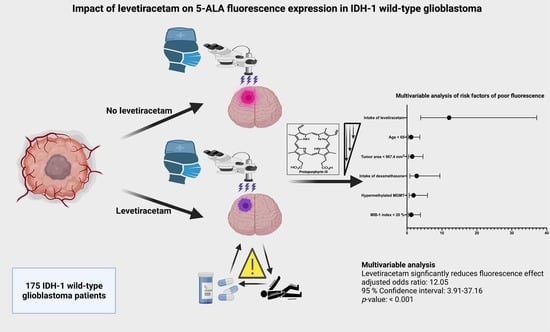
Impact of Levetiracetam Treatment on 5-Aminolevulinic Acid Fluorescence Expression in IDH1 Wild-Type Glioblastoma
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Patient Characteristics
2.2. Surgical Procedure
2.3. Histopathology
2.4. Clinical Data Recording and Analysis
2.5. Statistical Analysis
3. Results
3.1. Patient Characteristics
3.2. Clinical, Imaging, and Neuropathological Characteristics among Fluorescence Grades
3.3. Association between Antiepileptic Drug Treatment and Intraoperative 5-ALA Fluorescence
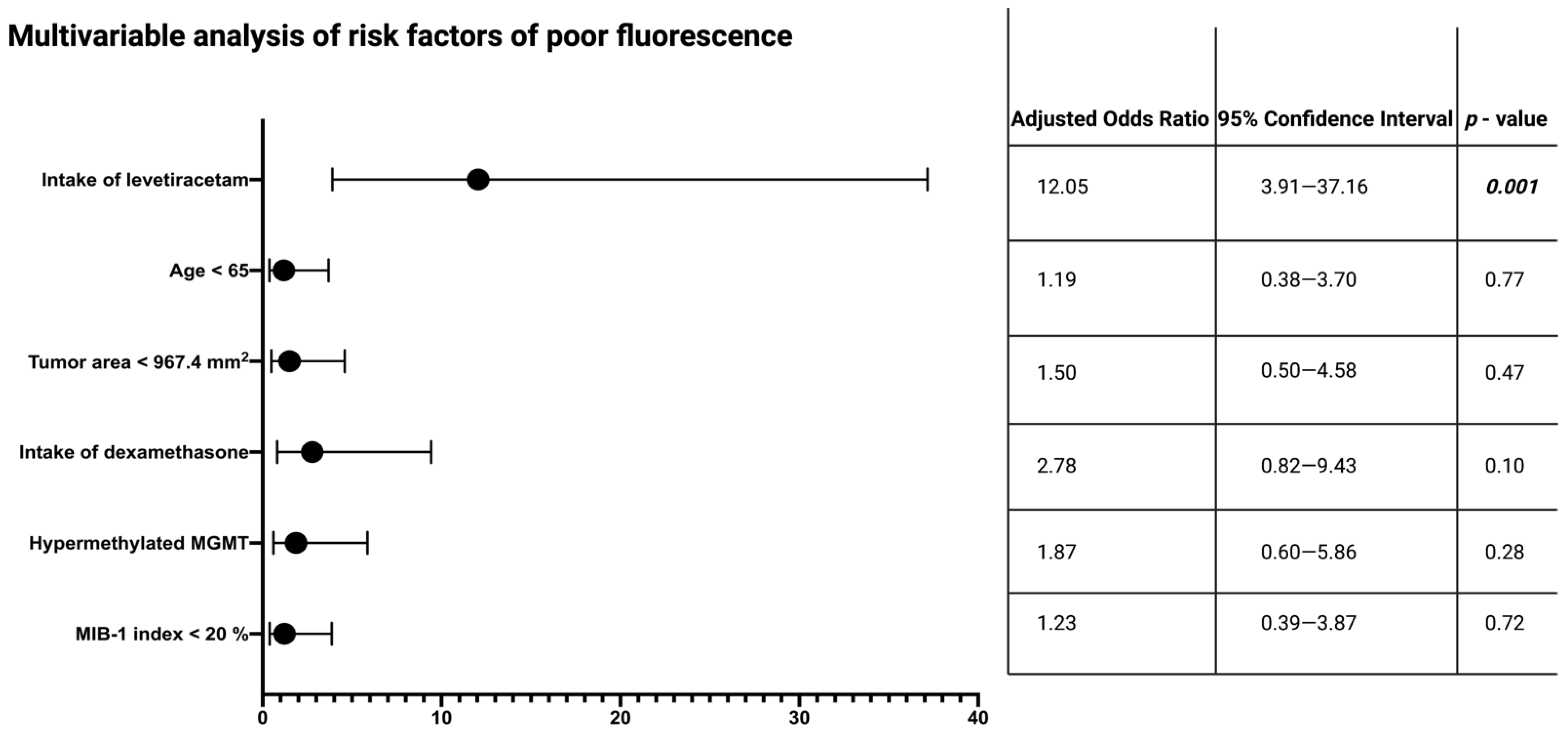
3.4. Specific Impact of Levetiracetam on Intraoperative 5-ALA Fluorescence
3.5. Impact of Intraoperative Fluorescence Quality on Extent of Resection and Influence of AEDs, and Levetiracetam on Overall Survival
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- DeAngelis, L.M. Brain tumors. N. Engl. J. Med. 2001, 344, 114–123. [Google Scholar] [CrossRef] [Green Version]
- Ostrom, Q.T.; Gittleman, H.; Fulop, J.; Liu, M.; Blanda, R.; Kromer, C.; Wolinsky, Y.; Kruchko, C.; Barnholtz-Sloan, J.S. CBTRUS Statistical Report: Primary Brain and Central Nervous System Tumors Diagnosed in the United States in 2008–2012. Neuro Oncol. 2015, 17 (Suppl. 4), iv1–iv62. [Google Scholar] [CrossRef]
- Louis, D.N.; Perry, A.; Wesseling, P.; Brat, D.J.; Cree, I.A.; Figarella-Branger, D.; Hawkings, C.; Ng, H.K.; Pfister, S.M.; Reifenberger, G.; et al. The 2021 WHO Classification of Tumors of the Central Nervous System: A summary. Neuro Oncol. 2021, 23, 1231–1251. [Google Scholar] [CrossRef]
- Weller, M.; van den Bent, M.; Presusser, M.; Le Rhun, E.; Tonn, J.C.; Minniti, G.; Bendszus, M.; Balana, C.; Chinot, O.; Dirven, L.; et al. EANO guidelines on the diagnosis and treatment of diffuse gliomas of adulthood. Nat. Rev. Clin. Oncol. 2021, 18, 170–186. [Google Scholar] [CrossRef]
- Stupp, R.; Hegi, M.E.; Mason, W.P.; van den Bent, M.J.; Taphoorn, M.J.; Janzer, R.C.; Ludwin, S.K.; Allgeier, A.; Fisher, B.; Belanger, K.; et al. Effects of radiotherapy with concomitant and adjuvant temozolomide versus radiotherapy alone on survival in glioblastoma in a randomized phase III study: 5-year analysis of the EORTC-NCIC trial. Lancet Oncol. 2009, 10, 459–466. [Google Scholar] [CrossRef]
- Herrlinger, U.; Tzaridis, T.; Mack, F.; Steinbach, J.P.; Schlegel, U.; Sabel, M.; Hau, P.; Kortmann, R.D.; Krex, D.; Grauer, O.; et al. Neurooncology Working Group of the German Cancer Society. Lomustine-temozolomide combination therapy versus standard temozolomide therapy in patients with newly diagnosed glioblastoma with methylated MGMT promoter (CeTeG/NOA-09): A randomized, open-label, phase 3 trial. Lancet 2019, 393, 678–688. [Google Scholar]
- Wach, J.; Apallas, S.; Schneider, M.; Güresir, A.; Schuss, P.; Herrlinger, U.; Vatter, H.; Güresir, E. Baseline Serum C-reactive Protein and Plasma Fibrinogen-Based Score in the Prediction of Survival in Glioblastoma. Front. Oncol. 2021, 11, 653614. [Google Scholar] [CrossRef]
- Smrdel, U.; Vidmar, M.S.; Smrdel, A. Glioblastoma in patients over 70 years of age. Radiol. Oncol. 2018, 52, 167–172. [Google Scholar] [CrossRef] [Green Version]
- Lamborn, K.R.; Chang, S.M.; Prados, M.D. Prognostic factors for survival of patients with glioblastoma: Recursive partitioning analysis. Neuro Oncol. 2004, 6, 227–235. [Google Scholar] [CrossRef] [Green Version]
- Kuhnt, D.; Becker, A.; Ganslandt, O.; Bauer, M.; Buchfelder, M.; Nimsky, C. Correlation of the extent of tumor volume resection and patient survival in surgery for glioblastoma multiforme with high-field intraoperative MRI guidance. Neuro Oncol. 2011, 13, 1339–1348. [Google Scholar] [CrossRef] [Green Version]
- Lacroix, M.; Abi-Said, D.; Fourney, D.R.; Gokaslan, Z.L.; Shi, W.; DeMonte, F.; Lang, F.F.; McCutcheon, I.E.; Hassenbusch, S.J.; Holland, E.; et al. A multivariate analysis of 416 patients with glioblastoma multiforme: Prognosis, extent of resection, and survival. J. Neurosurg. 2001, 95, 190–198. [Google Scholar] [CrossRef] [Green Version]
- Sanai, N.; Polley, M.Y.; McDermott, M.W.; Parsa, A.T.; Berger, M.S. An extent of resection threshold for newly diagnosed glioblastomas. J. Neurosurg. 2011, 115, 3–8. [Google Scholar] [CrossRef] [Green Version]
- Senft, C.; Bink, S.; Franz, K.; Vatter, H.; Gasser, T.; Seifert, V. Intraoperative MRI guidance and extent of resection in glioma surgery: A randomized, controlled trial. Lancet Oncol. 2011, 12, 997–1003. [Google Scholar] [CrossRef]
- Wu, J.S.; Gong, X.; Song, Y.Y.; Zhuang, D.X.; Yao, C.J.; Qiu, T.M.; Lu, J.F.; Zhang, J.; Zhu, W.; Mao, Y.; et al. 3.0-T intraoperative magnetic resonance imaging-guided resection in cerebral glioma surgery: Interim analysis of a prospective, randomized, triple-blind, parallel-controlled trial. Neurosurgery 2014, 61 (Suppl. 1), 145–154. [Google Scholar] [CrossRef]
- Wach, J.; Goetz, C.; Shareghi, K.; Scholz, T.; Heßelmann, V.; Mager, A.K.; Gottschalk, J.; Vatter, H.; Kremer, P. Dual-Use Intraoperative MRI in Glioblastoma Surgery: Results of Resection, Histopathologic Assessment, and Surgical Site Infections. J. Neurol. Surg. A Cent. Eur. Neurosurg. 2019, 80, 413–422. [Google Scholar] [CrossRef] [Green Version]
- Stummer, W.; Pichlmeier, U.; Meinel, T.; Wiestler, O.D.; Zanella, F.; Reulen, H.J.; ALA-Glioma Study Group. Fluorescence-guided surgery with 5-aminolevulinic acid for resection of malignant glioma: A randomized controlled multicentre phase III trial. Lancet Oncol. 2006, 7, 392–401. [Google Scholar] [CrossRef]
- Nabavi, A.; Thurm, H.; Zountsas, B.; Pietsch, T.; Lanfermann, H.; Pichlmeier, U.; Mehdorn, M.; 5-ALA Recurrent Glioma Study Group. Five-aminolevulenic acid for fluorescence-guided resection of recurrent malignant gliomas: A phase ii study. Neurosurgery 2009, 65, 1070–1077. [Google Scholar] [CrossRef]
- Pichlmeier, U.; Bink, A.; Schackert, G.; Stummer, W. Resection and survival in glioblastoma multiforme: An RTOG recursive partitioning analysis of ALA study patients. Neuro Oncol. 2008, 10, 1025–1034. [Google Scholar] [CrossRef] [Green Version]
- Roberts, D.W.; Valdés, P.A.; Harris, B.T.; Hartov, A.; Fan, X.; Ji, S.; Leblond, F.; Tosteson, T.D.; Wilson, B.C.; Paulsen, K.D. Glioblastoma multiforme treatment with clinical trials for surgical resection (aminolevulinic acid). Neurosurg. Clin. 2012, 23, 371–377. [Google Scholar] [CrossRef] [Green Version]
- Roberts, D.W.; Valdés, P.A.; Harris, B.T.; Fontaine, K.M.; Hartov, A.; Fan, X.; Ji, S.; Lollis, S.S.; Pogue, B.W.; Leblond, F.; et al. Coregistered fluorescence-enhanced tumor resection of malignant glioma: Relationships between δ-aminolevulinic acid–induced protoporphyrin IX fluorescence, magnetic resonance imaging enhancement, and neuropathological parameters. J. Neurosurg. 2011, 114, 595–603. [Google Scholar] [CrossRef] [Green Version]
- Stummer, W.; Reulen, H.J.; Meinel, T.; Pichlmeier, U.; Schumacher, W.; Tonn, J.C.; Rohde, V.; Oppel, F.; Turowski, B.; Woiciechowsky, C.; et al. ALA-Glioma Study Group. Extent of resection and survival in glioblastoma multiforme: Identification of and adjustment for bias. Neurosurgery 2008, 62, 564–576. [Google Scholar]
- Peng, Q.; Warloe, T.; Berg, K.; Moan, J.; Kongshaug, M.; Giercksky, K.E.; Nesland, J.M. 5-Aminolevulinic acid-based photodynamic therapy. Clinical research and future challenges. Cancer 1997, 79, 2282–2308. [Google Scholar] [CrossRef]
- Qian, Z.M.; Chang, Y.Z.; Leung, G.; Du, J.R.; Zhu, L.; Wang, Q.; Niu, L.; Xu, Y.J.; Yang, L.; Ho, K.P. Expression of ferroportin1, hephaestin and ceruloplasmin in rat heart. Biochim. Biophys. Acta 2007, 1772, 527–532. [Google Scholar] [CrossRef] [Green Version]
- Kondo, M.; Hirota, N.; Takaoka, T.; Kajiwara, M. Heme-biosynthetic enzyme activities and porphyrin accumulation in normal liver and hepatoma cell lines of rat. Cell Biol. Toxicol. 1993, 9, 95–105. [Google Scholar] [CrossRef]
- Shimura, M.; Nozawa, N.; Ogawa-Tominaga, M.; Fushimi, T.; Tajika, M.; Ichimoto, K.; Matsunaga, A.; Tsuruoka, T.; Kishita, Y.; Ishii, T.; et al. Effects of 5-aminolevulinic acid and sodium ferrous citrate on fibroblasts from individuals with mitochondrial diseases. Sci. Rep. 2019, 9, 10549. [Google Scholar] [CrossRef] [Green Version]
- Jiang, M.; Hong, K.; Mao, Y.; Ma, H.; Chen, T.; Wang, Z. Natural 5-Aminolevulinic Acid: Sources, Biosynthesis, Detection and Applications. Front. Bioeng. Biotechnol. 2022, 10, 841443. [Google Scholar] [CrossRef]
- Hefti, M.; Albert, I.; Luginbuehl, V. Phenytoin reduces 5-aminolevulinic acid-induced protoporphyrin IX accumulation in malignant glioma cells. J. Neurooncol. 2012, 108, 443–450. [Google Scholar] [CrossRef]
- Lawrence, J.E.; Steele, C.J.; Rovin, R.A.; Belton, R.J.; Belton, R.J., Jr.; Winn, R.J. Dexamethasone alone and in combination with desipramine, phenytoin, valproic acid or levetiracetam interferes with 5-ALA-mediated PpIX production and cellular retention in glioblastoma cells. J. Neurooncol. 2016, 127, 15–21. [Google Scholar] [CrossRef]
- Toldeo, M.; Sarria-Estrada, S.; Quintana, M.; Maldonado, X.; Martinez-Ricarte, F.; Rodon, J.; Auger, C.; Salas-Puig, J.; Santamraina, E.; Martinez-Saez, E. Prognostic implications of epilepsy in glioblastomas. Clin. Neurol. Neurosurger. 2015, 139, 166–171. [Google Scholar] [CrossRef]
- Glauser, T.; Ben-Menachem, E.; Bourgeois, B.; Cnaan, A.; Guerreiro, C.; Kälviäinen, R.; Mattson, R.; French, J.A.; Perucca, E.; Tomson, T. ILAE Subcommission on AED Guidelines. Updated ILAE evidence review of antiepileptic drug efficacy and effectiveness as initial monotherapy for epileptic seizures and syndromes. Epilepsia 2013, 54, 551–563. [Google Scholar] [CrossRef]
- Wach, J.; Hamed, M.; Schuss, P.; Güresir, E.; Herrlinger, U.; Vatter, H.; Schneider, M. Impact of initial midline-shift in glioblastoma on survival. Neurosurg. Rev. 2021, 44, 1401–1409. [Google Scholar] [CrossRef]
- Panciani, P.P.; Fontanella, M.; Garbossa, D.; Agnoletti, A.; Ducati, A.; Lanotte, M. 5-aminolevulinic acid and neuronavigation in high-grade glioma surgery: Results of combined approach. Neurocirugia 2012, 23, 23–28. [Google Scholar] [CrossRef]
- Kamp, M.A.; Felsberg, J.; Sadat, H.; Kuzibaev, J.; Steiger, H.J.; Rapp, M.; Reifenberger, G.; Dibue, M.; Sabel, M. 5-ALA-induced fluorescence behavior of reactive tissue changes following glioblastoma treatment with radiation and chemotherapy. Acta Neurochir. 2015, 157, 207–214. [Google Scholar] [CrossRef]
- Specchia, F.M.C.; Monticelli, M.; Zeppa, P.; Bianconi, A.; Zenga, F.; Altieri, R.; Pugliese, B.; Di Perna, G.; Cofano, F.; Tartara, F.; et al. Let Me See: Correlation between 5-ALA Fluorescence and Molecular Pathways in Glioblastoma: A Single Center Experience. Brain Sci. 2021, 11, 795. [Google Scholar] [CrossRef]
- Stummer, W.; Stepp, H.; Wiestler, O.D.; Pichlmeier, U. Randomized, Prospective Double-Blinded Study Comparing 3 Different Doses of 5-Aminolevulinic Acid for Fluorescence-Guided Resections of Malignant Gliomas. Neurosurgery 2017, 81, 230–239. [Google Scholar] [CrossRef] [Green Version]
- Forster, M.T.; Behrens, M.; Lortz, I.; Conradi, N.; Senft, C.; Voss, M.; Rauch, M.; Seifert, V. Benefits of glioma resection in the corpus callosum. Sci. Rep. 2020, 10, 16630. [Google Scholar] [CrossRef]
- Louis, D.N.; Perry, A.; Reifenberger, G.; von Deimling, A.; Figarella-Branger, D.; Cavenee, W.K.; Ohgaki, H.; Wiestler, O.D.; Kleihues, P.; Ellison, D.W. The 2016 World Health Organization classification of tumors of the central nervous system: A summary. Acta Neuropathol. 2016, 131, 803–820. [Google Scholar] [CrossRef] [Green Version]
- Hegi, M.E.; Diserens, A.C.; Gorlia, T.; Hamou, M.F.; de Tribolet, N.; Weller, M.; Kros, J.M.; Hainfellner, J.A.; Mason, W.; Mariani, L.; et al. MGMT gene silencing and benefit from temozolomide in glioblastoma. N. Engl. J. Med. 2005, 352, 997–1003. [Google Scholar] [CrossRef] [Green Version]
- Mikeska, T.; Bock, C.; El-Maarri, O.; Hübner, A.; Ehrentraut, D.; Schramm, J.; Felsberg, J.; Kahl, P.; Büttner, R.; Pietsch, T.; et al. Optimization of quantitative MGMT promoter methylation analysis using pyrosequencing and combined bisulfite restriction analysis. J. Mol. Diagn. 2007, 9, 368–381. [Google Scholar] [CrossRef] [Green Version]
- Leu, S.; Boulay, J.L.; Thommen, S.; Bucher, H.C.; Stippich, C.; Mariani, L.; Bink, A. Preoperative two-dimensional size of glioblastoma is associated with patient survival. World Neurosurg. 2018, 115, e448–e463. [Google Scholar] [CrossRef]
- Schoenegger, K.; Oberndorfer, S.; Wuschitz, B.; Struhal, W.; Hainfellner, J.; Prayer, D.; Heinzol, H.; Lahrmann, H.; Marosi, C.; Grisold, W. Peritumoral edema on MRI at initial diagnosis: An independent prognostic factor for glioblastoma? Eur. J. Neurol. 2009, 16, 874–878. [Google Scholar] [CrossRef]
- Morgan, E.R.; Norman, A.; Laing, K.; Seal, M.D. Treatment and outcomes for glioblastoma in elderly compared with non-elderly patients: A population-based study. Curr. Oncol. 2017, 24, e92–e98. [Google Scholar] [CrossRef] [Green Version]
- Henker, C.; Kriesen, T.; Schneider, B.; Glass, Ä.; Scherer, M.; Langner, S.; Erbersdobler, A.; Piek, J. Correlation of Ki-67 Index with Volumetric Segmentation and its Value as a Prognostic Marker in Glioblastoma. World Neurosurg. 2019, 125, e1093–e1103. [Google Scholar] [CrossRef]
- Yoshida, Y.; Nakada, M.; Harada, T.; Tanaka, S.; Furuta, T.; Hayashi, Y.; Kita, D.; Uchiyama, N.; Hayashi, Y.; Hamada, J. The expression level of sphingosine-1-phosphate receptor type 1 is related to MIB-1 labeling index and predicts survival of glioblastoma patients. J. Neurooncol. 2010, 98, 41–47. [Google Scholar] [CrossRef]
- Leibetseder, A.; Ackerl, M.; Flechl, B.; Wöhrer, A.; Widhalm, G.; Dieckmann, K.; Kreinecker, S.S.; Pichler, J.; Hainfellner, J.; Preussner, M.; et al. Outcome and molecular characteristics of adolescent and young adult patients with newly diagnosed primary glioblastoma: A study of the Society of Austrian Neurooncology (SANO). Neuro Oncol. 2013, 15, 112–121. [Google Scholar] [CrossRef]
- Widhalm, G.; Wolfsberger, S.; Minchev, G.; Woehrer, A.; Krssak, M.; Czech, T.; Prayer, D.; Asenbaum, S.; Hainfellner, J.A.; Knosp, E. 5-Aminolevulinic Acid is a Promising Marker for Detection of Anaplastic Foci in Diffusely Infiltrating Gliomas with Nonsignificant Contrast Enhancement. Cancer 2010, 116, 1545–1552. [Google Scholar] [CrossRef]
- Mercea, P.A.; Mischkulnig, M.; Kiesel, B.; Wadiura, L.I.; Roetzer, T.; Prihoda, R.; Heicappell, P.; Kreminger, J.; Furtner, J.; Woehrer, A.; et al. Prognostic Value of 5-ALA Fluorescence, Tumor Cell Infiltration and Angiogenesis in the Peritumoral Brain Tissue of Brain Metastase. Cancers 2021, 13, 603. [Google Scholar] [CrossRef]
- Kamp, M.A.; Fischer, I.; Bühner, J.; Turowski, B.; Cornelius, J.F.; Steiger, H.J.; Rapp, M.; Slotty, P.J.; Sabel, M. 5-ALA fluorescence of cerebral metastases and its impact for the local-in-brain progression. Oncotarget 2016, 7, 66776–66789. [Google Scholar] [CrossRef] [Green Version]
- Della Puppa, A.; Rustemi, O.; Gioffre, G.; Troncon, I.; Lombardi, G.; Rolma, G.; Sergi, M.; Munari, M.; Cecchin, D.; Gardiman, M.P.; et al. Predictive value of intraoperative 5-aminolevulinic acid-induced fluorescence for detecting bone invasion in meningioma surgery. J. Neurosurg. 2014, 120, 840–845. [Google Scholar] [CrossRef] [Green Version]
- Sawaya, R.; Hammoud, M.; Schoppa, D.; Hess, K.R.; Wu, S.Z.; Shi, W.M.; Wildrick, D.M. Neurosurgical outcomes in a modern series of 400 craniotomies for treatment of parenchymal tumors. Neurosurgery 1998, 42, 1044–1055. [Google Scholar] [CrossRef]
- Goryaynov, S.A.; Widhalm, G.; Goldberg, M.F.; Chelushkin, D.; Spallone, A.; Chernyshov, K.A.; Ryzhova, M.; Pavlova, G.; Revischin, A.; Shishkina, L.; et al. The Role of 5-ALA in Low-Grade Gliomas and the Influence of Antiepileptic Drugs on Intraoperative Fluorescence. Front. Oncol. 2019, 9, 423. [Google Scholar] [CrossRef] [Green Version]
- McGirt, M.J.; Chaichana, K.L.; Attenello, F.J.; Weingart, J.D.; Than, K.; Burger, P.C.; Olivi, A.; Brem, H.; Quinones-Hinojosa, A. Extent of surgical resection is independently associated with survival in patients with hemispheric infiltrating low-grade gliomas. Neurosurgery 2008, 63, 700–707. [Google Scholar] [CrossRef] [Green Version]
- Ewelt, C.; Floeth, F.W.; Felsberg, J.; Steiger, H.J.; Sabel, M.; Langen, K.J.; Stoffels, G.; Stummer, W. Finding the anaplastic focus in diffuse gliomas: The value of Gd-DTPA enhanced MRI, FET-PET, and intraoperative, ALA-derived tissue fluorescence. Clin. Neurol. Neurosurg. 2011, 113, 541–547. [Google Scholar] [CrossRef]
- Sanai, N.; Snyder, L.A.; Honea, N.J.; Coons, S.W.; Eschbacher, J.M.; Smith, K.A.; Spetzler, R.F. Intraoperative confocal microscopy in the visualization of 5-aminolevulinic acid fluorescence in low-grade gliomas. J. Neurosurg. 2011, 115, 740–748. [Google Scholar] [CrossRef]
- Rogers, S.K.; Shapiro, L.A.; Tobin, R.P.; Tow, B.; Zuzek, A.; Mukherjee, S.; Newell-Rogers, M.K. Levetiracetam Differentially Alters CD95 Expression of Neuronal Cells and the Mitochondrial Membrane Potential of Immune and Neuronal Cells in vitro. Front. Neurol. 2014, 18, 5–17. [Google Scholar] [CrossRef] [Green Version]
- Jaber, M.; Wölfer, J.; Ewelt, C.; Holling, M.; Hasselblatt, M.; Niederstadt, T.; Zoubi, T.; Weckesser, M.; Stummer, W. The Value of 5-Aminolevulinic Acid in Low-grade Gliomas and High-grade Gliomas Lacking Glioblastoma Imaging Features: An Analysis Based on Fluorescence, Magnetic Resonance Imaging, 18F-Fluoroethyl Tyrosine Positron Emission Tomography, and Tumor Molecular Factors. Neurosurgery 2016, 78, 401–411. [Google Scholar]
- Utsuki, S.; Oka, H.; Kijima, C.; Inukai, M.; Fujii, K.; Ishizuka, M.; Takahashi, K.; Inoue, K. Preoperative prediction of whether intraoperative fluorescence of protoporphyrin IX can be achieved by 5-aminolevulinic acid administration. Int. J. Clin. Med. 2012, 3, 132. [Google Scholar] [CrossRef] [Green Version]
- Van der Meer, P.B.; Dirven, L.; Fiocco, M.; Vos, M.J.; Kouwenhoven, M.C.M.; van den Bent, M.J.; Taphoorn, M.J.B.; Koekkoek, J.A.F. First-line antiepileptic drug treatment in glioma patients with epilepsy: Levetiracetam vs valproic acid. Epilepsia 2021, 62, 1119–1129. [Google Scholar] [CrossRef]
- Acerbi, F.; Broggi, M.; Schebesch, K.M.; Höhne, J.; Cavallo, C.; De Laurentis, C.; Eoli, M.; Anghileri, E.; Servida, M.; Boffano, C.; et al. Fluorescein-Guided Surgery for Resection of High-Grade Gliomas: A Multicentric Prospective Phase II Study (FLUOGLIO). Clin. Cancer Res. 2018, 24, 52–61. [Google Scholar] [CrossRef] [Green Version]
- Zeh, R.; Sheikh, S.; Xia, L.; Pierce, J.; Newton, A.; Predina, J.; Cho, S.; Nasrallah, M.; Singhal, S.; Dorsey, J.; et al. The second window ICG technique demonstrates a broad plateau period for near infrared fluorescence tumor contrast in glioblastoma. PLoS ONE 2017, 12, e018203. [Google Scholar] [CrossRef]
- Kremer, P.; Fardanesh, M.; Ding, R.; Pritsch, M.; Zoubaa, S.; Frei, E. Intraoperative fluorescence staining of malignant brain tumors using 5-aminofluorescein-labeled albumin. Neurosurgery 2009, 64 (Suppl. 3), 53–60. [Google Scholar] [CrossRef] [PubMed]
- Kong, X.; Guan, J.; Yang, Y.; Li, Y.; Ma, W.; Wang, R. A meta-analysis: Do prophylactic antiepileptic drugs in patients with brain tumors decrease the incidence of seizures? Clin. Neurol. Neurosurg. 2015, 134, 98–103. [Google Scholar] [CrossRef]
- Valdés, P.A.; Leblond, F.; Kim, A.; Harris, B.T.; Wilson, B.C.; Fan, X.; Tosteson, T.D.; Hartov, A.; Ji, S.; Erkmen, K.; et al. Quantitative fluorescence in intracranial tumor: Implications for ALA-induced PpIX as an intraoperative biomarker. J. Neurosurg. 2011, 115, 11–17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Knudsen-Baas, K.M.; Engeland, A.; Gilhus, N.E.; Storstein, A.M.; Owe, J.F. Does the choice of antiepileptic drug affect survival in glioblastoma patients? J. Neurooncol. 2016, 129, 461–469. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roh, T.H.; Moon, J.H.; Park, H.H.; Kim, E.H.; Hong, C.K.; Kim, S.H.; Kang, S.G.; Chang, J.H. Association between survival and levetiracetam use in glioblastoma patients treated with temozolomide chemoradiotherapy. Sci. Rep. 2020, 10, 10783. [Google Scholar] [CrossRef] [PubMed]
- Cucchiara, F.; Pasqualetti, F.; Giorgi, F.S.; Danesi, R.; Bocci, G. Epileptogenesis and oncogenesis: An antineoplastic role for antiepileptic drugs in brain tumours? Pharmacol. Res. 2020, 156, 104786. [Google Scholar] [CrossRef]
- Darryl, L.; Shawn, L.H.-J.; Susan, C.; Annette, M.M.; Michael, W.M.; Joanna, J.P.; Mitchel, S.B. A prospective Phase II clinical trial of 5-aminolevulinic acid to assess the correlation of intraoperative fluorescence intensity and degree of histologic cellularity during resection of high-grade gliomas. J. Neurosurg. 2016, 124, 1300–1309. [Google Scholar]


| Median age (IQR) (in years) | 66 (56–73) |
| Sex | |
| Female | 66 (37.7%) |
| Male | 109 (62.3%) |
| Median preoperative KPS (IQR) | 90 (80–90) |
| Median body mass index (IQR) | 25.7 (23.4–28.7) |
| Preoperative epilepsy | 52 (29.7%) |
| Generalized | 24 (13.7%) |
| Complex partial | 9 (5.1%) |
| Simple partial | 19 (10.9%) |
| Type of antiepileptic medication | |
| Levetiracetam | 43 (24.6%) |
| Lamotrigine | 2 (1.1%) |
| Valproate | 1 (0.6%) |
| Carbamazepine | 1 (0.6%) |
| Phenytoin | 1 (0.6%) |
| Benzodiazepines | 1 (0.6%) |
| Dexamethasone intake | |
| Yes | 75 (42.9%) |
| No | 100 (57.1%) |
| Median tumor area (IQR), mm2 | 1268 (724.8–2113) |
| Median peritumoral edema (IQR), mm | 21.3 (15.3–29.7) |
| MGMT promoter hypermethylation | 69 (39.4%) |
| Median MIB-1 labeling index (IQR) | 15 (10–20) |
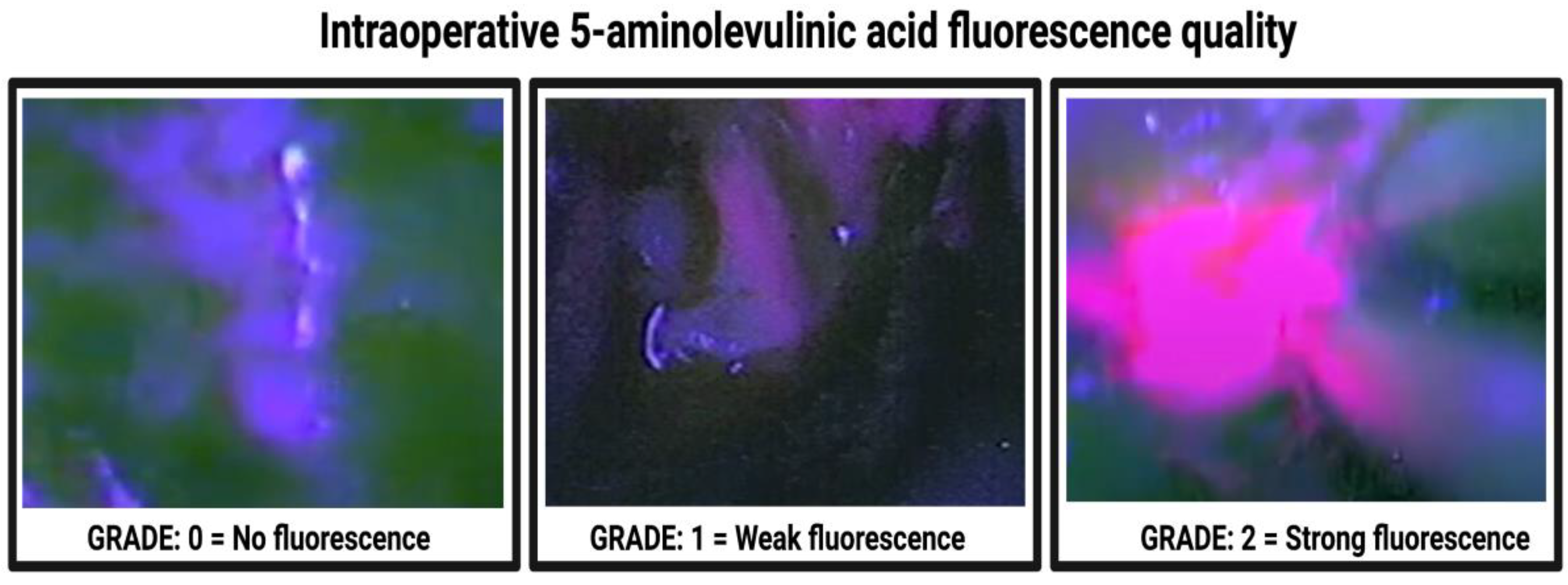
| 5-ALA fluorescence grade | |
| Grade 0—no fluorescence | 16 (9.1%) |
| Grade 1—weak fluorescence | 17 (9.7%) |
| Grade 2—strong fluorescence | 142 (81.1%) |
| Characteristics | Fluorescence Grade 0 (n = 16) | Fluorescence Grade 1 (n = 17) | Fluorescence Grade 2 (n = 142) | p-Value |
|---|---|---|---|---|
| Age (years), mean ± SD | 56.3 ± 15.9 | 61.5 ± 11.0 | 64.1 ± 12.7 | 0.06 |
| Sex | 0.26 | |||
| Female | 3 (18.8%) | 6 (35.3%) | 57 (40.1%) | |
| Male | 13 (81.2%) | 11 (64.7%) | 85 (59.9%) | |
| Preoperative AED | 0.001 | |||
| Yes | 14 (87.5%) | 12 (70.6%) | 26 (18.3%) | |
| No | 2 (12.5%) | 5 (29.4%) | 116 (81.7%) | |
| Dexamethasone intake | 0.023 | |||
| Yes | 11 (68.8%) | 10 (58.8%) | 54 (38.0%) | |
| No | 5 (32.2%) | 7 (42.2%) | 88 (62.0%) | |
| Body mass index, mean ± SD | 27.3 ± 6.7 | 26.1 ± 5.5 | 26.4 ± 3.9 | 0.72 |
| Tumor area, mean ± SD, mm2 | 834.1 ± 533.8 | 1197.6 ± 921.4 | 1553.7 ± 1047.8 | 0.03 |
| Peritumoral edema, mean ± SD, mm | 21.1 ± 9.7 | 21.7 ± 10.6 | 23.7 ± 11.7 | 0.64 |
| MGMT promoter status [available in 166 patients] | 0.19 | |||
| Hypermethylated | 3 (20.0%) | 6 (37.5%) | 60 (44.4%) | |
| Non-hypermethylated | 12 (80.0%) | 10 (62.5%) | 75 (55.6%) | |
| MIB-1 index, mean ± SD | 15.9 ± 6.5 | 19.7 ± 10.8 | 17.7 ± 8.2 | 0.48 |
| Characteristics | Fluorescence Grade 0 (n = 12) | Fluorescence Grade 1 (n = 16) | Fluorescence Grade 2 (n = 141) | p-Value |
|---|---|---|---|---|
| Age (years), mean ± SD | 55.0 ± 16.0 | 61.5 ± 11.4 | 64.1 ± 12.7 | 0 vs. 1: 0.22 |
| 1 vs. 2: 0.44 | ||||
| 0 vs. 2: 0.02 | ||||
| Sex | 0.49 | |||
| Female | 3 (25.0%) | 5 (31.3%) | 57 (40.4%) | |
| Male | 9 (75.0%) | 11 (68.8%) | 84 (59.6%) | |
| Preoperative Levetiracetam | 0.001 | |||
| Yes | 10 (83.3%) | 12 (75.0%) | 21 (14.9%) | |
| No | 2 (16.7%) | 4 (25.0%) | 120 (85.1%) | |
| Dexamethasone intake | 0.001 | |||
| Yes | 11 (91.7%) | 11 (68.8%) | 54 (38.3%) | |
| No | 1 (8.3%) | 5 (31.1%) | 87 (61.7%) | |
| Body mass index, mean ± SD | 27.0 ± 7.5 | 26.3 ± 5.6 | 26.4 ± 3.9 | 0 vs. 1: |
| 0.80 | ||||
| 1 vs. 2: | ||||
| 0.95 | ||||
| 0 vs. 2: | ||||
| 0.81 | ||||
| Tumor area, mean ± SD, mm2 | 830.7 ± 531.8 | 1274.0 ± 905.5 | 1550.9 ± 1048.5 | 0 vs. 1: |
| 0.18 | ||||
| 0 vs. 2: | ||||
| 0.002 | ||||
| 1 vs. 2: | ||||
| 0.33 | ||||
| Peritumoral edema, mean ± SD, mm | 21.6 ± 11.1 | 21.3 ± 10.8 | 23.7 ± 11.7 | 0 vs. 1: |
| 0.95 | ||||
| 0 vs. 2: | ||||
| 0.57 | ||||
| 1 vs. 2: | ||||
| 0.45 | ||||
| MGMT promoter status [available in 160 patients] | 0.18 | |||
| Hypermethylated | 2 (18.2%) | 5 (33.3%) | 60 (44.8%) | |
| Non-hypermethylated | 9 (81.8%) | 10 (66.7%) | 74 (55.2%) | |
| MIB-1 index, mean ± SD | 16.6 ± 6.8 | 19.7 ± 10.8 | 17.7 ± 8.2 | 0 vs. 1: |
| 0.42 | ||||
| 0 vs. 2: | ||||
| 0.67 | ||||
| 1 vs. 2: | ||||
| 0.40 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wach, J.; Güresir, Á.; Hamed, M.; Vatter, H.; Herrlinger, U.; Güresir, E. Impact of Levetiracetam Treatment on 5-Aminolevulinic Acid Fluorescence Expression in IDH1 Wild-Type Glioblastoma. Cancers 2022, 14, 2134. https://doi.org/10.3390/cancers14092134
Wach J, Güresir Á, Hamed M, Vatter H, Herrlinger U, Güresir E. Impact of Levetiracetam Treatment on 5-Aminolevulinic Acid Fluorescence Expression in IDH1 Wild-Type Glioblastoma. Cancers. 2022; 14(9):2134. https://doi.org/10.3390/cancers14092134
Chicago/Turabian StyleWach, Johannes, Ági Güresir, Motaz Hamed, Hartmut Vatter, Ulrich Herrlinger, and Erdem Güresir. 2022. "Impact of Levetiracetam Treatment on 5-Aminolevulinic Acid Fluorescence Expression in IDH1 Wild-Type Glioblastoma" Cancers 14, no. 9: 2134. https://doi.org/10.3390/cancers14092134
APA StyleWach, J., Güresir, Á., Hamed, M., Vatter, H., Herrlinger, U., & Güresir, E. (2022). Impact of Levetiracetam Treatment on 5-Aminolevulinic Acid Fluorescence Expression in IDH1 Wild-Type Glioblastoma. Cancers, 14(9), 2134. https://doi.org/10.3390/cancers14092134