalinda Bruno, Domenico Pirritano, Alfredo Petrone, Francesco Del Giudice, Jorge Ojeda, Sonia Vega, Oscar Sanchez-Gracia, and et al. 2021. "Thermal Liquid Biopsy (TLB) of Blood Plasma as a Potential Tool to Help in the Early Diagnosis of Multiple Sclerosis" Journal of Personalized Medicine 11, no. 4: 295. https://doi.org/10.3390/jpm11040295








