The Bateman-Type Soft Tissue Reconstruction around Proximal or Total Humeral Megaprostheses in Patients with Primary Malignant Bone Tumors—Functional Outcome and Endoprosthetic Complications
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Data Collection
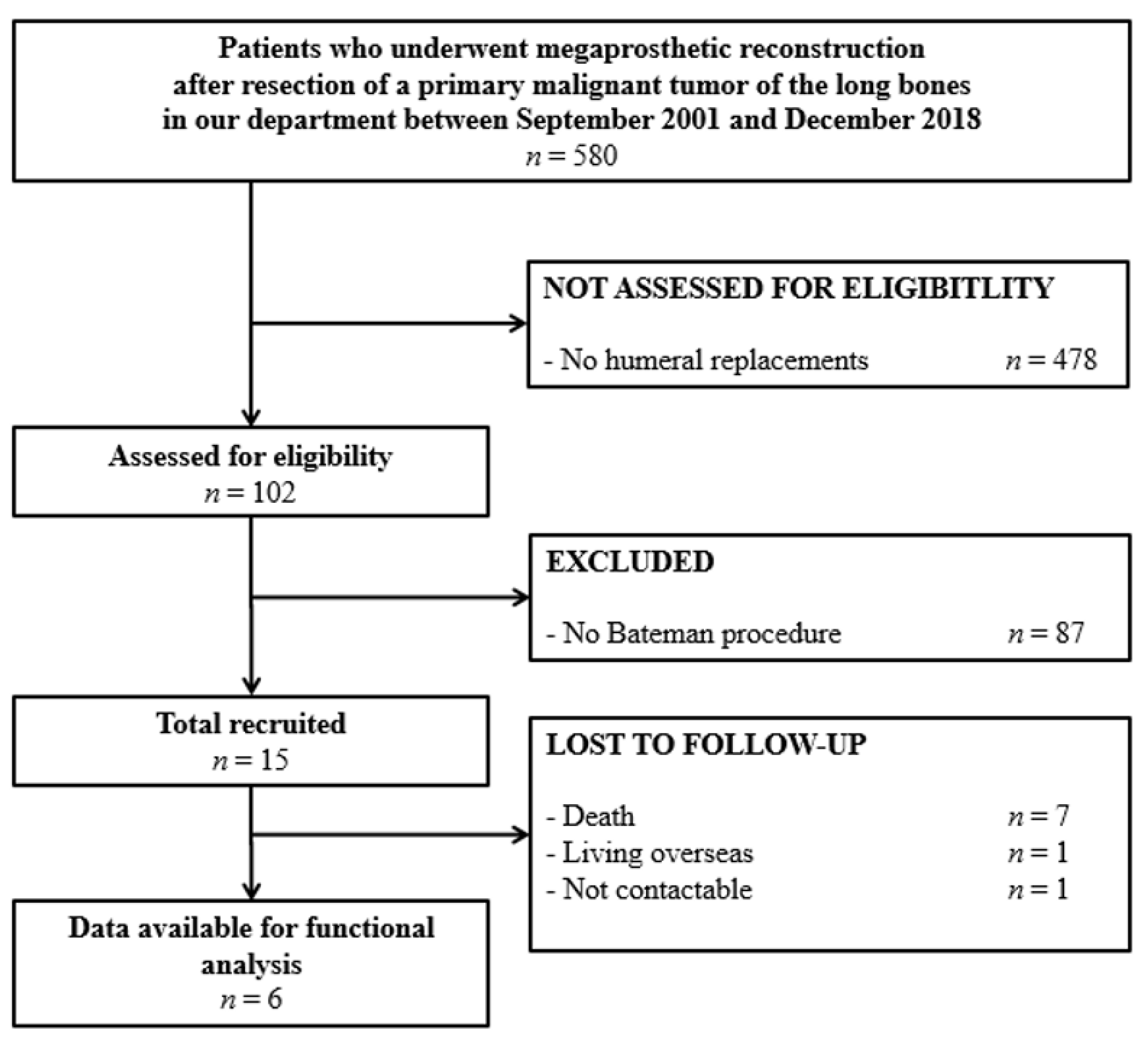
2.2. Study Population
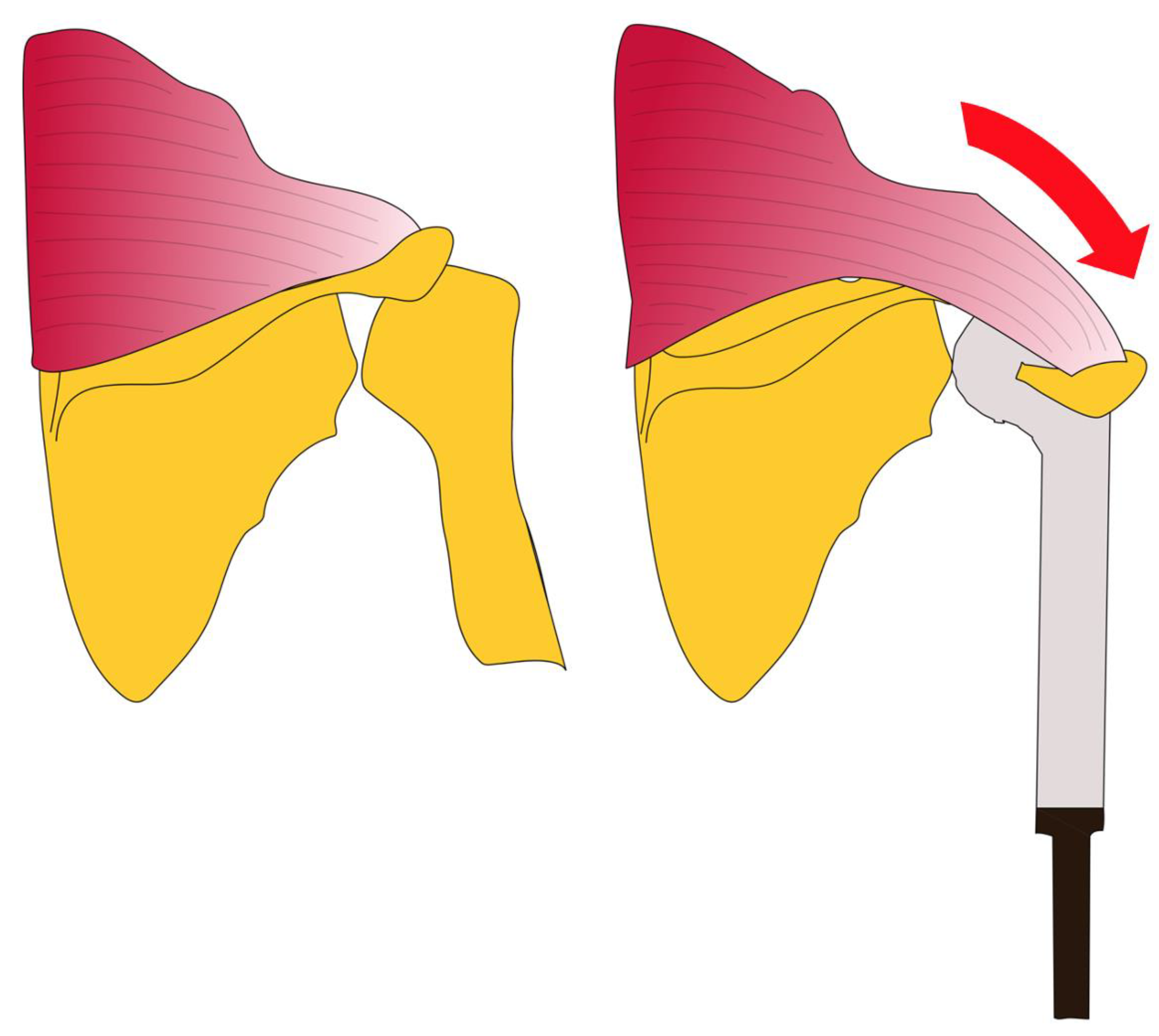
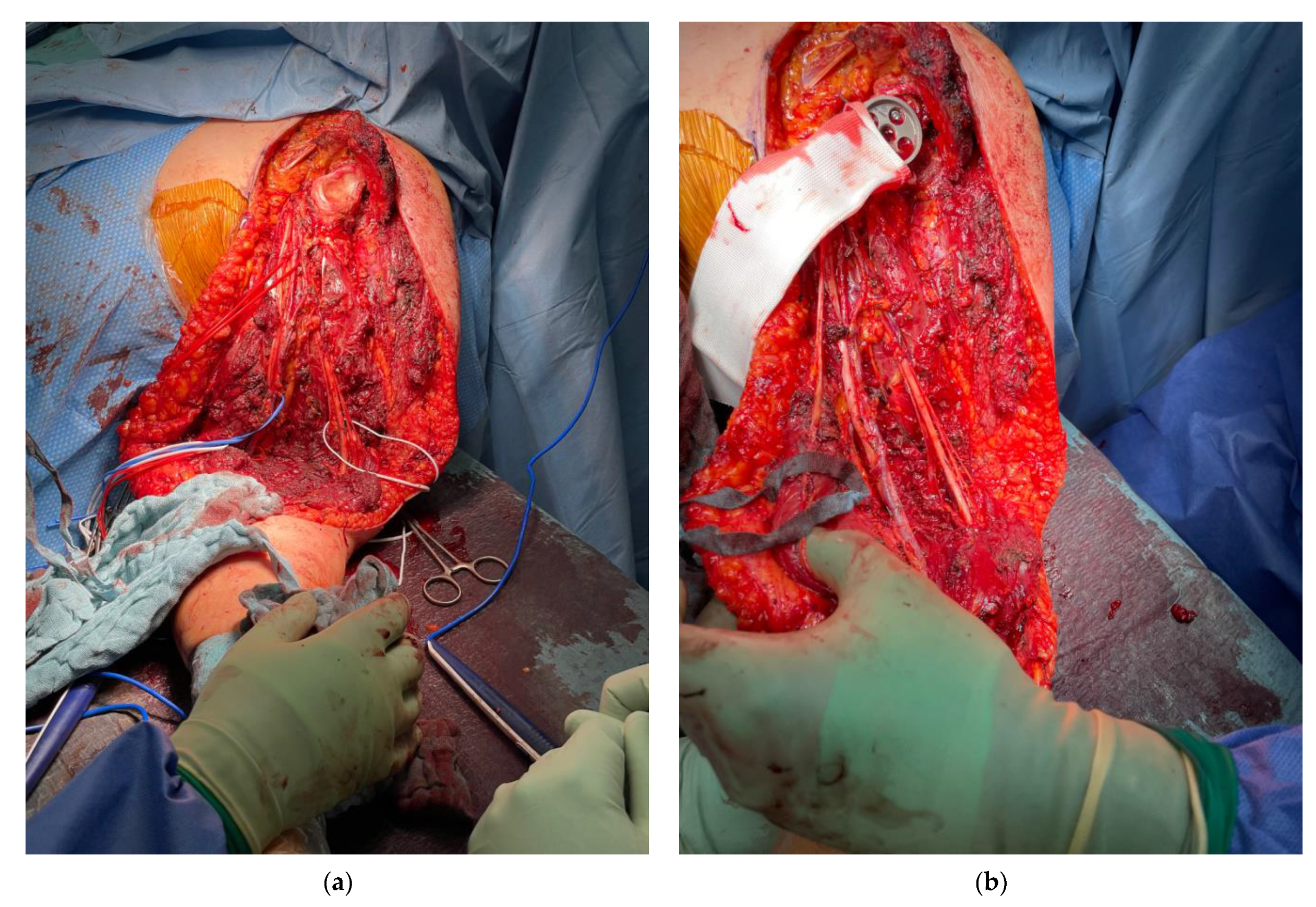
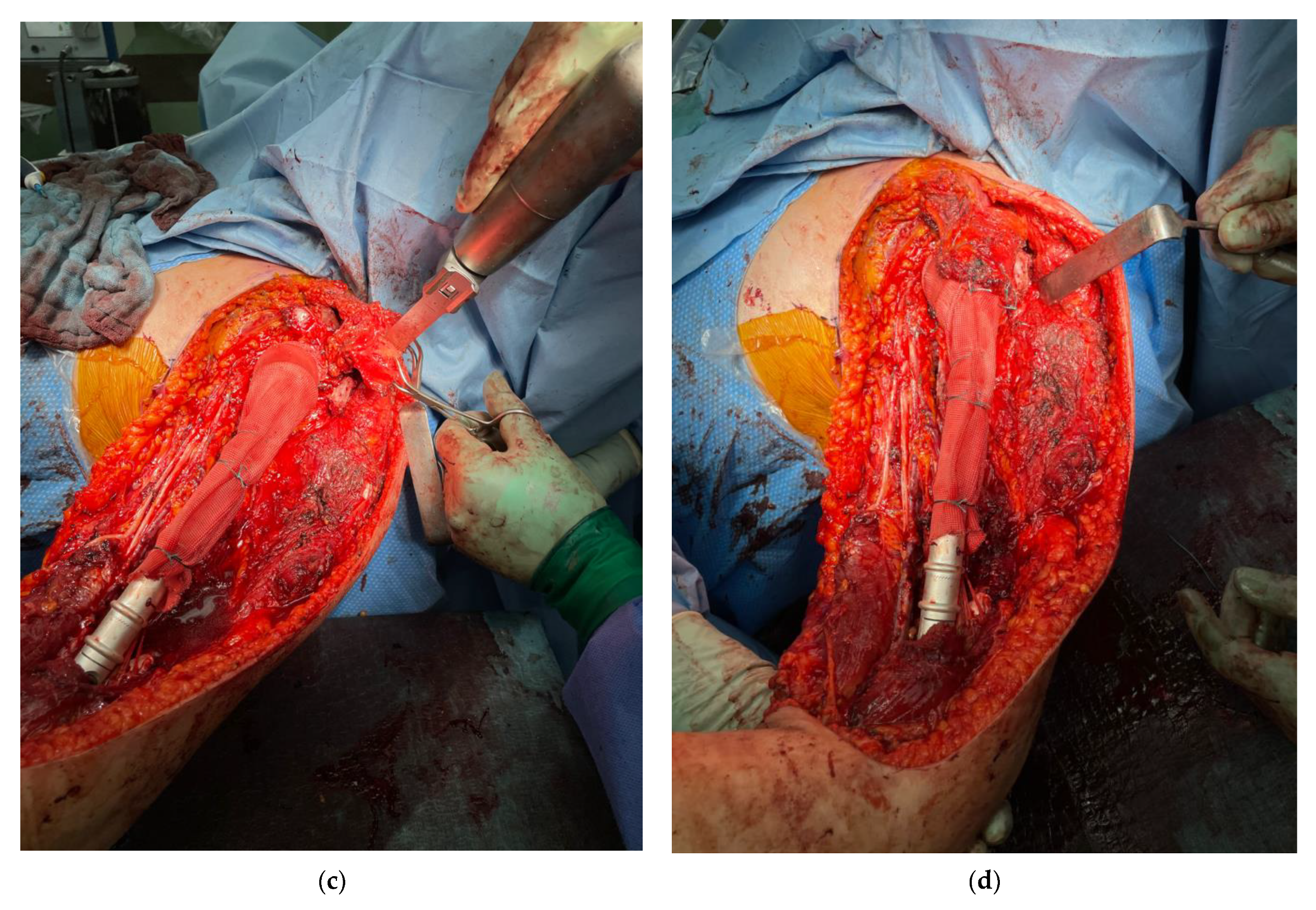
2.3. Surgical Technique
2.4. Classification of Endoprosthetic Failure and Assessment of Functional Outcome
2.5. Statistical Analysis
2.6. Ethics and Funding
3. Results
3.1. Functional Outcome
3.2. Endoprosthetic Complications
4. Discussion
Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Below, C. Dahlins Bone Tumors: General Aspects and Data on 11,087 Cases, 5th ed.; Lippincott-Raven: Philadelphia, PA, USA, 1996. [Google Scholar]
- Hameed, M.; Dorfman, H. Primary Malignant Bone Tumors—Recent Developments. Semin. Diagn. Pathol. 2011, 28, 86–101. [Google Scholar] [CrossRef]
- Böhler, C.; Brönimann, S.; Kaider, A.; Puchner, S.E.; Sigmund, I.K.; Windhager, R.; Funovics, P.T. Surgical and Functional Outcome after Endoprosthetic Reconstruction in Patients with Osteosarcoma of the Humerus. Sci. Rep. UK 2018, 8, 16148. [Google Scholar] [CrossRef]
- Nota, S.; Teunis, T.; Kortlever, J.; Ferrone, M.; Ready, J.; Gebhardt, M.; Raskin, K.; Hornicek, F.; Schwab, J.; Calderon, S.L. Functional Outcomes and Complications After Oncologic Reconstruction of the Proximal Humerus. J. Am. Acad. Orthop. Sur. 2018, 26, 403–409. [Google Scholar] [CrossRef]
- Kumar, D.; Grimer, R.J.; Abudu, A.; Carter, S.R.; Tillman, R.M. Endoprosthetic Replacement of the Proximal Humerus. Long-Term Results. J. Bone Jt. Surg. Br. Vol. 2003, 85, 717–722. [Google Scholar] [CrossRef] [Green Version]
- Fiore, M.; Sambri, A.; Giannini, C.; Zucchini, R.; Cristofaro, R.D.; Paolis, M.D. Anatomical and Reverse Megaprosthesis in Proximal Humerus Reconstructions after Oncologic Resections: A Systematic Review and Meta-Analysis. Arch. Orthop. Traum. Surg. 2021, 1–11. [Google Scholar] [CrossRef]
- Henderson, E.R.; Groundland, J.S.; Pala, E.; Dennis, J.A.; Wooten, R.; Cheong, D.; Windhager, R.; Kotz, R.I.; Mercuri, M.; Funovics, P.T.; et al. Failure Mode Classification for Tumor Endoprostheses. J. Bone Jt. Surg. 2011, 93, 418–429. [Google Scholar] [CrossRef]
- Grimer, R.J.; Aydin, B.K.; Wafa, H.; Carter, S.R.; Jeys, L.; Abudu, A.; Parry, M. Very Long-Term Outcomes after Endoprosthetic Replacement for Malignant Tumours of Bone. Bone Jt. J. 2016, 98-B, 857–864. [Google Scholar] [CrossRef]
- Smolle, M.A.; Andreou, D.; Tunn, P.-U.; Leithner, A. Advances in Tumour Endoprostheses: A Systematic Review. EFORT Open Rev. 2019, 4, 445–459. [Google Scholar] [CrossRef]
- Theil, C.; Röder, J.; Gosheger, G.; Deventer, N.; Dieckmann, R.; Schorn, D.; Hardes, J.; Andreou, D. What Is the Likelihood That Tumor Endoprostheses Will Experience a Second Complication after First Revision in Patients with Primary Malignant Bone Tumors and What Are Potential Risk Factors? Clin. Orthop. Relat. Res. 2019, 477, 2705–2714. [Google Scholar] [CrossRef]
- Sirveaux, F. Reconstruction Techniques after Proximal Humerus Tumour Resection. Orthop. Traumatol. Surg. Res. 2018, 105, S153–S164. [Google Scholar] [CrossRef] [PubMed]
- Schneider, K.N.; Bröking, J.N.; Gosheger, G.; Lübben, T.; Hardes, J.; Schorn, D.; Smolle, M.A.; Theil, C.; Andreou, D. What Is the Implant Survivorship and Functional Outcome after Total Humeral Replacement in Patients with Primary Bone Tumors? Clin. Orthop. Relat. Res. 2021. [Google Scholar] [CrossRef]
- Voggenreiter, G.; Assenmacher, S.; Schmit-Neuerburg, K.-P. Tikhoff-Linberg Procedure for Bone and Soft Tissue Tumors of the Shoulder Girdle. Arch. Surg. Chic. 1999, 134, 252–257. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Capanna, R.; van Horn, J.R.; Biagini, R.; Ruggieri, P.; Ferruzzi, A.; Campanacci, M. The Tikhoff-Linberg Procedure for Bone Tumors of the Proximal Humerus: The Classical “Extensive” Technique versus a Modified “Transglenoid” Resection. Arch. Orthop. Traum. Surg. 1990, 109, 63–67. [Google Scholar] [CrossRef]
- Nakagawa, M.; Nagamatsu, S.; Kayano, S.; Koizumi, T.; Matsui, T.; Katsuragi, Y.; Yamamoto, Y.; Iida, T.; Takahashi, M.; Katagiri, H.; et al. Strategy of Limb Salvage Surgery Using Free Flap for Malignant Tumors of the Extremities. J. Jpn. Soc. Reconstr. Microsurg. 2011, 24, 367–376. [Google Scholar] [CrossRef]
- Selber, J.C.; Treadway, C.; Lopez, A.; Lewis, V.O.; Chang, D.W. The Use of Free Flap for Limb Salvage in Children with Tumors of the Extremities. J. Pediatr. Surg. 2011, 46, 736–744. [Google Scholar] [CrossRef]
- Gosheger, G.; Hardes, J.; Ahrens, H.; Gebert, C.; Winkelmann, W. Endoprosthetic Replacement of the Humerus Combined with Trapezius and Latissimus Dorsi Transfer: A Report of Three Patients. Arch. Orthop. Traum. Surg. 2005, 125, 62–65. [Google Scholar] [CrossRef] [PubMed]
- Bateman, J.C. The Shoulder and Its Environs; The C.V. Mosby Company: St. Louis, MO, USA, 1955. [Google Scholar]
- Gerber, C. Latissimus Dorsi Transfer for the Treatment of Irreparable Tears of the Rotator Cuff. Clin. Orthop. Relat. Res. 1992, 275, 152–160. [Google Scholar] [CrossRef]
- Enneking, W.F.; Dunham, W.; Gebhardt, M.C.; Malawar, M.; Pritchard, D.J. A System for the Functional Evaluation of Reconstructive Procedures after Surgical Treatment of Tumors of the Musculoskeletal System. Clin. Orthop. Relat. Res. 1993, 286, 241–246. [Google Scholar] [CrossRef]
- Davis, A.M.; Wright, J.G.; Williams, J.I.; Bombardier, C.; Griffin, A.; Bell, R.S. Development of a Measure of Physical Function for Patients with Bone and Soft Tissue Sarcoma. Qual. Life Res. Int. J. Qual. Life Asp. Treat. Care Rehabil. 1996, 5, 508–516. [Google Scholar] [CrossRef]
- Richards, R.R.; An, K.-N.; Bigliani, L.U.; Friedman, R.J.; Gartsman, G.M.; Gristina, A.G.; Iannotti, J.P.; Mow, V.C.; Sidles, J.A.; Zuckerman, J.D. A Standardized Method for the Assessment of Shoulder Function. J. Shoulder Elb. Surg. 1994, 3, 347–352. [Google Scholar] [CrossRef]
- Dieckmann, R.; Liem, D.; Gosheger, G.; Henrichs, M.-P.; Höll, S.; Hardes, J.; Streitbürger, A. Evaluation of a Reconstruction Reverse Shoulder for Tumour Surgery and Tribological Comparision with an Anatomical Shoulder Arthroplasty. Int. Orthop. 2013, 37, 451–456. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Streitbürger, A.; Henrichs, M.; Hardes, J.; Dieckmann, R.; Hoell, S.; Gosheger, G. Proximaler Humerusersatz bei malignen Schultergelenktumoren. Oper. Orthopädie Und Traumatol. 2012, 24, 174–185. [Google Scholar] [CrossRef]
- Gosheger, G.; Hillmann, A.; Lindner, N.; Rödl, R.; Hoffmann, C.; Bürger, H.; Winkelmann, W. Soft Tissue Reconstruction of Megaprostheses Using a Trevira Tube. Clin. Orthop. Relat. Res. 2001, 393, 264–271. [Google Scholar] [CrossRef]
- Hardes, J.; Ahrens, H.; Nottrott, M.; Dieckmann, R.; Gosheger, G.; Henrichs, M.-P.; Streitbürger, A. Der Anbindungsschlauch zur Weichteilrekonstruktion nach Megaprothesenimplantation. Oper. Orthopädie Traumatol. 2012, 24, 227–234. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Sun, M.; Sun, W.; Qin, X.; Zhu, Z.; Wang, S. Cross-Cultural Adaptation and Validation of the Chinese Version of Toronto Extremity Salvage Score for Patients with Extremity Sarcoma. Springerplus 2016, 5, 1118. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Agrawal, N.K. Transfer of Upper Trapezius with Clavicular Segment for Restoration of Shoulder Movements Following Injury to the Brachial Plexus. Plast. Aesthetic Res. 2015, 2, 346–349. [Google Scholar] [CrossRef]
- Aziz, W.; Singer, R.; Wolff, T. Transfer of the Trapezius for Flail Shoulder after Brachial Plexus Injury. J. Bone Jt. Surg. Br. Vol. 1990, 72-B, 701–704. [Google Scholar] [CrossRef]
- Jeys, L.M.; Grimer, R.J.; Carter, S.R.; Tillman, R.M. Risk of Amputation Following Limb Salvage Surgery with Endoprosthetic Replacement, in a Consecutive Series of 1261 Patients. Int. Orthop. 2003, 27, 160–163. [Google Scholar] [CrossRef]
- Jeys, L.; Grimer, R. Treatment of Bone and Soft Tissue Sarcomas. Recent Results Canc. 2009, 179, 75–84. [Google Scholar] [CrossRef]
| Name | Surgical Technique |
|---|---|
| Bateman procedure [18] | Mobilization of the trapezius muscle and its bony attachments at the acromion and the lateral part of the scapular spine, fixing it to the lateral proximal humerus |
| Bateman-type reconstruction [17] | Mobilization of the trapezius muscle and its bony attachment at the acromion, fixing it to the attachment tube of the endoprothetic proximal humeral replacement |
| Gerber procedure [19] | Mobilization of the latissimus dorsi muscle and fixing it to the lateral proximal humerus |
| Tikhoff-Linberg procedure [13] | Extra-articular en bloc removal of the scapula, the lateral clavicle and the proximal humerus |
| Modified Tikhoff-Linberg procedure [14] | Resection of the glenoid, the lateral clavicle and the proximal humerus and endoprosthetic replacement of the proximal humerus |
| Sever-L’Episcopo procedure [11] | Mobilization of the latissimus dorsi and teres major muscles and transfer to the lateral proximal humerus posteriorly |
| Variable | n (%) |
|---|---|
| Gender | |
| Female | 6 (40%) |
| Male | 9 (60%) |
| Tumor Entity | |
| Osteosarcoma | 10 (67%) |
| Chondrosarcoma | 4 (27%) |
| Clear Cell Chondrosarcoma | 1 (7%) |
| Type of Tumor Resection | |
| Intra-articular | 6 (40%) |
| Extra-articular | 9 (60%) |
| Type of Reconstruction | |
| Proximal Humeral Replacement | 9 (60%) |
| Total Humeral Replacement | 6 (40%) |
| Type of Shoulder Reconstruction | |
| Anatomic | 12 (80%) |
| Reverse | 3 (20%) |
| MSTS | TESS | ASES | |
|---|---|---|---|
| Type of Tumor Resection | |||
| Intra-articular | 20 | 73 | 72 |
| Extra-articular | 20 | 66 | 73 |
| p = 0.7 | p = 1.0 | p = 0.7 | |
| Type of Shoulder Reconstruction | |||
| Anatomic | 18 | 52 | 65 |
| Reverse | 26 | 73 | 86 |
| p = 0.2 | p = 0.1 | p = 0.2 |
| Ante- Version | Retro- Version | Abduction | Adduction | Internal Rotation | External Rotation | |
|---|---|---|---|---|---|---|
| Type of Shoulder Reconstruction | 10 | 10 | 20 | 30 | 15 | |
| Anatomic | 10 | 10 | 10 | 20 | 30 | 15 |
| Reverse | 80 | 30 | 40 | 40 | 40 | 40 |
| p= 0.049 | p= 0.049 | p= 0.049 | p= 0.007 | p= 0.112 | p= 0.028 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ahrens, H.; Theil, C.; Gosheger, G.; Rödl, R.; Deventer, N.; Rickert, C.; Ackmann, T.; Schwarze, J.; Klingebiel, S.; Schneider, K.N. The Bateman-Type Soft Tissue Reconstruction around Proximal or Total Humeral Megaprostheses in Patients with Primary Malignant Bone Tumors—Functional Outcome and Endoprosthetic Complications. Cancers 2021, 13, 3971. https://doi.org/10.3390/cancers13163971
Ahrens H, Theil C, Gosheger G, Rödl R, Deventer N, Rickert C, Ackmann T, Schwarze J, Klingebiel S, Schneider KN. The Bateman-Type Soft Tissue Reconstruction around Proximal or Total Humeral Megaprostheses in Patients with Primary Malignant Bone Tumors—Functional Outcome and Endoprosthetic Complications. Cancers. 2021; 13(16):3971. https://doi.org/10.3390/cancers13163971
Chicago/Turabian StyleAhrens, Helmut, Christoph Theil, Georg Gosheger, Robert Rödl, Niklas Deventer, Carolin Rickert, Thomas Ackmann, Jan Schwarze, Sebastian Klingebiel, and Kristian Nikolaus Schneider. 2021. "The Bateman-Type Soft Tissue Reconstruction around Proximal or Total Humeral Megaprostheses in Patients with Primary Malignant Bone Tumors—Functional Outcome and Endoprosthetic Complications" Cancers 13, no. 16: 3971. https://doi.org/10.3390/cancers13163971






