Mechanical Fractionation of Adipose Tissue—A Scoping Review of Procedures to Obtain Stromal Vascular Fraction
Abstract
:1. Introduction
2. Procedures
2.1. Filtration Procedures
2.2. Centrifugation Procedures
2.3. Procedures Using a Combination of Centrifugation and Filtration
2.4. Studies Describing Direct Comparisons between Procedures
3. Clinical Applications
3.1. Skin and Volume Enhancement
3.2. Wound Healing
3.3. Osteoarthritis
3.4. Other
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ghiasloo, M.; Lobato, R.C.; Díaz, J.M.; Singh, K.; Verpaele, A.; Tonnard, P. Expanding Clinical Indications of Mechanically Isolated Stromal Vascular Fraction: A Systematic Review. Aesthetic Surg. J. 2020, 40, NP546–NP560. [Google Scholar] [CrossRef] [PubMed]
- Van Dongen, J.A.; Van Boxtel, J.; Harmsen, M.C.; Stevens, H.P. The Development of Facial Lipofilling from a Historical Point of View. Facial Plast. Surg. 2019, 35, 358–367. [Google Scholar] [CrossRef]
- Zuk, P.A.; Zhu, M.; Mizuno, H.; Huang, J.; Futrell, J.W.; Katz, A.J.; Benhaim, P.; Lorenz, H.P.; Hedrick, M.H. Multilineage Cells from Human Adipose Tissue: Implications for Cell-Based Therapies. Tissue Eng. 2001, 7, 211–228. [Google Scholar] [CrossRef] [PubMed]
- Eto, H.; Suga, H.; Matsumoto, D.; Inoue, K.; Aoi, N.; Kato, H.; Araki, J.; Yoshimura, K. Characterization of Structure and Cellular Components of Aspirated and Excised Adipose Tissue. Plast. Reconstr. Surg. 2009, 124, 1087–1097. [Google Scholar] [CrossRef] [PubMed]
- van Dongen, J.A.; Tuin, A.J.; Spiekman, M.; Jansma, J.; van der Lei, B.; Harmsen, M.C. Comparison of Intraoperative Procedures for Isolation of Clinical Grade Stromal Vascular Fraction for Regenerative Purposes: A Systematic Review. J. Tissue Eng. Regen. Med. 2018, 12, e261–e274. [Google Scholar] [CrossRef]
- Guo, J.; Nguyen, A.; Banyard, D.A.; Fadavi, D.; Toranto, J.D.; Wirth, G.A.; Paydar, K.Z.; Evans, G.R.D.; Widgerow, A.D. Stromal Vascular Fraction: A Regenerative Reality? Part 2: Mechanisms of Regenerative Action. J. Plast. Reconstr. Aesthetic Surg. 2016, 69, 180–188. [Google Scholar] [CrossRef]
- Sun, M.; He, Y.; Zhou, T.; Zhang, P.; Gao, J.; Lu, F. Adipose Extracellular Matrix/Stromal Vascular Fraction Gel Secretes Angiogenic Factors and Enhances Skin Wound Healing in a Murine Model. Biomed Res. Int. 2017, 2017, 3105780. [Google Scholar] [CrossRef] [PubMed]
- Bowles, A.C.; Wise, R.M.; Gerstein, B.Y.; Thomas, R.C.; Ogelman, R.; Febbo, I.; Bunnell, B.A. Immunomodulatory Effects of Adipose Stromal Vascular Fraction Cells Promote Alternative Activation Macrophages to Repair Tissue Damage. Stem Cells 2017, 35, 2198–2207. [Google Scholar] [CrossRef]
- Bourin, P.; Bunnell, B.A.; Casteilla, L.; Dominici, M.; Katz, A.J.; March, K.L.; Redl, H.; Rubin, J.P.; Yoshimura, K.; Gimble, J.M. Stromal Cells from the Adipose Tissue-Derived Stromal Vascular Fraction and Culture Expanded Adipose Tissue-Derived Stromal/Stem Cells: A Joint Statement of the International Federation for Adipose Therapeutics and Science (IFATS) and the International So. Cytotherapy 2013, 15, 641–648. [Google Scholar] [CrossRef]
- van Dongen, J.A.; Getova, V.; Brouwer, L.A.; Liguori, G.R.; Sharma, P.K.; Stevens, H.P.; van der Lei, B.; Harmsen, M.C. Adipose Tissue-Derived Extracellular Matrix Hydrogels as a Release Platform for Secreted Paracrine Factors. J. Tissue Eng. Regen. Med. 2019, 13, 973–985. [Google Scholar] [CrossRef]
- Hynes, R.O. The Extracellular Matrix: Not Just Pretty Fibrils. Science 2009, 326, 1216–1219. [Google Scholar] [CrossRef] [PubMed]
- Chaput, B.; Bertheuil, N.; Escubes, M.; Grolleau, J.-L.; Garrido, I.; Laloze, J.; Espagnolle, N.; Casteilla, L.; Sensebé, L.; Varin, A. Mechanically Isolated Stromal Vascular Fraction Provides a Valid and Useful Collagenase-Free Alternative Technique: A Comparative Study. Plast. Reconstr. Surg. 2016, 138, 807–819. [Google Scholar] [CrossRef] [PubMed]
- Aronowitz, J.A.; Lockhart, R.A.; Hakakian, C.S.; Hicok, K.C. Clinical Safety of Stromal Vascular Fraction Separation at the Point of Care. Ann. Plast. Surg. 2015, 75, 666–671. [Google Scholar] [CrossRef]
- Aronowitz, J.A.; Lockhart, R.A.; Hakakian, C.S. Mechanical versus Enzymatic Isolation of Stromal Vascular Fraction Cells from Adipose Tissue. Springerplus 2015, 4, 713. [Google Scholar] [CrossRef] [PubMed]
- Tonnard, P.; Verpaele, A.; Peeters, G.; Hamdi, M.; Cornelissen, M.; Declercq, H. Nanofat Grafting: Basic Research and Clinical Applications. Plast. Reconstr. Surg. 2013, 132, 1017–1026. [Google Scholar] [CrossRef]
- van Dongen, J.A.; Stevens, H.P.; Parvizi, M.; van der Lei, B.; Harmsen, M.C. The Fractionation of Adipose Tissue Procedure to Obtain Stromal Vascular Fractions for Regenerative Purposes. Wound Repair Regen. 2016, 24, 994–1003. [Google Scholar] [CrossRef] [PubMed]
- Tiryaki, T.; Condé-Green, A.; Cohen, S.R.; Canikyan, S.; Kocak, P. A 3-Step Mechanical Digestion Method to Harvest Adipose-Derived Stromal Vascular Fraction. Plast. Reconstr. Surg.-Glob. Open 2020, 8, e2652. [Google Scholar] [CrossRef] [PubMed]
- Lo Furno, D.; Tamburino, S.; Mannino, G.; Gili, E.; Lombardo, G.; Tarico, M.S.; Vancheri, C.; Giuffrida, R.; Perrotta, R.E. Nanofat 2.0: Experimental Evidence for a Fat Grafting Rich in Mesenchymal Stem Cells. Physiol. Res. 2017, 66, 663–671. [Google Scholar] [CrossRef]
- Sesé, B.; Sanmartín, J.M.; Ortega, B.; Matas-Palau, A.; Llull, R. Nanofat Cell Aggregates: A Nearly Constitutive Stromal Cell Inoculum for Regenerative Site-Specific Therapies. Plast. Reconstr. Surg. 2019, 144, 1079–1088. [Google Scholar] [CrossRef]
- Cohen, S.R.; Tiryaki, T.; Womack, H.A.; Canikyan, S.; Schlaudraff, K.U.; Scheflan, M. Cellular Optimization of Nanofat: Comparison of Two Nanofat Processing Devices in Terms of Cell Count and Viability. Aesthetic Surg. J. Open Forum 2019, 1, ojz028. [Google Scholar] [CrossRef]
- Van Dongen, J.A.; Gostelie, O.F.E.; Vonk, L.A.; De Bruijn, J.J.; Van Der Lei, B.; Harmsen, M.C.; Stevens, H.P. Fractionation of Adipose Tissue Procedure with a Disposable One-Hole Fractionator. Aesthetic Surg. J. 2020, 40, NP194–NP201. [Google Scholar] [CrossRef] [PubMed]
- Mashiko, T.; Wu, S.H.; Feng, J.; Kanayama, K.; Kinoshita, K.; Sunaga, A.; Narushima, M.; Yoshimura, K.; van Dongen, J.A.; Stevens, H.P.; et al. Mechanical Micronization of Lipoaspirates: Squeeze and Emulsification Techniques. Plast. Reconstr. Surg. 2017, 139, 79–90. [Google Scholar] [CrossRef] [PubMed]
- Tiryaki, K.T.; Cohen, S.; Kocak, P.; Canikyan Turkay, S.; Hewett, S. In-Vitro Comparative Examination of the Effect of Stromal Vascular Fraction Isolated by Mechanical and Enzymatic Methods on Wound Healing. Aesthetic Surg. J. 2020, 40, 1232–1240. [Google Scholar] [CrossRef] [PubMed]
- Gentile, P.; Scioli, M.G.; Bielli, A.; Orlandi, A.; Cervelli, V. Comparing Different Nanofat Procedures on Scars: Role of the Stromal Vascular Fraction and Its Clinical Implications. Regen. Med. 2017, 12, 939–952. [Google Scholar] [CrossRef] [PubMed]
- Copcu, H.E.; Oztan, S. New Mechanical Fat Separation Technique: Adjustable Regenerative Adipose-Tissue Transfer (ARAT) and Mechanical Stromal Cell Transfer (MEST). Aesthetic Surg. J. Open Forum 2020, 2, ojaa035. [Google Scholar] [CrossRef]
- Yao, Y.; Dong, Z.; Liao, Y.; Zhang, P.; Ma, J.; Gao, J.; Lu, F. Adipose Extracellular Matrix/Stromal Vascular Fraction Gel: A Novel Adipose Tissue-Derived Injectable for Stem Cell Therapy. Plast. Reconstr. Surg. 2017, 139, 867–879. [Google Scholar] [CrossRef]
- An, R.; Li, S.; Sheng, L.; Cao, W. Emulsified Fat Grafting Accelerates Tissue Expansion: An Experimental Study in a Rat Model. Ann. Plast. Surg. 2020, 85, 61–67. [Google Scholar] [CrossRef]
- Wang, J.; Liao, Y.; Xia, J.; Wang, Z.; Mo, X.; Feng, J.; He, Y.; Chen, X.; Li, Y.; Lu, F.; et al. Mechanical Micronization of Lipoaspirates for the Treatment of Hypertrophic Scars. Stem Cell Res. Ther. 2019, 10, 42. [Google Scholar] [CrossRef]
- Li, Q.; Zhao, F.; Li, Z.; Duan, X.; Cheng, J.; Zhang, J.; Fu, X.; Zhang, J.; Shao, Z.; Guo, Q.; et al. Autologous Fractionated Adipose Tissue as a Natural Biomaterial and Novel One-Step Stem Cell Therapy for Repairing Articular Cartilage Defects. Front. Cell Dev. Biol. 2020, 8, 684. [Google Scholar] [CrossRef]
- Corvera, S. Cellular Heterogeneity in Adipose Tissues. Annu. Rev. Physiol. 2021, 83, 257–278. [Google Scholar] [CrossRef]
- Hirsch, J.; Batchelor, B. Adipose Tissue Cellularity in Human Obesity. Clin. Endocrinol. Metab. 1976, 5, 299–311. [Google Scholar] [CrossRef] [PubMed]
- Ramaut, L.; Moonen, L.; Laeremans, T.; Aerts, J.L.; Geeroms, M.; Hamdi, M. Push-Through Filtration of Emulsified Adipose Tissue Over a 500-Μm Mesh Significantly Reduces the Amount of Stromal Vascular Fraction and Mesenchymal Stem Cells. Aesthetic Surg. J. 2023, 43, NP696–NP703. [Google Scholar] [CrossRef] [PubMed]
- Cicione, C.; Di Taranto, G.; Barba, M.; Isgrò, M.A.; D’Alessio, A.; Cervelli, D.; Sciarretta, F.V.; Pelo, S.; Michetti, F.; Lattanzi, W. In Vitro Validation of a Closed Device Enabling the Purification of the Fluid Portion of Liposuction Aspirates. Plast. Reconstr. Surg. 2016, 137, 1157–1167. [Google Scholar] [CrossRef]
- van Dongen, J.A.; Harmsen, M.C.; Stevens, H.P. Isolation of Stromal Vascular Fraction by Fractionation of Adipose Tissue. Methods Mol. Biol. 2019, 1993, 91–103. [Google Scholar] [CrossRef] [PubMed]
- Osinga, R.; Menzi, N.R.; Tchang, L.A.H.; Caviezel, D.; Kalbermatten, D.F.; Martin, I.; Schaefer, D.J.; Scherberich, A.; Largo, R.D. Effects of Intersyringe Processing on Adipose Tissue and Its Cellular Components: Implications in Autologous Fat Grafting. Plast. Reconstr. Surg. 2015, 135, 1618–1628. [Google Scholar] [CrossRef] [PubMed]
- Qiu, H.; Jiang, Y.; Chen, C.; Wu, K.; Wang, H. The Effect of Different Diameters of Fat Converters on Adipose Tissue and Its Cellular Components: Selection for Preparation of Nanofat. Aesthetic Surg. J. 2021, 41, NP1734–NP1744. [Google Scholar] [CrossRef]
- Che, D.; Zhou, Y.; Wang, J.; Liu, Y.; Gao, F.; Lv, T.; Cui, C.; Xiao, Z. Experimental Study on the Influence of Different Aperture Connectors on Nanofat. J. Plast. Reconstr. Aesthetic Surg. 2022, 75, 3595–3602. [Google Scholar] [CrossRef]
- van Dongen, J.A.; Tuin, A.J.; Harmsen, M.C.; van der Lei, B.; Stevens, H.P. The Difference between Stromal Vascular Fraction Isolation and Fat Emulsification: A Crucial Role for Centrifugation. Plast. Reconstr. Surg. 2020, 145, 232e–233e. [Google Scholar] [CrossRef]
- Yang, Z.; Jin, S.; He, Y.; Zhang, X.; Han, X.; Li, F. Comparison of Microfat, Nanofat, and Extracellular Matrix/Stromal Vascular Fraction Gel for Skin Rejuvenation: Basic Research and Clinical Applications. Aesthetic Surg. J. 2021, 41, NP1557–NP1570. [Google Scholar] [CrossRef]
- Pallua, N.; Grasys, J.; Kim, B.S. Enhancement of Progenitor Cells by Two-Step Centrifugation of Emulsified Lipoaspirates. Plast. Reconstr. Surg. 2018, 142, 99–109. [Google Scholar] [CrossRef]
- Banyard, D.A.; Sarantopoulos, C.N.; Borovikova, A.A.; Qiu, X.; Wirth, G.A.; Paydar, K.Z.; Haun, J.B.; Evans, G.R.D.; Widgerow, A.D. Phenotypic Analysis of Stromal Vascular Fraction after Mechanical Shear Reveals Stress-Induced Progenitor Populations. Plast. Reconstr. Surg. 2016, 138, 237e–247e. [Google Scholar] [CrossRef]
- Bianchi, F.; Maioli, M.; Leonardi, E.; Olivi, E.; Pasquinelli, G.; Valente, S.; Mendez, A.J.; Ricordi, C.; Raffaini, M.; Tremolada, C.; et al. A New Nonenzymatic Method and Device to Obtain a Fat Tissue Derivative Highly Enriched in Pericyte-like Elements by Mild Mechanical Forces from Human Lipoaspirates. Cell Transplant. 2013, 22, 2063–2077. [Google Scholar] [CrossRef]
- Tarallo, M.; Fino, P.; Ribuffo, D.; Casella, D.; Toscani, M.; Spalvieri, C.; Lattanzi, W.; Di Taranto, G. Liposuction Aspirate Fluid Adipose-Derived Stem Cell Injection and Secondary Healing in Fingertip Injury: A Pilot Study. Plast. Reconstr. Surg. 2018, 142, 136–147. [Google Scholar] [CrossRef]
- Gentile, P.; Scioli, M.G.; Orlandi, A.; Cervelli, V. Breast Reconstruction with Enhanced Stromal Vascular Fraction Fat Grafting: What Is the Best Method? Plast. Reconstr. Surg.-Glob. Open 2015, 3, 237e–247e. [Google Scholar] [CrossRef]
- Van Dongen, J.A.; Boxtel, J.V.; Willemsen, J.C.; Brouwer, L.A.; Vermeulen, K.M.; Tuin, A.J.; Harmsen, M.C.; Van Der Lei, B.; Stevens, H.P. The Addition of Tissue Stromal Vascular Fraction to Platelet-Rich Plasma Supplemented Lipofilling Does Not Improve Facial Skin Quality: A Prospective Randomized Clinical Trial. Aesthetic Surg. J. 2021, 41, NP1000–NP1013. [Google Scholar] [CrossRef]
- Zhang, Y.; Zou, J.; Yuan, Y.; Gao, J.; Chen, X. Contouring and Augmentation of the Temple Using Stromal Vascular Fraction Gel Grafting. Front. Surg. 2022, 9, 893219. [Google Scholar] [CrossRef] [PubMed]
- Cai, J.; Wang, J.; Hu, W.; Lu, F. Mechanical Micronization of Lipoaspirates for the Treatment of Horizontal Neck Lines. Plast. Reconstr. Surg. 2020, 145, 345–353. [Google Scholar] [CrossRef]
- Wang, Q.; Chen, X.; Wang, X.; Du, Y.; Li, Y.; Bai, R.; Luo, S.; Hao, L. A Reliable Method for Chin Augmentation by Mechanical Micronization of Lipoaspirates. Aesthetic Plast. Surg. 2021, 45, 1507–1517. [Google Scholar] [CrossRef] [PubMed]
- Ding, F.; Shen, Y.; Lu, L.; Sun, D.; Luo, X.; Liang, X.; Yang, J.; Jin, R. Correction of Mild-to-Moderate Sunken Upper Eyelids of Asians with Stromal Vascular Fraction Gel. Ophthalmol. Ther. 2023, 12, 535–548. [Google Scholar] [CrossRef] [PubMed]
- Xia, X.; Sun, S.; Zou, J.; Li, L.; Chen, R.; Cotofana, S.; Moellhoff, N. Clinical Application of Stromal Vascular Fraction Gel in Temple Augmentation Using Deep Injection and Shallow Pave Filling. Aesthetic Plast. Surg. 2022, 46, 1893–1899. [Google Scholar] [CrossRef]
- Luo, S.; Zhang, X.; Dong, H.; Wen, C.; Hao, L. Correction of the Tear Trough Deformity and Concomitant Infraorbital Hollows with Extracellular Matrix/Stromal Vascular Fraction Gel. Dermatologic Surg. 2020, 46, E118–E125. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Hao, L.; Chen, X.; Bai, R.; Luo, S. An Efficacy Study of a New Radical Treatment for Acne Vulgaris Using Fat Injection. Aesthetic Surg. J. 2021, 41, NP1061–NP1072. [Google Scholar] [CrossRef]
- Cao, Z.; Li, H.; Wang, Z.H.; Liang, X.Q. High-Density Fat Grafting Assisted Stromal Vascular Fraction Gel in Facial Deformities. J. Craniofac. Surg. 2022, 33, 108–111. [Google Scholar] [CrossRef]
- Liang, Z.J.; Lu, X.; Li, D.Q.; Liang, Y.D.; Zhu, D.D.; Wu, F.X.; Yi, X.L.; He, N.; Huang, Y.Q.; Tang, C.; et al. Precise Intradermal Injection of Nanofat-Derived Stromal Cells Combined with Platelet-Rich Fibrin Improves the Efficacy of Facial Skin Rejuvenation. Cell Physiol Biochem 2018, 47, 316–329. [Google Scholar] [CrossRef] [PubMed]
- Wei, H.; Gu, S.X.; Liang, Y.D.; Liang, Z.J.; Chen, H.; Zhu, M.G.; Xu, F.T.; He, N.; Wei, X.J.; Li, H.M. Nanofat-Derived Stem Cells with Platelet-Rich Fibrin Improve Facial Contour Remodeling and Skin Rejuvenation after Autologous Structural Fat Transplantation. Oncotarget 2017, 8, 68542–68556. [Google Scholar] [CrossRef] [PubMed]
- Menkes, S.; Luca, M.; Soldati, G.; Polla, L. Subcutaneous Injections of Nanofat Adipose-Derived Stem Cell Grafting in Facial Rejuvenation. Plast. Reconstr. Surg.-Glob. Open 2020, 8, e2550. [Google Scholar] [CrossRef]
- Menkes, S.; Sidahmed-Mezi, M.; Meningaud, J.P.; Benadiba, L.; Magalon, G.; Hersant, B. Microfat and Nanofat Grafting in Genital Rejuvenation. Aesthetic Surg. J. 2021, 41, 1060–1067. [Google Scholar] [CrossRef]
- Uyulmaz, S.; Sanchez Macedo, N.; Rezaeian, F.; Giovanoli, P.; Lindenblatt, N. Nanofat Grafting for Scar Treatment and Skin Quality Improvement. Aesthet Surg J 2018, 38, 421–428. [Google Scholar] [CrossRef]
- Zhu, Y.; Yang, F.; Yang, Y.; Cheng, B.; Zuo, J. A Retrospective Study of SVF-Gel Compared With Nanofat Combined With High-Density Fat in the Treatment of Early Periorbital Aging. Ophthal. Plast. Reconstr. Surg. 2022, 38, 340–347. [Google Scholar] [CrossRef]
- Yao, Y.; Cai, J.; Zhang, P.; Liao, Y.; Yuan, Y.; Dong, Z.; Lu, F. Adipose Stromal Vascular Fraction Gel Grafting: A New Method for Tissue Volumization and Rejuvenation. Dermatol Surg 2018, 44, 1278–1286. [Google Scholar] [CrossRef]
- van Dongen, J.A.; van Boxtel, J.; Uguten, M.; Brouwer, L.A.; Vermeulen, K.M.; Melenhorst, W.B.; Niessen, F.B.; Harmsen, M.C.; Stevens, H.P.; van der Lei, B. Tissue Stromal Vascular Fraction Improves Early Scar Healing: A Prospective Randomized Multicenter Clinical Trial. Aesthetic Surg. J. 2022, 42, NP477–NP488. [Google Scholar] [CrossRef]
- Abouzaid, A.M.; El Mokadem, M.E.; Aboubakr, A.K.; Kassem, M.A.; Al Shora, A.K.; Solaiman, A. Effect of Autologous Fat Transfer in Acute Burn Wound Management: A Randomized Controlled Study. Burns 2022, 48, 1368–1385. [Google Scholar] [CrossRef]
- Gu, Z.C.; Li, Y.R.; Li, H. Use of Condensed Nanofat Combined With Fat Grafts to Treat Atrophic Scars. JAMA Facial Plast. Surg. 2018, 20, 128–135. [Google Scholar] [CrossRef]
- Bhooshan, L.S.; Geetha Devi, M.; Aniraj, R.; Binod, P.; Lekshmi, M. Autologous Emulsified Fat Injection for Rejuvenation of Scars: A Prospective Observational Study. Indian J. Plast. Surg. 2018, 51, 77–83. [Google Scholar] [CrossRef] [PubMed]
- Hung, M.J.; Tsai, C.P.; Ying, T.H.; Chen, G.D.; Su, H.L.; Tseng, C.J. Improved Symptoms and Signs of Refractory Interstitial Cystitis in Women after Intravesical Nanofat plus Platelet-Rich Plasma Grafting: A Pilot Study. J. Chin. Med. Assoc. 2022, 85, 730–735. [Google Scholar] [CrossRef]
- Rageh, M.A.; El-Khalawany, M.; Ibrahim, S.M.A. Autologous Nanofat Injection in Treatment of Scars: A Clinico-Histopathological Study. J. Cosmet. Dermatol. 2021, 20, 3198–3204. [Google Scholar] [CrossRef]
- Huang, R.; Jiao, H.; Fan, J.; Liu, L.; Tian, J.; Gan, C.; Yang, Z.; Zhang, T.; Zeng, Y.; Su, Z. Nanofat Injection for the Treatment of Depressed Facial Scars. Aesthetic Plast. Surg. 2021, 45, 1762–1771. [Google Scholar] [CrossRef]
- Cantarella, G.; Mazzola, R.F. Management of Vocal Fold Scars by Concurrent Nanofat and Microfat Grafting. J. Craniofac. Surg. 2019, 30, 692–695. [Google Scholar] [CrossRef] [PubMed]
- Tenna, S.; Cogliandro, A.; Barone, M.; Panasiti, V.; Tirindelli, M.; Nobile, C.; Persichetti, P. Comparative Study Using Autologous Fat Grafts Plus Platelet-Rich Plasma With or Without Fractional CO2 Laser Resurfacing in Treatment of Acne Scars: Analysis of Outcomes and Satisfaction With FACE-Q. Aesthetic Plast Surg 2017, 41, 661–666. [Google Scholar] [CrossRef] [PubMed]
- Deng, C.; Wang, L.; Feng, J.; Lu, F. Treatment of Human Chronic Wounds with Autologous Extracellular Matrix/Stromal Vascular Fraction Gel: A STROBE-Compliant Study. Medicine 2018, 97, e11667. [Google Scholar] [CrossRef]
- Stevens, H.P.; Donners, S.; De Bruijn, J. Introducing Platelet-Rich Stroma: Platelet-Rich Plasma (PRP) and Stromal Vascular Fraction (SVF) Combined for the Treatment of Androgenetic Alopecia. Aesthetic Surg. J. 2018, 38, 811–822. [Google Scholar] [CrossRef] [PubMed]
- Gutierrez-Ontalvilla, P.; Vidal, L.; Ruiz-Valls, A.; Iborra, M. Letter to the Editor on The Effect of Lipofilling and Platelet-Rich Plasma on Patients with Moderate–Severe Vulvar Lichen Sclerosus Who Were Non-Responders to Topical Clobetasol Propionate: A Randomized Pilot Study. Aesthetic Plast. Surg. 2023, 47, 66–68. [Google Scholar] [CrossRef] [PubMed]
- Sun, Z.; Huang, D.; Cai, M.; Wang, W.; Meng, W.; Zhang, Q.; He, S. Clinical Application of Stromal Vascular Fraction Gel in Unilateral Vocal Fold Paralysis. J. Voice 2021, 37, 800.e17–800.e22. [Google Scholar] [CrossRef] [PubMed]
- Akgul, Y.; Constantine, R.; Bartels, M.; Scherer, P.; Davis, K.; Kenkel, J.M. Utility of Adipocyte Fractions in Fat Grafting in an Athymic Rat Model. Aesthetic Surg. J. 2018, 38, 1363–1373. [Google Scholar] [CrossRef]
- Zhu, S.; Cai, J.; Wang, J.; Feng, J.; Lu, F. Protective Effect of Fat-Tissue-Derived Products against Ultraviolet Irradiation-Induced Photoaging in Mouse Skin. Plast. Reconstr. Surg. 2021, 148, 1290–1299. [Google Scholar] [CrossRef]
- Yu, Q.; Cai, Y.; Huang, H.; Wang, Z.; Xu, P.; Wang, X.; Zhang, L.; Zhang, W.; Li, W. Co-Transplantation of Nanofat Enhances Neovascularization and Fat Graft Survival in Nude Mice. Aesthetic Surg. J. 2018, 38, 667–675. [Google Scholar] [CrossRef]
- VinayKumar, D.M.; Mohsina, S.; Tripathy, S.; Sharma, R.K.; Bhatia, A. Histological Analysis of the Effect of Nanofat Grafting in Scar Rejuvenation. J. Cutan. Aesthet. Surg. 2022, 15, 147–153. [Google Scholar] [CrossRef]
- Xu, P.; Yu, Q.; Huang, H.; Zhang, W.J.; Li, W. Nanofat Increases Dermis Thickness and Neovascularization in Photoaged Nude Mouse Skin. Aesthetic Plast. Surg. 2018, 42, 343–351. [Google Scholar] [CrossRef]
- Liu Fang, Z.Y. Effect of Co-Transplanting Stromal Vascular Fraction-Gelatin and Platelet-Rich Fibrin on the Long-Term Maintenance of Fat Volume. Aesthetic Plast. Surg. 2022, 46, 2612–2613. [Google Scholar] [CrossRef]
- Zhang, P.; Feng, J.; Liao, Y.; Cai, J.; Zhou, T.; Sun, M.; Gao, J.; Gao, K. Ischemic Flap Survival Improvement by Composition-Selective Fat Grafting with Novel Adipose Tissue Derived Product-Stromal Vascular Fraction Gel. Biochem. Biophys. Res. Commun. 2018, 495, 2249–2256. [Google Scholar] [CrossRef]
- Chen, L.; Wang, Z.C.; Ma, J.J.; Sun, W.J.; Wang, S.W.; Gu, Z.C.; Yang, X. Autologous Nanofat Transplantation Accelerates Foot Wound Healing in Diabetic Rats. Regen. Med. 2019, 14, 231–241. [Google Scholar] [CrossRef] [PubMed]
- Ye, Y.; Zou, J.; Tan, M.; Hu, K.; Jiang, J. Phenotypic and Cellular Characteristics of a Stromal Vascular Fraction/Extracellular Matrix Gel Prepared Using Mechanical Shear Force on Human Fat. Front. Bioeng. Biotechnol. 2021, 9, 638415. [Google Scholar] [CrossRef] [PubMed]
- Weinzierl, A.; Harder, Y.; Schmauss, D.; Menger, M.D.; Laschke, M.W. Boosting Tissue Vascularization: Nanofat as a Potential Source of Functional Microvessel Segments. Front. Bioeng. Biotechnol. 2022, 10, 820835. [Google Scholar] [CrossRef]
- Li, Z.; Zhang, J.; Li, M.; Tang, L.; Liu, H. Concentrated Nanofat: A Modified Fat Extraction Promotes Hair Growth in Mice via the Stem Cells and Extracellular Matrix Components Interaction. Ann. Transl. Med. 2020, 8, 1184. [Google Scholar] [CrossRef] [PubMed]
- Albano, D.; Messina, C.; Usuelli, F.G.; De Girolamo, L.; Grassi, M.; Maccario, C.; Bignotti, B.; Tagliafico, A.; Sconfienza, L.M. Magnetic Resonance and Ultrasound in Achilles Tendinopathy: Predictive Role and Response Assessment to Platelet-Rich Plasma and Adipose-Derived Stromal Vascular Fraction Injection. Eur. J. Radiol. 2017, 95, 130–135. [Google Scholar] [CrossRef]
- Usuelli, F.G.; Grassi, M.; Maccario, C.; Vigano’, M.; Lanfranchi, L.; Alfieri Montrasio, U.; de Girolamo, L. Intratendinous Adipose-Derived Stromal Vascular Fraction (SVF) Injection Provides a Safe, Efficacious Treatment for Achilles Tendinopathy: Results of a Randomized Controlled Clinical Trial at a 6-Month Follow-Up. Knee Surg. Sport. Traumatol. Arthrosc. 2018, 26, 2000–2010. [Google Scholar] [CrossRef]
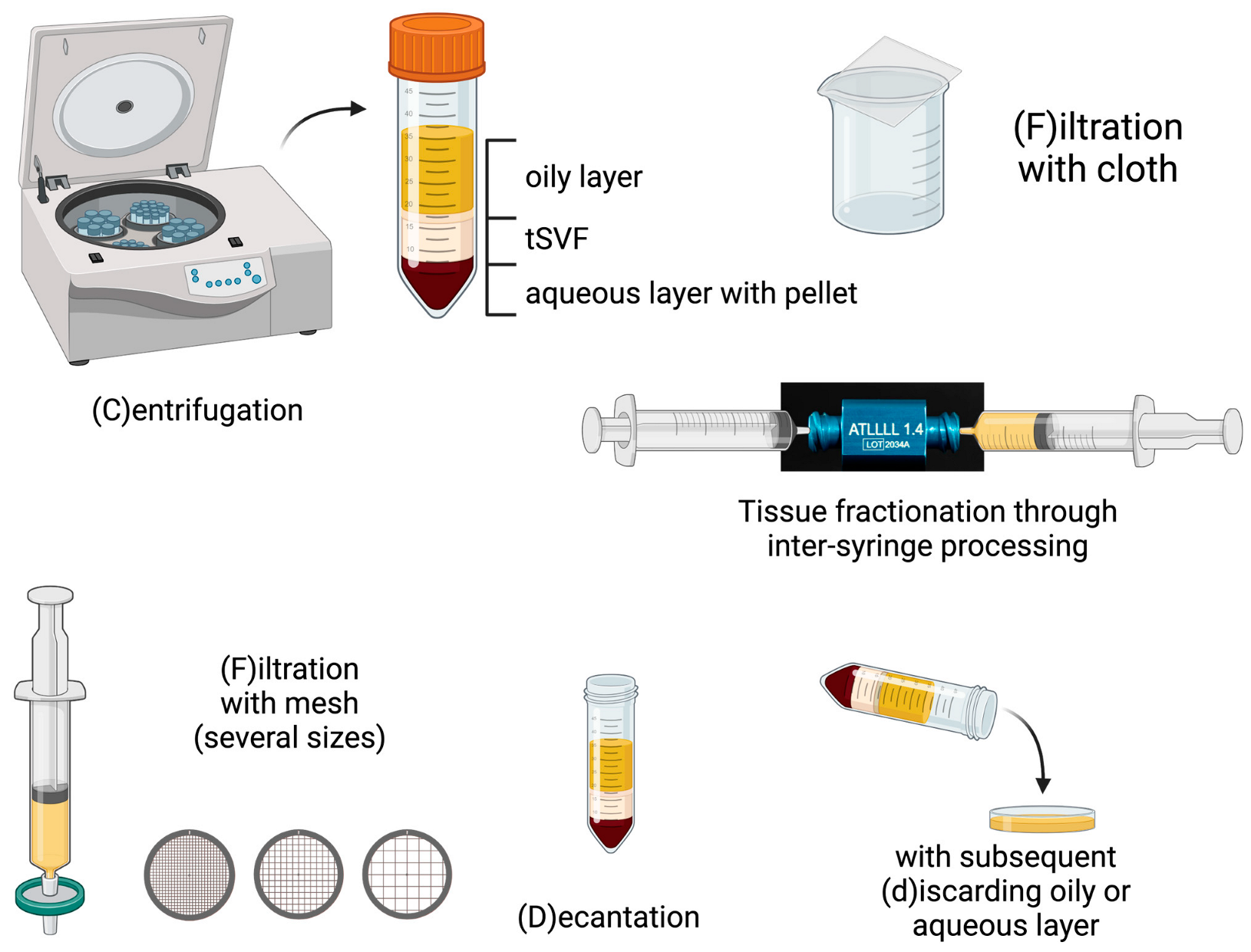 | |||
|---|---|---|---|
| Step 1 Tissue Separation | Step 2 Tissue Fractionation | Step 3 Tissue Separation | |
| Filtration | |||
| Nanofat [15] | F (cloth) | One-hole fractionator | F (cloth) |
| Nanofat 2.0 [18] | F (cloth) | One-hole fractionator | - |
| Nanofat cell aggregates [19] | F (mesh) | One-hole fractionator, 2.4 mm then 1.4 mm | F (mesh) |
| LipocubeNANO [20] | D + F | Undefined connector | F (mesh) |
| Centrifugation | |||
| FAT procedure [16] | C + d | Three-hole fractionator (1.4 mm) | C + d |
| FAT procedure 2.0 [21] | C + d | One-hole fractionator (1.4 mm) | C + d |
| Mechanical micronization: squeeze [22] | C + d | Slicer with spinning blade | C + d |
| Lipocube [17,23] | D | Through blade grid (1000–750–500 µm) | Add buffer + incubation C |
| Centrifuge-modified nanofat [24] | C + d | Fractionator | |
| Evo-modified nanofat [24] | C + d | Fractionator | |
| ARAT [25] | C + d | Mesh (4000–2400–1200–600–400–200–100 µm) | |
| MEST [25] | C + d | Mesh (4000–2400–1200–600–400–200–100 µm) | C |
| Centrifugation and filtration | |||
| SVF gel [26] | d + C + d + collect oil | One-hole fractionator (1.4 mm) | F (mesh) Add oil C + d |
| Mechanical micronization: Emulsification [22] | C + d | Three-hole fractionator (1.4 mm) | C + d F (mesh) |
| Supercharge-modified nanofat [24] | (1) F (mesh) + C | (2) fractionator | Add (1) + (2) |
| Step 1 Tissue Separation | Step 2 Tissue Fractionation | Step 3 Tissue Separation | Reduction in Volume (%) * | |
|---|---|---|---|---|
| Filtration | ||||
| Nanofat [15] | Filtering over nylon cloth 500 µm | 30× through one-hole (undefined size) connector between 10 cc syringes | Filtering over nylon cloth 500 µm | NR |
| Nanofat 2.0 [18] | Filtering over nylon cloth 500 µm | 30× through one-hole (undefined size) connector | - | NR |
| Nanofat cell aggregates [19,20] | Fluids expelled by manual pressure through filter device (Fat press, Tulip medical; undefined mesh size) | 1. 30× through one-hole 2.4 mm 2. 30× through one-hole 1.2 mm between 20 cc syringes | Filtering through 600 µm to 400 µm mesh screen | 15% |
| LipocubeNANO: [20] | Decantation 1× through undefined filter (port 1) | 10× between undefined holes (port 2 and 3) | 1× through a 500 µm filter (port 4) | NR |
| Centrifugation | ||||
| FAT procedure [16] | Centrifugation (3000 rpm; radius 9.5 cm; 2.5 min) Oil + liquid discarded | 30× through three-hole 1.4 mm | Centrifugation (3000 rpm; radius 9.5 cm; 2.5 min) Oil+liquid discarded | 90% [83–93%] |
| FAT procedure 2.0 [21] | Centrifugation (3000 rpm; radius 9.5 cm; 2.5 min) Oil + liquid discarded | 30× through one-hole 1.4 mm | Centrifugation (3000 rpm; radius 9.5 cm; 2.5 min) Oil + liquid discarded | 89 ± 4% |
| Mechanical micronization: squeeze [22] | Centrifugation (1200× g for 3 min) Oil + liquid discarded | Automated slicer with a spinning sharp blade | Centrifugation (1200× g for 3 min) Oil + liquid discarded | 52 ± 6% ** |
| Lipocube [17,23] | Decantation | 10× through blade grid 1000 µm holes 10× through blade grid 750 µm holes 10× through blade grid 500 µm holes between 20 cc metallic pistons | Add Ca-Mg balanced buffer solution (ratio 1:3) Incubation 10 min Centrifugation (2000× g for 10 min) Collect the pellet and resuspend | NR |
| Centrifuge-modified nanofat [24] | Centrifugation (1300 rpm for 10 min) and fat is collected (pellet is discarded) | 30× through Luer lock connector (undefined size) | - | NR |
| Evo-modified nanofat [24] | Centrifugation (80 RPM × 3 min) and fat is collected (pellet was discarded) | 30× through Luer lock connector (undefined size) | - | NR |
| ARAT [25] | Centrifugation (500× g for 2 min) Lower liquid layer was discarded | Shuffling 10 mL/s between 10 cc or 20 cc syringes with a blade mesh in between:
| - | NR |
| MEST [25] | Same as ARAT | Same as ARAT | Centrifugation (1200 g for 6 min) and bottom two layers are used (Stromal cell solution and stromal cell aggregate) | NR |
| Centrifugation and filtration | ||||
| SVF gel [26] | Liquid discarded Centrifugation (1200× g for 3 min) Liquid discarded Collect oil layer | 0.5–5 min through one-hole Luer lock connector 1.4 mm *** between syringes (undefined size) | Filtering over mesh filter 500-µm Add 0.5 mL of collected oil and mix by shifting 3–5× Centrifugation (2000× g for 3 min) Discard oil layer | 85–90% |
| Mechanical micronization: Emulsification [22] | Centrifugation (1200× g for 3 min) Oil+liquid discarded | 30× through three-hole 1.4 mm Luer lock connector between 2.5 cc syringes | Centrifugation (1200× g for 3 min) Oil discarded Filtering over mesh filter 500 μm | (tissue in the filter) 61 ± 5% ** (fluid after the filter) 90 ± 3% ** |
| Supercharge-modified nanofat [24] | Lipoaspirate divided in two parts Part 1 Automatic filtration (120 µm filter) Centrifugation (1300 rpm for 10 min) Pellet was collected | Part 2 30× through Luer lock connector between 10 cc syringes | Part 1 is then added to part 2 | NR |
| Other methods | ||||
| Emulsified fat by An et al. [27] | Washed with phosphate-buffered saline (PBS) Minced with scissors | 30× through three-hole 1.0mm connector between 2 cc syringes | NR | |
| tSVF gel by Wang et al. [28] | Washed with saline Mincing with scissors | 10 mL/s for 1 min between syringes (undefined size) | Centrifugation (2000× g for 3 min) Oil+liquid discarded | NR |
| ECM/tSVF gel by Li et al. [29] | Mincing with scissors | 90× through one-hole 2.0 mm connector between 10 cc syringes | Centrifugation (2000× g for 3 min) Oil+liquid discarded | NR |
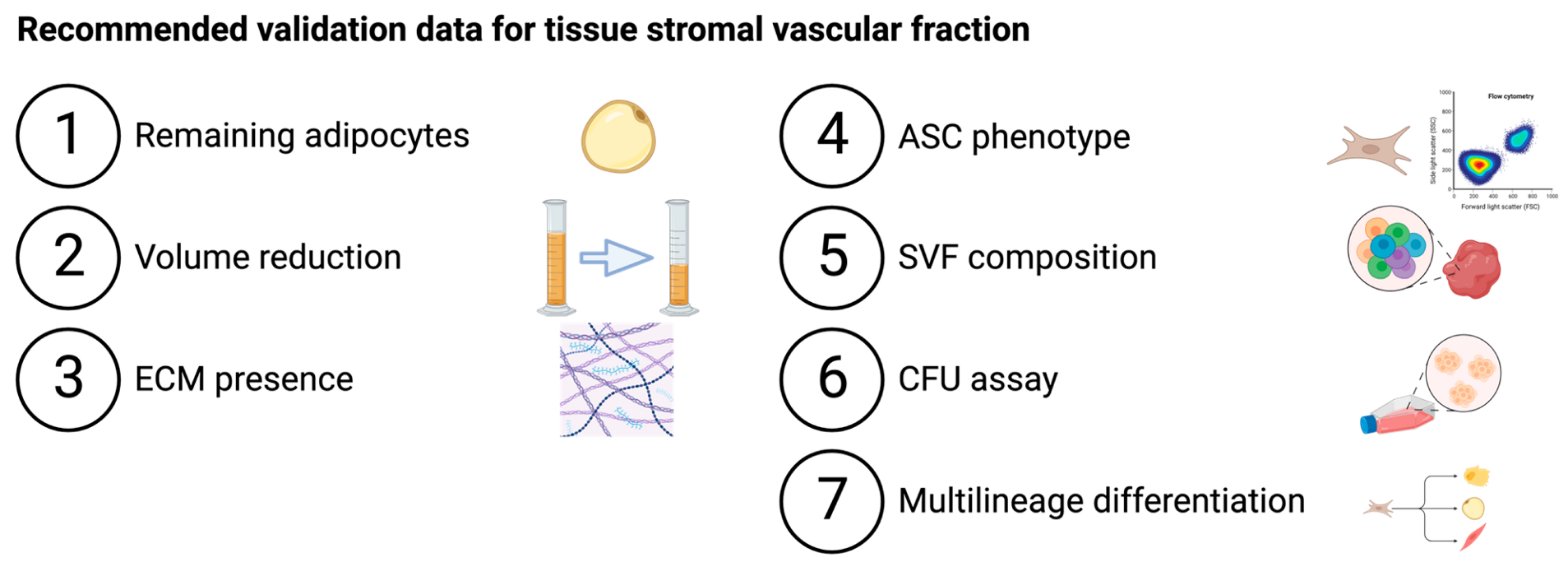 | |||||||
|---|---|---|---|---|---|---|---|
| Adipocytes Removal | ECM Presence | SVF Composition, Cultured ASC Phenotype and Characterization | |||||
| Adipocytes | Volume | ECM | ASC Phenotype | SVF Composition | CFU Assay | Multilineage Differentiation | |
| Filtration | |||||||
| Nanofat | - | - | - | ✓ | ✓ | - | Adipo+ |
| Nanofat 2.0 | - | - | - | ✓ | - | - | - |
| Nanofat cell aggregates | - | ✓ | - | - | - | - | Adipo+ |
| LipocubeNANO | - | - | - | - | ✓ | - | Adipo+ |
| Centrifugation | |||||||
| FAT procedure | Immunohistochemistry | ✓ | Histology | ✓ | ✓ * | ✓ * | Adipo+ Osteo+ SMC+ |
| FAT procedure 2.0 | Immunohistochemistry | ✓ | Histology | - | ✓ | ✓ | - |
| Mechanical micronization: squeeze | Confocal microscopy | ✓ | SEM | - | ✓ | - | - |
| Lipocube | - | - | - | ✓ | ✓ | ✓ | - |
| Centrifuge-modified nanofat | - | - | - | - | ✓ | - | - |
| Evo-modified nanofat | - | - | - | - | ✓ | - | - |
| ARAT | Histology | - | - | - | ✓ ** | ✓ ** | - |
| MEST | Histology | - | - | - | ✓ ** | ✓ ** | - |
| Centrifugation and filtration | |||||||
| SVF gel | Confocal microscopy | ✓ | SEM | - | ✓ ** | - | Adipo+ Osteo+ Chondro+ |
| Mechanical micronization: emulsification | Confocal microscopy | ✓ | SEM | - | ✓ | - | - |
| Supercharge-modified nanofat | - | - | - | - | ✓ | - | - |
| Histology/Immunohistochemistry | Viability | Other | |
|---|---|---|---|
| Filtration | |||
| Nanofat [15] | - | Fluorescence live/dead staining: no viable adipocytes are visible | - |
| Nanofat 2.0 [18] | Oil-red O staining: Showed marked damage at cellular level. | - | - |
| Nanofat cell aggregates [19,20] | - | - | - |
| LipocubeNANO: [20] | - | - | - |
| Centrifugation | |||
| FAT procedure [16] | Quantified (immuno)histochemistry: Alpha-SMA: 6.2% ± 5.5 vWF 7.0% ± 4.2 Masson’s Trichrome SVF more than control (no quantification) | Fluorescence live/dead staining: 100% | - |
| FAT procedure one hole [21] | Quantified immunohistochemistry: Alpha-SMA: 0.83% ± 0.33 Perilipin A: devoid of adipocytes More collagen than in control | - | - |
| Mechanical micronization: squeeze [22] | BODIPY/Lectin/Hoechst staining: Showed structural damage compared to normal fat and exhibited occasional aggregations of capillary fragments. Perilipin/Lectin/Hoechst staining: Irregular-sized/shaped adipocytes, and lipid droplets and fragmented capillaries. The irregular-sized/shaped adipocytes seemed to be damaged or dead, although they still expressed perilipin. | - | Scanning electron microscopy: Damaged adipocytes. |
| Lipocube [17,23] | - | - | - |
| Centrifuge-modified nanofat [24] | - | - | - |
| Evo-modified nanofat [24] | - | - | - |
| ARAT/MEST [25] | HE staining after 4000 micron, 2400 micron, 1200 micron, 600 micron, 400 micron and 200 micron adinizing: Intact adipocytes could be seen after all adinizing sessions. | - | Secretome after explant culture: (pg/mL): VEGF-A: 43.52 ± 12.21 EGF-A: 16.44 ± 2.67 FGF-2 8519.31 ± 3122.42 PDGF: 64.60 ± 21.43 NGF: 26.12 ± 14.78 TGFB1: 840.94 ± 115.77 Anti-inflammatory IL-10: 246.77 ± 116.86 IL-1ra: 417.21 ± 211.37 Inflammatory IFNg: 2.20 ± 1.85 IL-1b: 1221.44 ± 664.37 IL-6: 17,338.21 ± 3224.60 TNFa: 68.12 ± 21.44 |
| Centrifugation and filtration | |||
| SVF gel [26] | Confocal Microscopy using Hoechst(blue)/Lectin(red)/BODIPY(yellow): After mechanical process multiple lipid droplets. After centrifugation most of the free lipid drops were discarded leaving very few flat, fragmented adipocytes. Also, density of vessel-associated connective tissue increased. | - | Scanning electron microscopy: Level of fragmentation of ECM increased with the duration of processing time (p < 0.05 for 5 min processing). |
| Mechanical micronization: Emulsification [22] | BODIPY/Lectin/Hoechst staining: Showed structural damage compared to normal fat and exhibited occasional aggregations of capillary fragments. Perilipin/Lectin/Hoechst staining: Irregular-sized/shaped adipocytes, and lipid droplets and fragmented capillaries. The irregular-sized/shaped adipocytes seemed to be damaged or dead, although they still expressed perilipin. | - | Scanning electron microscopy: Filtrated fluid of emulsified fat showed more damaged adipocytes compared to centrifuged fat. Filtrated fluid of emulsified fat was filled with extracellular matrix fragments and contained few intact adipocytes. |
| Supercharge-modified nanofat [24] | - | - | - |
| Other methods | |||
| Emulsified fat by An et al. [27] | - | - | - |
| tSVF gel by Wang et al. [28] | - | - | - |
| ECM/tSVF gel by Li et al. [29] | - | - | - |
| Viability | Cell Number | Culture/Differentiation | Colony Formation Assay | ASC Phenotype | SVF Composition | Other | |
|---|---|---|---|---|---|---|---|
| Filtration | |||||||
| Nanofat [15] | Fluorescence microscopy: Only very few dead cells. | 1.975 × 106/100 mL lipoaspirate CD34+ 1 × 105/100 mL | Adipogenic+ | - | CD44 96.32% ± 1.32 CD90 67.38% ± 2.45 CD105 82.65% ± 2.07 CD14 6.67% ± 1.99 CD34 20.01% ± 1.63 CD45 13.68% ± 2.09 * | CD34+ 0.1 to 0.2 × 106 cells/100 mL lipoaspirate (4.5–6.5%) | - |
| Nanofat 2.0 [18] | - | - | - | - | CD44 98.88% ± 0.71 CD90 65.58% ± 2.95 CD105 75.83% ± 2.88 CD14 5.45 ± 1.87 CD34 14.86% ± 2.09 CD45 3.45% ± 2.72 | - | MTT assay: No difference between microfat/nanofat/nanofat 2.0 |
| Nanofat cell aggregates [19,20] | Image cytometry: 76.80% - Muse flow cytometer: 96.05% | DNA quantification: 7.3 million cells/g Cell yield: 6.63 million cells/g lipoaspirate Muse flow cytometer: 1.44 × 106/cc | Adipogenic+ | - | - | - | Gene expression profiles (mRNA levels): PPAR2 7.1-fold higher than NanoTransfer Adiponectin 1.7-fold higher than NanoTransfer |
| LipocubeNANO: [20] | Muse flow cytometer: 96.75% | Muse flow cytometer: 2.24 × 106/mL | Adipogenic+ | - | - | CD45− CD90+ 7.92% CD73+, CD90+ 37.29% CD45− CD31+ 11.99% CD45+ CD14+ 2.43% CD13+ 42.04% CD73+ 53.5% (extracted from figure) CD90+ 55.82% CD146+ 53.2% CD34+ 18.84% | - |
| Centrifugation | |||||||
| FAT procedure [16] | - | Bürker Turk counting chamber: 2.7 × 106 ± 1.1 cells/mL | Adipogenic+ Osteogenic+ Smooth muscle cell+ | 1.29% ± 0.045 * | CD90 CD31− CD45− 99.8% ± 0.2; CD29 99.8% ± 0.2 CD44 99.0% ± 0.7 CD105 95.9% ± 4.5 | CD45− CD90+ CD105+ 41.4% ± 16.5% CD31+ CD34+ 12.0% ± 4.5% CD45+ CD34− 5.3% ± 3.6% CD34+/− CD31−CD146+ 0.3% ± 0.3% CD45+ CD34+ 0.1 ± 0.2% CD34 bright CD31− CD146− uncountably low * | - |
| FAT procedure one hole [21] | - | Bürker Turk counting chamber: 2.35 × 106 ± 0.30 | - | 1.29% ± 0.038 | - | CD45− CD90+ CD105+ 44.9% ± 18.2% CD31+ CD34+ 19.1% ± 2.3% CD45+ CD34− 5.3% ± 3.6% CD34+/− CD31− CD146+ 0.5% ± 0.5% CD45+ CD34+ 0.2% ± 0.3% CD34 bright CD31− CD146− uncountably low | - |
| Mechanical micronization: squeeze [22] | Nucleocounter NC-100: 89.9 ± 4.6 percent | - | Culture: After 1 week normalized number of cultured adipose-derived stromal cells 1.1 ± 0.1 × 106 | - | - | Number of cells/1mL of fat CD45− CD31− CD34+ 1.5 × 105 CD45− CD31+ CD34− 1.1 × 105 | Mass fraction: 75% ECM 25% adipocytes |
| Lipocube [17,23] | 85.82% ± 5.74% 97.55% ** | 1.34 × 106/mL ±1.69 0.94 × 106 ** | - | Cell proliferation test: On day 7, more cluster formation in mechanically digested SVF. A490 value after 72 h 201 ± 0.1 (p ≤ 0.05) | CD90 11.39% CD44 21.45% CD105 9.0% CD73 6.16% | CD45− CD73+ 42.37% CD90+ CD73+ 52.08% CD45− CD31+ 21.06% CD45+ CD14+ 7.28% | Gene expression analysis: PPAR2/adiponectin 1.43- and 1.32-fold higher in mechanically digested SVF; Col1A PCR test: 12.5 [1.2] × 106; p ≤ 0.05, (1.5-fold higher compared to enzymatically digested SVF) |
| Centrifuge-modified nanofat [24] | - | Hemocytometer: 53,334 ± 8000 nucleated cells/mL | - | - | - | CD44 65.6% ± 9.1% CD90 59.7% ± 6.9% CD73 27.2% ± 4.8% CD34 55.3% ± 7.5% CD31 21.5% ± 5.1% CD146 19.7% ± 3.9% | - |
| Evo-modified nanofat [24] | - | Hemocytometer: 125,000 ± 12,000 nucleated cells/mL | - | - | - | CD44 68.3% ± 7.8% CD90 56.9% ± 6.1% CD73 32.3% ± 5.5% CD34 50.4% ± 5.2% CD31 17.8% ± 4.8% CD146 20.7% ± 5.1% | - |
| ARAT/MEST [25] | Flow cytometry: IP 1 94% ± 2 IP 2 93% ± 2 IP 3 93% ± 2 IP 4 91% ± 4 *** | Flow cytometry: IP 1 0.91 106/mL IP 2 0.88 106/mL IP 3 1.61 106/mL IP 4 1.47 106/mL *** | - | CFU-F assay: Results not reported | - | Quantification not reported | - |
| Centrifugation and filtration | |||||||
| SVF gel [26] | - | 0.5-min SVF gel: 2.7 ± 0.3 × 105 cells/mL 1 min SVF gel: 4.1 ± 0.3 × 105 cells/ml | Adipogenic+ Osteogenic+ Chondrogenic+ | - | - | Quantification not reported, only figure. | - |
| Mechanical micronization: emulsification [22] | Nucleocounter NC-100: residual tissue 90.6% ± 2.8%, filtrated fluid 39.3% ± 9.1% | - | After 1 week of culture, number of cells in residual tissue of emulsified fat (5.1 ± 0.7 × 105) | - | - | Number of cells/1 mL of fat CD45− CD31− CD34+ 1.4 × 105 CD45− CD31+ CD34− 0.8 × 105 | - |
| Supercharge-modified nanofat [24] | - | Hemocytometer: 200,000 ± 15,000 nucleated cells/mL | - | - | - | CD44 71.2% ± 8.0% CD90 62.8% ± 7.2% CD73 30.1% ± 5.4% CD34 58.1% ± 6.3% CD31 19.9% ± 4.4% CD146 22.1% ± 4.5% | - |
| Other methods | |||||||
| Emulsified fat by An et al. [27] | Trypan blue staining: 58.2% | Nucleocounter NC-100: 4.53 × 106 | - | - | - | CD45− CD34+ 12.40% ± 0.86% | - |
| tSVF gel by Wang et al. [28] | 7-AAD staining: 80% | - | - | - | - | CD34+ CD31− CD45− 64% CD34+ CD31+ CD45− 28% | - |
| ECM/tSVF gel by Li et al. [29] | - | - | Chondrogenic+ Osteogenic+ Adipogenic+ | - | CD29+ 97.3% CD90+ 98.7% CD105+ 99.7% CD34+ 1.3% CD45+ 1.2% | - | - |
| Study | Year | Study Design | N | Max. FU (m) | Categorized Method % | Additional Product | Clinical Endpoints | Clinical Results * (Index vs. Control) | Overall Result | |
|---|---|---|---|---|---|---|---|---|---|---|
| Skin and volume enhancement | ||||||||||
| 1 | van Dongen et al. (2021) [45] | 2016–2019 | RCT | 28 | 12 | FAT procedure | PRP + tSVF vs. PRP + saline | VISIA, FACE-Q, complications | No superior result in skin quality or satisfaction. No major complications | +/− |
| 2 | Zhang et al. (2022) [46] | 2018–2020 | RCT | 63 (34 vs. 29) | 12 | FAT procedure % | tSVF vs. Coleman’s | Volume ratio on 3D imaging, retention rate, satisfaction 5p Likert | Increased contour ratio 0.87 ± 0.02 to 0.89 ± 0.03 *, higher retention * 41.2 ± 8.4% vs. 32.6 ± 8.8%, higher satisfaction (4/5p) 79% vs. 62% * | + |
| 3 | Cai et al. (2019) [47] | ? | P | 50 (28 vs. 22) | 12 | FAT procedure % (with 2.4 mm fractionator) | tSVF vs. BTXa | Global Aesthetic Improvement Scale (GAIS), patient satisfaction, histological analysis (n = 1) | Higher GIAS and satisfaction in high-grade wrinkles group *, increased collagen density | + |
| 4 | Wang et al. (2021) [48] | 2017–2019 | P | 18 (6 vs. 12) | 12 | FAT procedure % | tSVF vs. PBS | Ultrasonogram, volume measurement | Similar elasticity, increased volume of 2.3 ± 0.3 mL | +/− |
| 5 | Ding et al. (2023) [49] | 2020–2021 | P | 31 | 15 | FAT procedure % | tSVF | Survival, volume, GAIS Reoperation rate | 65.3% ± 6.1 2.2 ± 0.8 12.9% | +/− |
| 6 | Xia et al. (2022) [50] | 2017–2021 | P | 33 (66 temples) | 6 | FAT procedure % | tSVF | Hollowness Severity Rating Scale, satisfaction 3p Likert | 91% absent hollowness, 94% satisfied | + |
| 7 | Luo et al. (2020) [51] | 2017–2018 | P | 33 (66 eyes) | 13 | FAT procedure % | tSVF | GAIS, depth measurement, retention rate | 2.5 [0.5] Improvement in all depth measurements 73 ± 10% | + |
| 8 | Zhao et al. (2021) [52] | 2018 | P | 18 | 12 | FAT procedure, 1.4 mm % | tSVF | Number of inflammatory lesions, Investors Global Assessment scale, biopsies at 1-month FU | Decrease in lesions, 7.3(2.7) vs. 0.7(0.7) *, 2.5(0.5) vs. 0.6(0.5) *, decrease in CD4+ T cells after 4 weeks | + |
| 9 | Cao et al. (2022) [53] | 2017–2019 | P | 13 | 10 | FAT procedure % | tSVF | Satisfaction | Improvement 84 ± 3 vs. 31 ± 3 * | + |
| 10 | Liang et al. (2018) [54] | 2014–2016 | P | 231 (103 vs. 128) | 24 | Nanofat | tSVF + PRF vs. hyaluronic acid | VISIA, SOFT5.5, satisfaction | Facial skin texture improved in both groups *, higher satisfaction rate | + |
| 11 | Wei et al. (2017) [55] | 2014–2016 | P | 139 (62 vs 77) | 24 | Nanofat | tSVF + PRF vs. Coleman’s procedure | VISIA, SOFT5.5, satisfaction | Skin quality improvement *, higher satisfaction 90% vs. 70% * | + |
| 12 | Menkes et al. (2020) [56] | 2018 | P | 50 | 6 | Nanofat | Nanofat + PRP | Satisfaction (10p Likert), biopsies | Improvement in texture, elasticity, glow Increase in collagen and elastic fibers *, higher cellularity and vascular density * | + |
| 13 | Menkes et al. (2021) [57] | 2017–2018 | P | 50 | 18 | Nanofat | Nanofat + PRP | Vaginal health index, Female Sexual Distress Scale Revised | Improvement in VHI, 9.2 ± 1.7 vs. 3.4 ± 1.5 *, FSDS-R, 3.4±3.7 vs. 32.9±9.5* | + |
| 14 | Uyulmaz et al. (2018) [58] | 2013–2016 | R | 52 | 5 | Nanofat | - | Photographs, satisfaction (yes/no) | Improvement in skin appearance 93%, satisfactory result 18% rater vs. 92% patient | + |
| 15 | Zhu et al. (2022) [59] | 2016–2020 | R | 103 (58 vs. 48) | 9 | FAT procedure % | tSVF (FAT %) vs. tSVF (nanofat) | Clinical data, satisfaction | Comparable improvement Less reoperations in FAT-treated patients*, higher satisfaction in FAT-treated patients * | + |
| 16 | Yao Yao et al. (2018) [60] | 2015–2017 | R | 204 (126 vs. 78) | 11 | tSVF gel | tSVF gel vs. Coleman’s procedure | Photo analysis, satisfaction (5p Likert scale), histological analysis (n = 1) | Higher Likert tSVF *, lower rate of 2nd surgery tSVF 10.9% (11/101) vs. 32.1% (25/78) * No cysts, fibrosis or calcification | + |
| Wound healing | ||||||||||
| 17 | van Dongen et al. (2022) [61] | 2016–2020 | RCT | 40 (20 vs. 20) | 12 | FAT procedure | tSVF vs. saline | Histological biopsies, POSAS, photographs, blinded analysis with VAS | Equal collagen alignment, depth, width, POSAS patient 14.4 ± 7.6 vs. 15.3 ± 9.0, observer 14.5 ± 6.4 vs. 14.6 ± 8.8, no differences in VAS scores, low agreement | +/− |
| 18 | Abouzaid et al. (2022) [62] | 2019–2020 | RCT | 100 (50 vs. 50) | 3 | Centrifuged-modified nanofat % | Coleman’s fat + tSVF vs. conventional dressings | Photo + clinical analysis, biopsies | Decrease in hospital stay and reoperation *, less contractures *, rapid collagen deposition * | + |
| 19 | Gu et al. (2018) [63] | 2014–2016 | P | 20 | 6 | FAT procedure % | - | POSAS and photographs, biopsies | Patient total 28.8 (1.0) vs. 12.2 (0.8) *, observer total 18.0 (0.7) vs. 9.2 (0.4) *, melanin AOD basal cell layer 0.8 (0.1) vs. 0.7 (0.1), no difference in elastic fibers | + |
| 20 | Bhooshan et al. (2019) [64] | 2015–2016 | P | 34 | 3 | Nanofat (without first filtration step) | - | POSAS and photographs, aesthetic result (total POSAS score) | Patient total 14 ± 4.4 vs. 27.4 ± 7.5 *, observer total 18 ± 6.8 vs. 31 ± 8.5 *, 77% good outcome | + |
| 21 | Hung et al. (2022) [65] | 2019 | P | 6 | 6 | Nanofat | Nanofat + PRP | Pain-VAS, PROM, cystoscopy | Improvement in PROMs *, 100% remission of lesions | + |
| 22 | Rageh et al. (2021) [66] | ? | P | 30 | 6 | Nanofat | Vancouver scar scale, biopsies | Lower VSS scores in height and pliability *, improved epidermal thickness *, increased collagen (52%) and elastic fibers (50%) * | + | |
| 23 | Huang et al. (2021) [67] | 2017–2020 | R | 44 | 12 | Nanofat | - | FACE-Q, assessment of photographs | Overall satisfied, 30% complete healing, 41% obvious improvement, 9% no effect | +/− |
| 24 | Cantarella et al. (2019) [68] | ? | Pi | 7 | 6 | Centrifuged- modified nanofat % | - | Videolaryngo- stroboscopy, max phonation time, VHI, EAT10 | Improvement in glottic closure, longer phonation *, reduction in VHI *, improved swallowing | + |
| 25 | Tenna et al. (2017) [69] | 2014–2015 | P | 30 (15 vs. 15) | 12 | Centrifuged- modified nanofat % | tSVF + PRP vs. tSVF + PRP + CO2 laser | Ultrasound, FACE-Q | Improvement in subcutaneous tissue of 0.67 vs. 0.63 cm, comparable FACE-Q | + |
| 26 | Deng et al. (2018) [70] | 2016–2017 | P | 20 (10 vs. 10) | 0.5 | SVF gel | tSVF vs. control | Wound healing rate, biopsies | 35 ± 11% vs. 10 ± 3% *, decreased lymphocyte infiltration, more * and thicker collagen deposition, more new vessels * | + |
| Other indications | ||||||||||
| 27 | Stevens et al. (2018) [71] | 2016–2016 | P | 10 | 3 | FAT procedure | tSVF + PRP | Hair density | 30.7 hairs/cm2 (range 5–59), regrowth observed | + |
| 28 | Gutierrez et al. (2022) [72] | 2017–2019 | Pi | 19 (9 vs. 10) | 12 | SVF gel | tSVF + PRP vs steroid injection | Skin elasticity, VAS, QoL (Skindex-29), biopsies | No elasticity improvement. Improvement in symptoms, but not in pain *. Improvement in QoL *, 79.7 ± 33.2 to 59.7 ± 24.9. Decrease in all inflammatory cells *. | + |
| 29 | Sun et al. (2021) [73] | 2017–2018 | P | 22 | 18 | SVF gel | tSVF | Glottis closure, GRBAS voice quality | Improvement in vocal cord shape and closure, improvement in 19/22 patients * | + |
| Study | Year | Study Design | N | Max. FU (m) | Categorized Method % | Additional Product | Clinical Endpoints | Clinical Results * | Overall Result | |
|---|---|---|---|---|---|---|---|---|---|---|
| Skin and volume enhancement | ||||||||||
| 1 | Akgul et al. (2018) [74] | 2013–2015 | P | 14 rats/6 XG | 1.5 | FAT procedure % | tSVF enriched with adipocyte fragments vs. controls | Histological biopsies | Viable adipocyte architecture, collagen accumulation, CD68 + CD44+ | + |
| 2 | Zhu et al. (2021) [75] | NR | P | 60 mice (15 vs. 45/17 XG) | 3 | FAT procedure % | tSVF vs. control | Histological biopsies | Dermal thickness * 184.4 ± 2.8, higher collagen deposition, increased TGF-b1 and Smad 2 expression *, lower MMP2/9 *, more fibroblasts | + |
| 3 | Yu et al. (2018) [76] | 2017 | P | 30 (20 vs. 10) mice/5 XG | 3 | Nanofat | tSVF vs. control | Histological biopsies, integrity, cysts/vacuoles, fibrosis, inflammation, capillary density (CD3+ vessels) | Better survival and morphological integrity, 3.6 ± 0.5 vs. 2.7 ± 0.9 *, 2.6 ± 0.7 vs. 3.2 ± 0.8 *, 2.1 ± 0.6 vs. 2.9 ± 0.8 *, 2.1 ± 0.6 vs. 2.6 ± 0.5 *, 24.6 ± 4.7 vs. 10.4 ± 2.9 * | + |
| 4 | An et al. (2020) [27] | NR | P | 24 rats (16 vs. 8) | 1 | Other % | tSVF vs. control | Histological biopsy, collagen AOD, anti-PCNA (cell proliferation), anti-CD31 (vascularization degree) | Higher AOD in 1mL SVF application *, 102 ± 12 vs. 55 ± 8 *, 95 ± 4.3 vs. 63 ± 2.7/mm2 * | + |
| 5 | VinayKumar et al. (2022) [77] | 2018 | P | 9 guinea-pigs (9 vs. 9) | 6 | Nanofat | tSVF vs. control | Histological biopsies, polarized light microscopy | Similar inflammatory infiltrate and collagen fiber orientation, increase in collagen distribution * | +/− |
| 6 | Xu et al. (2018) [78] | NR | P | 18 mice (6 vs. 12, 10 XG) | 2 | Nanofat | tSVF vs control and enzymatic tSVF | Histological biopsies | Increased dermal thickness *, higher capillary density and epidermal proliferation index *; high VEGF, EFG, bFGF, IGF and IL-6 * | + |
| 7 | Liu et al. (2021) [79] | NR | P | 12 rabbits | 1.5 | SVF gel | tSVF and PRF vs. tSVF | Histological biopsies | Slightly more volume combined with PRF, larger adipocytes and more ordered fibroblast distribution | +/− |
| Wound healing | ||||||||||
| 8 | Zhang et al. (2017) [80] | NR | P | 10 mice /NR XG | 0.5 | FAT procedure % | tSVF vs. control | Photographs, necrosis rate, histological biopsies | Thicker fatty layer, 22.1 ± 0.1 vs. 53.8 ± 0.1% *, 7/10× more VEGF and bFGF *, more human-derived vessels *, 43% more CD31+ vasculature * | + |
| 9 | Chen et al. (2019) [81] | NR | P | 10 rats | 0.5 | FAT procedure % | tSVF vs. control | Wound healing, biopsies | Faster and complete wound healing *, more vesicular structures and inflammatory cells, higher capillary density, MCP-1 and VEGF * | + |
| 10 | Sun et al. (2017) [7] | NR | P | 54 (18 vs. 36) | 0.5 | FAT procedure % | tSVF vs. control | Wound healing, capillary density, inflammatory reaction | Complete healing at 14 days FU, more vascularization *, sharp increase and later decrease in inflammatory cells | + |
| 11 | Yao Yao et al. (2016) [26] | NR | P | 52 mice/17 XG | 0.5 | SVF gel | tSVF vs. control | Photographs | Wound healing and closure | + |
| 12 | Wang et al. (2019) [28] | NR | P | 15 rabbits | 3 | Other % | tSVF gel | Size, color, texture, dermal thickness, histological biopsies | Improvement, 0.5 ± 0.3 vs. 1.4 ± 0.3 mm *, CD206+ macrophages dermal layer, lower IL-6 and MCP-1 *, lower collagen density *, less alpha-SMA, myofibroblasts and COL-1 * | + |
| Osteoarthritis | ||||||||||
| 13 | Li et al. (2020) [29] | 2019 | P | 30 rabbits | 3 | Other % | tSVF vs. control | Radiology (MRI), histology, immunohistochemistry, total histological outcome score, ICRS | Cartilage repair, Filled defect, strong glycosaminoglycan staining, COL-II up, COL-I down, 10.2 ± 0.8 vs. 8.4 ± 1.1 *, 9.8 ± 1.3 vs. 7.4 ± 1.1 * | + |
| Other indications | ||||||||||
| 14 | Ye et al. (2021) [82] | NR | P | 50 (25 vs. 25)/7 XG | 2 | Nanofat | tSVF vs. Coleman’s | Histological biopsies | Perilipin + cell density * | + |
| 15 | Weinzierl et al. (2022) [83] | NR | P | 16 mice | 0.5 | Nanofat | Histological biopsies | High functional microvessel density | + | |
| 16 | Li et al. (2020) [84] | NR | P | 6 mice (6 XG) | 0.5 | FAT procedure % | tSVF vs. decellularized tSVF | Hair growth Biopsies | Increased hair growth, increased proliferation, migration, cell cycle progression | + |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schipper, J.A.M.; van Laarhoven, C.J.H.C.M.; Schepers, R.H.; Tuin, A.J.; Harmsen, M.C.; Spijkervet, F.K.L.; Jansma, J.; van Dongen, J.A. Mechanical Fractionation of Adipose Tissue—A Scoping Review of Procedures to Obtain Stromal Vascular Fraction. Bioengineering 2023, 10, 1175. https://doi.org/10.3390/bioengineering10101175
Schipper JAM, van Laarhoven CJHCM, Schepers RH, Tuin AJ, Harmsen MC, Spijkervet FKL, Jansma J, van Dongen JA. Mechanical Fractionation of Adipose Tissue—A Scoping Review of Procedures to Obtain Stromal Vascular Fraction. Bioengineering. 2023; 10(10):1175. https://doi.org/10.3390/bioengineering10101175
Chicago/Turabian StyleSchipper, Jan Aart M., Constance J. H. C. M. van Laarhoven, Rutger H. Schepers, A. Jorien Tuin, Marco C. Harmsen, Fred K. L. Spijkervet, Johan Jansma, and Joris A. van Dongen. 2023. "Mechanical Fractionation of Adipose Tissue—A Scoping Review of Procedures to Obtain Stromal Vascular Fraction" Bioengineering 10, no. 10: 1175. https://doi.org/10.3390/bioengineering10101175
APA StyleSchipper, J. A. M., van Laarhoven, C. J. H. C. M., Schepers, R. H., Tuin, A. J., Harmsen, M. C., Spijkervet, F. K. L., Jansma, J., & van Dongen, J. A. (2023). Mechanical Fractionation of Adipose Tissue—A Scoping Review of Procedures to Obtain Stromal Vascular Fraction. Bioengineering, 10(10), 1175. https://doi.org/10.3390/bioengineering10101175







