Impact of Volatile Organic Compounds on the Growth of Aspergillus flavus and Related Aflatoxin B1 Production: A Review
Abstract
:1. Introduction
- -
- Which VOCs are emitted by A. flavus and are specific to TS or NTS?
- -
- Which bioactive VOCs or volatolomes of various origins affect the growth of A. flavus and/or its production of AFB1?
- -
- What are the modes of action of these bioactive VOCs?
- -
- How can we exploit these VOCs to our advantage to control the growth of A. flavus and its AFB1 production?
2. Which VOCs Are Produced by A. flavus and Are Specific to TS or NTS?
2.1. The Diversity of VOCs Emitted by A. flavus
2.2. VOCs Emission of A. flavus Influenced by Biotic and Abiotic Factors
3. Which Bioactive VOCs or Volatolome of Various Origins Affect the Growth of A. flavus and/or Its Production of AFB1?
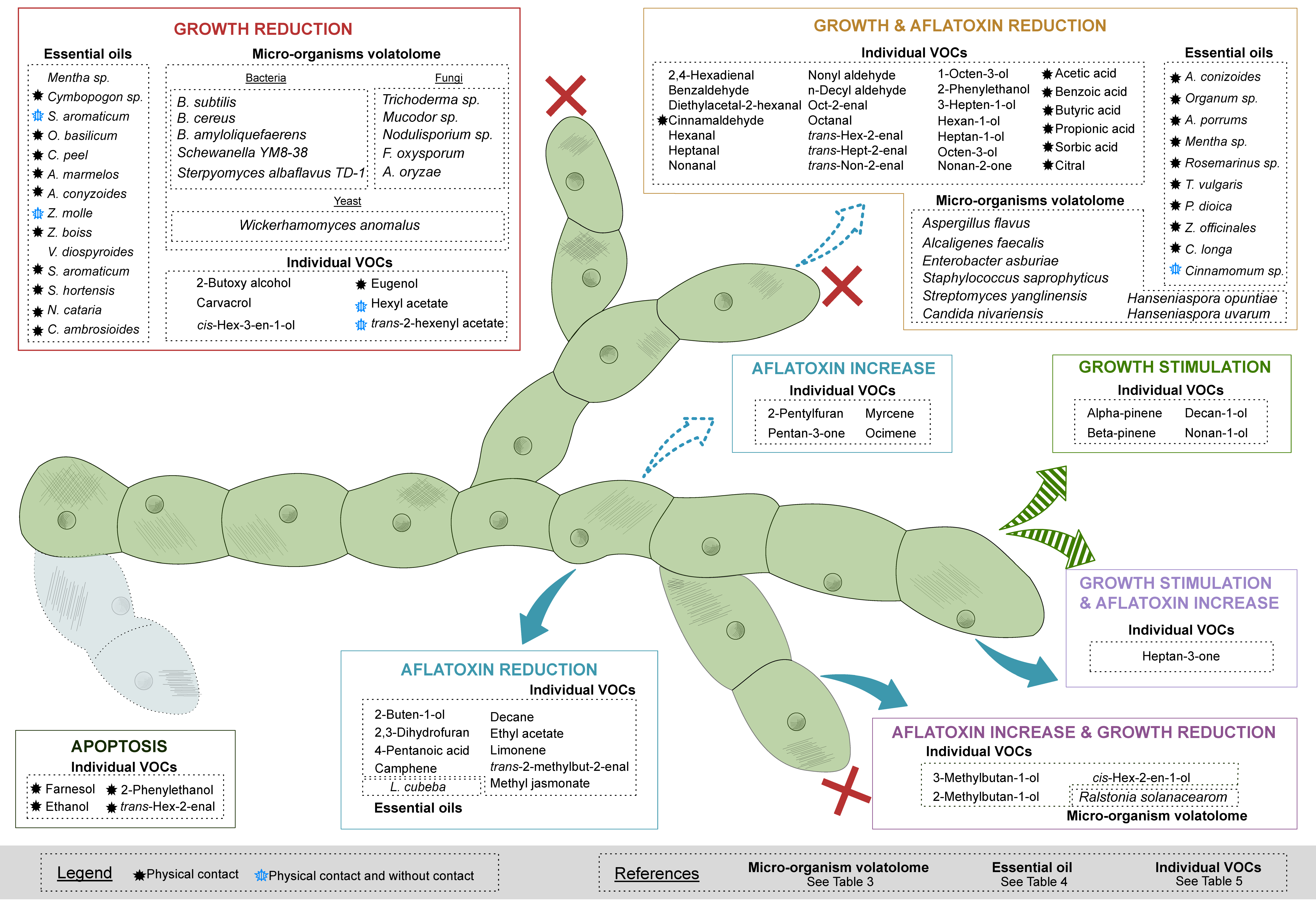
3.1. The Volatolomes from Bacteria, Yeast and Fungi Reduce the Growth of A. flavus
| Species and Main VOCs | Impact | References | |||
|---|---|---|---|---|---|
| Growth | Aflatoxin | ||||
| Bacteria | Alcaligenes faecalis Dimethyl disulfide Methyl 3-methylbutanoate | - | - | Gong et al., 2019 | [59] |
| Bacillus subtilis | - | NA | Chaves-López et al., 2015 | [57] | |
| Bacillus cereus | - | NA | Chaves-López et al., 2015 | [57] | |
| Bacillus amyloliquefaciens | - | NA | Chaves-López et al., 2015 | [57] | |
| Enterobacter asburiae 1-Methoxy-3-methylbutane Pentan-1-ol 2-Phenylethanol | - | - | Gong et al., 2019 | [60] | |
| Ralstonia Solanacearum | - | + | Spraker et al., 2014 Singh et al., 2020 Suwannarach et al., 2013 | [44] [55] [64] | |
| Schewanella algae Dodecan-2-ol 2,4-bis(1,1-Dimethylethyl)-phenol 2,2-Dimethyl-oxazole Butylated hydoxytoluene Nonane Dimethyl trisulfide | - | Gong et al., 2015 | [66] | ||
| Staphylococcus saprophyticus 3,3-dimethyl-1,2-epoxybutane | - | - | Gong et al., 2020 | [61] | |
| Streptomyces philanthi | - | Boukaew and Prasertsan, 2020 | [56] | ||
| Streptomyces yanglinensis | - | - | Lyu et al., 2020 | [67] | |
| Yeast | Candida nivariensis 2-Methylpropan-1-ol 3-Methylbutan-1-ol Pentan-1-ol | - | - | Jaibangyang et al., 2020 | [68] |
| Hanseniaspora opuntiae Acetic acid 2-Methylbutanoic acid 2-Phenylethyl acetate | - | - | Tejero et al., 2021 | [69] | |
| Hanseniaspora uvarum Ethyl acetate 3-Methylbutan-1-ol 2-Methylbutan-1-ol 2-Phenylethyl acetate | - | - | Tejero et al., 2021 | [69] | |
| Wickerhamomyces anomalus 2-Phenylethanol | - | NA | Tilocca et al., 2020 | [65] | |
| Fungi | Streptomyces alboflavus | - | NA | Yang et al., 2019 | [70] |
| Fusarium oxysporum Limonene | - | NA | Suwannarach et al., 2013 | [64] | |
| Muscodor genus 2-Methylpropanoic acid 2- Methylbutan-1-ol 3-Methylbutan-1-ol | - | NA | Braun et al., 2012 Singh et al., 2020 Suwannarach et al., 2013 | [63] [55] [64] | |
| Nodulisporium sp. 1,8 Cineole Terpinen-4-ol | - | NA | Suwannarach et al., 2013 | [64] | |
| Trichomderma genus | - | NA | Singh et al., 2020 | [55] | |
| Aspergillus flavus | - | - | Sweany and Damann, 2020 | [62] | |
| Aspergillus oryzae Octa-1,3-diene Octa-1,5-diene-3-ol 1-Octene-3-ol Octan-3-one Octanal Oct-2-enal 1-Octene-1-ol Octa-2,4-dieneal | - | NA | Singh et al., 2020 | [55] | |
3.2. Blends of VOCs from Essential Oils Show Antifungal Properties and Regulation Effects on AFB1 Production in A. flavus
| (a) Latin Name and Major VOCs | (b) Impact | (c) Application Mode | References | ||
|---|---|---|---|---|---|
| Growth | Aflatoxin | ||||
| Aegle marmelos D and L-Limonene * | - | NA | Contact | Adorjan and Buchbauer, 2010 | [75] |
| Ageratum conyzoides Precocene I and II Dimetoxy ageratocromene Ageratocromene | - | - | Contact | Adorjan and Buchbauer, 2010 Esper et al., 2014 | [75] [72] |
| Allium porrums Diallyl trisulfide Diallyl disulfide Methyl allyl trisulfide 5-Ethylthiazole | - | - | Contact | Kocevski et al., 2013 Abd El-Aziz et al., 2015 | [73] [76] |
| Capsicum Not available | - | NA | No contact | Boukaew et al., 2017 | [77] |
| Chenopodium ambrosioides (Z)-Ascaridole | - | NA | Contact | Adorjan and Buchbauer, 2010 | [75] |
| Cinnamomum Cinnamaldehyde (E)-2-methoxycinnamaldehyde Carveol α-Cadinol | - | - | Contact No contact | Abd El-Aziz et al., 2015 Boukaew et al., 2017 Manso et al., 2013 Kocevski et al., 2013 Xiang et al., 2020 | [76] [77] [78] [73] [74] |
| Citrus peel Limonene * Linalool Citral | - | NA | Contact | Taguchi et al., 2015 | [79] |
| Curcuma longa L. Ar-Tumerone α –Tumerone β-Tumerone Ar-Curcumene β -Sesquiphellandrene | - | - | Contact | Ferreira et al., 2013 Hu et al., 2017 | [80] [81] |
| Cymbopogon (Z)-Citral (E)-Citral Limonene * | - | NA | Contact | Xiang et al., 2020 | [74] |
| Litsea cubeba essential (Z) and (E)-Limonene oxide D-Limonene * | NA | - | Contact No contact | Li et al., 2016 | [82] |
| Mentha Menthol Menthone Menthyl acetate Menthofurane | - | - | Contact | Abd El-Aziz et al., 2015 Beyki et al., 2014 Taguchi et al., 2015 | [76] [83] [79] |
| Nepeta cataria 4aa,7a,7ab-Nepetalactone | - | NA | Contact | Adorjan and Buchbauer, 2010 | [75] |
| Ocimum basilicum Linalool Methylchalvicol Eugenol Methyl eugenol Methyl cinnamate 1,8- Cineole Caryophyllene * | - | NA | Contact | Taguchi et al., 2015 Xiang et al., 2020 | [79] [74] |
| Origanum Carvacrol Thymol 4-Terpineol Linalool γ-Terpinene α-Terpineol | - | - | Contact | Esper et al., 2014 Xiang et al., 2020 | [72] [74] |
| Pimenta dioica α-Terpinoel β-Linalool γ-Terpinene Eucalyptol | - | - | Contact | Kumar Chaudhari et al., 2022 | [84] |
| Pogostemon cablin Patchouli alcohol 4-Oxo-14-norvitrane δ-Guaiene | - | NA | Contact | Kocevski et al., 2013 | [73] |
| Rosemary Camphor 1,8-Cineole α-Pinene * Verbenone Camphene Limonene * Bornyl acetate α-Terpineol β-Pinene | - | - | Contact | Abd El-Aziz et al., 2015 Taguchi et al., 2015 | [76] [79] |
| Satureja hortensis Thymol Carvacrol | - | NA | Contact | Adorjan and Buchbauer, 2010 | [75] |
| Syzygium aromaticum Eugenol Eugenyl acetate Caryophyllene Benzenemethanol | - | NA | Contact No contact | Adorjan and Buchbauer, 2010 Boukaew et al., 2017 Taguchi et al., 2015 Xiang et al., 2020 | [75] [77] [79] [74] |
| Thymus vulgaris p-Cymene γ-Terpinene Thymol | - | - | Contact | Abd El-Aziz et al., 2015 Khalili et al., 2015 | [76] [85] |
| Vatica diospyroides Symington Benzyl acetate Benzyl benzoate Isoeugenol α-Terpineol | - | NA | No contact | Boukaew et al., 2017 | [77] |
| Zanthoxylum molle Undecan-2-one Limonene * Terpinen-4-ol | - | NA | Contact No contact | Tian et al., 2014 | [86] |
| Zataria multiflora Boiss Carvacrol | - | NA | Contact | Adorjan and Buchbauer, 2010 | [75] |
| Zingiber officinale β-Phellandrene Zingiberene Geranial Neral | - | - | Contact | Adorjan and Buchbauer, 2010 Nerilo et al., 2016 Taguchi et al., 2015 | [75] [87] [79] |
3.3. Single Bioactive VOCs Affecting the Growth and/or the AFB1 Production of A. flavus
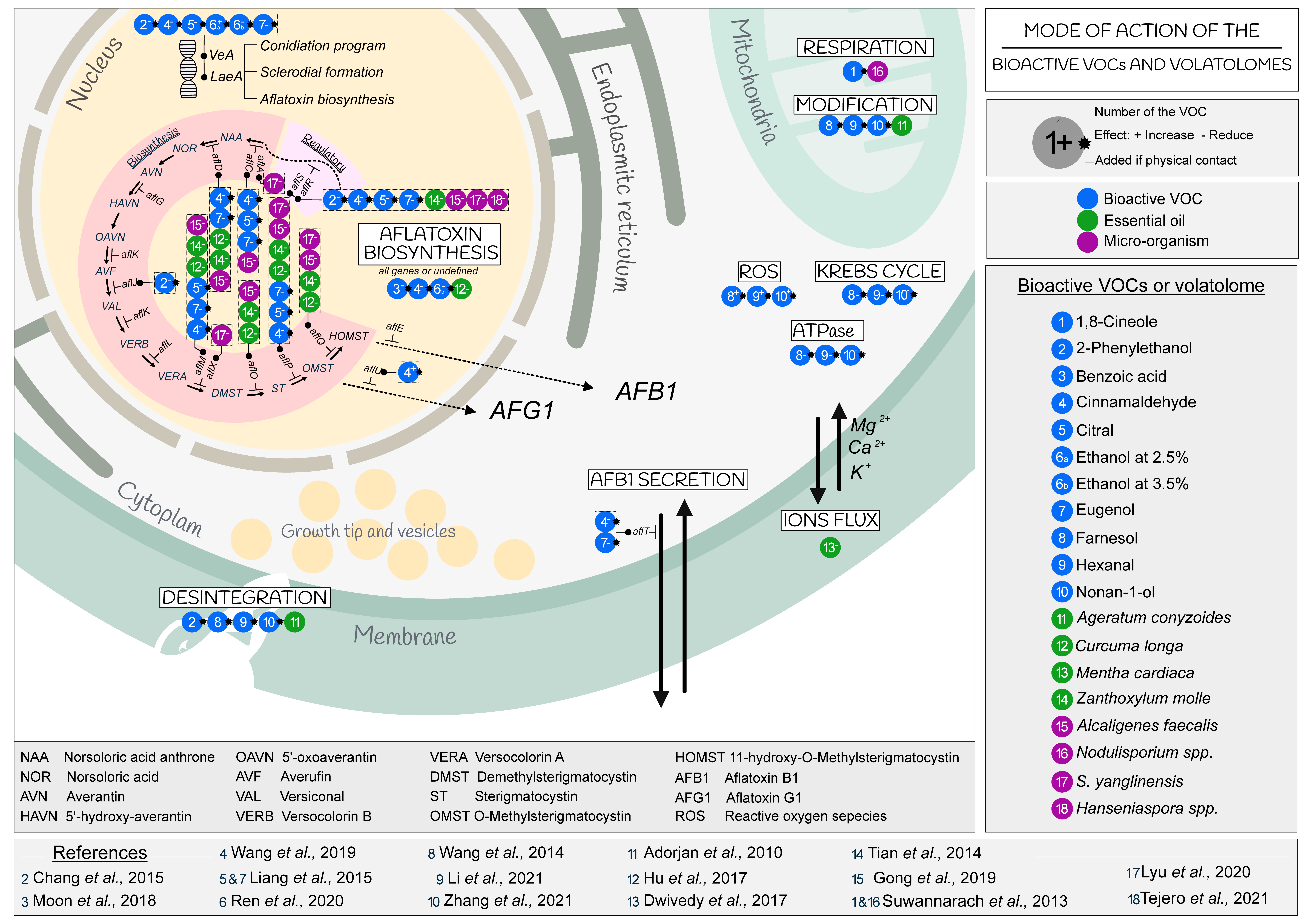
4. What Are the Modes of Action of These Bioactive VOCs?
4.1. Loss of Membrane Integrity of A. flavus
4.2. Modification of Afl Gene Expression
4.3. Impact on the Fungal Growth and Ergosterol Production
5. How Can We Exploit These VOCs to our Advantage to Control the Growth of A. flavus and Its AFB1 Production?
6. Conclusions
7. Perspectives
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Weisskopf, L.; Schulz, S.; Garbeva, P. Microbial volatile organic compounds in intra-kingdom and inter-kingdom interactions. Nat. Rev. Microbiol. 2021, 19, 391–404. [Google Scholar] [CrossRef] [PubMed]
- Minerdi, D.; Maggini, V.; Fani, R. Volatile organic compounds: From figurants to leading actors in fungal symbiosis. FEMS Microbiol. Ecol. 2021, 97, fiab067. [Google Scholar] [CrossRef]
- Cottier, F.; Mühlschlegel, F.A. Communication in fungi. Int. J. Microbiol. 2012, 2012, 351832. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Farbo, M.G.; Urgeghe, P.P.; Fiori, S.; Marcello, A.; Oggiano, S.; Balmas, V.; Hassan, Z.U.; Jaoua, S.; Migheli, Q. Effect of yeast volatile organic compounds on ochratoxin A-producing Aspergillus carbonarius and A. ochraceus. Int. J. Food Microbiol. 2018, 284, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Morath, S.U.; Hung, R.; Bennett, J.W. Fungal volatile organic compounds: A review with emphasis on their biotechnological potential. Fungal Biol. Rev. 2012, 26, 73–83. [Google Scholar] [CrossRef]
- Citron, C.A.; Gleitzmann, J.; Laurenzano, G.; Pukall, R.; Dickschat, J.S. Terpenoids are Widespread in Actinomycetes: A Correlation of Secondary Metabolism and Genome Data. ChemBioChem 2012, 13, 202–214. [Google Scholar] [CrossRef]
- Moore, G.G.; Lebar, M.D.; Carter-Wientjes, C.H.; Gilbert, M.K. The potential role of fungal volatile organic compounds in Aspergillus flavus biocontrol efficacy. Biol. Control 2021, 160, 104686. [Google Scholar] [CrossRef]
- Piotrowska, M. Microbiological decontamination of mycotoxins: Opportunities and limitations. Toxins 2021, 13, 819. [Google Scholar] [CrossRef]
- Klich, M.A. Aspergillus flavus: The major producer of aflatoxin. Mol. Plant Pathol. 2007, 8, 713–722. [Google Scholar] [CrossRef]
- Moretti, A.; Logrieco, A.F.; Susca, A. Mycotoxins: An underhand food problem. Methods Mol. Biol. 2017, 1542, 3–12. [Google Scholar] [CrossRef]
- Barkai-Golan, R. Aspergillus Mycotoxins. In Mycotoxins in Fruits and Vegetables; Academic Press: Cambridge, MA, USA, 2008; pp. 115–151. [Google Scholar] [CrossRef]
- Gourama, H.; Bullerman, L.B. Aspergillus flavus and Aspergillus parasiticus: Aflatoxigenic fungi of concern in foods and feeds†: A review. J. Food Prot. 1995, 58, 1395–1404. [Google Scholar] [CrossRef] [PubMed]
- Navale, V.; Vamkudoth, K.R.; Ajmera, S.; Dhuri, V. Aspergillus derived mycotoxins in food and the environment: Prevalence, detection, and toxicity. Toxicol. Rep. 2021, 8, 1008–1030. [Google Scholar] [CrossRef] [PubMed]
- Reddy, K.; Salleh, B.; Saad, B.; Abbas, H.K. An overview of mycotoxin contamination in foods and its implications for human health. Toxin Rev. 2010, 29, 2–26. [Google Scholar] [CrossRef]
- Kępińska-Pacelik, J.; Biel, W. Alimentary risk of mycotoxins for humans and animals. Toxins 2021, 13, 822. [Google Scholar] [CrossRef] [PubMed]
- Van Egmond, H.P.; Schothorst, R.C.; Jonker, M.A. Regulations relating to mycotoxins in food: PPPPerspectives in a global and European context. Anal. Bioanal. Chem. 2007, 389, 147–157. [Google Scholar] [CrossRef] [Green Version]
- Miklós, G.; Angeli, C.; Ambrus, Á.; Nagy, A.; Kardos, V.; Zentai, A.; Kerekes, K.; Farkas, Z.; Jóźwiak, Á.; Bartók, T. Detection of Aflatoxins in Different Matrices and Food-Chain Positions. Front. Microbiol. 2020, 11, 1916. [Google Scholar] [CrossRef]
- Wacoo, A.P.; Wendiro, D.; Vuzi, P.C.; Hawumba, J.F. Methods for Detection of Aflatoxins in Agricultural Food Crops. J. Appl. Chem. 2014, 2014, 706291. [Google Scholar] [CrossRef] [Green Version]
- Kabak, B.; Dobson, A.D.W.; Var, I. Strategies to prevent mycotoxin contamination of food and animal feed: A review. Crit. Rev. Food Sci. Nutr. 2006, 46, 593–619. [Google Scholar] [CrossRef]
- Susca, A.; Villani, A.; Moretti, A.; Stea, G.; Logrieco, A. Identification of toxigenic fungal species associated with maize ear rot: Calmodulin as single informative gene. Int. J. Food Microbiol. 2020, 319, 108491. [Google Scholar] [CrossRef]
- Tittlemier, S.A.; Brunkhorst, J.; Cramer, B.; DeRosa, M.C.; Lattanzio, V.M.T.; Malone, R.; Maragos, C.; Stranska, M.; Sumarah, M.W. Developments in mycotoxin analysis: An update for 2019–2020. World Mycotoxin J. 2021, 14, 3–26. [Google Scholar] [CrossRef]
- Caceres, I.; Al Khoury, A.; El Khoury, R.; Lorber, S.; Oswald, I.P.; El Khoury, A.; Atoui, A.; Puel, O.; Bailly, J.D. Aflatoxin biosynthesis and genetic regulation: A review. Toxins 2020, 12, 150. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peterson, S.W. Phylogenetic analysis of Aspergillus species using DNA sequences from four loci. Mycologia 2008, 100, 205–226. [Google Scholar] [CrossRef] [PubMed]
- Gallo, A.; Stea, G.; Battilani, P.; Logrieco, A.F.; Perrone, G. Molecular characterization of an Aspergillus flavus population isolated from maize during the first outbreak of aflatoxin contamination in Italy. Phytopathol. Mediterr. 2012, 51, 198–206. [Google Scholar]
- Tran-Dinh, N.; Pitt, J.I.; Markwell, P.J. Selection of non-toxigenic strains of Aspergillus flavus for biocontrol of aflatoxins in maize in Thailand. Biocontrol Sci. Technol. 2014, 24, 652–661. [Google Scholar] [CrossRef]
- Kesselmeier, J.; Staudt, M. Biogenic Volatile Organic Compounds (VOC): An Overview on Emission, Biogenic Volatile Organic Compounds (VOC): An Overview on Emission, Physiology and Ecology. J. Atmos. Chem. 2015, 33, 22–88. [Google Scholar]
- Kegge, W.; Pierik, R. Biogenic volatile organic compounds and plant competition. Trends Plant Sci. 2010, 15, 126–132. [Google Scholar] [CrossRef]
- Korpi, A.; Järnberg, J.; Pasanen, A.L. Microbial volatile organic compounds. Crit. Rev. Toxicol. 2009, 39, 139–193. [Google Scholar] [CrossRef]
- Inamdar, A.A.; Morath, S.; Bennett, J.W. Fungal Volatile Organic Compounds: More Than Just a Funky Smell? Annu. Rev. Microbiol. 2020, 74, 101–116. [Google Scholar] [CrossRef]
- Farh, M.E.A.; Jeon, J. Roles of fungal volatiles from perspective of distinct lifestyles in filamentous fungi. Plant Pathol. J. 2020, 36, 193–203. [Google Scholar] [CrossRef]
- Skoczek, A.; Piesik, D.; Wenda-Piesik, A.; Buszewski, B.; Bocianowski, J.; Wawrzyniak, M. Volatile organic compounds released by maize following herbivory or insect extract application and communication between plants. J. Appl. Entomol. 2017, 141, 630–643. [Google Scholar] [CrossRef]
- Padder, S.A.; Prasad, R.; Shah, A.H. Quorum sensing: A less known mode of communication among fungi. Microbiol. Res. 2018, 210, 51–58. [Google Scholar] [CrossRef] [PubMed]
- Mehmood, A.; Liu, G.; Wang, X.; Meng, G.; Wang, C.; Liu, Y. Fungal quorum-sensing molecules and inhibitors with potential antifungal activity: A review. Molecules 2019, 24, 1950. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Lucca, A.J.; Boué, S.; Carter-Wientjes, C.H.; Bland, J.M.; Bhatnagar, D.; Cleveland, T.E. Volatile profiles of toxigenic and non-toxigenic Aspergillus flavus using SPME for solid phase extraction. Ann. Agric. Environ. Med. 2010, 17, 301–308. [Google Scholar]
- De Lucca, A.J.; Boué, S.M.; Carter-Wientjes, C.; Bhatnagar, D. Volatile profiles and aflatoxin production by toxigenic and non-toxigenic isolates of Aspergillus flavus grown on sterile and non-sterile cracked corn. Ann. Agric. Environ. Med. 2012, 19, 91–98. [Google Scholar]
- Josselin, L.; De Clerck, C.; De Boevre, M.; Moretti, A.; Haïssam Jijakli, M.; Soyeurt, H.; Fauconnier, M.L. Volatile organic compounds emitted by Aspergillus flavus strains producing or not aflatoxin B1. Toxins 2021, 13, 705. [Google Scholar] [CrossRef]
- Müller, A.; Faubert, P.; Hagen, M.; zu Castell, W.; Polle, A.; Schnitzler, J.P.; Rosenkranz, M. Volatile profiles of fungi—Chemotyping of species and ecological functions. Fungal Genet. Biol. 2013, 54, 25–33. [Google Scholar] [CrossRef]
- Polizzi, V.; Adams, A.; Malysheva, S.V.; De Saeger, S.; Van Peteghem, C.; Moretti, A.; Picco, A.M.; De Kimpe, N. Identification of volatile markers for indoor fungal growth and chemotaxonomic classification of Aspergillus species. Fungal Biol. 2012, 116, 941–953. [Google Scholar] [CrossRef]
- Sun, D.; Wood-Jones, A.; Wang, W.; Vanlangenberg, C.; Jones, D.; Gower, J.; Simmons, P.; Baird, R.E.; Mlsna, T.E. Monitoring MVOC Profiles over Time from Isolates of Aspergillus flavus Using SPME GC-MS. J. Agric. Chem. Environ. 2014, 3, 48–63. [Google Scholar] [CrossRef] [Green Version]
- Sun, D.; She, J.; Gower, J.L.; Stokes, C.E.; Windham, G.L.; Baird, R.E.; Mlsna, T.E. Effects of Growth Parameters on the Analysis of Aspergillus flavus Volatile Metabolites. Separations 2016, 3, 13. [Google Scholar] [CrossRef] [Green Version]
- Jeleń, H.; Wąsowicz, E. Volatile fungal metabolites and their relation to the spoilage of agricultural commodities. Food Rev. Int. 1998, 14, 391–426. [Google Scholar] [CrossRef]
- Gao, P.; Korley, F.; Martin, J.; Chen, B.T. Determination of unique microbial volatile organic compounds produced by five Aspergillus species commonly found in problem buildings. Am. Ind. Hyg. Assoc. J. 2002, 63, 135–140. [Google Scholar] [CrossRef] [PubMed]
- Kamiński, E.; Libbey, L.M.; Stawicki, S.; Wasowicz, E. Identification of the predominant volatile compounds produced by Aspergillus flavus. Appl. Microbiol. 1972, 24, 721–726. [Google Scholar] [CrossRef] [PubMed]
- Spraker, J.E.; Jewell, K.; Roze, L.V.; Scherf, J.; Ndagano, D.; Beaudry, R.; Linz, J.E.; Allen, C.; Keller, N.P. A Volatile Relationship: Profiling an Inter-Kingdom Dialogue Between two Plant Pathogens, Ralstonia solanacearum and Aspergillus flavus. J. Chem. Ecol. 2014, 40, 502–513. [Google Scholar] [CrossRef] [PubMed]
- Zeringue, H.J.; Bhatnagar, D.; Cleveland, T.E. C15H24 Volatile Compounds Unique to Aflatoxigenic Strains of Aspergillus flavus. Appl. Environ. Microbiol. 1993, 59, 2264–2270. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pennerman, K.K.; Al-Maliki, H.S.; Lee, S.; Bennett, J.W. Fungal Volatile Organic Compounds (VOCs) and the Genus Aspergillus; Elsevier B.V.: Amsterdam, The Netherlands, 2016; ISBN 9780444635136. [Google Scholar]
- Kosegarten, C.E.; Ramírez-Corona, N.; Mani-López, E.; Palou, E.; López-Malo, A. Description of Aspergillus flavus growth under the influence of different factors (water activity, incubation temperature, protein and fat concentration, pH, and cinnamon essential oil concentration) by kinetic, probability of growth, and time-to-detectio. Int. J. Food Microbiol. 2017, 240, 115–123. [Google Scholar] [CrossRef]
- Keller, N.P.; Nesbitt, C.; Sarr, B.; Phillips, T.D.; Burow, G.B. pH Regulation of Sterigmatocystin and Aflatoxin Biosynthesis in Aspergillus spp. Postharvest Pathol. Mycotoxins 1997, 87, 643–648. [Google Scholar] [CrossRef] [Green Version]
- Medina, A.; Gilbert, M.K.; Mack, B.M.; OBrian, G.R.; Rodríguez, A.; Bhatnagar, D.; Payne, G.; Magan, N. Interactions between water activity and temperature on the Aspergillus flavus transcriptome and aflatoxin B1 production. Int. J. Food Microbiol. 2017, 256, 36–44. [Google Scholar] [CrossRef] [Green Version]
- Zeringue, H.J.; McCormick, S.P. Aflatoxin production in cultures of Aspergillus flavus incubated in atmospheres containing selected cotton leaf-derived volatiles. Toxicon 1990, 28, 445–448. [Google Scholar] [CrossRef]
- Cleveland, T.E.; Carter-Wientjes, C.H.; De Lucca, A.J.; Boué, S.M. Effect of soybean volatile compounds on Aspergillus flavus growth and aflatoxin production. J. Food Sci. 2009, 74, H83–H87. [Google Scholar] [CrossRef]
- Piechulla, B.; Lemfack, M.C.; Kai, M. Effects of discrete bioactive microbial volatiles on plants and fungi. Plant Cell Environ. 2017, 40, 2042–2067. [Google Scholar] [CrossRef]
- Ren, Y.; Jin, J.; Zheng, M.; Yang, Q.; Xing, F. Ethanol Inhibits Aflatoxin B1 Biosynthesis in Aspergillus flavus by Up-Regulating Oxidative Stress-Related Genes. Front. Microbiol. 2020, 10, 2946. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, S.B.; Qin, Y.L.; Li, S.F.; Lv, Y.Y.; Zhai, H.C.; Hu, Y.S.; Cai, J.P. Antifungal mechanism of 1-nonanol against Aspergillus flavus growth revealed by metabolomic analyses. Appl. Microbiol. Biotechnol. 2021, 105, 7871–7888. [Google Scholar] [CrossRef] [PubMed]
- Singh, D.; Son, S.Y.; Lee, C.H. Critical thresholds of 1-Octen-3-ol shape inter-species Aspergillus interactions modulating the growth and secondary metabolism. Sci. Rep. 2020, 10, 11116. [Google Scholar] [CrossRef] [PubMed]
- Boukaew, S.; Prasertsan, P. Efficacy of volatile compounds from Streptomyces philanthi RL-1-178 as a biofumigant for controlling growth and aflatoxin production of the two aflatoxin-producing fungi on stored soybean seeds. J. Appl. Microbiol. 2020, 129, 652–664. [Google Scholar] [CrossRef]
- Chaves-López, C.; Serio, A.; Gianotti, A.; Sacchetti, G.; Ndagijimana, M.; Ciccarone, C.; Stellarini, A.; Corsetti, A.; Paparella, A. Diversity of food-borne Bacillus volatile compounds and influence on fungal growth. J. Appl. Microbiol. 2015, 119, 487–499. [Google Scholar] [CrossRef]
- Rashmi, M.; Kushveer, J.S.; Sarma, V.V. A worldwide list of endophytic fungi with notes on ecology and diversity. Mycosphere 2019, 10, 798–1079. [Google Scholar] [CrossRef]
- Gong, A.D.; Wu, N.N.; Kong, X.W.; Zhang, Y.M.; Hu, M.J.; Gong, S.J.; Dong, F.Y.; Wang, J.H.; Zhao, Z.Y.; Liao, Y.C. Inhibitory effect of volatiles emitted from Alcaligenes faecalis N1-4 on Aspergillus flavusand aflatoxins in storage. Front. Microbiol. 2019, 10, 1419. [Google Scholar] [CrossRef] [Green Version]
- Gong, A.D.; Dong, F.Y.; Hu, M.J.; Kong, X.W.; Wei, F.F.; Gong, S.J.; Zhang, Y.M.; Zhang, J.B.; Wu, A.B.; Liao, Y.C. Antifungal activity of volatile emitted from Enterobacter asburiae Vt-7 against Aspergillus flavus and aflatoxins in peanuts during storage. Food Control 2019, 106, 106718. [Google Scholar] [CrossRef]
- Gong, A.D.; Sun, G.J.; Zhao, Z.Y.; Liao, Y.C.; Zhang, J.B. Staphylococcus saprophyticus L-38 produces volatile 3,3-dimethyl-1,2-epoxybutane with strong inhibitory activity against Aspergillus flavus germination and aflatoxin production. World Mycotoxin J. 2020, 13, 247–258. [Google Scholar] [CrossRef]
- Sweany, R.R.; Damann, K.E. Influence of Neighboring Clonal-Colonies on Aflatoxin Production by Aspergillus flavus. Front. Microbiol. 2020, 10, 3038. [Google Scholar] [CrossRef] [Green Version]
- Braun, G.; Vailati, M.; Prange, R.; Bevis, E. Muscodor albus volatiles control toxigenic fungi under controlled atmosphere (CA) storage conditions. Int. J. Mol. Sci. 2012, 13, 15848–15858. [Google Scholar] [CrossRef] [PubMed]
- Suwannarach, N.; Kumla, J.; Bussaban, B.; Nuangmek, W.; Matsui, K.; Lumyong, S. Biofumigation with the endophytic fungus Nodulisporium spp. CMU-UPE34 to control postharvest decay of citrus fruit. Crop Prot. 2013, 45, 63–70. [Google Scholar] [CrossRef]
- Tilocca, B.; Cao, A.; Migheli, Q. Scent of a Killer: Microbial Volatilome and Its Role in the Biological Control of Plant Pathogens. Front. Microbiol. 2020, 11, 41. [Google Scholar] [CrossRef] [PubMed]
- Gong, A.D.; Li, H.P.; Shen, L.; Zhang, J.B.; Wu, A.B.; He, W.J.; Yuan, Q.S.; He, J.D.; Liao, Y.C. The Shewanella algae strain YM8 produces volatiles with strong inhibition activity against Aspergillus pathogens and aflatoxins. Front. Microbiol. 2015, 6, 1091. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lyu, A.; Yang, L.; Wu, M.; Zhang, J.; Li, G. High Efficacy of the Volatile Organic Compounds of Streptomyces yanglinensis 3-10 in Suppression of Aspergillus Contamination on Peanut Kernels. Front. Microbiol. 2020, 11, 142. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jaibangyang, S.; Nasanit, R.; Limtong, S. Biological control of aflatoxin-producing Aspergillus flavus by volatile organic compound-producing antagonistic yeasts. BioControl 2020, 65, 377–386. [Google Scholar] [CrossRef]
- Tejero, P.; Martín, A.; Rodríguez, A.; Galván, A.I.; Ruiz-Moyano, S.; Hernández, A. In vitro biological control of Aspergillus flavus by Hanseniaspora opuntiae l479 and Hanseniaspora uvarum l793, producers of antifungal volatile organic compounds. Toxins 2021, 13, 663. [Google Scholar] [CrossRef]
- Yang, M.; Lu, L.; Pang, J.; Hu, Y.; Guo, Q.; Li, Z.; Wu, S.; Liu, H.; Wang, C. Biocontrol activity of volatile organic compounds from Streptomyces alboflavus TD-1 against Aspergillus flavus growth and aflatoxin production. J. Microbiol. 2019, 57, 396–404. [Google Scholar] [CrossRef]
- Dai, Q.; Zhang, F.L.; Feng, T. Sesquiterpenoids specially produced by fungi: Structures, biological activities, chemical and biosynthesis (2015–2020). J. Fungi 2021, 7, 1026. [Google Scholar] [CrossRef]
- Esper, R.H.; Gonçalez, E.; Marques, M.O.M.; Felicio, R.C.; Felicio, J.D. Potential of essential oils for protection of grains contaminated by aflatoxin produced by Aspergillus flavus. Front. Microbiol. 2014, 5, 269. [Google Scholar] [CrossRef]
- Kocevski, D.; Du, M.; Kan, J.; Jing, C.; Lačanin, I.; Pavlović, H. Antifungal effect of Allium tuberosum, Cinnamomum cassia, and Pogostemon cablin essential oils and their components against population of aspergillus species. J. Food Sci. 2013, 78, 731–737. [Google Scholar] [CrossRef] [PubMed]
- Xiang, F.; Zhao, Q.; Zhao, K.; Pei, H.; Tao, F. The Efficacy of Composite Essential Oils against Aflatoxigenic Fungus Aspergillus flavus in Maize. Toxins 2020, 12, 562. [Google Scholar] [CrossRef] [PubMed]
- Adorjan, B.; Buchbauer, G. Biological properties of essential oils: An updated review. Flavour Fragr. J. 2010, 25, 407–426. [Google Scholar] [CrossRef]
- Abd El-Aziz, A.R.M.; Mahmoud, M.A.; Al-Othman, M.R.; Al-Gahtani, M.F. Use of selected essential oils to control aflatoxin contaminated stored cashew and detection of aflatoxin biosynthesis gene. Sci. World J. 2015, 2015, 958192. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boukaew, S.; Prasertsan, P.; Sattayasamitsathit, S. Evaluation of antifungal activity of essential oils against aflatoxigenic Aspergillus flavus and their allelopathic activity from fumigation to protect maize seeds during storage. Ind. Crops Prod. 2017, 97, 558–566. [Google Scholar] [CrossRef]
- Manso, S.; Cacho-Nerin, F.; Becerril, R.; Nerín, C. Combined analytical and microbiological tools to study the effect on Aspergillus flavus of cinnamon essential oil contained in food packaging. Food Control 2013, 30, 370–378. [Google Scholar] [CrossRef]
- Taguchi, T.; Ishihara, A.; Nakajima, H. Effects of volatile compounds produced by plants on fungal growth and the production of mycotoxins. JSM Mycotoxins 2015, 65, 131–142. [Google Scholar] [CrossRef] [Green Version]
- Ferreira, F.D.; Kemmelmeier, C.; Arrotéia, C.C.; Da Costa, C.L.; Mallmann, C.A.; Janeiro, V.; Ferreira, F.M.D.; Mossini, S.A.G.; Silva, E.L.; Machinski, M. Inhibitory effect of the essential oil of Curcuma longa L. and curcumin on aflatoxin production by Aspergillus flavus Link. Food Chem. 2013, 136, 789–793. [Google Scholar] [CrossRef] [Green Version]
- Hu, Y.; Zhang, J.; Kong, W.; Zhao, G.; Yang, M. Mechanisms of antifungal and anti-aflatoxigenic properties of essential oil derived from turmeric (Curcuma longa L.) on Aspergillus flavus. Food Chem. 2017, 220, 1–8. [Google Scholar] [CrossRef]
- Li, Y.; Kong, W.; Li, M.; Liu, H.; Zhao, X.; Yang, S.; Yang, M. Litsea cubeba essential oil as the potential natural fumigant: Inhibition of Aspergillus flavus and AFB1 production in licorice. Ind. Crops Prod. 2016, 80, 186–193. [Google Scholar] [CrossRef]
- Beyki, M.; Zhaveh, S.; Khalili, S.T.; Rahmani-Cherati, T.; Abollahi, A.; Bayat, M.; Tabatabaei, M.; Mohsenifar, A. Encapsulation of Mentha piperita essential oils in chitosan-cinnamic acid nanogel with enhanced antimicrobial activity against Aspergillus flavus. Ind. Crops Prod. 2014, 54, 310–319. [Google Scholar] [CrossRef]
- Kumar Chaudhari, A.; Kumar Singh, V.; Das, S.; Deepika; Kishore Dubey, N. Fabrication, characterization, and bioactivity assessment of chitosan nanoemulsion containing allspice essential oil to mitigate Aspergillus flavus contamination and aflatoxin B1 production in maize. Food Chem. 2022, 372, 131221. [Google Scholar] [CrossRef]
- Khalili, S.T.; Mohsenifar, A.; Beyki, M.; Zhaveh, S.; Rahmani-Cherati, T.; Abdollahi, A.; Bayat, M.; Tabatabaei, M. Encapsulation of Thyme essential oils in chitosan-benzoic acid nanogel with enhanced antimicrobial activity against Aspergillus flavus. LWT-Food Sci. Technol. 2015, 60, 502–508. [Google Scholar] [CrossRef]
- Tian, J.; Zeng, X.; Feng, Z.; Miao, X.; Peng, X.; Wang, Y. Zanthoxylum molle Rehd. essential oil as a potential natural preservative in management of Aspergillus flavus. Ind. Crops Prod. 2014, 60, 151–159. [Google Scholar] [CrossRef]
- Nerilo, S.B.; Rocha, G.H.O.; Tomoike, C.; Mossini, S.A.G.; Grespan, R.; Mikcha, J.M.G.; Machinski, M. Antifungal properties and inhibitory effects upon aflatoxin production by Zingiber officinale essential oil in Aspergillus flavus. Int. J. Food Sci. Technol. 2016, 51, 286–292. [Google Scholar] [CrossRef]
- Chang, P.K.; Hua, S.S.T.; Sarreal, S.B.L.; Li, R.W. Suppression of Aflatoxin Biosynthesis in Aspergillus flavus By 2-Phenylethanol Is Associated With Stimulated Growth and Decreased Degradation of Branched-Chain Amino Acids. Toxins 2015, 7, 3887–3902. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hua, S.S.T.; Beck, J.J.; Sarreal, S.B.L.; Gee, W. The major volatile compound 2-phenylethanol from the biocontrol yeast, Pichia anomala, inhibits growth and expression of aflatoxin biosynthetic genes of Aspergillus flavus. Mycotoxin Res. 2014, 30, 71–78. [Google Scholar] [CrossRef] [PubMed]
- Ma, W.; Zhao, L.; Xie, Y. Inhibitory effect of (E)-2-hexenal as a potential natural fumigant on Aspergillus flavus in stored peanut seeds. Ind. Crops Prod. 2017, 107, 206–210. [Google Scholar] [CrossRef]
- Moon, Y.S.; Kim, H.M.; Chun, H.S.; Lee, S.E. Organic acids suppress aflatoxin production via lowering expression of aflatoxin biosynthesis-related genes in Aspergillus flavus. Food Control 2018, 88, 207–216. [Google Scholar] [CrossRef]
- Zeringue, H.J.; Brown, R.L.; Neucere, J.N.; Cleveland, T.E. Relationships between C 6-C 12 Alkanal and Alkenal Volatile Contents and Resistance of Maize Genotypes to Aspergillus flavus and Aflatoxin Production. J. Agric. Food Chem. 1996, 44, 403–407. [Google Scholar] [CrossRef]
- Ma, W.; Johnson, E.T. Natural flavour (E,E)-2,4-heptadienal as a potential fumigant for control of Aspergillus flavus in stored peanut seeds: Finding new antifungal agents based on preservative sorbic acid. Food Control 2021, 124, 107938. [Google Scholar] [CrossRef]
- Liang, D.; Xing, F.; Selvaraj, J.N.; Liu, X.; Wang, L.; Hua, H.; Zhou, L.; Zhao, Y.; Wang, Y.; Liu, Y. Inhibitory effect of cinnamaldehyde, citral, and eugenol on aflatoxin biosynthetic gene expression and aflatoxin b1 biosynthesis in aspergillus flavus. J. Food Sci. 2015, 80, M2917–M2924. [Google Scholar] [CrossRef] [PubMed]
- Yin, H.B.; Chen, C.H.; Kollanoor-Johny, A.; Darre, M.J.; Venkitanarayanan, K. Controlling Aspergillus flavus and Aspergillus parasiticus growth and aflatoxin production in poultry feed using carvacrol and trans-cinnamaldehyde. Poult. Sci. 2015, 94, 2183–2190. [Google Scholar] [CrossRef]
- Wang, P.; Ma, L.; Jin, J.; Zheng, M.; Pan, L.; Zhao, Y.; Sun, X.; Liu, Y.; Xing, F. The anti-aflatoxigenic mechanism of cinnamaldehyde in Aspergillus flavus. Sci. Rep. 2019, 9, 10499. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wright, M.S.; Greene-Mcdowelle, D.M.; Zeringue, H.J.; Bhatnagar, D.; Cleveland, T.E. Effects of volatile aldehydes from Aspergillus-resistant varieties of corn on Aspergillus parasiticus growth and aflatoxin biosynthesis. Toxicon 2000, 38, 1215–1223. [Google Scholar] [CrossRef] [PubMed]
- Zeringue, H.J. Identification and effects of maize silk volatiles on cultures of Aspergillus flavus. J. Agric. Food Chem. 2000, 48, 921–925. [Google Scholar] [CrossRef] [PubMed]
- Li, S.F.; Zhang, S.B.; Lv, Y.Y.; Zhai, H.C.; Li, N.; Hu, Y.S.; Cai, J.P. Metabolomic analyses revealed multifaceted effects of hexanal on Aspergillus flavus growth. Appl. Microbiol. Biotechnol. 2021, 105, 3745–3757. [Google Scholar] [CrossRef]
- De Lucca, A.J.; Carter-Wientjes, C.H.; Boué, S.; Bhatnagar, D. Volatile Trans-2-Hexenal, a Soybean Aldehyde, Inhibits Aspergillus flavus Growth and Aflatoxin Production in Corn. J. Food Sci. 2011, 76, 3–8. [Google Scholar] [CrossRef]
- Moore, G.G.; Lebar, M.D.; Carter-Wientjes, C.H. Cumulative Effects of Non-Aflatoxigenic Aspergillus flavus Volatile Organic Compounds to Abate Toxin Production by Mycotoxigenic Aspergilli. Toxins 2022, 14, 340. [Google Scholar] [CrossRef]
- Wang, X.; Wang, Y.; Zhou, Y.; Wei, X. Farnesol induces apoptosis-like cell death in the pathogenic fungus Aspergillus flavus. Mycologia 2014, 106, 881–888. [Google Scholar] [CrossRef]
- Goodrich-Tanrikulu, M.; Mahoney, N.E.; Rodriguez, S.B. The plant growth regulator methyl jasmonate inhibits aflatoxin production by Aspergillus flavus. Microbiology 1995, 141, 2831–2837. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dwivedy, A.K.; Prakash, B.; Chanotiya, C.S.; Bisht, D.; Dubey, N.K. Chemically characterized Mentha cardiaca L. essential oil as plant based preservative in view of efficacy against biodeteriorating fungi of dry fruits, aflatoxin secretion, lipid peroxidation and safety profile assessment. Food Chem. Toxicol. 2017, 106, 175–184. [Google Scholar] [CrossRef] [PubMed]
- Nogueira, J.H.C.; Gonçalez, E.; Galleti, S.R.; Facanali, R.; Marques, M.O.M.; Felício, J.D. Ageratum conyzoides essential oil as aflatoxin suppressor of Aspergillus flavus. Int. J. Food Microbiol. 2010, 137, 55–60. [Google Scholar] [CrossRef] [PubMed]
- Basak, S.; Guha, P. A review on antifungal activity and mode of action of essential oils and their delivery as nano-sized oil droplets in food system. J. Food Sci. Technol. 2018, 55, 4701–4710. [Google Scholar] [CrossRef] [PubMed]
- Ogunade, I.M.; Martinez-Tuppia, C.; Queiroz, O.C.M.; Jiang, Y.; Drouin, P.; Wu, F.; Vyas, D.; Adesogan, A.T. Silage review: Mycotoxins in silage: Occurrence, effects, prevention, and mitigation. J. Dairy Sci. 2018, 101, 4034–4059. [Google Scholar] [CrossRef]
- Storm, I.M.L.D. Post-Harvest Fungal Spoilage of Maize Silage: Species, Growth Conditions and Mycotoxin Detection; Technical University of Denmark: Kongens Lyngby, Denmark, 2009. [Google Scholar]
- Sharon, A.; Finkelstein, A.; Shlezinger, N.; Hatam, I. Fungal apoptosis: Function, genes and gene function. FEMS Microbiol. Rev. 2009, 33, 833–854. [Google Scholar] [CrossRef]
- Hamann, A.; Brust, D.; Osiewacz, H.D. Apoptosis pathways in fungal growth, development and ageing. Trends Microbiol. 2008, 16, 276–283. [Google Scholar] [CrossRef]
- Maes, C.; Bouquillon, S.; Fauconnier, M.L. Encapsulation of essential oils for the development of biosourced pesticides with controlled release: A review. Molecules 2019, 24, 2539. [Google Scholar] [CrossRef]
| (a) | Total Number of VOCs | Chemical Family of VOCs Reported in Literature | |||||||
|---|---|---|---|---|---|---|---|---|---|
| (b) TS VOCs | (c) NTS VOCs | (d) VOCs Shared by TS and NTS | (e) US VOCs | ||||||
| Alcohol | 51 | De Lucca et al., 2010 De Lucca et al., 2012 Josselin et al., 2021 Müller et al., 2013 Polizzi et al., 2012 Sun et al., 2014 Sun et al., 2016 | [34] [35] [36] [37] [38] [39] [40] | De Lucca et al., 2010 Josselin et al., 2021 Jeleń and Wąsowicz, 1998 | [34] [36] [41] | De Lucca et al., 2010 Gao et al., 2002 Josselin et al., 2021 Kamiński et al., 1972 Polizzi et al., 2012 Spraker et al., 2014 Sun et al., 2014 | [34] [42] [36] [43] [38] [44] [39] | Jeleń and Wąsowicz, 1998 Kamiński et al., 1972 | [41] [43] |
| Aldehyde | 23 | De Lucca et al., 2010 De Lucca et al., 2012 Josselin et al., 2021 Müller et al., 2013 Sun et al., 2014 Sun et al., 2016 | [34] [35] [36] [37] [39] [40] | De Lucca et al., 2010 Sun et al., 2014 | [34] [39] | De Lucca et al., 2010 Josselin et al., 2021 Sun et al., 2014 | [34] [36] [39] | ||
| Alkane | 96 | De Lucca et al., 2010 De Lucca et al., 2012 Josselin et al., 2021 Müller et al., 2013 Spraker et al., 2014 | [34] [35] [36] [37] [44] | De Lucca et al., 2010 De Lucca et al., 2012 Josselin et al., 2021 Spraker et al., 2014 Sun et al., 2014 | [34] [35] [36] [44] [39] | De Lucca et al., 2010 De Lucca et al., 2012 Farh and Jeon, 2020 Josselin et al., 2021 Sun et al., 2014 | [34] [35] [30] [36] [39] | De Lucca et al., 2012 | [35] |
| Alkene | 65 | De Lucca et al., 2010 De Lucca et al., 2012 Jeleń and Wąsowicz, 1998 Josselin et al., 2021 Polizzi et al., 2012 Sun et al., 2016 | [34] [35] [41] [36] [38] [40] | De Lucca et al., 2010 De Lucca et al., 2012 Sun et al., 2014 | [34] [35] [39] | De Lucca et al., 2012 Josselin et al., 2021 | [35] [36] | Jeleń and Wąsowicz, 1998 | [41] |
| Alkyne | 9 | De Lucca et al., 2012 | [35] | De Lucca et al., 2012 | [35] | ||||
| Amine | 8 | De Lucca et al., 2010 De Lucca et al., 2012 Spraker et al., 2014 | [34] [35] [44] | ||||||
| Amide | 3 | De Lucca et al., 2010 De Lucca et al., 2012 | [34] [35] | ||||||
| Acid | 13 | De Lucca et al., 2010 De Lucca et al., 2012 Josselin et al., 2021 | [34] [35] [36] | De Lucca et al., 2010 Sun et al., 2014 | [34] [39] | Sun et al., 2014 | [39] | ||
| Ester | 20 | De Lucca et al., 2010 De Lucca et al., 2012 Josselin et al., 2021 | [34] [35] [36] | De Lucca et al., 2010 De Lucca et al., 2012 Sun et al., 2014 | [34] [35] [39] | De Lucca et al., 2010 | [34] | ||
| Ether | 1 | De Lucca et al., 2012 | [35] | ||||||
| Furan | 10 | De Lucca et al., 2010 De Lucca et al., 2012 Jeleń and Wąsowicz, 1998 Josselin et al., 2021 Sun et al., 2014 Sun et al., 2016 | [34] [35] [41] [36] [39] [40] | De Lucca et al., 2012 Sun et al., 2014 | [35] [39] | De Lucca et al., 2010 Josselin et al., 2021 Sun et al., 2014 | [34] [36] [39] | Jeleń and Wąsowicz, 1998 | [41] |
| Ketone | 29 | De Lucca et al., 2010 De Lucca et al., 2012 Josselin et al., 2021 Spraker et al., 2014 Sun et al., 2016 | [34] [35] [36] [44] [40] | De Lucca et al., 2010 De Lucca et al., 2012 Sun et al., 2014 | [34] [35] [39] | De Lucca et al., 2010 De Lucca et al., 2012 Gao et al., 2002 Josselin et al., 2021 Kamiński et al., 1972 Sun et al., 2014 | [34] [35] [42] [36] [43] [39] | Polizzi et al., 2012 | [38] |
| Halogen | 4 | De Lucca et al., 2010 | [34] | Jeleń and Wąsowicz, 1998 | [41] | ||||
| Terpene | 69 | De Lucca et al., 2010 De Lucca et al., 2012 Josselin et al., 2021 Polizzi et al., 2012 Sun et al., 2016 Zeringue et al., 1993 | [34] [35] [36] [38] [40] [45] | De Lucca et al., 2012 Sun et al., 2014 | [35] [39] | De Lucca et al., 2010 Josselin et al., 2021 | [34] [36] | Gao et al., 2002 Pennerman et al., 2016 Polizzi et al., 2012 | [42] [46] [38] |
| Others | 9 | De Lucca et al., 2010 De Lucca et al., 2012 Spraker et al., 2014 Sun et al., 2014 | [34] [35] [44] [39] | De Lucca et al., 2010 Josselin et al., 2021 | [34] [36] | Gao et al., 2002 | [42] | ||
| Application Mode | Contact | No Contact | |||
|---|---|---|---|---|---|
| Source of Bioactive VOCs | Growth of A. flavus | AFB1 Production | Growth of A. flavus | AFB1 Production | |
| Microorganisms | Bacteria | NA | NA | ↓ | ↓/↑ |
| Yeast | NA | NA | ↓ | ↓ | |
| Fungi | NA | NA | ↓ | NA/↓ | |
| Essential oil | ↓ | ↓ | ↓ | NA/↓ | |
| Individual VOC | Acid | ↓ | ↓ | ↓ | ↓ |
| Alcohol | ↓/↑ | ↓ | ↓ | ↓/↑ | |
| Aldehyde | ↓ | ↓ | ↓ | ↓ | |
| Alkane | NA | NA | NA | ↓ | |
| Ester | ↓ | ↓ | ↓ | ↓ | |
| Furan | NA | ↓/↑ | NA | ↓/↑ | |
| Ketone | NA | NA | ↓/↑ | ↓/↑ | |
| Terpene | ↓ | ↓ | ↓/↑ | ↓/↑ | |
| Other | NA | NA | NA | ↓ | |
| (a) | (b) Name | (c) Source | (d) Impact | (e) Application Mode | References | |||
|---|---|---|---|---|---|---|---|---|
| Growth | Aflatoxin | |||||||
| Alcohol | 1-Octen-3-ol | ◌ | ● | - | - | No contact | Singh et al., 2020 | [55] |
| 2-Buten-1-ol | ◌ | NA | - | No contact | Zeringue and McCormick, 1990 | [50] | ||
| 2-Butoxy alcohol | ◌ | - | NA | No contact | Zeringue and McCormick, 1990 | [50] | ||
| 2-Methylbutan-1-ol | ◌ | ● | - | + | No contact | Braun et al., 2012 Zeringue and McCormick, 1990 | [63] [50] | |
| 2-Phenylethanol | ◌ | - | - | No contact Contact | Chang et al., 2015 Gong et al., 2019 Hua et al., 2014 | [88] [60] [89] | ||
| 3-Hepten-1-ol | ◌ | - | - | No contact | Zeringue and McCormick, 1990 | [50] | ||
| Cis-hex-3-en-1-ol | ◌ | - | NA | No contact Contact | Ma et al., 2017 | [90] | ||
| 3-Methylbutan-1-ol | ◌ | ● | - | + | No contact | Braun et al., 2012 Zeringue and McCormick, 1990 | [63] [50] | |
| Cis-hex-2-en-1-ol | ◌ | - | + | No contact Contact | Ma et al., 2017 Zeringue and McCormick, 1990 | [90] [50] | ||
| Decan-1-ol | ◌ | ● | + | NA | No contact | Zeringue and McCormick, 1990 | [50] | |
| Ethanol | ◌ | ● | - | - | Contact | Ren et al., 2020 | [53] | |
| Heptan-1-ol | ◌ | - | - | No contact | Zeringue and McCormick, 1990 | [50] | ||
| Hexan-1-ol | ◌ | ● | - | - | No contact Contact | Cleveland et al., 2009 Ma et al., 2017 | [51] [90] | |
| Nonan-1-ol | ◌ | + | NA | No contact Contact | Zeringue and McCormick, 1990 Zhang et al., 2021 | [50] [54] | ||
| Octan-3-ol | ◌ | ● | - | - | No contact | Cleveland et al., 2009 | [51] | |
| Pentan-1-ol | ◌ | +/- | +/- | No contact | Cleveland et al., 2009 Zeringue and McCormick, 1990 | [51] [50] | ||
| Acid | 2-Methylpropanoic acid | ◌ | ● | - | NA | No contact | Braun et al., 2012 | [63] |
| 4-Pentanoic acid | ◌ | NA | - | No contact | Zeringue and McCormick, 1990 | [50] | ||
| Benzoic acid | ◌ | ● | - | - | Contact | Moon et al., 2018 | [91] | |
| Sorbic acid | ◌ | - | - | Contact | Moon et al., 2018 | [91] | ||
| Acetic acid | ◌ | ● | - | - | Contact | Moon et al., 2018 | [91] | |
| Propionic acid | ◌ | - | - | Contact | Moon et al., 2018 | [91] | ||
| Butyric acid | ◌ | - | - | Contact | Moon et al., 2018 | [91] | ||
| Aldehyde | Trans-hept-2-enal | ◌ | - | - | No contact | Cleveland et al., 2009 Zeringue and McCormick, 1990 Zeringue et al., 1996 | [51] [50] [92] | |
| 2,4-Hexadienal | ◌ | - | - | No contact | Cleveland et al., 2009 Zeringue and McCormick, 1990 | [51] [50] | ||
| (E,E)-2,4-Heptadienal | ◌ | - | NA | No contact | Ma and Johnson, 2021 | [93] | ||
| Oct-2-enal | ◌ | - | - | No contact | Cleveland et al., 2009 Zeringue and McCormick, 1990 Zeringue et al., 1996 | [51] [50] [92] | ||
| Benzaldehyde | ◌ | ● | - | - | No contact | Cleveland et al., 2009 | [51] | |
| Cinnamaldehyde | ◌ | - | - | Contact | Liang et al., 2015 Yin et al., 2015 Wang et al., 2019 | [94] [95] [96] | ||
| Citral | ◌ | - | - | Contact | Liang et al., 2015 | [94] | ||
| Diethylacetal 2-hexenal | ◌ | - | - | No contact | Zeringue and McCormick, 1990 | [50] | ||
| n-Decyl aldehyde | ◌ | - | - | No contact | Wright et al., 2000 | [97] | ||
| Furfural | ◌ | ● | - | NA | No contact | Zeringue, 2000 | [98] | |
| Heptanal | ◌ | ● | - | - | No contact | Zeringue and McCormick, 1990 Zeringue et al., 1996 | [50] [92] | |
| Hexanal | ◌ | ● | - | - | No contact Contact | Cleveland et al., 2009 Li et al., 2021 Ma et al., 2017 Wright et al., 2000 Zeringue and McCormick, 1990 Zeringue et al., 1996 | [51] [99] [90] [97] [50] [92] | |
| Nonanal | ◌ | ● | - | - | No contact | Cleveland et al., 2009 | [51] | |
| Nonyl aldehyde | ◌ | - | - | No contact | Zeringue and McCormick, 1990 Zeringue et al., 1996 | [50] [92] | ||
| Octanal | ◌ | ● | - | +/- | No contact | Cleveland et al., 2009 Wright et al., 2000 Zeringue and McCormick, 1990 Zeringue et al., 1996 | [51] [97] [50] [92] | |
| Sorbaldehyde | ◌ | - | NA | No contact | Ma and Johnson, 2021 | [93] | ||
| Trans-hex-2-enal | ◌ | ● | - | - | No contact | Cleveland et al., 2009 De Lucca et al., 2011 Ma et al., 2017 Zeringue and McCormick, 1990 Zeringue et al., 1996 | [51] [100] [90] [50] [92] | |
| Trans-2-methylbut-2-enal | ◌ | ● | NA | - | No contact | Moore et al., 2021 | [7] | |
| Trans-non-2-enal | ◌ | - | - | No contact | Zeringue and McCormick, 1990 Zeringue et al., 1996 | [50] [92] | ||
| Ester | Ethyl acetate | ◌ | ● | NA | - | No contact | Zeringue and McCormick, 1990 | [50] |
| Hexyl acetate | ◌ | - | NA | No contact Contact | Ma et al., 2017 | [90] | ||
| Trans-2-hexenyl acetate | ◌ | - | NA | No contact Contact | Ma et al., 2017 | [90] | ||
| Furan | 2,3-Dihydrofuran | ● | NA | - | No contact | Moore et al., 2021 Moore et al., 2022 | [7] [101] | |
| 2-Pentylfuran | ◌ | ● | = | + | No contact | Cleveland et al., 2009 | [51] | |
| Alkane | Decane | ◌ | ● | NA | - | No contact | Moore et al., 2021 | [7] |
| Ketone | Heptan-3-one | ◌ | + | + | No contact | Cleveland et al., 2009 | [51] | |
| Hexan-3-one | ◌ | = | - | No contact | Cleveland et al., 2009 | [51] | ||
| Nonan-2-one | ◌ | ● | - | - | No contact | Zeringue and McCormick, 1990 | [50] | |
| Octan-3-one | ◌ | ● | - | - | No contact | Moore et al., 2021 Moore et al., 2022 Zeringue and McCormick, 1990 | [7] [101] [50] | |
| 3-Octen-2-one | ◌ | ● | - | - | No contact | Cleveland et al., 2009 | [51] | |
| Pentan-3-one | ◌ | NA | + | No contact | Zeringue and McCormick, 1990 | [50] | ||
| Terpene | Alpha-pinene | ◌ | ● | + | (-) | No contact | Zeringue and McCormick, 1990 | [50] |
| Beta-pinene | ◌ | + | (-) | No contact | Zeringue and McCormick, 1990 | [50] | ||
| Camphene | ◌ | NA | - | No contact | Zeringue and McCormick, 1990 | [50] | ||
| Carvacrol | ◌ | - | NA | Contact | Yin et al., 2015 | [95] | ||
| Eugenol | ◌ | - | NA | Contact | Liang et al., 2015 | [94] | ||
| Farnesol | ◌ | - | - | Contact | Wang et al., 2014 | [102] | ||
| Limonene | ◌ | ● | NA | - | No contact | Zeringue and McCormick, 1990 | [50] | |
| Myrcene | ◌ | NA | + | No contact | Zeringue and McCormick, 1990 | [50] | ||
| Ocimene | ◌ | NA | + | No contact | Zeringue and McCormick, 1990 | [50] | ||
| Other | Methyl jasmonate | ◌ | NA | - | No contact | Goodrich-Tanrikulu et al., 1995 | [103] | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Josselin, L.; De Clerck, C.; De Boevre, M.; Moretti, A.; Fauconnier, M.-L. Impact of Volatile Organic Compounds on the Growth of Aspergillus flavus and Related Aflatoxin B1 Production: A Review. Int. J. Mol. Sci. 2022, 23, 15557. https://doi.org/10.3390/ijms232415557
Josselin L, De Clerck C, De Boevre M, Moretti A, Fauconnier M-L. Impact of Volatile Organic Compounds on the Growth of Aspergillus flavus and Related Aflatoxin B1 Production: A Review. International Journal of Molecular Sciences. 2022; 23(24):15557. https://doi.org/10.3390/ijms232415557
Chicago/Turabian StyleJosselin, Laurie, Caroline De Clerck, Marthe De Boevre, Antonio Moretti, and Marie-Laure Fauconnier. 2022. "Impact of Volatile Organic Compounds on the Growth of Aspergillus flavus and Related Aflatoxin B1 Production: A Review" International Journal of Molecular Sciences 23, no. 24: 15557. https://doi.org/10.3390/ijms232415557