Pathophysiology and Clinical Management of Bile Acid Diarrhea
Abstract
:1. Introduction
2. Search Strategy
3. Pathophysiology
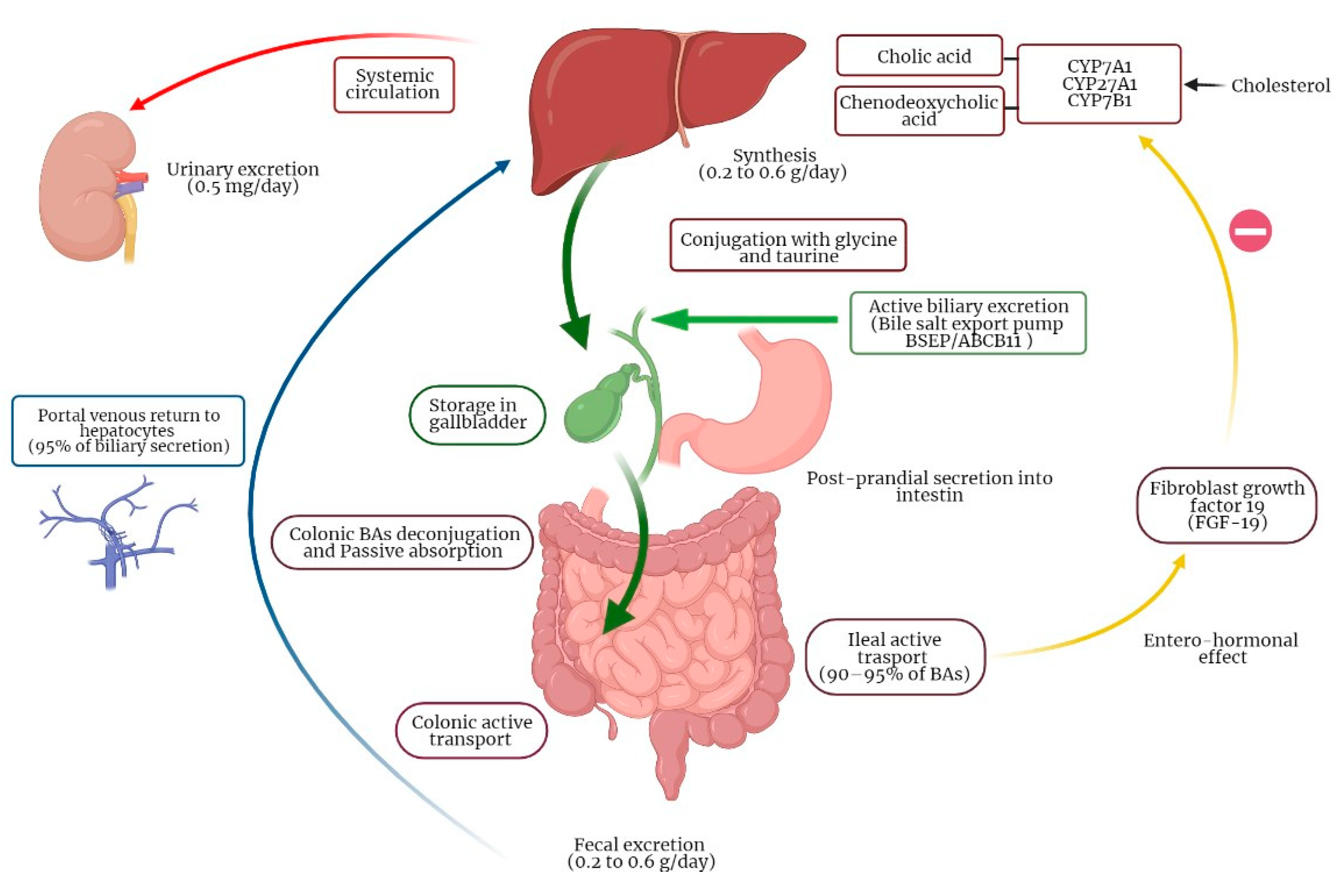
3.1. Physiology of Bile Acids Synthesis and Circulation
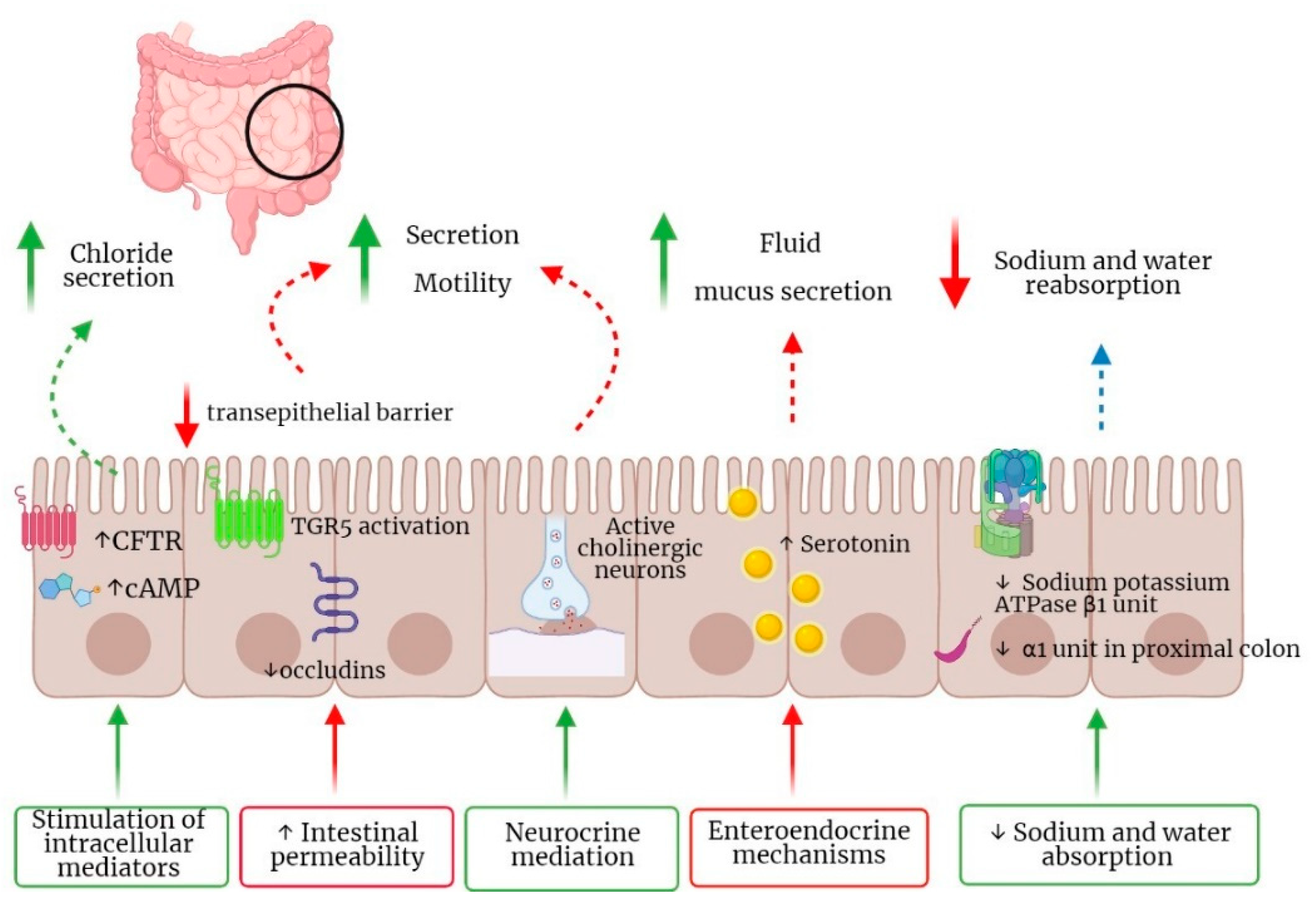
3.2. Molecular Mechanisms of BAs Enterohepatic Circulation
3.3. Pathogenetic Mechanisms of BAM and Clinical Manifestations
4. Classification
4.1. Type 1
4.2. Type 2
4.3. Type 3
5. Clinical Manifestations
6. Diagnosis
6.1. Selenium HomotauroCholic Acid Test (75SeHCAT)
6.2. Hour Fecal Bile Acid Test
6.3. Fasting Serum 7α-hydroxy-4-cholesten-3-one (C4) and Fibroblast Growth Factor 19 (FGF19)
6.4. BAST Empirical Trial
7. Bile Acids Diarrhea Treatment
7.1. Dietary Modifications
7.2. BAs Sequestrants
7.3. Farnesoid X Receptors (FXR) Agonists
7.4. Microbiota Modulation
8. Conclusions and Future Strategies
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hofmann, A.F.; Small, D. Detergent Properties of Bile Salts: Correlation with Physiological Function. Annu. Rev. Med. 1967, 18, 333–376. [Google Scholar] [CrossRef] [PubMed]
- Vijayvargiya, P.; Camilleri, M. Current Practice in the Diagnosis of Bile Acid Diarrhea. Gastroenterology 2019, 156, 1233–1238. [Google Scholar] [CrossRef] [Green Version]
- Wedlake, L.; A’Hern, R.; Russell, D.; Thomas, K.; Walters, J.R.F.; Andreyev, H.J.N. Systematic review: The prevalence of idiopathic bile acid malabsorption as diagnosed by SeHCAT scanning in patients with diarrhoea-predominant irritable bowel syndrome. Aliment. Pharmacol. Ther. 2009, 30, 707–717. [Google Scholar] [CrossRef] [PubMed]
- Walters, J.R. Defining primary bile acid diarrhea: Making the diagnosis and recognizing the disorder. Expert Rev. Gastroenterol. Hepatol. 2010, 4, 561–567. [Google Scholar] [CrossRef] [PubMed]
- Walters, J.R.F.; Pattni, S.S. Managing bile acid diarrhoea. Ther. Adv. Gastroenterol. 2010, 3, 349–357. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mekhjian, H.S.; Phillips, S.F.; Hofmann, A.F. Colonic Secretion of Water and Electrolytes Induced by Bile Acids: Perfusion Studies in Man. J. Clin. Investig. 1971, 50, 1569–1577. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McJunkin, B.; Fromm, H.; Sarva, R.P.; Amin, P. Factors in the mechanism of diarrhea in bile acid malabsorption: Fecal pH—A key determinant. Gastroenterology 1981, 80, 1454–1464. [Google Scholar] [CrossRef]
- Pattni, S.; Walters, J.R.F. Recent advances in the understanding of bile acid malabsorption. Br. Med. Bull. 2009, 92, 79–93. [Google Scholar] [CrossRef] [Green Version]
- Ridlon, J.M.; Alves, J.M.; Hylemon, P.B.; Bajaj, J.S. Cirrhosis, bile acids and gut microbiota: Unraveling a complex relationship. Gut Microbes 2013, 4, 382–387. [Google Scholar] [CrossRef] [Green Version]
- Camilleri, M. Bile Acid Diarrhea: Prevalence, Pathogenesis, and Therapy. Gut Liver 2015, 9, 332–339. [Google Scholar] [CrossRef]
- Balzer, K.; Breuer, N.; Quebe-Fehling, E. Postprandial serum bile acid level and 75SeHCAT retention in diagnosis of bile acid malabsorption syndrome. A comparative study. Med. Klin. 1993, 88 (Suppl. 1), 23–28. [Google Scholar]
- Walters, J.R.F.; Arasaradnam, R.; Andreyev, H.J.N.; Network, F.T.U.B.A.R.D. Diagnosis and management of bile acid diarrhoea: A survey of UK expert opinion and practice. Front. Gastroenterol. 2019, 11, 358–363. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bannaga, A.; Kelman, L.; O'Connor, M.; Pitchford, C.; Walters, J.R.F.; Arasaradnam, R.P. How bad is bile acid diarrhoea: An online survey of patient-reported symptoms and outcomes. BMJ Open Gastroenterol. 2017, 4, e000116. [Google Scholar] [CrossRef] [PubMed]
- Shrank, W.H.; Rogstad, T.L.; Parekh, N. Waste in the US Health Care System: Estimated Costs and Potential for Savings. JAMA 2019, 322, 1501–1509. [Google Scholar] [CrossRef]
- Björkhem, I.; Leoni, V.; Meaney, S. Genetic connections between neurological disorders and cholesterol metabolism. J. Lipid Res. 2010, 51, 2489–2503. [Google Scholar] [CrossRef] [Green Version]
- Di Ciaula, A.; Garruti, G.; Baccetto, R.L.; Molina-Molina, E.; Bonfrate, L.; Wang, D.Q.-H.; Portincasa, P. Bile Acid Physiology. Ann. Hepatol. 2017, 16, S4–S14. [Google Scholar] [CrossRef]
- Chiang, J.Y.L. Bile acids: Regulation of synthesis. J. Lipid Res. 2009, 50, 1955–1966. [Google Scholar] [CrossRef] [Green Version]
- Johnston, I.; Nolan, J.; Pattni, S.S.; Walters, J.R.F. New Insights into Bile Acid Malabsorption. Curr. Gastroenterol. Rep. 2011, 13, 418–425. [Google Scholar] [CrossRef]
- Pandak, W.M.; Kakiyama, G. The acidic pathway of bile acid synthesis: Not just an alternative pathway. Liver Res. 2019, 3, 88–98. [Google Scholar] [CrossRef]
- Myant, N.B.; Mitropoulos, K.A. Cholesterol 7 alpha-hydroxylase. J. Lipid Res. 1977, 18, 135–153. [Google Scholar] [CrossRef]
- Li, T.; Francl, J.M.; Boehme, S.; Chiang, J.Y.L. Regulation of cholesterol and bile acid homeostasis by the cholesterol 7α-hydroxylase/steroid response element-binding protein 2/microRNA-33a axis in mice. Hepatology 2013, 58, 1111–1121. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kubitz, R.; Dröge, C.; Stindt, J.; Weissenberger, K.; Häussinger, D. The bile salt export pump (BSEP) in health and disease. Clin. Res. Hepatol. Gastroenterol. 2012, 36, 536–553. [Google Scholar] [CrossRef] [PubMed]
- Camilleri, M. Bile acid detergency: Permeability, inflammation, and effects of sulfation. Am. J. Physiol. Gastrointest. Liver Physiol. 2022, 322, G480–G488. [Google Scholar] [CrossRef] [PubMed]
- Mekhjian, H.S.; Phillips, S.F.; Hofmann, A.F. Colonic absorption of unconjugated bile acids. Am. J. Dig. Dis. 1979, 24, 545–550. [Google Scholar] [CrossRef] [PubMed]
- Moschetta, A.; Bookout, A.L.; Mangelsdorf, D.J. Prevention of cholesterol gallstone disease by FXR agonists in a mouse model. Nat. Med. 2004, 10, 1352–1358. [Google Scholar] [CrossRef] [PubMed]
- Camilleri, M.; Shin, A.; Busciglio, I.; Carlson, P.; Acosta, A.; Bharucha, A.E.; Burton, D.; Lamsam, J.; Lueke, A.; Donato, L.J.; et al. Genetic variation in GPBAR1 predisposes to quantitative changes in colonic transit and bile acid excretion. Am. J. Physiol. Liver Physiol. 2014, 307, G508–G516. [Google Scholar] [CrossRef] [Green Version]
- Camilleri, M.; Vijayvargiya, P. The Role of Bile Acids in Chronic Diarrhea. Am. J. Gastroenterol. 2020, 115, 1596–1603. [Google Scholar] [CrossRef]
- Bunnett, N.W. Neuro-humoral signalling by bile acids and the TGR5 receptor in the gastrointestinal tract. J. Physiol. 2014, 592, 2943–2950. [Google Scholar] [CrossRef]
- Portincasa, P.; Di Ciaula, A.; Wang, H.H.; Palasciano, G.; van Erpecum, K.J.; Moschetta, A.; Wang, D.Q.-H. Coordinate regulation of gallbladder motor function in the gut-liver axis. Hepatology 2008, 47, 2112–2126. [Google Scholar] [CrossRef]
- Keitel, V.; Cupisti, K.; Ullmer, C.; Knoefel, W.T.; Kubitz, R.; Häussinger, D. The membrane-bound bile acid receptor TGR5 is localized in the epithelium of human gallbladders. Hepatology 2009, 50, 861–870. [Google Scholar] [CrossRef]
- Keitel, V.; Görg, B.; Bidmon, H.J.; Zemtsova, I.; Spomer, L.; Zilles, K.; Häussinger, D. The bile acid receptor TGR5 (Gpbar-1) acts as a neurosteroid receptor in brain. Glia 2010, 58, 1794–1805. [Google Scholar] [CrossRef] [PubMed]
- Schaap, F.G.; Trauner, M.; Jansen, P.L.M. Bile acid receptors as targets for drug development. Nat. Rev. Gastroenterol. Hepatol. 2013, 11, 55–67. [Google Scholar] [CrossRef] [PubMed]
- Beuers, U.; Hohenester, S.; Wenniger, L.J.M.D.B.; Kremer, A.E.; Jansen, P.L.M.; Elferink, R.P.J.O. The biliary HCO3− umbrella: A unifying hypothesis on pathogenetic and therapeutic aspects of fibrosing cholangiopathies. Hepatology 2010, 52, 1489–1496. [Google Scholar] [CrossRef] [PubMed]
- Brighton, C.; Rievaj, J.; Kuhre, R.E.; Glass, L.; Schoonjans, K.; Holst, J.J.; Gribble, F.; Reimann, F. Bile Acids Trigger GLP-1 Release Predominantly by Accessing Basolaterally Located G Protein–Coupled Bile Acid Receptors. Endocrinology 2015, 156, 3961–3970. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yde, J.; Keely, S.; Wu, Q.; Borg, J.F.; Lajczak, N.; O’Dwyer, A.; Dalsgaard, P.; Fenton, R.A.; Moeller, H.B. Characterization of AQPs in Mouse, Rat, and Human Colon and Their Selective Regulation by Bile Acids. Front. Nutr. 2016, 3, 46. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Suhail, M. Na+, K+-ATPase: Ubiquitous Multifunctional Transmembrane Protein and its Relevance to Various Pathophysiological Conditions. J. Clin. Med. Res. 2010, 2, 1–17. [Google Scholar] [CrossRef] [Green Version]
- Yde, J.; Borg, J.; Fenton, R.A.; Moeller, H.B. Altered expression of aquaporin water channels in a rat model of chronic diarrhea due to bile acid malabsorption. FASEB J. 2017, 31 (Suppl. 1), 703. [Google Scholar]
- Zimmerman, T.W.; Binder, H.J. Serotonin-induced alteration of colonic electrolyte transport in the rat. Gastroenterology 1984, 86, 310–317. [Google Scholar] [CrossRef]
- Bardhan, P.K.; Rahman, A.S.M.; Islam, S.; Rahman, M.; Gyr, K. Effects of Tropisetron, a 5-Hydroxytryptamine Type 3 Receptor Blocker, on Intestinal Secretion Induced by Cholera Toxin or Deoxycholic Acid in Rabbits In Vivo. J. Int. Med. Res. 1993, 21, 323–333. [Google Scholar] [CrossRef]
- Camilleri, M.; Murphy, R.; Chadwick, V.S. Pharmacological inhibition of chenodeoxycholate-induced fluid and mucus secretion and mucosal injury in the rabbit colon. Am. J. Dig. Dis. 1982, 27, 865–869. [Google Scholar] [CrossRef]
- Ward, J.B.J.; Mroz, M.S.; Keely, S.J. The bile acid receptor, TGR5, regulates basal and cholinergic-induced secretory responses in rat colon. Neurogastroenterol. Motil. 2013, 25, 708–711. [Google Scholar] [CrossRef] [PubMed]
- Cipriani, S.; Mencarelli, A.; Chini, M.G.; Distrutti, E.; Renga, B.; Bifulco, G.; Baldelli, F.; Donini, A.; Fiorucci, S. The Bile Acid Receptor GPBAR-1 (TGR5) Modulates Integrity of Intestinal Barrier and Immune Response to Experimental Colitis. PLoS ONE 2011, 6, e25637. [Google Scholar] [CrossRef]
- Sarathy, J.; Detloff, S.J.; Ao, M.; Khan, N.; French, S.; Sirajuddin, H.; Nair, T.; Rao, M.C. The Yin and Yang of bile acid action on tight junctions in a model colonic epithelium. Physiol. Rep. 2017, 5, e13294. [Google Scholar] [CrossRef] [PubMed]
- Bampton, P.A.; Dinning, P.; Kennedy, M.L.; Lubowski, D.Z.; Cook, I.J. The proximal colonic motor response to rectal mechanical and chemical stimulation. Am. J. Physiol. Liver Physiol. 2002, 282, G443–G449. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dior, M.; Delagrèverie, H.; Duboc, H.; Jouet, P.; Coffin, B.; Brot, L.; Humbert, L.; Trugnan, G.; Seksik, P.; Sokol, H.; et al. Interplay between bile acid metabolism and microbiota in irritable bowel syndrome. Neurogastroenterol. Motil. 2016, 28, 1330–1340. [Google Scholar] [CrossRef] [PubMed]
- Jeffery, I.; O'Toole, P.; Öhman, L.; Claesson, M.; Deane, J.; Quigley, E.M.M.; Simrén, M. An irritable bowel syndrome subtype defined by species-specific alterations in faecal microbiota. Gut 2011, 61, 997–1006. [Google Scholar] [CrossRef]
- Rajilic-Stojanovic, M.; Biagi, E.; Heilig, H.G.; Kajander, K.; Kekkonen, R.A.; Tims, S.; de Vos, W.M. Global and Deep Molecular Analysis of Microbiota Signatures in Fecal Samples From Patients With Irritable Bowel Syndrome. Gastroenterology 2011, 141, 1792–1801. [Google Scholar] [CrossRef]
- Duboc, H.; Rainteau, D.; Rajca, S.; Humbert, L.; Farabos, D.; Maubert, M.; Grondin, V.; Jouet, P.; Bouhassira, D.; Seksik, P.; et al. Increase in fecal primary bile acids and dysbiosis in patients with diarrhea-predominant irritable bowel syndrome. Neurogastroenterol. Motil. 2012, 24, 513-e247. [Google Scholar] [CrossRef]
- Zhao, L.; Yang, W.; Chen, Y.; Huang, F.; Lu, L.; Lin, C.; Huang, T.; Ning, Z.; Zhai, L.; Zhong, L.L.; et al. A Clostridia-rich microbiota enhances bile acid excretion in diarrhea-predominant irritable bowel syndrome. J. Clin. Investig. 2019, 130, 438–450. [Google Scholar] [CrossRef] [Green Version]
- Sagar, N.M.; Duboc, H.; Kay, G.L.; Alam, M.T.; Wicaksono, A.N.; Covington, J.A.; Quince, C.; Kokkorou, M.; Svolos, V.; Palmieri, L.J.; et al. The pathophysiology of bile acid diarrhoea: Differences in the colonic microbiome, metabolome and bile acids. Sci. Rep. 2020, 10, 20436. [Google Scholar] [CrossRef]
- Jeffery, I.B.; Das, A.; O’Herlihy, E.; Coughlan, S.; Cisek, K.; Moore, M.; Bradley, F.; Carty, T.; Pradhan, M.; Dwibedi, C.; et al. Differences in Fecal Microbiomes and Metabolomes of People With vs Without Irritable Bowel Syndrome and Bile Acid Malabsorption. Gastroenterology 2020, 158, 1016–1028.e8. [Google Scholar] [CrossRef] [PubMed]
- Walters, J.R.; Marchesi, J.R. Chronic diarrhea, bile acids, and Clostridia. J. Clin. Investig. 2019, 130, 77–79. [Google Scholar] [CrossRef] [PubMed]
- Walters, J.R.; Tasleem, A.M.; Omer, O.S.; Brydon, W.G.; Dew, T.; le Roux, C.W. A New Mechanism for Bile Acid Diarrhea: Defective Feedback Inhibition of Bile Acid Biosynthesis. Clin. Gastroenterol. Hepatol. 2009, 7, 1189–1194. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Johnston, I.M.; Nolan, J.D.; Pattni, S.S.; Appleby, R.; Zhang, J.H.; Kennie, S.L.; Madhan, G.K.; Jameie-Oskooei, S.; Pathmasrirengam, S.; Lin, J.; et al. Characterizing Factors Associated With Differences in FGF19 Blood Levels and Synthesis in Patients With Primary Bile Acid Diarrhea. Am. J. Gastroenterol. 2016, 111, 423–432. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, J.M.; Ong, J.R.; Vergnes, L.; Vallim, T.Q.D.A.; Nolan, J.; Cantor, R.M.; Walters, J.R.; Reue, K. Diet1, bile acid diarrhea, and FGF15/19: Mouse model and human genetic variants. J. Lipid Res. 2018, 59, 429–438. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wong, B.S.; Camilleri, M.; Carlson, P.J.; Guicciardi, M.E.; Burton, D.; McKinzie, S.; Rao, A.S.; Zinsmeister, A.R.; Gores, G.J. A Klothoβ Variant Mediates Protein Stability and Associates With Colon Transit in Irritable Bowel Syndrome With Diarrhea. Gastroenterology 2011, 140, 1934–1942. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fromm, H.; Malavolti, M. Bile Acid-Induced Diarrhoea. Clin. Gastroenterol. 1986, 15, 567–582. [Google Scholar] [CrossRef]
- Caspary, W.F.; Zavada, I.; Reimold, W.; Deuticke, U.; Emrich, D.; Willms, B. Alteration of Bile Acid Metabolism and Vitamin-B12-Absorption in Diabetics on Biguanides. Diabetologia 1977, 193, 187–193. [Google Scholar] [CrossRef] [Green Version]
- Wu, S.; Li, X.; Zhang, H. Effects of metformin on endothelial function in type 2 diabetes. Exp. Ther. Med. 2014, 7, 1349–1353. [Google Scholar] [CrossRef]
- Vijayvargiya, P.; Camilleri, M. Update on Bile Acid Malabsorption: Finally Ready for Prime Time? Curr. Gastroenterol. Rep. 2018, 20, 10. [Google Scholar] [CrossRef]
- Borghede, M.K.; Schlütter, J.M.; Agnholt, J.; Christensen, L.A.; Gormsen, L.C.; Dahlerup, J.F. Bile acid malabsorption investigated by selenium-75-homocholic acid taurine (75SeHCAT) scans: Causes and treatment responses to cholestyramine in 298 patients with chronic watery diarrhoea. Eur. J. Intern. Med. 2011, 22, e137–e140. [Google Scholar] [CrossRef] [PubMed]
- Barkun, A.N.; Love, J.; Gould, M.; Pluta, H.; Steinhart, A.H. Bile Acid Malabsorption in Chronic Diarrhea: Pathophysiology and Treatment. Can. J. Gastroenterol. 2013, 27, 653–659. [Google Scholar] [CrossRef] [PubMed]
- Lenicek, M.; Duricova, D.; Komarek, V.; Gabrysova, B.; Lukas, M.; Smerhovsky, Z.; Vitek, L. Bile acid malabsorption in inflammatory bowel disease. Inflamm. Bowel Dis. 2011, 17, 1322–1327. [Google Scholar] [CrossRef] [PubMed]
- Gracie, D.J.; Kane, J.S.; Mumtaz, S.; Scarsbrook, A.F.; Chowdhury, F.U.; Ford, A.C. Prevalence of, and predictors of, bile acid malabsorption in outpatients with chronic diarrhea. Neurogastroenterol. Motil. 2012, 24, 983-e538. [Google Scholar] [CrossRef] [PubMed]
- Mottacki, N.; Simrén, M.; Bajor, A. Review article: Bile acid diarrhoea—Pathogenesis, diagnosis and management. Aliment. Pharmacol. Ther. 2016, 43, 884–898. [Google Scholar] [CrossRef] [Green Version]
- Yeoh, E.K.; Lui, D.; Lee, N.Y. The mechanism of diarrhoea resulting from pelvic and abdominal radiotherapy; a prospective study using selenium-75 labelled conjugated bile acid and cobalt-58 labelled cyanocobalamin. Br. J. Radiol. 1984, 57, 1131–1136. [Google Scholar] [CrossRef]
- Bajor, A.; Törnblom, H.; Rudling, M.; Ung, K.-A.; Simrén, M. Increased colonic bile acid exposure: A relevant factor for symptoms and treatment in IBS. Gut 2014, 64, 84–92. [Google Scholar] [CrossRef]
- Nyhlin, H.; Merrick, M.V.; Eastwood, M.A. Bile acid malabsorption in Crohn's disease and indications for its assessment using SeHCAT. Gut 1994, 35, 90–93. [Google Scholar] [CrossRef] [Green Version]
- Smith, M.J.; Cherian, P.; Raju, G.S.; Dawson, B.F.; Mahon, S.; Bardhan, K.D. Bile acid malabsorption in persistent diarrhoea. J. R. Coll. Physicians Lond. 2000, 34, 448–451. [Google Scholar]
- Kurien, M.; Evans, K.E.; Leeds, J.S.; Hopper, A.D.; Harris, A.; Sanders, D.S. Bile acid malabsorption: An under-investigated differential diagnosis in patients presenting with diarrhea predominant irritable bowel syndrome type symptoms. Scand. J. Gastroenterol. 2011, 46, 818–822. [Google Scholar] [CrossRef]
- Khalid, U.; Lalji, A.; Stafferton, R.; Andreyev, J. Bile acid malabsoption: A forgotten diagnosis? Clin. Med. 2010, 10, 124–126. [Google Scholar] [CrossRef] [PubMed]
- Vijayvargiya, P.; Izundegui, D.G.; Calderon, G.; Tawfic, S.; Batbold, S.; Camilleri, M. Fecal Bile Acid Testing in Assessing Patients With Chronic Unexplained Diarrhea: Implications for Healthcare Utilization. Am. J. Gastroenterol. 2020, 115, 1094–1102. [Google Scholar] [CrossRef] [PubMed]
- Slattery, S.A.; Niaz, O.; Aziz, Q.; Ford, A.; Farmer, A.D. Systematic review with meta-analysis: The prevalence of bile acid malabsorption in the irritable bowel syndrome with diarrhoea. Aliment. Pharmacol. Ther. 2015, 42, 3–11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Valentin, N.; Camilleri, M.; Altayar, O.; Vijayvargiya, P.; Acosta, A.; Nelson, A.D.; Murad, M.H. Biomarkers for bile acid diarrhoea in functional bowel disorder with diarrhoea: A systematic review and meta-analysis. Gut 2015, 65, 1951–1959. [Google Scholar] [CrossRef] [PubMed]
- Niaz, S. Bile acid malabsorption and postinfective diarrhoea. Clin. Med. 2001, 1, 156. [Google Scholar] [CrossRef] [PubMed]
- Menon, S.; Jones, B.J. Postinfective bile acid malabsorption. Eur. J. Gastroenterol. Hepatol. 2011, 23, 308–310. [Google Scholar] [CrossRef]
- Camilleri, M. Editorial: Dissecting Molecular Mechanisms in Bile Acid Diarrhea. Am. J. Gastroenterol. 2016, 111, 433–435. [Google Scholar] [CrossRef]
- Shin, A.; Camilleri, M.; Vijayvargiya, P.; Busciglio, I.; Burton, D.; Ryks, M.; Rhoten, D.; Lueke, A.; Saenger, A.; Girtman, A.; et al. Bowel Functions, Fecal Unconjugated Primary and Secondary Bile Acids, and Colonic Transit in Patients With Irritable Bowel Syndrome. Clin. Gastroenterol. Hepatol. 2013, 11, 1270–1275. [Google Scholar] [CrossRef] [Green Version]
- Ford, G.A.; Preece, J.D.; Davies, I.H.; Wilkinson, S.P. Use of the SeHCAT test in the investigation of diarrhoea. Postgrad. Med. J. 1992, 68, 272–276. [Google Scholar] [CrossRef] [Green Version]
- Farahmandfar, M.R.; Chabok, M.; Alade, M.; Bouhelal, A.; Patel, B. Post Cholecystectomy Diarrhoea—A Systematic Review. Surg. Sci. 2012, 3, 332–338. [Google Scholar] [CrossRef] [Green Version]
- Al-Hadrani, A.; Lavelle-Jones, M.; Kennedy, N.; Neill, G.; Sutton, D.; Cuschieri, A. Bile acid male absorption in patients with post-vagotomy diarrhoea. Ann. Chir. Gynaecol. 1992, 81, 351–353. [Google Scholar] [PubMed]
- Nakamura, T.; Kikuchi, H.; Takebe, K.; Ishii, M.; Imamura, K.-I.; Yamada, N.; Kudoh, K.; Terada, A. Correlation Between Bile Acid Malabsorption and Pancreatic Exocrine Dysfunction in Patients with Chronic Pancreatitis. Pancreas 1994, 9, 580–584. [Google Scholar] [CrossRef] [PubMed]
- Lanzini, A.; Lanzarotto, F. Thèmechanical Pumps’ and the Enterohepatic Circulation of Bile Acids ± Defects in Coeliac Disease. Aliment. Pharmacol. Ther. 2000, 14, 58–61. [Google Scholar] [CrossRef] [PubMed]
- Marteau, P.; Lavergne-Slove, A.; Lemann, M.; Bouhnik, Y.; Bertheau, P.; Becheur, H.; Galian, A.; Rambaud, J.C. Primary ileal villous atrophy is often associated with microscopic colitis. Gut 1997, 41, 561–564. [Google Scholar] [CrossRef] [Green Version]
- Ung, K.-A.; Gillberg, R.; Kilander, A.; Abrahamsson, H. Role of bile acids and bile acid binding agents in patients with collagenous colitis. Gut 2000, 46, 170–175. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tysk, C.; Wickbom, A.; Nyhlin, N.; Eriksson, S.; Bohr, J. Recent advances in diagnosis and treatment of microscopic colitis. Ann. Gastroenterol. 2011, 24, 253–262. [Google Scholar]
- Dandona, P.; Fonseca, V.; Mier, A.; Beckett, A.G. Diarrhea and Metformin in a Diabetic Clinic. Diabetes Care 1983, 6, 472–474. [Google Scholar] [CrossRef] [Green Version]
- Appleby, R.; Moghul, I.; Khan, S.; Yee, M.; Manousou, P.; Neal, T.D.; Walters, J.R.F. Non-alcoholic fatty liver disease is associated with dysregulated bile acid synthesis and diarrhea: A prospective observational study. PLoS ONE 2019, 14, e0211348. [Google Scholar] [CrossRef] [Green Version]
- Wong, B.S.; Camilleri, M.; Carlson, P.; McKinzie, S.; Busciglio, I.; Bondar, O.; Dyer, R.B.; Lamsam, J.; Zinsmeister, A.R. Increased Bile Acid Biosynthesis Is Associated With Irritable Bowel Syndrome With Diarrhea. Clin. Gastroenterol. Hepatol. 2012, 10, 1009–1015.e3. [Google Scholar] [CrossRef] [Green Version]
- Bajor, A.; Kilander, A.; Fae, A.; Gälman, C.; Jonsson, O.; Ohman, L.; Rudling, M.; Sjövall, H.; Stotzer, P.-O.; Ung, K.-A. Normal or increased bile acid uptake in isolated mucosa from patients with bile acid malabsorption. Eur. J. Gastroenterol. Hepatol. 2006, 18, 397–403. [Google Scholar] [CrossRef]
- BouSaba, J.; Sannaa, W.; McKinzie, S.; Vijayvargiya, P.; Chedid, V.; Wang, X.J.; Atieh, J.; Zheng, T.; Brandler, J.; Taylor, A.L.; et al. Impact of Bile Acid Diarrhea in Patients With Diarrhea-Predominant Irritable Bowel Syndrome on Symptoms and Quality of Life. Clin. Gastroenterol. Hepatol. 2021. [Google Scholar] [CrossRef] [PubMed]
- Lyutakov, I.; Ursini, F.; Penchev, P.; Caio, G.; Carroccio, A.; Volta, U.; De Giorgio, R. Methods for diagnosing bile acid malabsorption: A systematic review. BMC Gastroenterol. 2019, 19, 185. [Google Scholar] [CrossRef] [Green Version]
- Sciarretta, G.; Vicini, G.; Fagioli, G.; Verri, A.; Ginevra, A.; Malaguti, P. Use of 23-selena-25-homocholyltaurine to detect bile acid malabsorption in patients with ileal dysfunction or diarrhea. Gastroenterology 1986, 91, 1–9. [Google Scholar] [CrossRef]
- Brydon, W.G.; Culbert, P.; Kingstone, K.; Jarvie, A.; Iacucci, M.; Tenhage, M.; Ghosh, S. An evaluation of the use of serum 7-alpha-hydroxycholestenone as a diagnostic test of bile acid malabsorption causing watery diarrhea. Can. J. Gastroenterol. 2011, 25, 319–323. [Google Scholar] [CrossRef] [PubMed]
- Bares, L.M.; Asenjo, E.C.; Sánchez, M.G.; Ortega, E.M.; Muret, F.M.; Moreno, M.G.; Bueno, A.S.; Flores, E.I.; Cantero, J.B.; Casas, J.V. Gammagrafía con 75SeHCAT en la diarrea crónica por malabsorción de ácidos biliares. Rev. Española Med. Nucl. Imagen Mol. 2017, 36, 37–47. [Google Scholar] [CrossRef] [PubMed]
- Brydon, W.G.; Nyhlin, H.; Eastwood, M.A.; Merrick, M.V. Serum 7α-hydroxy-4-cholesten-3-one and selenohomocholyltaurine (SeHCAT) whole body retention in the assessment of bile acid induced diarrhoea. Eur. J. Gastroenterol. Hepatol. 1996, 8, 117–124. [Google Scholar] [CrossRef]
- Vijayvargiya, P.; Camilleri, M.; Chedid, V.; Carlson, P.; Busciglio, I.; Burton, D.; Donato, L.J. Analysis of Fecal Primary Bile Acids Detects Increased Stool Weight and Colonic Transit in Patients With Chronic Functional Diarrhea. Clin. Gastroenterol. Hepatol. 2018, 17, 922–929.e2. [Google Scholar] [CrossRef]
- Vijayvargiya, P.; Camilleri, M.; Carlson, P.; Lueke, A.; O’Neill, J.; Burton, D.; Busciglio, I.; Donato, L. Performance characteristics of serum C4 and FGF19 measurements to exclude the diagnosis of bile acid diarrhoea in IBS-diarrhoea and functional diarrhoea. Aliment. Pharmacol. Ther. 2017, 46, 581–588. [Google Scholar] [CrossRef]
- Camilleri, M.; Shin, A.; Busciglio, I.; Carlson, P.; Acosta, A.; Bharucha, A.E.; Burton, D.; Lamsam, J.; Lueke, A.; Donato, L.J.; et al. Validating biomarkers of treatable mechanisms in irritable bowel syndrome. Neurogastroenterol. Motil. 2014, 26, 1677–1685. [Google Scholar] [CrossRef]
- Odunsi–Shiyanbade, S.T.; Camilleri, M.; McKinzie, S.; Burton, D.; Carlson, P.; Busciglio, I.A.; Lamsam, J.; Singh, R.; Zinsmeister, A.R. Effects of Chenodeoxycholate and a Bile Acid Sequestrant, Colesevelam, on Intestinal Transit and Bowel Function. Clin. Gastroenterol. Hepatol. 2010, 8, 159–165.e5. [Google Scholar] [CrossRef] [Green Version]
- Camilleri, M. Physiological underpinnings of irritable bowel syndrome: Neurohormonal mechanisms. J. Physiol. 2014, 592, 2967–2980. [Google Scholar] [CrossRef] [PubMed]
- Gälman, C.; Angelin, B.; Rudling, M. Bile Acid Synthesis in Humans Has a Rapid Diurnal Variation That Is Asynchronous With Cholesterol Synthesis. Gastroenterology 2005, 129, 1445–1453. [Google Scholar] [CrossRef] [PubMed]
- Sauter, G.H.; Munzing, W.; Von Ritter, C.; Paumgartner, G. Bile Acid Malabsorption as a Cause of Chronic Diarrhea Diagnostic Value of 7α-Hydroxy-4-Cholesten-3-One in Serum. Am. J. Dig. Dis. 1999, 44, 14–19. [Google Scholar] [CrossRef]
- Pattni, S.S.; Brydon, W.G.; Dew, T.; Johnston, I.M.; Nolan, J.D.; Srinivas, M.; Basumani, P.; Bardhan, K.D.; Walters, J.R.F. Fibroblast growth factor 19 in patients with bile acid diarrhoea: A prospective comparison of FGF19 serum assay and SeHCAT retention. Aliment. Pharmacol. Ther. 2013, 38, 967–976. [Google Scholar] [CrossRef] [PubMed]
- Vijayvargiya, P.; Camilleri, M.; Shin, A.; Saenger, A. Methods for Bile Acid Malabsorption in Clinical Practice. Clin. Gastroenterol. Hepatol. 2013, 11, 1232–1239. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Camilleri, M.; Nadeau, A.; Tremaine, W.J.; Lamsam, J.; Burton, D.; Odunsi, S.; Sweetser, S.; Singh, R. Measurement of serum 7α-hydroxy-4-cholesten-3-one (or 7αC4), a surrogate test for bile acid malabsorption in health, ileal disease and irritable bowel syndrome using liquid chromatography-tandem mass spectrometry. Neurogastroenterol. Motil. 2009, 21, 734-e43. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Battat, R.; Duijvestein, M.; Casteele, N.V.; Singh, S.; Dulai, P.S.; Valasek, M.A.; Mimms, L.; McFarland, J.; Hester, K.D.; Renshaw, M.; et al. Serum Concentrations of 7α-hydroxy-4-cholesten-3-one Are Associated with Bile Acid Diarrhea in Patients With Crohn’s Disease. Clin. Gastroenterol. Hepatol. 2018, 17, 2722–2730. [Google Scholar] [CrossRef]
- Sadowski, D.C.; Camilleri, M.; Chey, W.D.; Leontiadis, G.I.; Marshall, J.; Shaffer, E.A.; Tse, F.; Walters, J.R. Canadian Association of Gastroenterology Clinical Practice Guideline on the Management of Bile Acid Diarrhea. Clin. Gastroenterol. Hepatol. 2019, 18, 24–41.e4. [Google Scholar] [CrossRef] [Green Version]
- Orekoya, O.; McLaughlin, J.; Leitao, E.; Johns, W.; Lal, S.; Paine, P. Quantifying bile acid malabsorption helps predict response and tailor sequestrant therapy. Clin. Med. 2015, 15, 252–257. [Google Scholar] [CrossRef] [Green Version]
- Jackson, A.; Lalji, A.; Kabir, M.; Muls, A.; Gee, C.; Vyoral, S.; Shaw, C.; Andreyev, H.J.N. The efficacy of a low-fat diet to manage the symptoms of bile acid malabsorption—Outcomes in patients previously treated for cancer. Clin. Med. 2017, 17, 412–418. [Google Scholar] [CrossRef] [Green Version]
- Watson, L.; Lalji, A.; Bodla, S.; Muls, A.; Andreyev, H.J.N.; Shaw, C. Management of bile acid malabsorption using low-fat dietary interventions: A useful strategy applicable to some patients with diarrhoea-predominant irritable bowel syndrome? Clin. Med. 2015, 15, 536–540. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Camilleri, M.; Acosta, A.; Busciglio, I.; Boldingh, A.; Dyer, R.B.; Zinsmeister, A.R.; Lueke, A.; Gray, A.; Donato, L.J. Effect of colesevelam on faecal bile acids and bowel functions in diarrhoea-predominant irritable bowel syndrome. Aliment. Pharmacol. Ther. 2015, 41, 438–448. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Bañares, F.; Rosinach, M.; Piqueras, M.; Ruiz-Cerulla, A.; Modolell, I.; Zabana, Y.; Guardiola, J.; Esteve, M. Randomised clinical trial: Colestyramine vs. hydroxypropyl cellulose in patients with functional chronic watery diarrhoea. Aliment. Pharmacol. Ther. 2015, 41, 1132–1140. [Google Scholar] [CrossRef] [PubMed]
- Hiatt, J.G.; Shamsie, S.G.; Schectman, G. Discontinuation rates of cholesterol-lowering medications: Implications for primary care. Am. J. Manag. Care 1999, 5, 437–444. [Google Scholar]
- Sjöberg, B.G.; Straniero, S.; Angelin, B.; Rudling, M. Cholestyramine treatment of healthy humans rapidly induces transient hypertriglyceridemia when treatment is initiated. Am. J. Physiol. Metab. 2017, 313, E167–E174. [Google Scholar] [CrossRef]
- Jacobson, T.A.; Armani, A.; McKenney, J.M.; Guyton, J.R. Safety Considerations with Gastrointestinally Active Lipid-Lowering Drugs. Am. J. Cardiol. 2007, 99, S47–S55. [Google Scholar] [CrossRef]
- Knodel, L.C.; Talbert, R.L. Adverse Effects of Hypolipidaemic Drugs. Med. Toxicol. 1987, 2, 10–32. [Google Scholar] [CrossRef]
- Riaz, S.; John, S. Cholestyramine Resin; StatPearls Publishing: Treasure Island, FL, USA, 2021. [Google Scholar]
- Kamar, F.B.; McQuillan, R.F. Hyperchloremic Metabolic Acidosis due to Cholestyramine: A Case Report and Literature Review. Case Rep. Nephrol. 2015, 2015, 309791. [Google Scholar] [CrossRef]
- Eaves, E.R.; Korman, M.G. Cholestyramine induced hyperchloremic metabolic acidosis. Aust. N. Z. J. Med. 1984, 14, 670–672. [Google Scholar] [CrossRef]
- Davidson, M.H.; Dillon, M.A.; Gordon, B.; Jones, P.; Samuels, J.; Weiss, S.; Isaacsohn, J.; Toth, P.; Burke, S.K. Colesevelam Hydrochloride (Cholestagel). Arch. Intern. Med. 1999, 159, 1893–1900. [Google Scholar] [CrossRef] [Green Version]
- Lewis, S.J.; Heaton, K.W. Stool Form Scale as a Useful Guide to Intestinal Transit Time. Scand. J. Gastroenterol. 1997, 32, 920–924. [Google Scholar] [CrossRef]
- Beigel, F.; Teich, N.; Howaldt, S.; Lammert, F.; Maul, J.; Breiteneicher, S.; Rust, C.; Göke, B.; Brand, S.; Ochsenkühn, T. Colesevelam for the treatment of bile acid malabsorption-associated diarrhea in patients with Crohn's disease: A randomized, double-blind, placebo-controlled study. J. Crohn's Colitis 2014, 8, 1471–1479. [Google Scholar] [CrossRef] [PubMed]
- Walters, J.R.F.; Johnston, I.M.; Nolan, J.D.; Vassie, C.; Pruzanski, M.E.; Shapiro, D.A. The response of patients with bile acid diarrhoea to the farnesoid X receptor agonist obeticholic acid. Aliment. Pharmacol. Ther. 2014, 41, 54–64. [Google Scholar] [CrossRef] [PubMed]
- Mroz, M.S.; Keating, N.; Ward, J.B.; Sarker, R.; Amu, S.; Aviello, G.; Donowitz, M.; Fallon, P.; Keely, S. Farnesoid X receptor agonists attenuate colonic epithelial secretory function and prevent experimental diarrhoea in vivo. Gut 2013, 63, 808–817. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Camilleri, M.; Nord, S.L.; Burton, D.; Oduyebo, I.; Zhang, Y.; Chen, J.; Im, K.; Bhad, P.; Badman, M.K.; Sanders, D.S.; et al. Randomised clinical trial: Significant biochemical and colonic transit effects of the farnesoid X receptor agonist tropifexor in patients with primary bile acid diarrhoea. Aliment. Pharmacol. Ther. 2020, 52, 808–820. [Google Scholar] [CrossRef]
- Wei, W.; Wang, H.-F.; Zhang, Y.; Zhang, Y.-L.; Niu, B.-Y.; Yao, S.-K. Altered metabolism of bile acids correlates with clinical parameters and the gut microbiota in patients with diarrhea-predominant irritable bowel syndrome. World J. Gastroenterol. 2020, 26, 7153–7172. [Google Scholar] [CrossRef]
| Study | Year | Nation | Total Number of Patients with Ileal Disease, n | Patients with BAM, n (%) | Clinical Feature | Diagnostic Method | Treatment |
|---|---|---|---|---|---|---|---|
| Nyhlin et al. [68] | 1994 | UK | 51 | 34 (67%) | Diarrhea | SeHCAT retention < 10% | Cholestyramine |
| Smith et al. [69] | 2000 | UK | 81 | 60 (74%) | Diarrhea | SeHCAT retention < 10% | Antidiarrheals BAS |
| Borghede et al. [61] | 2011 | Denmark | 87 | 77 (88%) | Diarrhea | SeHCAT retention < 15% | Cholestyramine |
| Kurien et al. [70] | 2011 | UK | 47 | 40 (85%) | Diarrhea | SeHCAT retention < 10% | n/a |
| Lenicek et al. [63] | 2011 | Czech Republic | 232 | 112 (48%) | Inflammatory bowel disease-related | Serum C4 FGF19 | n/a |
| Gracie et al. [64] | 2012 | UK | 90 | 62 (69%) | Diarrhea | SeHCAT retention < 15% | n/a |
| Study | Year | Nation | Total Number of Patients, n | Patients with BAM, n (%) | Clinical Feature | Diagnostic Method | Treatment |
|---|---|---|---|---|---|---|---|
| Ford et al. [79] | 1992 | UK | 74 | 20 (27%) | Diarrhea | SeHCAT retention < 15% | Cholestyramine |
| Smith et al. [69] | 2000 | UK | 197 | 65 (33%) | IBS-D | SeHCAT retention < 10% | Antidiarrheals BAS |
| Borghede et al. [61] | 2011 | Denmark | 114 | 68 (60%) | Diarrhea | SeHCAT retention < 15% | Cholestyramine |
| Kurien et al. [70] | 2011 | UK | 102 | 36 (34%) | Diarrhea | SeHCAT retention < 10% | n/a |
| Gracie et al. [64] | 2012 | UK | 77 | 21 (27%) | IBS-D | SeHCAT retention < 15% | n/a |
| Vijayvargiya et al. [72] | 2020 | USA | 936 | 476 (51%) | Diarrhea | 48-h fecal BA excretion | Cholestyramine Colesevelam Colestipol |
| Study | Year | Nation | Total Number of Patients, n | Patients with BAM, n (%) | Clinical Feature | Diagnostic Method | Treatment |
|---|---|---|---|---|---|---|---|
| Ford et al. [79] | 1992 | UK | 30 | 24 (80%) | Cholecystectomy | SeHCAT retention < 15% | Cholestyramine |
| 11 | 4 (36%) | Vagotomy | |||||
| Ung et al. [85] | 2000 | Sweden | 27 | 12 (44%) | Collagenous colitis | SeHCAT retention < 10% | Cholestyramine Colestipol |
| Borghede et al. [61] | 2011 | Denmark | 36 | 31 (86%) | Cholecystectomy | SeHCAT retention < 15% | Cholestyramine |
| 12 | 4 (33%) | Microscopic colitis | |||||
| Kurien et al. [70] | 2011 | UK | 31 | 21 (68%) | Cholecystectomy | SeHCAT retention < 10% | n/a |
| 12 | 4 (33%) | Celiac disease | |||||
| 1 | 1 (100%) | Vagotomy | |||||
| 11 | 3 (27%) | Connective tissue disease | |||||
| 8 | 2 (25%) | Pancreatic insufficiency | |||||
| Gracie et al. [64] | 2012 | UK | 76 | 52 (68%) | Cholecystectomy | SeHCAT retention < 15% | n/a |
| 6 | 1 (17%) | Celiac disease | |||||
| 18 | 6 (33%) | Collagenous colitis | |||||
| 6 | 3 (50%) | Lymphocytic colitis | |||||
| Farahmandfar et al. [80] | 2012 | UK | 55 | 36 (65%) | Post-cholecystectomy diarrhea | SeHCAT | Cholestyramine |
| Appleby et al. [88] | 2019 | UK | 92 | 11 (12%) | NAFLD | Serum C4 FGF19 | n/a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marasco, G.; Cremon, C.; Barbaro, M.R.; Falangone, F.; Montanari, D.; Capuani, F.; Mastel, G.; Stanghellini, V.; Barbara, G. Pathophysiology and Clinical Management of Bile Acid Diarrhea. J. Clin. Med. 2022, 11, 3102. https://doi.org/10.3390/jcm11113102
Marasco G, Cremon C, Barbaro MR, Falangone F, Montanari D, Capuani F, Mastel G, Stanghellini V, Barbara G. Pathophysiology and Clinical Management of Bile Acid Diarrhea. Journal of Clinical Medicine. 2022; 11(11):3102. https://doi.org/10.3390/jcm11113102
Chicago/Turabian StyleMarasco, Giovanni, Cesare Cremon, Maria Raffaella Barbaro, Francesca Falangone, Davide Montanari, Federica Capuani, Giada Mastel, Vincenzo Stanghellini, and Giovanni Barbara. 2022. "Pathophysiology and Clinical Management of Bile Acid Diarrhea" Journal of Clinical Medicine 11, no. 11: 3102. https://doi.org/10.3390/jcm11113102
APA StyleMarasco, G., Cremon, C., Barbaro, M. R., Falangone, F., Montanari, D., Capuani, F., Mastel, G., Stanghellini, V., & Barbara, G. (2022). Pathophysiology and Clinical Management of Bile Acid Diarrhea. Journal of Clinical Medicine, 11(11), 3102. https://doi.org/10.3390/jcm11113102







