Immunotherapy for HER2-Positive Breast Cancer: Clinical Evidence and Future Perspectives
Abstract
:Simple Summary
Abstract
1. Introduction
2. Monoclonal Antibodies
3. Antibody–Drug Conjugates
3.1. Trastuzmab-Emtansine (T-DM1)
3.2. Trastuzumab Deruxtecan (T-DXd)
3.3. Trastuzumab Duocarmazine (SYD985)
3.4. Other ADCs
4. Immune-Checkpoint Inhibitors
5. Chimeric Antigen Receptor (CAR) T Cells
6. Vaccines
7. Discussion and Future Perspectives
8. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Loibl, S.; Poortmans, P.; Morrow, M.; Denkert, C.; Curigliano, G. Breast Cancer. Lancet 2021, 397, 1750–1769. [Google Scholar] [CrossRef]
- Loibl, S.; Gianni, L. HER2-Positive Breast Cancer. Lancet 2017, 389, 2415–2429. [Google Scholar] [CrossRef]
- Bradley, R.; Braybrooke, J.; Gray, R.; Hills, R.; Liu, Z.; Peto, R.; Davies, L.; Dodwell, D.; McGale, P.; Pan, H.; et al. Trastuzumab for Early-Stage, HER2-Positive Breast Cancer: A Meta-Analysis of 13,864 Women in Seven Randomised Trials. Lancet Oncol. 2021, 22, 1139–1150. [Google Scholar] [CrossRef]
- Ribas, A.; Wolchok, J.D. Cancer Immunotherapy Using Checkpoint Blockade. Science 2018, 359, 1350–1355. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pusztai, L.; Karn, T.; Safonov, A.; Abu-Khalaf, M.M.; Bianchini, G. New Strategies in Breast Cancer: Immunotherapy. Clin. Cancer Res. 2016, 22, 2105–2110. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Criscitiello, C.; Curigliano, G. Immunotherapy of Breast Cancer. Prog. Tumor Res. 2015, 42, 30–43. [Google Scholar] [CrossRef] [PubMed]
- Bianchini, G.; Balko, J.M.; Mayer, I.A.; Sanders, M.E.; Gianni, L. Triple-Negative Breast Cancer: Challenges and Opportunities of a Heterogeneous Disease. Nat. Rev. Clin. Oncol. 2016, 13, 674–690. [Google Scholar] [CrossRef] [PubMed]
- Luen, S.; Virassamy, B.; Savas, P.; Salgado, R.; Loi, S. The Genomic Landscape of Breast Cancer and Its Interaction with Host Immunity. Breast 2016, 29, 241–250. [Google Scholar] [CrossRef] [PubMed]
- Solinas, C.; Carbognin, L.; De Silva, P.; Criscitiello, C.; Lambertini, M. Tumor-Infiltrating Lymphocytes in Breast Cancer According to Tumor Subtype: Current State of the Art. Breast 2017, 35, 142–150. [Google Scholar] [CrossRef] [PubMed]
- Agostinetto, E.; Eiger, D.; Punie, K.; de Azambuja, E. Emerging Therapeutics for Patients with Triple-Negative Breast Cancer. Curr. Oncol. Rep. 2021, 23, 57. [Google Scholar] [CrossRef] [PubMed]
- Agostinetto, E.; Losurdo, A.; Nader-Marta, G.; Santoro, A.; Punie, K.; Barroso, R.; Popovic, L.; Solinas, C.; Kok, M.; de Azambuja, V.; et al. Progress and Pitfalls in the Use of Immunotherapy for Patients with Triple Negative Breast Cancer. Expert Opin. Investig. Drugs 2022, 9, 1–25. [Google Scholar] [CrossRef]
- Savas, P.; Salgado, R.; Denkert, C.; Sotiriou, C.; Darcy, P.K.; Smyth, M.J.; Loi, S. Clinical Relevance of Host Immunity in Breast Cancer: From TILs to the Clinic. Nat. Rev. Clin. Oncol. 2016, 13, 228–241. [Google Scholar] [CrossRef] [PubMed]
- Gianni, L.; Pienkowski, T.; Im, Y.-H.; Tseng, L.-M.; Liu, M.-C.; Lluch, A.; Starosławska, E.; de la Haba-Rodriguez, J.; Im, S.-A.; Pedrini, J.L.; et al. 5-Year Analysis of Neoadjuvant Pertuzumab and Trastuzumab in Patients with Locally Advanced, Inflammatory, or Early-Stage HER2-Positive Breast Cancer (NeoSphere): A Multicentre, Open-Label, Phase 2 Randomised Trial. Lancet. Oncol. 2016, 17, 791–800. [Google Scholar] [CrossRef]
- Piccart, M.; Procter, M.; Fumagalli, D.; de Azambuja, E.; Clark, E.; Ewer, M.S.; Restuccia, E.; Jerusalem, G.; Dent, S.; Reaby, L.; et al. Adjuvant Pertuzumab and Trastuzumab in Early HER2-Positive Breast Cancer in the APHINITY Trial: 6 Years’ Follow-Up. J. Clin. Oncol. 2021, 39, 1448–1457. [Google Scholar] [CrossRef] [PubMed]
- Swain, S.M.; Miles, D.; Kim, S.-B.; Im, Y.-H.; Im, S.-A.; Semiglazov, V.; Ciruelos, E.; Schneeweiss, A.; Loi, S.; Monturus, E.; et al. Pertuzumab, Trastuzumab, and Docetaxel for HER2-Positive Metastatic Breast Cancer (CLEOPATRA): End-of-Study Results from a Double-Blind, Randomised, Placebo-Controlled, Phase 3 Study. Lancet Oncol. 2020, 21, 519–530. [Google Scholar] [CrossRef]
- Wolff, A.C.; Hammond, M.E.H.; Allison, K.H.; Harvey, B.E.; Mangu, P.B.; Bartlett, J.M.S.; Bilous, M.; Ellis, I.O.; Fitzgibbons, P.; Hanna, W.; et al. Human Epidermal Growth Factor Receptor 2 Testing in Breast Cancer: American Society of Clinical Oncology/College of American Pathologists Clinical Practice Guideline Focused Update. Arch. Pathol. Lab. Med. 2018, 142, 1364–1382. [Google Scholar] [CrossRef] [Green Version]
- Demonty, G.; Bernard-Marty, C.; Puglisi, F.; Mancini, I.; Piccart, M. Progress and New Standards of Care in the Management of HER-2 Positive Breast Cancer. Eur. J. Cancer 2007, 43, 497–509. [Google Scholar] [CrossRef]
- Gómez Román, V.R.; Murray, J.C.; Weiner, L.M. Antibody-Dependent Cellular Cytotoxicity (ADCC). Antib. Fc Link. Adapt. Innate Immun. 2013, 1–27. [Google Scholar] [CrossRef]
- Lo Nigro, C.; Macagno, M.; Sangiolo, D.; Bertolaccini, L.; Aglietta, M.; Merlano, M.C. NK-Mediated Antibody-Dependent Cell-Mediated Cytotoxicity in Solid Tumors: Biological Evidence and Clinical Perspectives. Ann. Transl. Med. 2019, 7, 105. [Google Scholar] [CrossRef] [Green Version]
- Bang, Y.J.; Giaccone, G.; Im, S.A.; Oh, D.Y.; Bauer, T.M.; Nordstrom, J.L.; Li, H.; Chichili, G.R.; Moore, P.A.; Hong, S.; et al. First-in-Human Phase 1 Study of Margetuximab (MGAH22), an Fc-Modified Chimeric Monoclonal Antibody, in Patients with HER2-Positive Advanced Solid Tumors. Ann. Oncol. Off. J. Eur. Soc. Med. Oncol. 2017, 28, 855–861. [Google Scholar] [CrossRef] [PubMed]
- Rugo, H.S.; Im, S.-A.; Cardoso, F.; Cortés, J.; Curigliano, G.; Musolino, A.; Pegram, M.D.; Wright, G.S.; Saura, C.; Escrivá-de-Romaní, S.; et al. Efficacy of Margetuximab vs. Trastuzumab in Patients With Pretreated ERBB2-Positive Advanced Breast Cancer: A Phase 3 Randomized Clinical Trial. JAMA Oncol. 2021, 7, 573–584. [Google Scholar] [CrossRef] [PubMed]
- Rugo, H.; Im, S.; Cardoso, F. Phase 3 SOPHIA Study of Margetuximab (M) + Chemotherapy (CTX) vs. Trastuzumab (T) + CTX in Patients (Pts) with HER2+ Metastatic Breast Cancer (MBC) after Prior Anti-HER2 Therapies: Final Overall Survival (OS) Analysis. In Proceedings of the San Antonio Breast Cancer Symposium, San Antonio, TX, USA, 7–10 December 2021. [Google Scholar]
- Park, S.; Jiang, Z.; Mortenson, E.D.; Deng, L.; Radkevich-Brown, O.; Yang, X.; Sattar, H.; Wang, Y.; Brown, N.K.; Greene, M.; et al. The Therapeutic Effect of Anti-HER2/Neu Antibody Depends on Both Innate and Adaptive Immunity. Cancer Cell 2010, 18, 160–170. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bianchini, G.; Gianni, L. The Immune System and Response to HER2-Targeted Treatment in Breast Cancer. Lancet. Oncol. 2014, 15, e58–e68. [Google Scholar] [CrossRef]
- Bianchini, G.; Pusztai, L.; Pienkowski, T.; Im, Y.-H.; Bianchi, G.V.; Tseng, L.-M.; Liu, M.-C.; Lluch, A.; Galeota, E.; Magazzù, D.; et al. Immune Modulation of Pathologic Complete Response after Neoadjuvant HER2-Directed Therapies in the NeoSphere Trial. Ann. Oncol. Off. J. Eur. Soc. Med. Oncol. 2015, 26, 2429–2436. [Google Scholar] [CrossRef] [PubMed]
- Birrer, M.J.; Moore, K.N.; Betella, I.; Bates, R.C. Antibody-Drug Conjugate-Based Therapeutics: State of the Science. J. Natl. Cancer Inst. 2019, 111, 538–549. [Google Scholar] [CrossRef] [PubMed]
- Lewis Phillips, G.D.; Li, G.; Dugger, D.L.; Crocker, L.M.; Parsons, K.L.; Mai, E.; Blättler, W.A.; Lambert, J.M.; Chari, R.V.J.; Lutz, R.J.; et al. Targeting HER2-Positive Breast Cancer with Trastuzumab-DM1, an Antibody-Cytotoxic Drug Conjugate. Cancer Res. 2008, 68, 9280–9290. [Google Scholar] [CrossRef] [Green Version]
- Verma, S.; Miles, D.; Gianni, L.; Krop, I.E.; Welslau, M.; Baselga, J.; Pegram, M.; Oh, D.-Y.; Diéras, V.; Guardino, E.; et al. Trastuzumab Emtansine for HER2-Positive Advanced Breast Cancer. N. Engl. J. Med. 2012, 367, 1783–1791. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Diéras, V.; Miles, D.; Verma, S.; Pegram, M.; Welslau, M.; Baselga, J.; Krop, I.E.; Blackwell, K.; Hoersch, S.; Xu, J.; et al. Trastuzumab Emtansine versus Capecitabine plus Lapatinib in Patients with Previously Treated HER2-Positive Advanced Breast Cancer (EMILIA): A Descriptive Analysis of Final Overall Survival Results from a Randomised, Open-Label, Phase 3 Trial. Lancet. Oncol. 2017, 18, 732–742. [Google Scholar] [CrossRef]
- von Minckwitz, G.; Huang, C.-S.; Mano, M.S.; Loibl, S.; Mamounas, E.P.; Untch, M.; Wolmark, N.; Rastogi, P.; Schneeweiss, A.; Redondo, A.; et al. Trastuzumab Emtansine for Residual Invasive HER2-Positive Breast Cancer. N. Engl. J. Med. 2019, 380, 617–628. [Google Scholar] [CrossRef]
- Emens, L.A.; Esteva, F.J.; Beresford, M.; Saura, C.; De Laurentiis, M.; Kim, S.-B.; Im, S.-A.; Wang, Y.; Salgado, R.; Mani, A.; et al. Trastuzumab Emtansine plus Atezolizumab versus Trastuzumab Emtansine plus Placebo in Previously Treated, HER2-Positive Advanced Breast Cancer (KATE2): A Phase 2, Multicentre, Randomised, Double-Blind Trial. Lancet Oncol. 2020, 21, 1283–1295. [Google Scholar] [CrossRef]
- Hurvitz, S.A.; Martin, M.; Jung, K.H.; Huang, C.-S.; Harbeck, N.; Valero, V.; Stroyakovskiy, D.; Wildiers, H.; Campone, M.; Boileau, J.-F.; et al. Neoadjuvant Trastuzumab Emtansine and Pertuzumab in Human Epidermal Growth Factor Receptor 2-Positive Breast Cancer: Three-Year Outcomes from the Phase III KRISTINE Study. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2019, 37, 2206–2216. [Google Scholar] [CrossRef]
- Ogitani, Y.; Aida, T.; Hagihara, K.; Yamaguchi, J.; Ishii, C.; Harada, N.; Soma, M.; Okamoto, H.; Oitate, M.; Arakawa, S.; et al. DS-8201a, A Novel HER2-Targeting ADC with a Novel DNA Topoisomerase I Inhibitor, Demonstrates a Promising Antitumor Efficacy with Differentiation from T-DM1. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2016, 22, 5097–5108. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cortés, J. LBA1—Trastuzumab Deruxtecan (T-DXd) vs. Trastuzumab Emtansine (T-DM1) in Patients (Pts) with HER2+ Metastatic Breast Cancer (MBC): Results of the Randomized Phase III DESTINY-Breast03 Study. In Proceedings of the ESMO Congress, Virtual Meeting, 16–21 September 2021. [Google Scholar]
- Modi, S.; Saura, C.; Yamashita, T.; Park, Y.H.; Kim, S.B.; Tamura, K.; Andre, F.; Iwata, H.; Ito, Y.; Tsurutani, J.; et al. Trastuzumab Deruxtecan in Previously Treated HER2-Positive Breast Cancer. N. Engl. J. Med. 2020, 382, 610–621. [Google Scholar] [CrossRef] [PubMed]
- Modi, S.; Park, H.; Murthy, R.K.; Iwata, H.; Tamura, K.; Tsurutani, J.; Moreno-Aspitia, A.; Doi, T.; Sagara, Y.; Redfern, C.; et al. Antitumor Activity and Safety of Trastuzumab Deruxtecan in Patients with HER2-Low–Expressing Advanced Breast Cancer: Results from a Phase Ib Study. J. Clin. Oncol. 2020, 38, 1887. [Google Scholar] [CrossRef] [PubMed]
- Narayan, P.; Osgood, C.L.; Singh, H.; Chiu, H.-J.; Ricks, T.K.; Chiu Yuen Chow, E.; Qiu, J.; Song, P.; Yu, J.; Namuswe, F.; et al. FDA Approval Summary: Fam-Trastuzumab Deruxtecan-Nxki for the Treatment of Unresectable or Metastatic HER2-Positive Breast Cancer. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2021, 27, 4478–4485. [Google Scholar] [CrossRef] [PubMed]
- van der Lee, M.M.C.; Groothuis, P.G.; Ubink, R.; van der Vleuten, M.A.J.; van Achterberg, T.A.; Loosveld, E.M.; Damming, D.; Jacobs, D.C.H.; Rouwette, M.; Egging, D.F.; et al. The Preclinical Profile of the Duocarmycin-Based HER2-Targeting ADC SYD985 Predicts for Clinical Benefit in Low HER2-Expressing Breast Cancers. Mol. Cancer Ther. 2015, 14, 692–703. [Google Scholar] [CrossRef] [Green Version]
- Manich, C.S. LBA15—Primary Outcome of the Phase III SYD985.002/TULIP Trial Comparing [Vic-]Trastuzumab Duocarmazine to Physician’s Choice Treatment in Patients with Pre-Treated HER2-Positive Locally Advanced or Metastatic Breast Cancer. In Proceedings of the ESMO Congress, Virtual Meeting, 16–21 September 2021; Annals of Oncology (2021). Volume 32 (Suppl. S5), p. S1288. [Google Scholar]
- Banerji, U.; van Herpen, C.M.L.; Saura, C.; Thistlethwaite, F.; Lord, S.; Moreno, V.; Macpherson, I.R.; Boni, V.; Rolfo, C.; de Vries, E.G.E.; et al. Trastuzumab Duocarmazine in Locally Advanced and Metastatic Solid Tumours and HER2-Expressing Breast Cancer: A Phase 1 Dose-Escalation and Dose-Expansion Study. Lancet Oncol. 2019, 20, 1124–1135. [Google Scholar] [CrossRef] [Green Version]
- Hamilton, E.P.; Barve, M.A.; Bardia, A.; Beeram, M.; Bendell, J.C.; Mosher, R.; Hailman, E.; Bergstrom, D.A.; Burris, H.A.; Soliman, H.H. Phase 1 Dose Escalation of XMT-1522, a Novel HER2-Targeting Antibody-Drug Conjugate (ADC), in Patients (Pts) with HER2-Expressing Breast, Lung and Gastric Tumors. J. Clin. Oncol. 2018, 36, 2546. [Google Scholar] [CrossRef] [Green Version]
- Pegram, M.D.; Hamilton, E.P.; Tan, A.R.; Storniolo, A.M.; Balic, K.; Rosenbaum, A.I.; Liang, M.; He, P.; Marshall, S.; Scheuber, A.; et al. First-in-Human, Phase 1 Dose-Escalation Study of Biparatopic Anti-HER2 Antibody–Drug Conjugate MEDI4276 in Patients with HER2-Positive Advanced Breast or Gastric Cancer. Mol. Cancer Ther. 2021, 20, 1442–1453. [Google Scholar] [CrossRef]
- Hamblett, K.J.; Barnscher, S.D.; Davies, R.H.; Hammond, P.W.; Hernandez, A.; Wickman, G.R.; Fung, V.K.; Ding, T.; Garnett, G.; Galey, A.S.; et al. Abstract P6-17-13: ZW49, a HER2 Targeted Biparatopic Antibody Drug Conjugate for the Treatment of HER2 Expressing Cancers. Cancer Res. 2019, 79, P6-17-13. [Google Scholar] [CrossRef]
- Corti, C.; Giugliano, F.; Nicolò, E.; Ascione, L.; Curigliano, G. Antibody–Drug Conjugates for the Treatment of Breast Cancer. Cancers 2021, 13, 2898. [Google Scholar] [CrossRef]
- Stagg, J.; Loi, S.; Divisekera, U.; Ngiow, S.F.; Duret, H.; Yagita, H.; Teng, M.W.; Smyth, M.J. Anti-ErbB-2 MAb Therapy Requires Type I and II Interferons and Synergizes with Anti-PD-1 or Anti-CD137 MAb Therapy. Proc. Natl. Acad. Sci. USA 2011, 108, 7142–7147. [Google Scholar] [CrossRef] [Green Version]
- Loi, S.; Giobbie-Hurder, A.; Gombos, A.; Bachelot, T.; Hui, R.; Curigliano, G.; Campone, M.; Biganzoli, L.; Bonnefoi, H.; Jerusalem, G.; et al. Pembrolizumab plus Trastuzumab in Trastuzumab-Resistant, Advanced, HER2-Positive Breast Cancer (PANACEA): A Single-Arm, Multicentre, Phase 1b-2 Trial. Lancet Oncol. 2019, 20, 371–382. [Google Scholar] [CrossRef]
- Chia, S.K.L.; Bedard, P.L.; Hilton, J.; Amir, E.; Gelmon, K.A.; Goodwin, R.A.; Villa, D.; Cabanero, M.; Ritter, H.; Tu, D.; et al. A Phase I Study of a PD-L1 Antibody (Durvalumab) in Combination with Trastuzumab in HER-2 Positive Metastatic Breast Cancer (MBC) Progressing on Prior Anti HER-2 Therapies (CCTG IND.229)[NCT02649686]. J. Clin. Oncol. 2018, 36, 1029. [Google Scholar] [CrossRef]
- Dirix, L.Y.; Takacs, I.; Jerusalem, G.; Nikolinakos, P.; Arkenau, H.T.; Forero-Torres, A.; Boccia, R.; Lippman, M.E.; Somer, R.; Smakal, M.; et al. Avelumab, an Anti-PD-L1 Antibody, in Patients with Locally Advanced or Metastatic Breast Cancer: A Phase 1b JAVELIN Solid Tumor Study. Breast Cancer Res. Treat. 2018, 167, 671–686. [Google Scholar] [CrossRef] [Green Version]
- Huober, J.; Barrios, C.H.; Niikura, N.; Jarzab, M.; Chang, Y.-C.; Huggins-Puhalla, S.L.; Graupner, V.; Eiger, D.; Henschel, V.; Gochitashvili, N.; et al. VP6-2021: IMpassion050: A Phase III Study of Neoadjuvant Atezolizumab + Pertuzumab + Trastuzumab + Chemotherapy (Neoadj A + PH + CT) in High-Risk, HER2-Positive Early Breast Cancer (EBC). Ann. Oncol. 2021, 32, 1061–1062. [Google Scholar] [CrossRef]
- Ni, Y.; Tsang, J.Y.; Shao, Y.; Poon, I.K.; Tam, F.; Shea, K.-H.; Tse, G.M. Combining Analysis of Tumor-Infiltrating Lymphocytes (TIL) and PD-L1 Refined the Prognostication of Breast Cancer Subtypes. Oncologist 2022, 27, oyab063. [Google Scholar] [CrossRef] [PubMed]
- Emens, L.A.; Cruz, C.; Eder, J.P.; Braiteh, F.; Chung, C.; Tolaney, S.M.; Kuter, I.; Nanda, R.; Cassier, P.A.; Delord, J.P.; et al. Long-Term Clinical Outcomes and Biomarker Analyses of Atezolizumab Therapy for Patients with Metastatic Triple-Negative Breast Cancer: A Phase 1 Study. JAMA Oncol. 2019, 5, 74–82. [Google Scholar] [CrossRef]
- Adams, S.; Schmid, P.; Rugo, H.S.; Winer, E.P.; Loirat, D.; Awada, A.; Cescon, D.W.; Iwata, H.; Campone, M.; Nanda, R.; et al. Pembrolizumab Monotherapy for Previously Treated Metastatic Triple-Negative Breast Cancer: Cohort A of the Phase II KEYNOTE-086 Study. Ann. Oncol. 2019, 30, 397–404. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Adams, S.; Loi, S.; Toppmeyer, D.; Cescon, D.W.; De Laurentiis, M.; Nanda, R.; Winer, E.P.; Mukai, H.; Tamura, K.; Armstrong, A.; et al. Pembrolizumab Monotherapy for Previously Untreated, PD-L1-Positive, Metastatic Triple-Negative Breast Cancer: Cohort B of the Phase II KEYNOTE-086 Study. Ann. Oncol. 2019, 30, 405–411. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ogiya, R.; Niikura, N.; Kumaki, N.; Bianchini, G.; Kitano, S.; Iwamoto, T.; Hayashi, N.; Yokoyama, K.; Oshitanai, R.; Terao, M.; et al. Comparison of Tumor-Infiltrating Lymphocytes between Primary and Metastatic Tumors in Breast Cancer Patients. Cancer Sci. 2016, 107, 1730–1735. [Google Scholar] [CrossRef] [PubMed]
- Luen, S.J.; Salgado, R.; Fox, S.; Savas, P.; Eng-Wong, J.; Clark, E.; Kiermaier, A.; Swain, S.M.; Baselga, J.; Michiels, S.; et al. Tumour-Infiltrating Lymphocytes in Advanced HER2-Positive Breast Cancer Treated with Pertuzumab or Placebo in Addition to Trastuzumab and Docetaxel: A Retrospective Analysis of the CLEOPATRA Study. Lancet. Oncol. 2017, 18, 52–62. [Google Scholar] [CrossRef] [Green Version]
- Dieci, M.V.; Tsvetkova, V.; Orvieto, E.; Piacentini, F.; Ficarra, G.; Griguolo, G.; Miglietta, F.; Giarratano, T.; Omarini, C.; Bonaguro, S.; et al. Immune Characterization of Breast Cancer Metastases: Prognostic Implications. Breast Cancer Res. 2018, 20, 62. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Szekely, B.; Bossuyt, V.; Li, X.; Wali, V.B.; Patwardhan, G.A.; Frederick, C.; Silber, A.; Park, T.; Harigopal, M.; Pelekanou, V.; et al. Immunological Differences between Primary and Metastatic Breast Cancer. Ann. Oncol. Off. J. Eur. Soc. Med. Oncol. 2018, 29, 2232–2239. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Blake, S.J.; Yong, M.C.R.; Harjunpää, H.; Ngiow, S.F.; Takeda, K.; Young, A.; O’Donnell, J.S.; Allen, S.; Smyth, M.J.; et al. Improved Efficacy of Neoadjuvant Compared to Adjuvant Immunotherapy to Eradicate Metastatic Disease. Cancer Discov. 2016, 6, 1382–1399. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kroemer, G.; Galluzzi, L.; Kepp, O.; Zitvogel, L. Immunogenic Cell Death in Cancer Therapy. Annu. Rev. Immunol. 2013, 31, 51–72. [Google Scholar] [CrossRef] [PubMed]
- Priceman, S.J.; Tilakawardane, D.; Jeang, B.; Aguilar, B.; Murad, J.P.; Park, A.K.; Chang, W.-C.; Ostberg, J.R.; Neman, J.; Jandial, R.; et al. Regional Delivery of Chimeric Antigen Receptor-Engineered T Cells Effectively Targets HER2(+) Breast Cancer Metastasis to the Brain. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2018, 24, 95–105. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Globerson-Levin, A.; Waks, T.; Eshhar, Z. Elimination of Progressive Mammary Cancer by Repeated Administrations of Chimeric Antigen Receptor-Modified T Cells. Mol. Ther. 2014, 22, 1029–1038. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, X.-L.; Wang, X.-L.; Ma, B.; Jia, J.; Yan, Y.; Di, L.-J.; Yuan, Y.-H.; Wan, F.-L.; Lu, Y.-L.; Liang, X.; et al. HER2-Specific T Lymphocytes Kill Both Trastuzumab-Resistant and Trastuzumab-Sensitive Breast Cell Lines in Vitro. Chin. J. Cancer Res. 2012, 24, 143–150. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Szöőr, Á.; Tóth, G.; Zsebik, B.; Szabó, V.; Eshhar, Z.; Abken, H.; Vereb, G. Trastuzumab Derived HER2-Specific CARs for the Treatment of Trastuzumab-Resistant Breast Cancer: CAR T Cells Penetrate and Eradicate Tumors That Are Not Accessible to Antibodies. Cancer Lett. 2020, 484, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.-F.; Huang, Y.; Liang, X.; Li, D.; Jiang, L.; Yang, X.; Zhu, M.; Gou, H.-F.; Gong, Y.-L.; Wei, Y.-Q.; et al. Enhancement of the Antitumor Effect of HER2-Directed CAR-T Cells through Blocking Epithelial-Mesenchymal Transition in Tumor Cells. FASEB J. 2020, 34, 11185–11199. [Google Scholar] [CrossRef]
- Li, H.; Yuan, W.; Bin, S.; Wu, G.; Li, P.; Liu, M.; Yang, J.; Li, X.; Yang, K.; Gu, H. Overcome Trastuzumab Resistance of Breast Cancer Using Anti-HER2 Chimeric Antigen Receptor T Cells and PD1 Blockade. Am. J. Cancer Res. 2020, 10, 688–703. [Google Scholar]
- Heczey, A.; Louis, C.U.; Savoldo, B.; Dakhova, O.; Durett, A.; Grilley, B.; Liu, H.; Wu, M.F.; Mei, Z.; Gee, A.; et al. CAR T Cells Administered in Combination with Lymphodepletion and PD-1 Inhibition to Patients with Neuroblastoma. Mol. Ther. 2017, 25, 2214–2224. [Google Scholar] [CrossRef] [Green Version]
- Morgan, R.A.; Johnson, L.A.; Davis, J.L.; Zheng, Z.; Woolard, K.D.; Reap, E.A.; Feldman, S.A.; Chinnasamy, N.; Kuan, C.-T.; Song, H.; et al. Recognition of Glioma Stem Cells by Genetically Modified T Cells Targeting EGFRvIII and Development of Adoptive Cell Therapy for Glioma. Hum. Gene Ther. 2012, 23, 1043–1053. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ahmed, N.; Brawley, V.S.; Hegde, M.; Robertson, C.; Ghazi, A.; Gerken, C.; Liu, E.; Dakhova, O.; Ashoori, A.; Corder, A.; et al. Human Epidermal Growth Factor Receptor 2 (HER2) -Specific Chimeric Antigen Receptor-Modified T Cells for the Immunotherapy of HER2-Positive Sarcoma. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2015, 33, 1688–1696. [Google Scholar] [CrossRef] [PubMed]
- Razavi, A.; Keshavarz-Fathi, M.; Pawelek, J.; Rezaei, N. Chimeric Antigen Receptor T-Cell Therapy for Melanoma. Expert Rev. Clin. Immunol. 2021, 17, 209–223. [Google Scholar] [CrossRef] [PubMed]
- Castellarin, M.; Sands, C.; Da, T.; Scholler, J.; Graham, K.; Buza, E.; Fraietta, J.A.; Zhao, Y.; June, C.H. A Rational Mouse Model to Detect On-Target, off-Tumor CAR T Cell Toxicity. JCI Insight 2020, 5, e136012. [Google Scholar] [CrossRef]
- Dai, H.; Wang, Y.; Lu, X.; Han, W. Chimeric Antigen Receptors Modified T-Cells for Cancer Therapy. J. Natl. Cancer Inst. 2016, 108, djv439. [Google Scholar] [CrossRef] [Green Version]
- Liu, X.; Jiang, S.; Fang, C.; Yang, S.; Olalere, D.; Pequignot, E.C.; Cogdill, A.P.; Li, N.; Ramones, M.; Granda, B.; et al. Affinity-Tuned ErbB2 or EGFR Chimeric Antigen Receptor T Cells Exhibit an Increased Therapeutic Index against Tumors in Mice. Cancer Res. 2015, 75, 3596–3607. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Corti, C.; Giachetti, P.P.M.B.; Eggermont, A.M.M.; Delaloge, S.; Curigliano, G. Therapeutic Vaccines for Breast Cancer: Has the Time Finally Come? Eur. J. Cancer 2022, 160, 150–174. [Google Scholar] [CrossRef] [PubMed]
- Brown, T.A., 2nd; Mittendorf, E.A.; Hale, D.F.; Myers, J.W., 3rd; Peace, K.M.; Jackson, D.O.; Greene, J.M.; Vreeland, T.J.; Clifton, G.T.; Ardavanis, A.; et al. Prospective, Randomized, Single-Blinded, Multi-Center Phase II Trial of Two HER2 Peptide Vaccines, GP2 and AE37, in Breast Cancer Patients to Prevent Recurrence. Breast Cancer Res. Treat. 2020, 181, 391–401. [Google Scholar] [CrossRef] [PubMed]
- Patel, S.; McWilliams, D.; Fischette, C.T.; Thompson, J.; Patel, M.; Daugherty, F.J. Final Five-Year Median Follow-up Safety Data from a Prospective, Randomized, Placebo-Controlled, Single-Blinded, Multicenter, Phase IIb Study Evaluating the Use of HER2/Neu Peptide GP2 + GM-CSF vs. GM-CSF Alone after Adjuvant Trastuzumab in HER2-Positive. J. Clin. Oncol. 2021, 39, 542. [Google Scholar] [CrossRef]
- Clifton, G.T.; Hale, D.; Vreeland, T.J.; Hickerson, A.T.; Litton, J.K.; Alatrash, G.; Murthy, R.K.; Qiao, N.; Philips, A.V.; Lukas, J.J.; et al. Results of a Randomized Phase IIb Trial of Nelipepimut-S + Trastuzumab versus Trastuzumab to Prevent Recurrences in Patients with High-Risk HER2 Low-Expressing Breast Cancer. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2020, 26, 2515–2523. [Google Scholar] [CrossRef] [Green Version]
- Eiger, D.; Agostinetto, E.; Saúde-Conde, R.; de Azambuja, E. The Exciting New Field of HER2-Low Breast Cancer Treatment. Cancers 2021, 13, 1015. [Google Scholar] [CrossRef] [PubMed]
- Benavides, L.C.; Gates, J.D.; Carmichael, M.G.; Patil, R.; Holmes, J.P.; Hueman, M.T.; Mittendorf, E.A.; Craig, D.; Stojadinovic, A.; Ponniah, S.; et al. The Impact of HER2/Neu Expression Level on Response to the E75 Vaccine: From U.S. Military Cancer Institute Clinical Trials Group Study I-01 and I-02. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2009, 15, 2895–2904. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gall, V.A.; Philips, A.V.; Qiao, N.; Clise-Dwyer, K.; Perakis, A.A.; Zhang, M.; Clifton, G.T.; Sukhumalchandra, P.; Ma, Q.; Reddy, S.M.; et al. Trastuzumab Increases HER2 Uptake and Cross-Presentation by Dendritic Cells. Cancer Res. 2017, 77, 5374–5383. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hickerson, A.; Clifton, G.T.; Hale, D.F.; Peace, K.M.; Holmes, J.P.; Vreeland, T.J.; Litton, J.K.; Murthy, R.K.; Lukas, J.J.; Mittendorf, E.A.; et al. Final Analysis of Nelipepimut-S plus GM-CSF with Trastuzumab versus Trastuzumab Alone to Prevent Recurrences in High-Risk, HER2 Low-Expressing Breast Cancer: A Prospective, Randomized, Blinded, Multicenter Phase IIb Trial. J. Clin. Oncol. 2019, 37, 1. [Google Scholar] [CrossRef]
- Mittendorf, E.A.; Lu, B.; Melisko, M.; Price Hiller, J.; Bondarenko, I.; Brunt, A.M.; Sergii, G.; Petrakova, K.; Peoples, G.E. Efficacy and Safety Analysis of Nelipepimut-S Vaccine to Prevent Breast Cancer Recurrence: A Randomized, Multicenter, Phase III Clinical Trial. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2019, 25, 4248–4254. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Patel, S.S.; McWilliams, D.B.; Patel, M.S.; Fischette, C.T.; Thompson, J.; Daugherty, F.J. Abstract PS10-23: Five Year Median Follow-up Data from a Prospective, Randomized, Placebo-Controlled, Single-Blinded, Multicenter, Phase IIb Study Evaluating the Reduction of Recurrences Using HER2/Neu Peptide GP2 + GM-CSF vs. GM-CSF Alone after Adjuvant. Cancer Res. 2021, 81, PS10–PS23. [Google Scholar] [CrossRef]
- McCarthy, P.M.; Clifton, G.T.; Vreeland, T.J.; Adams, A.M.; O’Shea, A.E.; Peoples, G.E. AE37: A HER2-Targeted Vaccine for the Prevention of Breast Cancer Recurrence. Expert Opin. Investig. Drugs 2021, 30, 5–11. [Google Scholar] [CrossRef]
- Arab, A.; Yazdian-Robati, R.; Behravan, J. HER2-Positive Breast Cancer Immunotherapy: A Focus on Vaccine Development. Arch. Immunol. Ther. Exp. 2020, 68, 2. [Google Scholar] [CrossRef] [PubMed]
- Al-Awadhi, A.; Lee Murray, J.; Ibrahim, N.K. Developing Anti-HER2 Vaccines: Breast Cancer Experience. Int. J. Cancer 2018, 143, 2126–2132. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, J.W.; Melisko, M.E.; Esserman, L.J.; Jones, L.A.; Wollan, J.B.; Sims, R. Treatment with Autologous Antigen-Presenting Cells Activated with the HER-2 Based Antigen Lapuleucel-T: Results of a Phase I Study in Immunologic and Clinical Activity in HER-2 Overexpressing Breast Cancer. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2007, 25, 3680–3687. [Google Scholar] [CrossRef] [PubMed]
- Emens, L.A.; Asquith, J.M.; Leatherman, J.M.; Kobrin, B.J.; Petrik, S.; Laiko, M.; Levi, J.; Daphtary, M.M.; Biedrzycki, B.; Wolff, A.C.; et al. Timed Sequential Treatment with Cyclophosphamide, Doxorubicin, and an Allogeneic Granulocyte-Macrophage Colony-Stimulating Factor-Secreting Breast Tumor Vaccine: A Chemotherapy Dose-Ranging Factorial Study of Safety and Immune Activation. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2009, 27, 5911–5918. [Google Scholar] [CrossRef] [Green Version]
- Li, L.; Saade, F.; Petrovsky, N. The Future of Human DNA Vaccines. J. Biotechnol. 2012, 162, 171–182. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Norell, H.; Poschke, I.; Charo, J.; Wei, W.Z.; Erskine, C.; Piechocki, M.P.; Knutson, K.L.; Bergh, J.; Lidbrink, E.; Kiessling, R. Vaccination with a Plasmid DNA Encoding HER-2/Neu Together with Low Doses of GM-CSF and IL-2 in Patients with Metastatic Breast Carcinoma: A Pilot Clinical Trial. J. Transl. Med. 2010, 8, 53. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Disis, M.L.; Coveler, A.L.; Higgins, D.; Fintak, P.; Waisman, J.R.; Reichow, J.; Slota, M.; Childs, J.; Dang, Y.; Salazar, L.G. A Phase I Trial of the Safety and Immunogenicity of a DNA-Based Vaccine Encoding the HER2/Neu (HER2) Intracellular Domain in Subjects with HER2+ Breast Cancer. J. Clin. Oncol. 2014, 32, 616. [Google Scholar] [CrossRef]
- Diaz, C.M.; Chiappori, A.; Aurisicchio, L.; Bagchi, A.; Clark, J.; Dubey, S.; Fridman, A.; Fabregas, J.C.; Marshall, J.; Scarselli, E.; et al. Phase 1 Studies of the Safety and Immunogenicity of Electroporated HER2/CEA DNA Vaccine Followed by Adenoviral Boost Immunization in Patients with Solid Tumors. J. Transl. Med. 2013, 11, 62. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Larocca, C.; Schlom, J. Viral Vector-Based Therapeutic Cancer Vaccines. Cancer J. 2011, 17, 359–371. [Google Scholar] [CrossRef] [PubMed]
- Miles, D.; Roché, H.; Martin, M.; Perren, T.J.; Cameron, D.A.; Glaspy, J.; Dodwell, D.; Parker, J.; Mayordomo, J.; Tres, A.; et al. Phase III Multicenter Clinical Trial of the Sialyl-TN (STn)-Keyhole Limpet Hemocyanin (KLH) Vaccine for Metastatic Breast Cancer. Oncologist 2011, 16, 1092–1100. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dafni, U.; Martín-Lluesma, S.; Balint, K.; Tsourti, Z.; Vervita, K.; Chenal, J.; Coukos, G.; Zaman, K.; Sarivalasis, A.; Kandalaft, L.E. Efficacy of Cancer Vaccines in Selected Gynaecological Breast and Ovarian Cancers: A 20-Year Systematic Review and Meta-Analysis. Eur. J. Cancer 2021, 142, 63–82. [Google Scholar] [CrossRef] [PubMed]
- Antonarelli, G.; Corti, C.; Tarantino, P.; Ascione, L.; Cortes, J.; Romero, P.; Mittendorf, E.A.; Disis, M.L.; Curigliano, G. Therapeutic Cancer Vaccines Revamping: Technology Advancements and Pitfalls. Ann. Oncol. Off. J. Eur. Soc. Med. Oncol. 2021, 32, 1537–1551. [Google Scholar] [CrossRef] [PubMed]
- Müller, P.; Kreuzaler, M.; Khan, T.; Thommen, D.S.; Martin, K.; Glatz, K.; Savic, S.; Harbeck, N.; Nitz, U.; Gluz, O.; et al. Trastuzumab Emtansine (T-DM1) Renders HER2+ Breast Cancer Highly Susceptible to CTLA-4/PD-1 Blockade. Sci. Transl. Med. 2015, 7, 315ra188. [Google Scholar] [CrossRef] [PubMed]
- Nakasone, E.S.; Hurvitz, S.A.; McCann, K.E. Harnessing the Immune System in the Battle against Breast Cancer. Drugs Context 2018, 7, 212520. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gatti-Mays, M.E.; Balko, J.M.; Gameiro, S.R.; Bear, H.D.; Prabhakaran, S.; Fukui, J.; Disis, M.L.; Nanda, R.; Gulley, J.L.; Kalinsky, K.; et al. If We Build It They Will Come: Targeting the Immune Response to Breast Cancer. Jpn. Breast Cancer 2019, 5, 37. [Google Scholar] [CrossRef] [Green Version]
- Conforti, F.; Pala, L.; Sala, I.; Oriecuia, C.; De Pas, T.; Specchia, C.; Graffeo, R.; Pagan, E.; Queirolo, P.; Pennacchioli, E.; et al. Evaluation of Pathological Complete Response as Surrogate Endpoint in Neoadjuvant Randomised Clinical Trials of Early Stage Breast Cancer: Systematic Review and Meta-Analysis. BMJ 2021, 375, e066381. [Google Scholar] [CrossRef] [PubMed]
- Loibl, S.; Schneeweiss, A.; Huober, J.B.; Braun, M.; Rey, J.; Blohmer, J.U.; Furlanetto, J.; Zahm, D.M.; Hanusch, C.; Thomalla, J.; et al. Durvalumab Improves Long-Term Outcome in TNBC: Results from the Phase II Randomized GeparNUEVO Study Investigating Neodjuvant Durvalumab in Addition to an Anthracycline/Taxane Based Neoadjuvant Chemotherapy in Early Triple-Negative Breast Cancer (TNBC). J. Clin. Oncol. 2021, 39, 506. [Google Scholar] [CrossRef]
- Schmid, P.; Cortes, J.; Dent, R.; Pusztai, L.; McArthur, H.; Kümmel, S.; Bergh, J.; Denkert, C.; Park, Y.H.; Hui, R.; et al. Event-Free Survival with Pembrolizumab in Early Triple-Negative Breast Cancer. N. Engl. J. Med. 2022, 386, 556–567. [Google Scholar] [CrossRef] [PubMed]
- Dieras, V.; Deluche, E.; Lusque, A. Trastuzumab Deruxtecan for Advanced Breast Cancer Patients, Regardless of HER2 Status: A Phase II Study with Biomarkers Analysis (DAISY). In Proceedings of the San Antonio Breast Cancer Symposium, San Antonio, TX, USA, 7–10 December 2021; p. Abstract PD8-02. [Google Scholar]
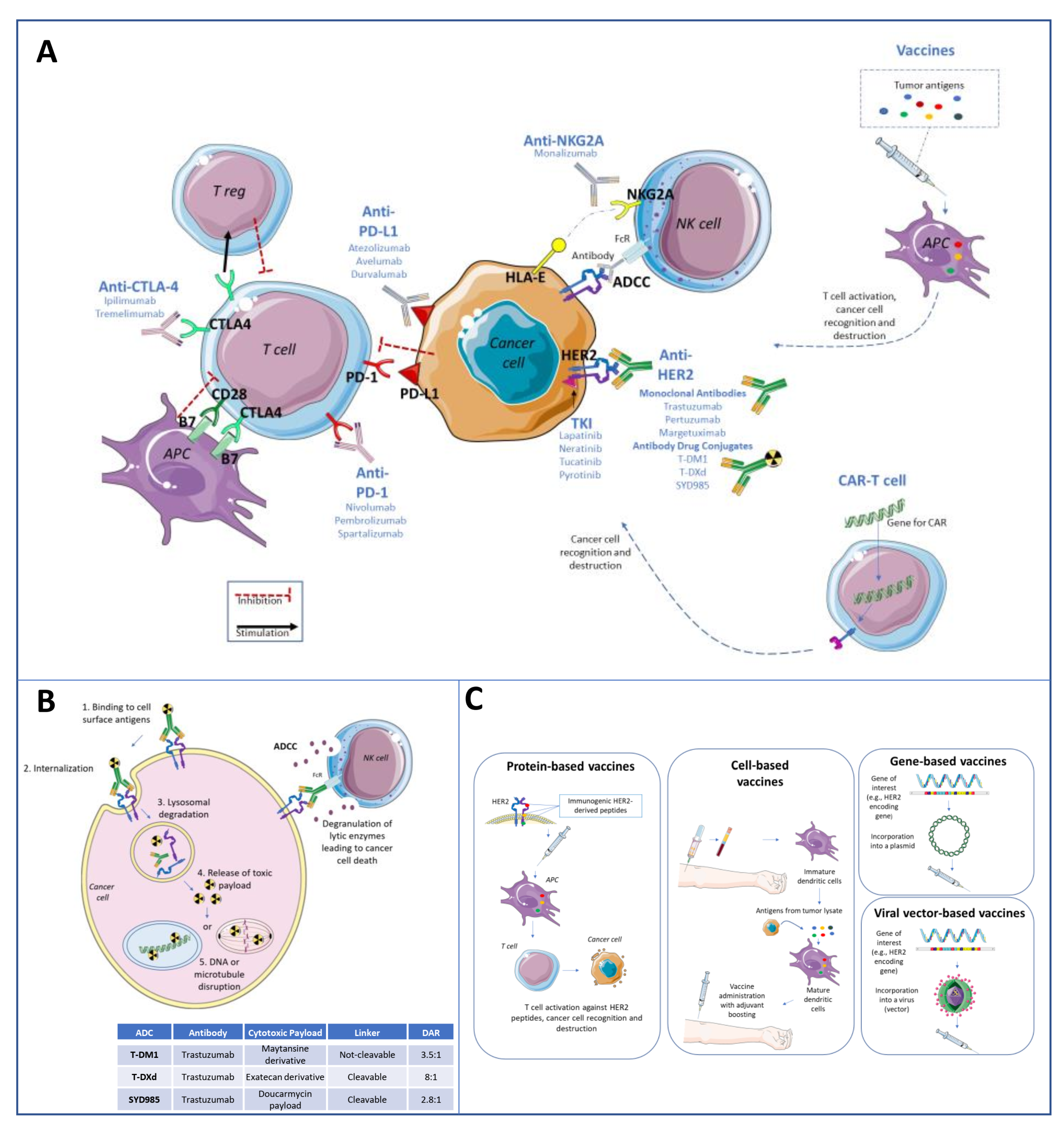
| Study | Phase | Population | Treatment | Main Results |
|---|---|---|---|---|
| Advanced Setting | ||||
| PANACEA [46] | I/II, single arm | HER2+ ABC, progressed to trastuzumab (n = 52, PD-L1+ n = 40) | Pembrolizumab + trastuzumab | ORR: 15% of PD-L1+ pts No ORs among PD-L1− pts mPFS: 2.7 mos (90% CI 2.6–4.0) in PD-L1+ mPFS: 2.5 mos (90% CI 1.4–2.7) in PD-L1− |
| KATE-2 [31] | II, randomized | HER2+ ABC, previously treated with trastuzumab and a taxane (n = 202, PD-L1+ n = 84) | Atezolizumab + T-DM1 vs. Placebo + T-DM1 | ORR: 54% vs. 33% in PD-L1+ pts; ORR: 39% vs. 50% in PD-L1− pts mPFS: 8.2 vs. 6.8 mos in ITT (HR 0.82, 95% CI 0.55–1.23) mPFS: 8.5 vs. 4.1 mos in PD-L1+ (HR 0.60, 95% CI 0.32–1.1) |
| NCT02649686 [47] | I | HER-2 positive ABC, previously treated with trastuzumab and taxanes (n = 15, PD-L1+ n = 0) | Durvalumab + trastuzumab | ORR: 0/15 |
| JAVELIN Solid Tumors [48] | Ib | ABC refractory to or progressing after standard-of-care therapy (n = 26) | Avelumab | ORR: 0/26 |
| Early setting | ||||
| IMpassion050 [49] | III | Stage II-III HER2+ EBC (n = 454, PD-L1+ n = 218) | (ddAC → paclitaxel + pertuzumab + trastuzumab) +/− atezolizumab → surgery → (trastuzumab + pertuzumab) +/− atezolizumab | pCR: 62.4% in atezolizumab arm vs. 62.7% in placebo arm (p = 0.9551) in ITT pCR: 64.2% in atezolizumab arm vs. 72.5% in placebo arm (p = 0.1846) in PD-L1+ pts |
| Study (NCT ID) | Phase | Setting | Population | Experimental Treatment | Status |
|---|---|---|---|---|---|
| Pembrolizumab | |||||
| NCT04512261 TOPAZ | I/II | Advanced | Patients with brain metastases | Pembrolizumab + tucatinib + trastuzumab | Suspended (enrollment temporarily on hold pending amendment to the protocol) |
| NCT04789096 TUGETHER | II | Advanced | Any line, but prior treatment with pembrolizumab and T-DM1 (in any setting) is required | Pembrolizumab + tucatinib + trastuzumab (+/− capecitabine) | Not yet recruiting |
| NCT03032107 | I | Advanced | At least 1 prior line for advanced disease, or PD during or within 6 months from the adjuvant treatment | Pembrolizumab + T-DM1 | Active, not recruiting |
| NCT03841110 | I | Advanced | Not available standard treatments | FT500 + immune checkpoint inhibitors (including pembrolizumab) +/− IL2 | Recruiting |
| NCT03632941 | II | Advanced | HER2-positive BC, prior pertuzumab + trastuzumab is required | VRP-HER2 vaccination + pembrolizumab | Recruiting |
| NCT04348747 | II | Advanced | Patients with asymptomatic brain metastases | Anti-HER2/HER3 dendritic cell vaccine + pembrolizumab | Not yet recruiting |
| NCT04042701 | I | Advanced | Patients with advanced BC (HER2-positive or HER2-low) or HER2 expressing/mutant NSCLC | Pembrolizumab + trastuzumab deruxtecan | Recruiting |
| NCT01042379 I-SPY | II | Neoadjuvant | T > 2.5 cm, no prior treatment | Personalized Adaptive novel agents including pembrolizumab | Recruiting |
| NCT03747120 | II | Neoadjuvant | T > 2 cm and/or N+, no prior treatment | Pembrolizumab + trastuzumab + pertuzumab + weekly paclitaxel | Recruiting |
| NCT03988036 Keyriched-1 | II | Neoadjuvant | T1c, N0-N2; T2, N0-N2; T3, N0-N2 with molecular HER2-enriched intrinsic subtype tested by PAM50 | Pembrolizumab + trastuzumab + pertuzumab | Recruiting |
| Nivolumab | |||||
| NCT03841110 | I | Advanced | Not available standard treatments | FT500 + immune checkpoint inhibitors (including nivolumab) +/− IL2 | Recruiting |
| NCT03523572 | I | Advanced | Advanced BC (HER2-positive and HER2-low) and urothelial Cancer | Nivolumab + trastuzumab deruxtecan | Active, not recruiting |
| Atezolizumab | |||||
| NCT03125928 | II | Advanced | 1st line | Atezolizumab + paclitaxel + trastuzumab + pertuzumab | Recruiting |
| NCT03650348 | I | Advanced | Any line | PRS-343 + atezolizumab | Active, not recruiting |
| NCT03417544 | II | Advanced | Patients with brain metastases | atezolizumab + pertuzumab + trastuzumab | Active, not recruiting |
| NCT04759248 ATREZZO | II | Advanced | Any line, prior pertuzumab/trastuzumab and T-DM1 is required | Atezolizumab + trastuzumab + vinorelbine | Recruiting |
| NCT03841110 | I | Advanced | Not available standard treatments | FT500 + immune checkpoint inhibitors (including atezolizumab) +/− IL2 | Recruiting |
| NCT03199885 NRG BR004 | III | Advanced | 1st line | Atezolizumab + taxane + trastuzumab + pertuzumab | Recruiting |
| NCT04740918 KATE3 | III | Advanced | Prior Trastuzumab- (+/− Pertuzumab) and Taxane-Based Therapy is required | Atezolizumab + T-DM1 | Recruiting |
| NCT03726879 IMpassion050 | III | Neoadjuvant | T2-T4, N1-N3, M0 | Atezolizumab + doxorubicin + cyclophosphamide → paclitaxel + trastuzumab + pertuzumab | Active, not recruiting |
| NCT03881878 | I/II | (Neo)adjuvant | T2-3N0-3 or T1cN1 | Atezolizumab + docetaxel + trastuzumab + pertuzumab → surgery → adjuvant atezolizumab + trastuzumab + pertuzumab (+ doxorubicine and cyclophosphamide for non-pCR patients) | Not yet recruiting |
| NCT03595592 APTneo | III | (Neo)adjuvant | T1cN1, T2N1, T3N0, or locally advanced and inflammatory breast cancers | Atezolizumab + trastuzumab + pertuzumab + carboplatin + paclitaxel → surgery → adjuvant atezolizumab + trastuzumab + pertuzumab | Recruiting |
| NCT04873362 Astefania | III | Adjuvant | cT4/anyN/M0, any cT/N2-3/M0 or cT1-3/N0-1/M0 at presentation, treated with neoadjuvant therapy and surgery | Atezolizumab + T-DM1 | Recruiting |
| Avelumab | |||||
| NCT03414658 AVIATOR | II | Advanced | Prior T-DM1 (in any setting) and prior pertuzumab and trastuzumab are required | Avelumab + trastuzumab +/− vinorelbine +/− utomilumab | Recruiting |
| Durvalumab | |||||
| NCT04538742 DB-07 | I/II | Advanced | ≥ 2nd line (phase I); ≥ 1st line (phase II) | Trastuzumab deruxtecan + durvalumab + paclitaxel | Recruiting |
| NCT01042379 I-SPY | II | Neoadjuvant | T > 2.5 cm, no prior treatment | Personalized adaptive novel agents including durvalumab | Recruiting |
| Spartalizumab | |||||
| NCT04802876 ACROPOLI | II | Advanced | PD1-high mRNA expressing solid tumors | Spartalizumab | Recruiting |
| Other immunotherapies | |||||
| HER2-CAR-T | |||||
| NCT01219907 | I | Advanced | Any line | Ex vivo-expanded HER2-specific T cells and cyclophosphamide after vaccine therapy | Withdrawn |
| NCT03696030 | I | Advanced | Patients with brain or leptomeningeal metastases | Chimeric antigen receptor (CAR) T-cell therapy | Recruiting |
| NCT04660929 | I | Advanced | No available standard treatment options | CAR-macrophages (CT-0508) | Recruiting |
| NCT03319459 | I | Advanced | Solid tumors including HER2-positive breast cancer | FATE-NK100 + trastuzumab | Completed |
| NCT02843126 | I/II | Advanced | No available standard treatment options | Trastuzumab + NK immunotherapy | Completed |
| NCT04650451 | I | Advanced | Subjects with HER2-positive solid tumors | HER2-targeted dual-switch CAR-T cells (BPX-603) | Recruiting |
| NCT04511871 | I | Advanced | Patients with relapsed or refractory HER2 positive solid tumors | Autologous T cell modified chimeric antigen receptor (CAR) (CCT303-406) | Recruiting |
| NCT02491697 | II | Advanced | Stage IV BC (any subtype) | DC-CIK immunotherapy + capecitabine | Active, not recruiting |
| Vaccines | |||||
| NCT00194714 | I/II | Advanced | Stable disease on trastuzumab monotherapy | HER-2/neu peptide vaccine + trastuzumab | Active, not recruiting |
| NCT00436254 | I | Advanced | Stage III–IV HER2-positive breast cancer with metastasis in remission | DNA plasmid-based vaccine encoding the HER-2/Neu Intracellular domain + GM-CSF | Active, not recruiting |
| NCT02297698 | II | Adjuvant | HER2-positive BC at high risk of relapse | NeuVax vaccine (nelipepimut-S/GM-CSF) + trastuzumab | Active, not recruiting |
| NCT00393783 | I | Advanced | Stage III–IV HER2- positive BC | Xenogeneic HER2/Neu DNA immunization | Active, not recruiting |
| NCT04521764 | I | Advanced | Stage IV BC (any subtype) | Oncolytic measles virus Encoding Helicobacter pylori neutrophil-activating protein (MV-s-NAP) vaccine | Recruiting |
| NCT01376505 | I | Advanced | Solid tumors including BC | MVF-HER-2 Vaccine | Recruiting |
| NCT03328026 | I/II | Advanced | Stage IV BC (any subtype) | SV-BR-1-GM in combination With INCMGA00012 and epacadostat | Recruiting |
| NCT04246671 | I/II | Advanced | Stage IV HER2-positive BC | Intravenous TAEK-VAC-HerBy vaccine | Recruiting |
| NCT03630809 | II | Early and advanced | Patients with metastatic and early HER2-positive breast cancer | HER2-pulsed DC1 vaccine | Suspended for protocol revision |
| NCT03387553 | I | Neoadjuvant | Patients candidate to receive neoadjuvant therapy | HER2 directed dendritic cell vaccine + neoadjuvant standard therapy | Active, not recruiting |
| NCT04329065 | II | Neoadjuvant | Stage I–III ER-negative/HER2- positive BC | WOKVAC vaccination (pUMVC3-IGFBP2-HER2-IGF1R plasmid DNA vaccine) + pertuzumab + trastuzumab + paclitaxel | Recruiting |
| NCT04197687 | II | Post-neoadjuvant | Stage II/III in patients with residual disease after chemotherapy and surgery | T-DM1 + TPIV100 (multi-epitope HER2 peptide vaccine) + GM-CSF | Recruiting |
| NCT02061423 | I | Post-neoadjuvant | Stage I–III HER2-positive BC with residual disease post-neoadjuvant CT | HER-2 pulsed dendritic cell vaccine | Active, not recruiting |
| NCT03384914 | II | Adjuvant | Residual invasive disease after neoadjuvant therapy | DC1 vaccine vs. WOKVAC vaccine | Recruiting |
| Others | |||||
| NCT00684983 | II | Advanced | Prior treatment with trastuzumab and anthracycline or taxane is required | Capecitabine + lapatinib ditosylate +/− cixutumumab | Completed |
| NCT04120246 | I | Advanced | Any line | Alpha-tocopheryloxyacetic acid (TEA) + trastuzumab | Recruiting |
| NCT04307329 MIMOSA | II | Advanced | At least one and maximum 3 prior lines of palliative chemotherapy | Monalizumab + trastuzumab | Recruiting |
| NCT03571633 BREASTIMMU02 | II | Neoadjuvant | T > 20 mm, cN0 or cN1, M0, previously treated with 4 cycles of standard adriamycine/cyclophosphamide | Pegfilgrastim + trastuzumab + paclitaxel → surgery → adjuvant trastuzumab (+/− endocrine therapy) | Recruiting |
| NCT03620201 | I | Neoadjuvant | Stage II–III | M7824 (Bintrafusp Alfa) + neoadjuvant standard therapy | Recruiting |
| NCT01042379 I-SPY | II | Neoadjuvant | T > 2.5 cm, no prior treatment | Personalized adaptive novel agents including immunotherapy | Recruiting |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Agostinetto, E.; Montemurro, F.; Puglisi, F.; Criscitiello, C.; Bianchini, G.; Del Mastro, L.; Introna, M.; Tondini, C.; Santoro, A.; Zambelli, A. Immunotherapy for HER2-Positive Breast Cancer: Clinical Evidence and Future Perspectives. Cancers 2022, 14, 2136. https://doi.org/10.3390/cancers14092136
Agostinetto E, Montemurro F, Puglisi F, Criscitiello C, Bianchini G, Del Mastro L, Introna M, Tondini C, Santoro A, Zambelli A. Immunotherapy for HER2-Positive Breast Cancer: Clinical Evidence and Future Perspectives. Cancers. 2022; 14(9):2136. https://doi.org/10.3390/cancers14092136
Chicago/Turabian StyleAgostinetto, Elisa, Filippo Montemurro, Fabio Puglisi, Carmen Criscitiello, Giampaolo Bianchini, Lucia Del Mastro, Martino Introna, Carlo Tondini, Armando Santoro, and Alberto Zambelli. 2022. "Immunotherapy for HER2-Positive Breast Cancer: Clinical Evidence and Future Perspectives" Cancers 14, no. 9: 2136. https://doi.org/10.3390/cancers14092136
APA StyleAgostinetto, E., Montemurro, F., Puglisi, F., Criscitiello, C., Bianchini, G., Del Mastro, L., Introna, M., Tondini, C., Santoro, A., & Zambelli, A. (2022). Immunotherapy for HER2-Positive Breast Cancer: Clinical Evidence and Future Perspectives. Cancers, 14(9), 2136. https://doi.org/10.3390/cancers14092136










