Serum Metabolomics Reveals Distinct Profiles during Ischemia and Reperfusion in a Porcine Model of Myocardial Ischemia–Reperfusion
Abstract
:1. Introduction
2. Methods
2.1. Swine MI/R Model
2.2. Metabolomic Profiling
2.3. Workflow and Analyses of Metabolome Data
3. Results and Discussion
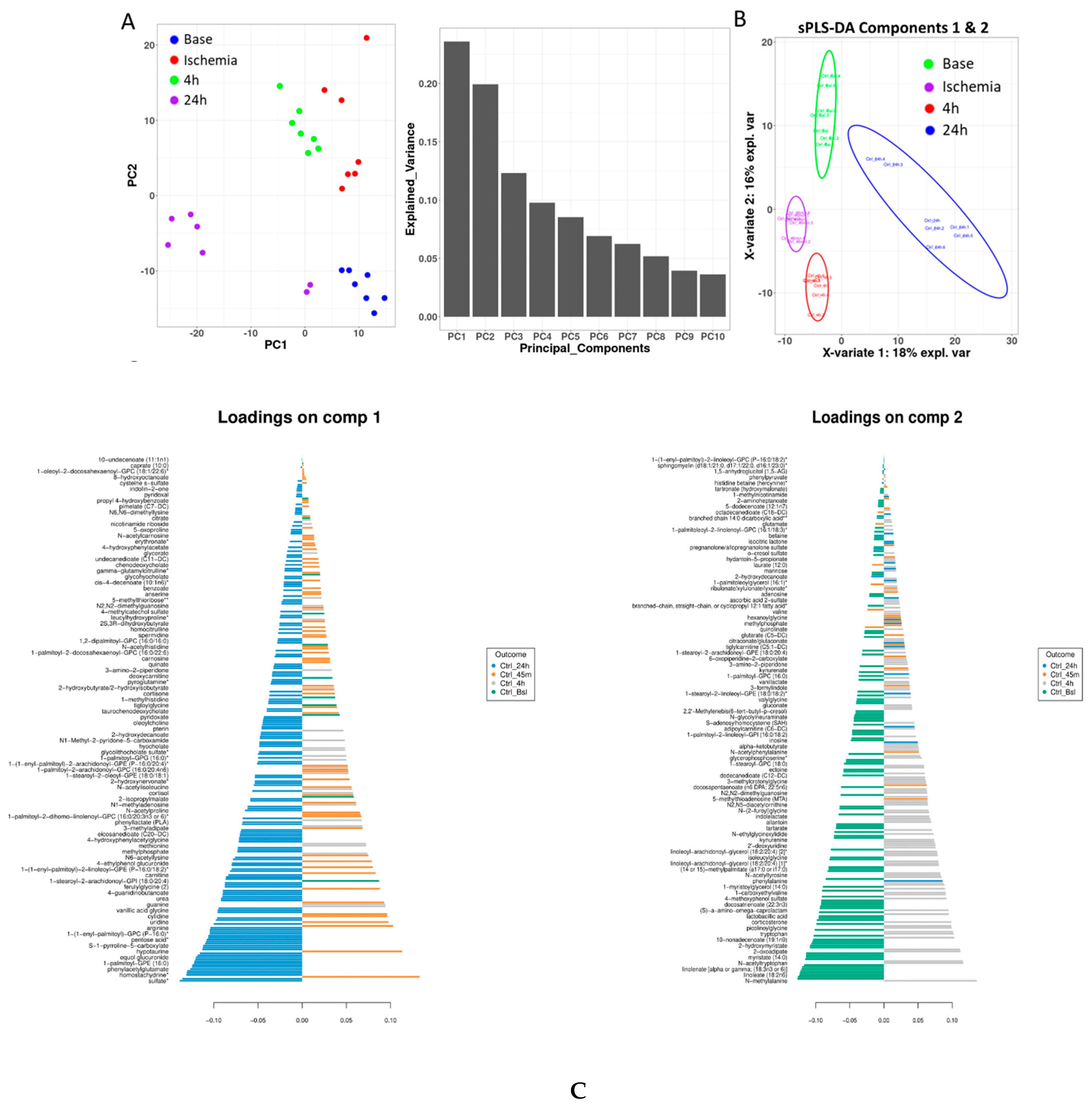
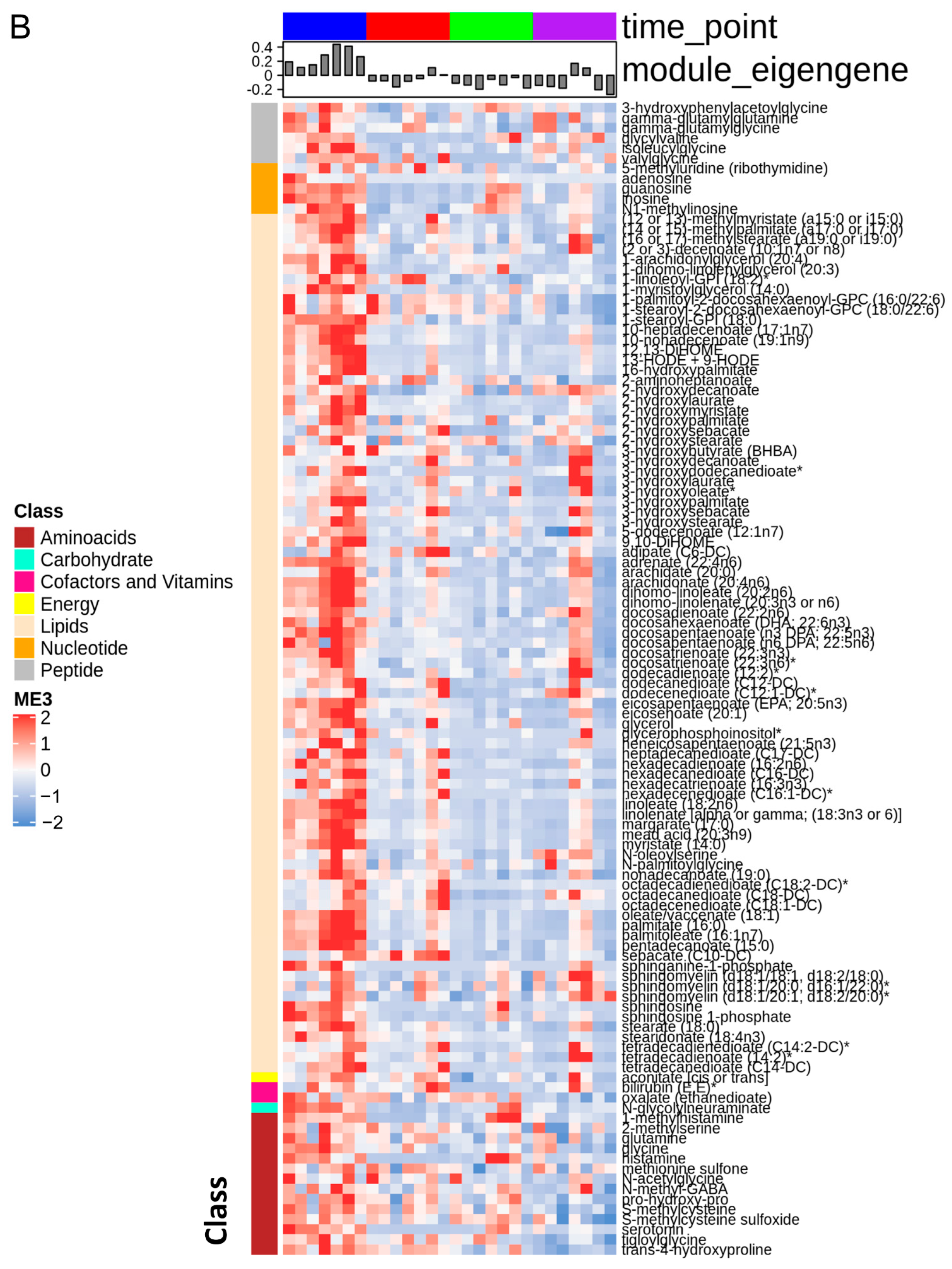
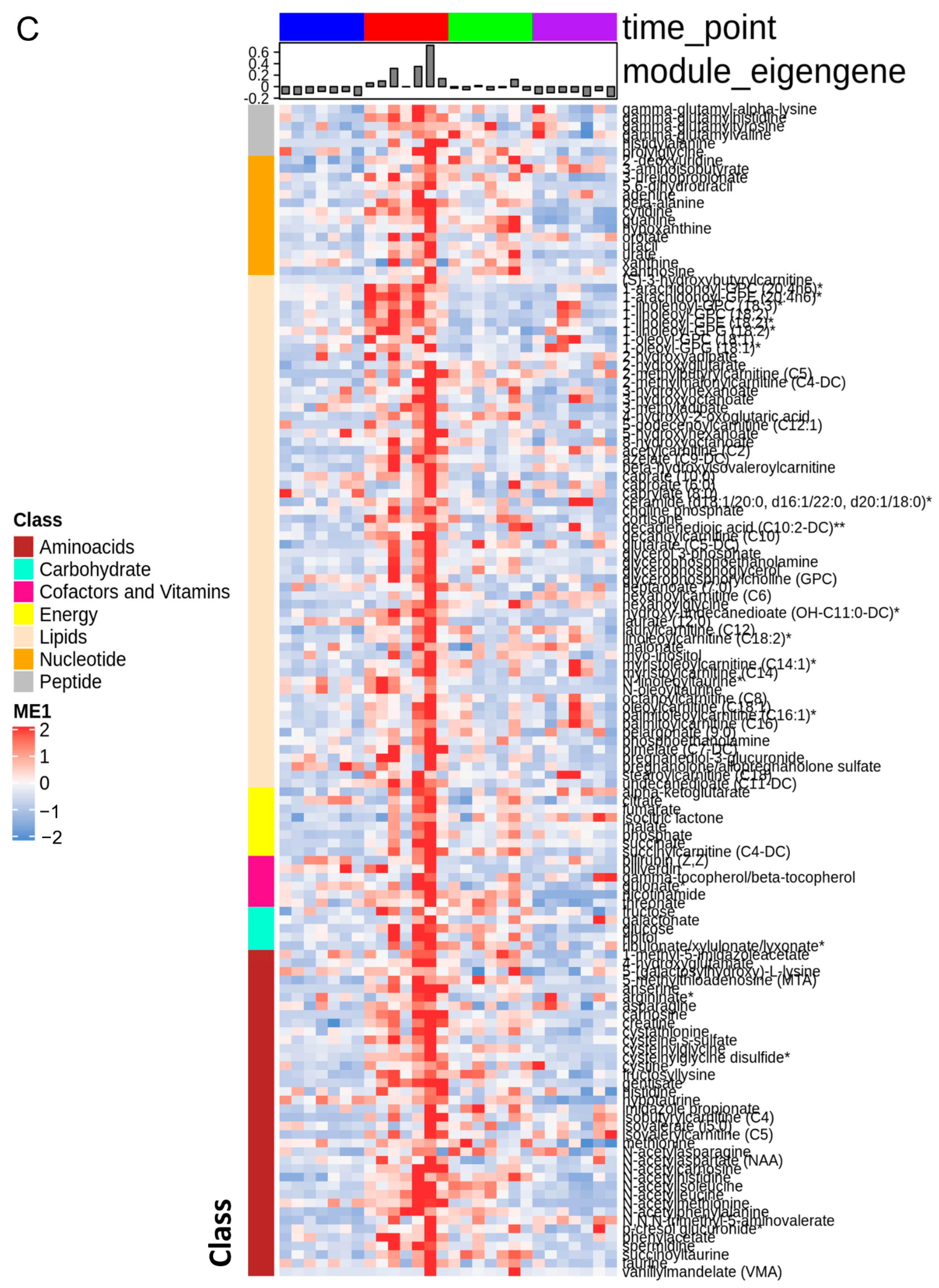
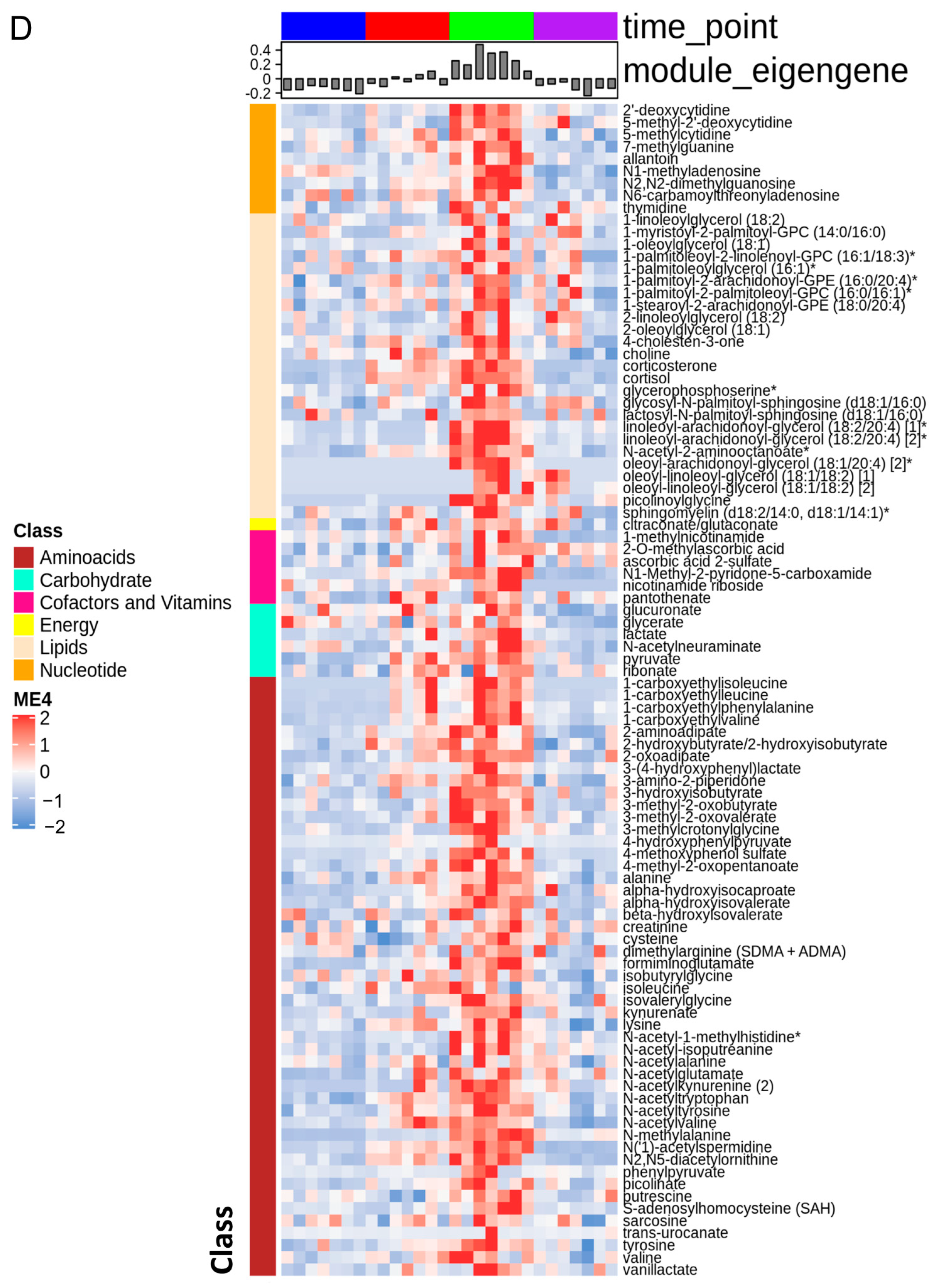
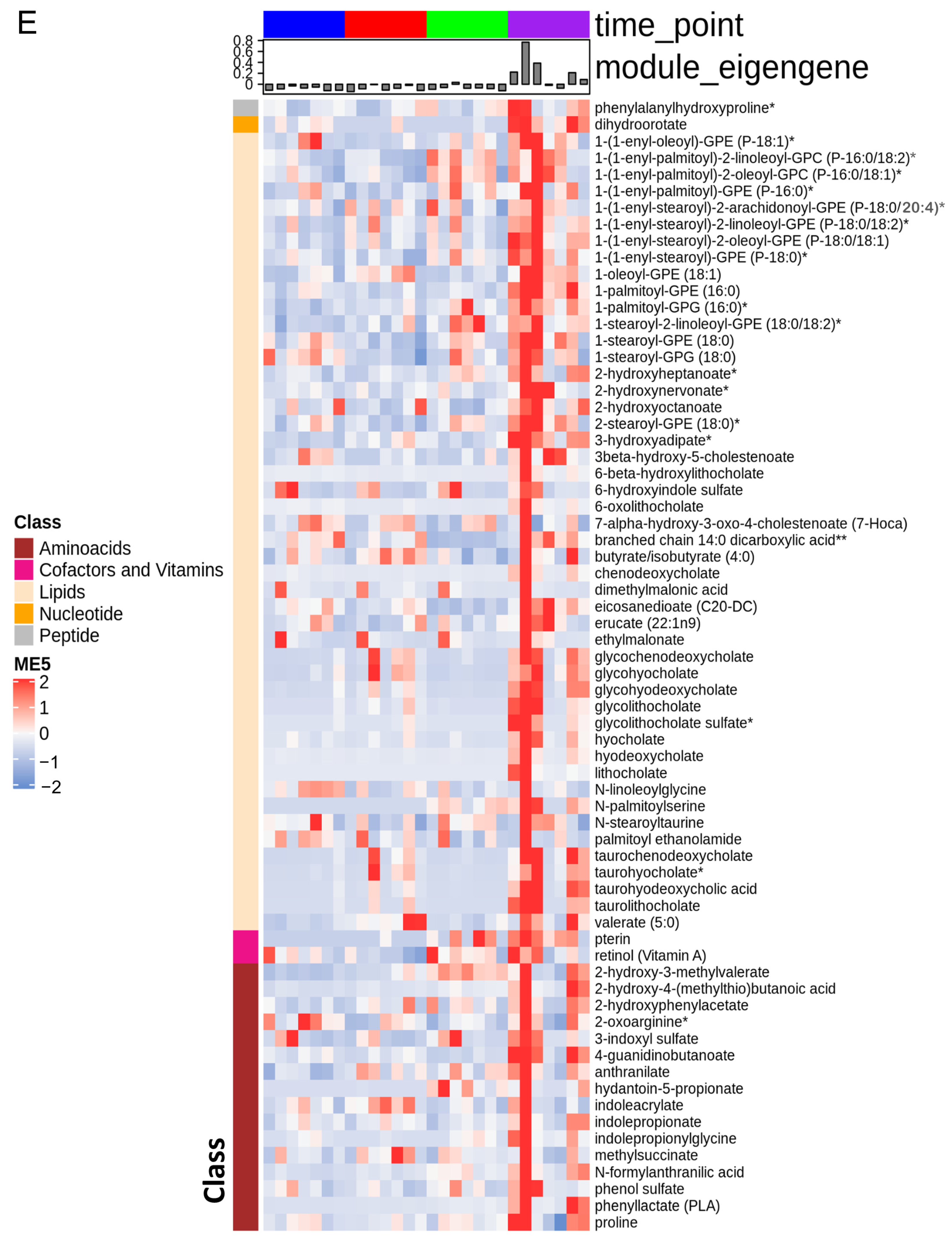
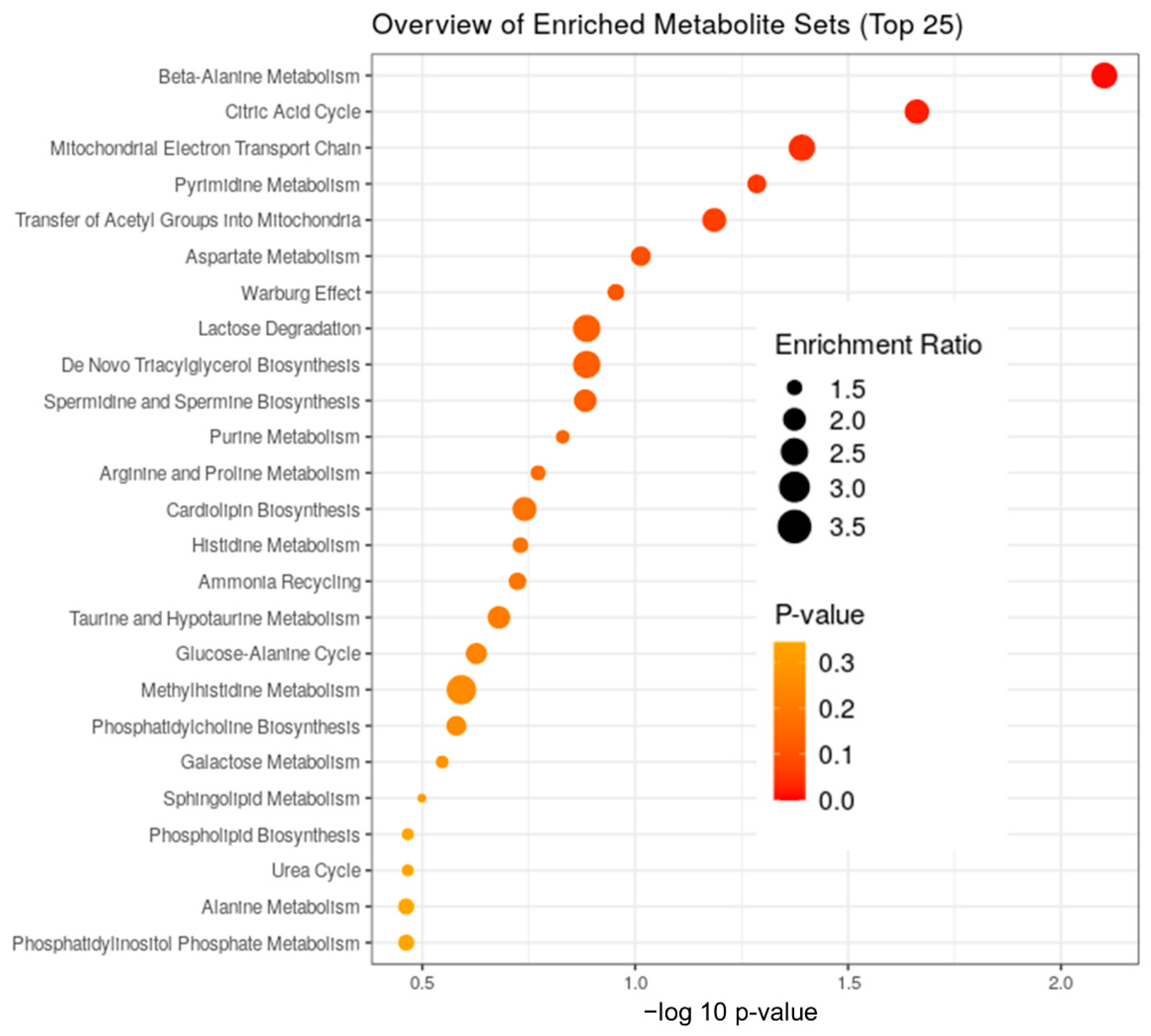
3.1. The Stages of IR Injury Show Distinct Serum Metabolite Profiles
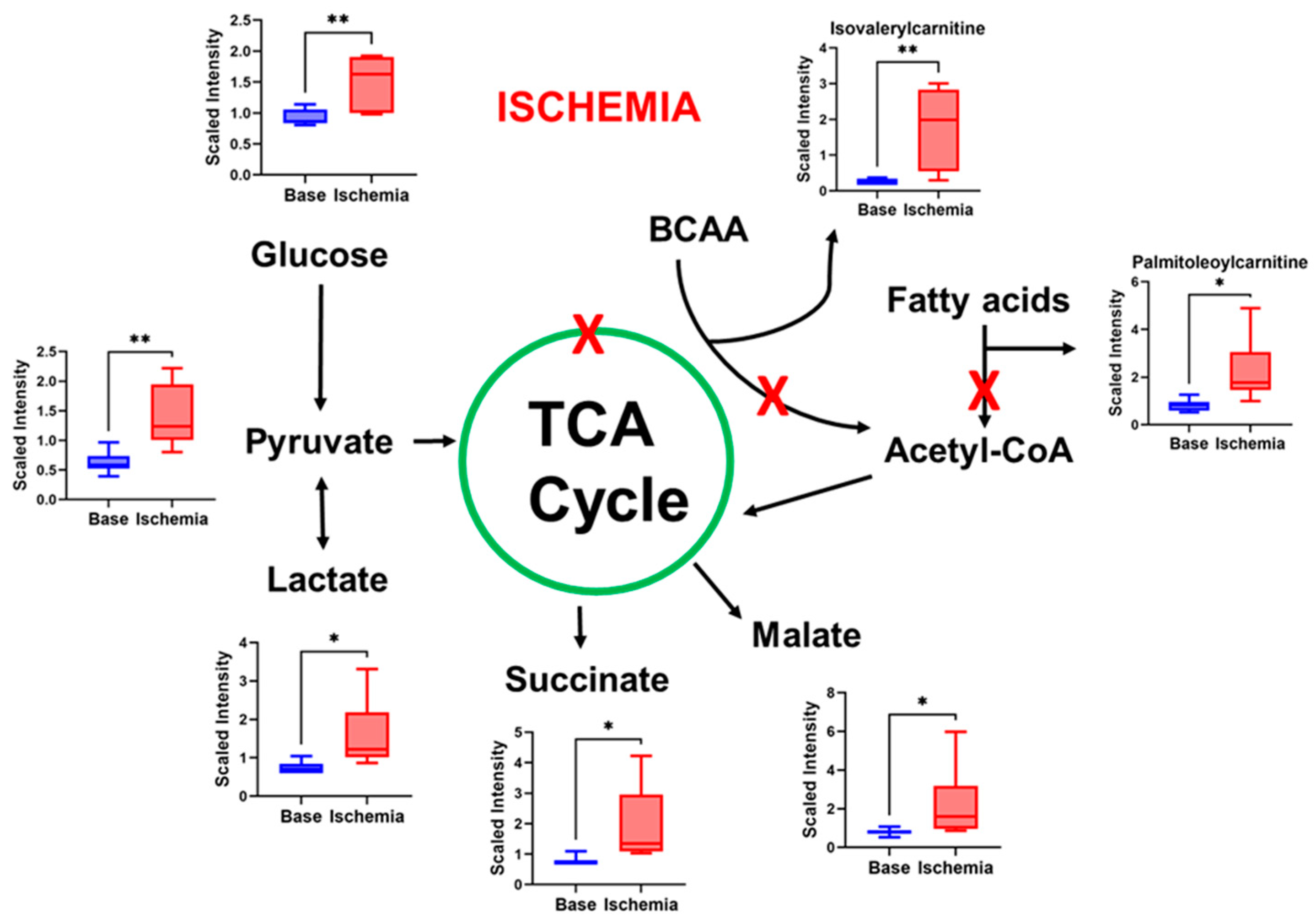
3.2. Ischemia Induces Changes in Metabolites That Indicate Impaired Mitochondrial Function
3.3. FFAs Related to Energy Metabolism Are Reduced by Ischemia
3.4. Polyunsaturated Fatty Acids (PUFAs) Are Suppressed by I–R Injury
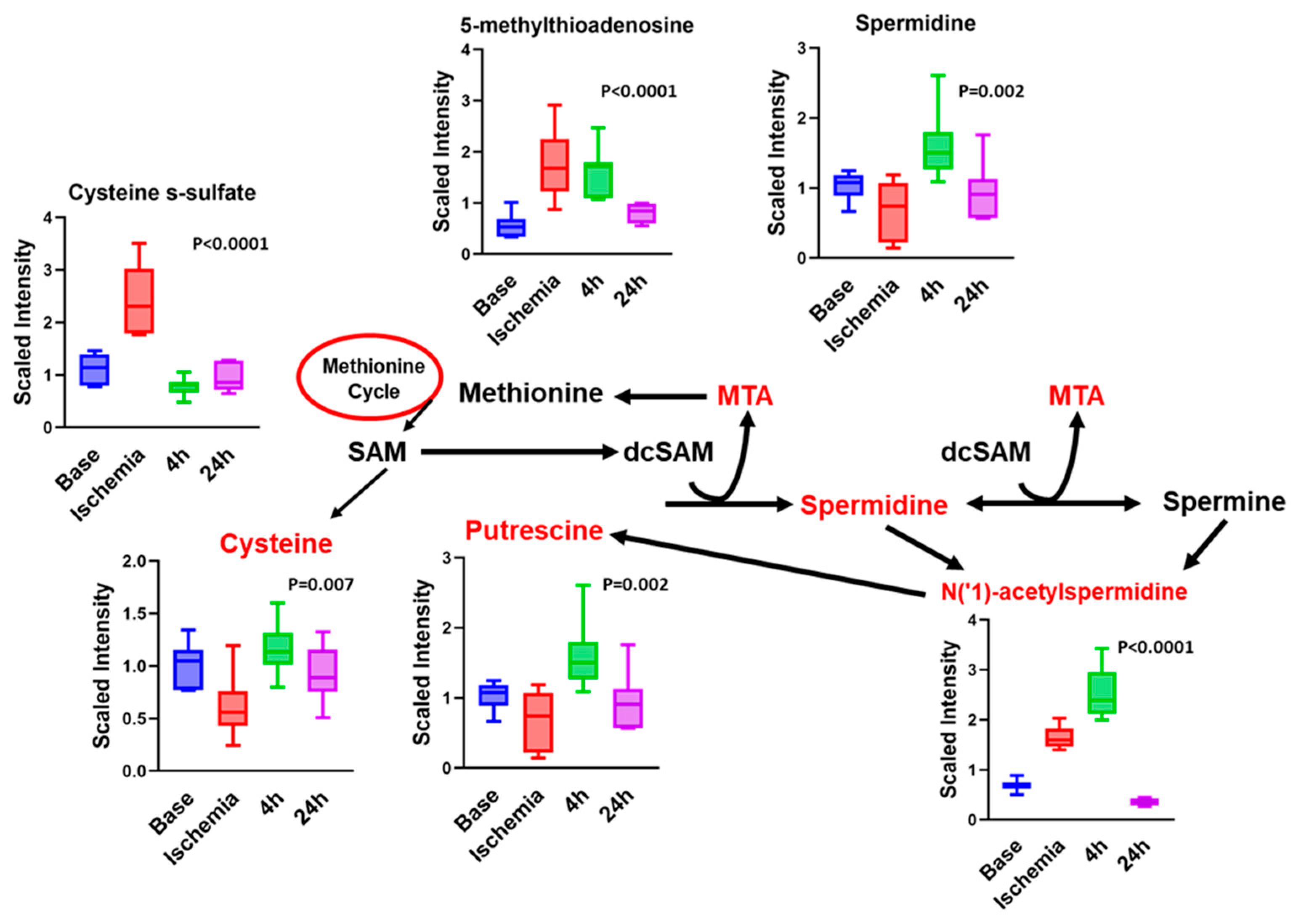
3.5. Ischemia Increases Serum Levels of N-Acetylated Amino Acids
3.6. Other Notable Changes in Amino-Acid Metabolism
3.7. BCAA Metabolites and Glycerols Are Predominant during the Early Reperfusion Phase
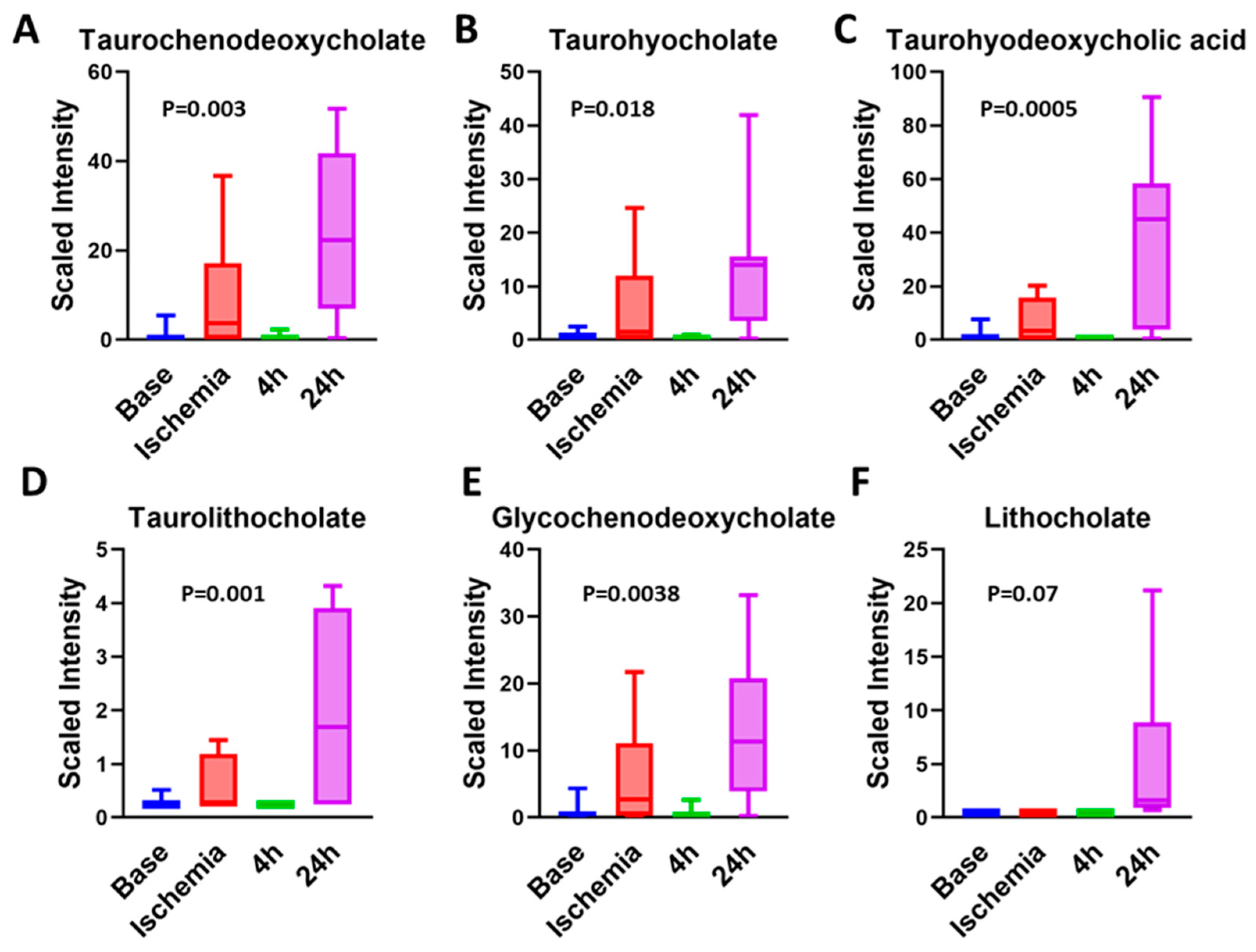
3.8. Bile Acids Are Key Metabolites during Late Reperfusion Phase
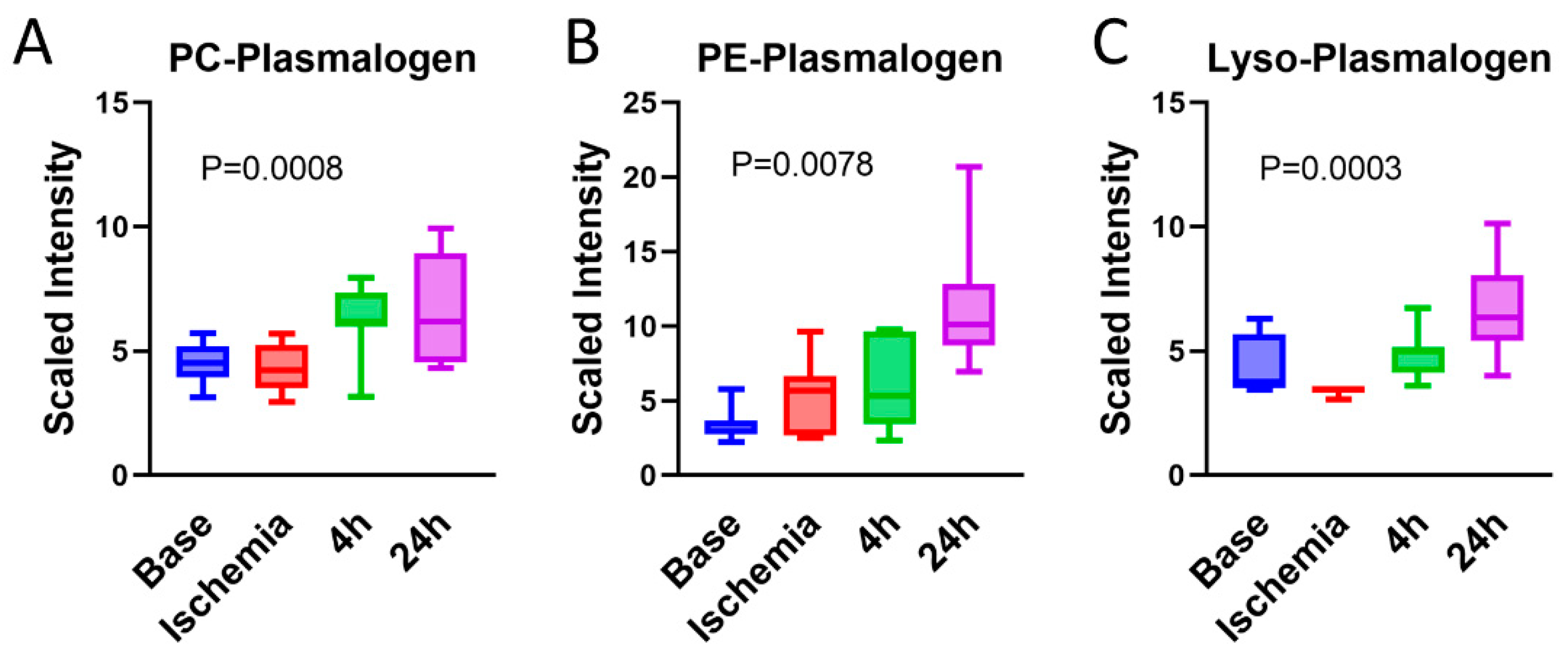
3.9. Serum Plasmalogens Increase during the Reperfusion Phase
4. Strengths and Limitations
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Benjamin, E.J.; Muntner, P.; Alonso, A.; Bittencourt, M.S.; Callaway, C.W.; Carson, A.P.; Chamberlain, A.M.; Chang, A.R.; Cheng, S.; Das, S.R.; et al. Heart Disease and Stroke Statistics-2019 Update: A Report From the American Heart Association. Circulation 2019, 139, e56–e528. [Google Scholar] [CrossRef] [PubMed]
- Law, M.R.; Watt, H.C.; Wald, N.J. The Underlying Risk of Death After Myocardial Infarction in the Absence of Treatment. Arch. Intern. Med. 2002, 162, 2405–2410. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aghajanian, H.; Kimura, T.; Rurik, J.G.; Hancock, A.S.; Leibowitz, M.S.; Li, L.; Scholler, J.; Monslow, J.; Lo, A.; Han, W.; et al. Targeting cardiac fibrosis with engineered T cells. Nature 2019, 573, 430–433. [Google Scholar] [CrossRef] [PubMed]
- Kochar, A.; Chen, A.Y.; Sharma, P.P.; Pagidipati, N.J.; Fonarow, G.C.; Cowper, P.A.; Roe, M.T.; Peterson, E.D.; Wang, T.Y. Long-Term Mortality of Older Patients with Acute Myocardial Infarction Treated in US Clinical Practice. J. Am. Heart Assoc. 2018, 7, e007230. [Google Scholar] [CrossRef] [Green Version]
- Jaswal, J.S.; Keung, W.; Wang, W.; Ussher, J.R.; Lopaschuk, G.D. Targeting fatty acid and carbohydrate oxidation—A novel therapeutic intervention in the ischemic and failing heart. Biochim. Biophys. Acta 2011, 1813, 1333–1350. [Google Scholar] [CrossRef] [Green Version]
- Dambrova, M.; Zuurbier, C.J.; Borutaite, V.; Liepinsh, E.; Makrecka-Kuka, M. Energy substrate metabolism and mitochondrial oxidative stress in cardiac ischemia/reperfusion injury. Free Radic. Biol. Med. 2021, 165, 24–37. [Google Scholar] [CrossRef]
- Lopaschuk, G.D.; Stanley, W.C. Glucose Metabolism in the Ischemic Heart. Circulation 1997, 95, 313–315. [Google Scholar] [CrossRef]
- Abel, E.D. Glucose transport in the heart. Front. Biosci. 2004, 9, 201–215. [Google Scholar] [CrossRef]
- Vik-Mo, H.; Mjøs, O.D.; Neely, J.R.; Maroko, P.R.; Ribeiro, L.G. Limitation of myocardial infarct size by metabolic interventions that reduce accumulation of fatty acid metabolites in ischemic myocardium. Am. Heart J. 1986, 111, 1048–1054. [Google Scholar] [CrossRef]
- Pell, V.R.; Chouchani, E.T.; Frezza, C.; Murphy, M.P.; Krieg, T. Succinate metabolism: A new therapeutic target for myocardial reperfusion injury. Cardiovasc. Res. 2016, 111, 134–141. [Google Scholar] [CrossRef] [Green Version]
- Mehta, S.R.; Yusuf, S.; Diaz, R.; Zhu, J.; Pais, P.; Xavier, D.; Paolasso, E.; Ahmed, R.; Xie, C.; Kazmi, K.; et al. Effect of glucose-insulin-potassium infusion on mortality in patients with acute ST-segment elevation myocardial infarction: The CREATE-ECLA randomized controlled trial. JAMA 2005, 293, 437–446. [Google Scholar] [PubMed]
- Klein, L.J.; van Campen, C.M.C.; Kamp, O.; Visser, F. Effects of high-dose insulin infusion on left ventricular function in normal subjects. Neth. Heart J. 2010, 18, 183–189. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ussher, J.R.; Wang, W.; Gandhi, M.; Keung, W.; Samokhvalov, V.; Oka, T.; Wagg, C.S.; Jaswal, J.S.; Harris, R.A.; Clanachan, A.S.; et al. Stimulation of glucose oxidation protects against acute myocardial infarction and reperfusion injury. Cardiovasc. Res. 2012, 94, 359–369. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lionetti, V.; Stanley, W.C.; Recchia, F.A. Modulating fatty acid oxidation in heart failure. Cardiovasc. Res. 2011, 90, 202–209. [Google Scholar] [CrossRef] [PubMed]
- Walters, A.M.; Porter, G.; Brookes, P.S. Mitochondria as a Drug Target in Ischemic Heart Disease and Cardiomyopathy. Circ. Res. 2012, 111, 1222–1236. [Google Scholar] [CrossRef]
- Bersin, R.M.; Wolfe, C.; Kwasman, M.; Lau, D.; Klinski, C.; Tanaka, K.; Khorrami, P.; Henderson, G.N.; de Marco, T.; Chatterjee, K. Improved hemodynamic function and mechanical efficiency in congestive heart failure with sodium dichloroacetate. J. Am. Coll. Cardiol. 1994, 23, 1617–1624. [Google Scholar] [CrossRef]
- Korvald, C.; Elvenes, O.P.; Myrmel, T. Myocardial substrate metabolism influences left ventricular energetics in vivo. Am. J. Physiol. Circ. Physiol. 2000, 278, H1345–H1351. [Google Scholar] [CrossRef] [Green Version]
- Chandler, M.P.; Stanley, W.C.; Morita, H.; Suzuki, G.; Roth, B.A.; Blackburn, B.; Wolff, A.; Sabbah, H.N. Short-Term Treatment with Ranolazine Improves Mechanical Efficiency in Dogs with Chronic Heart Failure. Circ. Res. 2002, 91, 278–280. [Google Scholar] [CrossRef] [Green Version]
- Zacharowski, K.; Blackburn, B.; Thiemermann, C. Ranolazine, a partial fatty acid oxidation inhibitor, reduces myocardial infarct size and cardiac troponin T release in the rat. Eur. J. Pharmacol. 2001, 418, 105–110. [Google Scholar] [CrossRef]
- Sesti, C.; Simkhovich, B.Z.; Kalvinsh, I.; Kloner, R.A. Mildronate, a novel fatty acid oxidation inhibitor and antianginal agent, reduces myocardial infarct size without affecting hemodynamics. J. Cardiovasc. Pharmacol. 2006, 47, 493–499. [Google Scholar] [CrossRef]
- Aksentijević, D.; McAndrew, D.J.; Karlstädt, A.; Zervou, S.; Sebag-Montefiore, L.; Cross, R.; Douglas, G.; Regitz-Zagrosek, V.; Lopaschuk, G.D.; Neubauer, S.; et al. Cardiac dysfunction and peri-weaning mortality in malonyl-coenzyme A decarboxylase (MCD) knockout mice as a consequence of restricting substrate plasticity. J. Mol. Cell. Cardiol. 2014, 75, 76–87. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kantor, P.F.; Dyck, J.R.; Lopaschuk, G.D. Fatty acid oxidation in the reperfused ischemic heart. Am. J. Med. Sci. 1999, 318, 3–14. [Google Scholar] [CrossRef]
- Kuznetsov, A.V.; Javadov, S.; Margreiter, R.; Grimm, M.; Hagenbuchner, J.; Ausserlechner, M.J. The Role of Mitochondria in the Mechanisms of Cardiac Ischemia-Reperfusion Injury. Antioxidants 2019, 8, 454. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sharp, T.E., 3rd; Polhemus, D.J.; Li, Z.; Spaletra, P.; Jenkins, J.S.; Reilly, J.P.; White, C.J.; Kapusta, D.R.; Lefer, D.J.; Goodchild, T.T. Renal Denervation Prevents Heart Failure Progression Via Inhibition of the Renin-Angiotensin System. J. Am. Coll. Cardiol. 2018, 72, 2609–2621. [Google Scholar] [CrossRef]
- Jones, S.P.; Tang, X.L.; Guo, Y.; Steenbergen, C.; Lefer, D.J.; Kukreja, R.C.; Kong, M.; Li, Q.; Bhushan, S.; Zhu, X.; et al. The NHLBI-sponsored Consortium for preclinicAl assESsment of cARdioprotective therapies (CAESAR): A new paradigm for rigorous, accurate, and reproducible evaluation of putative infarct-sparing interventions in mice, rabbits, and pigs. Circ. Res. 2015, 116, 572–586. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sharp, T.E., 3rd; Gong, Z.; Scarborough, A.; Goetzman, E.S.; Ali, M.J.; Spaletra, P.; Lefer, D.J.; Muzumdar, R.H.; Goodchild, T.T. Efficacy of a Novel Mitochondrial-Derived Peptide in a Porcine Model of Myocardial Ischemia/Reperfusion Injury. JACC Basic Transl. Sci. 2020, 5, 699–714. [Google Scholar] [CrossRef]
- Evans, A.M.; Bridgewater, B.R.; Liu, Q.; Mitchell, M.W.; Robinson, R.J.; Dai, H.; Stewart, S.J.; DeHaven, C.D.; Miller, L.A.D. High Resolution Mass Spectrometry Improves Data Quantity and Quality as Compared to Unit Mass Resolution Mass Spectrometry in High-Throughput Profiling Metabolomics. Metabolomics 2014, 4, 32. [Google Scholar]
- Rohart, F.; Gautier, B.; Singh, A.; Lê Cao, K.-A. mixOmics: An R package for ‘omics feature selection and multiple data integration. PLoS Comput. Biol. 2017, 13, e1005752. [Google Scholar] [CrossRef] [Green Version]
- Langfelder, P.; Horvath, S. WGCNA: An R package for weighted correlation network analysis. BMC Bioinform. 2008, 9, 559. [Google Scholar] [CrossRef] [Green Version]
- Pang, Z.; Chong, J.; Zhou, G.; de Lima Morais, D.A.; Chang, L.; Barrette, M.; Gauthier, C.; Jacques, P.-É.; Li, S.; Xia, J. MetaboAnalyst 5.0: Narrowing the gap between raw spectra and functional insights. Nucleic Acids Res. 2021, 49, W388–W396. [Google Scholar] [CrossRef]
- Kemp, R.G.; Fox, R.W.; Latshaw, S.P. Amino acid sequence at the citrate allosteric site of rabbit muscle phosphofructokinase. Biochemistry 1987, 26, 3443–3446. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Xiong, Z.; Yan, W.; Gao, E.; Cheng, H.; Wu, G.; Liu, Y.; Zhang, L.; Li, C.; Wang, S.; et al. Branched chain amino acids exacerbate myocardial ischemia/reperfusion vulnerability via enhancing GCN2/ATF6/PPAR-alpha pathway-dependent fatty acid oxidation. Theranostics 2020, 10, 5623–5640. [Google Scholar] [CrossRef] [PubMed]
- Uddin, G.M.; Zhang, L.; Shah, S.; Fukushima, A.; Wagg, C.S.; Gopal, K.; Al Batran, R.; Pherwani, S.; Ho, K.L.; Boisvenue, J.; et al. Impaired branched chain amino acid oxidation contributes to cardiac insulin resistance in heart failure. Cardiovasc. Diabetol. 2019, 18, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Ferdinandusse, S.; Denis, S.; Van Roermund, C.W.; Wanders, R.J.; Dacremont, G. Identification of the peroxisomal beta-oxidation enzymes involved in the degradation of long-chain dicarboxylic acids. J. Lipid Res. 2004, 45, 1104–1111. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, X.; Gao, T.; Deng, S.; Shang, L.; Chen, X.; Chen, K.; Li, P.; Cui, X.; Zeng, J. Fasting induces hepatic lipid accumulation by stimulating peroxisomal dicarboxylic acid oxidation. J. Biol. Chem. 2021, 296, 100622. [Google Scholar] [CrossRef] [PubMed]
- Tan, S.T.; Ramesh, T.; Toh, X.R.; Nguyen, L.N. Emerging roles of lysophospholipids in health and disease. Prog. Lipid Res. 2020, 80, 101068. [Google Scholar] [CrossRef]
- Desnoyers, M.; Gilbert, K.; Rousseau, G. Cardioprotective Effects of Omega-3 Polyunsaturated Fatty Acids: Dichotomy between Experimental and Clinical Studies. Mar. Drugs 2018, 16, 234. [Google Scholar] [CrossRef] [Green Version]
- Linster, E.; Stephan, I.; Bienvenut, W.V.; Maple-Grødem, J.; Myklebust, L.M.; Huber, M.; Reichelt, M.; Sticht, C.; Møller, S.G.; Meinnel, T.; et al. Downregulation of N-terminal acetylation triggers ABA-mediated drought responses in Arabidopsis. Nat. Commun. 2015, 6, 7640. [Google Scholar] [CrossRef] [Green Version]
- Ree, R.; Varland, S.; Arnesen, T. Spotlight on protein N-terminal acetylation. Exp. Mol. Med. 2018, 50, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Herr, D.J.; Singh, T.; Dhammu, T.; Menick, D.R. Regulation of metabolism by mitochondrial enzyme acetylation in cardiac ischemia-reperfusion injury. Biochim. Biophys. Acta (BBA)—Mol. Basis Dis. 2020, 1866, 165728. [Google Scholar] [CrossRef]
- Wei, C.; Wang, Y.; Li, M.; Li, H.; Lu, X.; Shao, H.; Xu, C. Spermine inhibits Endoplasmic Reticulum Stress—Induced Apoptosis: A New Strategy to Prevent Cardiomyocyte Apoptosis. Cell. Physiol. Biochem. 2016, 38, 531–544. [Google Scholar] [CrossRef] [PubMed]
- Duan, Q.; Yang, W.; Jiang, D.; Tao, K.; Dong, A.; Cheng, H. Spermine ameliorates ischemia/reperfusion injury in cardiomyocytes via regulation of autophagy. Am. J. Transl. Res. 2016, 8, 3976–3985. [Google Scholar] [PubMed]
- Tantini, B.; Fiumana, E.; Cetrullo, S.; Pignatti, C.; Bonavita, F.; Shantz, L.M.; Giordano, E.; Muscari, C.; Flamigni, F.; Guarnieri, C.; et al. Involvement of polyamines in apoptosis of cardiac myoblasts in a model of simulated ischemia. J. Mol. Cell. Cardiol. 2006, 40, 775–782. [Google Scholar] [CrossRef] [PubMed]
- Nayak, A.; Liu, C.; Mehta, A.; Ko, Y.; Tahhan, A.S.; Dhindsa, D.S.; Uppal, K.; Jones, D.P.; Butler, J.; Morris, A.A.; et al. N8-Acetylspermidine: A Polyamine Biomarker in Ischemic Cardiomyopathy with Reduced Ejection Fraction. J. Am. Heart Assoc. 2020, 9, e016055. [Google Scholar] [CrossRef]
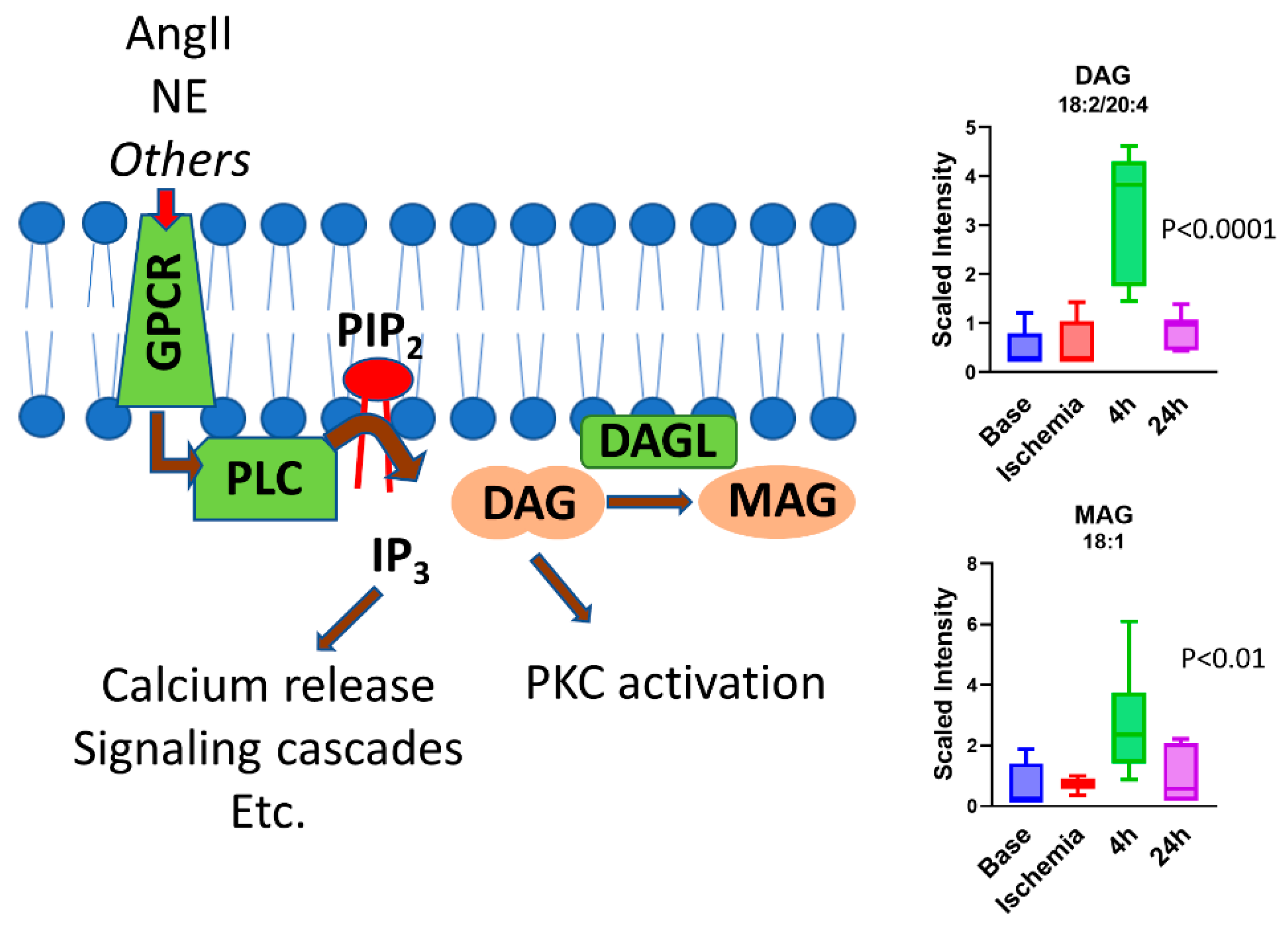
- Miyamae, M.; Domae, N.; Zhou, H.-Z.; Sugioka, S.; Diamond, I.; Figueredo, V.M. Phospholipase C activation is required for cardioprotection by ethanol consumption. Exp. Clin. Cardiol. 2003, 8, 184–188. [Google Scholar]
- Kong, L.; Andrassy, M.; Chang, J.S.; Huang, C.; Asai, T.; Szabolcs, M.J.; Homma, S.; Liu, R.; Zou, Y.S.; Leitges, M.; et al. PKCbeta modulates ischemia-reperfusion injury in the heart. American journal of physiology. Heart Circ. Physiol. 2008, 294, H1862–H1870. [Google Scholar] [CrossRef]
- Boni, L.T.; Rando, R.R. The nature of protein kinase C activation by physically defined phospholipid vesicles and diacylglycerols. J. Biol. Chem. 1985, 260, 10819–10825. [Google Scholar] [CrossRef]
- Ekser, B.; Gridelli, B.; Cooper, D.K. Porcine alanine transaminase after liver allo-and xenotransplantation. Xenotransplantation 2012, 19, 52–55. [Google Scholar] [CrossRef] [Green Version]
- Nguyen, C.C.; Duboc, D.; Rainteau, D.; Sokol, H.; Humbert, L.; Seksik, P.; Bellino, A.; Abdoul, H.; Bouazza, N.; Treluyer, J.-M.; et al. Circulating bile acids concentration is predictive of coronary artery disease in human. Sci. Rep. 2021, 11, 1–10. [Google Scholar] [CrossRef]
- Feng, X.; Zhai, G.; Yang, J.; Liu, Y.; Zhou, Y.; Guo, Q. Myocardial Infarction and Coronary Artery Disease in Menopausal Women with Type 2 Diabetes Mellitus Negatively Correlate with Total Serum Bile Acids. Front. Endocrinol. 2021, 12, 754006. [Google Scholar] [CrossRef]
- Huang, L.; Xu, G.; Zhang, R.; Wang, Y.; Ji, J.; Long, F.; Sun, Y. Increased admission serum total bile acids can be associated with decreased 3-month mortality in patients with acute ischemic stroke. Lipids Health Dis. 2022, 21, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.-C.; Chen, J.-H.; Xiang, C.-H.; Su, M.-Y.; Zhang, X.-S.; Ma, Y.-F. Increased serum bile acid level is associated with high-risk coronary artery plaques in an asymptomatic population detected by coronary computed tomography angiography. J. Thorac. Dis. 2019, 11, 5063–5070. [Google Scholar] [CrossRef] [PubMed]
- Shoaib, M.; Choudhary, R.C.; Choi, J.; Kim, N.; Hayashida, K.; Yagi, T.; Yin, T.; Nishikimi, M.; Stevens, J.F.; Becker, L.B.; et al. Plasma metabolomics supports the use of long-duration cardiac arrest rodent model to study human disease by demonstrating similar metabolic alterations. Sci. Rep. 2020, 10, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Bozelli, J.C.J.; Azher, S.; Epand, R.M. Plasmalogens and Chronic Inflammatory Diseases. Front. Physiol. 2021, 12. [Google Scholar] [CrossRef]
- Maulik, N.; Tosaki, A.; Engelman, R.M.; Cordis, G.A.; Das, D.K. Myocardial Salvage by Chimyl Alcohol: Possible Role of Peroxisomal Dysfunction in Reperfusion Injury. Ann. N. Y. Acad. Sci. 1994, 723, 380–384. [Google Scholar] [CrossRef]
- Davies, N.J.; Schulz, R.; Olley, P.M.; Strynadka, K.D.; Panas, D.L.; Lopaschuk, G.D. Lysoplasmenylethanolamine accumulation in ischemic/reperfused isolated fatty acid-perfused hearts. Circ. Res. 1992, 70, 1161–1168. [Google Scholar] [CrossRef] [Green Version]
- Honsho, M.; Abe, Y.; Fujiki, Y. Plasmalogen biosynthesis is spatiotemporally regulated by sensing plasmalogens in the inner leaflet of plasma membranes. Sci. Rep. 2017, 7, srep43936. [Google Scholar] [CrossRef] [Green Version]



| Ischemia | 4 h | 24 h | p-Value | Effect Size | |
|---|---|---|---|---|---|
| Saturated Fatty Acids | |||||
| Myristate (14:0) | 0.29 | 0.22 | 0.24 | 1.4 × 10−11 | 0.67 |
| Pentadecanoate (15:0) | 0.64 | 0.53 | 0.57 | 5.5 × 10−11 | 0.65 |
| Palmitate (16:0) | 0.44 | 0.30 | 0.31 | 6.5 × 10−11 | 0.66 |
| Margarate (17:0) | 0.46 | 0.29 | 0.27 | 1.0 × 10−11 | 0.67 |
| Monounsaturated Fatty Acids | |||||
| Palmitoleate (16:1n7) | 0.25 | 0.15 | 0.18 | 2.0 × 10−12 | 0.69 |
| 10-Heptadecenoate (17:1n7) | 0.25 | 0.14 | 0.16 | 2.0 × 10−12 | 0.70 |
| Oleate/vaccenate (18:1) | 0.40 | 0.22 | 0.22 | 3.4 × 10−11 | 0.72 |
| 10-Nonadecenoate (19:1n9) | 0.36 | 0.24 | 0.28 | 1.0 × 10−10 | 0.65 |
| Polyunsatured Fatty Acids | |||||
| Linoleate (18:2n6) | 0.34 | 0.19 | 0.24 | 7.0 × 10−12 | 0.69 |
| Linolenate (alpha or gamma; (18:3n3 or 6)) | 0.27 | 0.15 | 0.20 | 8.0 × 10−12 | 0.65 |
| Mead acid (20:3n9) | 0.36 | 0.21 | 0.21 | 3.7 × 10−11 | 0.58 |
| Dihomo-linolenate (20:3n3 or n6) | 0.37 | 0.27 | 0.26 | 1.2 × 10−10 | 0.68 |
| Arachidonate (20:4n6) | 0.47 | 0.30 | 0.36 | 6.6 × 10−10 | 0.70 |
| Eicosapentaenoate (EPA; 20:5n3) | 0.51 | 0.25 | 0.25 | 1.0 × 10−11 | 0.66 |
| Adrenate (22:4n6) | 0.44 | 0.32 | 0.44 | 1.0 × 10−7 | 0.61 |
| Docosapentaenoate (n3 DPA; 22:5n3) | 0.41 | 0.29 | 0.37 | 1.5 × 10−9 | 0.66 |
| Metabolite Name | F-Statistic | Effect Size | Changes with Ischemia | p-Value |
|---|---|---|---|---|
| N-Acetyl serine | 10.755 | 0.59 | Decreased | 0.0088 |
| N-Acetyl histidine | 11.438 | 0.73 | Increased | 0.0215 |
| N-Acetyl valine | 22.487 | 0.78 | Increased | 0.0015 |
| N-Acetyl leucine | 14.858 | 0.69 | Increased | 0.0002 |
| N-Acetyl isoleucine | 8.821 | 0.57 | Increased | 0.0143 |
| N-Acetyl methionine | 31.556 | 0.97 | Increased | 0.0462 |
| N-Acetyl phenylalanine | 19.688 | 0.71 | Increased | 0.0000 |
| N-Acetyl tryptophan | 31.036 | 0.77 | Increased | 0.0001 |
| Metabolite_Name | F-Statistic | Effect Size | Changes with Ischemia | p-Value |
|---|---|---|---|---|
| N(‘1)-Acetyl spermidine | 88.846 | 0.912 | Increased | 2.37812 × 10−5 |
| Spermidine | 15.366 | 0.631 | Increased | 8.84902 × 10−5 |
| 5-Methylthioadenosine (MTA) | 15.348 | 0.609 | Increased | 0.000146238 |
| Biochemical Name | 45 min | 4 h | 24 h | |
|---|---|---|---|---|
| Primary bile acid metabolism | Glycochenodeoxycholate | 6.46 | 0.61 | 13.60 |
| Taurochenodeoxycholate | 8.39 | 0.52 | 21.49 | |
| Secondary bile acid metabolism | Deoxycholate | 0.77 | 0.74 | 1.55 |
| 6-beta-Hydroxylithocholate | 0.48 | 0.29 | 11.03 | |
| Lithocholate | 1.00 | 1.00 | 12.05 | |
| Glycolithocholate | 2.63 | 0.74 | 11.33 | |
| Glycolithocholate sulfate * | 1.19 | 0.91 | 4.68 | |
| Taurolithocholate | 1.99 | 0.86 | 7.45 | |
| 6-Oxolithocholate | 0.36 | 0.22 | 7.41 | |
| Hyocholate | 1.06 | 0.46 | 7.09 | |
| Glycohyocholate | 4.17 | 0.24 | 7.80 | |
| Taurohyocholate * | 10.22 | 0.49 | 20.89 | |
| Hyodeoxycholate | 0.76 | 0.57 | 20.39 | |
| Taurohyodeoxycholic acid | 4.47 | 0.33 | 26.68 | |
| glycohyodeoxycholate | 3.08 | 0.34 | 12.54 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Goetzman, E.; Gong, Z.; Rajasundaram, D.; Muzumdar, I.; Goodchild, T.; Lefer, D.; Muzumdar, R. Serum Metabolomics Reveals Distinct Profiles during Ischemia and Reperfusion in a Porcine Model of Myocardial Ischemia–Reperfusion. Int. J. Mol. Sci. 2022, 23, 6711. https://doi.org/10.3390/ijms23126711
Goetzman E, Gong Z, Rajasundaram D, Muzumdar I, Goodchild T, Lefer D, Muzumdar R. Serum Metabolomics Reveals Distinct Profiles during Ischemia and Reperfusion in a Porcine Model of Myocardial Ischemia–Reperfusion. International Journal of Molecular Sciences. 2022; 23(12):6711. https://doi.org/10.3390/ijms23126711
Chicago/Turabian StyleGoetzman, Eric, Zhenwei Gong, Dhivyaa Rajasundaram, Ishan Muzumdar, Traci Goodchild, David Lefer, and Radhika Muzumdar. 2022. "Serum Metabolomics Reveals Distinct Profiles during Ischemia and Reperfusion in a Porcine Model of Myocardial Ischemia–Reperfusion" International Journal of Molecular Sciences 23, no. 12: 6711. https://doi.org/10.3390/ijms23126711






