Central Neuropathic Pain Development Modulation Using Coffee Extract Major Polyphenolic Compounds in Spinal-Cord-Injured Female Mice
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Design
2.2. Animals and Ethical Regulations
2.3. Surgical Procedure and Spinal Cord Contusion
2.4. Polyphenolic Treatments
2.5. Functional Evaluation
2.5.1. Locomotor Activity
2.5.2. Reflexive Pain Response Assessment: Thermal Hyperalgesia and Mechanical Allodynia
2.5.3. Nonreflexive Pain Response Assessment: Depression-Like Behavior
2.6. Tissue and Serum Samples Collection
2.7. Immunohistochemical Staining and Image Analysis
2.8. Biochemical Analysis of Hepatotoxicity and Nephrotoxicity
2.9. Statistical Analysis
3. Results
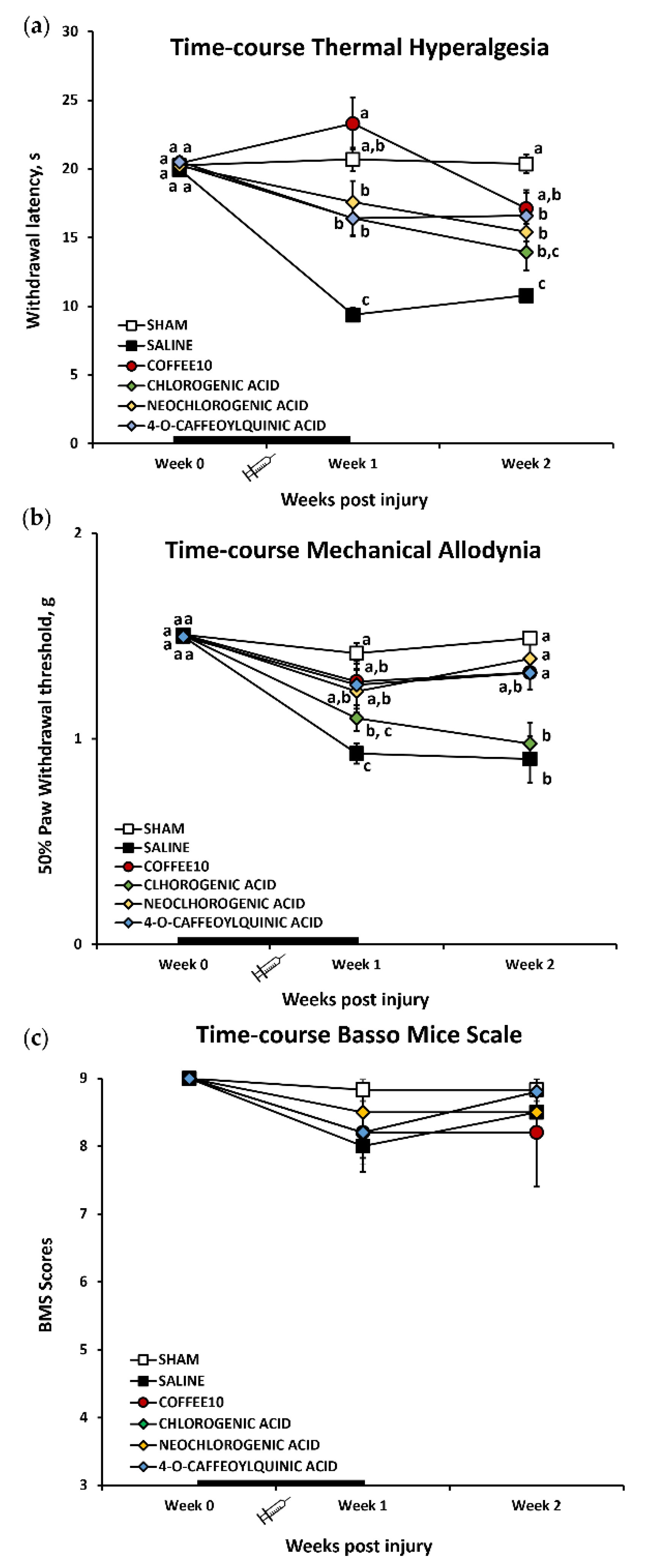
3.1. Major Coffee Extract Polyphenolic Compounds Significantly Attenuate Spinal-Cord-Injury-Induced Reflexive Pain Responses
3.2. Major Coffee Extract Polyphenolic Compounds Prevent the Development of Depression-Like Behavior after Spinal Cord Contusion
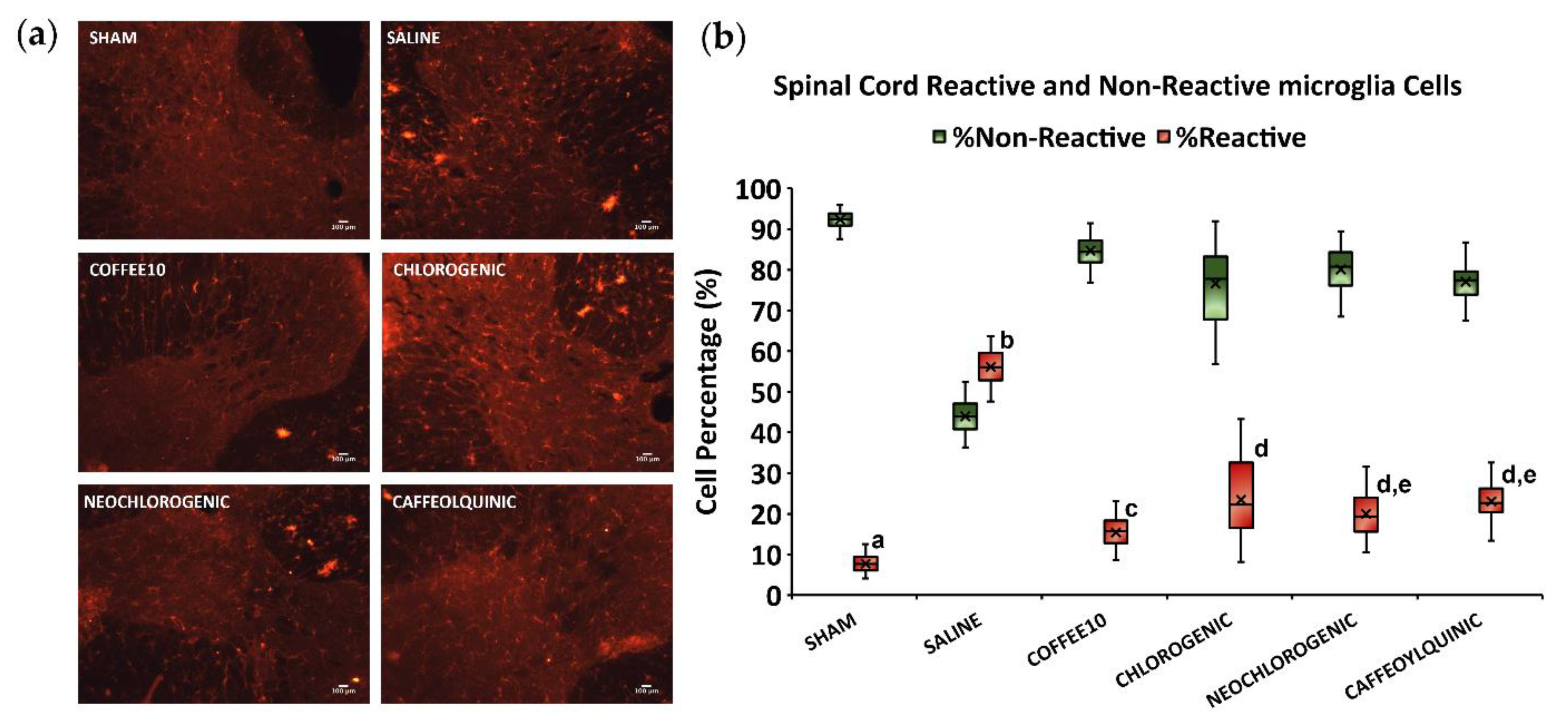
3.3. Major Coffee Extract Polyphenolic Compounds Significantly Attenuate Spinal Cord Gliosis, but the Whole Coffee Extract Exerts the Most Effective Effect
3.4. Major Coffee Extract Polyphenolic Compounds Significantly Attenuate Spinal-Cord-Injury-Induced Sprouting of Afferent Fibers in the Dorsal Horn, but the Whole Coffee Extract Exerts the Most Effective Effect
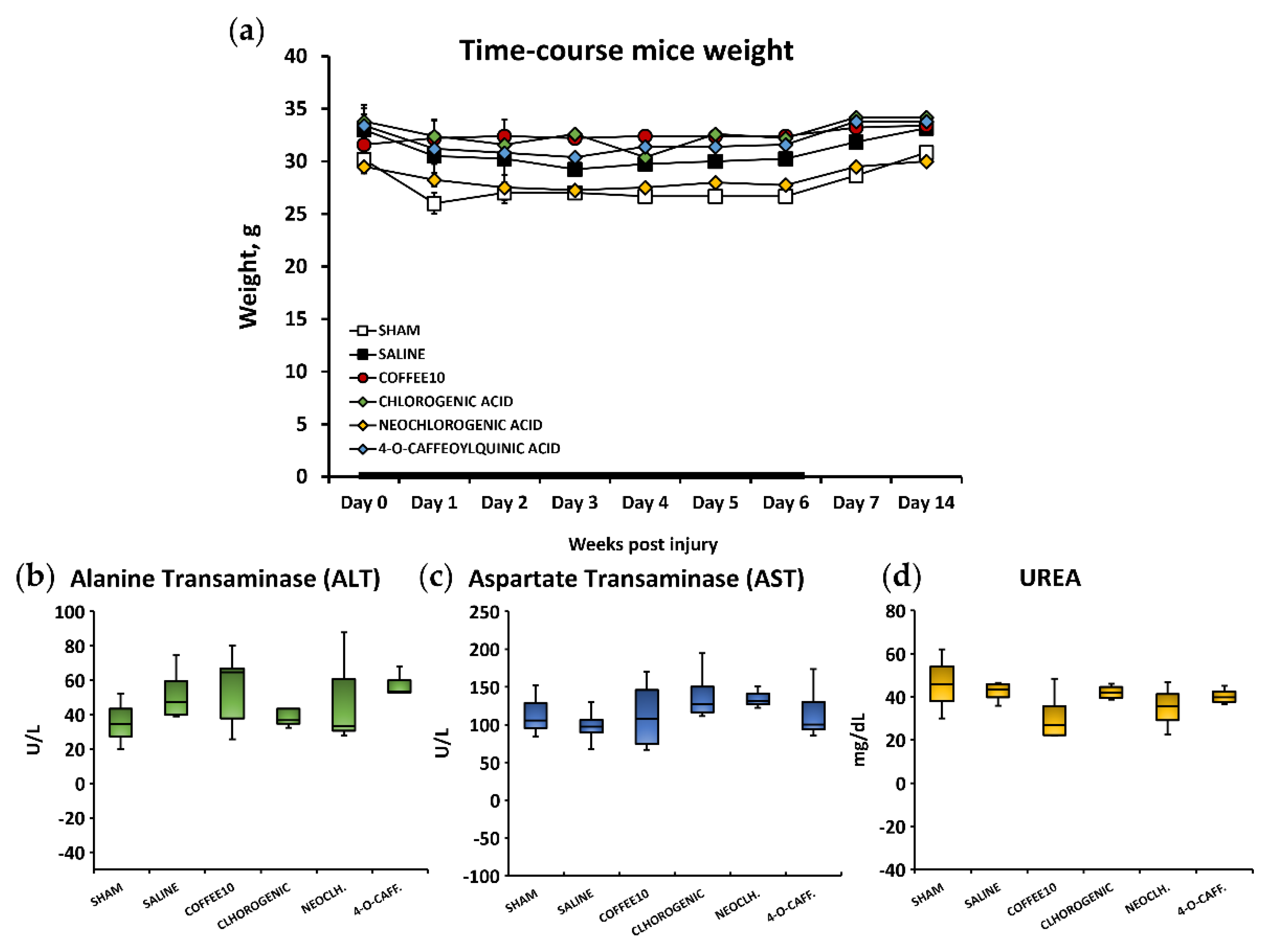
3.5. No Signs of Systemic Toxicity, Hepatotoxicity, or Nephrotoxicity Were Associated with the Polyphenolic Treatments
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bennett, J.; Das, J.M.; Emmady, P.D. Spinal Cord Injuries. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2022. [Google Scholar]
- Burke, D.; Fullen, B.M.; Stokes, D.; Lennon, O. Neuropathic pain prevalence following spinal cord injury: A systematic review and meta-analysis. Eur. J. Pain. 2017, 21, 29–44. [Google Scholar] [CrossRef] [PubMed]
- Attal, N. Pharmacological treatments of neuropathic pain: The latest recommendations. Rev. Neurol. 2019, 175, 46–50. [Google Scholar] [CrossRef] [PubMed]
- Pirvulescu, I.; Biskis, A.; Candido, K.D.; Knezevic, N.N. Overcoming clinical challenges of refractory neuropathic pain. Expert. Rev. Neurother. 2022, 22, 595–622. [Google Scholar] [CrossRef] [PubMed]
- Jahromi, B.; Pirvulescu, I.; Candido, K.D.; Knezevic, N.N. Herbal Medicine for Pain Management: Efficacy and Drug Interactions. Pharmaceutics 2021, 13, 251. [Google Scholar] [CrossRef] [PubMed]
- Boadas-Vaello, P.; Vela, J.M.; Verdu, E. New Pharmacological Approaches Using Polyphenols on the Physiopathology of Neuropathic Pain. Curr. Drug Targets 2017, 18, 160–173. [Google Scholar] [CrossRef]
- Hassler, S.N.; Johnson, K.M.; Hulsebosch, C.E. Reactive oxygen species and lipid peroxidation inhibitors reduce mechanical sensitivity in a chronic neuropathic pain model of spinal cord injury in rats. J. Neurochem. 2014, 131, 413–417. [Google Scholar] [CrossRef]
- Ma, L.; Mu, Y.; Zhang, Z.; Sun, Q. Eugenol promotes functional recovery and alleviates inflammation, oxidative stress, and neural apoptosis in a rat model of spinal cord injury. Restor. Neurol. Neurosci. 2018, 36, 659–668. [Google Scholar] [CrossRef]
- Renno, W.M.; Al-Khaledi, G.; Mousa, A.; Karam, S.M.; Abul, H.; Asfar, S. (-)-Epigallocatechin-3-gallate (EGCG) modulates neurological function when intravenously infused in acute and, chronically injured spinal cord of adult rats. Neuropharmacology 2014, 77, 100–119. [Google Scholar] [CrossRef]
- Álvarez-Pérez, B.; Homs, J.; Bosch-Mola, M.; Puig, T.; Reina, F.; Verdú, E.; Boadas-Vaello, P. Epigallocatechin-3-gallate treatment reduces thermal hyperalgesia after spinal cord injury by down-regulating RhoA expression in mice. Eur. J. Pain. 2016, 20, 341–352. [Google Scholar] [CrossRef]
- Bagó-Mas, A.; Korimová, A.; Deulofeu, M.; Verdú, E.; Fiol, N.; Svobodová, V.; Dubový, P.; Boadas-Vaello, P. Polyphenolic grape stalk and coffee extracts attenuate spinal cord injury-induced neuropathic pain development in ICR-CD1 female mice. Sci. Rep. 2022, 12, 14980. [Google Scholar] [CrossRef]
- Schmidt, B.M.; Ribnicky, D.M.; Lipsky, P.E.; Raskin, I. Revisiting the ancient concept of botanical therapeutics. Nat. Chem. Biol. 2007, 3, 360–366. [Google Scholar] [CrossRef]
- Atanasov, A.G.; Zotchev, S.B.; Dirsch, V.M.; International Natural Product Sciences Taskforce; Supuran, C.T. Natural products in drug discovery: Advances and opportunities. Nat. Rev. Drug Discov. 2021, 20, 200–216. [Google Scholar] [CrossRef]
- Atanasov, A.G.; Waltenberger, B.; Pferschy-Wenzig, E.M.; Linder, T.; Wawrosch, C.; Uhrin, P.; Temml, V.; Wang, L.; Schwaiger, S.; Heiss, E.H.; et al. Discovery and resupply of pharmacologically active plant-derived natural products: A review. Biotechnol. Adv. 2015, 33, 1582–1614. [Google Scholar] [CrossRef]
- Zimmermann, M. Ethical guidelines for investigations of experimental pain in conscious animals. Pain 1983, 16, 109–110. [Google Scholar] [CrossRef]
- Castany, S.; Gris, G.; Vela, J.M.; Verdú, E.; Boadas-Vaello, P. Critical role of sigma-1 receptors in central neuropathic pain-related behaviours after mild spinal cord injury in mice. Sci. Rep. 2018, 8, 3873. [Google Scholar] [CrossRef]
- Castany, S.; Codony, X.; Zamanillo, D.; Merlos, M.; Verdú, E.; Boadas-Vaello, P. Repeated Sigma-1 Receptor Antagonist MR309 Administration Modulates Central Neuropathic Pain Development After Spinal Cord Injury in Mice. Front. Pharmacol. 2019, 10, 222. [Google Scholar] [CrossRef]
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventós, R.M. Analysis of total phenols and other oxidation substrates and antioxidants by means of Folin-Ciocalteu reagent. Methods Enzymol. 1999, 299, 152–178. [Google Scholar] [CrossRef]
- Basso, D.M.; Fisher, L.C.; Anderson, A.J.; Jakeman, L.B.; McTigue, D.M.; Popovich, P.G. Basso Mouse Scale for locomotion detects differences in recovery after spinal cord injury in five common mouse strains. J. Neurotrauma. 2006, 23, 635–659. [Google Scholar] [CrossRef]
- Hargreaves, K.; Dubner, R.; Brown, F.; Flores, C.; Joris, J. A new and sensitive method for measuring thermal nociception in cutaneous hyperalgesia. Pain 1988, 32, 77–88. [Google Scholar] [CrossRef]
- Dixon, W.J. Efficient analysis of experimental observations. Annu. Rev. Pharmacol. Toxicol. 1980, 20, 441–462. [Google Scholar] [CrossRef]
- Porsolt, R.D.; Bertin, A.; Jalfre, M. Behavioral despair in mice: A primary screening test for antidepressants. Arch. Int. Pharm. Ther. 1977, 229, 327–336. [Google Scholar]
- Álvarez-Pérez, B.; Deulofeu, M.; Homs, J.; Merlos, M.; Vela, J.M.; Verdú, E.; Boadas-Vaello, P. Long-lasting reflexive and nonreflexive pain responses in two mouse models of fibromyalgia-like condition. Sci. Rep. 2022, 12, 9719. [Google Scholar] [CrossRef] [PubMed]
- Zamboni, L.; De Martino, C. Buffered picric acid-formaldehyde: A new, rapid, fixative for electron microscopy. J. Cell Biol. 1967, 35, 148A. [Google Scholar]
- Morton, D.B.; Griffiths, P.H. Guidelines on the recognition of pain, distress and discomfort in experimental animals and an hypothesis for assessment. Vet. Rec. 1985, 116, 431–436. [Google Scholar] [CrossRef] [PubMed]
- Anwar, M.A.; Al Shehabi, T.S.; Eid, A.H. Inflammogenesis of Secondary Spinal Cord Injury. Front. Cell. Neurosci. 2016, 10, 98. [Google Scholar] [CrossRef]
- Gwak, Y.S.; Hulsebosch, C.E.; Leem, J.W. Neuronal-Glial Interactions Maintain Chronic Neuropathic Pain after Spinal Cord Injury. Neural Plast. 2017, 2480689. [Google Scholar] [CrossRef]
- National Center for Biotechnology Information. PubChem Compound Summary for CID 1794427, Chlorogenic Acid. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Chlorogenic-acid (accessed on 14 September 2022).
- Bagdas, D.; Cinkilic, N.; Ozboluk, H.Y.; Ozyigit, M.O.; Gurun, M.S. Antihyperalgesic activity of chlorogenic acid in experimental neuropathic pain. J. Nat. Med. 2013, 67, 698–704. [Google Scholar] [CrossRef]
- Hara, K.; Haranishi, Y.; Kataoka, K.; Takahashi, Y.; Terada, T.; Nakamura, M.; Sata, T. Chlorogenic acid administered intrathecally alleviates mechanical and cold hyperalgesia in a rat neuropathic pain model. Eur. J. Pharmacol. 2014, 723, 459–464. [Google Scholar] [CrossRef]
- Bagdas, D.; Ozboluk, H.Y.; Cinkilic, N.; Gurun, M.S. Antinociceptive effect of chlorogenic acid in rats with painful diabetic neuropathy. J. Med. Food. 2014, 17, 730–732. [Google Scholar] [CrossRef]
- Bagdas, D.; Gul, Z.; Meade, J.A.; Cam, B.; Cinkilic, N.; Gurun, M.S. Pharmacologic Overview of Chlorogenic Acid and its Metabolites in Chronic Pain and Inflammation. Curr. Neuropharmacol. 2020, 18, 216–228. [Google Scholar] [CrossRef]
- Liang, N.; Kitts, D.D. Role of Chlorogenic Acids in Controlling Oxidative and Inflammatory Stress Conditions. Nutrients 2015, 8, 16. [Google Scholar] [CrossRef]
- Naveed, M.; Hejazi, V.; Abbas, M.; Kamboh, A.A.; Khan, G.J.; Shumzaid, M.; Ahmad, F.; Babazadeh, D.; FangFang, X.; Modarresi-Ghazani, F.; et al. Chlorogenic acid (CGA): A pharmacological review and call for further research. Biomed. Pharmacother. 2018, 97, 67–74. [Google Scholar] [CrossRef]
- National Center for Biotechnology Information. PubChem Compound Summary for CID 5280633, Neochlorogenic Acid. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Neochlorogenic-acid (accessed on 14 September 2022).
- Park, S.Y.; Jin, M.L.; Yi, E.H.; Kim, Y.; Park, G. Eochlorogenic acid inhibits against LPS-activated inflammatory responses through up-regulation of Nrf2/HO-1 and involving AMPK pathway. Environ. Toxicol. Pharmacol. 2018, 62, 1–10. [Google Scholar] [CrossRef]
- Gao, X.H.; Zhang, S.D.; Wang, L.T.; Yu, L.; Zhao, X.L.; Ni, H.Y.; Wang, Y.Q.; Wang, J.D.; Shan, C.H.; Fu, Y.J. Anti-Inflammatory Effects of Neochlorogenic Acid Extract from Mulberry Leaf (Morus alba L.) against LPS-Stimulated Inflammatory Response through Mediating the AMPK/Nrf2 Signaling Pathway in A549 Cells. Molecules 2020, 25, 1385. [Google Scholar] [CrossRef]
- Basbaum, A.I.; Bautista, D.M.; Scherrer, G.; Julius, D. Cellular and molecular mechanisms of pain. Cell 2009, 139, 267–284. [Google Scholar] [CrossRef]
- National Center for Biotechnology Information. PubChem Compound Summary for CID 9798666, Cryptochlorogenic Acid. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Cryptochlorogenic-acid (accessed on 14 September 2022).
- Ganzon, J.G.; Chen, L.G.; Wang, C.C. 4-O-Caffeoylquinic acid as an antioxidant marker for mulberry leaves rich in phenolic compounds. J. Food Drug Anal. 2018, 26, 985–993. [Google Scholar] [CrossRef]
- Shen, W.; Qi, R.; Zhang, J.; Wang, Z.; Wang, H.; Hu, C.; Zhao, Y.; Bie, M.; Wang, Y.; Fu, Y.; et al. Chlorogenic acid inhibits LPS-induced microglial activation and improves survival of dopaminergic neurons. Brain Res. Bull. 2012, 88, 487–494. [Google Scholar] [CrossRef]
- Guo, Y.J.; Luo, T.; Wu, F.; Mei, Y.W.; Peng, J.; Liu, H.; Li, H.R.; Zhang, S.L.; Dong, J.H.; Fang, Y.; et al. Involvement of TLR2 and TLR9 in the anti-inflammatory effects of chlorogenic acid in HSV-1-infected microglia. Life Sci. 2015, 127, 12–18. [Google Scholar] [CrossRef]
- Cásedas, G.; Bennett, A.C.; González-Burgos, E.; Gómez-Serranillos, M.P.; López, V.; Smith, C. Polyphenol-associated oxidative stress and inflammation in a model of LPS-induced inflammation in glial cells: Do we know enough for responsible compounding? Inflammopharmacology 2019, 27, 189–197. [Google Scholar] [CrossRef]
- Kim, M.; Choi, S.Y.; Lee, P.; Hur, J. Neochlorogenic Acid Inhibits Lipopolysaccharide-Induced Activation and Pro-inflammatory Responses in BV2 Microglial Cells. Neurochem. Res. 2015, 40, 1792–1798. [Google Scholar] [CrossRef]
- Lukitasari, M.; Nugroho, D.A.; Widodo, N. Chlorogenic Acid: The Conceivable Chemosensitizer Leading to Cancer Growth Suppression. J. Evid. Based Integr. Med. 2018, 23, 2515690X18789628. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.K.; Ngoc, N.; Chang, H.W.; Su, Y.F.; Chen, C.H.; Goan, Y.G.; Chen, J.Y.; Tung, C.W.; Hour, T.C. Chlorogenic Acid Inhibition of Esophageal Squamous Cell Carcinoma Metastasis via EGFR/p-Akt/Snail Signaling Pathways. Anticancer Res. 2022, 42, 3389–3402. [Google Scholar] [CrossRef] [PubMed]
- Ferrer, I.; Alcántara, S.; Ballabriga, J.; Olivé, M.; Blanco, R.; Rivera, R.; Carmona, M.; Berruezo, M.; Pitarch, S.; Planas, A.M. Transforming growth factor-alpha (TGF-alpha) and epidermal growth factor-receptor (EGF-R) immunoreactivity in normal and pathologic brain. Prog. Neurobiol. 1996, 49, 99–123. [Google Scholar] [CrossRef]
- Planas, A.M.; Justicia, C.; Soriano, M.A.; Ferrer, I. Epidermal growth factor receptor in proliferating reactive glia following transient focal ischemia in the rat brain. Glia 1998, 23, 120–129. [Google Scholar] [CrossRef]
- Qu, W.S.; Tian, D.S.; Guo, Z.B.; Fang, J.; Zhang, Q.; Yu, Z.Y.; Xie, M.J.; Zhang, H.Q.; Lü, J.G.; Wang, W. Inhibition of EGFR/MAPK signaling reduces microglial inflammatory response and the associated secondary damage in rats after spinal cord injury. J Neuroinflammation 2012, 9, 178. [Google Scholar] [CrossRef]
- Xu, W.; Luo, T.; Chai, J.; Jing, P.; Xiong, L. Chlorogenic Acid Alleviates the Inflammatory Stress of LPS-Induced BV2 Cell via Interacting with TLR4-Mediated Downstream Pathway. Comput. Math. Methods Med. 2022, 2022, 6282167. [Google Scholar] [CrossRef]
- Shi, A.; Shi, H.; Wang, Y.; Liu, X.; Cheng, Y.; Li, H.; Zhao, H.; Wang, S.; Dong, L. Activation of Nrf2 pathway and inhibition of NLRP3 inflammasome activation contribute to the protective effect of chlorogenic acid on acute liver injury. Int. Immunopharmacol. 2018, 54, 125–130. [Google Scholar] [CrossRef]
- Huang, X.; Liu, Y.; Shen, H.; Fu, T.; Guo, Y.; Qiu, S. Chlorogenic acid attenuates inflammation in LPS-induced Human gingival fibroblasts via CysLT1R/Nrf2/NLRP3 signaling. Int. Immunopharmacol. 2022, 107, 108706. [Google Scholar] [CrossRef]
- He, W.; Long, T.; Pan, Q.; Zhang, S.; Zhang, Y.; Zhang, D.; Qin, G.; Chen, L.; Zhou, J. Microglial NLRP3 inflammasome activation mediates IL-1β release and contributes to central sensitization in a recurrent nitroglycerin-induced migraine model. J. Neuroinflammation. 2019, 16, 78. [Google Scholar] [CrossRef]
- Starobova, H.; Nadar, E.I.; Vetter, I. The NLRP3 Inflammasome: Role and Therapeutic Potential in Pain Treatment. Front. Physiol. 2020, 11, 1016. [Google Scholar] [CrossRef]
- Wang, J.; Li, F.; Zeng, K.; Li, Q.; Zhao, X.; Zheng, X. Bioactive compounds of Shuang-Huang-Lian prescription and an insight into its binding mechanism by β2 -adrenoceptor chromatography coupled with site-directed molecular docking. J. Sep. Sci. 2017, 40, 4357–4365. [Google Scholar] [CrossRef]
- Wohleb, E.S.; Hanke, M.L.; Corona, A.W.; Powell, N.D.; Stiner, L.M.; Bailey, M.T.; Nelson, R.J.; Godbout, J.P.; Sheridan, J.F. β-Adrenergic receptor antagonism prevents anxiety-like behavior and microglial reactivity induced by repeated social defeat. J. Neurosci. 2011, 31, 6277–6288. [Google Scholar] [CrossRef]
- Johnson, J.D.; Zimomra, Z.R.; Stewart, L.T. Beta-adrenergic receptor activation primes microglia cytokine production. J. Neuroimmunol. 2013, 254, 161–164. [Google Scholar] [CrossRef]
- Sharma, M.; Arbabzada, N.; Flood, P.M. Mechanism underlying β2-AR agonist-mediated phenotypic conversion of LPS-activated microglial cells. J. Neuroimmunol. 2019, 332, 37–48. [Google Scholar] [CrossRef]
- Nees, T.A.; Finnerup, N.B.; Blesch, A.; Weidner, N. Neuropathic pain after spinal cord injury: The impact of sensorimotor activity. Pain 2017, 158, 371–376. [Google Scholar] [CrossRef]
- Sliwinski, C.; Nees, T.A.; Puttagunta, R.; Weidner, N.; Blesch, A. Sensorimotor Activity Partially Ameliorates Pain and Reduces Nociceptive Fiber Density in the Chronically Injured Spinal Cord. J. Neurotrauma. 2018, 35, 2222–2238. [Google Scholar] [CrossRef]
- Hutchinson, K.J.; Gómez-Pinilla, F.; Crowe, M.J.; Ying, Z.; Basso, D.M. Three exercise paradigms differentially improve sensory recovery after spinal cord contusion in rats. Brain 2004, 127, 1403–1414. [Google Scholar] [CrossRef]
- Detloff, M.R.; Smith, E.J.; Quiros Molina, D.; Ganzer, P.D.; Houlé, J.D. Acute exercise prevents the development of neuropathic pain and the sprouting of non-peptidergic (GDNF- and artemin-responsive) c-fibers after spinal cord injury. Exp. Neurol. 2014, 255, 38–48. [Google Scholar] [CrossRef]
- Saeed, A.W.; Ribeiro-da-Silva, A. Non-peptidergic primary afferents are presynaptic to neurokinin-1 receptor immunoreactive lamina I projection neurons in rat spinal cord. Mol. Pain 2012, 8, 64. [Google Scholar] [CrossRef]
- Mika, J.; Zychowska, M.; Popiolek-Barczyk, K.; Rojewska, E.; Przewlocka, B. Importance of glial activation in neuropathic pain. Eur. J. Pharmacol. 2013, 716, 106–119. [Google Scholar] [CrossRef]
- Bennett, D.L.; Michael, G.J.; Ramachandran, N.; Munson, J.B.; Averill, S.; Yan, Q.; McMahon, S.B.; Priestley, J.V. A distinct subgroup of small DRG cells express GDNF receptor components and GDNF is protective for these neurons after nerve injury. J. Neurosci. 1998, 18, 3059–3072. [Google Scholar] [CrossRef] [PubMed]
- Bennett, D.L.; French, J.; Priestley, J.V.; McMahon, S.B. NGF but not NT-3 or BDNF prevents the A fiber sprouting into lamina II of the spinal cord that occurs following axotomy. Mol. Cell Neurosci. 1996, 8, 211–220. [Google Scholar] [CrossRef] [PubMed]
- Bresjanac, M.; Antauer, G. Reactive astrocytes of the quinolinic acid-lesioned rat striatum express GFRalpha1 as well as GDNF in vivo. Exp. Neurol. 2000, 164, 53–59. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.Y.; Zhao, H.K.; Chen, L.W.; Yao, X.Y.; Wang, Y.L.; Huang, Z.W.; Li, G.P.; Wang, Z.; Chen, B.Y. Reactive astrocytes increase expression of proNGF in the mouse model of contused spinal cord injury. Neurosci. Res. 2020, 157, 34–43. [Google Scholar] [CrossRef] [PubMed]
- Iravani, M.M.; Sadeghian, M.; Leung, C.C.; Jenner, P.; Rose, S. Lipopolysaccharide-induced nigral inflammation leads to increased IL-1β tissue content and expression of astrocytic glial cell line-derived neurotrophic factor. Neurosci. Lett. 2012, 510, 138–142. [Google Scholar] [CrossRef]
- Krenz, N.R.; Weaver, L.C. Sprouting of primary afferent fibers after spinal cord transection in the rat. Neuroscience 1998, 85, 443–458. [Google Scholar] [CrossRef]
- Schaffer, S.; Halliwell, B. Do polyphenols enter the brain and does it matter? Some theoretical and practical considerations. Genes Nutr. 2012, 7, 99–109. [Google Scholar] [CrossRef]
- Williams, C.M.; El Mohsen, M.A.; Vauzour, D.; Rendeiro, C.; Butler, L.T.; Ellis, J.A.; Whiteman, M.; Spencer, J.P. Blueberry-induced changes in spatial working memory correlate with changes in hippocampal CREB phosphorylation and brain-derived neurotrophic factor (BDNF) levels. Free Radic. Biol. Med. 2008, 45, 295–305. [Google Scholar] [CrossRef]
- Wang, Q.; Sun, A.Y.; Simonyi, A.; Jensen, M.D.; Shelat, P.B.; Rottinghaus, G.E.; MacDonald, R.S.; Miller, D.K.; Lubahn, D.E.; Weisman, G.A.; et al. Neuroprotective mechanisms of curcumin against cerebral ischemia-induced neuronal apoptosis and behavioral deficits. J. Neurosci. Res. 2005, 82, 138–148. [Google Scholar] [CrossRef]
- Kalt, W.; Blumberg, J.B.; McDonald, J.E.; Vinqvist-Tymchuk, M.R.; Fillmore, S.A.; Graf, B.A.; O’Leary, J.M.; Milbury, P.E. Identification of anthocyanins in the liver, eye, and brain of blueberry-fed pigs. J. Agric. Food Chem. 2008, 56, 705–712. [Google Scholar] [CrossRef]
- Begum, A.N.; Jones, M.R.; Lim, G.P.; Morihara, T.; Kim, P.; Heath, D.D.; Rock, C.L.; Pruitt, M.A.; Yang, F.; Hudspeth, B.; et al. urcumin structure-function, bioavailability, and efficacy in models of neuroinflammation and Alzheimer’s disease. J. Pharmacol. Exp. Ther. 2008, 326, 196–208. [Google Scholar] [CrossRef]
- Milbury, P.E.; Kalt, W. Xenobiotic metabolism and berry flavonoid transport across the blood-brain barrier. J. Agric. Food Chem. 2010, 58, 3950–3956. [Google Scholar] [CrossRef]
- Suresh, D.; Srinivasan, K. Tissue distribution & elimination of capsaicin, piperine & curcumin following oral intake in rats. Indian J. Med. Res. 2010, 131, 682–691. [Google Scholar]
- Miller, L.R.; Cano, A. Comorbid chronic pain and depression: Who is at risk? J. Pain 2009, 10, 619–627. [Google Scholar] [CrossRef]
- Goesling, J.; Clauw, D.J.; Hassett, A.L. Pain and depression: An integrative review of neurobiological and psychological factors. Curr. Psychiatry Rep. 2013, 15, 421. [Google Scholar] [CrossRef]
- Fakhri, S.; Abbaszadeh, F.; Moradi, S.Z.; Cao, H.; Khan, H.; Xiao, J. Effects of polyphenols on oxidative stress, inflammation, and interconnected pathways during spinal cord injury. Oxid. Med. Cell. Longev. 2022, 2022, 8100195. [Google Scholar] [CrossRef]
- Teixeira-Santos, L.; Albino-Teixeira, A.; Pinho, D. Neuroinflammation, oxidative stress and their interplay in neuropathic pain: Focus on specialized pro-resolving mediators and NADPH oxidase inhibitors as potential therapeutic strategies. Pharmacol. Res. 2020, 162, 105280. [Google Scholar] [CrossRef]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Soler-Martínez, R.; Deulofeu, M.; Bagó-Mas, A.; Dubový, P.; Verdú, E.; Fiol, N.; Boadas-Vaello, P. Central Neuropathic Pain Development Modulation Using Coffee Extract Major Polyphenolic Compounds in Spinal-Cord-Injured Female Mice. Biology 2022, 11, 1617. https://doi.org/10.3390/biology11111617
Soler-Martínez R, Deulofeu M, Bagó-Mas A, Dubový P, Verdú E, Fiol N, Boadas-Vaello P. Central Neuropathic Pain Development Modulation Using Coffee Extract Major Polyphenolic Compounds in Spinal-Cord-Injured Female Mice. Biology. 2022; 11(11):1617. https://doi.org/10.3390/biology11111617
Chicago/Turabian StyleSoler-Martínez, Roger, Meritxell Deulofeu, Anna Bagó-Mas, Petr Dubový, Enrique Verdú, Núria Fiol, and Pere Boadas-Vaello. 2022. "Central Neuropathic Pain Development Modulation Using Coffee Extract Major Polyphenolic Compounds in Spinal-Cord-Injured Female Mice" Biology 11, no. 11: 1617. https://doi.org/10.3390/biology11111617
APA StyleSoler-Martínez, R., Deulofeu, M., Bagó-Mas, A., Dubový, P., Verdú, E., Fiol, N., & Boadas-Vaello, P. (2022). Central Neuropathic Pain Development Modulation Using Coffee Extract Major Polyphenolic Compounds in Spinal-Cord-Injured Female Mice. Biology, 11(11), 1617. https://doi.org/10.3390/biology11111617







