Human Alveolar Echinococcosis—A Neglected Zoonotic Disease Requiring Urgent Attention
Abstract
:1. Introduction
2. Life Cycle and Transmission of E. multilocularis
3. Pathogenesis and Clinical Progression of Disease
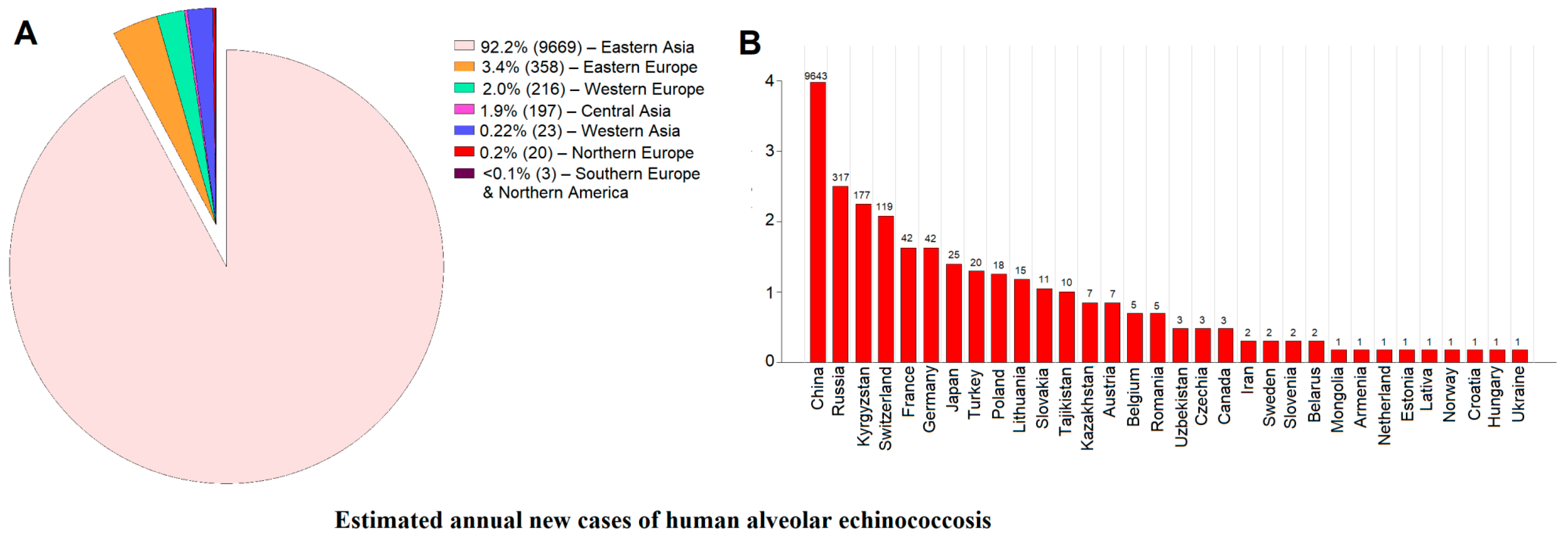
4. Recent Epidemiological Insights into the Incidence and Prevalence Trends of Human AE
5. Comprehensive Diagnosis of Disease Requires a Multimodal Approach
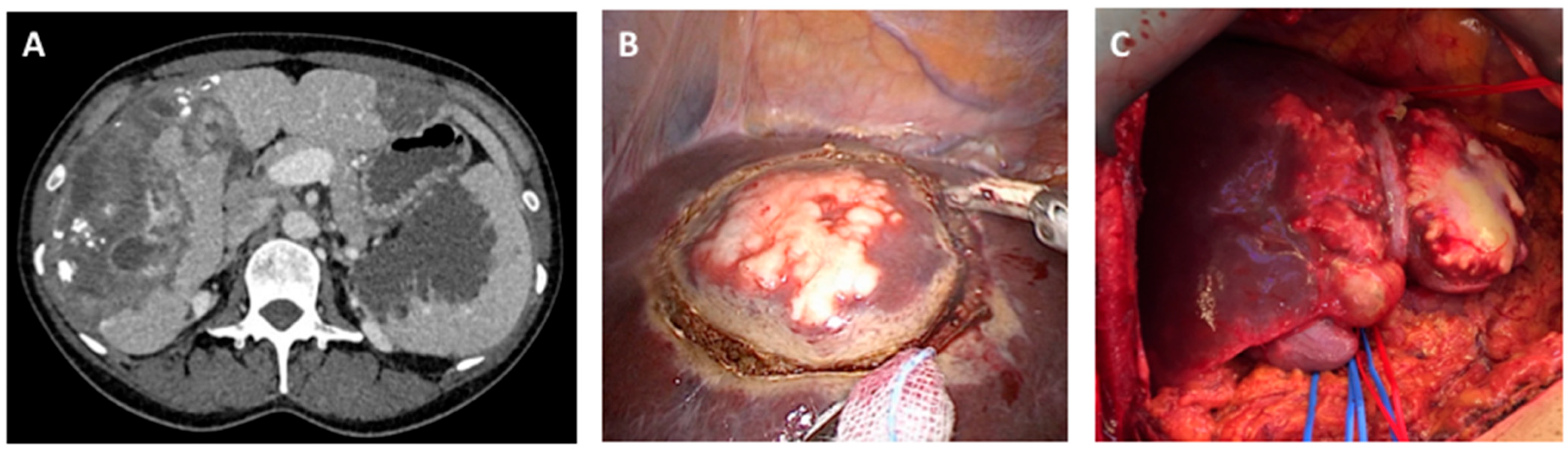
6. Clinical Management of Human Disease—Surgical and Medical Interventions
7. Insights into Host–Parasite Interactions at the Molecular Level—Implications for Novel Interventions
8. Concluding Remarks—Need to Strengthen Surveillance and Control of a Neglected Disease
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- World Health Organization (WHO). Echinococcosis. Available online: https://www.who.int/health-topics/echinococcosis#tab=tab_1 (accessed on 5 March 2024).
- Casulli, A. Recognising the substantial burden of neglected pandemics cystic and alveolar echinococcosis. Lancet Glob. Health 2020, 8, e470–e471. [Google Scholar] [CrossRef] [PubMed]
- Engels, D.; Zhou, X.-N. Neglected tropical diseases: An effective global response to local poverty-related disease priorities. Infect. Dis. Poverty 2020, 9, 9–17. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Dai, G.; Li, M.; Jia, W.; Guo, Z.; Lu, J. Prevalence of human alveolar echinococcosis in China: A systematic review and meta-analysis. BMC Public Health 2020, 20, 1105. [Google Scholar] [CrossRef] [PubMed]
- Deplazes, P.; Rinaldi, L.; Rojas, C.A.; Torgerson, P.; Harandi, M.F.; Romig, T.; Antolova, D.; Schurer, J.; Lahmar, S.; Cringoli, G. Global distribution of alveolar and cystic echinococcosis. Adv. Parasitol. 2017, 95, 315–493. [Google Scholar]
- Rossi, P.; Tamarozzi, F.; Galati, F.; Pozio, E.; Akhan, O.; Cretu, C.M.; Vutova, K.; Siles-Lucas, M.; Brunetti, E.; Casulli, A. The first meeting of the European Register of Cystic Echinococcosis (ERCE). Parasit. Vectors 2016, 9, 243. [Google Scholar] [CrossRef]
- Torgerson, P.R.; Devleesschauwer, B.; Praet, N.; Speybroeck, N.; Willingham, A.L.; Kasuga, F.; Rokni, M.B.; Zhou, X.-N.; Fèvre, E.M.; Sripa, B. World Health Organization estimates of the global and regional disease burden of 11 foodborne parasitic diseases, 2010: A data synthesis. PLoS Med. 2015, 12, e1001920. [Google Scholar] [CrossRef]
- Gottstein, B.; Stojkovic, M.; Vuitton, D.A.; Millon, L.; Marcinkute, A.; Deplazes, P. Threat of alveolar echinococcosis to public health—A challenge for Europe. Trends Parasitol. 2015, 31, 407–412. [Google Scholar] [CrossRef]
- Trotz-Williams, L.; Mercer, N.; Walters, J.; Wallace, D.; Gottstein, B.; Osterman-Lind, E.; Boggild, A.; Peregrine, A. Public health follow-up of suspected exposure to Echinococcus multilocularis in southwestern Ontario. Zoonoses Public Health 2017, 64, 460–467. [Google Scholar] [CrossRef]
- Bebezov, B.; Mamashev, N.; Umetaliev, T.; Ziadinov, I.; Craig, P.S.; Joekel, D.E.; Deplazes, P.; Grimm, F.; Torgerson, P.R. Intense focus of alveolar echinococcosis, South Kyrgyzstan. Emerg. Infect. Dis. 2018, 24, 1119–1122. [Google Scholar] [CrossRef]
- Gottstein, B.; Deplazes, P. Alveolar echinococcosis: What triggers emergence in North America, Central Europe and Asia? Curr. Opin. Infect. Dis. 2021, 34, 440–446. [Google Scholar] [CrossRef]
- Bresson-Hadni, S. Alveolar echinococcosis in Switzerland. Universimed. 2023. Available online: https://www.universimed.com/ch/article/gastroenterologie/alveolar-switzerland-265336 (accessed on 21 March 2024).
- Craig, P.; Hegglin, D.; Lightowlers, M.; Torgerson, P.R.; Wang, Q. Echinococcosis: Control and prevention. Adv. Parasitol. 2017, 96, 55–158. [Google Scholar] [PubMed]
- Budke, C.M.; Casulli, A.; Kern, P.; Vuitton, D.A. Cystic and alveolar echinococcosis: Successes and continuing challenges. PLoS Negl. Trop. Dis. 2017, 11, e0005477. [Google Scholar] [CrossRef] [PubMed]
- Bulakçı, M.; Kartal, M.G.; Yılmaz, S.; Yılmaz, E.; Yılmaz, R.; Şahin, D.; Aşık, M.; Erol, O.B. Multimodality imaging in diagnosis and management of alveolar echinococcosis: An update. Diagn. Interv. Radiol. 2016, 22, 247–256. [Google Scholar] [CrossRef] [PubMed]
- Torgerson, P.R.; Schweiger, A.; Deplazes, P.; Pohar, M.; Reichen, J.; Ammann, R.W.; Tarr, P.E.; Halkik, N.; Müllhaupt, B. Alveolar echinococcosis: From a deadly disease to a well-controlled infection. Relative survival and economic analysis in Switzerland over the last 35 years. J. Hepatol. 2008, 49, 72–77. [Google Scholar] [CrossRef]
- Kapel, C.; Torgerson, P.; Thompson, R.; Deplazes, P. Reproductive potential of Echinococcus multilocularis in experimentally infected foxes, dogs, raccoon dogs and cats. Int. J. Parasitol. 2006, 36, 79–86. [Google Scholar] [CrossRef]
- Romig, T.; Wassermann, M. Echinococcus species in wildlife. Int. J. Parasitol. Parasites Wildl. 2024, 23, 100913. [Google Scholar] [CrossRef]
- Howell, M.; Smyth, J. Maintenance and cultivation of Echinococcus species in vivo and in vitro. In Echinococcus and Hydatid Disease; Thompson, R.C.A., Lymbery, A.J., Eds.; CAB International: Wallingford, UK, 1995; pp. 201–232. [Google Scholar]
- Thompson, R.; Kapel, C.; Hobbs, R.; Deplazes, P. Comparative development of Echinococcus multilocularis in its definitive hosts. Parasitology 2006, 132, 709–716. [Google Scholar] [CrossRef]
- Corsini, M.; Geissbühler, U.; Howard, J.; Gottstein, B.; Spreng, D.; Frey, C. Clinical presentation, diagnosis, therapy and outcome of alveolar echinococcosis in dogs. Vet. Rec. 2015, 177, 569. [Google Scholar] [CrossRef]
- Tappe, D.; Zidowitz, S.; Demmer, P.; Kern, P.; Barth, T.F.; Frosch, M. Three-dimensional reconstruction of Echinococcus multilocularis larval growth in human hepatic tissue reveals complex growth patterns. Am. J. Trop. Med. Hyg. 2010, 82, 126–127. [Google Scholar] [CrossRef]
- Reinehr, M.; Micheloud, C.; Grimm, F.; Kronenberg, P.A.; Grimm, J.; Beck, A.; Nell, J.; Zu Schwabedissen, C.M.; Furrer, E.; Müllhaupt, B. Pathology of echinococcosis: A morphologic and immunohistochemical study on 138 specimens with focus on the differential diagnosis between cystic and alveolar echinococcosis. Am. J. Surg. Pathol. 2020, 44, 43–54. [Google Scholar] [CrossRef]
- Gottstein, B.; Soboslay, P.; Ortona, E.; Wang, J.; Siracusano, A.; Vuitton, D. Immunology of alveolar and cystic echinococcosis (AE and CE). Adv. Parasitol. 2017, 96, 1–54. [Google Scholar] [PubMed]
- Lundström-Stadelmann, B.; Rostami, A.; Frey, C.F.; Torgerson, P.R.; Riahi, S.M.; Bagheri, K.; Kaethner, M.; Lachenmayer, A.; Beldi, G.; Gasser, R.B.; et al. Human alveolar echinococcosis—Global, regional and national annual incidence and prevalence rates. Clin. Microbiol. Infect. 2025, in press. [Google Scholar] [CrossRef] [PubMed]
- Torgerson, P.R.; Keller, K.; Magnotta, M.; Ragland, N. The global burden of alveolar echinococcosis. PLoS Negl. Trop. Dis. 2010, 4, e722. [Google Scholar] [CrossRef] [PubMed]
- Conraths, F.J.; Probst, C.; Possenti, A.; Boufana, B.; Saulle, R.; La Torre, G.; Busani, L.; Casulli, A. Potential risk factors associated with human alveolar echinococcosis: Systematic review and meta-analysis. PLoS Negl. Trop. Dis. 2017, 11, e0005801. [Google Scholar] [CrossRef]
- Schmidberger, J.; Weimer, H.; Schlingeloff, P.; Kratzer, W.; Grüner, B.; Echinococcosis Working Group, Ulm. Health-related quality of life in patients with alveolar echinococcosis: A cross-sectional study. Infection 2019, 47, 67–75. [Google Scholar] [CrossRef]
- Nikendei, C.; Greinacher, A.; Berkunova, A.; Junghanss, T.; Stojkovic, M. Psychological burden and resilience factors in patients with Alveolar echinococcosis—A cross-sectional study. PLoS Negl. Trop. Dis. 2019, 13, e0007082. [Google Scholar] [CrossRef]
- Brunetti, E.; Kern, P.; Vuitton, D.A. Expert consensus for the diagnosis and treatment of cystic and alveolar echinococcosis in humans. Acta Trop. 2010, 114, 1–16. [Google Scholar] [CrossRef]
- Kern, P.; Da Silva, A.M.; Akhan, O.; Müllhaupt, B.; Vizcaychipi, K.; Budke, C.; Vuitton, D. The echinococcoses: Diagnosis, clinical management and burden of disease. Adv. Parasitol. 2017, 96, 259–369. [Google Scholar]
- Wen, H.; Vuitton, L.; Tuxun, T.; Li, J.; Vuitton, D.A.; Zhang, W.; McManus, D.P. Echinococcosis: Advances in the 21st century. Clin. Microbiol. Rev. 2019, 32, e00075-18. [Google Scholar] [CrossRef]
- Lightowlers, M.W.; Gasser, R.B.; Hemphill, A.; Romig, T.; Tamarozzi, F.; Deplazes, P.; Torgerson, P.R.; Garcia, H.H.; Kern, P. Advances in the treatment, diagnosis, control and scientific understanding of taeniid cestode parasite infections over the past 50 years. Int. J. Parasitol. 2021, 51, 1167–1192. [Google Scholar] [CrossRef]
- Kronenberg, P.A.; Deibel, A.; Gottstein, B.; Grimm, F.; Müllhaupt, B.; Meyer zu Schwabedissen, C.; Aitbaev, S.; Omorov, R.A.; Abdykerimov, K.K.; Minbaeva, G. Serological assays for alveolar and cystic echinococcosis—A comparative multi-test study in Switzerland and Kyrgyzstan. Pathogens 2022, 11, 518. [Google Scholar] [CrossRef] [PubMed]
- Díaz, Á.; Barrios, A.A.; Grezzi, L.; Mouhape, C.; Jenkins, S.J.; Allen, J.E.; Casaravilla, C. Immunology of a unique biological structure: The Echinococcus laminated layer. Protein Cell 2023, 14, 87–104. [Google Scholar] [CrossRef] [PubMed]
- Kronenberg, P.A.; Reinehr, M.; Eichenberger, R.M.; Hasler, S.; Laurimäe, T.; Weber, A.; Deibel, A.; Müllhaupt, B.; Gottstein, B.; Müller, N. Monoclonal antibody-based localization of major diagnostic antigens in metacestode tissue, excretory/secretory products, and extracellular vesicles of Echinococcus species. Front. Cell. Infect. Microbiol. 2023, 13, 1162530. [Google Scholar] [CrossRef] [PubMed]
- Barth, T.F.; Herrmann, T.S.; Tappe, D.; Stark, L.; Grüner, B.; Buttenschoen, K.; Hillenbrand, A.; Juchems, M.; Henne-Bruns, D.; Kern, P. Sensitive and specific immunohistochemical diagnosis of human alveolar echinococcosis with the monoclonal antibody Em2G11. PLoS Negl. Trop. Dis. 2012, 6, e1877. [Google Scholar] [CrossRef]
- Salm, L.; Lachenmayer, A.; Perrodin, S.F.; Candinas, D.; Beldi, G. Surgical treatment strategies for hepatic alveolar echinococcosis. Food Waterborne Parasitol. 2019, 15, e00050. [Google Scholar] [CrossRef]
- Beldi, G.; Vuitton, D.; Lachenmayer, A.; Heyd, B.; Dufour, J.-F.; Richou, C.; Candinas, D.; Bresson-Hadni, S. Is ex vivo liver resection and autotransplantation a valid alternative treatment for end-stage hepatic alveolar echinococcosis in Europe? J. Hepatol. 2019, 70, 1030–1031. [Google Scholar] [CrossRef]
- Lundström-Stadelmann, B.; Rufener, R.; Ritler, D.; Zurbriggen, R.; Hemphill, A. The importance of being parasiticidal… An update on drug development for the treatment of alveolar echinococcosis. Food Waterborne Parasitol. 2019, 15, e00040. [Google Scholar] [CrossRef]
- Lacey, E. Mode of action of benzimidazoles. Parasitol. Today 1990, 6, 112–115. [Google Scholar] [CrossRef]
- Kern, P. Echinococcus granulosus infection: Clinical presentation, medical treatment and outcome. Langenbeck’s Arch. Surg. 2003, 388, 413–420. [Google Scholar] [CrossRef]
- Brehm, K.; Koziol, U. On the importance of targeting parasite stem cells in anti-echinococcosis drug development. Parasite 2014, 21, 72. [Google Scholar] [CrossRef]
- Grüner, B.; Kern, P.; Mayer, B.; Gräter, T.; Hillenbrand, A.; Barth, T.E.; Muche, R.; Henne-Bruns, D.; Kratzer, W.; Kern, P. Comprehensive diagnosis and treatment of alveolar echinococcosis: A single-center, long-term observational study of 312 patients in Germany. GMS Infect. Dis. 2017, 5, Doc01. [Google Scholar] [PubMed]
- Eberhardt, N.; Peters, L.; Kapp-Schwoerer, S.; Beer, M.; Beer, A.J.; Grüner, B.; Thaiss, W.M. 18F-FDG-PET/MR in alveolar echinococcosis: Multiparametric imaging in a real-world setting. Pathogens 2022, 11, 348. [Google Scholar] [CrossRef] [PubMed]
- Peters, L.; Jiang, W.; Eberhardt, N.; Hagemann, J.B.; Grüner, B.; Tappe, D. 18FDG-PET/CT-scans and biomarker levels predicting clinical outcome in patients with alveolar echinococcosis—A single-center cohort study with 179 patients. Pathogens 2023, 12, 1041. [Google Scholar] [CrossRef]
- Schweizer, M.; Schmidberger, J.; Schlingeloff, P.; Kratzer, W. Contrast-enhanced ultrasound (CEUS) in patients with metastasis-like hepatic alveolar echinococcosis: A cohort study. J. Ultrasound 2023, 26, 129–136. [Google Scholar] [CrossRef]
- Gloor, S.; Jiang, W.; Maurer, M.H.; Gottstein, B.; Oberli, A.; Hagemann, J.B.; Hotz, J.F.; Candinas, D.; Lachenmayer, A.; Grüner, B. The trajectory of anti-recEm18 antibody levels determines follow-up after curative resection of hepatic alveolar echinococcosis. HPB 2024, 26, 224–233. [Google Scholar] [CrossRef]
- Lachenmayer, A.; Gebbers, D.; Gottstein, B.; Candinas, D.; Beldi, G. Elevated incidence of alveolar echinococcosis in immunocompromised patients. Food Waterborne Parasitol. 2019, 16, e00060. [Google Scholar] [CrossRef]
- Tsai, I.J.; Zarowiecki, M.; Holroyd, N.; Garciarrubio, A.; Sanchez-Flores, A.; Brooks, K.L.; Tracey, A.; Bobes, R.J.; Fragoso, G.; Sciutto, E. The genomes of four tapeworm species reveal adaptations to parasitism. Nature 2013, 496, 57–63. [Google Scholar] [CrossRef]
- Herz, M.; Zarowiecki, M.; Wessels, L.; Pätzel, K.; Herrmann, R.; Braun, C.; Holroyd, N.; Huckvale, T.; Bergmann, M.; Spiliotis, M. Genome-wide transcriptome analysis of Echinococcus multilocularis larvae and germinative cell cultures reveals genes involved in parasite stem cell function. Front. Cell. Infect. Microbiol. 2024, 14, 1335946. [Google Scholar] [CrossRef]
- Belton, J.-M.; McCord, R.P.; Gibcus, J.H.; Naumova, N.; Zhan, Y.; Dekker, J. Hi–C: A comprehensive technique to capture the conformation of genomes. Methods 2012, 58, 268–276. [Google Scholar] [CrossRef]
- Rao, S.S.; Huntley, M.H.; Durand, N.C.; Stamenova, E.K.; Bochkov, I.D.; Robinson, J.T.; Sanborn, A.L.; Machol, I.; Omer, A.D.; Lander, E.S. A 3D map of the human genome at kilobase resolution reveals principles of chromatin looping. Cell 2014, 159, 1665–1680. [Google Scholar] [CrossRef]
- Young, N.D.; Stroehlein, A.J.; Kinkar, L.; Wang, T.; Sohn, W.-M.; Chang, B.C.; Kaur, P.; Weisz, D.; Dudchenko, O.; Aiden, E.L. High-quality reference genome for Clonorchis sinensis. Genomics 2021, 113, 1605–1615. [Google Scholar] [CrossRef] [PubMed]
- Stroehlein, A.J.; Korhonen, P.K.; Lee, V.V.; Ralph, S.A.; Mentink-Kane, M.; You, H.; McManus, D.P.; Tchuenté, L.-A.T.; Stothard, J.R.; Kaur, P. Chromosome-level genome of Schistosoma haematobium underpins genome-wide explorations of molecular variation. PLoS Pathog. 2022, 18, e1010288. [Google Scholar] [CrossRef]
- Romig, T.; Deplazes, P.; Jenkins, D.; Giraudoux, P.; Massolo, A.; Craig, P.S.; Wassermann, M.; Takahashi, K.; De La Rue, M. Ecology and life cycle patterns of Echinococcus species. Adv. Parasitol. 2017, 95, 213–314. [Google Scholar]
- Brehm, K. The role of evolutionarily conserved signalling systems in Echinococcus multilocularis development and host-parasite interaction. Med. Microbiol. Immunol. 2010, 199, 247–259. [Google Scholar] [CrossRef]
- Hemphill, A.; Stadelmann, B.; Scholl, S.; Müller, J.; Spiliotis, M.; Müller, N.; Gottstein, B.; Siles-Lucas, M. Echinococcus metacestodes as laboratory models for the screening of drugs against cestodes and trematodes. Parasitology 2010, 137, 569–587. [Google Scholar] [CrossRef]
- Brehm, K.; Koziol, U. Echinococcus–host interactions at cellular and molecular levels. Adv. Parasitol. 2017, 95, 147–212. [Google Scholar]
- Kaethner, M.; Preza, M.; Kaempfer, T.; Zumstein, P.; Tamponi, C.; Varcasia, A.; Hemphill, A.; Brehm, K.; Lundström-Stadelmann, B. Establishment and application of unbiased in vitro drug screening assays for the identification of compounds against Echinococcus granulosus sensu stricto. PLoS Negl. Trop. Dis. 2023, 17, e0011343. [Google Scholar] [CrossRef]
- Campos, T.L.; Korhonen, P.K.; Hofmann, A.; Gasser, R.B.; Young, N.D. Harnessing model organism genomics to underpin the machine learning-based prediction of essential genes in eukaryotes—Biotechnological implications. Biotechnol. Adv. 2022, 54, 107822. [Google Scholar] [CrossRef]
- Spiliotis, M.; Mizukami, C.; Oku, Y.; Kiss, F.; Brehm, K.; Gottstein, B. Echinococcus multilocularis primary cells: Improved isolation, small-scale cultivation and RNA interference. Mol. Biochem. Parasitol. 2010, 174, 83–87. [Google Scholar] [CrossRef]
- Mizukami, C.; Spiliotis, M.; Gottstein, B.; Yagi, K.; Katakura, K.; Oku, Y. Gene silencing in Echinococcus multilocularis protoscoleces using RNA interference. Parasitol. Int. 2010, 59, 647–652. [Google Scholar] [CrossRef]
- Arunsan, P.; Ittiprasert, W.; Smout, M.J.; Cochran, C.J.; Mann, V.H.; Chaiyadet, S.; Karinshak, S.E.; Sripa, B.; Young, N.D.; Sotillo, J. Programmed knockout mutation of liver fluke granulin attenuates virulence of infection-induced hepatobiliary morbidity. eLife 2019, 8, e41463. [Google Scholar] [CrossRef] [PubMed]
- Arunsan, P.; Chaidee, A.; Cochran, C.J.; Mann, V.H.; Tanno, T.; Kumkhaek, C.; Smout, M.J.; Karinshak, S.E.; Rodpai, R.; Sotillo, J. Liver fluke granulin promotes extracellular vesicle-mediated crosstalk and cellular microenvironment conducive to cholangiocarcinoma. Neoplasia 2020, 22, 203–216. [Google Scholar] [CrossRef] [PubMed]
- You, H.; Mayer, J.U.; Johnston, R.L.; Sivakumaran, H.; Ranasinghe, S.; Rivera, V.; Kondrashova, O.; Koufariotis, L.T.; Du, X.; Driguez, P. CRISPR/Cas9-mediated genome editing of Schistosoma mansoni acetylcholinesterase. FASEB J. 2021, 35, e21205. [Google Scholar] [CrossRef] [PubMed]
- Chaiyadet, S.; Tangkawattana, S.; Smout, M.J.; Ittiprasert, W.; Mann, V.H.; Deenonpoe, R.; Arunsan, P.; Loukas, A.; Brindley, P.J.; Laha, T. Knockout of liver fluke granulin, Ov-grn-1, impedes malignant transformation during chronic infection with Opisthorchis viverrini. PLoS Pathog. 2022, 18, e1010839. [Google Scholar] [CrossRef] [PubMed]
- Hagen, J.; Young, N.D.; Every, A.L.; Pagel, C.N.; Schnoeller, C.; Scheerlinck, J.-P.Y.; Gasser, R.B.; Kalinna, B.H. Omega-1 knockdown in Schistosoma mansoni eggs by lentivirus transduction reduces granuloma size in vivo. Nat. Commun. 2014, 5, 5375. [Google Scholar] [CrossRef]
- Hagen, J.; Scheerlinck, J.-P.Y.; Young, N.D.; Gasser, R.B.; Kalinna, B.H. Prospects for vector-based gene silencing to explore immunobiological features of schistosoma mansoni. Adv. Parasitol. 2015, 88, 85–122. [Google Scholar]
- Hagen, J.; Scheerlinck, J.-P.Y.; Gasser, R.B. Knocking down schistosomes—Promise for lentiviral transduction in parasites. Trends Parasitol. 2015, 31, 324–332. [Google Scholar] [CrossRef]
- Du, X.; McManus, D.P.; French, J.D.; Jones, M.K.; You, H. CRISPR/Cas9: A new tool for the study and control of helminth parasites. BioEssays 2021, 43, e2000185. [Google Scholar] [CrossRef]
- Du, X.; McManus, D.P.; French, J.D.; Sivakumaran, H.; Johnston, R.L.; Kondrashova, O.; Fogarty, C.E.; Jones, M.K.; You, H. Lentiviral Transduction-based CRISPR/Cas9 Editing of Schistosoma mansoni Acetylcholinesterase. Curr. Genom. 2023, 24, 155–170. [Google Scholar] [CrossRef]
- Rufener, R.; Dick, L.; D’Ascoli, L.; Ritler, D.; Hizem, A.; Wells, T.N.; Hemphill, A.; Lundström-Stadelmann, B. Repurposing of an old drug: In vitro and in vivo efficacies of buparvaquone against Echinococcus multilocularis. Int. J. Parasitol. Drugs Drug Resist. 2018, 8, 440–450. [Google Scholar] [CrossRef]
- Stadelmann, B.; Scholl, S.; Müller, J.; Hemphill, A. Application of an in vitro drug screening assay based on the release of phosphoglucose isomerase to determine the structure–activity relationship of thiazolides against Echinococcus multilocularis metacestodes. J. Antimicrob. Chemother. 2010, 65, 512–519. [Google Scholar] [CrossRef]
- Stadelmann, B.; Aeschbacher, D.; Huber, C.; Spiliotis, M.; Müller, J.; Hemphill, A. Profound activity of the anti-cancer drug bortezomib against Echinococcus multilocularis metacestodes identifies the proteasome as a novel drug target for cestodes. PLoS Negl. Trop. Dis. 2014, 8, e3352. [Google Scholar] [CrossRef] [PubMed]
- Stadelmann, B.; Rufener, R.; Aeschbacher, D.; Spiliotis, M.; Gottstein, B.; Hemphill, A. Screening of the open source malaria box reveals an early lead compound for the treatment of alveolar echinococcosis. PLoS Negl. Trop. Dis. 2016, 10, e0004535. [Google Scholar] [CrossRef] [PubMed]
- Kaethner, M.; Rennar, G.; Gallinger, T.; Kämpfer, T.; Hemphill, A.; Mäder, P.; Luque-Gómez, A.; Schlitzer, M.; Lundström-Stadelmann, B. In vitro activities of dithiocarbamate derivatives against Echinococcus multilocularis metacestode vesicles. Trop. Med. Infect. Dis. 2023, 8, 517. [Google Scholar] [CrossRef] [PubMed]
- Hemer, S.; Brehm, K. In vitro efficacy of the anticancer drug imatinib on Echinococcus multilocularis larvae. Int. J. Antimicrob. Agents 2012, 40, 458–462. [Google Scholar] [CrossRef]
- Hemer, S.; Konrad, C.; Spiliotis, M.; Koziol, U.; Schaack, D.; Förster, S.; Gelmedin, V.; Stadelmann, B.; Dandekar, T.; Hemphill, A. Host insulin stimulates Echinococcus multilocularis insulin signalling pathways and larval development. BMC Biol. 2014, 12, 5. [Google Scholar] [CrossRef]
- Ritler, D.; Rufener, R.; Sager, H.; Bouvier, J.; Hemphill, A.; Lundström-Stadelmann, B. Development of a movement-based in vitro screening assay for the identification of new anti-cestodal compounds. PLoS Negl. Trop. Dis. 2017, 11, e0005618. [Google Scholar] [CrossRef]
- Rufener, R.; Ritler, D.; Zielinski, J.; Dick, L.; da Silva, E.T.; da Silva Araujo, A.; Joekel, D.E.; Czock, D.; Goepfert, C.; Moraes, A.M. Activity of mefloquine and mefloquine derivatives against Echinococcus multilocularis. Int. J. Parasitol. Drugs Drug Resist. 2018, 8, 331–340. [Google Scholar] [CrossRef]
- Chaudhry, S.; Zurbriggen, R.; Preza, M.; Kämpfer, T.; Kaethner, M.; Memedovski, R.; Scorrano, N.; Hemphill, A.; Doggett, J.S.; Lundström-Stadelmann, B. Dual inhibition of the Echinococcus multilocularis energy metabolism. Front. Vet. Sci. 2022, 9, 981664. [Google Scholar] [CrossRef]
- Memedovski, R.; Preza, M.; Müller, J.; Kämpfer, T.; Rufener, R.; de Souza, M.V.N.; da Silva, E.T.; de Andrade, G.F.; Braga, S.; Uldry, A.-C. Investigation of the mechanism of action of mefloquine and derivatives against the parasite Echinococcus multilocularis. Int. J. Parasitol. Drugs Drug Resist. 2023, 21, 114–124. [Google Scholar] [CrossRef]
- Karpstein, T.; Chaudhry, S.; Bresson-Hadni, S.; Hayoz, M.; Boubaker, G.; Hemphill, A.; Rufener, R.; Kaethner, M.; Schindler, I.; Aebi, Y. Maca against echinococcosis?— A reverse approach from patient to in vitro testing. Pathogens 2021, 10, 1335. [Google Scholar] [CrossRef] [PubMed]
- Weingartner, M.; Stücheli, S.; Jebbawi, F.; Gottstein, B.; Beldi, G.; Lundström-Stadelmann, B.; Wang, J.; Odermatt, A. Albendazole reduces hepatic inflammation and endoplasmic reticulum-stress in a mouse model of chronic Echinococcus multilocularis infection. PLoS Negl. Trop. Dis. 2022, 16, e0009192. [Google Scholar] [CrossRef] [PubMed]
- Andrews, K.T.; Fisher, G.; Skinner-Adams, T.S. Drug repurposing and human parasitic protozoan diseases. Int. J. Parasitol. Drugs Drug Resist. 2014, 4, 95–111. [Google Scholar] [CrossRef] [PubMed]
- Panic, G.; Duthaler, U.; Speich, B.; Keiser, J. Repurposing drugs for the treatment and control of helminth infections. Int. J. Parasitol. Drugs Drug Resist. 2014, 4, 185–200. [Google Scholar] [CrossRef]
- Hernandez, H.W.; Soeung, M.; Zorn, K.M.; Ashoura, N.; Mottin, M.; Andrade, C.H.; Caffrey, C.R.; de Siqueira-Neto, J.L.; Ekins, S. High throughput and computational repurposing for neglected diseases. Pharm. Res. 2018, 36, 27. [Google Scholar] [CrossRef]
- Reuter, S.; Buck, A.; Grebe, O.; Nüssle-Kügele, K.; Kern, P.; Manfras, B.J. Salvage treatment with amphotericin B in progressive human alveolar echinococcosis. Antimicrob. Agents Chemother. 2003, 47, 3586–3591. [Google Scholar] [CrossRef]
- Reuter, S.; Merkle, M.; Brehm, K.; Kern, P.; Manfras, B. Effect of amphotericin B on larval growth of Echinococcus multilocularis. Antimicrob. Agents Chemother. 2003, 47, 620–625. [Google Scholar] [CrossRef]
- Reuter, S.; Manfras, B.; Merkle, M.; Härter, G.; Kern, P. In vitro activities of itraconazole, methiazole, and nitazoxanide versus Echinococcus multilocularis larvae. Antimicrob. Agents Chemother. 2006, 50, 2966–2970. [Google Scholar] [CrossRef]
- Stettler, M.; Fink, R.; Walker, M.; Gottstein, B.; Geary, T.G.; Rossignol, J.F.; Hemphill, A. In vitro parasiticidal effect of nitazoxanide against Echinococcus multilocularis metacestodes. Antimicrob. Agents Chemother. 2003, 47, 467–474. [Google Scholar] [CrossRef]
- Lundström-Stadelmann, B.; Rufener, R.; Hemphill, A. Drug repurposing applied: Activity of the anti-malarial mefloquine against Echinococcus multilocularis. Int. J. Parasitol. Drugs Drug Resist. 2020, 13, 121–129. [Google Scholar] [CrossRef]
- Albani, C.M.; Denegri, G.M.; Elissondo, M.C. Effect of different terpene-containing essential oils on the proliferation of Echinococcus granulosus larval cells. Interdiscip. Perspect. Infect. Dis. 2014, 2014, 746931. [Google Scholar] [CrossRef] [PubMed]
- Albani, C.M.; Pensel, P.E.; Elissondo, N.; Gambino, G.; Elissondo, M.C. In vivo activity of albendazole in combination with thymol against Echinococcus multilocularis. Vet. Parasitol. 2015, 212, 193–199. [Google Scholar] [CrossRef] [PubMed]
- Yuan, M.; Luo, Y.; Xin, Q.; Gao, H.; Zhang, G.; Jing, T. Efficacy of osthole for Echinococcus granulosus in vitro and Echinococcus multilocularis in vivo. Vet. Parasitol. 2016, 226, 38–43. [Google Scholar] [CrossRef] [PubMed]
- Hizem, A.; Lundström-Stadelmann, B.; M’rad, S.; Souiai, S.; Jannet, H.B.; Flamini, G.; Ascrizzi, R.; Ghedira, K.; Babba, H.; Hemphill, A. Activity of Thymus capitatus essential oil components against in vitro cultured Echinococcus multilocularis metacestodes and germinal layer cells. Parasitology 2019, 146, 956–967. [Google Scholar] [CrossRef]
- Xin, Q.; Yuan, M.; Li, H.; Lu, J.; Song, X.; Jing, T. In vitro efficacy of ampelopsin against Echinococcus granulosus and Echinococcus multilocularis. J. Vet. Med. Sci. 2019, 81, 1853–1858. [Google Scholar] [CrossRef]
- Schweiger, A.; Ammann, R.W.; Candinas, D.; Clavien, P.-A.; Eckert, J.; Gottstein, B.; Halkic, N.; Muellhaupt, B.; Prinz, B.M.; Reichen, J. Human alveolar echinococcosis after fox population increase, Switzerland. Emerg. Infect. Dis. 2007, 13, 878–882. [Google Scholar] [CrossRef]
- Deplazes, P.; Hegglin, D.; Gloor, S.; Romig, T. Wilderness in the city: The urbanization of Echinococcus multilocularis. Trends Parasitol. 2004, 20, 77–84. [Google Scholar] [CrossRef]
- Catalano, S.; Lejeune, M.; Liccioli, S.; Verocai, G.G.; Gesy, K.M.; Jenkins, E.J.; Kutz, S.J.; Fuentealba, C.; Duignan, P.J.; Massolo, A. Echinococcus multilocularis in urban coyotes, Alberta, Canada. Emerg. Infect. Dis. 2012, 18, 1625–1628. [Google Scholar] [CrossRef]
- Hegglin, D.; Deplazes, P. Control of Echinococcus multilocularis: Strategies, feasibility and cost–benefit analyses. Int. J. Parasitol. 2013, 43, 327–337. [Google Scholar] [CrossRef]
- Wenker, C.; Hoby, S.; Wyss, F.; Mengiardi, B.; Vögtli, R.; Posthaus, H.; Deplazes, P.; Gottstein, B. Alveolar echinococcosis in western lowland gorillas (Gorilla gorilla gorilla): Albendazole was not able to stop progression of the disease. J. Zoo Wildl. Med. 2019, 50, 243–253. [Google Scholar]
- Meyer, A.; Olias, P.; Schüpbach, G.; Henzi, M.; Barmettler, T.; Hentrich, B.; Gottstein, B.; Frey, C.F. Combined cross-sectional and case-control study on Echinococcus multilocularis infection in pigs in Switzerland. Vet. Parasitol. 2020, 277, 100031. [Google Scholar] [CrossRef] [PubMed]
- Fu, M.; Han, S.; Xue, C.; Wang, X.; Liu, B.; Wang, Y.; Wang, L.; Wei, S.; Cui, X.; Zhang, T. Contribution to the echinococcosis control programme in China by NIPD-CTDR. Adv. Parasitol. 2020, 110, 107–144. [Google Scholar] [PubMed]
- Paternoster, G.; Boo, G.; Wang, C.; Minbaeva, G.; Usubalieva, J.; Raimkulov, K.M.; Zhoroev, A.; Abdykerimov, K.K.; Kronenberg, P.A.; Müllhaupt, B. Epidemic cystic and alveolar echinococcosis in Kyrgyzstan: An analysis of national surveillance data. Lancet Glob. Health 2020, 8, e603–e611. [Google Scholar] [CrossRef] [PubMed]
- Budke, C.M.; Jiamin, Q.; Craig, P.S.; Torgerson, P.R. Modeling the transmission of Echinococcus granulosus and Echinococcus multilocularis in dogs for a high endemic region of the Tibetan plateau. Int. J. Parasitol. 2005, 35, 163–170. [Google Scholar] [CrossRef]
- Ziadinov, I.; Mathis, A.; Trachsel, D.; Rysmukhambetova, A.; Abdyjaparov, T.; Kuttubaev, O.; Deplazes, P.; Torgerson, P.R. Canine echinococcosis in Kyrgyzstan: Using prevalence data adjusted for measurement error to develop transmission dynamics models. Int. J. Parasitol. 2008, 38, 1179–1190. [Google Scholar] [CrossRef]
- Dyachenko, V.; Pantchev, N.; Gawlowska, S.; Vrhovec, M.G.; Bauer, C. Echinococcus multilocularis infections in domestic dogs and cats from Germany and other European countries. Vet. Parasitol. 2008, 157, 244–253. [Google Scholar] [CrossRef]
- Hegglin, D.; Ward, P.I.; Deplazes, P. Anthelmintic baiting of foxes against urban contamination with Echinococcus multilocularis. Emerg. Infect. Dis. 2003, 9, 1266–1272. [Google Scholar] [CrossRef]
- Frey, C.F.; Oakley, J.R.; Lobanov, V.A.; Marreros, N.; Schurer, J.M.; Lalonde, L.F. A novel protocol to isolate, detect and differentiate taeniid eggs in leafy greens and berries using real-time PCR with melting curve analysis. Parasites Vectors 2019, 12, 590. [Google Scholar] [CrossRef]
- Hemphill, A.; Stadelmann, B.; Rufener, R.; Spiliotis, M.; Boubaker, G.; Müller, J.; Müller, N.; Gorgas, D.; Gottstein, B. Treatment of echinococcosis: Albendazole and mebendazole—What else? Parasite 2014, 21, 70. [Google Scholar] [CrossRef]
- Ritler, D.; Rufener, R.; Li, J.V.; Kämpfer, U.; Müller, J.; Bühr, C.; Schürch, S.; Lundström-Stadelmann, B. In vitro metabolomic footprint of the Echinococcus multilocularis metacestode. Sci. Rep. 2019, 9, 19438. [Google Scholar] [CrossRef]
- Müller, J.; Preza, M.; Kaethner, M.; Rufener, R.; Braga, S.; Uldry, A.-C.; Heller, M.; Lundström-Stadelmann, B. Targeted and non-targeted proteomics to characterize the parasite proteins of Echinococcus multilocularis metacestodes. Front. Cell. Infect. Microbiol. 2023, 13, 1170763. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rostami, A.; Lundström-Stadelmann, B.; Frey, C.F.; Beldi, G.; Lachenmayer, A.; Chang, B.C.H.; Norouzian, M.M.; Hemphill, A.; Gasser, R.B. Human Alveolar Echinococcosis—A Neglected Zoonotic Disease Requiring Urgent Attention. Int. J. Mol. Sci. 2025, 26, 2784. https://doi.org/10.3390/ijms26062784
Rostami A, Lundström-Stadelmann B, Frey CF, Beldi G, Lachenmayer A, Chang BCH, Norouzian MM, Hemphill A, Gasser RB. Human Alveolar Echinococcosis—A Neglected Zoonotic Disease Requiring Urgent Attention. International Journal of Molecular Sciences. 2025; 26(6):2784. https://doi.org/10.3390/ijms26062784
Chicago/Turabian StyleRostami, Ali, Britta Lundström-Stadelmann, Caroline F. Frey, Guido Beldi, Anja Lachenmayer, Bill C. H. Chang, Mohammad Mobin Norouzian, Andrew Hemphill, and Robin B. Gasser. 2025. "Human Alveolar Echinococcosis—A Neglected Zoonotic Disease Requiring Urgent Attention" International Journal of Molecular Sciences 26, no. 6: 2784. https://doi.org/10.3390/ijms26062784
APA StyleRostami, A., Lundström-Stadelmann, B., Frey, C. F., Beldi, G., Lachenmayer, A., Chang, B. C. H., Norouzian, M. M., Hemphill, A., & Gasser, R. B. (2025). Human Alveolar Echinococcosis—A Neglected Zoonotic Disease Requiring Urgent Attention. International Journal of Molecular Sciences, 26(6), 2784. https://doi.org/10.3390/ijms26062784





