Microbial Fuel Cell Performance Boost through the Use of Graphene and Its Modifications—Review
Abstract
1. Introduction
2. Methodology
3. Graphene as Material
3.1. Graphene Properties
3.2. Preparation of Graphene Methods
4. Microbial Fuel Cell Principles of Operation
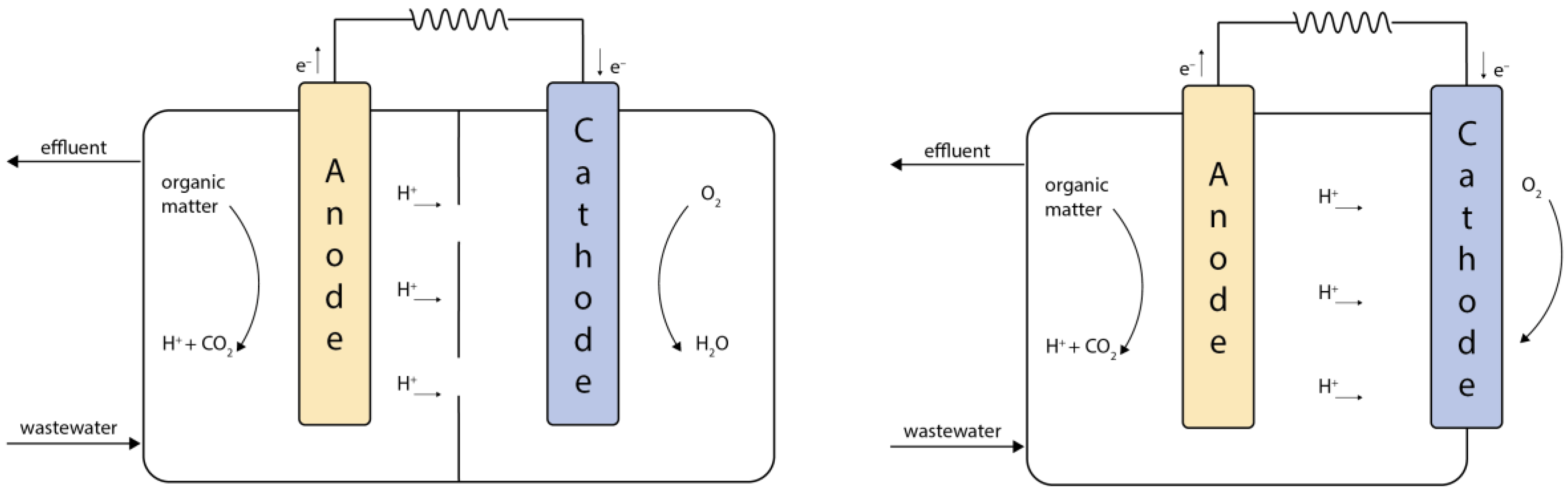
4.1. Constructrion of a Microbial Fuel Cell
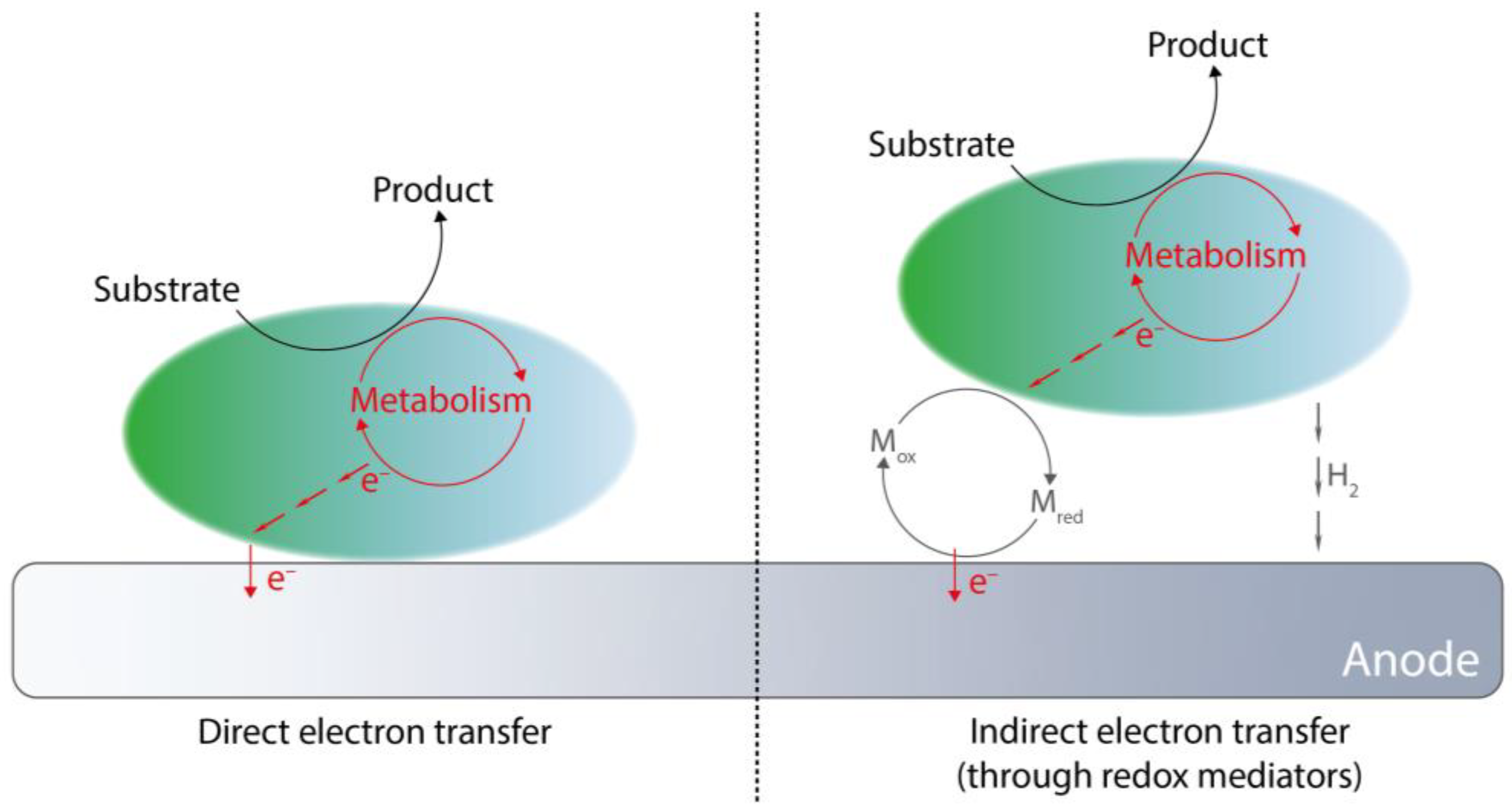
4.2. Extracellular Electron Transfer
5. Graphene Applications in Microbial Fuel Cells
5.1. Anodes Based on Graphene Used in MFC
5.2. Graphene Used in the Cathode Chamber of MFC
5.3. Graphene Advantages in Terms of Microbial Fuel Cells
6. Summary and Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Schäfer, M.; Gretzschel, O.; Steinmetz, H. The possible roles of wastewater treatment plants in sector coupling. Energies 2020, 13, 2088. [Google Scholar] [CrossRef]
- Slate, A.J.; Whitehead, K.A.; Brownson, D.A.C.; Banks, C.E. Microbial fuel cells: An overview of current technology. Renew. Sustain. Energy Rev. 2019, 101, 60–81. [Google Scholar] [CrossRef]
- Paucar, N.E.; Sato, C. Microbial fuel cell for energy production, nutrient removal and recovery from wastewater: A review. Processes 2021, 9, 1318. [Google Scholar] [CrossRef]
- Palanisamy, G.; Jung, H.Y.; Sadhasivam, T.; Kurkuri, M.D.; Kim, S.C.; Roh, S.H. A comprehensive review on microbial fuel cell technologies: Processes, utilization, and advanced developments in electrodes and membranes. J. Clean. Prod. 2019, 221, 598–621. [Google Scholar] [CrossRef]
- Heyrovska, R. Various Carbon to Carbon Bond Lengths Inter-related via the Golden Ratio, and their Linear Dependence on Bond Energies. Graphene 2008, 5, 35–38. [Google Scholar] [CrossRef][Green Version]
- Tiwari, S.K.; Sahoo, S.; Wang, N.; Huczko, A. Graphene research and their outputs: Status and prospect. J. Sci. Adv. Mater. Devices 2020, 5, 10–29. [Google Scholar] [CrossRef]
- Singh, S.B.; De, M. Effects of gaseous environments on physicochemical properties of thermally exfoliated graphene oxides for hydrogen storage: A comparative study. J. Porous Mater. 2021, 28, 875–888. [Google Scholar] [CrossRef]
- Trikkaliotis, D.G.; Christoforidis, A.K.; Mitropoulos, A.C.; Kyzas, G.Z. Graphene Oxide Synthesis, Properties and Characterization Techniques: A Comprehensive Review. ChemEngineering 2021, 5, 64. [Google Scholar] [CrossRef]
- Singh, S.; Hasan, M.R.; Sharma, P.; Narang, J. Graphene nanomaterials: The wondering material from synthesis to applications. Sens. Int. 2022, 3, 100190. [Google Scholar] [CrossRef]
- Li, X.; Tao, L.; Chen, Z.; Fang, H.; Li, X.; Wang, X.; Xu, J.; Zhu, H. Graphene and related two-dimensional materials: Structure-property relationships for electronics and optoelectronics. Appl. Phys. Rev. 2017, 4, 021306. [Google Scholar] [CrossRef]
- Singh, S.B.; De, M. Thermally exfoliated graphene oxide for hydrogen storage. Mater. Chem. Phys. 2020, 239, 122102. [Google Scholar] [CrossRef]
- Tarek, E.G.; Montassar, N.; Akermi, M.; Lassad, E.M. Graphene field-effect transistor for pH sensing application: Compact modelling and simulation study. AIP Conf. Proc. 2018, 1976, 020037. [Google Scholar] [CrossRef]
- Souri, H.; Bhattacharyya, D. Electrical conductivity of the graphene nanoplatelets coated natural and synthetic fibres using electrophoretic deposition technique. Int. J. Smart Nano Mater. 2018, 9, 167–183. [Google Scholar] [CrossRef]
- Li, A.; Zhang, C.; Zhang, Y.F. Thermal Conductivity of Graphene-Polymer Composites: Mechanisms, Properties, and Applications. Polymer 2017, 9, 437. [Google Scholar] [CrossRef] [PubMed]
- Zare, P.; Aleemardani, M.; Seifalian, A.; Bagher, Z.; Seifalian, A.M. Graphene Oxide: Opportunities and Challenges in Biomedicine. Nanomaterials 2021, 11, 1083. [Google Scholar] [CrossRef]
- Rafiee, R.; Eskandariyun, A. Comparative study on predicting Young’s modulus of graphene sheets using nano-scale continuum mechanics approach. Phys. E Low Dimens Syst. Nanostruct. 2017, 90, 42–48. [Google Scholar] [CrossRef]
- Shen, C.; Oyadiji, S.O. The processing and analysis of graphene and the strength enhancement effect of graphene-based filler materials: A review. Mater. Today Phys. 2020, 15, 100257. [Google Scholar] [CrossRef]
- Ke, Q.; Wang, J. Graphene-based materials for supercapacitor electrodes—A review. J. Mater. 2016, 2, 37–54. [Google Scholar] [CrossRef]
- Marchesini, S.; Turner, P.; Paton, K.R.; Reed, B.P.; Brennan, B.; Koziol, K.; Pollard, A.J. Gas physisorption measurements as a quality control tool for the properties of graphene/graphite powders. Carbon. N. Y. 2020, 167, 585–595. [Google Scholar] [CrossRef]
- Bolotin, K.I.; Sikes, K.J.; Jiang, Z.; Klima, M.; Fudenberg, G.; Hone, J.; Kim, P.; Stormer, H.L. Ultrahigh electron mobility in suspended graphene. Solid State Commun. 2008, 146, 351–355. [Google Scholar] [CrossRef]
- Han, Z.; Kimouche, A.; Kalita, D.; Allain, A.; Arjmandi-Tash, H.; Reserbat-Plantey, A.; Marty, L.; Pairis, S.; Reita, V.; Bendiab, N.; et al. Homogeneous Optical and Electronic Properties of Graphene Due to the Suppression of Multilayer Patches During CVD on Copper Foils. Adv. Funct. Mater. 2014, 24, 964–970. [Google Scholar] [CrossRef]
- Li, X.; Cai, W.; An, J.; Kim, S.; Nah, J.; Yang, D.; Piner, R.; Velamakanni, A.; Jung, I.; Tutuc, E.; et al. Large-area synthesis of high-quality and uniform graphene films on copper foils. Science 2009, 324, 1312–1314. [Google Scholar] [CrossRef] [PubMed]
- Mishra, N.; Boeckl, J.; Motta, N.; Iacopi, F. Graphene growth on silicon carbide: A review. Phys. Status Solid. A 2016, 213, 2277–2289. [Google Scholar] [CrossRef]
- Grafen, Czyli z Dużej Chmury Mały Deszcz. Available online: https://www.chemiaibiznes.com.pl/artykuly/grafen-czyli-z-duzej-chmury-maly-deszcz (accessed on 27 December 2022).
- Vieira, N.C.S.; Borme, J.; MacHado, G.; Cerqueira, F.; Freitas, P.P.; Zucolotto, V.; Peres, N.M.R.; Alpuim, P. Graphene field-effect transistor array with integrated electrolytic gates scaled to 200 mm. J. Phys. Condens. Matter 2016, 28, 085302. [Google Scholar] [CrossRef] [PubMed]
- Pawlak, R.; Lebioda, M.; Rymaszewski, J.; Szymanski, W.; Kolodziejczyk, L.; Kula, P. A Fully Transparent Flexible Sensor for Cryogenic Temperatures Based on High Strength Metallurgical Graphene. Sensors 2016, 17, 51. [Google Scholar] [CrossRef]
- Sheikh, R.; Karmaker, S.; Solayman, M.; Mayna, J. Bioelectricity from Anaerobic Co-Digestion of Organic Solid Wastes and Sewage Sludge Using Microbial Fuel Cells (MFCs). J. Sustain. Bioenergy Syst. 2018, 08, 95–106. [Google Scholar] [CrossRef]
- Al-Asheh, S.; Al-Assaf, Y.; Aidan, A. Single-chamber microbial fuel cells’ behavior at different operational scenarios. Energies 2020, 13, 5458. [Google Scholar] [CrossRef]
- Tatinclaux, M.; Gregoire, K.; Leininger, A.; Biffinger, J.C.; Tender, L.; Ramirez, M.; Torrents, A.; Kjellerup, B.V. Electricity generation from wastewater using a floating air cathode microbial fuel cell. Water-Energy Nexus 2018, 1, 97–103. [Google Scholar] [CrossRef]
- Kracke, F.; Vassilev, I.; Krömer, J.O. Microbial electron transport and energy conservation—The foundation for optimizing bioelectrochemical systems. Front. Microbiol. 2015, 6, 575. [Google Scholar] [CrossRef]
- Mahmoud, R.H.; Samhan, F.A.; Ibrahim, M.K.; Ali, G.H.; Hassan, R.Y.A. Formation of electroactive biofilms derived by nanostructured anodes surfaces. Bioprocess Biosyst. Eng. 2021, 44, 759–768. [Google Scholar] [CrossRef]
- Kalathil, S.; Patil, S.A.; Pant, D. Microbial fuel cells: Electrode materials. In Encyclopedia of Interfacial Chemistry: Surface Science and Electrochemistry; Elsevier: Amsterdam, The Netherlands, 2018; pp. 309–318. ISBN 9780128098943. [Google Scholar]
- Tiwari, S.K.; Mishra, R.K.; Ha, S.K.; Huczko, A. Evolution of Graphene Oxide and Graphene: From Imagination to Industrialization. ChemNanoMat 2018, 4, 598–620. [Google Scholar] [CrossRef]
- Tsang, C.H.A.; Huang, H.; Xuan, J.; Wang, H.; Leung, D.Y.C. Graphene materials in green energy applications: Recent development and future perspective. Renew. Sustain. Energy Rev. 2020, 120, 109656. [Google Scholar] [CrossRef]
- Chaturvedi, A.; Kundu, P.P. Nanostructured Graphene Utilization in Microbial Fuel Cells for Green Energy and Wastewater Treatment: Recent Developments and Future Perspectives. J. Hazard. Toxic Radioact. Waste 2022, 26, 03122002. [Google Scholar] [CrossRef]
- Ono, T.; Min, Y.; Shapira, P.; Zavan, B.; Kim, S.O.; Lammel, T. Advances in Graphene; Scientific Research Publishing: Wuhan, China, 2017. [Google Scholar]
- Yaqoob, A.A.; Ibrahim, M.N.M.; Rafatullah, M.; Chua, Y.S.; Ahmad, A.; Umar, K. Recent Advances in Anodes for Microbial Fuel Cells: An Overview. Materials 2020, 13, 2078. [Google Scholar] [CrossRef]
- Liu, Y.; Fan, W.; Xu, Z.; Peng, W.; Luo, S. Comparative effects of graphene and graphene oxide on copper toxicity to Daphnia magna: Role of surface oxygenic functional groups. Environ. Pollut. 2018, 236, 962–970. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Zhao, Z.; Li, S.; Li, B.; Weng, Z.; Fang, Y.; Lei, W.; Jiang, H. Fabrication of 3D graphene anode for improving performance of miniaturized microbial fuel cells. 3 Biotech 2022, 12, 302. [Google Scholar] [CrossRef]
- Sayed, E.T.; Alawadhi, H.; Olabi, A.G.; Jamal, A.; Almahdi, M.S.; Khalid, J.; Abdelkareem, M.A. Electrophoretic deposition of graphene oxide on carbon brush as bioanode for microbial fuel cell operated with real wastewater. Int. J. Hydrogen Energy 2021, 46, 5975–5983. [Google Scholar] [CrossRef]
- Shi, M.M.; Jiang, Y.G.; Shi, L. Electromicrobiology and biotechnological applications of the exoelectrogens Geobacter and Shewanella spp. Sci. China Technol. Sci. 2019, 62, 1670–1678. [Google Scholar] [CrossRef]
- Cao, B.; Zhao, Z.; Peng, L.; Shiu, H.-Y.; Ding, M.; Song, F.; Guan, X.; Lee, C.K.; Huang, J.; Zhu, D.; et al. Silver Nanoparticles Boost Charge-Extraction Efficiency in Shewanella Microbial Fuel Cells. Science 2021, 373, 1336–1340. [Google Scholar] [CrossRef]
- Yaqoob, A.A.; Ibrahim, M.N.M.; Yaakop, A.S.; Rafatullah, M. Utilization of biomass-derived electrodes: A journey toward the high performance of microbial fuel cells. Appl. Water Sci. 2022, 12, 99. [Google Scholar] [CrossRef]
- Song, R.B.; Zhou, S.; Guo, D.; Li, P.; Jiang, L.P.; Zhang, J.R.; Wu, X.; Zhu, J.J. Core/Satellite Structured Fe3O4/Au Nanocomposites Incorporated with Three-Dimensional Macroporous Graphene Foam as a High-Performance Anode for Microbial Fuel Cells. ACS Sustain. Chem. Eng. 2020, 8, 1311–1318. [Google Scholar] [CrossRef]
- Yu, F.; Wang, C.; Ma, J. Capacitance-enhanced 3D graphene anode for microbial fuel cell with long-time electricity generation stability. Electrochim Acta 2018, 259, 1059–1067. [Google Scholar] [CrossRef]
- Wang, R.; Yan, M.; Li, H.; Zhang, L.; Peng, B.; Sun, J.; Liu, D.; Liu, S.; Wang, R.W.; Yan, M.; et al. FeS2 Nanoparticles Decorated Graphene as Microbial-Fuel-Cell Anode Achieving High Power Density. Adv. Mater. 2018, 30, 1800618. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.Y.; Xie, P.; Zhang, Z.P. Reduced graphene oxide/polyacrylamide composite hydrogel scaffold as biocompatible anode for microbial fuel cell. Chem. Eng. J. 2019, 361, 615–624. [Google Scholar] [CrossRef]
- Paul, D.; Noori, M.T.; Rajesh, P.P.; Ghangrekar, M.M.; Mitra, A. Modification of carbon felt anode with graphene oxide-zeolite composite for enhancing the performance of microbial fuel cell. Sustain. Energy Technol. Assess. 2018, 26, 77–82. [Google Scholar] [CrossRef]
- Jawaharraj, K.; Sigdel, P.; Gu, Z.; Muthusamy, G.; Sani, R.K.; Gadhamshetty, V. Photosynthetic microbial fuel cells for methanol treatment using graphene electrodes. Environ. Res 2022, 215, 114045. [Google Scholar] [CrossRef]
- Yaqoob, A.A.; Ibrahim, M.N.M.; Umar, K.; Bhawani, S.A.; Khan, A.; Asiri, A.M.; Khan, M.R.; Azam, M.; Alammari, A.M. Cellulose Derived Graphene/Polyaniline Nanocomposite Anode for Energy Generation and Bioremediation of Toxic Metals via Benthic Microbial Fuel Cells. Polymers 2020, 13, 135. [Google Scholar] [CrossRef]
- Li, Z.L.; Yang, S.K.; Song, Y.; Xu, H.Y.; Wang, Z.Z.; Wang, W.K.; Dang, Z.; Zhao, Y.Q. In-situ modified titanium suboxides with polyaniline/graphene as anode to enhance biovoltage production of microbial fuel cell. Int. J. Hydrogen Energy 2019, 44, 6862–6870. [Google Scholar] [CrossRef]
- Lin, X.Q.; Li, Z.L.; Liang, B.; Nan, J.; Wang, A.J. Identification of biofilm formation and exoelectrogenic population structure and function with graphene/polyanliline modified anode in microbial fuel cell. Chemosphere 2019, 219, 358–364. [Google Scholar] [CrossRef]
- Li, Y.; Liu, J.; Chen, X.; Yuan, X.; Li, N.; He, W.; Feng, Y. Enhanced electricity generation and extracellular electron transfer by polydopamine–reduced graphene oxide (PDA–rGO) modification for high-performance anode in microbial fuel cell. Chem. Eng. J. 2020, 387, 123408. [Google Scholar] [CrossRef]
- Noori, M.T.; Bhowmick, G.D.; Tiwari, B.R.; Ghangrekar, O.M.; Ghangrekar, M.M.; Mukherjee, C.K. Carbon Supported Cu-Sn Bimetallic Alloy as an Excellent Low-Cost Cathode Catalyst for Enhancing Oxygen Reduction Reaction in Microbial Fuel Cell. J. Electrochem. Soc. 2018, 165, F621–F628. [Google Scholar] [CrossRef]
- Santoro, C.; Kodali, M.; Kabir, S.; Soavi, F.; Serov, A.; Atanassov, P. Three-dimensional graphene nanosheets as cathode catalysts in standard and supercapacitive microbial fuel cell. J. Power Sources 2017, 356, 371–380. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Chata, G.; Zhang, Y.; Peng, Y.; Lu, J.E.; Wang, N.; Mercado, R.; Li, J.; Chen, S. Graphene oxide-supported zinc cobalt oxides as effective cathode catalysts for microbial fuel cell: High catalytic activity and inhibition of biofilm formation. Nano Energy 2019, 57, 811–819. [Google Scholar] [CrossRef]
- Papiya, F.; Pattanayak, P.; Kumar, V.; Das, S.; Kundu, P.P. Sulfonated graphene oxide and titanium dioxide coated with nanostructured polyaniline nanocomposites as an efficient cathode catalyst in microbial fuel cells. Mater. Sci. Eng. C 2020, 108, 110498. [Google Scholar] [CrossRef] [PubMed]
- Pattanayak, P.; Papiya, F.; Kumar, V.; Pramanik, N.; Kundu, P.P. Deposition of Ni–NiO nanoparticles on the reduced graphene oxide filled polypyrrole: Evaluation as cathode catalyst in microbial fuel cells. Sustain. Energy Fuels 2019, 3, 1808–1826. [Google Scholar] [CrossRef]
- Xin, S.; Shen, J.; Liu, G.; Chen, Q.; Xiao, Z.; Zhang, G.; Xin, Y. Electricity generation and microbial community of single-chamber microbial fuel cells in response to Cu2O nanoparticles/reduced graphene oxide as cathode catalyst. Chem. Eng. J. 2020, 380, 122446. [Google Scholar] [CrossRef]
- Li, J.C.; Hou, P.X.; Liu, C. Heteroatom-Doped Carbon Nanotube and Graphene-Based Electrocatalysts for Oxygen Reduction Reaction. Small 2017, 13, 1702002. [Google Scholar] [CrossRef]
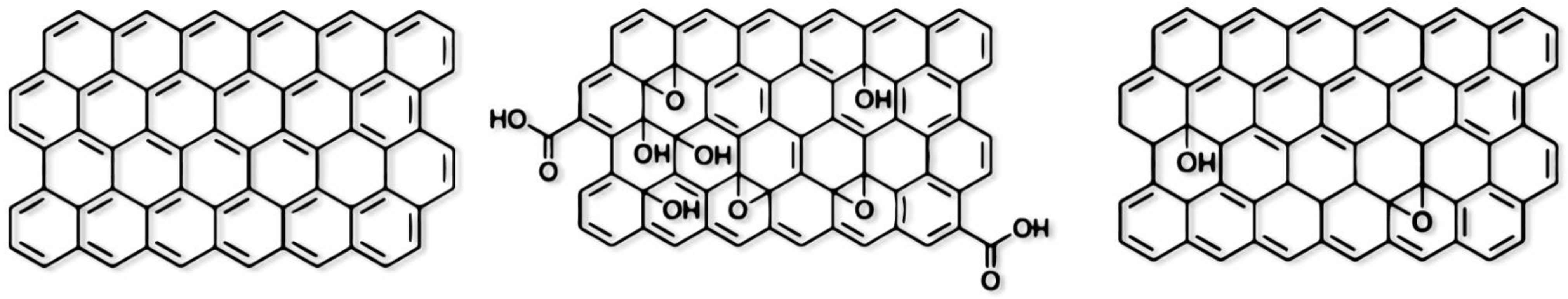
| Graphene Type | Type of Electrode | MFC Type | Bacteria Type | Substrate Type | MFC Performance | Ref. |
|---|---|---|---|---|---|---|
| Graphene-coated carbon brush (RGO-CB) | Anode | Double chamber | - | Real industrial wastewater | 381 mW/m2 | [40] |
| Reduced graphene oxide (rGO) | Anode | Double chamber | Shewanella | - | 1300 mW/m2 | [42] |
| Carbon paper with silver nanoparticles added (rGO/Ag) | Anode | Double chamber | Shewanella | - | 6600 ± 300 mW/m2 | [42] |
| Lignin-derived graphene oxide GO-ZnO composite | Anode | Double chamber | Biofilm with majority of Klebsiella pneumoniae sp. and Enterobacter sp. | Wastewater with cobalt addition | 1.214 mW/m2 | [43] |
| Lignin-derived graphene oxide GO-TiO2 composite | Anode | Double chamber | Biofilm with majority of Klebsiella pneumoniae sp. and Enterobacter sp. | Wastewater with cobalt addition | 0.784 mW/m2 | [43] |
| Lignin-derived graphene oxide (GO) | Anode | Double chamber | Biofilm with majority of Klebsiella pneumoniae sp. and Enterobacter sp. | Wastewater with cobalt addition | 0.148 mW/m2 | [43] |
| Three-dimensional (3D) macroporous graphene foam core/satellite structure with the addition of a nanocomposite Fe3O4/Au (Fe3O4/Au NCs-3DGF) | Anode | Double chamber | Shewanella oneidensis MR-1 | - | 2980 ± 54 mW/m2 | [44] |
| Graphene aerogel (GA) | Anode | Double chamber | Anaerobic sludge | Acetate solution | 2381.44 mW/m3 | [45] |
| Graphene anode coated with iron (II) persulfide nanoparticles (FeS2) | Anode | Double chamber | Mixed-bacteria with Geobacter | Acetate solution | 3220 mW/m2 | [46] |
| Graphene anode coated with iron (II) persulfide nanoparticles (FeS2) | Anode | Double chamber | Mixed-bacteria with Geobacter | Beer factory wastewater | 310 mW/m2 | [46] |
| Three-dimensional composite hydrogel with reduced graphene oxide and polyacrylamide (rGO/PAM) in combination with graphite brush (GB) GB/rGO/PAM | Anode | Double chamber | Mixed bacteria | Culture medium | 758 mW/m2 | [47] |
| Carbon felt anode with graphene oxide-zeolite | Anode | Single chamber | Pre-treated mixed anaerobic sludge | Synthetic wastewater with sodium acetate | 280.56 mW/m2 | [48] |
| Carbon felt anode with graphene oxide | Anode | Single chamber | Pre-treated mixed anaerobic sludge | Synthetic wastewater with sodium acetate | 77.82 mW/m2 | [48] |
| 3D nickel (Ni) foam modified with plasma-grown graphene | Anode | Double chamber | Rhodobacter sphaeroides | Nitrate mineral salts media (NMS) supplemented with 0.1% CH3OH | 141 mW/m2 | [49] |
| Graphene oxide-polyaniline composite (GO-PANI) | Anode | Double chamber benthic MFC | - | Synthetic wastewater | 1.1 mW/m2 | [50] |
| Graphene oxide (GO) | Anode | Double chamber benthic MFC | - | Synthetic wastewater | 0.11 mW/m2 | [50] |
| Graphene polyaniline combined with titanium sub-oxides (GO-PANI-TS) | Anode | Double chamber | - | - | 2073 mW/m2 | [51] |
| Carbon cloth modified with polyaniline and reduced graphene oxide (CC/rGO-PANI) | Anode | Double chamber | Biofilm with Geobacter predomination | Wastewater | 1.9 times higher than CC anode | [52] |
| Carbon cloth with polydopamine–reduced graphene oxide (CC/rGO-PDA) | Anode | Double chamber | Mixed culture | Acetate solution | 2047 mW/m2 | [53] |
| Carbon cloth reduced graphene oxide (CC/rGO) | Anode | Double chamber | Mixed culture | Acetate solution | 1062 mW/m2 | [53] |
| Three-dimensional graphene nanosheets (3D-GNS) | Cathode | Double chamber | Activated sludge | Acetate solution | 2059 ± 3 mW/m2 | [55] |
| Graphene oxide-supported zinc cobalt oxides | Cathode | Double chamber | Anaerobic digester sludge | Acetate solution | 773 mW/m2 | [56] |
| Sulfonated graphene oxide (SGO) with TiO2 and Polyaniline (PAni) nanoparticles | Cathode | Single chamber | Prepared inoculum | - | 904.18 mW/m2 | [57] |
| Graphene oxide (GO) with TiO2 and Polyaniline (PAni) nanoparticles | Cathode | Single chamber | Prepared inoculum | - | 734.12 mW/m2 | [57] |
| Pyrrole (Py) on reduced graphene oxide (rGO) with nickel–nickel oxide (Ni–NiO) nanoparticles (Ni-NiO/PPy-rGO) | Cathode | Single chamber | Mixed bacterial culture | - | 679 ± 34 mW/m2 | [58] |
| Reduced graphene oxide with copper (I) oxide nanoparticles (Cu2O/rGO) | Cathode | Single chamber | Biofilm with Geobacter dominance | - | Output voltage of 0.223 V | [59] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Starowicz, A.; Zieliński, M.; Rusanowska, P.; Dębowski, M. Microbial Fuel Cell Performance Boost through the Use of Graphene and Its Modifications—Review. Energies 2023, 16, 576. https://doi.org/10.3390/en16020576
Starowicz A, Zieliński M, Rusanowska P, Dębowski M. Microbial Fuel Cell Performance Boost through the Use of Graphene and Its Modifications—Review. Energies. 2023; 16(2):576. https://doi.org/10.3390/en16020576
Chicago/Turabian StyleStarowicz, Adam, Marcin Zieliński, Paulina Rusanowska, and Marcin Dębowski. 2023. "Microbial Fuel Cell Performance Boost through the Use of Graphene and Its Modifications—Review" Energies 16, no. 2: 576. https://doi.org/10.3390/en16020576
APA StyleStarowicz, A., Zieliński, M., Rusanowska, P., & Dębowski, M. (2023). Microbial Fuel Cell Performance Boost through the Use of Graphene and Its Modifications—Review. Energies, 16(2), 576. https://doi.org/10.3390/en16020576








