Cyclic GMP in Liver Cirrhosis—Role in Pathophysiology of Portal Hypertension and Therapeutic Implications
Abstract
:1. Introduction
2. Current Research Directions in Components of the NO-cGMP Pathway in Experimental Liver Damage
3. Role of the NO-cGMP Pathway in PH Pathophysiology
- These drugs are potential therapeutic alternatives for portal hypertension.
- PDE-5 inhibitors or sGC modulators may be useful for reversal of liver fibrosis/cirrhosis, as shown in animal studies.
- PDE-5 inhibitors are a promising therapy of hepatic encephalopathy in liver cirrhosis.
- Serum levels of cGMP can be used as a simple non-invasive marker of clinically significant portal hypertension.
4. Modulation of the NO-cGMP Pathway in a Healthy and Cirrhotic Liver
4.1. Effect in Portal Hypertension
4.2. Targeting the NO-cGMP Pathway May Contribute to Reversal of Liver Fibrosis/Cirrhosis
4.3. Role of Inhibitors of PDE-5 or Modulators of sGC in Hepatic Encephalopathy
4.4. Plasma/Serum cGMP as a Potential Marker of Clinically Significant Portal Hypertension (CSPH)
5. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| NO | nitric oxide |
| GTP | guanosine triphosphate |
| cGMP | cyclic guanosine monophosphate |
| PKG | protein kinase G |
| CaMKII | calcium/calmodulin-dependent protein kinase II |
| eNOS | endothelial NO synthase |
| nNOS | neuronal NO synthase |
| sGC | soluble guanylate cyclase |
| PDE-5 | phosphodiesterase-5 |
| PDE-5-I | phosphodiesterase-5-inhibitor |
| CSPH | clinically significant portal hypertension |
| ANP | atria natriuretic factor |
| MELD | Model End Stage Liver Disease |
| HE | hepatic encephalopathy |
| PVP | portal venous pressure |
| MAP | mean arterial pressure |
| PPHTN | porto-pulmonary hypertension |
References
- Tsochatzis, E.A.; Bosch, J.; Burroughs, A.K. Liver cirrhosis. Lancet 2014, 383, 1749–1761. [Google Scholar] [CrossRef]
- Turco, L.; Garcia-Tsao, G. Portal Hypertension: Pathogenesis and Diagnosis. Clin. Liver Dis. 2019, 23, 573–587. [Google Scholar] [CrossRef] [PubMed]
- Berzigotti, A.; Seijo, S.; Reverter, E.; Bosch, J. Assessing portal hypertension in liver diseases. Expert Rev. Gastroenterol. Hepatol. 2013, 7, 141–155. [Google Scholar] [CrossRef] [PubMed]
- Bosch, J.; Pizcueta, P.; Feu, F.; Fernández, M.; Garcia-Pagan, J.C. Pathophysiology of portal hypertension. Gastroenterol. Clin. N. Am. 1992, 21, 1–14. [Google Scholar] [CrossRef]
- Bosch, J. Vascular deterioration in cirrhosis: The big picture. J. Clin. Gastroenterol. 2007, 41 (Suppl. S3), S247–S253. [Google Scholar] [CrossRef] [PubMed]
- Bosch, J.; Gonzalez-Abraldes, J.; Berzigotti, A.; Garcia-Pagan, J.C. The clinical use of HVPG measurements in chronic liver disease. Nat. Rev. Gastroenterol. Hepatol. 2009, 6, 573–582. [Google Scholar] [CrossRef]
- Bosch, J.; Groszmann, R.J.; Shah, V.H. Evolution in the understanding of the pathophysiological basis of portal hypertension: How changes in paradigm are leading to successful new treatments. J. Hepatol. 2015, 62, S121–S130. [Google Scholar] [CrossRef] [Green Version]
- Wongcharatrawee, S.; Groszmann, R.J. Diagnosing portal hypertension. Baillieres. Best Pract. Res. Clin. Gastroenterol. 2000, 14, 881–894. [Google Scholar] [CrossRef]
- Gracia-Sancho, J.; Maeso-Díaz, R.; Bosch, J. Pathophysiology and a Rational Basis of Therapy. Dig. Dis. 2015, 33, 508–514. [Google Scholar] [CrossRef]
- De Franchis, R.; Dell’Era, A.; Primignani, M. Diagnosis and monitoring of portal hypertension. Dig. Liver Dis. 2008, 40, 312–317. [Google Scholar] [CrossRef]
- De Franchis, R.; Baveno, V.I. Faculty. Expanding consensus in portal hypertension: Report of the Baveno VI Consensus Workshop: Stratifying risk and individualizing care for portal hypertension. J. Hepatol. 2015, 63, 743–752. [Google Scholar] [CrossRef] [Green Version]
- La Mura, V.; Abraldes, J.G.; Raffa, S.; Retto, O.; Berzigotti, A.; García-Pagán, J.C.; Bosch, J. Prognostic value of acute hemodynamic response to i.v. propranolol in patients with cirrhosis and portal hypertension. J. Hepatol. 2009, 51, 279–287. [Google Scholar] [CrossRef]
- Villanueva, C.; Aracil, C.; Colomo, A.; Hernández-Gea, V.; López-Balaguer, J.M.; Alvarez-Urturi, C.; Torras, X.; Balanzó, J.; Guarner, C. Acute hemodynamic response to beta-blockers and prediction of long-term outcome in primary prophylaxis of variceal bleeding. Gastroenterology 2009, 137, 119–128. [Google Scholar] [CrossRef]
- Alvarado Tapias, E.; Ardevol, A.; Garcia-Guix, M.; Montañés, R.; Pavel, O.; Cuyas, B.; Graupera, I.; Brujats, A.; Vilades, D.; Colomo, A.; et al. Short-term hemodynamic effects of β-blockers influence survival of patients with decompensated cirrhosis. J. Hepatol. 2020, 73, 829–841. [Google Scholar] [CrossRef] [PubMed]
- Fernandez, M. Molecular pathophysiology of portal hypertension. Hepatology 2015, 61, 1406–1415. [Google Scholar] [CrossRef]
- Mccuskey, R.S. The Hepatic Microvascular System in Health and Its Response to Toxicants. Anat. Rec. Adv. Integr. Anat. Evol. Biol. 2008, 291, 661–671. [Google Scholar] [CrossRef] [PubMed]
- Sørensen, K.K.; Simon-Santamaria, J.; McCuskey, R.S.; Smedsrød, B. Liver Sinusoidal Endothelial Cells. Compr. Physiol. 2015, 5, 1751–1774. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Iwakiri, Y.; Groszmann, R.J. Vascular endothelial dysfunction in cirrhosis. J. Hepatol. 2007, 46, 927–934. [Google Scholar] [CrossRef] [PubMed]
- Iwakiri, Y. Pathophysiology of Portal Hypertension. Clin. Liver Dis. 2014, 18, 281–291. [Google Scholar] [CrossRef] [Green Version]
- Bosch, J.; Iwakiri, Y. The portal hypertension syndrome: Etiology, classification, relevance, and animal models. Hepatol. Int. 2018, 12, 1–10. [Google Scholar] [CrossRef]
- Iwakiri, Y.; Trebicka, J. Portal hypertension in cirrhosis: Pathophysiological mechanisms and therapy. JHEP Rep. 2021, 3, 100316. [Google Scholar] [CrossRef] [PubMed]
- Bolognesi, M.; Di Pascoli, M.; Verardo, A.; Gatta, A. Splanchnic vasodilation and hyperdynamic circulatory syndrome in cirrhosis. World J. Gastroenterol. 2014, 20, 2555–2563. [Google Scholar] [CrossRef] [PubMed]
- Vallance, P.; Moncada, S. Hyperdynamic circulation in cirrhosis: A role for nitric oxide? Lancet 1991, 337, 776–778. [Google Scholar] [CrossRef]
- Iwakiri, Y. Nitric oxide in liver fibrosis: The role of inducible nitric oxide synthase. Clin. Mol. Hepatol. 2015, 21, 319–325. [Google Scholar] [CrossRef] [PubMed]
- Corbin, J.D.; Francis, S.H. Cyclic GMP Phosphodiesterase-5: Target of Sildenafil. J. Biol. Chem. 1999, 274, 13729–13732. [Google Scholar] [CrossRef] [Green Version]
- Rybalkin, S.D.; Yan, C.; Bornfeldt, K.E.; Beavo, J.A. Cyclic GMP Phosphodiesterases and Regulation of Smooth Muscle Function. Circ. Res. 2003, 93, 280–291. [Google Scholar] [CrossRef]
- Wall, M.E.; Francis, S.H.; Corbin, J.D.; Grimes, K.; Richie-Jannetta, R.; Kotera, J.; Macdonald, B.A.; Gibson, R.R.; Trewhella, J. Mechanisms associated with cGMP binding and activation of cGMP-dependent protein kinase. Proc. Natl. Acad. Sci. USA 2003, 100, 2380–2385. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, J.; Clark, J.W.; Bryan, R.M.; Robertson, C.S. Mathematical modeling of the nitric oxide/cGMP pathway in the vascular smooth muscle cell. Am. J. Physiol. Circ. Physiol. 2005, 289, H886–H897. [Google Scholar] [CrossRef]
- Shah, V.; Lyford, G.; Gores, G.; Farrugia, G. Nitric oxide in gastrointestinal health and disease. Gastroenterology 2004, 126, 903–913. [Google Scholar] [CrossRef]
- Rockey, D.C.; Shah, V. Nitric oxide biology and the liver: Report of an AASLD research workshop. Hepatology 2004, 39, 250–257. [Google Scholar] [CrossRef]
- Iwakiri, Y.; Grisham, M.; Shah, V. Vascular biology and pathobiology of the liver: Report of a single-topic symposium. Hepatology 2008, 47, 1754–1763. [Google Scholar] [CrossRef]
- Langer, D.A.; Shah, V.H. Nitric oxide and portal hypertension: Interface of vasoreactivity and angiogenesis. J. Hepatol. 2005, 44, 209–216. [Google Scholar] [CrossRef]
- Kreisel, W.; Schaffner, D.; Lazaro, A.; Trebicka, J.; Merfort, I.; Schmitt-Graeff, A.; Deibert, P. Phosphodiesterases in the Liver as Potential Therapeutic Targets of Cirrhotic Portal Hypertension. Int. J. Mol. Sci. 2020, 21, 6223. [Google Scholar] [CrossRef] [PubMed]
- Iwakiri, Y.; Kim, M.Y. Nitric oxide in liver diseases. Trends Pharmacol. Sci. 2015, 36, 524–536. [Google Scholar] [CrossRef] [Green Version]
- Trebicka, J.; Hennenberg, M.; Odenthal, M.; Shir, K.; Klein, S.; Granzow, M.; Vogt, A.; Dienes, H.-P.; Lammert, F.; Reichen, J.; et al. Atorvastatin attenuates hepatic fibrosis in rats after bile duct ligation via decreased turnover of hepatic stellate cells. J. Hepatol. 2010, 53, 702–712. [Google Scholar] [CrossRef] [PubMed]
- Hu, L.S.; George, J.; Wang, J.H. Current concepts on the role of nitric oxide in portal hypertension. World J. Gastroenterol. 2013, 19, 1707–1717. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.-C.; Wang, S.-S.; Chan, C.-Y.; Chen, Y.-C.; Lee, F.-Y.; Chang, F.-Y.; Chu, C.-J.; Lin, H.-C.; Lu, R.-H.; Lee, S.-D. Role of Hepatic Nitric Oxide Synthases in Rats with Thioacetamide-induced Acute Liver Failure and Encephalopathy. J. Chin. Med. Assoc. 2007, 70, 16–23. [Google Scholar] [CrossRef] [Green Version]
- Leung, T.-M.; Tipoe, G.L.; Liong, E.C.; Lau, T.Y.; Fung, M.-L.; Nanji, A.A. Endothelial nitric oxide synthase is a critical factor in experimental liver fibrosis. Int. J. Exp. Pathol. 2008, 89, 241–250. [Google Scholar] [CrossRef] [PubMed]
- Trebicka, J.; Hennenberg, M.; Laleman, W.; Shelest, N.; Biecker, E.; Schepke, M.; Nevens, F.; Sauerbruch, T.; Heller, J. Atorvastatin lowers portal pressure in cirrhotic rats by inhibition of RhoA/Rho-kinase and activation of endothelial nitric oxide synthase. Hepatology 2007, 46, 242–253. [Google Scholar] [CrossRef] [PubMed]
- McNaughton, L.; Puttagunta, L.; Martinez-Cuesta, M.A.; Kneteman, N.; Mayers, I.; Moqbel, R.; Hamid, Q.; Radomski, M.W. Distribution of nitric oxide synthase in normal and cirrhotic human liver. Proc. Natl. Acad. Sci. USA 2002, 99, 17161–17166. [Google Scholar] [CrossRef] [Green Version]
- Schwabl, P.; Brusilovskaya, K.; Supper, P.; Bauer, D.; Königshofer, P.; Riedl, F.; Hayden, H.; Fuchs, C.; Stift, J.; Oberhuber, G.; et al. The soluble guanylate cyclase stimulator riociguat reduces fibrogenesis and portal pressure in cirrhotic rats. Sci. Rep. 2018, 8, 9372. [Google Scholar] [CrossRef] [PubMed]
- Schaffner, D.; Lazaro, A.; Deibert, P.; Hasselblatt, P.; Stoll, P.; Fauth, L.; Baumstark, M.W.; Merfort, I.; Schmitt-Graeff, A.; Kreisel, W. Analysis of the nitric oxide-cyclic guanosine monophosphate pathway in experimental liver cirrhosis suggests phosphodiesterase-5 as potential target to treat portal hypertension. World J. Gastroenterol. 2018, 24, 4356–4368. [Google Scholar] [CrossRef]
- Uschner, F.E.; Glückert, K.; Paternostro, R.; Gnad, T.; Schierwagen, R.; Mandorfer, M.; Magdaleno, F.; Ortiz, C.; Schwarzkopf, K.; Kamath, P.S.; et al. Combination of phosphodiesterase-5-inhibitors and beta blockers improves experimental portal hypertension and erectile dysfunction. Liver Int. 2020, 40, 2228–2241. [Google Scholar] [CrossRef] [PubMed]
- Theilig, F.; Bostanjoglo, M.; Pavenstädt, H.; Grupp, C.; Holland, G.; Slosarek, I.; Gressner, A.M.; Russwurm, M.; Koesling, D.; Bachmann, S. Cellular distribution and function of soluble guanylyl cyclase in rat kidney and liver. J. Am. Soc. Nephrol. 2001, 12, 2209–2220. [Google Scholar] [CrossRef] [PubMed]
- Davies, N.A.; Hodges, S.J.; Pitsillides, A.A.; Mookerjee, R.; Jalan, R.; Mehdizadeh, S. Hepatic guanylate cyclase activity is decreased in a model of cirrhosis: A quantitative cytochemistry study. FEBS Lett. 2006, 580, 2123–2128. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Loureiro-Silva, M.R.; Iwakiri, Y.; Abraldes, J.G.; Haq, O.; Groszmann, R.J. Increased phosphodiesterase-5 expression is involved in the decreased vasodilator response to nitric oxide in cirrhotic rat livers. J. Hepatol. 2006, 44, 886–893. [Google Scholar] [CrossRef]
- Lee, K.-C.; Yang, Y.-Y.; Huang, Y.-T.; Lee, F.-Y.; Hou, M.-C.; Lin, H.-C.; Lee, S.-D. Administration of a low dose of sildenafil for 1 week decreases intrahepatic resistance in rats with biliary cirrhosis: The role of NO bioavailability. Clin. Sci. 2010, 119, 45–55. [Google Scholar] [CrossRef]
- Brusilovskaya, K.; Königshofer, P.; Lampach, D.; Szodl, A.; Supper, P.; Bauer, D.; Beer, A.; Stift, J.; Timelthaler, G.; Oberhuber, G.; et al. Soluble guanylyl cyclase stimulation and phosphodiesterase-5 inhibition improve portal hypertension and reduce liver fibrosis in bile duct–ligated rats. United Eur. Gastroenterol. J. 2020, 8, 1174–1185. [Google Scholar] [CrossRef]
- Hall, K.C.; Bernier, S.G.; Jacobson, S.; Liu, G.; Zhang, P.Y.; Sarno, R.; Catanzano, V.; Currie, M.G.; Masferrer, J.L. sGC stimulator praliciguat suppresses stellate cell fibrotic transformation and inhibits fibrosis and inflammation in models of NASH. Proc. Natl. Acad. Sci. USA 2019, 116, 11057–11062. [Google Scholar] [CrossRef] [Green Version]
- Perri, R.E.; Langer, D.A.; Chatterjee, S.; Gibbons, S.J.; Gadgil, J.; Cao, S.; Farrugia, G.; Shah, V.H. Defects in cGMP-PKG pathway contribute to impaired NO-dependent responses in hepatic stellate cells upon activation. Am. J. Physiol. Gastrointest. Liver Physiol. 2006, 290, G535–G542. [Google Scholar] [CrossRef]
- Niederberger, M.; Ginès, P.; Tsai, P.; Martin, P.-Y.; Morris, K.; Weigert, A.; McMurtry, I.; Schrier, R.W. Increased aortic cyclic guanosine monophosphate concentration in experimental cirrhosis in rats: Evidence for a role of nitric oxide in the pathogenesis of arterial vasodilation in cirrhosis. Hepatology 1995, 21, 1625–1631. [Google Scholar] [CrossRef]
- Niederberger, M.; Martin, P.-Y.; Ginès, P.; Morris, K.; Tsai, P.; Xu, D.-L.; McMurtry, I.; Schrier, R.W. Normalization of nitric oxide production corrects arterial vasodilation and hyperdynamic circulation in cirrhotic rats. Gastroenterology 1995, 109, 1624–1630. [Google Scholar] [CrossRef]
- Tahseldar-Roumieh, R.; Keravis, T.; Maarouf, S.; Justiniano, H.; Sabra, R.; Lugnier, C. PDEs1-5 activity and expression in tissues of cirrhotic rats reveal a role for aortic PDE3 in NO desensitization. Int. J. Exp. Pathol. 2009, 90, 605–614. [Google Scholar] [CrossRef] [PubMed]
- Halverscheid, L.; Deibert, P.; Schmidt, R.; Blum, H.E.; Dunkern, T.; Pannen, B.H.J.; Kreisel, W. Phosphodiesterase-5 inhibitors have distinct effects on the hemodynamics of the liver. BMC Gastroenterol. 2009, 9, 69. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Choi, S.-M.; Shin, J.-H.; Kim, J.-M.; Lee, C.-H.; Kang, K.-K.; Ahn, B.-O.; Yoo, M. Effect of Udenafil on Portal Venous Pressure and Hepatic Fibrosis in Rats. Arzneimittelforschung 2009, 59, 641–646. [Google Scholar] [CrossRef]
- Deibert, P.; Schumacher, Y.-O.; Ruecker, G.; Opitz, O.G.; Blum, H.E.; Rossle, M.; Kreisel, W. Effect of vardenafil, an inhibitor of phosphodiesterase-5, on portal haemodynamics in normal and cirrhotic liver—Results of a pilot study. Aliment. Pharmacol. Ther. 2006, 23, 121–128. [Google Scholar] [CrossRef] [PubMed]
- Bremer, H.C.; Kreisel, W.; Roecker, K.; Dreher, M.; Koenig, D.; Kurz-Schmieg, A.K.; Blum, H.E.; Roessle, M.; Deibert, P. Phosphodiesterase 5 inhibitors lower both portal and pulmonary pressure in portopulmonary hypertension: A case report. J. Med. Case Rep. 2007, 1, 46. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, K.-C.; Yang, Y.-Y.; Wang, Y.-W.; Hou, M.-C.; Lee, F.-Y.; Lin, H.-C.; Lee, S.-D. Acute administration of sildenafil enhances hepatic cyclic guanosine monophosphate production and reduces hepatic sinusoid resistance in cirrhotic patients. Hepatol. Res. 2008, 38, 1186–1193. [Google Scholar] [CrossRef]
- Clemmesen, J.O.; Giraldi, A.; Ott, P.; Dalhoff, K.; Hansen, B.A.; Larsen, F.S. Sildenafil does not influence hepatic venous pressure gradient in patients with cirrhosis. World J. Gastroenterol. 2008, 14, 6208–6212. [Google Scholar] [CrossRef] [PubMed]
- Tandon, P.; Inayat, I.; Tal, M.; Spector, M.; Shea, M.; Groszmann, R.J.; Garcia–Tsao, G. Sildenafil Has No Effect on Portal Pressure but Lowers Arterial Pressure in Patients with Compensated Cirrhosis. Clin. Gastroenterol. Hepatol. 2010, 8, 546–549. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kreisel, W.; Deibert, P.; Kupcinskas, L.; Sumskiene, J.; Appenrodt, B.; Roth, S.; Neagu, M.; Rössle, M.; Zipprich, A.; Caca, K.; et al. The phosphodiesterase-5-inhibitor udenafil lowers portal pressure in compensated preascitic liver cirrhosis. A dose-finding phase-II-study. Dig. Liver Dis. 2015, 47, 144–150. [Google Scholar] [CrossRef]
- Deibert, P.; Lazaro, A.; Stankovic, Z.; Schaffner, D.; Rössle, M.; Kreisel, W. Beneficial long term effect of a phosphodiesterase-5-inhibitor in cirrhotic portal hypertension: A case report with 8 years follow-up. World J. Gastroenterol. 2018, 24, 438–444. [Google Scholar] [CrossRef]
- Colle, I.; De Vriese, A.; Van Vlierberghe, H.; Lameire, N.H.; DeVos, M. Systemic and splanchnic haemodynamic effects of sildenafil in an in vivo animal model of cirrhosis support for a risk in cirrhotic patients. Liver Int. 2004, 24, 63–68. [Google Scholar] [CrossRef]
- Jung, Y.K.; Yim, H.J. Reversal of liver cirrhosis: Current evidence and expectations. Korean J. Intern. Med. 2017, 32, 213–228. [Google Scholar] [CrossRef] [Green Version]
- Rockey, D.C. Fibrosis reversal after hepatitis C virus elimination. Curr. Opin. Gastroenterol. 2019, 35, 137–144. [Google Scholar] [CrossRef]
- Rockey, D.C.; Friedman, S.L. Fibrosis Regression After Eradication of Hepatitis C Virus: From Bench to Bedside. Gastroenterology 2021, 160, 1502–1520.e1. [Google Scholar] [CrossRef]
- Selicean, S.; Wang, C.; Guixé-Muntet, S.; Stefanescu, H.; Kawada, N.; Gracia-Sancho, J. Regression of portal hypertension: Underlying mechanisms and therapeutic strategies. Hepatol. Int. 2021, 15, 36–50. [Google Scholar] [CrossRef]
- Zoubek, M.E.; Trautwein, C.; Strnad, P. Reversal of liver fibrosis: From fiction to reality. Best Pract. Res. Clin. Gastroenterol. 2017, 31, 129–141. [Google Scholar] [CrossRef]
- Schuppan, D.; Ashfaq-Khan, M.; Yang, A.T.; Kim, Y.O. Liver fibrosis: Direct antifibrotic agents and targeted therapies. Matrix Biol. 2018, 68–69, 435–451. [Google Scholar] [CrossRef]
- Trautwein, C.; Friedman, S.L.; Schuppan, D.; Pinzani, M. Hepatic fibrosis: Concept to treatment. J. Hepatol. 2015, 62, S15–S24. [Google Scholar] [CrossRef] [Green Version]
- Su, T.-H.; Hu, T.-H.; Chen, C.-Y.; Huang, Y.-W.; Chuang, W.-L.; Lin, C.-C.; Wang, C.-C.; Su, W.-W.; Chen, M.-Y.; Peng, C.-Y.; et al. Four-year entecavir therapy reduces hepatocellular carcinoma, cirrhotic events and mortality in chronic hepatitis B patients. Liver Int. 2016, 36, 1755–1764. [Google Scholar] [CrossRef]
- Marcellin, P.; Gane, E.; Buti, M.; Afdhal, N.; Sievert, W.; Jacobson, I.M.; Washington, M.K.; Germanidis, G.; Flaherty, J.F.; Schall, R.A.; et al. Regression of cirrhosis during treatment with tenofovir disoproxil fumarate for chronic hepatitis B: A 5-year open-label follow-up study. Lancet 2013, 381, 468–475. [Google Scholar] [CrossRef]
- Lampertico, P.; Invernizzi, F.; Viganò, M.; Loglio, A.; Mangia, G.; Facchetti, F.; Primignani, M.; Jovani, M.; Iavarone, M.; Fraquelli, M.; et al. The long-term benefits of nucleos(t)ide analogs in compensated HBV cirrhotic patients with no or small esophageal varices: A 12-year prospective cohort study. J. Hepatol. 2015, 63, 1118–1125. [Google Scholar] [CrossRef]
- Mandorfer, M.; Kozbial, K.; Schwabl, P.; Freissmuth, C.; Schwarzer, R.; Stern, R.; Chromy, D.; Stättermayer, A.F.; Reiberger, T.; Beinhardt, S.; et al. Sustained virologic response to interferon-free therapies ameliorates HCV-induced portal hypertension. J. Hepatol. 2016, 65, 692–699. [Google Scholar] [CrossRef]
- Schwabl, P.; Mandorfer, M.; Steiner, S.; Scheiner, B.; Chromy, D.; Herac, M.; Bucsics, T.; Hayden, H.; Grabmeier-Pfistershammer, K.; Ferlitsch, A.; et al. Interferon-free regimens improve portal hypertension and histological necroinflammation in HIV/HCV patients with advanced liver disease. Aliment. Pharmacol. Ther. 2016, 45, 139–149. [Google Scholar] [CrossRef]
- Lens, S.; Alvarado-Tapias, E.; Mariño, Z.; Londoño, M.-C.; LLop, E.; Martinez, J.; Fortea, J.I.; Ibañez, L.; Ariza, X.; Baiges, A.; et al. Effects of all-oral anti-viral therapy on HVPG and systemic hemodynamics in patients with hepatitis C virus-associated cirrhosis. Gastroenterology 2017, 153, 1273–1283.e1. [Google Scholar] [CrossRef] [Green Version]
- Laursen, T.L.; Hagemann, C.A.; Wei, C.; Kazankov, K.; Thomsen, K.L.; Knop, F.K.; Grønbæk, H. Bariatric surgery in patients with non-alcoholic fatty liver disease—From pathophysiology to clinical effects. World J. Hepatol. 2019, 11, 138–149. [Google Scholar] [CrossRef]
- Seymour, K.A.; Abdelmalek, M.F. The role of bariatric surgery in the management of nonalcoholic steatohepatitis. Curr. Opin. Gastroenterol. 2021, 37, 208–215. [Google Scholar] [CrossRef]
- Lee, N.; Suk, K. The Role of the Gut Microbiome in Liver Cirrhosis Treatment. Int. J. Mol. Sci. 2020, 22, 199. [Google Scholar] [CrossRef]
- Tsuchida, T.; Friedman, S.L. Mechanisms of hepatic stellate cell activation. Nat. Rev. Gastroenterol. Hepatol. 2017, 14, 397–411. [Google Scholar] [CrossRef]
- Tacke, F.; Trautwein, C. Mechanisms of liver fibrosis resolution. J. Hepatol. 2015, 63, 1038–1039. [Google Scholar] [CrossRef] [Green Version]
- Ramachandran, P.; Iredale, J.P.; Fallowfield, J.A. Resolution of Liver Fibrosis: Basic Mechanisms and Clinical Relevance. Semin. Liver Dis. 2015, 35, 119–131. [Google Scholar] [CrossRef] [PubMed]
- Kisseleva, T.; Brenner, D. Molecular and cellular mechanisms of liver fibrosis and its regression. Nat. Rev. Gastroenterol. Hepatol. 2021, 18, 151–166. [Google Scholar] [CrossRef]
- Knorr, A.; Hirth-Dietrich, C.; Alonso-Alija, C.; Härter, M.; Hahn, M.; Keim, Y.; Wunder, F.; Stasch, J.-P. Nitric Oxide-independent Activation of Soluble Guanylate Cyclase by BAY 60-2770 in Experimental Liver Fibrosis. Arzneimittelforschung 2008, 58, 71–80. [Google Scholar] [CrossRef]
- Xie, G.; Wang, X.; Wang, L.; Wang, L.; Atkinson, R.D.; Kanel, G.C.; Gaarde, W.A.; DeLeve, L.D. Role of Differentiation of Liver Sinusoidal Endothelial Cells in Progression and Regression of Hepatic Fibrosis in Rats. Gastroenterology 2012, 142, 918–927.e6. [Google Scholar] [CrossRef] [Green Version]
- Flores-Costa, R.; Alcaraz-Quiles, J.; Titos, E.; López-Vicario, C.; Casulleras, M.; Duran-Güell, M.; Rius, B.; Diaz, A.; Hall, K.; Shea, C.; et al. The soluble guanylate cyclase stimulator IW-1973 prevents inflammation and fibrosis in experimental non-alcoholic steatohepatitis. Br. J. Pharmacol. 2018, 175, 953–967. [Google Scholar] [CrossRef]
- Jayakumar, A.; Norenberg, M.D. Hyperammonemia in Hepatic Encephalopathy. J. Clin. Exp. Hepatol. 2018, 8, 272–280. [Google Scholar] [CrossRef] [PubMed]
- Hadjihambi, A.; Khetan, V.; Jalan, R. Pharmacotherapy for hyperammonemia. Expert Opin. Pharmacother. 2014, 15, 1685–1695. [Google Scholar] [CrossRef]
- Ochoa-Sanchez, R.; Rose, C.F. Pathogenesis of Hepatic Encephalopathy in Chronic Liver Disease. J. Clin. Exp. Hepatol. 2018, 8, 262–271. [Google Scholar] [CrossRef]
- DeMorrow, S.; Cudalbu, C.; Davies, N.; Jayakumar, A.R.; Rose, C.F. 2021 ISHEN guidelines on animal models of hepatic encephalopathy. Liver Int. 2021, 41, 1474–1488. [Google Scholar] [CrossRef]
- Rudler, M.; Weiss, N.; Bouzbib, C.; Thabut, D. Diagnosis and Management of Hepatic Encephalopathy. Clin. Liver Dis. 2021, 25, 393–417. [Google Scholar] [CrossRef]
- Alsahhar, J.S.; Rahimi, R. Updates on the pathophysiology and therapeutic targets for hepatic encephalopathy. Curr. Opin. Gastroenterol. 2019, 35, 145–154. [Google Scholar] [CrossRef]
- Butterworth, R.F. Hepatic Encephalopathy in Cirrhosis: Pathology and Pathophysiology. Drugs 2019, 79, 17–21. [Google Scholar] [CrossRef] [Green Version]
- Vilstrup, H.; Amodio, P.; Bajaj, J.; Cordoba, J.; Ferenci, P.; Mullen, K.D.; Weissenborn, K.; Wong, P. Hepatic encephalopathy in chronic liver disease: 2014 Practice Guideline by the American Association for the Study of Liver Diseases and the European Association for the Study of the Liver: Vilstrup et al. Hepatology 2014, 60, 715–735. [Google Scholar] [CrossRef]
- Angeli, P.; Bernardi, M.; Villanueva, C.; Francoz, C.; Mookerjee, R.; Trebicka, J.; Krag, A.; Laleman, W.; Gines, P. EASL Clinical Practice Guidelines for the management of patients with decompensated cirrhosis. J. Hepatol. 2018, 69, 406–460. [Google Scholar] [CrossRef] [Green Version]
- Ferenci, P. Hepatic encephalopathy. Gastroenterol. Rep. 2017, 5, 138–147. [Google Scholar] [CrossRef] [Green Version]
- Rose, C.F.; Amodio, P.; Bajaj, J.S.; Dhiman, R.K.; Montagnese, S.; Taylor-Robinson, S.D.; Vilstrup, H.; Jalan, R. Hepatic encephalopathy: Novel insights into classification, pathophysiology and therapy. J. Hepatol. 2020, 73, 1526–1547. [Google Scholar] [CrossRef]
- Bajaj, J.S. Diagnosis and Treatment of Hepatic Encephalopathy. Gastroenterol. Hepatol. 2019, 15, 434–436. [Google Scholar]
- Kircheis, G.; Lüth, S. Pharmacokinetic and Pharmacodynamic Properties of l-Ornithine l-Aspartate (LOLA) in Hepatic Encephalopathy. Drugs 2019, 79, 23–29. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Butterworth, R.F. Ammonia Removal by Metabolic Scavengers for the Prevention and Treatment of Hepatic Encephalopathy in Cirrhosis. Drugs R&D 2021, 21, 123–132. [Google Scholar] [CrossRef]
- Levitt, D.G.; Levitt, M.D. A model of blood-ammonia homeostasis based on a quantitative analysis of nitrogen metabolism in the multiple organs involved in the production, catabolism, and excretion of ammonia in humans. Clin. Exp. Gastroenterol. 2018, 11, 193–215. [Google Scholar] [CrossRef] [Green Version]
- Erceg, S.; Monfort, P.; Hernandez-Viadel, M.; Llansola, M.; Montoliu, C.; Felipo, V. Restoration of learning ability in hyperammonemic rats by increasing extracellular cGMP in brain. Brain Res. 2005, 1036, 115–121. [Google Scholar] [CrossRef]
- Cabrera-Pastor, A.; Hernandez-Rabaza, V.; Taoro-Gonzalez, L.; Balzano, T.; Llansola, M.; Felipo, V. In vivo administration of extracellular cGMP normalizes TNF-α and membrane expression of AMPA receptors in hippocampus and spatial reference memory but not IL-1β, NMDA receptors in membrane and working memory in hyperammonemic rats. Brain Behav. Immun. 2016, 57, 360–370. [Google Scholar] [CrossRef]
- Rodrigo, R.; Monfort, P.; Cauli, O.; Erceg, S.; Felipo, V. Pharmacological manipulation of cyclic GMP levels in brain restores learning ability in animal models of hepatic encephalopathy: Therapeutic implications. Neuropsychiatr. Dis. Treat. 2006, 2, 53–63. [Google Scholar]
- Felipo, V. Hepatic encephalopathy: Effects of liver failure on brain function. Nat. Rev. Neurosci. 2013, 14, 851–858. [Google Scholar] [CrossRef] [PubMed]
- Erceg, S.; Monfort, P.; Hernández-Viadel, M.; Rodrigo, R.; Montoliu, C.; Felipo, V. Oral administration of sildenafil restores learning ability in rats with hyperammonemia and with portacaval shunts. Hepatology 2005, 41, 299–306. [Google Scholar] [CrossRef]
- Cauli, O.; Rodrigo, R.; Piedrafita, B.; Boix, J.; Felipo, V. Inflammation and hepatic encephalopathy: Ibuprofen restores learning ability in rats with portacaval shunts. Hepatology 2007, 46, 514–519. [Google Scholar] [CrossRef]
- Gonzalez-Usano, A.; Cauli, O.; Agusti, A.; Felipo, V. Pregnenolone sulfate restores the glutamate-nitric-oxide-cGMP pathway and extracellular GABA in cerebellum and learning and motor coordination in hyperammonemic rats. ACS Chem. Neurosci. 2014, 5, 100–105. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Agusti, A.; Cauli, O.; Rodrigo, R.; Llansola, M.; Hernández-Rabaza, V.; Felipo, V. p38 MAP kinase is a therapeutic target for hepatic encephalopathy in rats with portacaval shunts. Gut 2011, 60, 1572–1579. [Google Scholar] [CrossRef]
- Cauli, O.; Mansouri, M.T.; Agusti, A.; Felipo, V. Hyperammonemia Increases GABAergic Tone in the Cerebellum but Decreases It in the Rat Cortex. Gastroenterology 2009, 136, 1359–1367.e2. [Google Scholar] [CrossRef] [PubMed]
- Johansson, M.; Agusti, A.; Llansola, M.; Montoliu, C.; Strömberg, J.; Malinina, E.; Ragagnin, G.; Doverskog, M.; Backstrom, T.; Felipo, V. GR3027 antagonizes GABAA receptor-potentiating neurosteroids and restores spatial learning and motor coordination in rats with chronic hyperammonemia and hepatic encephalopathy. Am. J. Physiol. Liver Physiol. 2015, 309, G400–G409. [Google Scholar] [CrossRef] [Green Version]
- Prickaerts, J.; van Staveren, W.; Şik, A.; Ittersum, M.M.-V.; Niewöhner, U.; van der Staay, F.; Blokland, A.; de Vente, J. Effects of two selective phosphodiesterase type 5 inhibitors, sildenafil and vardenafil, on object recognition memory and hippocampal cyclic GMP levels in the rat. Neuroscience 2002, 113, 351–361. [Google Scholar] [CrossRef]
- Baratti, C.M.; Boccia, M.M. Effects of sildenafil on long-term retention of an inhibitory avoidance response in mice. Behav. Pharmacol. 1999, 10, 731–737. [Google Scholar] [CrossRef]
- Venkat, P.; Chopp, M.; Zacharek, A.; Cui, C.; Landschoot-Ward, J.; Qian, Y.; Chen, Z.; Chen, J. Sildenafil treatment of vascular dementia in aged rats. Neurochem. Int. 2019, 127, 103–112. [Google Scholar] [CrossRef]
- Chalasani, N.; Younossi, Z.; LaVine, J.E.; Charlton, M.; Cusi, K.; Rinella, M.; Harrison, S.A.; Brunt, E.M.; Sanyal, A.J. The diagnosis and management of nonalcoholic fatty liver disease: Practice guidance from the American Association for the Study of Liver Diseases. Hepatology 2017, 67, 328–357. [Google Scholar] [CrossRef]
- Lala, V.; Goyal, A.; Bansal, P.; Minter, D.A. Liver Function Tests; StatPearls Publishing: Treasure Island, FL, USA, 2021. Available online: http://www.ncbi.nlm.nih.gov/books/NBK482489/ (accessed on 22 July 2021).
- Tripodi, A.; Mannucci, P.M. The Coagulopathy of Chronic Liver Disease. N. Engl. J. Med. 2011, 365, 147–156. [Google Scholar] [CrossRef] [Green Version]
- Kwo, P.Y.; Cohen, S.M.; Lim, J.K. ACG Clinical Guideline: Evaluation of Abnormal Liver Chemistries. Am. J. Gastroenterol. 2017, 112, 18–35. [Google Scholar] [CrossRef]
- Nababan, S.; Kalista, K.; Jasirwan, C.; Kurniawan, J.; Lesmana, C.; Sulaiman, A.; Hasan, I.; Gani, R. Mac-2 Binding Protein Glycosylation Isomer for Screening High-Risk Esophageal Varices in Liver Cirrhotic Patient. Livers 2021, 1, 6. [Google Scholar] [CrossRef]
- Karatzas, A.; Konstantakis, C.; Aggeletopoulou, I.; Kalogeropoulou, C.; Thomopoulos, K.; Triantos, C. Νon-invasive screening for esophageal varices in patients with liver cirrhosis. Ann. Gastroenterol. 2018, 31, 305–314. [Google Scholar] [CrossRef]
- Pons, M.; Augustin, S.; Scheiner, B.; Guillaume, M.; Rosselli, M.; Rodrigues, S.G.; Stefanescu, H.; Ma, M.M.; Mandorfer, M.; Mergeay-Fabre, M.; et al. Noninvasive Diagnosis of Portal Hypertension in Patients with Compensated Advanced Chronic Liver Disease. Am. J. Gastroenterol. 2021, 116, 723–732. [Google Scholar] [CrossRef]
- Procopet, B.; Berzigotti, A. Diagnosis of cirrhosis and portal hypertension: Imaging, non-invasive markers of fibrosis and liver biopsy. Gastroenterol. Rep. 2017, 5, 79–89. [Google Scholar] [CrossRef] [Green Version]
- Mandorfer, M.; Hernández-Gea, V.; García-Pagán, J.C.; Reiberger, T. Noninvasive Diagnostics for Portal Hypertension: A Comprehensive Review. Semin. Liver Dis. 2020, 40, 240–255. [Google Scholar] [CrossRef]
- Kirstetter, P.; Moreau, R.; Vachiery, F.; Gadano, A.; Soupison, T.; Pilette, C.; Pussard, E.; Cailmail, S.; Takahashi, H.; Lebrec, D. Plasma concentrations of cyclic 3′,5′-guanosine monophosphate in patients with cirrhosis: Relationship with atrial natriuretic peptide and haemodynamics. J. Gastroenterol. Hepatol. 1997, 12, 233–236. [Google Scholar] [CrossRef]
- Montoliu, C.; Kosenko, E.; Del Olmo, J.A.; Serra, M.A.; Rodrigo, J.M.; Felipo, V. Correlation of nitric oxide and atrial natriuretic peptide changes with altered cGMP homeostasis in liver cirrhosis. Liver Int. 2005, 25, 787–795. [Google Scholar] [CrossRef]
- Montoliu, C.; Rodrigo, R.; Monfort, P.; Llansola, M.; Cauli, O.; Boix, J.; Elmlili, N.; Agusti, A.; Felipo, V. Cyclic GMP pathways in hepatic encephalopathy. Neurological and therapeutic implications. Metab. Brain Dis. 2010, 25, 39–48. [Google Scholar] [CrossRef] [PubMed]
- Siqueira, C.; de Moura, M.C.; Pedro, A.J.; Rocha, P. Elevated nitric oxide and 3′,5′ cyclic guanosine monophosphate levels in patients with alcoholic cirrhosis. World J. Gastroenterol. 2008, 14, 236–242. [Google Scholar] [CrossRef]
- Felipo, V.; Urios, A.; Giménez-Garzó, C.; Cauli, O.; Andrés-Costa, M.-J.; González, O.; Serra, M.A.; Sánchez-González, J.; Aliaga, R.; Giner-Durán, R.; et al. Non invasive blood flow measurement in cerebellum detects minimal hepatic encephalopathy earlier than psychometric tests. World J. Gastroenterol. 2014, 20, 11815–11825. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sturm, L.; Roth, L.; Bettinger, D.; Zoldan, K.; Boettler, T.; Huber, J.; Kaeser, R.; Kreisel, W.; Thimme, R.; Schultheiss, M. Blood cyclic guanosine monophosphate levels as potential marker of portal hypertension in patients with liver cirrhosis. Z. Gastroenterol. 2021, 59, 2–31. [Google Scholar] [CrossRef]
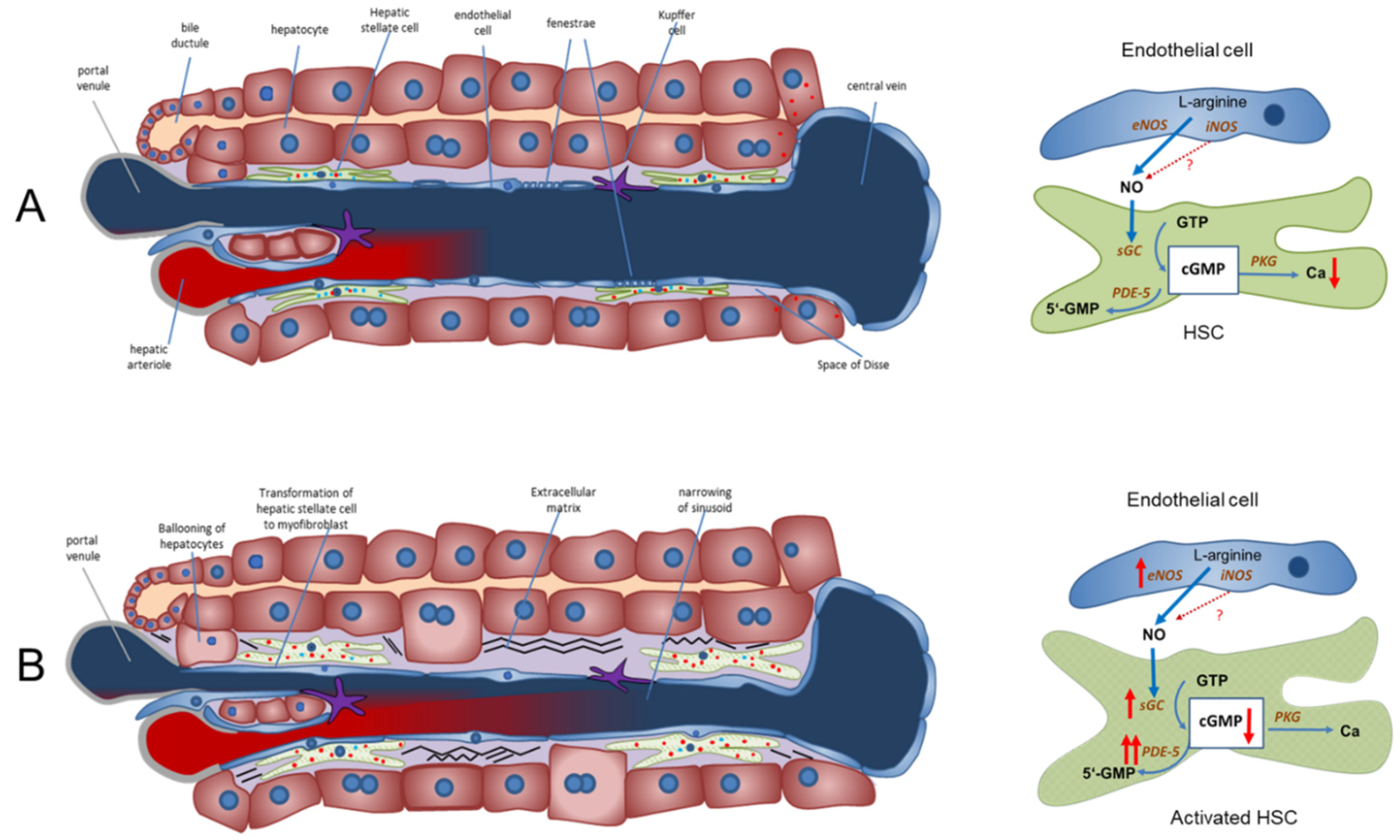
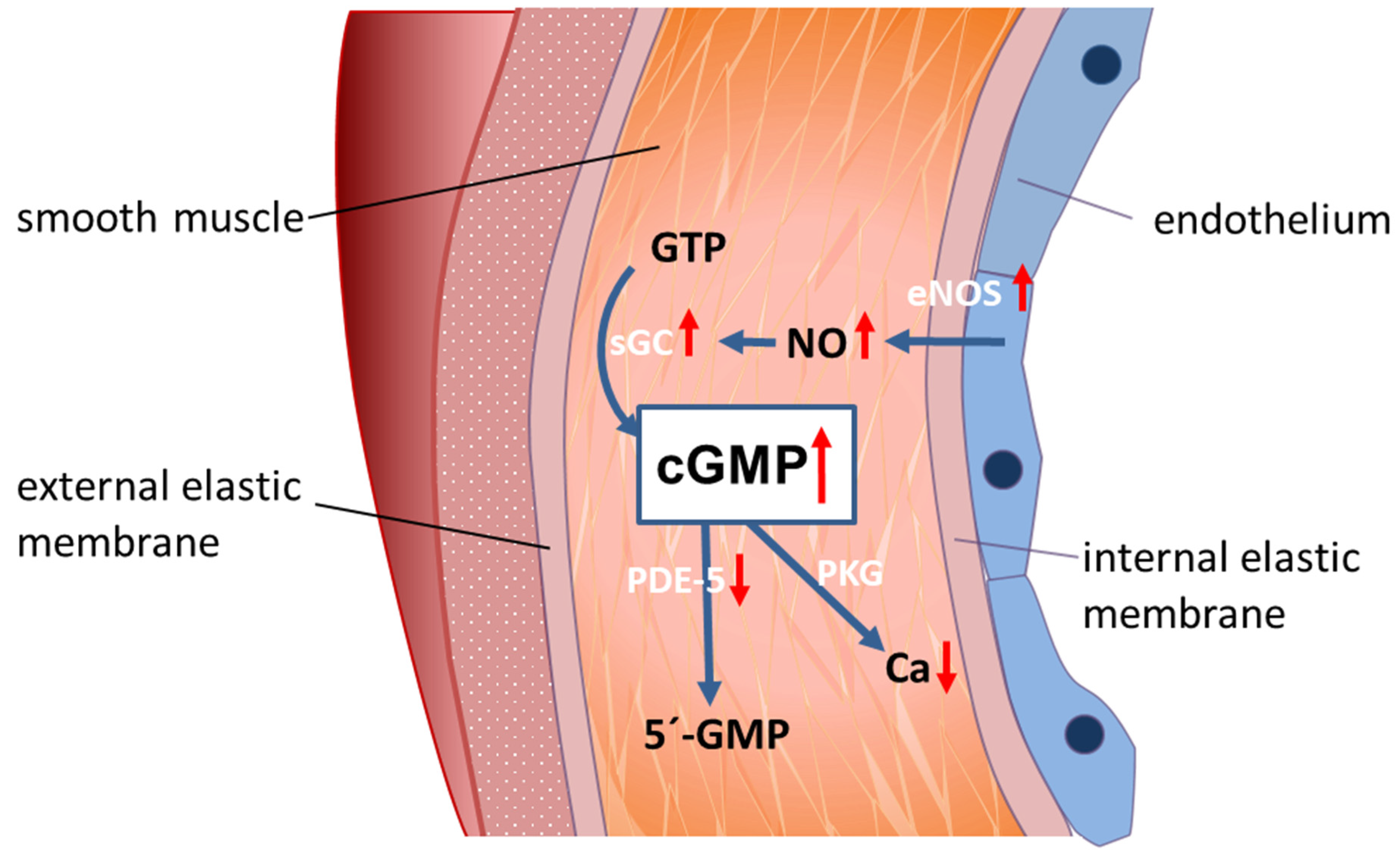
 inhibition;
inhibition;  stimulation/activation.
stimulation/activation.
 inhibition;
inhibition;  stimulation/activation.
stimulation/activation.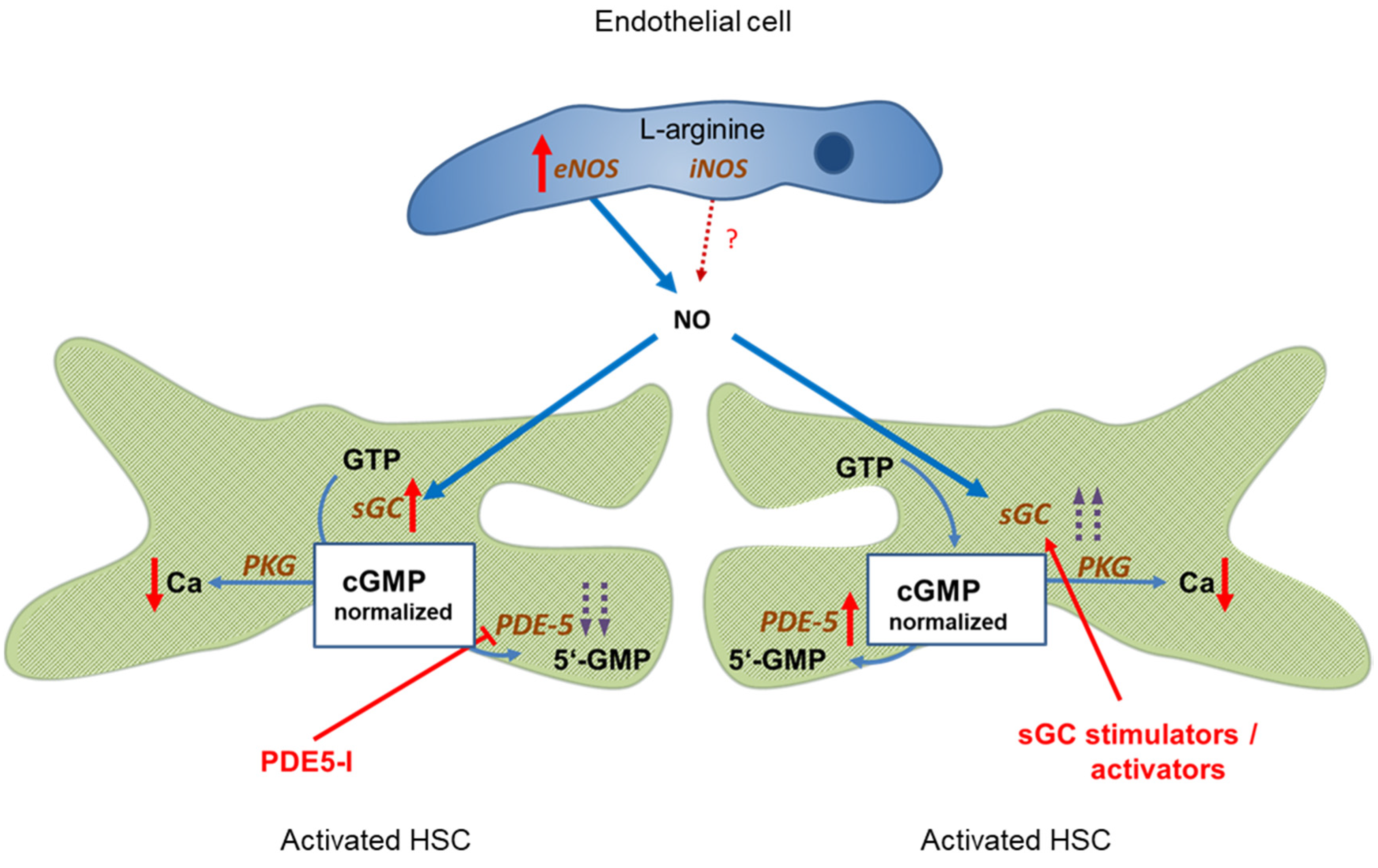

 NMDA-receptor; ↑ increased expression; ↑↑ markedly increased expression; ↑ increased concentration; ↓ decreased concentration.
NMDA-receptor; ↑ increased expression; ↑↑ markedly increased expression; ↑ increased concentration; ↓ decreased concentration.
 NMDA-receptor; ↑ increased expression; ↑↑ markedly increased expression; ↑ increased concentration; ↓ decreased concentration.
NMDA-receptor; ↑ increased expression; ↑↑ markedly increased expression; ↑ increased concentration; ↓ decreased concentration.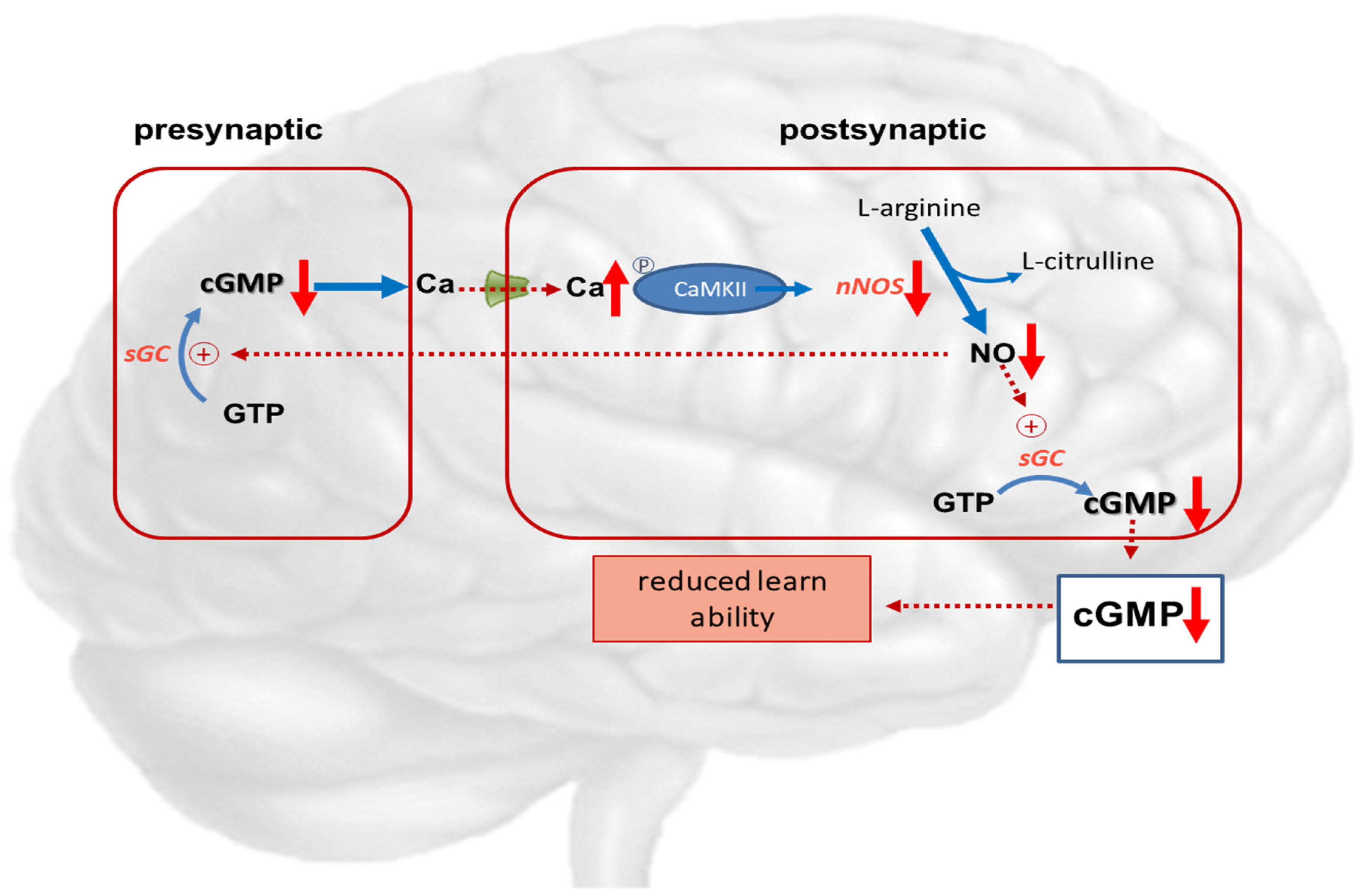
| Model | Compound | Dosage and Route | ΔMAP | ΔPVP | Remarks | |
|---|---|---|---|---|---|---|
| Colle 2004 [63] | Wistar rats, BDL | Sildenafil | 0.01–10 mg/kg, i.v. | −1–20% even more in sham rats | +2– +6% even more in sham rats | |
| Halverscheid 2009 [54] | Sprague-Dawley rats, non-cirrhotic | Sildenafil Vardenafil | 1, 10, or 100 µg/kg, i.v. 1, 10, or 100 µg/kg, i.v. | 1.1; −3.9; −2.6% −11.0; −8.7; −7.4% | In all groups no increase, but decrease over time | 3.3; 24.1; 18.3% 15.9; 29.2; 23.9% increase in portal flow |
| Schaffner 2018 [42] | Wistar rats, TAA | Sildenafil | 0.1–1.0 mg/kg, i.v. | −14–−17% | −13–−19% | |
| Uschner 2020 [43] | Sprague-Dawley rats, BDL or CCL4 | Udenafil Udenafil/propranolol Udenafil 1 or 5 mg/kg | 1 or 5 mg/kg 1 mg/kg | 1 mg/kg: −20%; 5 mg/kg: −22% −7.5% 1 mg/kg: −31%; 5 mg/kg: −34% | −30–−23% −40% −30–−0% | Significant reduction of portal pressure |
| Lee 2010 [47] | Sprague-Dawley rats, BDL | Sildenafil, 1 week | 0.25 mg/kg twice daily p.o. | −25% | ||
| Choi 2009 [55] | Sprague-Dawley rats, BDL | Udenafil for 3 weeks | 1, 5, or 25 mg/kg p.o. | −14, −13, −31% | ||
| Deibert 2006 [56] | Human, cirrhotic (n = 18) | Vardenafil | 10 mg, p.o. | −19% (n = 5) | Hepatic arterial resistance and portal flow increased significantly | |
| Bremer 2007 [57] | Human, cirrhotic PPHTN (n = 1) | Tadalafil | 10 mg, p.o. | −30% | Pulmonary arterial pressure −25% | |
| Lee 2008 [58] | Human, cirrhotic (n = 7) | Sildenafil | 50 mg, p.o. | Unchanged | +1% | Pulmonary arterial and sinusoidal resistance significantly reduced |
| Clemmesen 2008 [59] | Human, cirrhotic (n = 10) | Sildenafil | 50 mg, p.o. | −14% | −11% | |
| Tandon 2010 [60] | Human, Cirrhotic (n = 12) | Sildenafil | 25 mg, p.o. | −8% | −4% n.s. | Dose of Sildenafil too low |
| Kreisel 2015 [61] | Human, cirrhotic (n = 30) | Udenafil | 12.5; 25; 50; 75; 100 mg p.o. acute 6 days | −3.5; −4.5; −7.5; −25.1; −17.3%−14.4; 3.1; −14.0; −13.5; −16.8% | Significant reduction of HVPG with ≥75 mg Udenafil in the acute setting and after 6 days | |
| Deibert 2018 [62] | Human, cirrhotic (n = 1) | Vardenafil Tadalafil | 10 mg 5 mg | −11% | −14% −15% | Sustaining reduction of HVPG > 10 months |
| Schwabl [41] | BDL rats, CCl4, BDL | Riociguat | −20% in early cirrhosis | Improvement offibrosis | ||
| Brusilovskaya [48] | BDL cirrhosis | Tadalafil Riociguat Cineciguat | 1.5 mg/kg bw 0.5 mg/kg bw 1 mg/kg bw | −20% −40% no effect | cGMP + 40% cGMP + 239% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kreisel, W.; Lazaro, A.; Trebicka, J.; Grosse Perdekamp, M.; Schmitt-Graeff, A.; Deibert, P. Cyclic GMP in Liver Cirrhosis—Role in Pathophysiology of Portal Hypertension and Therapeutic Implications. Int. J. Mol. Sci. 2021, 22, 10372. https://doi.org/10.3390/ijms221910372
Kreisel W, Lazaro A, Trebicka J, Grosse Perdekamp M, Schmitt-Graeff A, Deibert P. Cyclic GMP in Liver Cirrhosis—Role in Pathophysiology of Portal Hypertension and Therapeutic Implications. International Journal of Molecular Sciences. 2021; 22(19):10372. https://doi.org/10.3390/ijms221910372
Chicago/Turabian StyleKreisel, Wolfgang, Adhara Lazaro, Jonel Trebicka, Markus Grosse Perdekamp, Annette Schmitt-Graeff, and Peter Deibert. 2021. "Cyclic GMP in Liver Cirrhosis—Role in Pathophysiology of Portal Hypertension and Therapeutic Implications" International Journal of Molecular Sciences 22, no. 19: 10372. https://doi.org/10.3390/ijms221910372






