Simulation and Techno-Economic Assessment of Hydrogen Production from Biomass Gasification-Based Processes: A Review
Abstract
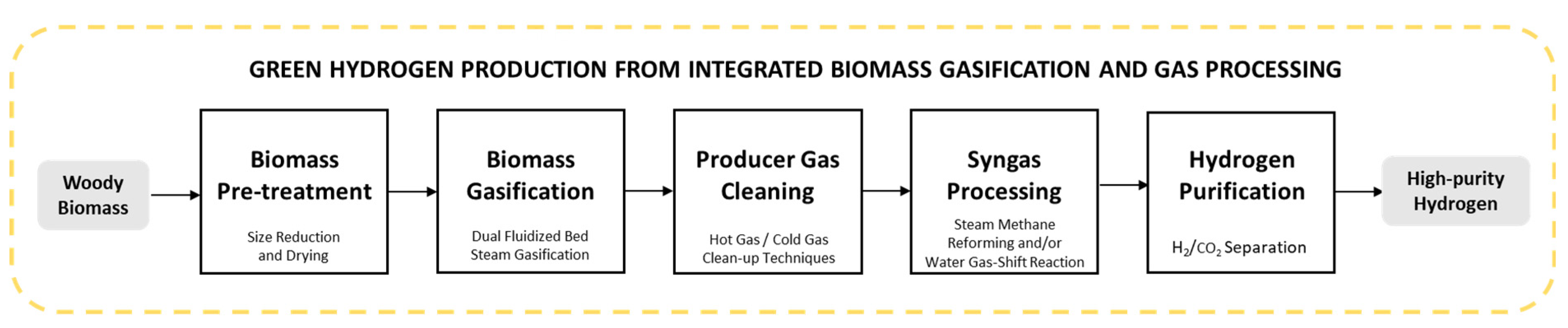
1. Introduction
2. Woody Biomass
2.1. Woody Biomass Chemical Composition
2.2. Pre-Treatment and Handling
2.2.1. Biomass Size Reduction
2.2.2. Biomass Drying
3. Biomass Gasification
3.1. Steam Gasification
3.1.1. Drying
3.1.2. Pyrolysis
3.1.3. Gasification Reactions
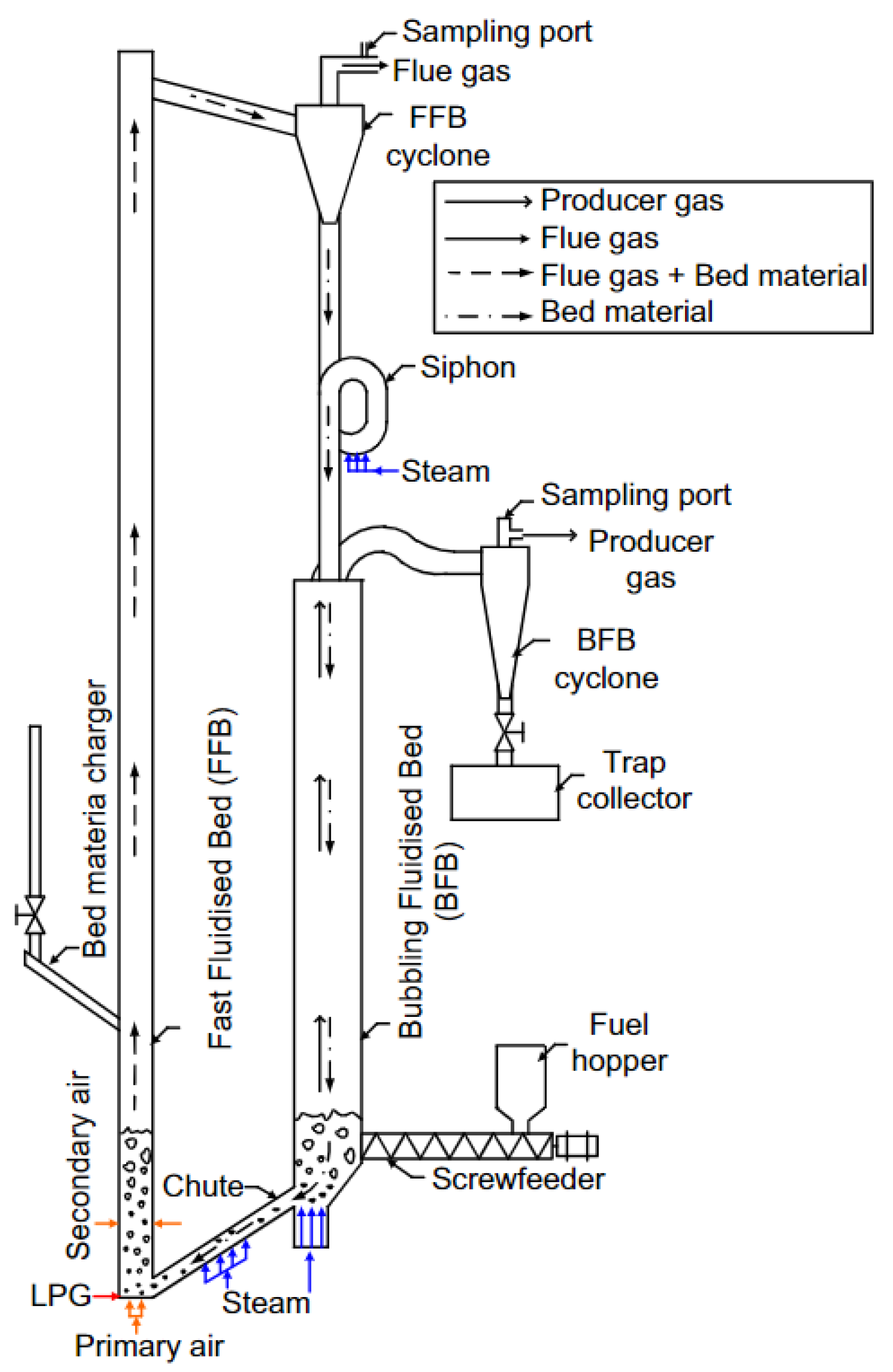
3.2. Dual Fluidised-Bed Gasifiers
3.3. Effect of Operating Conditions
3.3.1. Gasification Agent
3.3.2. Gasification Temperature
3.3.3. Steam-to-Biomass Ratio
4. Producer Gas Processing
4.1. Producer Gas Cleaning
4.2. Producer Gas Processing: Steam Methane Reforming
4.3. Producer Gas Processing: Water–Gas Shift Reaction
4.4. Separation of H2 and CO2
5. Simulation-Based Modelling of Biomass Gasification
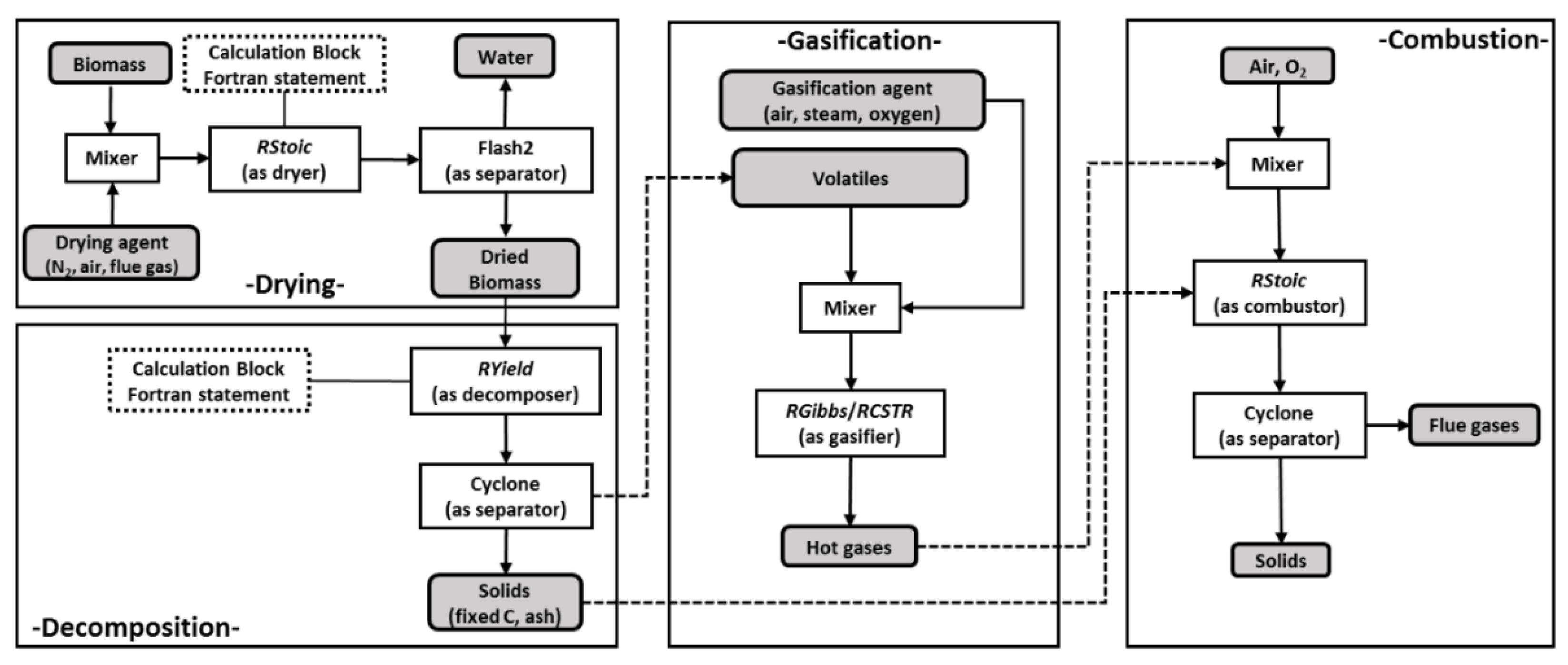
5.1. Simulation of Biomass Gasification with Aspen Plus
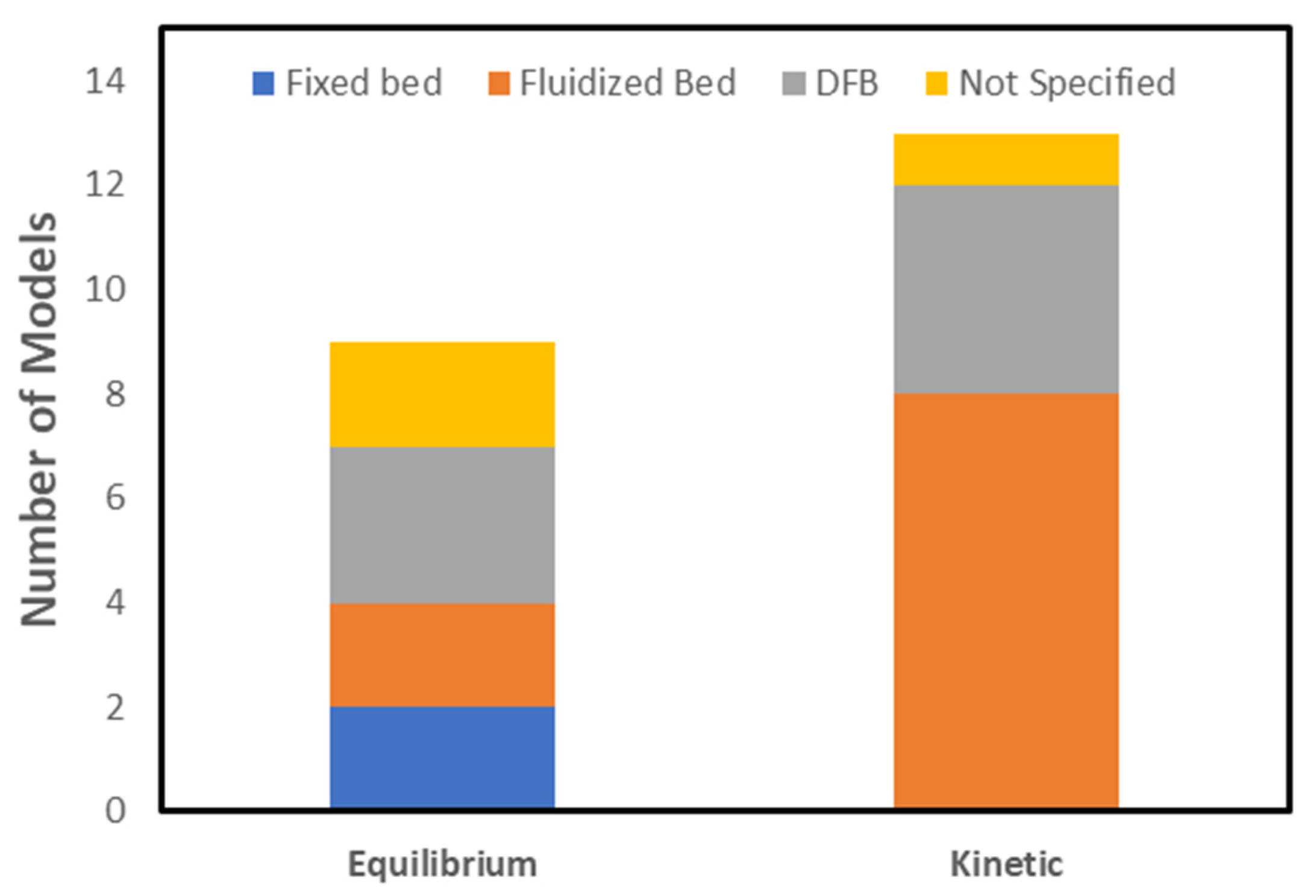
5.2. Thermodynamic Equilibrium Models
5.3. Kinetic Models
5.4. Gasification with Subsequent Producer Gas Processing Models
6. Techno-Economic Assessment of Biomass Gasification
7. Conclusions and Recommendation
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- International Energy Agency (IEA). World Energy Outlook 2021. Available online: https://www.iea.org/reports/world-energy-outlook-2021 (accessed on 28 March 2022).
- Dincer, I. Green Methods for Hydrogen Production. Int. J. Hydrogen Energy 2012, 37, 1954–1971. [Google Scholar] [CrossRef]
- Parthasarathy, P.; Narayanan, K.S. Hydrogen Production from Steam Gasification of Biomass: Influence of Process Parameters on Hydrogen Yield—A Review. Renew. Energy 2014, 66, 570–579. [Google Scholar] [CrossRef]
- Safarian, S.; Unnþórsson, R.; Richter, C. A Review of Biomass Gasification Modelling. Renew. Sust. Energy Rev. 2019, 378–391. [Google Scholar] [CrossRef]
- Baruah, D.; Baruah, D.C. Modeling of Biomass Gasification: A Review. Renew. Sust. Energy Rev. 2014, 39, 806–815. [Google Scholar] [CrossRef]
- Safarian, S.; Saryazdi, S.M.E.; Unnthorsson, R.; Richter, C. Gasification of Woody Biomasses and Forestry Residues: Simulation, Performance Analysis, and Environmental Impact. Fermentation 2021, 7, 61. [Google Scholar] [CrossRef]
- Akhator, P.; Asibor, J. Simulation of Air-Gasification of Wood Wastes Using Aspen Plus. Int. J. Eng. Sci. Appl. 2021, 5, 86–97. [Google Scholar]
- Montiel-Bohórquez, N.D.; Pérez, J.F. Energy Valorization Strategies of Fallen Leaves and Woody Biomass in a Based Downdraft Gasification-Engine Power Plant. Sustain. Energy Technol. Assess. 2022, 49, 101749. [Google Scholar] [CrossRef]
- Tavares, R.; Monteiro, E.; Tabet, F.; Rouboa, A. Numerical Investigation of Optimum Operating Conditions for Syngas and Hydrogen Production from Biomass Gasification Using Aspen Plus. Renew. Energy 2020, 146, 1309–1314. [Google Scholar] [CrossRef]
- Čeković, I.; Manić, N.; Stojiljković, D.; Trninić, M.; Todorović, D.; Jovović, A. Modeling of Wood Chips Gasification Process in Aspen plus with Multiple Validation Approach. Chem. Ind. Chem. Eng. Q. 2019, 25, 217–228. [Google Scholar] [CrossRef]
- Gagliano, A.; Nocera, F.; Bruno, M.; Cardillo, G. Development of an Equilibrium-Based Model of Gasification of Biomass by Aspen Plus. Renew. Energy 2017, 111, 1010–1019. [Google Scholar] [CrossRef]
- Formica, M.; Frigo, S.; Gabbrielli, R. Development of a New Steady State Zero-Dimensional Simulation Model for Woody Biomass Gasification in a Full Scale Plant. Energy Convers. Manag. 2016, 120, 358–369. [Google Scholar] [CrossRef]
- Huang, F.; Jin, S. Investigation of Biomass (Pine Wood) Gasification: Experiments and Aspen Plus Simulation. Energy Sci. Eng. 2019, 7, 1178–1187. [Google Scholar] [CrossRef]
- Safarian, S.; Richter, C.; Unnthorsson, R. Waste Biomass Gasification Simulation Using Aspen Plus: Performance Evaluation of Wood Chips, Sawdust and Mixed Paper Wastes. J. Energy Eng. 2019, 7, 12–30. [Google Scholar] [CrossRef]
- Cao, Y.; Bai, Y.; Du, J. Co-Gasification of Rice Husk and Woody Biomass Blends in a CFB System: A Modeling Approach. Renew. Energy 2022, 188, 849–858. [Google Scholar] [CrossRef]
- Dang, Q.; Zhang, X.; Zhou, Y.; Jia, X. Prediction and Optimization of Syngas Production from a Kinetic-Based Biomass Gasification Process Model. Fuel Process. Technol. 2021, 212. [Google Scholar] [CrossRef]
- Puig-Gamero, M.; Pio, D.T.; Tarelho, L.A.C.; Sánchez, P.; Sanchez-Silva, L. Simulation of Biomass Gasification in Bubbling Fluidized Bed Reactor Using Aspen Plus®. Energy Convers. Manag. 2021, 235, 113981. [Google Scholar] [CrossRef]
- Nguyen, N.M.; Alobaid, F.; Epple, B. Process Simulation of Steam Gasification of Torrefied Woodchips in a Bubbling Fluidized Bed Reactor Using Aspen Plus. Appl. Sci. 2021, 11, 2877. [Google Scholar] [CrossRef]
- Kaushal, P.; Tyagi, R. Advanced Simulation of Biomass Gasification in a Fluidized Bed Reactor Using ASPEN PLUS. Renew. Energy 2017, 101, 629–636. [Google Scholar] [CrossRef]
- Beheshti, S.M.; Ghassemi, H.; Shahsavan-Markadeh, R. Process Simulation of Biomass Gasification in a Bubbling Fluidized Bed Reactor. Energy Convers. Manag. 2015, 94, 345–352. [Google Scholar] [CrossRef]
- Rafati, M.; Hashemisohi, A.; Wang, L.; Shahbazi, A. Sequential Modular Simulation of Hydrodynamics and Reaction Kinetics in a Biomass Bubbling Fluidized-Bed Gasifier Using Aspen Plus. Energy Fuels 2015, 29, 8261–8272. [Google Scholar] [CrossRef]
- Begum, S.; Rasul, M.G.; Akbar, D.; Cork, D. An Experimental and Numerical Investigation of Fluidized Bed Gasification of Solid Waste. Energies 2014, 7, 43–61. [Google Scholar] [CrossRef]
- Abdelouahed, L.; Authier, O.; Mauviel, G.; Corriou, J.P.; Verdier, G.; Dufour, A. Detailed Modeling of Biomass Gasification in Dual Fluidized Bed Reactors under Aspen Plus. Energy Fuels 2012, 26, 3840–3855. [Google Scholar] [CrossRef]
- Yan, L.; Jim Lim, C.; Yue, G.; He, B.; Grace, J.R. One-Dimensional Modeling of a Dual Fluidized Bed for Biomass Steam Gasification. Energy Convers. Manag. 2016, 127, 612–622. [Google Scholar] [CrossRef]
- Babatabar, M.A.; Saidi, M. Hydrogen Production via Integrated Configuration of Steam Gasification Process of Biomass and Water-Gas Shift Reaction: Process Simulation and Optimization. Int. J. Energy Res. 2021, 45, 19378–19394. [Google Scholar] [CrossRef]
- Puig-Gamero, M.; Argudo-Santamaria, J.; Valverde, J.L.; Sánchez, P.; Sanchez-Silva, L. Three Integrated Process Simulation Using Aspen Plus®: Pine Gasification, Syngas Cleaning and Methanol Synthesis. Energy Convers. Manag. 2018, 177, 416–427. [Google Scholar] [CrossRef]
- Pala, L.P.R.; Wang, Q.; Kolb, G.; Hessel, V. Steam Gasification of Biomass with Subsequent Syngas Adjustment Using Shift Reaction for Syngas Production: An Aspen Plus Model. Renew. Energy 2017, 101, 484–492. [Google Scholar] [CrossRef]
- Li, G.; Wang, S.; Zhao, J.; Qi, H.; Ma, Z.; Cui, P.; Zhu, Z.; Gao, J.; Wang, Y. Life Cycle Assessment and Techno-Economic Analysis of Biomass-to-Hydrogen Production with Methane Tri-Reforming. Energy 2020, 199, 117488. [Google Scholar] [CrossRef]
- Sara, H.R.; Enrico, B.; Mauro, V.; Andrea, D.C.; Vincenzo, N. Techno-Economic Analysis of Hydrogen Production Using Biomass Gasification—A Small Scale Power Plant Study. Energy Procedia 2016, 101, 806–813. [Google Scholar] [CrossRef]
- Salkuyeh, Y.K.; Saville, B.A.; MacLean, H.L. Techno-Economic Analysis and Life Cycle Assessment of Hydrogen Production from Different Biomass Gasification Processes. Int. J. Hydrogen Energy 2018, 43, 9514–9528. [Google Scholar] [CrossRef]
- Doherty, W.; Reynolds, A.; Kennedy, D.; Doherty, W.; Reynolds, A.; Kennedy, D. Aspen Plus Simulation of Biomass Gasification in a Steam Blown Dual Fluidised Bed. In Materials and Processes for Energy: Communicating Current Research and Technological Developments; Méndez-Vilas, A., Ed.; Formatex Research Centre: Dublin, Ireland, 2013; pp. 212–220. [Google Scholar]
- Liao, C.H.; Summers, M.; Seiser, R.; Cattolica, R.; Herz, R. Simulation of a Pilot-Scale Dual-Fluidized-Bed Gasifier for Biomass. Environ. Prog. Sustain. 2014, 33, 732–736. [Google Scholar] [CrossRef]
- Zhai, M.; Guo, L.; Wang, Y.; Zhang, Y.; Dong, P.; Jin, H. Process Simulation of Staging Pyrolysis and Steam Gasification for Pine Sawdust. Int. J. Hydrogen Energy 2016, 41, 21926–21935. [Google Scholar] [CrossRef]
- Tapasvi, D.; Kempegowda, R.S.; Tran, K.Q.; Skreiberg, Ø.; Grønli, M. A Simulation Study on the Torrefied Biomass Gasification. Energy Convers. Manag. 2015, 90, 446–457. [Google Scholar] [CrossRef]
- Wang, Y.; Li, G.; Liu, Z.; Cui, P.; Zhu, Z.; Yang, S. Techno-Economic Analysis of Biomass-to-Hydrogen Process in Comparison with Coal-to-Hydrogen. Process. Energy 2019, 185, 1063–1075. [Google Scholar] [CrossRef]
- Al-Qahtani, A.; Parkinson, B.; Hellgardt, K.; Shah, N.; Guillen-Gosalbez, G. Uncovering the True Cost of Hydrogen Production Routes Using Life Cycle Monetisation. Appl. Energy 2021, 281, 115958. [Google Scholar] [CrossRef]
- Liu, Z. Economic Analysis of Energy Production from Coal/Biomass Upgrading; Part 1: Hydrogen Production. Energy Sources B Econ. Plan. Policy 2018, 13, 132–136. [Google Scholar] [CrossRef]
- Yao, J.; Kraussler, M.; Benedikt, F.; Hofbauer, H. Techno-Economic Assessment of Hydrogen Production Based on Dual Fluidized Bed Biomass Steam Gasification, Biogas Steam Reforming, and Alkaline Water Electrolysis Processes. Energy Convers. Manag. 2017, 145, 278–292. [Google Scholar] [CrossRef]
- Dai, J.; Saayman, J.; Grace, J.R.; Ellis, N. Gasification of Woody Biomass. Annu. Rev. Chem. Biomol. Eng. 2015, 6, 77–99. [Google Scholar] [CrossRef]
- Basu, P. Chapter 1: Introduction. In Biomass Gasification Design Handbook; Elsevier: Amsterdam, The Netherlands, 2010; pp. 1–25. [Google Scholar] [CrossRef]
- Solarte-Toro, J.C.; González-Aguirre, J.A.; Poveda Giraldo, J.A.; Cardona Alzate, C.A. Thermochemical Processing of Woody Biomass: A Review Focused on Energy-Driven Applications and Catalytic Upgrading. Renew. Sustain. Energ. Rev. 2021, 136, 110376. [Google Scholar] [CrossRef]
- Molino, A.; Chianese, S.; Musmarra, D. Biomass Gasification Technology: The State of the Art Overview. J. Energy Chem. 2016, 25, 10–25. [Google Scholar] [CrossRef]
- Zhao, C.; Jiang, E.; Chen, A. Volatile Production from Pyrolysis of Cellulose, Hemicellulose and Lignin. J. Energy Inst. 2017, 90, 902–913. [Google Scholar] [CrossRef]
- Bach, Q.V.; Gye, H.R.; Song, D.; Lee, C.J. High Quality Product Gas from Biomass Steam Gasification Combined with Torrefaction and Carbon Dioxide Capture Processes. Int. J. Hydrogen Energy 2019, 44, 14387–14394. [Google Scholar] [CrossRef]
- Liu, S. A Synergetic Pretreatment Technology for Woody Biomass Conversion. Appl. Energy 2015, 144, 114–128. [Google Scholar] [CrossRef]
- Yaghoubi, E.; Xiong, Q.; Doranehgard, M.H.; Yeganeh, M.M.; Shahriari, G.; Bidabadi, M. The Effect of Different Operational Parameters on Hydrogen Rich Syngas Production from Biomass Gasification in a Dual Fluidized Bed Gasifier. Chem. Eng. Process. 2018, 126, 210–221. [Google Scholar] [CrossRef]
- Mirmoshtaghi, G.; Skvaril, J.; Campana, P.E.; Li, H.; Thorin, E.; Dahlquist, E. The Influence of Different Parameters on Biomass Gasification in Circulating Fluidized Bed Gasifiers. Energy Convers. Manag. 2016, 126, 110–123. [Google Scholar] [CrossRef]
- Erkiaga, A.; Lopez, G.; Amutio, M.; Bilbao, J.; Olazar, M. Influence of Operating Conditions on the Steam Gasification of Biomass in a Conical Spouted Bed Reactor. J. Chem. Eng. 2014, 237, 259–267. [Google Scholar] [CrossRef]
- Sikarwar, V.S.; Zhao, M.; Clough, P.; Yao, J.; Zhong, X.; Memon, M.Z.; Shah, N.; Anthony, E.J.; Fennell, P.S. An Overview of Advances in Biomass Gasification. Energy Environ. Sci. 2016, 9, 2939–2977. [Google Scholar] [CrossRef]
- Pang, S.; Mujumdar, A.S. Drying of Woody Biomass for Bioenergy: Drying Technologies and Optimization for an Integrated Bioenergy Plant. Dry. Technol. 2010, 28, 690–701. [Google Scholar] [CrossRef]
- Pang, S. Advances in Thermochemical Conversion of Woody Biomass to Energy, Fuels and Chemicals. Biotechnol. Adv. 2019, 37, 589–597. [Google Scholar] [CrossRef]
- Patra, T.K.; Sheth, P.N. Biomass Gasification Models for Downdraft Gasifier: A State-of-the-Art Review. Renew. Sustain. Energy Rev. 2015, 50, 583–593. [Google Scholar] [CrossRef]
- Zhang, Z.; Pang, S. Experimental Investigation of Biomass Devolatilization in Steam Gasification in a Dual Fluidised Bed Gasifier. Fuel 2017, 188, 628–635. [Google Scholar] [CrossRef]
- Karl, J.; Pröll, T. Steam Gasification of Biomass in Dual Fluidized Bed Gasifiers: A Review. Renew. Sustain. Energy Rev. 2018, 98, 64–78. [Google Scholar] [CrossRef]
- Göransson, K.; Söderlind, U.; He, J.; Zhang, W. Review of Syngas Production via Biomass DFBGs. Renew. Sustain. Energy Rev. 2011, 15, 482–492. [Google Scholar] [CrossRef]
- Hanchate, N.; Ramani, S.; Mathpati, C.S.; Dalvi, V.H. Biomass Gasification Using Dual Fluidized Bed Gasification Systems: A Review. J. Clean. Prod. 2021, 280, 123148. [Google Scholar] [CrossRef]
- Saw, W.L.; Pang, S. The Influence of Calcite Loading on Producer Gas Composition and Tar Concentration of Radiata Pine Pellets in a Dual Fluidised Bed Steam Gasifier. Fuel 2012, 102, 445–452. [Google Scholar] [CrossRef]
- Aghaalikhani, A.; Schmid, J.C.; Borello, D.; Fuchs, J.; Benedikt, F.; Hofbauer, H.; Rispoli, F.; Henriksen, U.B.; Sárossy, Z.; Cedola, L. Detailed Modelling of Biomass Steam Gasification in a Dual Fluidized Bed Gasifier with Temperature Variation. Renew. Energy 2019, 143, 703–718. [Google Scholar] [CrossRef]
- Pfeifer, C.; Koppatz, S.; Hofbauer, H. Steam Gasification of Various Feedstocks at a Dual Fluidised Bed Gasifier: Impacts of Operation Conditions and Bed Materials. Biomass Convers. Biorefinery 2011, 1, 39–53. [Google Scholar] [CrossRef]
- Rashidi, H.; Duffy, A.; Doherty, W. A Detailed General Model of the Gasification Zone of a Dual Fluidised Bed Gasifier. Biomass Convers. Biorefinery 2022, 1–16. [Google Scholar] [CrossRef]
- Schmid, J.C.; Wolfesberger, U.; Koppatz, S.; Pfeifer, C.; Hofbauer, H. Variation of Feedstock in a Dual Fluidized Bed Steam Gasifier-Influence on Product Gas, Tar Content, and Composition. Environ. Prog. Sustain. Energy 2012, 31, 205–215. [Google Scholar] [CrossRef]
- Zhang, Z.; Pang, S. Experimental Investigation of Tar Formation and Producer Gas Composition in Biomass Steam Gasification in a 100 kW Dual Fluidised Bed Gasifier. Renew. Energy 2019, 132, 416–424. [Google Scholar] [CrossRef]
- Shahbaz, M.; Yusup, S.; Inayat, A.; Patrick, D.O.; Ammar, M. The Influence of Catalysts in Biomass Steam Gasification and Catalytic Potential of Coal Bottom Ash in Biomass Steam Gasification: A Review. Renew. Sustain. Energy Rev. 2017, 73, 468–476. [Google Scholar] [CrossRef]
- Bach, Q.V.; Nguyen, D.D.; Lee, C.J. Effect of Torrefaction on Steam Gasification of Biomass in Dual Fluidized Bed Reactor—A Process Simulation Study. Bioenergy Res 2019, 12, 1042–1051. [Google Scholar] [CrossRef]
- Ahmad, A.A.; Zawawi, N.A.; Kasim, F.H.; Inayat, A.; Khasri, A. Assessing the Gasification Performance of Biomass: A Review on Biomass Gasification Process Conditions, Optimization and Economic Evaluation. Renew. Sustain. Energy Rev. 2016, 53, 1333–1347. [Google Scholar] [CrossRef]
- Samimi, F.; Marzoughi, T.; Rahimpour, M.R. Energy and Exergy Analysis and Optimization of Biomass Gasification Process for Hydrogen Production (Based on Air, Steam and Air/Steam Gasifying Agents). Int. J. Hydrogen Energy 2020, 45, 33185–33197. [Google Scholar] [CrossRef]
- Zaman, S.A.; Roy, D.; Ghosh, S. Process Modeling and Optimization for Biomass Steam-Gasification Employing Response Surface Methodology. Biomass Bioenergy 2020, 143, 105847. [Google Scholar] [CrossRef]
- Woolcock, P.J.; Brown, R.C. A Review of Cleaning Technologies for Biomass-Derived Syngas. Biomass Bioenergy 2013, 52, 54–84. [Google Scholar] [CrossRef]
- Anis, S.; Zainal, Z.A. Tar Reduction in Biomass Producer Gas via Mechanical, Catalytic and Thermal Methods: A Review. Renew. Sustain. Energy Rev. 2011, 15, 2355–2377. [Google Scholar] [CrossRef]
- Abdoulmoumine, N.; Adhikari, S.; Kulkarni, A.; Chattanathan, S. A Review on Biomass Gasification Syngas Cleanup. Appl. Energy. 2015, 155, 294–307. [Google Scholar] [CrossRef]
- Harb, R.; Rivera-Tinoco, R.; Nemer, M.; Zeghondy, B.; Bouallou, C. Process Simulation of Tar Removal from Gasification Producer Gas. Chem. Eng. Trans. 2020, 81, 931–936. [Google Scholar] [CrossRef]
- Zhang, X.; Pan, J.; Wang, L.; Qian, T.; Zhu, Y.; Sun, H.; Gao, J.; Chen, H.; Gao, Y.; Liu, C. COSMO-Based Solvent Selection and Aspen Plus Process Simulation for Tar Absorptive Removal. Appl. Energy 2019, 251, 113314. [Google Scholar] [CrossRef]
- Hai, I.U.; Sher, F.; Zarren, G.; Liu, H. Experimental Investigation of Tar Arresting Techniques and Their Evaluation for Product Syngas Cleaning from Bubbling Fluidized Bed Gasifier. J. Clean. Prod. 2019, 240, 118239. [Google Scholar] [CrossRef]
- Pranolo, S.H.; Waluyo, J.; Paryanto; Susanti, A.D.; Permana, R.B.; Erwanda, I.; Delayaski, N.; Alhaq, A.I.; Putro, F.A. Feasible Tar Cleaning Method of Producer Gas from Palm Kernel Shell and Mahogany Fruit Shell Gasification. Mater. Today Proc. 2022, 63, S237–S243. [Google Scholar] [CrossRef]
- Pallozzi, V.; di Carlo, A.; Bocci, E.; Carlini, M. Combined Gas Conditioning and Cleaning for Reduction of Tars in Biomass Gasification. Biomass Bioenergy 2018, 109, 85–90. [Google Scholar] [CrossRef]
- Nikolaidis, P.; Poullikkas, A. A Comparative Overview of Hydrogen Production Processes. Renew. Sustain. Energy Rev. 2017, 67, 597–611. [Google Scholar] [CrossRef]
- Minh, D.P.; Siang, T.J.; Vo, D.V.N.; Phan, T.S.; Ridart, C.; Nzihou, A.; Grouset, D. Hydrogen Production from Biogas Reforming: An Overview of Steam Reforming, Dry Reforming, Dual Reforming, and Tri-Reforming of Methane. In Hydrogen Supply Chain: Design, Deployment and Operation; Elsevier: Amsterdam, The Netherlands, 2018; pp. 111–166. [Google Scholar] [CrossRef]
- Kaiwen, L.; Bin, Y.; Tao, Z. Economic Analysis of Hydrogen Production from Steam Reforming Process: A Literature Review. Energy Sources B Econ. Plan. Policy 2018, 13, 109–115. [Google Scholar] [CrossRef]
- Antonini, C.; Treyer, K.; Streb, A.; van der Spek, M.; Bauer, C.; Mazzotti, M. Hydrogen Production from Natural Gas and Biomethane with Carbon Capture and Storage—A Techno-Environmental Analysis. Sustain. Energy Fuels 2020, 4, 2967–2986. [Google Scholar] [CrossRef]
- Savuto, E.; di Carlo, A.; Gallucci, K.; Natali, S.; Bocci, E. Characterization and Performance Analysis of an Innovative Ni/Mayenite Catalyst for the Steam Reforming of Raw Syngas. Fuel 2017, 194, 348–356. [Google Scholar] [CrossRef]
- Robinson, A.M.; Gin, M.E.; Yung, M.M. Methane Steam Reforming Kinetics on a Ni/Mg/K/Al2O3 Catalyst. Top. Catal. 2013, 56, 1708–1715. [Google Scholar] [CrossRef]
- Buchireddy, P.R.; Peck, D.; Zappi, M.; Bricka, R.M. Catalytic Hot Gas Cleanup of Biomass Gasification Producer Gas via Steam Reforming Using Nickel-Supported Clay Minerals. Energies 2021, 14, 1875. [Google Scholar] [CrossRef]
- Hiblot, H.; Ziegler-Devin, I.; Fournet, R.; Glaude, P.A. Steam Reforming of Methane in a Synthesis Gas from Biomass Gasification. Int. J. Hydrogen Energy 2016, 41, 18329–18338. [Google Scholar] [CrossRef]
- Vivanpatarakij, S.; Rulerk, D.; Assabumrungrat, S. Removal of Tar from Biomass Gasification Process by Steam Reforming over Nickel Catalysts. Chem. Eng. Trans. 2014, 37, 205–210. [Google Scholar] [CrossRef]
- Carapellucci, R.; Giordano, L. Steam, Dry and Autothermal Methane Reforming for Hydrogen Production: A Thermodynamic Equilibrium Analysis. J. Power Sources 2020, 469, 228391. [Google Scholar] [CrossRef]
- Izquierdo, U.; Barrio, V.L.; Cambra, J.F.; Requies, J.; Güemez, M.B.; Arias, P.L.; Kolb, G.; Zapf, R.; Gutiérrez, A.M.; Arraibi, J.R. Hydrogen Production from Methane and Natural Gas Steam Reforming in Conventional and Microreactor Reaction Systems. Int. J. Hydrogen Energy 2012, 37, 7026–7033. [Google Scholar] [CrossRef]
- Terrell, E.; Theegala, C.S. Thermodynamic Simulation of Syngas Production through Combined Biomass Gasification and Methane Reformation. Sustain. Energy Fuels 2019, 3, 1562–1572. [Google Scholar] [CrossRef]
- Ngo, T.N.L.T.; Chiang, K.Y.; Liu, C.F.; Chang, Y.H.; Wan, H.P. Hydrogen Production Enhancement Using Hot Gas Cleaning System Combined with Prepared Ni-Based Catalyst in Biomass Gasification. Int. J. Hydrogen Energy 2021, 46, 11269–11283. [Google Scholar] [CrossRef]
- Pal, D.B.; Chand, R.; Upadhyay, S.N.; Mishra, P.K. Performance of Water Gas Shift Reaction Catalysts: A Review. Renew. Sustain. Energy Rev. 2018, 93, 549–565. [Google Scholar] [CrossRef]
- Giuliano, A.; Freda, C.; Catizzone, E. Techno-Economic Assessment of Bio-Syngas Production for Methanol Synthesis: A Focus on the Water–Gas Shift and Carbon Capture Sections. Bioengineering 2020, 7, 70. [Google Scholar] [CrossRef]
- Patra, T.K.; Sheth, P.N. Biomass Gasification Coupled with Producer Gas Cleaning, Bottling and HTS Catalyst Treatment for H2-Rich Gas Production. Int. J. Hydrogen Energy 2019, 44, 11602–11616. [Google Scholar] [CrossRef]
- Chianese, S.; Loipersböck, J.; Malits, M.; Rauch, R.; Hofbauer, H.; Molino, A.; Musmarra, D. Hydrogen from the High Temperature Water Gas Shift Reaction with an Industrial Fe/Cr Catalyst Using Biomass Gasification Tar Rich Synthesis Gas. Fuel Process. Technol. 2015, 132, 39–48. [Google Scholar] [CrossRef]
- Voldsund, M.; Jordal, K.; Anantharaman, R. Hydrogen Production with CO2 Capture. Int. J. Hydrogen Energy 2016, 41, 4969–4992. [Google Scholar] [CrossRef]
- Luberti, M.; Ahn, H. Review of Polybed Pressure Swing Adsorption for Hydrogen Purification. Int. J. Hydrogen Energy 2022, 47, 10911–10933. [Google Scholar] [CrossRef]
- Mondal, M.K.; Balsora, H.K.; Varshney, P. Progress and Trends in CO2 Capture/Separation Technologies: A Review. Energy 2012, 46, 431–441. [Google Scholar] [CrossRef]
- Yu, C.H.; Huang, C.H.; Tan, C.S. A Review of CO2 Capture by Absorption and Adsorption. Aerosol Air Qual Res. 2012, 12, 745–769. [Google Scholar] [CrossRef]
- Oreggioni, G.D.; Brandani, S.; Luberti, M.; Baykan, Y.; Friedrich, D.; Ahn, H. CO2 Capture from Syngas by an Adsorption Process at a Biomass Gasification CHP Plant: Its Comparison with Amine-Based CO2 Capture. Int. J. Greenh. Gas Control 2015, 35, 71–81. [Google Scholar] [CrossRef]
- Streb, A.; Hefti, M.; Gazzani, M.; Mazzotti, M. Novel Adsorption Process for Co-Production of Hydrogen and CO2 from a Multicomponent Stream. Ind. Eng. Chem. Res. 2019, 58, 17489–17506. [Google Scholar] [CrossRef]
- Li, P.; Wang, Z.; Qiao, Z.; Liu, Y.; Cao, X.; Li, W.; Wang, J.; Wang, S. Recent Developments in Membranes for Efficient Hydrogen Purification. J. Membr. Sci. 2015, 495, 130–168. [Google Scholar] [CrossRef]
- Du, Z.; Liu, C.; Zhai, J.; Guo, X.; Xiong, Y.; Su, W.; He, G. A Review of Hydrogen Purification Technologies for Fuel Cell Vehicles. Catalysts 2021, 11, 393. [Google Scholar] [CrossRef]
- Dai, J.; Whitty, K.J. Chemical Looping Gasification and Sorption Enhanced Gasification of Biomass: A Perspective. Chem. Eng. Process. 2022, 174, 108902. [Google Scholar] [CrossRef]
- Parvez, A.M.; Hafner, S.; Hornberger, M.; Schmid, M.; Scheffknecht, G. Sorption Enhanced Gasification (SEG) of Biomass for Tailored Syngas Production with in-Situ CO2 Capture: Current Status, Process Scale-up Experiences and Outlook. Renew. Sustain. Energy Rev. 2021, 141, 110756. [Google Scholar] [CrossRef]
- Ji, G.; Yao, J.G.; Clough, P.T.; da Costa, J.C.D.; Anthony, E.J.; Fennell, P.S.; Wang, W.; Zhao, M. Enhanced Hydrogen Production from Thermochemical Processes. Energy Environ. Sci. 2018, 11, 2647–2672. [Google Scholar] [CrossRef]
- Soria, M.A.; Tosti, S.; Mendes, A.; Madeira, L.M. Enhancing the Low Temperature Water-Gas Shift Reaction through a Hybrid Sorption-Enhanced Membrane Reactor for High-Purity Hydrogen Production. Fuel 2015, 159, 854–863. [Google Scholar] [CrossRef]
- Detchusananard, T.; Im-orb, K.; Ponpesh, P.; Arpornwichanop, A. Biomass Gasification Integrated with CO2 Capture Processes for High-Purity Hydrogen Production: Process Performance and Energy Analysis. Energy Convers. Manag. 2018, 171, 1560–1572. [Google Scholar] [CrossRef]
- Streb, A.; Mazzotti, M. Novel Adsorption Process for Co-Production of Hydrogen and CO2from a Multicomponent Stream-Part 2: Application to Steam Methane Reforming and Autothermal Reforming Gases. Ind. Eng. Chem. Res. 2020, 59, 10093–10109. [Google Scholar] [CrossRef]
- Safarian, S.; Unnthorsson, R.; Richter, C. Performance Analysis of Power Generation by Wood and Woody Biomass Gasification in a Downdraft Gasifier. IJAPE 2021, 10, 80. [Google Scholar] [CrossRef]
- Chilev, C.; Simeonov, E.; Stoyanov, V.; Chaushev, S. A Computer Simulation of a Biomass Gasification Process. J. Chem. Technol. Metall. 2020, 55, 785–796. [Google Scholar]
- Sreejith, C.C.; Muraleedharan, C.; Arun, P. Performance Prediction of Steam Gasification of Wood Using an Aspen Plus Thermodynamic Equilibrium Model. Int. J. Sustain. Energy 2014, 33, 416–434. [Google Scholar] [CrossRef]
- Mutlu, O.C.; Zeng, T. Challenges and Opportunities of Modeling Biomass Gasification in Aspen Plus: A Review. Chem. Eng. Technol. 2020, 43, 1674–1689. [Google Scholar] [CrossRef]
- Song, Y.; Tian, Y.; Zhou, X.; Liang, S.; Li, X.; Yang, Y.; Yuan, L. Simulation of Air-Steam Gasification of Pine Sawdust in an Updraft Gasification System for Production of Hydrogen-Rich Producer Gas. Energy 2021, 226, 120380. [Google Scholar] [CrossRef]
- Adeyemi, I.; Janajreh, I. Modeling of the Entrained Flow Gasification: Kinetics-Based ASPEN Plus Model. Renew. Energy 2015, 82, 77–84. [Google Scholar] [CrossRef]
- Pauls, J.H.; Mahinpey, N.; Mostafavi, E. Simulation of Air-Steam Gasification of Woody Biomass in a Bubbling Fluidized Bed Using Aspen Plus: A Comprehensive Model Including Pyrolysis, Hydrodynamics and Tar Production. Biomass Bioenergy 2016, 95, 157–166. [Google Scholar] [CrossRef]
- Meramo-Hurtado, S.I.; Puello, P.; Cabarcas, A. Process Analysis of Hydrogen Production via Biomass Gasification under Computer-Aided Safety and Environmental Assessments. ACS Omega 2020, 5, 19667–19681. [Google Scholar] [CrossRef]
- Norouzi, N. Hydrogen Production in the Light of Sustainability: A Comparative Study on the Hydrogen Production Technologies Using the Sustainability Index Assessment Method. Nucl. Eng. Technol. 2022, 54, 1288–1294. [Google Scholar] [CrossRef]
- Dodds, P.E. Economics of Hydrogen Production. In Compendium of Hydrogen Energy; Woodhead Publishing: Sawston, UK, 2015; pp. 63–79. [Google Scholar] [CrossRef]
- Lepage, T.; Kammoun, M.; Schmetz, Q.; Richel, A. Biomass-to-Hydrogen: A Review of Main Routes Production, Processes Evaluation and Techno-Economical Assessment. Biomass Bioenergy 2021, 144, 105920. [Google Scholar] [CrossRef]
- Acar, C.; Dincer, I. Comparative Assessment of Hydrogen Production Methods from Renewable and Non-Renewable Sources. Int. J. Hydrog. Energy 2014, 39, 1–12. [Google Scholar] [CrossRef]
- Valente, A.; Iribarren, D.; Dufour, J. Life Cycle Sustainability Assessment of Hydrogen from Biomass Gasification: A Comparison with Conventional Hydrogen. Int. J. Hydrogen Energy 2019, 44, 21193–21203. [Google Scholar] [CrossRef]
- Wang, J.J.; Jing, Y.Y.; Zhang, C.F.; Zhao, J.H. Review on Multi-Criteria Decision Analysis Aid in Sustainable Energy Decision-Making. Renew. Sustain. Energy Rev. 2009, 13, 2263–2278. [Google Scholar] [CrossRef]
| Biomass Type | Cellulose | Hemicellulose | Lignin |
|---|---|---|---|
| Hardwood | 42–48 | 27–38 | 16–25 |
| Softwood | 40–45 | 24–29 | 26–33 |
| Straws | 36–40 | 21–45 | 15–20 |
| Properties | Woodchip | Wood Pellet | Sawdust | Forest Residues | Mixed Wood Wastes |
|---|---|---|---|---|---|
| Ultimate Analysis (wt%, dry-ash free basis) | |||||
| C | 51.82 | 50.60 | 50.82 | 43.0 | 57.74 |
| H | 6.15 | 6.39 | 7.12 | 5.0 | 5.21 |
| O | 41.81 | 42.92 | 41.34 | 49.6 | 37.10 |
| N | 0.20 | 0.0 | 0.15 | 2.4 | 0.11 |
| S | 0.02 | 0.09 | 0.57 | 0.0 | 0.04 |
| Proximate Analysis (wt%, dry basis) | |||||
| Volatile matter | 80.0 | 86.01 | 82.28 | 79.82 | 87.55 |
| Fixed carbon | 18.84 | 13.23 | 17.15 | 19.95 | 9.77 |
| Ash | 1.16 | 0.76 | 0.57 | 0.23 | 2.68 |
| Moisture | 20.0 | 3.91 | 8.0 | 11.3 | 25.0 |
| Lower heating value (MJ/kg) | 19.09 | 18.82 * | 18.88 | 13.88 * | 19.85 |
| References | [31] | [32] | [33] | [9] | [7] |
| Reaction I.D. | Reaction Type | Chemical Reactions | ΔH298K (kJ/mol) |
|---|---|---|---|
| Primary devolatilisation | |||
| (R1) | Biomass → CO, CO2, H2O, CH4, C2H4, C, Primary tars (CHxOy) | ||
| Tar cracking and reforming | |||
| (R2) | Primary tar → CO, CO2, CH4, C2H4, H2, Secondary tars | ||
| Homogeneous gas-phase reactions | |||
| (R3) | Secondary tars → C, CO, H2 | ||
| (R4) | Combustion (H2 oxidation) | H2 + 0.5O2 H2O | −242 |
| (R5) | Combustion (CO oxidation) | CO + 0.5O2 CO2 | −283 |
| (R6) | Combustion (CH4 oxidation) | CH4 + 0.5O2 CO + H2 | −110 |
| (R7) | Dry methane reforming | CH4 + CO2 2CO + 2H2 | +247 |
| (R8) | Steam methane reforming | CH4 + H2O CO + 3H2 | +206 |
| (R9) | Water–gas shift | CO + H2O CO2 + H2 | −40.9 |
| Heterogeneous reactions | |||
| (R10) | Carbon oxidation | C + O2 CO2 | −393.5 |
| (R11) | Partial oxidation | C + 0.5O2 CO | −123.1 |
| (R12) | Boudouard | C + CO2 2CO | +159.9 |
| (R13) | Water–gas (steam reforming) | C + H2O CO + H2 | +118.5 |
| (R14) | Methanation | C + 2H2 CH4 | −87.5 |
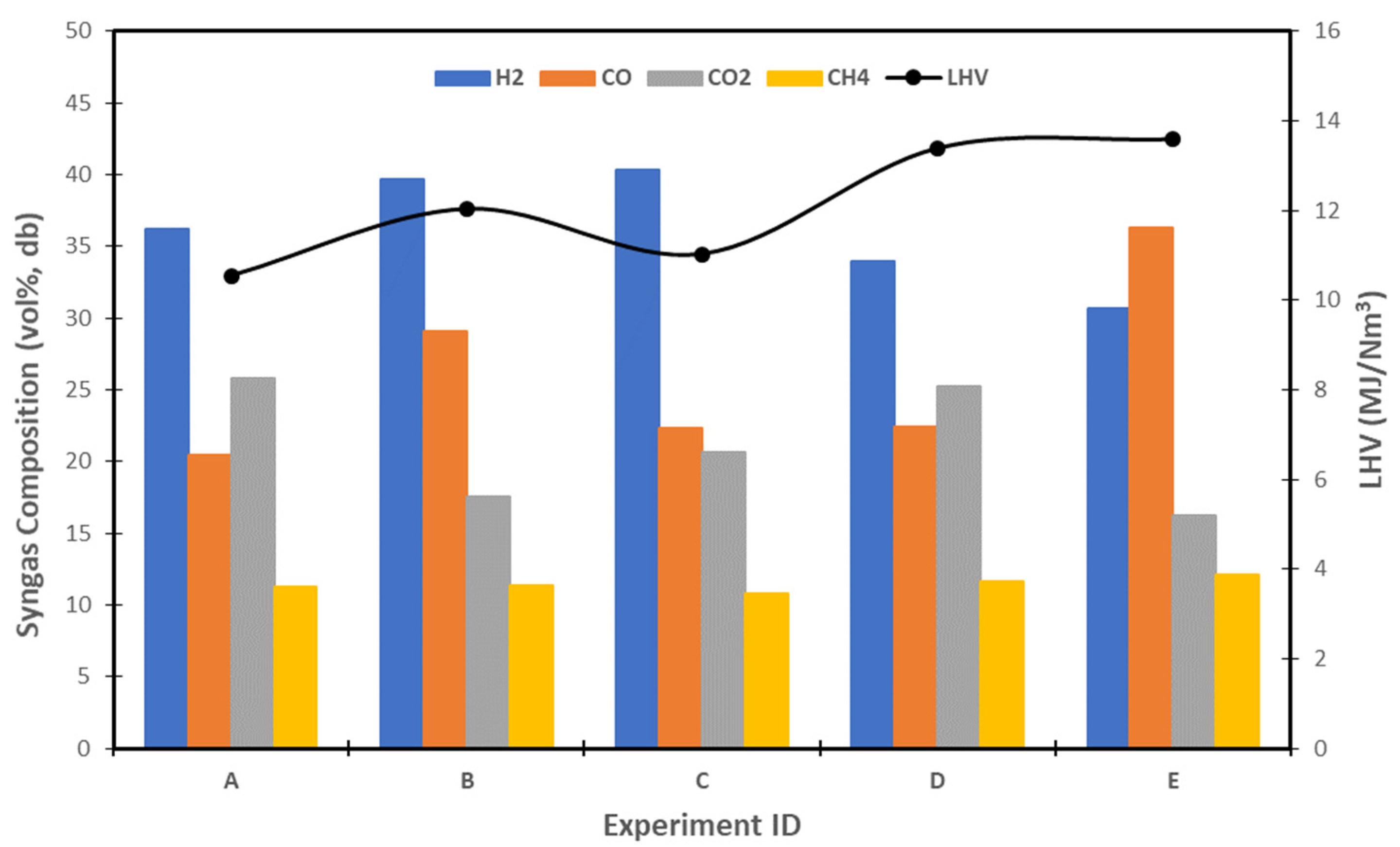
| Experiment ID | Woody Biomass | S/B | Temperature (°C) | References |
|---|---|---|---|---|
| A | Woodchip | 0.87 | 850 | [59] |
| B | Wood pellets | 0.91 | 850 | [24] |
| C | Softwood | 0.88 | 850 | [60] |
| D | Hardwood | 1.0 | 807 | [61] |
| E | Pine | 0.89 | 800 | [62] |
| Gasification Agent | Air | Oxygen | Steam |
|---|---|---|---|
| Product gas LHV, MJ/Nm3 | Low: 4–6 | High: 10–15 | High: 15–20 |
| Products | CO, H2, Water, CO2, HC, Tar, N2 | CO, H2, HC, CO2 | H2, CO, CO2, CH4, light HC, Tar |
| Product gas composition (vol./vol. or mol./mol.) | H2—15%, CO—20% CH4—2%, CO2—15% N2—48% H2:CO: 0.75 | H2—40% CO—40% CO2—20% H2:CO: 1 | H2—40%, CO—25% CH4—8%, CO2—25% N2—2% H2:CO: 1 |
| Gasification temperature, °C | 900–1100 | 1000–1400 | 700–1200 |
| Cost | Cheap | Costly | Medium |
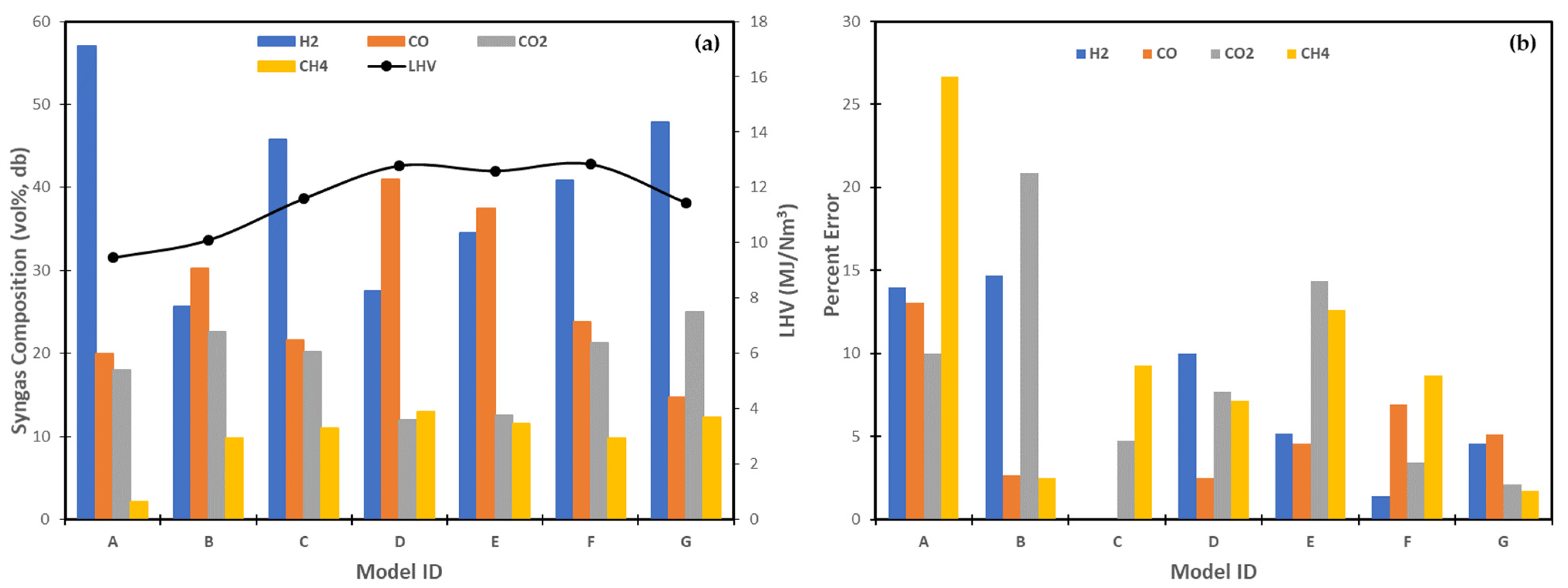
| MODEL ID No. | Modelling Approach | Feedstock | Operating Conditions | Remarks | Ref. | ||
|---|---|---|---|---|---|---|---|
| Biomass Feeding Rate (kg/h) | Gasifier Temp. (°C) | S/B | |||||
| A | Thermodynamic equilibrium | Pine | 100 | 831 | 0.2 | The reactions considered in minimizing the Gibb’s free energy to model the gasification stage were water–gas, water–gas shift, steam reforming, Boudouard, and tar reforming reactions. | [26] |
| B | Restricted chemical equilibrium | Commercial wood pellets | 104.3 | 820 | 0.54 | The reaction extent of the water–gas shift and hydrogenating gasification reactions were adjusted to 0.51 and 0.8 kmol/h, respectively, to match the experimental measurements of CO and CH4. | [32] |
| C | Restricted chemical equilibrium | Wood chip | 1508.64 | 850 | 0.75 | The extent of equilibrium for the steam methane reforming and water–gas shift reactions were restricted by inputting temperature approach values of −265 °C and −90 °C, respectively. | [31] |
| D | Kinetic | Wood | 3600 | 866 | 0.4 | The mass yields of the pyrolysis products were modelled with respect to temperature by a pyrolysis correlation. The gasification reactions, including catalytic conversion of lumped tar species, were modelled by a semi-detailed kinetic mechanism. | [23] |
| E | Kinetic | Wood pellets | 18.6 | 850 | 0.6 | The mass yields of the pyrolysis products were modelled with respect to temperature by a pyrolysis correlation. The gasification stage was modelled based on the two-phase assumptions with consideration of the heat and mass transfer between the two phases. | [24] |
| F | Kinetic | Beech wood | 21 | 850 | 0.88 | The mass yields and elemental compositions of char and tar, and pyrolytic gases yields were modelled by combining semi-empirical equations with numerical algorithms. | [60] |
| G | Kinetic | Beech wood | 100 | 850 | 1 | The gasification stage was modelled using a user-defined block. | [64] |
| Process Assumptions | References |
|---|---|
| [26,31,32] |
| [26,31,32,64] |
| [26,31,32,64] |
| [26,32] |
| [24,26] |
| [26,31] |
| [64] |
| [23,24] |
| [31] |
| [24] |
| Component assumptions | References |
| [26] |
| [31,64] |
| [26,64] |
| [26,32] |
| [26] |
| [31,64] |
| [32,60,64] |
| Hydrodynamics assumptions | References |
| [23] |
| [24] |
| [24] |
| [24] |
| Biomass Feedstock | Processes | Technology and Operating Conditions | Modelling Approach/Assumptions | Simulation Results (dry vol%) | References | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| H2 | CO | CO2 | CH4 | Others | ||||||
| Wood residue | (1) Biomass steam gasification | Fluidised bed (750 °C, S/B = 0.5) | Thermodynamic Equilibrium | 56.1 | 34.5 | 8.3 | 1.1 | [25] | ||
| (2) Producer Gas Cleaning | Tar reforming (800 °C, 1 bar) | Thermodynamic Equilibrium | ||||||||
| (3) H2/CO Enhancement | HT-WGS (450 °C, S/B = 0.5) and LT-WGS (250 °C) | Thermodynamic Equilibrium | 64.1 | 12 | 23.7 | 0.2 | ||||
| (4) Purification Process | Pressure Swing Adsorption (40 °C, 7 bar) | 70% efficiency of separator | Purity not specified in the study | |||||||
| Spruce wood branches | (1) Biomass Steam Gasification | Dual Fluidised Bed (800 °C, S/B = 1) | Kinetic modelling | 47.5 | 14.5 | 25.5 | 12.5 | [44] | ||
| (2) CO2 Capture | Adsorption with monoethanolamine (40 °C) | Uses an absorber for simulation | 60 | 18 | 5.5 | 16.5 | ||||
| Pine | (1) Biomass Steam Gasification | Dual Fluidised Bed (800 °C and S/B = 0.6) | Thermodynamic Equilibrium | 48 | 18 | 24 | 3 | 7 | [26] | |
| (2) Raw Syngas Purification | Tar Cracking (800 °C) | Thermodynamic Equilibrium | 47.1 | 41.2 | 6.5 | 2.5 | 2.7 | |||
| Pressure Swing Adsorption (35 °C and 30 atm) | Four-stage separation | H2/CO = 2.5 | ||||||||
| 30% CO2 captured | 95% CH4 captured | |||||||||
| (3) Methanol synthesis | 220 °C and 55 atm | Thermodynamic Equilibrium | ||||||||
| H2 | CO | CO2 | CH4 | Others | ||||||
| Wood residue | (1) Biomass Steam Gasification | Fluidised bed (900 °C, S/B = 0.2) | Thermodynamic Equilibrium | 55.7 | 40.8 | 3.3 | 0.08 | 0.12 | [27] | |
| (2) Syngas Adjustment | WGS Reaction (350 °C and S/CO = 0.28) | Thermodynamic Equilibrium | 59.7 | 27.6 | 12.4 | 0.20 | 0.1 | |||
| H2 | CO | CO2 | N2 | Others | ||||||
| Rice straw and cassava wastes | (1) Biomass pre-processing | Drying to <10% humidity | -- | [114] | ||||||
| (2) Biomass gasification | Air gasification (750 °C, 1 atm) | Thermodynamic Equilibrium | 25.1 | 18.8 | 5.1 | 44.8 | 6.1 | |||
| (3) Raw syngas purification | Cyclone separation and water scrubbing (15 °C) | -- | 54.9 | 45.1 | -- | -- | -- | |||
| (4) H2 maximisation | WGS reaction (205 °C, 32 bar) | Thermodynamic Equilibrium | 68.2 | 3.2 | 28.6 | -- | -- | |||
| (5) H2 purification | Membrane filtration (430 °C and 5 bar) | Target: 97% by vol H2 | 97.9 | -- | 2.09 | -- | -- | |||
| Biomass type not specified | (1) Biomass Gasification | Entrained-flow gasification (1300 °C, O/B = 0.39, S/B = 0.65) | Thermodynamic Equilibrium | 27.6 | 56.8 | 15.1 | 0.4 | 0.1 | [35] | |
| (2) Syngas Adjustment | WGS reaction (280 °C, 38 bar) | Thermodynamic Equilibrium | 53.5 | 0.08 | 45.5 | 0.54 | 0.38 | |||
| (3) Syngas Purification | Acid-Gas Removal (−58 °C) | 35 stages absorber | 98.2 | 0.14 | 0.70 | 0.98 | -- | |||
| PSA | Split fraction: H2 = 1; CO = 0; CO2, CH4, N2 = 0.02 | 99.97 | -- | 0.01 | 0.02 | -- | ||||
| Study | Technical Indicators | Economic Indicators | References | |||
|---|---|---|---|---|---|---|
| Technologies | H2 Yield (g/kg feed) | CAPEX (€) | H2 PC (US$/kg H2) | [117] | ||
| Fossil-based SMR | 40–130 | 170.95–240.20 M | 0.77 | |||
| Coal Gasification | -- | 257.60 M | 0.92–2.83 | |||
| Biomass Gasification | 40–190 | 11 M | 1.21–3.5 | |||
| Biomass Pyrolysis | 25–65 | 210–287 M | 1.21–2.57 | |||
| Water Electrolysis | -- | 257.60 M | 0.92–2.83 | |||
| Photo-fermentation | 9–49 | 115.6 | 2.83–3.89 | |||
| Technologies | H2 Yield (g/kg feed) | CO2 emission (kg/kg H2) | LcoH (US$/kg H2) | Total Cost of Hydrogen (US$/kg H2) | [36] | |
| Fossil-based SMR | 297 | 9.26 | 1.35 | 5.51 | ||
| Fossil-based SMR with CCS | 265 | 1.03 | 2.01 | 4.67 | ||
| Coal Gasification | 118 | 22.0 | 1.48 | 12.65 | ||
| Coal Gasification with CCS | 103 | 4.13 | 2.32 | 10.59 | ||
| Biomass Gasification | 27 | 32.84 | 2.4 | 12.63 | ||
| Biomass Gasification with CCS | 27 | 16.77 | 3.71 | 11.59 | ||
| Electrolysis from wind energy | 100 | -- | 5.61 | 6.52 | ||
| Electrolysis from nuclear energy | 100 | -- | 4.95 | 5.76 | ||
| Technologies | Energy Efficiency (%) | CO2 emission (t/t H2) | Material Consumption (t/t H2) | TCI a (US$) | PC (US$/kg H2) | [35] |
| Coal Gasification | 37.82 | 16.39 | 6.43 | 1.39 × 108 | 1.18 | |
| Biomass Gasification | 37.88 | 15.23 | 10.99 | 1.65 × 108 | 0.98 | |
| Technologies | Energy Efficiency (%) | H2 Conversion Rate (%) | TCI (€) | After Tax BEP b (€/kg H2) | NPV (€) | [38] |
| DFB Biomass Steam Gasification with CC | 38.9 | 51.4 | 12.1 M | 4.88 | −34.8 M | |
| Biogas Steam Reforming with CC | 47.0 | 27.2 | 9.9 M | 5.02 | −33.18 M | |
| Alkaline Electrolysis | 66 | 62.5 | 4.4 M | 6.30 | −33.64 M | |
| Technologies | Energy Efficiency (%) | Exergy Efficiency (%) | PC (US$/kg H2) | Social Cost of Carbon (US$/kg H2) | [118] | |
| Natural Gas SR | 37 | 32 | 1.5 | 1.9 | ||
| Coal Gasification with CCS | 35 | 32 | 1.8 | 2.75 | ||
| Biomass Gasification | 64 | 60 | 1.5 | 0.5 | ||
| Electrolysis (Wind) | 31 | 30 | 7.3 | 0.2 | ||
| Electrolysis (Solar) | 4 | 3 | 9 | 0.4 | ||
| Technologies | Energy Efficiency (%) | TCI (US$) | Min H2 Selling Price (US$/kg H2) | [30] | ||
| FB biomass gasification | 45 | 647 M | 3.1 | |||
| FB biomass gasification with CC | 41 | 852 M | 3.5 | |||
| EF biomass gasification | 56 | 1229 M | 3.4 | |||
| EF biomass gasification with CC | 50 | 1340 M | 3.5 | |||
| Natural gas reforming | 66 | -- | 1.1 | |||
| Natural gas reforming with CC | 48 | -- | 2.2 | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Castro, J.; Leaver, J.; Pang, S. Simulation and Techno-Economic Assessment of Hydrogen Production from Biomass Gasification-Based Processes: A Review. Energies 2022, 15, 8455. https://doi.org/10.3390/en15228455
Castro J, Leaver J, Pang S. Simulation and Techno-Economic Assessment of Hydrogen Production from Biomass Gasification-Based Processes: A Review. Energies. 2022; 15(22):8455. https://doi.org/10.3390/en15228455
Chicago/Turabian StyleCastro, Jhulimar, Jonathan Leaver, and Shusheng Pang. 2022. "Simulation and Techno-Economic Assessment of Hydrogen Production from Biomass Gasification-Based Processes: A Review" Energies 15, no. 22: 8455. https://doi.org/10.3390/en15228455
APA StyleCastro, J., Leaver, J., & Pang, S. (2022). Simulation and Techno-Economic Assessment of Hydrogen Production from Biomass Gasification-Based Processes: A Review. Energies, 15(22), 8455. https://doi.org/10.3390/en15228455








