Abstract
The use of thermal and non-thermal atmospheric pressure plasma to solve problems related to agriculture and biomedicine is the focus of this paper. Plasma in thermal equilibrium is used where heat is required. In agriculture, it is used to treat soil and land contaminated by the products of biomass, plastics, post-hospital and pharmaceutical waste combustion, and also by ecological phenomena that have recently been observed, such as droughts, floods and storms, leading to environmental pollution. In biomedical applications, thermal plasma is used in so-called indirect living tissue treatment. The sources of thermal plasma are arcs, plasma torches and microwave plasma reactors. In turn, atmospheric pressure cold (non-thermal) plasma is applied in agriculture and biomedicine where heat adversely affects technological processes. The thermodynamic imbalance of cold plasma makes it suitable for organic syntheses due its low power requirements and the possibility of conducting chemical reactions in gas at relatively low and close to ambient temperatures. It is also suitable in the treatment of living tissues and sterilisation of medical instruments made of materials that are non-resistant to high temperatures. Non-thermal and non-equilibrium discharges at atmospheric pressure that include dielectric barrier discharges (DBDs) and atmospheric pressure plasma jets (APPJs), as well as gliding arc (GAD), can be the source of cold plasma. This paper presents an overview of agriculture and soil protection problems and biomedical and health protection problems that can be solved with the aid of plasma produced with electrical discharges. In particular, agricultural processes related to water, sewage purification with ozone and with advanced oxidation processes, as well as those related to contaminated soil treatment and pest control, are presented. Among the biomedical applications of cold plasma, its antibacterial activity, wound healing, cancer treatment and dental problems are briefly discussed.
1. Introduction
Many problems in agriculture and biomedicine are the result of the generation, distribution, and conversion of multiple forms of energy. They not only cause climate change, as a result of environmental pollution and greenhouse gas emissions, but are also the cause of many of diseases such as asthma, cardiovascular diseases, respiratory diseases, lung and skin cancers, and new variants of previously unknown viruses and bacteria and their accompanying diseases. Fighting these problems is the most important challenge of the 21st century [1,2,3,4].
Agricultural and biomedical problems can be solved using plasma technology, which is still being exploited; ozone, UV radiation, and other excited and ionised plasma particles are used in disinfection and sterilisation processes, tissue treatment, and in healing processes. Some of these technologies are very mature, such as the treatment of drinking water, but they are still being used and developed along with technological advancements related to new materials and power electronics [5,6,7,8,9].
Although the effectiveness of other technologies has been confirmed in applied research, and reactor prototypes have been demonstrated in conditions similar to real conditions, such technologies have not yet been implemented. The reason for this is probably due to the existence of proven non-plasma-based technologies, which are often cheaper but sometimes less effective and more harmful to the environment, e.g., the treatment of sewage of various origins, or the treatment of water in swimming pools using chlorine and its derivatives [10,11].
The application of plasma technologies, such as in the treatment of contaminated soils and their fumigation [12,13,14,15,16,17,18,19,20,21]; cultivation and storage of agricultural products and food [22,23,24,25,26]; removal of insects, fungi, and mould [27,28]; sterilisation and disinfection of materials non-resistant to high temperatures [29,30,31,32]; and living tissue treatment [33,34,35,36,37], have a chance to be implemented in the near future. This is because of their features, such as reliability, ability to conduct plasma processes at atmospheric pressure, energy selectivity and progress in the field of power electronics, which allow for the construction of more efficient, smaller, and cheaper power supply plasma reactors [7,8,38,39,40,41,42,43,44,45].
Since the beginning of the 20th century, research on plasma technologies has mainly been devoted to discharge physics as a source of non-thermal atmospheric pressure plasma, power supply systems for plasma reactors, and applications in drinking water treatment and air pollution control [46,47,48,49,50,51,52,53,54,55,56,57,58,59]; while, research into technologies relating to agriculture and medicine started in the current century [12,14,17,19,22,23,28,32,60,61,62,63,64,65,66,67,68,69,70,71,72]. This paper presents an overview of the applications of plasma technology in agriculture and biomedicine. Selected technologies using both thermal and non-thermal plasma, produced at atmospheric pressure, are presented.
2. Plasma as a Medium for Application in Agriculture and Biomedicine
In the physical sciences, plasma–which is ubiquitous in the universe–refers to the fourth state of matter, in addition to solid, liquids and gas. It is an ionised gas containing positive and negative ions, as well as electrons and inert gas molecules, and it has a net electric charge that is equal to zero.
In the biomedical sciences, intercellular plasma is known as the non-cellular fluid component of blood. The unique physicochemical properties of biological plasma and the coagulation factors it contains are the basis of the haemostasis process—stopping bleeding when one cuts themselves or breaks the continuity of their blood vessels in some other way.
Due to the properties of physical plasma and its analogy to the ubiquity of ionic liquid in biology and medicine, Irving Langmuir [59] introduced the term “plasma” for ionised gas. As a technological medium, plasma can be produced in a wide range of temperatures and pressures. The moment of change in the physical properties of the gas, accompanied by the appearance of electrical conductivity and loss of insulating capacity, is considered as the most important. It is a state of matter with the broadest particle energy band. The electrons are partially or completely detached from the atoms and play a crucial role in initiating chemical reactions in the plasma. Other plasma particles may be weakly, partially or fully ionised including excited particles, positive and negative ions, neutral particles, and photons, the nature of which depends on the gas in which the electrical discharge occurs, have much lower energies than electrons. Their properties and interactions are used in a variety of technological processes.
An important criterion for the classification of plasma is the thermodynamic equilibrium of the particles, which means that electrons, ions, and neutral particles have an approximately equal energy. When talking about the thermodynamic equilibrium of a plasma, we usually mean the so-called local balance. Discharges, which are the source of plasma in local thermodynamic equilibrium, are characterised by relatively high temperatures and are used where heat is required. It should be emphasised that although high pressure usually means thermal and equilibrium plasma, and low pressure means non-thermal and non-equilibrium plasma, the final criteria for determining the classification of plasma as thermal or non-thermal are the products of the gas pressure, p, and the electrode gap, d, of the plasma reactor [46].
In recent years, research has been conducted on the use of thermal plasma in the combustion of municipal waste (biomass, plastics, post-hospital, pharmaceutical, etc.) containing hazardous organic compounds, such as phenols, chlorinated biphenols and dioxins [73,74,75,76], which can contaminate the soil, making it barren and incapable for plants to breed. The sources of thermal plasma are arcs, plasma torches and microwave reactors.
In biomedical applications, especially in the decontamination of heat-sensitive devices and the tissues of living organisms, thermal plasma is used in the so-called remote mode [56,77,78]. The plasma does not reach the contaminated object directly, but the radicals, chemical compounds and UV radiation produced in it enable the treatment of heat-sensitive objects.
Non-equilibrium and non-thermal (cold) plasmas are used in agriculture and biomedicine where heat may adversely affect technological processes. The thermodynamic imbalance of cold plasma makes it suitable for organic syntheses due to the low power requirements and the possibility of conducting chemical reactions in the gas at relatively low and close to ambient temperatures. This type of plasma is used directly to carry out plasma–chemical processes in facilities, such as living tissues and medical instruments, that are made of materials non-resistant to high temperatures. Such non-thermal and non-equilibrium discharges at atmospheric pressure most often include barrier dielectric discharges [18,29,60,61,62,63], atmospheric pressure plasma jets APPJs [35,62], and gliding arc GAD [25,39,40,45,49,50,51,56]; these are currently the subject of advanced research in many scientific centres around the world.
The antimicrobial agents generated in plasma, such as charged and excited molecules, reactive oxygen species (ROS), atomic oxygen (O), reactive forms of nitrogen (RNS), atomic nitrogen (N), nitric oxide (NO) and, in the case of electrical discharges with water admixtures, also hydroxyl ions (OH) and hydrogen peroxide (H2O2), can be used in agricultural and biomedical processes.
Physical and chemical phenomena, such as etching processes, high-energy UV radiation, heat and alternating electric fields, as well as their role in processes of decontamination, sterilisation, surface treatment and modification and their influence on biomedical and agriculture objects, should also be considered, and this makes the subject of the application of plasma in these areas highly interdisciplinary.
The high efficiency of plasma in decontamination, sterilisation and healing is due to, among others, the large selection of different types of plasma reactors that can be used in plasma medicine, the possibility of controlling their parameters to ensure the antimicrobial ability of plasma particles and easy access to narrow and closed spaces [34,64,77,78].
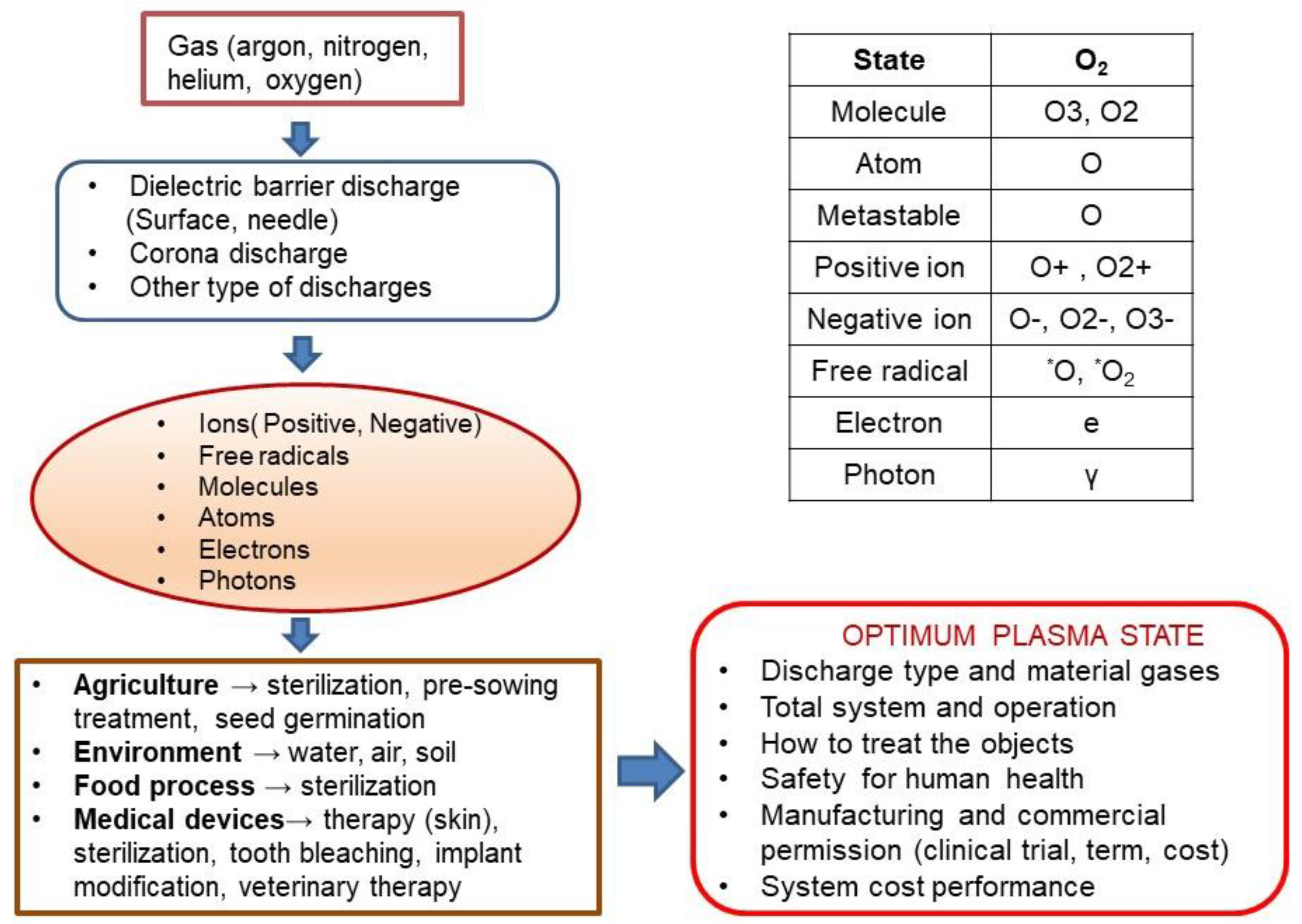
Figure 1 presents how many parameters should be taken into consideration when optimising plasma processes for application in agriculture and biomedicine. We mention only the most important of them, such as technological gases, types of electric discharges and active particles, molecules, atoms, and free radicals produced in the plasma, both in the neutral and excited states, and ions. Other important issues are plasma treatment methods for agricultural and biomedical media; used solutions of plasma installations and their power supply systems, their costs efficiency, and, above all, safety for living organisms.
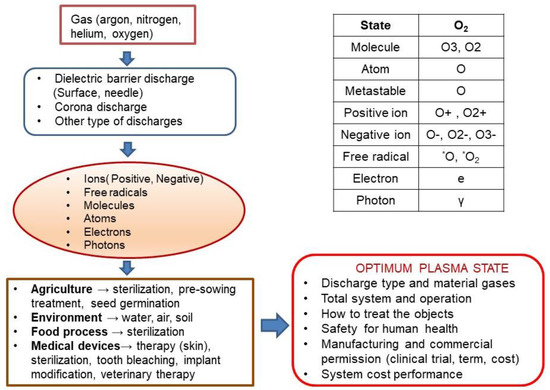
Figure 1.
The technological complexity of plasma applications in agriculture and biomedicine.
Research works on the use of non-thermal plasma in technologies related to agriculture [14,17,19,23,28,77] and biomedicine [72,75,76,78,79] are aimed at, inter alia:
- Assessing the suitability and effectiveness of various types of electrical discharges for plasma generation, especially at atmospheric pressure.
- Improving the process of generating electrons initiating the production of ions, oxygen and nitrogen radicals and other compounds useful in the processes of water, and soil treatment and in biomedicine.
- Achieving the desired composition of the final gas mixture and a high degree of conversion of harmful compounds by adding admixtures and catalysts (steam, ammonia).
- Minimising energy consumption.
- Matching the electric power source to the plasma reactor, including solar energy [21,67,80,81,82].
Table 1 presents a comparison of thermal and non-thermal plasma in terms of their properties, while Table 2 presents the most commonly tested and practically used types of plasma reactors with electrical discharges for the production of thermal and non-thermal plasma, their applications, and power supply systems, which determine their application on an industrial scale.

Table 1.
Comparison of thermal and non-thermal plasma properties *.

Table 2.
Plasma reactors, their applications, and their power supply **.
2.1. Agricultural and Soil Protection Problems
It is obvious that soil quality is a crucial factor for the prosperity and future of a region. Agriculture and soil protection include problems related to the overuse of chemicals to fertilise soil, which causes soil degradation; increases pesticide residues in soil, groundwater and food; and affects plant cultivation, seed quality and their germination capacity. An equally important problem is preventing the microbial contamination of soil and agricultural products, the latter during the processes of their processing, transport, and storage.
Recently, due to the growing emission of greenhouse gases into the atmosphere, and the related global warming of our planet, a significant increase in various ecological phenomena have been observed: droughts, floods, storms, and other disasters in areas where they have not previously been observed, leading to environment pollution. Apart from the direct destruction of crops, housing, and public facilities they inflict, floods are the cause of the massive contamination of soil and, thus, deterioration in soil quality. When a flood occurs, persistent odour and the contamination of buildings, such as farms, housing, and public institutions, are unavoidable. During restoration after a flood event, the most difficult challenges are related to:
- Epidemiological risk and lack of drinking water due to polluted wells and destruction of water treatment plants.
- Lack of simple, sufficient, and cheap drying and decontamination techniques for indoors.
- Severe deterioration of indoor air quality due to the pollution of housing by flood water. Because of inadequate drying and disinfection techniques, infestation by very persistent mould often takes place. A secondary consequence is the rotting odour.
- The presence of difficult-to-decompose sediments transported by flood waters, and chemical and biological pollution (bactericidal risk, secondary infestation by pests).
- Surface water contamination. Furthermore, sapropelic sediments may contribute to secondary oxygen deficiency.
- Risk of bactericidal and chemical soil and crop contamination. Some crops (depending on the flood season and growth phase) could possibly survive, but the dilemma remains whether they can be collected without risk.
All these make it necessary to search for new techniques to solve problems related to the pollution of the agricultural environment, in particular soils and surface water. Application of ozone produced by electrical discharges in agriculture has, therefore, become one of the most important techniques to ensure the safety of plant growing, and soil and food treatment. The objective of agricultural soil sterilisation is to destroy or eliminate microbial cells and insects in the soil, but only those that cause decreasing soil fertility and destroy the seeds, roots and leaves of the plants. Conventional agricultural soil treatment methods based on chemical compounds, especially methyl bromide (CH3Br), have contributed to ozone layer depletion, degradation of soil and chemical residues in food. Other methods, including autoclaving (moist heat), dry heat, and microwaves have been tried as alternatives to chemical fumigation. Soil treatment using pesticides is an effective method for resisting the spread of disease in soil. However, pesticide residues in groundwater, caused by the overuse of chemicals, have become a serious issue in agriculture. From the environmental point of view, alternative methods of soil treatment are being sought. Ozone is a powerful oxidising agent and a much more effective disinfectant than chlorine compounds. A mixture of water–mist with ozone produces very reactive intermediate hydroxyl free radicals, which are much stronger oxidising agents than ozone itself, and have exciting potential in soil and plant treatment, such as pest removal from agricultural soil [12,13,18,19,20,80,81,82,83,84].
2.2. Biomedical and Health Protection Problems
Over the last two decades of this century, plasma technologies have been used to detect, prevent, and solve many problems related to health protection. These include hygiene problems, especially hospital hygiene and related viral and bacterial diseases, mycoses and skin diseases, chronic and senile diseases, dental care, cosmetology, tissue engineering, and medical diagnostics [22,30,37,62,65,68,69,70,71,75,76,85,86,87].
The effects of plasma on biological material (tissues, skin, viruses, bacteria, and fungi) result from three basic techniques: direct plasma, indirect interaction, and a hybrid technique [88]. The direct interaction of plasma is based on the fact that a living organ/tissue plays the role of one of the plasma rector electrodes. Most often, the voltage is not directly connected to the living tissue, but some current may flow through it in the form of a small forward current, a displacement current, or both, because tissues have conductive and dielectric properties. The conduction current is usually limited to avoid thermal effects or electrical stimulation of the muscles. Such treatment makes it possible to use the influence of active and uncharged atoms and molecules, as well as ultraviolet (UV) radiation, on the surface of living tissue. The most important feature that distinguishes direct plasma treatment is that a significant stream of charges reaches the surface of the living tissue. The indirect (“current free”) plasma interaction mainly uses the uncharged atoms and molecules that are produced in the plasma and involves little, if any, flux of charges to the surface. The plasma generated between the two electrodes is transported to the application area by a gas stream (argon, helium, and oxygen). Various devices are used here, including very narrow devices, such as so-called plasma needles for larger plasma torches. An advantage of indirect treatment may be that the plasma device is always at a distance from the surface to be treated, so that there is no current flowing through the tissue. Hybrid plasma treatment combines both direct and indirect plasma generation techniques. The discharges used in the hybrid method are called barrier corona discharge (BCD) [60,88]. This is achieved by introducing a grounded mesh electrode on one of the electrodes, which has much lower electrical resistance than the skin. As a result, virtually all the electric discharge current flows through the mesh electrode. The listed techniques of non-thermal plasma treatment allow for a certain degree of fine-tuning of its properties to a given medical process. Thus, the amount of nitric oxide (NO) can be fine-tuned compared with the ozone (O3) produced in the plasma. It is also possible to influence the microstructure of the plasma discharge, which may be of particular importance in direct treatment [89]. The direct plasma treatment technique involves a significant charge flux, which provides greater flexibility in fine-tuning the non-thermal effects of the generated plasma for medical applications.
3. Review of Plasma Applications in Agriculture and Medicine
Since the beginning of 21st century, many reviews, articles, and conference papers were published on the biomedical and agricultural applications of plasma generated by reactors with electrical discharges in the air, in noble gases and their mixtures. Different designs of such plasma reactors are presented in Table 2 [5,6,22,23,26,28,30,31,32,33,36,59,66,72,75,77,79,83,85,88,89,90,91].
These studies show that viricidal, fungicidal, and bactericidal properties of non-thermal plasma make it effective in processes of sterilisation and disinfection, as well as in the treatment of many diseases, including:
- Sterilisation of human and animal tissues, blood, and surface wounds [33,34,35,36,37].
- Assisting skin cancer therapy [92,93,94,95].
- Treatment of viral, bacterial, and fungal infections due to antimicrobial plasma activity [31,88,96].
- Odontology—caries therapy [97,98].
- Coating of implants, contact optical lens, and dentures with biocompatible films [99,100,101].
- Live tissue engineering—fabrication of bioactive agents and medicines [29,30,35,90].
- Immobilisation of biological molecules, cell surface modification to control their behaviour, and improvement of blood adhesion.
- Sterilisation of medical and surgical instruments, especially those made of materials and fabrics not resistant to high temperature.
- Medical diagnostics—fabrication of biosensors based on polymers, and thin amorphous films for medical and biochemical analysis.
In the agricultural and food industry, both thermal and non-thermal plasma are used for:
- Water and wastewater purification [16,21,38,67,82].
- Utilisation and management of industrial waste [73,74,102,103,104,105,106].
- Soil treatment and pest control [12,13,14,15,16,17,18,19,20,21,63,80,81,82,83].
- Odour removal from air in agricultural production processes and waste utilisation [107].
- Food pasteurisation, disinfection, and preservation—O3 is used in common refrigerators as a deodorising and antimicrobial agent [25,26,108].
- Food storage and package sterilisation [22,23,24,25,109].
- Conditioning and microbiological decontamination of biomaterials, including food [24].
- Enhancement of seed germination, plant growth, and fruit formation processes [110,111,112,113,114,115,116,117,118,119,120].
- Ozone-aided corn-steeping processes to replace current SO2 application [121].
There are no general requirements for all possible plasma components and their safe use in agricultural and biomedical processes, especially in therapy. The current safety restrictions for all non-thermal plasma sources refer to general regulations for the operation of electrical devices, those generating ultraviolet (UV) radiation, and active compounds, and the limits of electric currents safe for humans. Research is still being carried out on the design of non-thermal plasma sources with good bactericidal and fungicidal properties that would not be harmful to humans and animals, both farmed and wild, which may live in the vicinity of the plasma-treated crops.
Over the last three years, with the worldwide SARS-CoV-2 pandemic, which started in 2019, and with which we are still struggling today, research has intensified on the influence of plasma and highly ozonated water on various types of viruses, including SARS-CoV-2 and their mutations [122,123].
3.1. Plasma Applications in Agriculture
Industrial and municipal waste, including toxic and post-hospital waste, resulting from the progressive development of civilisation throughout the world in recent centuries, is a serious problem for the natural environment and its main components: air, water, and soil. To counteract this, effective methods for their disposal and management are sought. Despite significant progress in this area, there are still technological difficulties that limit the achievement of the expected environmental and economic benefits. A review of plasma technologies in the management of solid and liquid industrial and municipal wastes was performed in [47]. Given are examples of the thermal technology of plasma generated in arc furnaces, used on an industrial scale in the metallurgical processing of materials. Arc furnaces, as sources of thermal plasma [106], are also used in the recovery of materials from the metallurgical industry and steel processing and of noble metals from the platinum group from used catalysts; waste disposal containing asbestos, radioactive, electronic, oil, petrochemical and mining pollutants. This waste contains both hazardous materials that can have a very detrimental effect on local ecosystems and also on valuable elements, usually precious metals.
The plasma process based on thermal arc discharge was designed in such a way as to separate metals and minerals from the waste material while destroying elements hazardous to the environment. The same thermal arc technology can also neutralise gaseous pollutants generated in the combustion of liquid and solid wastes. Despite the significant advancement of the arc plasma thermal technology in environmental protection, research is still being conducted around the world on processes to convert industrial and municipal waste into energy [106]; liquid and gaseous fuels [73,124]; the treatment of medical waste [103,125]; sewage sludge [126]; tanning sludge [127]; organochlorine waste [128]; and contaminated soil [15,19,80,83].
In Poland, research on thermal arc plasma technology is conducted on a large scale, e.g., at the Technical University of Łódź [15,105,123,126], Silesian Technical University [105], and Częstochowa Technical University [127]. This research concerns the disposal of solid waste by means of arc discharges, which leads to the thermal decomposition of the organic fraction and the melting of the inorganic fraction which, as a result of the cooling process, cause vitrification of the waste material. An example of such a process is the utilisation of ash and sedimentation wastes. At a temperature for the arc plasma at approximately 2300 K [15,105], the ash melts, and after cooling obtains the structure of glass. Many waste materials can be plasma vitrified. These include residues of combustion processes (ash, slag, and sedimentation deposits), inorganic hazardous waste (asbestos), and industrial sewage waste (galvanic residues). The most important advantages of the vitrification process include [10,105,123]:
- Release of the product from organic compounds and their destruction.
- Dissolving or freezing elements and toxic compounds (heavy metals) in the vitrified product.
- Mass and volume reduction in the process of degassing and decomposition of oxides.
- Resistance of the obtained product to the action of organic compounds, and its good physical properties (hardness, resistance to abrasion, and high temperatures).
Thermal plasma offers some unique advantages in destroying hazardous waste compared with classical incineration. The high energy density and temperature associated with thermal plasma and the high rates of plasma–chemical reactions make it possible to treat large amounts of waste in relatively small reactors. In addition, thermal plasma reactors (arc furnaces) can be easily integrated into a production process that generates hazardous waste, allowing for its disposal at the point of origin.
3.1.1. Water and Sewage Purification with Ozone and AOPs
In the processes of water and wastewater treatment, it uses non-thermal plasma technologies, mainly ozone generated during electrical discharges in air or oxygen or directly in water [16,21,38,67,81,82,123,128,129]. The treatment of drinking water and wastewater requires the production of large amounts of ozone; its advantage of which over commonly used chemical oxidants (chlorine and fluorine) is due to the fact of its high efficiency in removing a wide range of organic water impurities; improving its taste and colour; and the lack of by-products from the ozonation process.
Water treatment with ozone and AOTs is the most technologically advanced and used in practice. From the beginning of the 20th century, water treatment stations were established in Europe (e.g., Nice in 1907) and around the world (e.g., Los Angeles in 1987) in which chlorine, which is harmful to the environment, was replaced with ozone produced in barrier discharges.
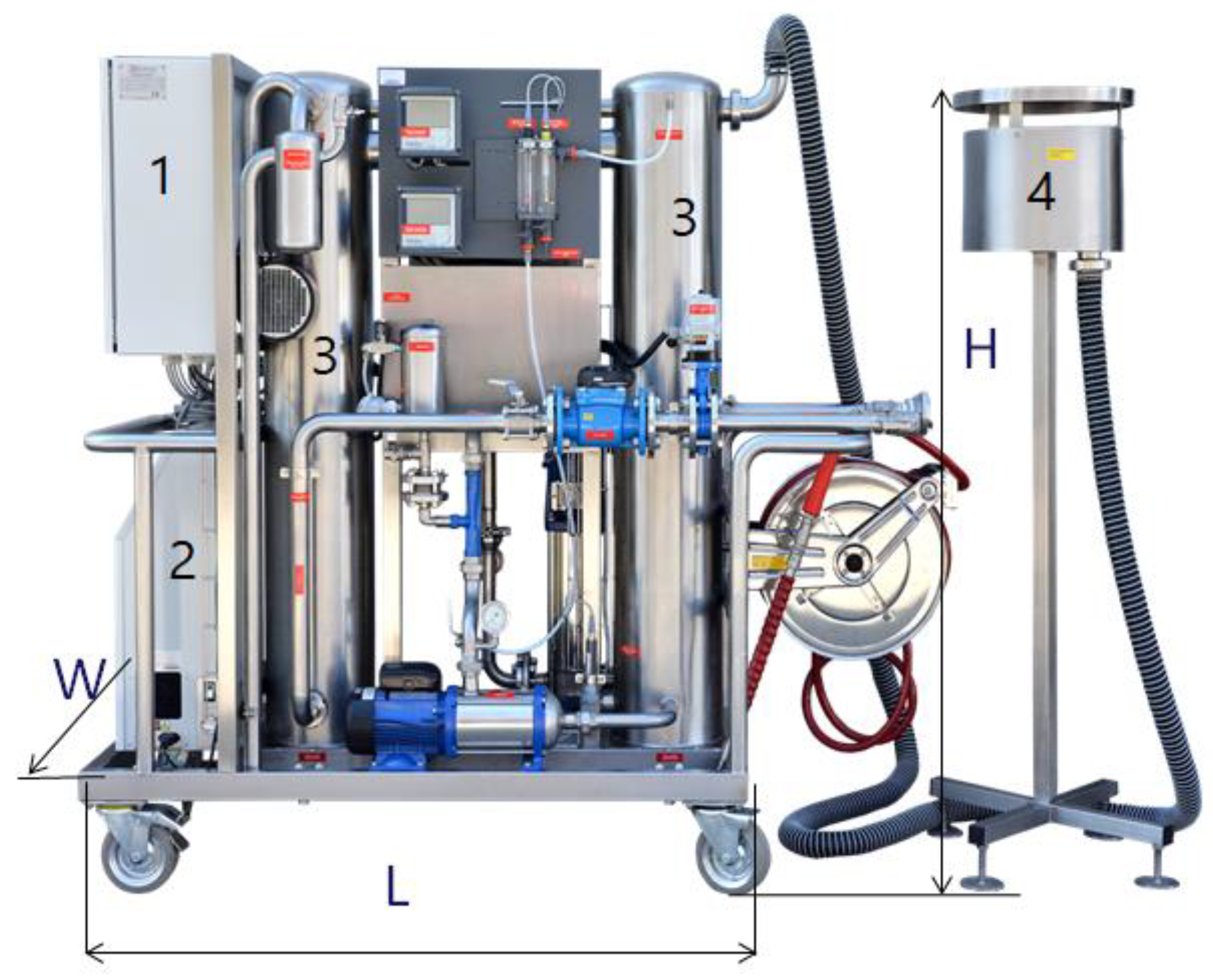
As an example of a water treatment installation, a mobile ozone rinse and disinfection system is presented; this was constructed by the Polish company WOFIL Ozone Technology, and it was designed to reduce the chlorine used in pools and the processes of water infrastructure facility disinfection such as fittings, pumps, technological installations, tanks, walls, and floors [16,67,123]. This autonomous device can prepare a disinfectant solution based on ozonated water, which will require only water and electricity supplied directly from the mains or from a power generator. A three-phase power supply of 400 V and 7 kW is required. The nominal capacity of the system was 4 m3/h of highly ozonated water, at a pressure of 12 bars. The SPID system received the Grand Prix main award at the 2017 fair in Bydgoszcz, Poland, for the most innovative solution. Figure 2 depicts the SPID system. This mobile device producing highly ozonated water was tested on various pollutants and under varying atmospheric conditions [16,123]. Applications of the SPID system also include sterilisation of agriculture product packaging, food product storage preservation, and fruit and vegetable disinfection. Its technological effectiveness in the removal of selected pharmaceuticals (i.e., diclofenac, carbamazepine, and sulfamethoxazole) from sewage was also analysed [11].
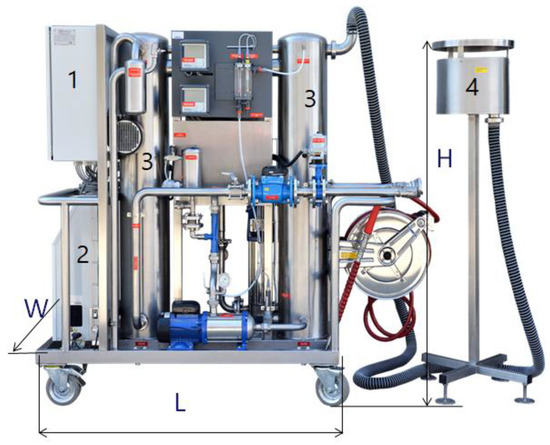
Figure 2.
Mobile ozone rinse and disinfection system, where H = 1.7 m, L = 2 m, W = 0.6 m and maximal ozone production = 80 g/h: (1) pulse power supply; (2) oxygen and ozone generator; (3) contact columns for the production of highly ozonated water; (4) vent system with residual ozone destructor [16].
The task of the research installation was to reduce the concentration of pharmaceuticals in wastewater in such a way as to reduce the formation of ozone by-products (e.g., bromates) and the toxicity of wastewater after the process, and to optimise the process in terms of economy and energy. The system consisted of three stages for the removal of pharmaceuticals: (1) with ionised air and prefiltration; (2) with highly ozonated water in multi-stage contact columns, and (3) removing residual contaminants on gravel–sand and coal filters. The technological efficiency of the removal of selected pharmaceuticals from wastewater allows for the recommendation for practical use of this innovative technology for the removal of pharmaceuticals from wastewater: the obtained effects were at the level of 80% to over 90%; the reduction in the BOD5 and COD ratios by 42% and 20%, respectively; and the determined electricity consumption at the level of 0.96 kWh/m3 in a pilot-scale facility [11]. The authors emphasised that the benefits of improving the quality of treated wastewater discharged into the environment without the need to dose chemicals into them should be considered in the aspect of improving the technological efficiency of the treatment plant. It is a waste-free technology which, compared with other methods of disposal of pharmaceuticals with similar energy consumption (e.g., membrane techniques 0.5–1.0 kWh/m3), makes it much more advantageous.
Recently, with the aid of using the SPID RS2 system [123], which is a variant of the mobile SPID device, producing degassed high-ozone water (OWWO) in the amount of 4 tons per hour and 96 tons per day; the possibilities and effectiveness of OWWO for virus inactivation were investigated including, in particular, for SARS-CoV-2. As shown by previous studies, viruses are resistant to disinfectants, and the effectiveness of their inactivation depends on the type, structure, and physical form in which they occur, e.g., on the surface of solid particles or surrounded by impurities in the form of street dust and soot, as well as on various surfaces, such as plastics, metals, cardboard, and leather [123,130].
The OWWO technology was developed in Poland 10 years ago. SPID devices have been produced since 2016. The results of research on this technology in the inactivation of viruses in urban infrastructure (bus shelters and street routes), based on the example of Warsaw, show that it is a promising technology, allowing for the disinfection of places frequented by thousands of people. It is also safe for the environment, because OWWO technology does not use any chemicals, only water, air, and electricity. For the above reasons, it is a technology supported by the Polish Ministry of Climate to be used to counter the pandemic caused by the coronavirus [123].
In [122], it was shown that in a plasma reactor of the APPJ type (plasma nozzle), SARS-CoV-2 is inactivated quickly and efficiently on various surfaces by means of electric discharges in argon. The effectiveness of inactivation depends on the absorbency and roughness of the surface, and argon plasma has much more reactive oxygen and nitrogen molecules than helium plasma. The results showed that treatment of the tested surfaces with plasma generated by the APPJ reactor, powered by a sinusoidal voltage with an input power of 12 W, argon flow rate of 6.4 L/min, voltage of U = 6.8 kV, frequency f = 12.9 kHz, and at a distance of the reactor from the surface of ∼15 mm, led to an almost 100% inactivation of the SARS-CoV-2 virus [122].
3.1.2. Agricultural Soil Treatment and Pest Control
Application of non-thermal plasma in agriculture has recently become an important technique to ensure the safety of plant growth, soil treatment, and food processing. The objective of agricultural soil sterilisation is to destroy or eliminate microbial cells in the soil.
A mixture of water–mist with ozone produces very reactive intermediate, hydroxyl free radicals, and reactive oxygen and nitrogen species (RONS), including atomic nitrogen, nitric oxide, peroxynitrite (ONOO-), atomic oxygen, hydroxyl radical, superoxide (O2−) and hydrogen peroxide, which are much stronger oxidising agents than ozone itself, and they have great potential in soil and plants treatment and pest removal in agricultural soil [14,17,19,28,63,83,84,131,132]. The use of atmospheric plasma as a treatment technique in agriculture has many advantages, including low cost and avoiding chemicals and undesirable changes to agricultural produce related to heat treatment.
Soil remediation is a difficult procedure because it must be performed on site and should cover a relatively large surface area with various geological settings, while also maintaining penetration depth. Organic matter has the greatest influence on the sorptive capacity of soils. Apart from organic matter, grain-size distribution is an important factor influencing the sorptive capacity of soils, especially the content of very fine and colloidal particles. The distribution of cations in the sorptive complex depends on pedogenesis (soil forming process), which is only their typology. The total exchange of cations in the sorptive complex and the base saturation depend on the type of the parent rock, including the presence of carbonates [133].
The processing of soil depends on several factors including:
- Type of soil (content of water, organic compounds, consistence, and structure).
- Type of pollutant.
- Treatment technique.
- Geological and atmospheric circumstances [134].
Pollutants might be distributed in soil in several ways, namely [135]:
- In soil matrix.
- In vapour phase.
- In non-aqueous phase.
- In groundwater.
Conventional agricultural methods of soil decontamination are based on chemical compounds, such as chlorine, and methyl and ethyl derivatives—trichloromethane, ethylene oxide, or methyl bromide. The latter, quite commonly applied to fungal and bactericidal decontamination, was banned from practical use (in developed countries since 2005, and in other countries, including Poland, since 2015).
Many techniques of soil remediation, such as heating, flushing with chemical additives (surfactants), irradiation, irradiation with catalyst, soil vapour extraction, land filling, incineration [15,105], aeration, oxidation, and bioremediation have been tested alone or in combination. Other methods, including autoclaving (moist heat), dry heat and microwave, have been attempted as alternatives to chemical fumigation [83]. Soil treatment using pesticides is one of the effective ways to resist the spread of diseases in the soil. However, pesticide residues in the groundwater, caused by the overuse of chemicals, has become a serious issue in agriculture. From the environmental point of view, alternative methods of soil treatment are being sought.
Oxidation techniques involve ozone, hydrogen peroxide, chlorine dioxide, and potassium permanganate. Trials employing the usage of ozone, alone or combined with AOPs, have been performed in the treatment of soil by international research groups [18]. Ozone generated during electrical discharges, because of its relatively good solubility in the aqueous phase, seems to especially have potential for soil treatment, as it can be applied in both the gaseous and aqueous phases.
Eco-oxidative techniques can improve soil aeration, thus inhibiting denitrification processes. Application of AOPs helps to preserve the natural structure of soil, preventing its acidification, leaching and the release of metallic compounds.
The ozone remediation process can be divided into two phases [136]:
- Instantaneous ozone demand phase, when rapid interactions with soil organic matter and metal oxides occur and most of the pollutant-removing processes take place.
- Relatively slow decay stage.
AOTs result in ring cleavage of poorly soluble aromatic compounds and insertion of oxygen, which increases their water solubility, thereby facilitating their degradation in the natural environment (www.H2O2.com accessed on 23 April 2022). Remediation was positively tested for such pollutants as diesel range organics, trichloroethylene, and PAHs (polycyclic aromatic hydrocarbons) [137,138]. In highly cultivated areas, soil and groundwater contamination with herbicides and pesticides causes severe health problems (bioaccumulation) and significantly reduces the amount of crop yield.
Non-thermal plasma with ozone and oxidative species can inactivate pathogens via:
- Direct destruction, volatisation, and etching of cells.
- Decreasing of biofilm adhesivity by decomposition of the polymer matrix.
- Oxidative stress due to the formation of radicals of various active agents (O3, OH, and O) and the influence of hydrogen peroxide, H2O2, or UV radiation during the electrical discharges.
- Nitrogen stress (research results suggest cell damage from reactive nitrogen intermediates, such as nitric oxide, peroxynitrite, nitrous acid, and nitrogen trioxide) [139].
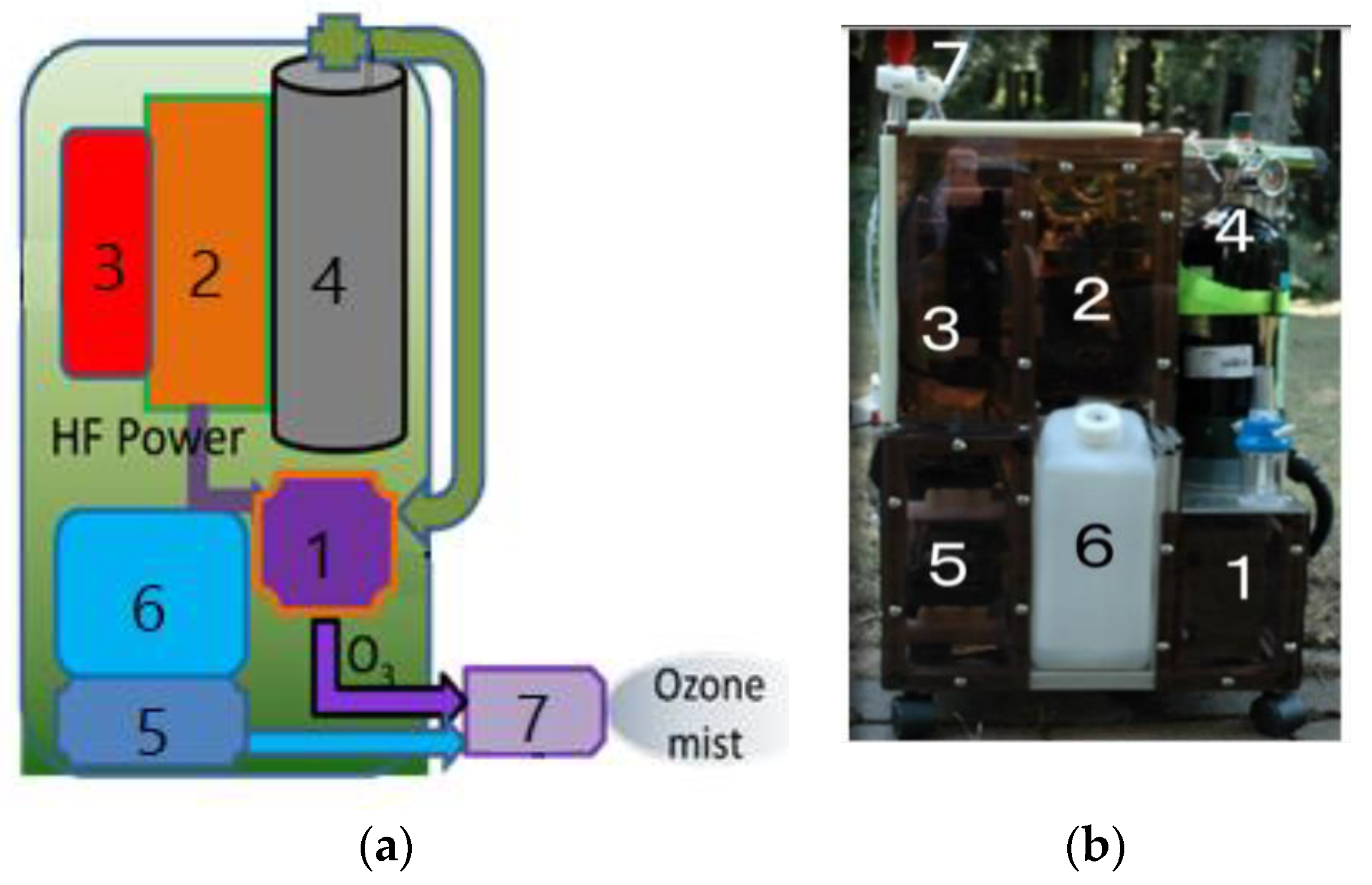
Japanese researchers [13,14,16,17,18,19,20,21,28,63,80,83,84,132,134] developed a portable backpack ozone–mist spray system that is environmentally friendly and intended for use in non-chemical agricultural management. The system, depicted in Figure 3, consists of an ozone generator, a water–mist spray, and a specific nozzle, which is used to kill harmful insects, bacteria and viruses on the surface of plants and in agricultural soil. The results of insect inactivation with the ozone–mist spray are presented in Table 3. Sterilisation efficiency is expressed as a percentage of the inactivation of all tested insects in a given sample.
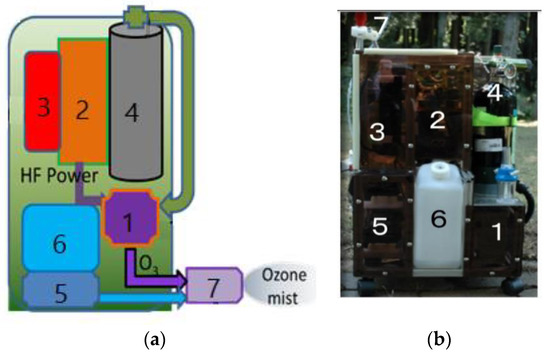
Figure 3.
Prototype of the mobile ozone–mist spray system (MOMSS): (a) sketch of MOMSS; (b) photo of the prototype, where (1) DBD ozone generator, (2) HF inverter, (3) Li-ion battery, (4) O2 vessel, (5) water pump, (6) water tank and (7) ozone–mist spray.

Table 3.
Results of the inactivation of plant insects ***.
The plasma inactivation conditions are expressed by the following parameters: mist–spray ozone concentration in g/m3; oxygen flow rate in litres per minute; spray time in seconds and ozone solubility in ppm. Inactivation studies were carried out on leaves of four different plants (i.e., tobacco, Canada goldenrod, orange, and green tea) and on five insects feeding on these plant’s leaves: green-peach aphid and green caterpillar—on tobacco; goldenrod aphid—on Canada goldenrod; black citrus aphid—on orange; plant lice—on green tea. Treatment parameters for the ozone–mist spray system were the same for all cases, with the exception of green caterpillar and plant lice (No. 2 and No. 5 in Table 3, respectively), for which the spraying time was not 10, but 20 s. As can be seen from Table 3, the sterilisation rate was relatively high in all cases of insect treatment by ozone–mist spray; the lowest for the green caterpillar insect on tobacco was equal to 50% at 20 s of treatment time; the biggest one was equal to 100% for black citrus aphid at 10 s of spraying time, while it was 90% for plant lice over a treatment time equal to 20 s. Using the ozone gas instead of the ozone-mist spray for 20 s allows for 100% inactivation of green tea plant lice insects [84,134].
The same authors [140] proposed and conducted preliminary studies of a mobile soil purification system, which was a project for the practical use of surface barrier discharge and ozone diffusion into the soil, and which was used to treat the soil in a greenhouse. Soil treatment with ozone increased soil acidity, which improved sprouting for some plants (radish) and worsened it for others (spinach). The acidity of the soil returned to the values from before the plasma treatment, which in actual cultivation requires optimisation of the sowing time of the grain, depending on the type of plants.
The presented experiment on a real agricultural field proved the possibility of practical use of soil treatment with ozone, both as a pesticide protecting crops against fungi, insects, weeds, and as a fertiliser [134].
3.1.3. Cold Plasma Applications in Pre-Sowing Seed Treatment
Cold atmospheric plasma (CAP) exposure in combination with electromagnetic field (EMF) treatment was found to be an innovative technique for the enhancement of seed germination (even up to 20%), removal of surface contamination, and early seedling growth [141,142,143]. This approach results in long-term changes in plant metabolism, increasing of biomass production; it also increases plant disease resistance [144,145,146,147]. Moreover, CAP application improves agricultural performance of crops as reported in [109,142].
Cold plasma is quite an effective tool for pre-sowing seed treatment [141,146,148,149]. Lithuanian and Belarusian scientists reported Norway spruce germination and growth after CAP seed treatment [141]. A DBD planar geometry plasma reactor with an RF power supply system was used in the experiment [148]. The researchers found that most treatments accelerated the germination rate of spruce seeds in cassettes, whereas in vitro germination decreased. The seeds were processed for 2, 5, and 7 min. Such treatment in combination with the application of EMF induces changes in H2O2 concentration in germinating seeds. Ivankov et al. used the same approach to study changes in the growth and production of non-psychotropic cannabinoids induced by pre-sowing treatment of hemp seeds [149]. In this case, plasma stimulated the in vitro germination rate under laboratory conditions even up to 25%. The authors noticed that the treatment accelerated growth in male plants and inhibited growth in female plants.
The Belarusian group investigated the effect of pre-sowing plasma treatment of maize, narrow-leaved lupine, and winter wheat seeds on germination, disease resistance during vegetation, and crop yield in laboratory conditions [146]. Filatova et al. employed the DBD technique using a capacitively coupled 5.28 MHz plasma reactor. The seeds were treated in an air atmosphere with an ambient pressure of 200 Pa, and a temperature of 37 °C. Treatment duration varied from 2 to 7 min. The researchers reported the decrease in contamination of maize seeds with Penicillium spp. by approx. 21% after plasma treatment. In addition, positive changes in germination and maize sprout length were observed. In the case of lupine seeds, the plasma treatment caused the death of all C. gloeosporioides and K. caulivora, and significantly reduced the amount of Cladosporium and Alternaria fungi. After plasma treatment, winter wheat seeds demonstrated a reduction in contamination with Alternaria and Fusarium fungi. Filatova et al. summarised the plasma treatment effects as stimulation in germination and growth of seeds, fungal contamination reduction, improvement in the yield of plants, and crop plant resistance enhancement.
3.2. Plasma Application in Biomedicine
Starting from the 1990s, the development of medical plasma has demonstrated remarkable progress due to the increasing involvement of representatives of various scientific fields, such as microbiology, bioengineering, biochemistry, bioelectronics, and theoretical and applied electrical engineering [150,151,152,153]. The first principal plasma-in-medicine experiments (late 1990s) were related to the examining of bactericidal properties of plasma. The experiments were based on the DBD approach to inactivating bacteria on different surfaces and liquids [154,155]. After the experiments, it was found that low-temperature plasma (LTP) can be used not only for inactivating pathogens, but also for disinfecting biological tissues and, in the long term, for wound healing. Due to the great interest in the new interdisciplinary field, around 2005, the establishment of a plasma community was noted [156].
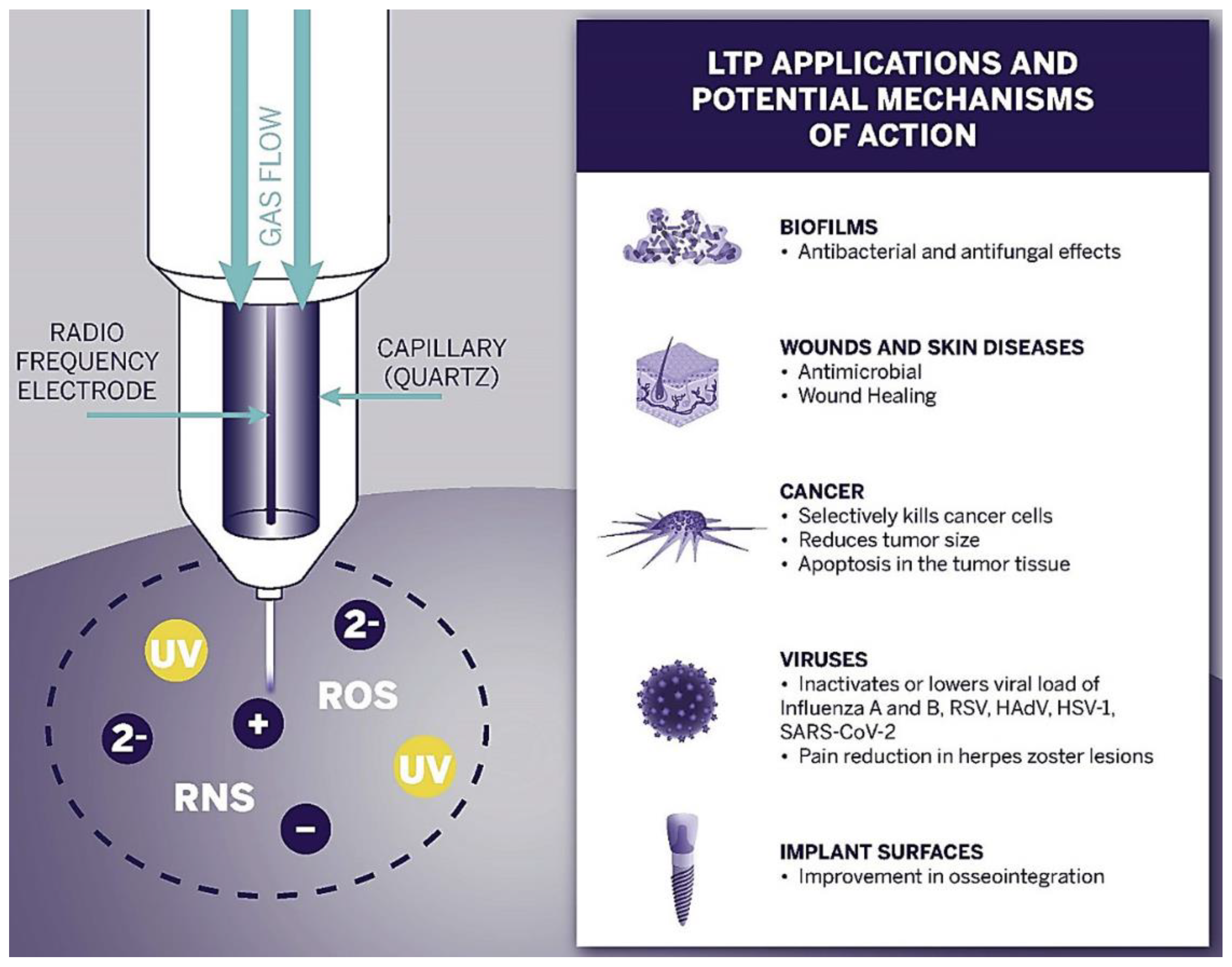
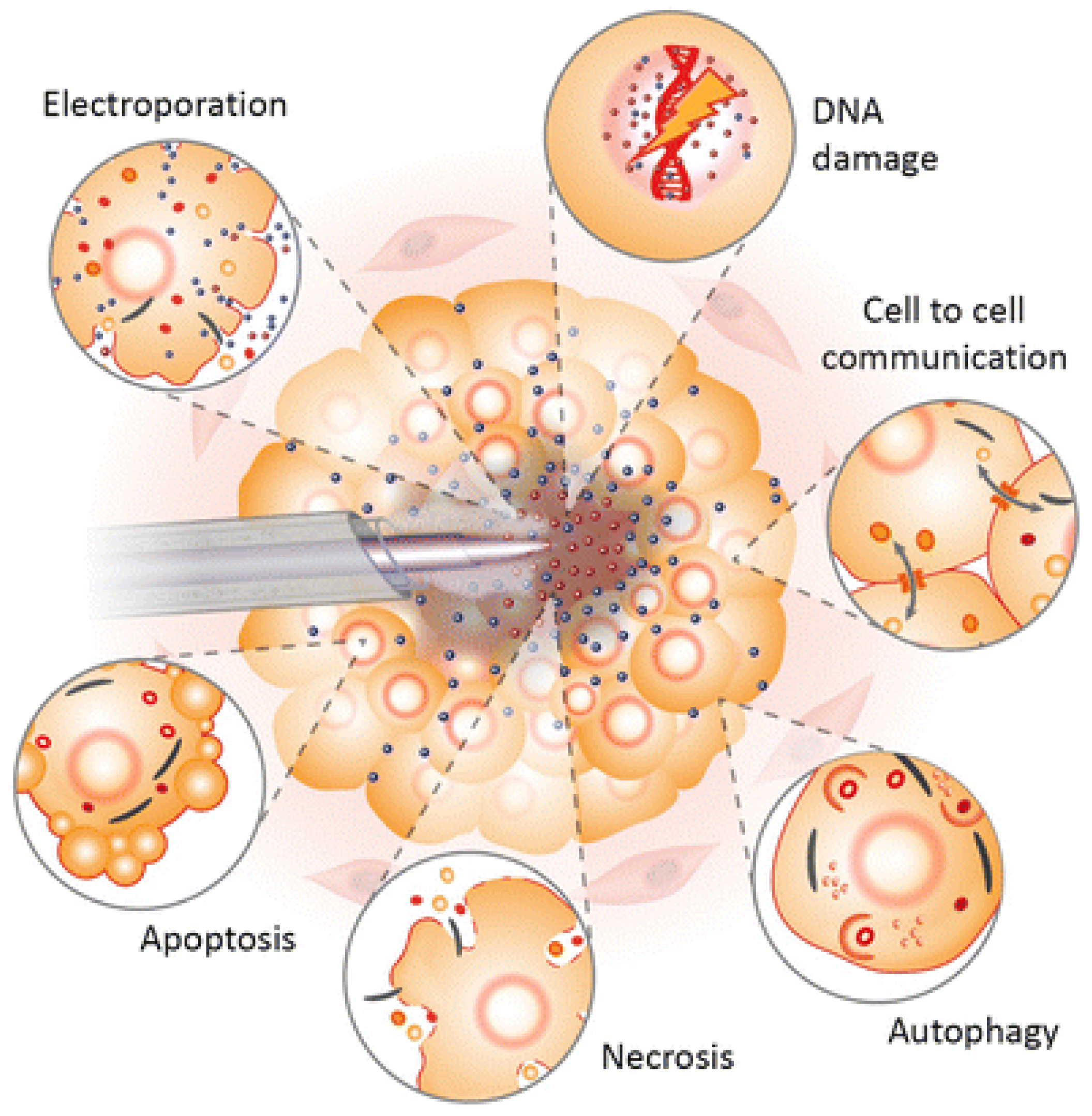
The first successful clinical trials relating to chronic sores were observed in 2010 [157,158,159]. These promising results began the new era of plasma oncology experiments and included in vitro and in vivo approaches for the assistance in killing cancer lines [160], therapy in oral biofilm-related diseases [161,162], wound and tumour treatment [163,164], virus and infection treatment [165,166]. Figure 4 presents the most important LTP applications in biomedicine and its expected effects.
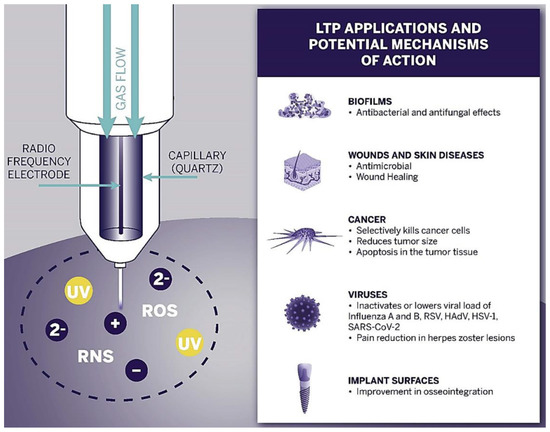
Figure 4.
Schematic diagram of the LTP application spectrum and observed effects in biofilms, wound, skin, cancer, and viruses, as well as implant surface treatment [166].
This section contains a brief review and exemplary experimental results from using both approaches—direct and indirect plasma exposures—for the antimicrobial, wound healing, tumour treatment, cell incubation and proliferation, dentistry, and veterinary medicine plasma applications.
3.2.1. Antimicrobial Applications
Cold atmospheric plasma (CAP) has been considered quite an effective technique for inactivation of pathogenic infections in humans and animals [166,167]. Numerous scientific attempts demonstrated that LTP treatment can lead to the structure modification of proteins, lipids of viral envelopes, and nucleic acids [168]. In 2010, Yasuda et al. investigated the general mechanism of plasma inactivating microorganisms with the use of DBD devices for CAP generation (described in detail in [169]). In this research, the tested samples included poly(ethylene terephthalate) (PET) films soaked in gelatin and air-dried under UV light. The discharge treatment of the sample caused a rapid decrease in the bacteriophages (λ phages), while total inactivation was observed after 20 s exposure. It was also found that protein DNA damage, gradually accumulating with an increase in the dose of plasma, is responsible for phage inactivation.
In 2009, Terrier et al. published a paper that described the inactivation of human and animal airborne respiratory viruses, such as respiratory syncytial virus (RSV), human parainfluenza virus (HPIV-3), and influenza A virus subtype H5N2 [170]. Their experiment was performed within the biozone technology, with the use of a cold-oxygen plasma-jet device. Plasma-generated ozone was recognised as the main factor in the viral titre decrease in all three studied viruses. The scientists suggested that ozone inactivation of viruses occurs primarily as a result of the peroxidation of both lipids and proteins. Moreover, the viruses demonstrated extreme sensitivity to ozone radicals.
Zimmermann et al. reported that plasma-generated ROS/RNS species have an effect on the DNA of adenoviruses and their immunogenicity [171]. In the study, a FlatPlaSter CAP device, based on surface micro-discharge plasma electrode allowing plasma generation under ambient conditions, was used. The researchers assumed that the ROS/RNS may dissolve in the virus embedding fluid and inactivate the adenoviruses afterwards. The tests also showed that 240 s plasma exposure was sufficient to significantly inhibit replication and caused a drastic reduction in adenovirus concentration.
Niedźwiedź et al. suggested that plasma-treated solutions, such as H2O, NaCl (0.9%), and H2O2 (0.3%), can assist in effective virus decontamination [168]. Together with Liao et al. [172] and Klämpfl T.G. et al. [173], they describe the sensitivity of microorganisms to plasma in the form of a pyramid, in which gram-negative bacteria show the lowest resistance to plasma, and bacterial spores the highest (see [168]). The researchers found that the sensitivity of certain groups of micro bio-objects highly depends on the conditions of the treatment.
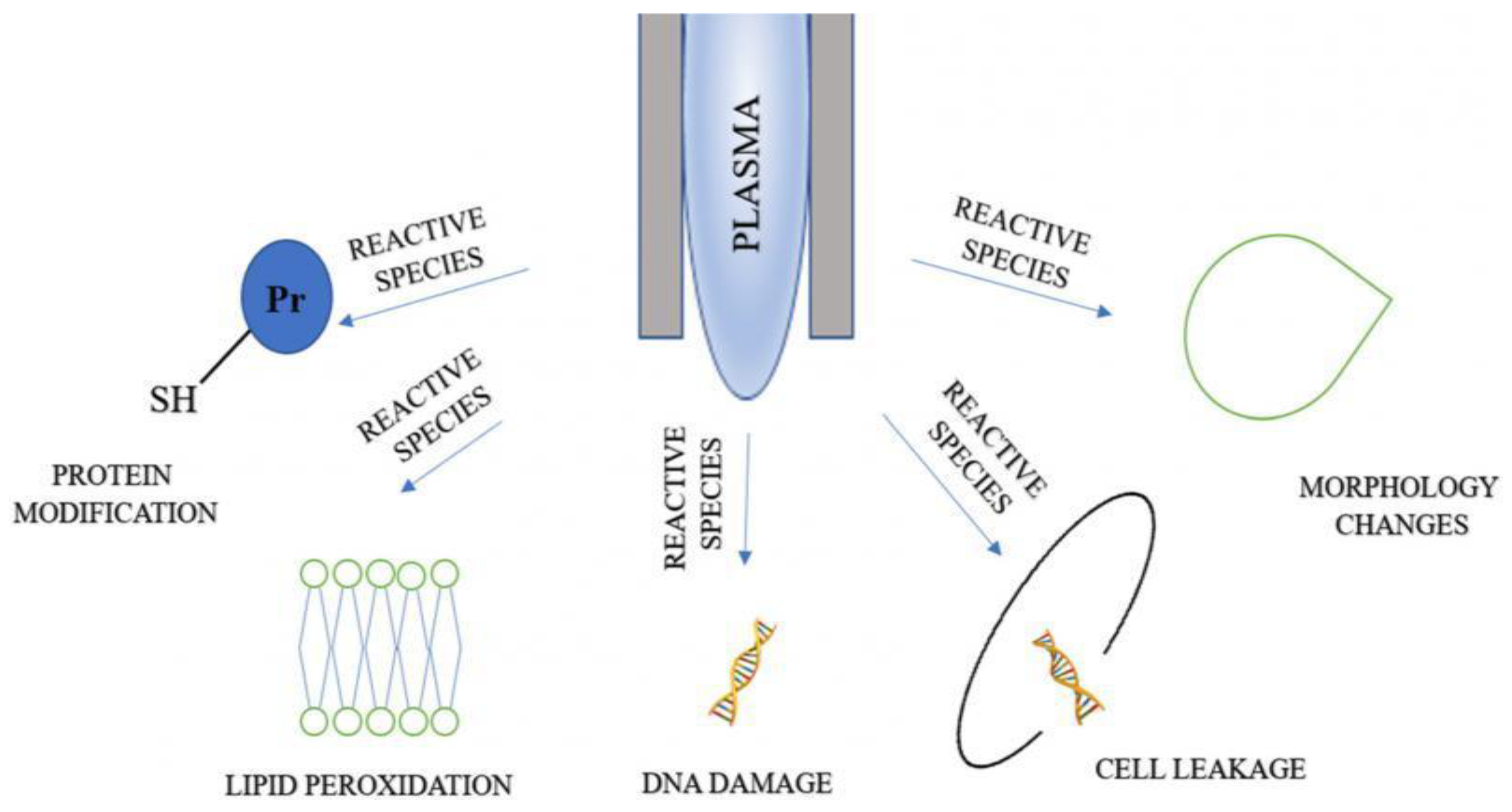
Figure 5 shows that the application of LTP on bacterial cells results in protein modification, lipid peroxidation, DNA damage and cell leakage, and changes in cell morphology. The reason w these changes take place is that plasma agents cause oxidative damage to intracellular proteins, potassium, and nucleic acids. According to [99,153,168,174], LTP may also cause the degradation and destruction of large protein and the peptide bonds of the cell, leading to a decrease in their enzymatic activity.
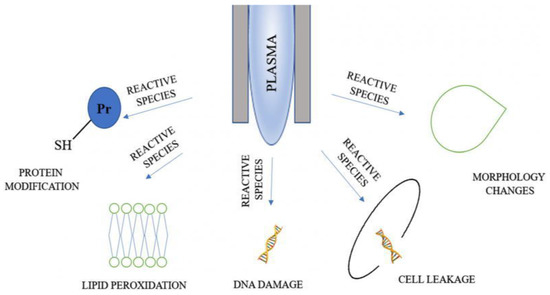
Figure 5.
Mechanisms of microbial inactivation with plasma agents [168].
The applications of LTP on bacterial biofilms are studied in [175,176,177]. It was assumed that the cell wall thicknesses may be correlated with the time duration of LTP treatment. Moreover, the plasma sterilants inactivate biofilms by destruction of the extracellular matrix and internal cell structure. However, ROS and RNS species could also cause undesirable effects, such as cellular component destruction and bacterial death [178].
The impact of LTP on yeast species were studied in [179,180,181,182,183]. In [179], the results of the inactivating effect of the DBD plasma of Enterococcus faecalis (Gram-positive), P. aeruginosa (Gram-negative) bacteria, and Candida albicans yeasts was described. The researchers reported that due to the differences in cell structure, C. albicans demonstrate higher plasma agent resistance than the Gram-positive bacteria. Research [180] conducted on S. cerevisiae cells with the use of dry air plasma generation exhibited a dependence between sterilisation effectiveness and the plasma synthesis parameters (applied voltage, gas composition, exposure time). References [181,182,183] inform that LTP can be efficient in inactivation of enzymes associated with plasma-reactive species interaction with the secondary structure of protein.
3.2.2. Wound Healing
The main aspects of LTP plasma wound treatment rely on two types of mechanism: physical, due to the effects of plasma-generated species, and biological, due to the processes of cell-membrane degradation and modification of its DNA. Plasma efficiency in this area is highly dependent on the possibility of inducing tissue regeneration and inactivating microbial cells.
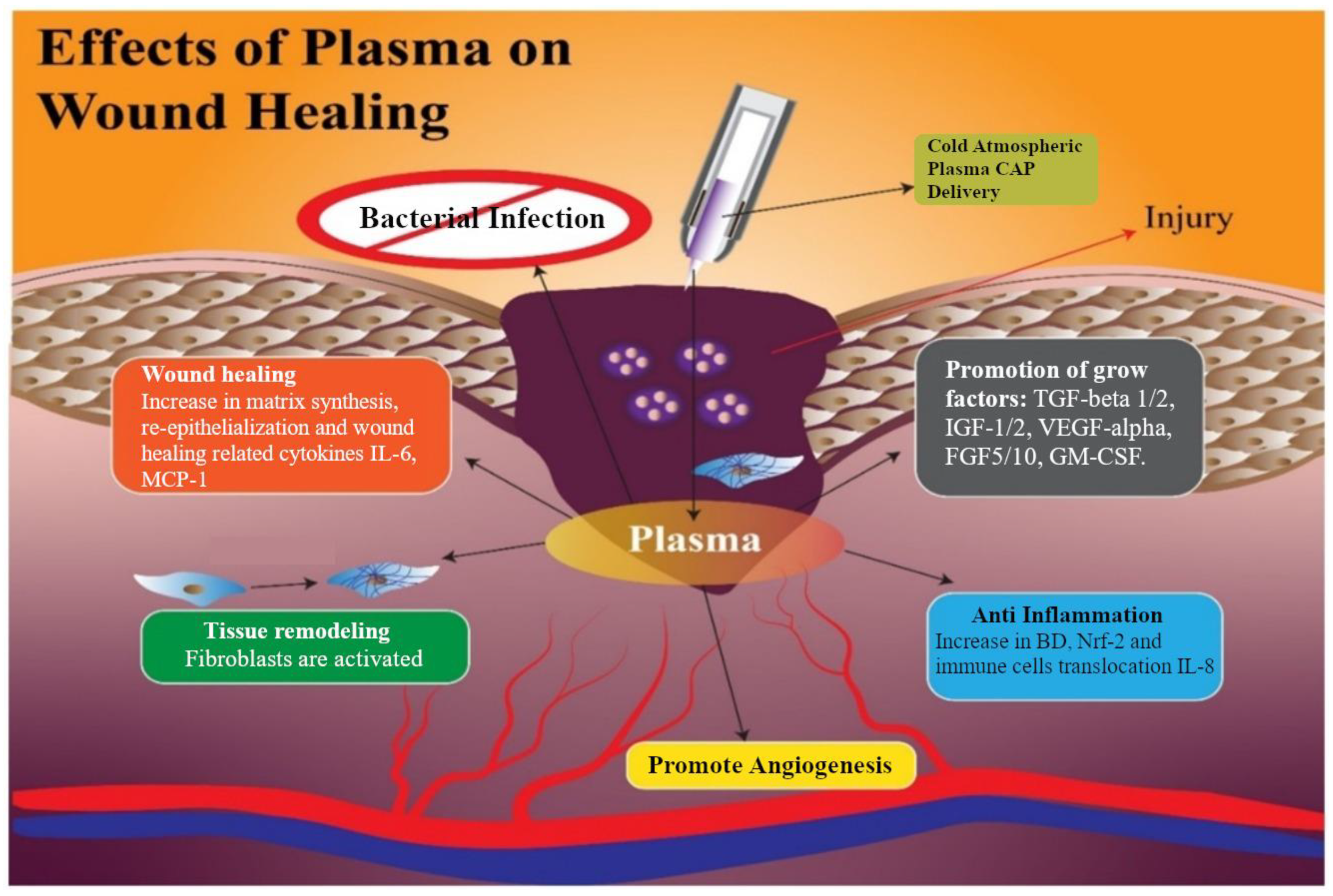
Dubey et al. [184] distinguished the following effects of plasma on wound healing: antimicrobial, self-healing, tissue remodelling, angiogenesis, anti-inflammatory, and growing factors (Figure 6). The scientists also suggested that the main advantages of nitride oxides generated from plasma are the prevention of tissue infection and promoting its healing [185,186], anti-inflammatory properties, and remodelling and angiogenesis [187]. According to [188,189], NO, ROS, and H2O2 species contribute to regulating re-epithelialisation and wound contraction. Plasma also causes the reduction in pH at the infected zone [190]. One of the key factors of understanding effective plasma treatment in wound healing is RONS interaction with cell membrane [189,191]. Plasma medicine in vitro and in vivo applications on the diabetic wound also demonstrates healing stimulation after helium gas plasma treatment [166].
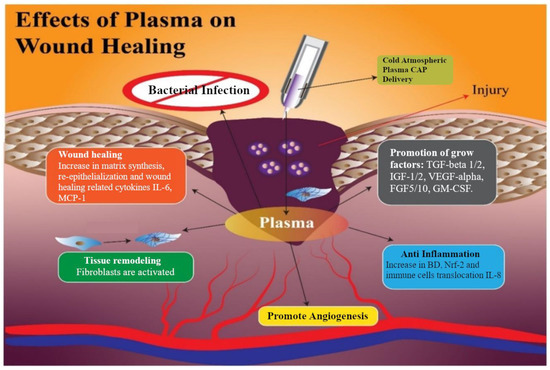
Figure 6.
Processes initiated by exposure of LTP and associated wound healing [184].
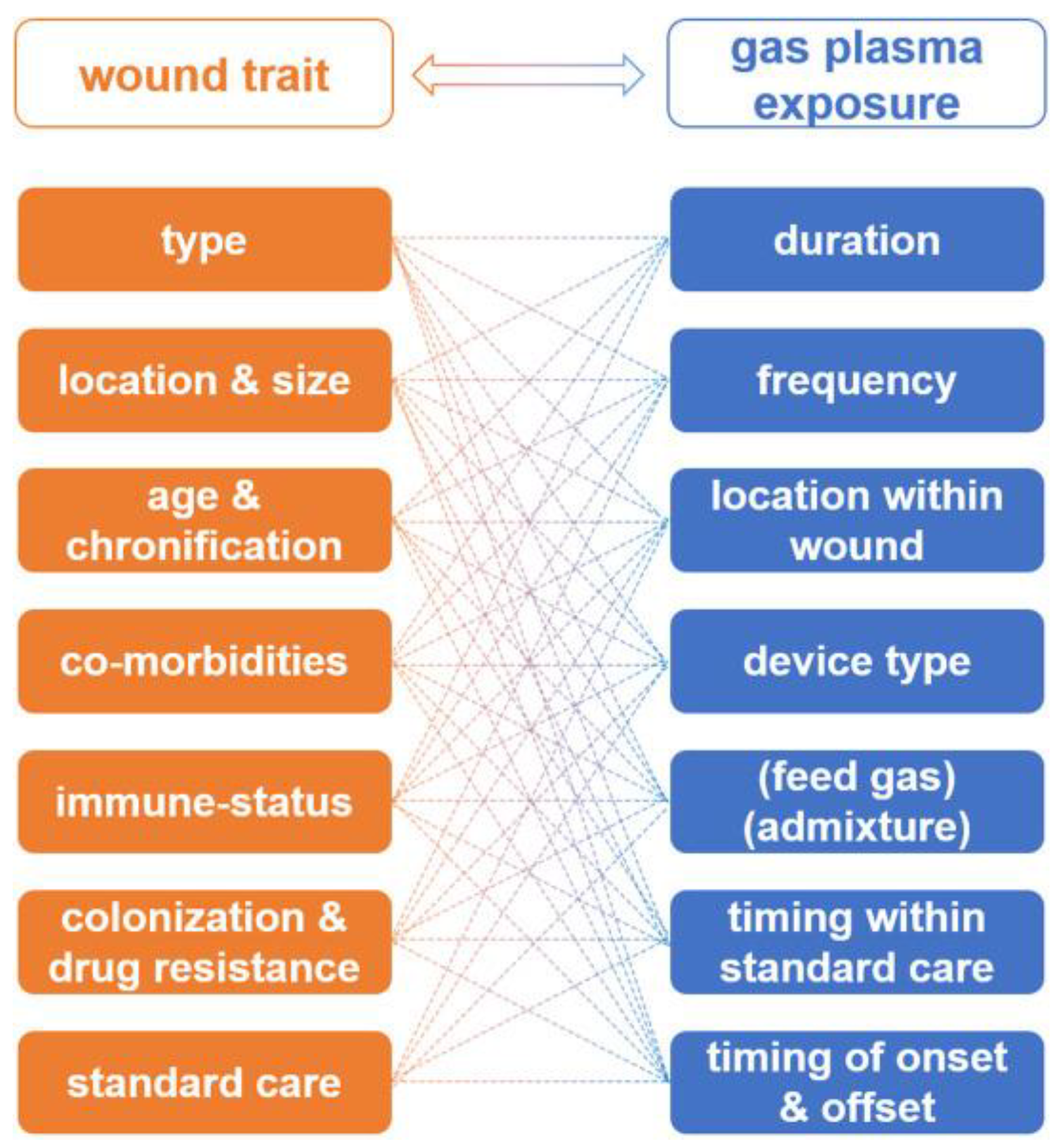
Bekeschus et al. in [62] developed the interdependency of wound characteristics, and unknown or non-standardised gas plasma therapeutic schemes, which can lead to the optimisation of wound therapy, but absolutely need clinical trials (Figure 7). The authors found that the issue of LTP application on wounds is complex and depends on a large number of factors such as: wound type, physical dimensions or volume, location, and possibility of proper plasma delivery.
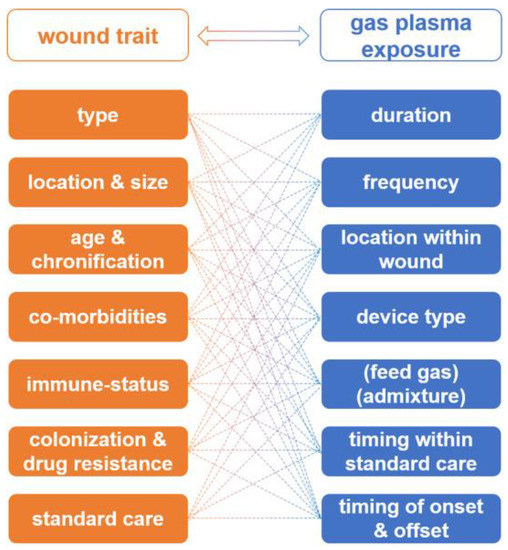
Figure 7.
The interdependency of wound characteristics and gas plasma therapeutic schemes [62].
The important factors playing a decisive role in wound treatment are also wound age and chronification, previous history of wound care [192,193], co-morbidities, immune status [194], wound colonisation profiles, and antimicrobial resistance against drugs [195]. Based on this information, a similar variety of gas plasma therapy parameters (duration, frequency, device type, gas mixture, etc.) must be taken into account to conduct safe and effective wound therapy.
3.2.3. Tumour Treatment
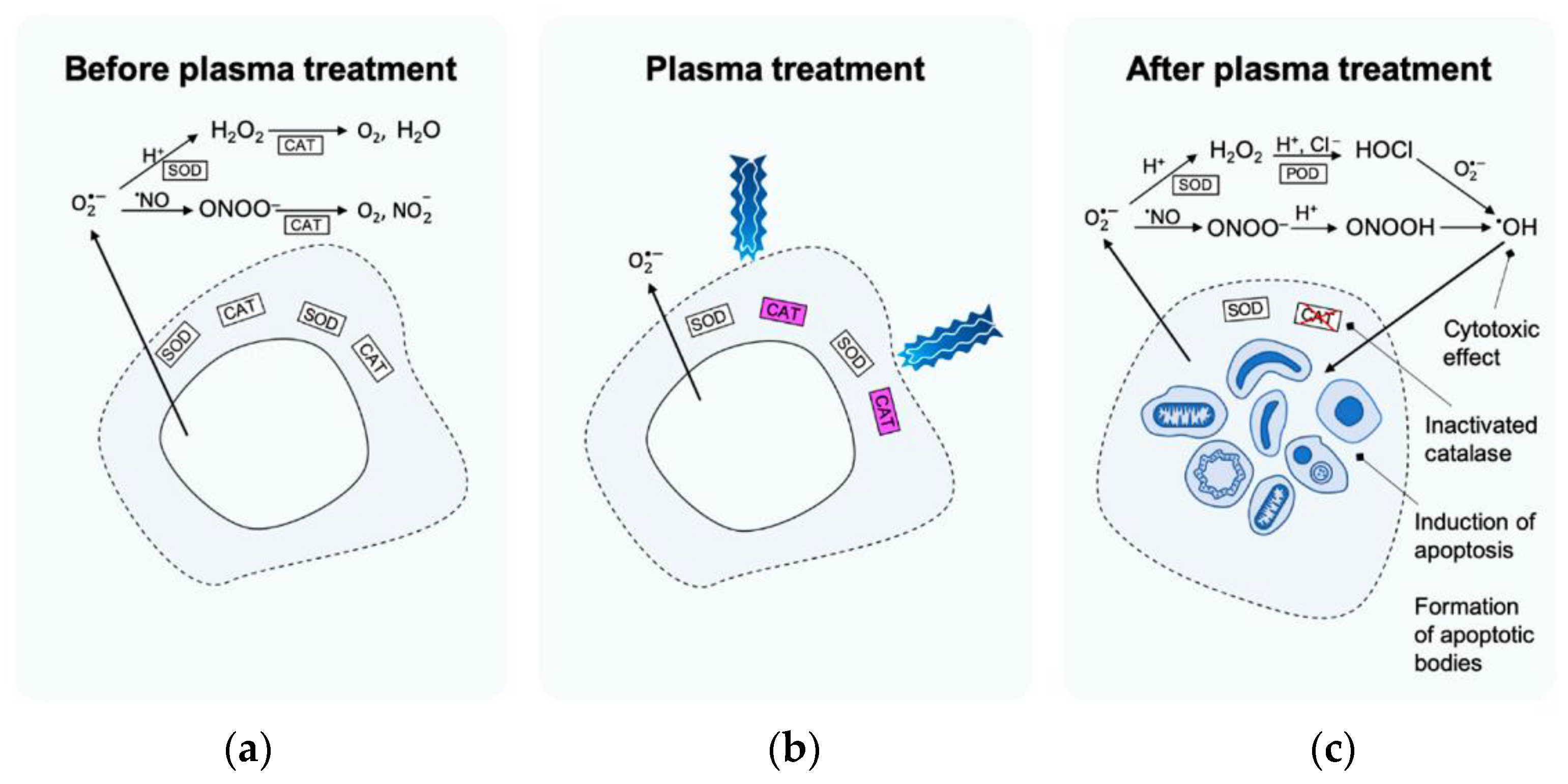
The most recent studies in the plasma oncology area are related to the direct application of different cold plasma techniques to tumour cells [196]. The most important task in tumour treatment lies in plasma-reactive species which are expected to penetrate several layers of cell membrane [197,198,199]. Privat-Maldonado et al. [200] proposed a set of possible events underlying the apoptosis mechanism (cell self-destruction when stimulated by the appropriate trigger) in cancer treatment after cold plasma applications (Figure 8). Before the treatment (Figure 8a), the catalyse (commonly widespread enzyme) protects the tumour cell from the damage of oxidative ROS species. LTP plasma application activates the catalyse by the singlet molecular oxygen 1O2 (Figure 8b). When plasma treatment is finished, the cytotoxic effect, formation of apoptosis bodies, and apoptosis induction can, afterwards, be observed (Figure 8c).
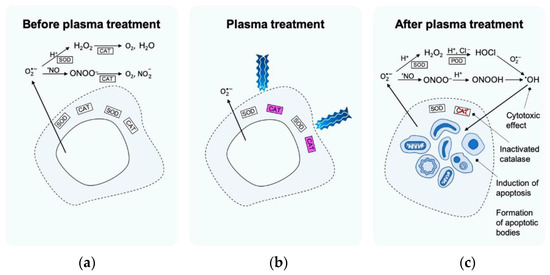
Figure 8.
Proposed set of events forming the basis of the mechanism of apoptosis induction in selective LTP cancer treatment [200]: (a) before plasma treatment, (b) plasma treatment, (c) after plasma treatment.
Figure 9 presents one the most successful approaches for plasma penetration into the tumour core. It allows the execution of an effective plasma application, focal with limited surgical invasion, and destroys the tumour radially from the inside. This concept was proposed in Hirst et al. [196,201]. The investigators suggested that this technique ensures enhanced targeted treatment of a tumour and controls tumour volume destruction. The efficiency of the concept depends on the plasma source and its synthesis parameters, such as voltage waveform, gas composition, and treatment duration.
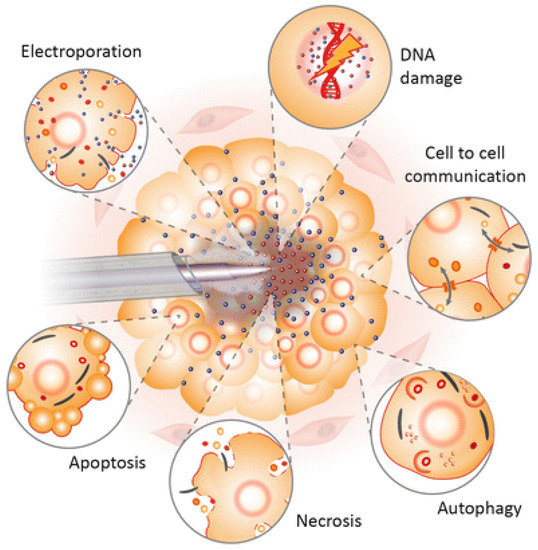
Figure 9.
LTP tumour treatment by inserting the probe into the tumour core [196].
A group of Korean scientists conducted research on T98G brain cancer cells [202,203,204]. They observed the cytogenetic damage in T98G brain cancer cells upon exposure of DBD plasma discharge. They also reported the influence of exposure and incubation times on the studied cells [202]. The experiments show that the micronucleus formation rate is highly dependent on the plasma exposure time. During the experiment, the cells were exposed to 30, 60, 120 and 240 s plasma treatment. The first two exposure times show less effectiveness than the longer times of 120 and 240 s. The maximal effect was observed with 240 s exposure, which inhibited the growth of cells even up to 80% at 48 and 72 h incubation after treatment [203]. Plasma-treated cell viability was found to be significantly dependent on incubation time. The authors also compared the impact of DBD plasma exposure on cancer cells and normal human embryonic kidney cells (HEK). They concluded that the treatment is much more toxic on T98G cells than on HEK cells. The best results were obtained in the case of exposure times less than 120 s, which demonstrated viability above 75%. Plasma treatment of normal cells for a longer time duration reduces their viability to approximately 45%. Moreover, the researchers found that plasma exposure can lead to irreversible changes in DNA, mitochondrial impairment, and caspase activation resulting in cancer cell death [204].
3.2.4. Dentistry Applications
Cold plasma exhibits a great potential for use in dental science. The first LTP application in dentistry was likely associated with sanitisation and disinfection of dental instruments [101,205]. Clinical equipment containing cold plasma devices or modules does not cause the vibrations, which leads to reduced pain perception by the patient [206]. In addition, the advantages of LTP use in dentistry are pathogenic bacteria degradation and non-inflammatory tissue alteration for dental cavities and composite restorations [207]. Furthermore, in comparison with conventional techniques, such as laser or drills, plasma strengthens the chemical bonding between teeth and fillings.
In addition, LTP is used for root canal disinfection and tooth bleaching [208]. Table 4 contains the different types of LTP plasma sources and their applications in dental science. Generally, cold plasma treatment processes in dentistry can be divided by their applications:
- Sterilisation: relies on killing viruses, bacteria, fungus, and bacterial endospores. Its effectiveness depends on plasma gas composition, microbial stain, or driving frequency. It is a well-established technique for the treatment of a wide range of instruments [209,210].
- Dental caries: using plasma in dental cavities without generation of heat, vibration, or noise. Plasma therapy is considered to be a popular tissue-saving approach for cleaning root canals within the damaged tooth; it generates bactericidal agents locally [211,212].
- Root canal disinfection: easy-to-use plasma jet devices generate plasma radiation inside the root canal without any pain. Such plasma treatment can effectively kill the bacteria responsible for failures of canal cleaning [213,214].
- Tooth bleaching: atmospheric pressure plasma used with OH radicals can erase coffee and alcohol staining from extracted teeth [100,215]. Low-frequency plasma sources in combination with OH can eliminate intrinsic stains [216]. They can also be used for preliminary treatment of deionised water for the target tooth [217].
- Clinical removal of biofilms: LTP makes possible the breaking down of biofilm matrices without harming the tissues [208], removing biofilms from dental implants [212]. It is also useful in decontamination of root canal biofilms and dental slices [213].
- Polymerisation: curing composite resin by using plasma. The LTP brush has already been tested for polymerised self-etch adhesives [214].
- Implant modification: LTP enhances surface roughness and wettability, leading to cell adhesion [218]. Plasma also helps in reducing the angle of implant contact and supports the spread of osteoblastic cells [219].
- Other processes: periodontal disease assistance [220,221], enhancing of bond strength between fibre-reinforced posts and resin composites for core building [222], mouth wound healing [223], intraoral disease curing, etc. [224].

Table 4.
Plasma applications in dentistry [205].
Table 4.
Plasma applications in dentistry [205].
| Dental Science Applications | Source of Plasma/Plasma Devices | Biological Models | References |
|---|---|---|---|
| Dental canal disinfection | Plasma jet device/He; He/O2 | Human extracted tooth | [225] |
| Dental canal disinfection | Plasma jet device/Ar/O2 | Human extracted tooth | [226] |
| Improvement of dental structures | Plasma brush/Ar; low pressure plasma device/O2, Ar, N2, and He + N2; HDBD device/Ar; plasma jet device | Human extracted tooth | [227,228,229] |
| Biofilm reduction | Kinpen MED® plasma jet/Ar | In vitro (bacteria, lab condition) | [161] |
| Biofilm reduction on titanium discs | Three different types of CAP devices: (a) kINPen plasma jet/Ar; (b) HDBD device/Ar; (c) VDBD device/Ar | In vitro (bacteria, lab condition), extracted tooth | [230] |
3.2.5. Veterinary Medicine
The vast majority of medical research, whether laboratory or clinical using non-thermal plasma, concerns research involving humans. A comparatively small number of articles report experiments already carried out on animals to improve their health problems related to pathogens, bacteria, wound healing, and other ailments for which human-aimed plasma-treatment methods have already been found. Nevertheless, dermatologists from Cummings School of Veterinary Medicine at Tufts University (Grafton, MA, USA) reported that the CAP can be used to treat skin wounds on all types of animals, from cats and dogs to horses, birds, farm animals, and even exotic animal species [231]. The scientists use the kINPen plasma jet for painless treatment of cat and dog superficial skin infections, benign skin growths, chronic wounds, etc.
Nonthermal atmospheric plasma can also be useful in canine osteosarcoma treatment, especially in large breeds [232]. This is a primary bone tumour which usually arises in the bones of the limbs, skull, spine, or ribcage, and in rare cases arising in non-boney tissues. Lee et al. presented research where D-17 and DSN canine osteosarcoma cell lines subdued to DBD CAP treatment were studied. The authors suggested both lines of DNA damage were caused by ROS plasma agents in a time-dependent manner. Moreover, further CAP treatment resulted in induction of cells apoptosis, inhibition of the invasion, and migration activity of cells.
The most widespread pathogens that cause bacterial skin and ear infections, such as canine pyoderma and otitis in dogs are Staphylococcus spp. (Gram-positive bacteria), and Pseudomonas aeruginosa, Proteus spp., and Escherichia coli (Gram-negative bacteria) [233,234]. Jin et al. [235] presented the application of cold atmospheric microwave plasma (CAMP) on Staphylococcus pseudintermedius field strains, obtained from dog skin with pyoderma and ears with otitis externa. Plasma-generation parameters included argon gas, a microwave energy range of 30–50 W, power supply frequency of 2450 Mhz, and gas flow rate after ionisation of 10–20 L/min. The experiments showed that 30 W CAMP-treatment of P. aeruginosa, E. coli, and S. aureus at 10, 30 and 60 s exposures caused a very significant survival rate reduction, even up to 0.7–0.8% (60 s of treatment). All studied organisms, except S. aureus, were completely killed after 60 s of plasma exposure in 50 W mode. To kill S. aureus cells, an additional 120 s of treatment was given.
A novel therapy method using non-invasive physical plasma (NIPP) treatment was developed by a German scientific group. Nitsch et al. presented research on NIPP exposure applied to a hedgehog with a head injury [236]. The experiment was carried out with a male European hedgehog with a head wound of unknown aetiology. NIPP treatment was performed in combination with conventional pharmacological wound therapy that relied on primary wound cleansing with octenidine, and antibiotic therapy with amoxicillin after the first plasma treatment. The Plasma Care NIPP device from Terraplasma Medical (Garchingen, Germany) was used for NIPP generation. The therapy lasted 12 days until complete wound closure. The plasma treatment was carried out every for 3–4 days with durations of 1 to 3 min. The authors concluded that the combination of common wound care and NIPP showed much better results than each of these approaches separately. This combined technique can also be applied for wound and tissue healing in dogs [237].
The optimisation of wound healing in sheep with the use of low-temperature atmospheric plasma was recognised as an innovative therapeutic method for extensive and chronic wound treatment [238]. Martines et al. studied square wounds (4 × 4 cm) on sheep backs under 2 min of daily indirect plasma treatment, using the radiofrequency plasma source described in [239]. They reported an increase in cell proliferation, a reduction in inflammation, a drastic reduction in bacterial load, and stimulation of blood vessel formation, after the completed plasma therapy.
4. Summary and Prospects
The antimicrobial properties of plasmas in the case of decontamination of water, ambient air and surfaces, as well as in biomedical treatment, is widely proven, as evidenced by numerous scientific publications, but also by the many examples of practical and, in the case of biomedicine, clinical applications.
AOTS using ozone in combination with other strong active species, such as hydrogen peroxide, hydroxyl radicals, and UV radiation, can be successfully applied to combat bactericidal pollution of soils and allow for a synergetic effect. The contamination of soils in public grounds and in agriculture with various microorganisms, including parasite eggs from pets and wild animals, in both developing and developed countries, raises public concern. The influence of ozone in the air and soil on seed and plant development has been broadly investigated. Ozone injected into previously oversaturated soil was reported to be beneficial for the growth of tomatoes and certain fungi, as well as for the treatment of seeds and bulbs, causing 15–20% disease immunity enhancement of cotton plants and an improvement in the morpho-biological and technological parameters of cotton fibre, 12–15% higher grain yield with better quality of corn grain, and over 60% improvement in bean seed germination. After-flood agricultural soil should not be cultivated for three or more years to prevent crop contamination. However, the application of AOT-based treatment techniques significantly shortened this period.
The use of non-thermal plasma in biomedical applications is very promising and has aroused great interest in recent years. Non-thermal plasma has been explored for its potential use in wound healing, skin cancer treatment and viral, bacterial, and fungal sterilisation. The advantage of plasma technology is its high degree of versatility and adaptability. Non-thermal plasma can be generated in a wide range of energies, in a variety of gases, and using an increasing variety of generators. This multitude of parameters related to plasma production allows for fine-tuning of the nature, quality, and intensity of the produced plasma to specific biomedical needs. Plasma reactors generating plasma at atmospheric pressure can be built in various shapes and sizes, with the use of various types of electrical discharges (i.e., DBD, APPJ, CD, PN, ARC and GAD, PT, and MWD).
Plasma biomedicine and agriculture are newly discovered, promising, and rapidly developing areas of science and engineering. Plasma in biomedicine focuses on the mechanisms of plasma agent interaction with cells and cellular structures. A deeper understanding of this topic should bring new results in tumour and skin disease, dental sciences, wound healing, antimicrobial treatments, dermatology, and oncology.
Cold atmospheric plasma demonstrates great potential for applications in seed germination, seed decontamination, plant disease control, water cleaning, water activation with minerals, and soil purification. In the food industry, plasma can contribute to inactivating different pathogens in food products, increasing lipid oxidation and acidity of products, reducing colour intensity, and increasing fruit firmness.
For the last 20 years, interdisciplinary teams have been working on plasma and its potential to solve agricultural and biomedical problems. They include scientists in the field of chemistry and physics, chemical technology, environmental engineering, agricultural engineering, biochemistry, microbiology, medicine, bioengineering, metrology, materials, and electrical engineering.
Funding
This research was funded by Lublin University of Technology grants numbers FD-20/EE-2/416, FD-20/EE-2/401 intended for research activities within the “Automatics, Electronics and Electrical Engineering” scientific discipline.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 2011–2013 Review of the EU Clean Air Policy. Available online: https://ec.europa.eu/environment/air/clean_air/review.htm (accessed on 23 March 2022).
- Halit, E. Environmental Impacts of Technology. In Wiley Encyclopedia of Electrical and Electronics Online; Webster, J., Ed.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2001. [Google Scholar]
- UNECE. Natural Resource Nexuses in the ECE Region; United Nations: Geneva, Switzerland, 2021. [Google Scholar]
- Harrison, R.M. Pollution-Causes, Effects and Control, 3rd ed.; The Royal Society of Chemistry: Cambridge, UK, 1996. [Google Scholar]
- Hipler, R.; Kersten, H.; Schmidt, M.; Schoenbach, K.H. (Eds.) Low Temperature Plasmas. Fundamentals, Technologies and Techniques; Second Revised and Enlarged Edition; WILEY-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2008; Volume 1, 945p, ISBN 978-3-527-40673-9. [Google Scholar]
- Attri, P.; Arora, B.; Choi, E.H. Retraction: Utility of plasma: A new road from physics to chemistry. RSC Adv. 2017, 7, 15735. [Google Scholar] [CrossRef]
- Stryczewska, H.D. Supply Systems of Non-Thermal Plasma Reactors. Construction Review with Examples of Applications. Appl. Sci. 2020, 10, 3242. [Google Scholar] [CrossRef]
- Stryczewska, H.D. Plasma Technologies in Energy and Environmental Engineering; Electrotechnical Committee of Polish Academy of Science PAN: Lublin, Poland, 2009; p. 212. ISBN 978-83-7497-070-9. (In Polish) [Google Scholar]
- Penetrante, M.; Schultheis, S.E. (Eds.) Non-thermal Plasma techniques for Pollution Control, Part A: Overview, Fundamentals and Supporting Technologies; NATO ASI Series G: Ecological Science; Springer: Berlin, Germany, 1993; Volume 34, part B. [Google Scholar]
- Jedrzejczyk, T.; Kolacinski, Z.; Koza, D.; Raniszewski, G.; Szymanski, L.; Wiak, S. Plasma recycling of chloroorganic wastes. Open Chem. 2015, 13, 156–160. [Google Scholar] [CrossRef]
- Pawełek, J.; Bergel, T.; Siedlecka, E.; Biń, A.K.; Szatkowska, B.; Muszański, R.M.; Kosiniak, M. Techniczne i ekonomiczne aspekty zastosowania innowacyjnej metody ozonowania do usuwania wybranych farmaceutyków ze ścieków–badania w skali ułamkowo-technologicznej w oczyszczalni ścieków Jaworzno Dąb. Technical and economic aspects of using the innovative ozonation method for removal of selected pharmaceuticals from sewage—Research on a fragmentary-technical scale in the Jaworzno Dąb wastewater treatment plant. Instal 2021, 7, 33–38. (In Polish) [Google Scholar] [CrossRef]
- Zhang, H.; Ma, D.; Qiu, R.; Tang, Y.; Du, C. Non-thermal plasma technology for organic contaminated soil remediation: A review. Chem. Eng. J. 2017, 313, 157–170. [Google Scholar] [CrossRef]
- Mitsugi, F.; Nagatomo, T.; Takigawa, K.; Sakai, T.; Ikegami, T.; Nagahama, K.; Ebihara, K.; Sung, T.; Teii, S. Properties of soil treated with ozone generated by surface discharge. IEEE Trans. Plasma Sci. 2014, 42, 3706–3711. [Google Scholar] [CrossRef]
- Mitsugi, F.; Ebihara, K.; Aoqui, S.; Stryczewska, H.D. Review of Developments in Application of Ozone in Agriculture. In Proceedings of the 16th High Pressure Low Temperature Plasma Chemistry Symposium HAKONE XVI, Beijing, China, 2–7 September 2018. [Google Scholar]
- Szymański, Ł.; Kolacinski, Z.; Raniszewski, G. Influence of Contaminants on Arc Properties during Treatment of Polluted Soils in Electric Arc Plasma. J. Adv. Oxid. Technol. 2012, 15, 34–40. [Google Scholar]
- Stryczewska, H.D.; Ebihara, K.; Muszański, R. Mobile installations of air, water and soil treatment with ozone. In Proceedings of the 23rd International Conference on Advanced Oxidation Technologies for Treatment of Water, Air and Soil-(AOTs-23), Clearwater Beach, FL, USA, 13–16 November 2017; pp. 43–44. [Google Scholar]
- Yamashita, Y.; Yamashita, T.; Hashimoto, Y.; Ebihara, K.; Mitsugi, F.; Ikegami, T.; Stryczewska, H.D.; Pawłat, J.; Teil, S.; Sung, T.L. Backpack-type ozone-mist sterilization system developed for non-chemical agriculture processes. In Proceedings of the 8th International Conference ELMECO-8 “Electromagnetic Devices and Processes in Environment Protection” joint with 11th Seminar AoS-11 “Applications of Superconductors”, Nałęczów, Poland, 28 September–1 October 2014. [Google Scholar]
- Ebihara, K.; Takayama, M.; Stryczewska, H.D.; Ikegami, T.; Gyoutoku, Y.; Tachibana, M. Wide range concentration control of dielectric barrier discharge generated ozone for soil sterilization. IEEJ Trans. Fundam. Mater. 2006, 126, 963–969. [Google Scholar] [CrossRef][Green Version]
- Pawłat, J.; Stryczewska, H.D.; Ebihara, K. Sterilization Techniques for Soil Remediation and Agriculture Based on Ozone and AOP. J. Adv. Oxid. Technol. 2010, 13, 138–145. [Google Scholar] [CrossRef]
- Stryczewska, H.D.; Pawłat, J.; Ebihara, K. Non-thermal plasma aided soil decontamination. J. Adv. Oxid. Technol. 2013, 16, 23–30. [Google Scholar] [CrossRef][Green Version]
- Pawłat, J.; Stryczewska, H.D.; Ebihara, K.; Mitsugi, F.; Sung, T. AOTs and solar energy for air, water and soil treatment. Trans. Mater. Res. Soc. Jpn. 2014, 39, 117–120. [Google Scholar] [CrossRef][Green Version]
- Machala, Z.; Hensel, K.; Akishev, Y. (Eds.) Plasma for Bio-Decontamination, Medicine and Food Security. In Proceedings of the NATO Advanced Research Workshop on Plasma for Bio-Decontamination, Medicine and Food Security, Demänovská Dolina, Slovakia, 15–18 March 2011; Springer: Dordrecht, The Netherlands, 2012. [Google Scholar] [CrossRef]
- Domonkos, M.; Tichá, P.; Trejbal, J.; Demo, P. Applications of Cold Atmospheric Pressure Plasma Technology in Medicine, Agriculture and Food Industry. Appl. Sci. 2021, 11, 4809. [Google Scholar] [CrossRef]
- Starek, A.; Pawłat, J.; Chudzik, B.; Kwiatkowski, M.; Terebun, P.; Sagan, A.; Andrejko, D. Evaluation of selected microbial and physicochemical parameters of fresh tomato juice after cold atmospheric pressure plasma treatment during refrigerated storage. Sci. Rep. 2019, 9, 8407. [Google Scholar] [CrossRef] [PubMed]
- Dasan, B.G.; Onal-Ulusoy, B.A.; Pawlat, J.; Diatczyk, J.; Sen, Y.S.; Mutlu, M. New and Simple Approach for Decontamination of Food Contact Surfaces with Gliding Arc Discharge Atmospheric Non-Thermal Plasma. Food Bioprocess Technol. 2017, 10, 650–661. [Google Scholar] [CrossRef]
- Hati, S.; Patel, M.; Yadav, D. Food bioprocessing by non-thermal plasma technology. Curr. Opin. Food Sci. 2018, 19, 85–91. [Google Scholar] [CrossRef]
- Yousefi, M.; Mohammadi, M.A.; Zabihzadeh Khajavi, M.; Ehsani, A.; Scholtz, V. Application of Novel Non-Thermal Physical Technologies to Degrade Mycotoxins. J. Fungi 2021, 7, 395. [Google Scholar] [CrossRef]
- Stryczewska, H.D.; Ebihara, K.; Mitsugi, F.; Pawlat, J. Application of non-thermal plasma in agriculture. In Workshop of Application of Advanced Plasma Technologies in CE Agriculture; Slovenian Society for Vacuum Technique (DVTS): Ljubljana, Slovenia, 2017. [Google Scholar]
- Fridman, G.; Ayan, H.; Fridman, A.; Gutsol, A.; Vasilets, V.; Friedman, G.; Shereshevsky, A.; Balasubramanian, M.; Peddinghaus, M.; Brooks, A. Sterilization of Living Human and Animal Tissue by Non-Thermal Atmospheric Pressure Dielectric Barrier Discharge Plasma. In Proceedings of the IEEE 34th International Conference on Plasma Science (ICOPS), Albuquerque, NM, USA, 17–22 June 2007; IEEE: Piscataway, NJ, USA, 2007. [Google Scholar] [CrossRef]
- Kuo, S.P. Air plasma for medical applications. J. Biomed. Sci. Eng. 2012, 5, 481–495. [Google Scholar] [CrossRef][Green Version]
- Sakudo, A.; Yagyu, Y.; Onodera, T. Disinfection and Sterilization Using Plasma Technology: Fundamentals and Future Perspectives for Biological Applications. Int. J. Mol. Sci. 2019, 20, 5216. [Google Scholar] [CrossRef]
- Michael, K. Adaptive Plasmas for Plasma Medicine. In Proceedings of the 74th Annual Gaseous Electronics Conference Monday–Friday, Huntsville, AL, USA, 4–8 October 2021. [Google Scholar]
- Stoffels, E. “Tissue Processing” with atmospheric plasmas. Contrib. Plasma Physics. 2007, 47, 40–48. [Google Scholar] [CrossRef]
- Lendeckel, D.; Eymann, C.; Emicke, P.; Daeschlein, G.; Darm, K.; O’Neil, S.; Beule, A.G.; von Woedtke, T.; Völker, U.; Weltmann, K.-D.; et al. Proteomic changes of tissue-tolerable plasma treated airway epithelial cells and their relation to wound healing. Proteomic changes of tissue-tolerable plasma treated airway epithelial cells and their relation to wound healing. Biomed Res. Int. 2015, 2015, 506059. [Google Scholar] [CrossRef] [PubMed]
- Hasse, S.; Hahn, O.; Kindler, S.; von Woedtke, T.; Metelmann, H.R.; Masur, K. Atmospheric pressure plasma jet application on human oral mucosa modulates tissue regeneration. Plasma Med. 2014, 4, 117–1129. [Google Scholar] [CrossRef]
- Plasmatis Initiative Group. Declaration of the 1st International Workshop on Plasma Tissue Interactions. GMS Krankenh. Interdiszip 2008, 3, Doc01. [Google Scholar]
- Hammann, A.; Huebner, N.-O.; Bender, C.; Ekkernkamp, A.; Hartmann, B.; Hinz, P.; Kindel, E.; Koban, I.; Koch, S.; Kohlmann, T.; et al. Antiseptic efficacy and tolerance of tissue-tolerable plasma compared with two wound antiseptics on artificially bacterially contaminated eyes from commercially slaughtered pigs. Skin Pharmacol. Physiol. 2010, 23, 328–332. [Google Scholar] [CrossRef] [PubMed]
- Komarzyniec, G.; Stryczewska, H.D.; Muszański, R. Autonomous Water Treatment Installation Energized from PV Panels. J. Adv. Oxid. Technol. 2010, 13, 146–152. [Google Scholar] [CrossRef]
- Stryczewska, H.D.; Komarzyniec, G.K. Properties of Gliding Arc (GA) Reactors Energized from AC/DC/AC Power Converters. In Proceedings of the IEEE Region 8 International Conference on Computational Technologies in Electrical and Electronics Engineering SIBIRCON-2010, Irkutsk, Russia, 11–15 July 2010; IEEE: Piscataway, NJ, USA, 2010. [Google Scholar] [CrossRef]
- Krupski, P.; Stryczewska, H.D. A Gliding Arc Microreactor Power Supply System Based on Push-Pull Converter Topology. Appl. Sci. 2020, 10, 3989. [Google Scholar] [CrossRef]
- Krupski, P.; Stryczewska, H.D. GLIDARC reactor power supply with ignition improvement. COMPEL—Int. J. Comput. Math. Electr. Electron. Eng. 2019, 38, 1274–1284. [Google Scholar] [CrossRef]
- Komarzyniec, G.; Stryczewska, H.D.; Krupski, P. The Influence of the Architecture of the Power System on the Operational Parameters of the Glidarc Plasma Reactor. In Proceedings of the 2019 IEEE Pulsed Power & Plasma Science (PPPS), Orlando, FL, USA, 23–29 June 2019; pp. 1–4. [Google Scholar] [CrossRef]
- Krupski, P.; Stryczewska, H. The Investigation of The Properties of High-voltage Transformer in Nonthermal Plasma Pulse Power Supply. In Proceedings of the 14th Selected Issues of Electrical Engineering and Electronics (WZEE), Szczecin, Poland, 19–21 November 2018; pp. 1–4. [Google Scholar]
- Krupski, P.; Stryczewska, H.D.; Komarzyniec, G. The Push-Pull Plasma Power Supply—A Combining Technique for Increased Stability. In Proceedings of the 2019 IEEE Pulsed Power & Plasma Science (PPPS), Orlando, FL, USA, 23–29 June 2019; pp. 1–4. [Google Scholar] [CrossRef]
- Komarzyniec, G.; Stryczewska, H.D.; Aftyka, M. Reduction of the Conducted Disturbances Generated by the Ignition Systems of Glidarc Plasma Reactors. In Proceedings of the 2019 IEEE Pulsed Power & Plasma Science (PPPS), Orlando, FL, USA, 23–29 June 2019; pp. 1–4. [Google Scholar] [CrossRef]
- Karl, T. Compton and Irving Langmuir, Electrical Discharges in Gases. Part I. Survey of Fundamental Processes. Rev. Mod. Phys. 1930, 2, 123. [Google Scholar]
- Boulos, M.I.; Fauchais, P.; Pfender, E. Thermal Plasma: Fundamental and Applications; Plenum Press: New York, NY, USA, 1994; Volume 1. [Google Scholar]
- Kanegae, H. Ecofriendly Ozone-Based Wet Processes for Electron Device Fabrication. Mitsubishi Electr. Adv. Tech. Rep. 1999, 86, 2–6. [Google Scholar]
- Lesueur, H.; Czernichowski, A.; Chapelle, J. Apparatus for generation of low temperature plasmas by the formation of gliding arc discharges. Pat. Appl. Fr. Natl. Regist. 1990, 8814932. [Google Scholar]
- Czernichowski, A. Gliding Discharge Reactor for H2S Valorization or Destruction. In Non-Thermal Plasma Techniques for Pollution Control, Part A: Overview, Fundamentals and Supporting Technologies, Proceedings of the NATO Advanced Research Workshop on Non-Thermal Plasma Techniques for Pollution Control, Cambridge, UK, 21–25 September 1992; Penetrante, M., Schultheis, S.E., Eds.; Springer: Berlin/Heidelberg, Germany, 1993. [Google Scholar] [CrossRef]
- Stryczewska, H.D. New supply system of non-thermal plasma reactor with gliding arc. Arch. Electr. Eng. 1997, 46, 379–399. [Google Scholar]
- Janowski, T.; Stryczewska, H.D. Application of the Third Magnetic Flux Harmonic of Transformer Cores in Plasma Reactors. In Studies in Applied Electromagnetics and Mechanics, Nonlinear Electromagnetic Systems; IOS Press: Amsterdam, The Netherlands, 1996; Volume 10, pp. 31–34. [Google Scholar]
- Janowski, T.; Stryczewska, D.; Mastalarczuk, M.; Yamada, S.; Bessho, K. Performances of medium frequency supplying system for plasma reactor. J. Jpn. Soc. Appl. Electromagn. 1993, 1, 19–22. [Google Scholar]
- Janowski, T.; Stryczewska, H.D. Magnetic Frequency Triplers: Another Way to Supply Ozone Generators. Ozone-Sci. Eng. 1992, 14, 139–152. [Google Scholar] [CrossRef]
- Stryczewska, H.; Janowski, T. Investigation of an Ozone Generation System with Magnetic Frequency Tripler. In Scientific Papers of the Lublin University of Technology; Lublin University of Technology: Lublin, Poland, 1990; pp. 63–69. [Google Scholar]
- Czernichowski, A.; Ranaivosoloarimanana, A.; Janowski, T.; Stryczewska, H.D.; Cojan, M. Chemical Processing of CO2 in a Gliding Arc Reactor Supplied at 50 or 150 Hz. In Proceedings of the International Conference on Electromagnetic Devices and Processes in Environment Protection ELMECO’94, Lublin, Poland, 8–9 September 1994; pp. 11–24. [Google Scholar]
- Janowski, T.; Stryczewska, H.D.; Czerwiński, D.; Bessho, K.; Yamada, S. Model of the plasma reactor supplying system with frequency tripler. In Proceedings of the Third MAGDA Conference, Osaka, Japan, 10–15 April 1994; pp. 219–222. [Google Scholar]
- Janowski, T.; Stryczewska, H.D. An electrical model of an ozone generator for computer simulation. In Proceedings of the Abstracts of International Conference on Modelling and Simulation, Shekou, China, 7–9 November 1988; p. 132. [Google Scholar]
- Franklin, R.N.; Braithwaite, N.S.J. 80 Years of Plasma. Plasma Sources Sci. Technol. 2009, 18, 010201. [Google Scholar] [CrossRef]
- Von Woedtke, T.; Reuter, S.; Masura, K.; Weltmanna, K.D. Plasmas for medicine. Phys. Rep. 2013, 530, 291–320. [Google Scholar] [CrossRef]
- Alekseev, O.; Donovan, K.; Limonnik, V.; Azizkhan-Clifford, J. Nonthermal dielectric barrier discharge (DBD) plasma suppresses herpes simplex virus type 1 (HSV-1) replication in corneal epithelium. Transl. Vis. Sci. Technol. 2014, 3, 2. [Google Scholar] [CrossRef] [PubMed]
- Daeschlein, G.; Napp, M.; von Podewils, S.; Lutze, S.; Emmert, S.; Lange, A.; Klare, I.; Haase, H.; Gümbel, D.; von Woedtke, T.; et al. In Vitro susceptibility of multidrug resistant skin and wound pathogens against low temperature atmospheric pressure plasma jet (APPJ) and dielectric barrier discharge plasma (DBD). Plasma Process Polym. 2014, 11, 175–183. [Google Scholar] [CrossRef]
- Ebihara, K.; Sugimoto, S.; Ikegami, T.; Mitsugi, F.; Stryczewska, H.D. Application of gaseous ozone to agricultural soil sterilization. In Proceedings of the sixth International Conference ELEMCO-6 Joint with 9th Seminar Applications of Superconductors AoS-9, Nałęczów, Poland, 24–27 June 2008; Lublin University of Technology: Lublin, Poland, 2008; pp. 11–13, ISBN 978-83-61301-20-2. [Google Scholar]
- Bekeschus, S.; von Woedtke, T.; Emmert, S.; Schmidt, A. Medical gas plasma-stimulated wound healing: Evidence and mechanisms. Redox Biol. 2021, 46, 102116. [Google Scholar] [CrossRef]
- Schmidt, A.; Bekeschus, S.; Wende, K.; Vollmar, B.; von Woedtke, T. A cold plasma jet accelerates wound healing in a murine model of full-thickness skin wounds. Exp. Dermatol. 2017, 26, 156–162. [Google Scholar] [CrossRef]
- Fridman, G.; Shereshevsky, A.; Peddinghaus, M.; Gutsol, A.; Vasilets, V.; Brooks, A.; Balasubramanian, M.; Friedman, G.; Fridman, A. Bio-Medical Applications of Non-Thermal Atmospheric Pressure Plasma. In Proceedings of the 37th AIAA Plasmadynamics and Lasers Conference, San Francisco, CA, USA, 5–8 June 2006. [Google Scholar]
- Jarosław, D.; Grzegorz, K.; Robert, M.; Henryka, D. Stryczewska, Solar powered ozone installation for water treatment in swimming pools. In Proceedings of the Workshop on Progress in New Methods of Water and Waste Water Cleaning, Gdańsk, Poland, 4–5 July 2011. [Google Scholar]
- Bogle, M.A.; Arndt, K.A.; Dover, J.S. Evaluation of plasma skin regeneration technology in low-energy full-facial rejuvenation. Arch. Dermatol. 2007, 143, 168–174. [Google Scholar] [CrossRef]
- Kilmer, S.; Semchyshyn, N.; Shag, G.; Fitzpatrick, R. A pilot study on the use of a plasma skin regeneration device (Portrait PSR3) in full facial rejuvenation procedures. Lasers Med. Sci. 2007, 22, 101–109. [Google Scholar] [CrossRef]
- Elsaie, M.L.; Kammer, J.N. Evaluation of plasma skin regeneration technology for cutaneous remodeling. J. Cosmet. Dermatol. 2008, 7, 309–311. [Google Scholar] [CrossRef] [PubMed]
- Potter, M.J.; Harrison, R.; Ramsden, A.; Bryan, B.; Andrews, P.; Gault, D. Facial acne and fine lines: Transforming patient outcomes with plasma skin regeneration. Ann. Plast. Surg. 2007, 58, 608–613. [Google Scholar] [CrossRef] [PubMed]
- Weltmann, K.D.; von Woedtke, T. Basic requirements for plasma sources in medicine. Eur. Phys. J. Appl. Phys. 2011, 55, 13807. [Google Scholar] [CrossRef]
- Mączka, T. Technologia Plazmowego Zgazowania Biomasy i Odpadów Organicznych dla Wytwarzania Paliw Płynnych (Technology of Plasma Gasification of Biomass and Organic Waste for the Production of Liquid Fuels); Wydawnictwo Książkowe Instytutu Elektrotechniki: Warszawa, Poland, 2014; ISBN 978-83-61956-32-7. e-ISBN 978-83-61956-33-4. (In Polish) [Google Scholar]
- Szałatkiewicz, J.; Zielono, G.; Obrzut, Ł.; Szałatkiewicz, M. Method of Control for Gasification Reactor in Ecological Technology of Biomass Waste Utilization. In Recent Advances in Systems, Control and Information Technology; Advances in Intelligent Systems and Computing; Szewczyk, R., Kaliczyńska, M., Eds.; Springer: Cham, Germany, 2017; Volume 543, pp. 252–259, SCIT 2016. [Google Scholar] [CrossRef]
- Kong, M.G.; Kroesen, G.; Morfill, G.; Nosenko, T.; Shimizu, T.; van Dijk, J.; Zimmermann, J.L. Plasma medicine: An introductory review. New J. Phys. 2009, 11, 115012. [Google Scholar] [CrossRef]
- Fridman, G.; Friedman, G.; Gutsol, A.; Shekhter, A.B.; Vasilets, V.N.; Fridman, A. Applied plasma medicine. Plasma Process. Polym. 2008, 5, 503–533. [Google Scholar] [CrossRef]
- Moreau, M.; Orange, N.; Feuilloley, M.G.J. Non-thermal plasma technologies: New tools for bio-decontamination. Biotechnol. Adv. 2008, 26, 610–617. [Google Scholar] [CrossRef]
- Haertel, B.; von Woedtke, T.; Weltmann, K.D.; Lindequist, U. Physical plasma—Possible application in wound healing. Biomol. Ther. 2014, 22, 477–490. [Google Scholar] [CrossRef]
- Graves, D.B. Low temperature plasma biomedicine: A tutorial review. Phys. Plasmas 2014, 21, 080901. [Google Scholar] [CrossRef]
- Pawłat, J.; Diatczyk, J.; Komarzyniec, G.; Giżewski, T.; Stryczewska Henryka, D.; Mitsugi, F.; Ebihara, K.; Aoqui, S.; Nakamiya, T. Solar energy for soil conditioning. In Proceedings of the EUROCON—International Conference on Computer as a Tool (EUROCON), 2011 IEEE, Lisbon, Portugal, 27–29 April 2011; pp. 1–4. [Google Scholar]
- Pawłat, J.; Stryczewska, H. Application of Solar Energy in the Processes of Gas, Water and Soil Treatment: W Solar Power [Red:]; Rugescu Radu, D., Ed.; InTech: Rijeka, Croatia, 2012; pp. 105–132. [Google Scholar] [CrossRef]
- Stryczewska, H.; Pawłat, J. Technologies of air, water and soil treatment based on solar energy and advanced oxidation processes. In w: Modern Power Engineering 1; Waldemar, W., Politechnika, L., Eds.; Lublin University of Technology: Lublin, Poland, 2011; pp. 176–197. [Google Scholar]
- Stryczewska, H.D.; Ebihara, K.; Takayama, M.; Gyuotoku, Y.; Tachibana, M. Non-Thermal Plasma-Based Technology for Soil Treatment. Plasma Processes Polym. 2005, 2, 238–245. [Google Scholar] [CrossRef]
- Ebihara, K.; Mitsugi, F.; Ikegami, T.; Nakamura, N.; Hashimoto, Y.; Yamashita, Y.; Baba, S.; Stryczewska, H.D.; Pawlat, J.; Teii, S.; et al. Ozone-mist spray sterilization for pest control in agricultural management. EPJ Appl. Phys. 2013, 61, 24318. [Google Scholar] [CrossRef]
- Von Woedtke, T.; Schmidt, A.; Bekeschus, S.; Wende, K. Introduction to plasma medicine. In Comprehensive Clinical Plasma Medicine; Metelmann, H.M., von Woedtke, T., Weltmann, K.D., Eds.; Springer: Berlin, Germany, 2018; pp. 3–21. [Google Scholar] [CrossRef]
- Daeschlein, G.; Scholz, S.; Emmert, S.; von Podewils, S.; Haase, H.; von Woedtke, T.; Jünger, M. Plasma medicine in dermatology: Basic antimicrobial efficacy testing as prerequisite to clinical plasma therapy. Plasma Med. 2012, 2, 33–69. [Google Scholar] [CrossRef]
- Metelmann, H.R.; Vu, T.T.; Do, H.T.; Le, T.N.B.; Hoang, T.H.A.; Phi, T.T.T.; Luong, T.M.L.; Doan, V.T.; Nguyen, T.T.H.; Nguyen, T.H.M.; et al. Scar formation of laser skin lesions after cold atmospheric pressure plasma (CAP) treatment: A clinical long term observation. Clin. Plasma Med. 2013, 1, 30–35. [Google Scholar] [CrossRef]
- Morfill, G.E.; Shimizu, T.; Steffes, B.; Schmidt, H.U. Nosocomical infections—A new approach towards preventive medicine using plasmas. New J. Phys. 2009, 11, 115019. [Google Scholar] [CrossRef]
- Weltmann, K.D.; Kindel, E.; von Woedtke, T.; Hähnel, M.; Stieber, M.; Brandenburg, R. Atmospheric-pressure plasma sources: Prospective tools for plasma medicine. Pure Appl. Chem. 2010, 82, 1223–1237. [Google Scholar] [CrossRef]
- Weltmann, K.D.; von Woedtke, T. Plasma medicine—Current state of research and medical application. Plasma Phys. Control. Fusion 2017, 59, 014031. [Google Scholar] [CrossRef]
- Emmert, S.; Isbary, G.; Klutschke, F.; Lademann, J.; Westermann, U.; Podmelle, F.; Metelmann, H.R.; Daeschlein, G.; Masur, K.; von Woedtke, T.; et al. Clinical plasma medicine—Position and perspectives. Clin. Plasma Med. 2013, 1, 3–4. [Google Scholar] [CrossRef]
- Ratovitski, E.A.; Cheng, X.; Yan, D.; Sherman, J.H.; Canady, J.; Trink, B.; Keidar, M. Anti-cancer therapies of the 21st century: Novel approach to treat human cancers using cold atmospheric plasma. Plasma Process Polym. 2014, 11, 1128–1137. [Google Scholar] [CrossRef]
- Schlegel, J.; Köritzer, J.; Boxhammer, V. Plasma in cancer treatment. Clin. Plasma Med. 2013, 1, 2–7. [Google Scholar] [CrossRef]
- Graves, D.B. Reactive species from cold atmospheric plasma: Implications for cancer therapy. Plasma Process Polym. 2014, 11, 1120–1127. [Google Scholar] [CrossRef]
- Tanaka, H.; Mizuno, M.; Ishikawa, K.; Kondo, H.; Takeda, K.; Hashizume, H.; Nakamura, K.; Utsumi, F.; Kajiyama, H.; Kano, H.; et al. Plasma with high electron density and plasma-activated medium for cancer treatment. Clin. Plasma Med. 2015, 3, 72–76. [Google Scholar] [CrossRef]
- Mai-Prochnow, A.; Murphy, A.B.; McLean, K.M.; Kong, M.G.; Ostrikov, K.K. Atmospheric pressure plasmas: Infection control and bacterial responses. Int. J. Antimicrob. Agents 2014, 43, 508–517. [Google Scholar] [CrossRef]
- Lee, H.W.; Kim, G.J.; Kim, J.M.; Park, J.K.; Lee, J.K.; Kim, G.C. Tooth bleaching with non-thermal atmospheric pressure plasma. J. Endod. 2009, 35, 587–591. [Google Scholar] [CrossRef]
- Singh, S.; Chandra, R.; Tripathi, S.; Rahman, H.; Tripathi, P.; Jain, A.; Gupta, P. The bright future of dentistry with cold plasma—Review. J. Dent. Med. Sci. 2014, 13, 6–13. [Google Scholar] [CrossRef]
- Agata, P.; Audemar, M.; Pawlat, J.; Canal, C.; Thomann, J.S.; Labay, C.; Wojcik, M.; Kwiatkowski, M.; Terebun, P.; Ginalska, G.; et al. Positive Effect of Cold Atmospheric Nitrogen Plasma on the Behavior of Mesenchymal Stem Cells Cultured on a Bone Scaffold Containing Iron Oxide-Loaded Silica Nanoparticles Catalyst. Int. J. Mol. Sci. 2020, 21, 4738. [Google Scholar] [CrossRef]
- Przekora, A.; Pawlat, J.; Terebun, P.; Duday, D.; Canal, C.; Hermans, S.; Audemar, M.; Labay, C.; Thomann, J.S.; Ginalska, G. The effect of low temperature atmospheric nitrogen plasma on MC3T3-E1 preosteoblast proliferation and differentiation in vitro. J. Phys. D Appl. Phys. 2019, 52, 275401. [Google Scholar] [CrossRef]
- Polak, M.; Winter, J.; Schnabel, U.; Ehlbeck, J.; Weltmann, K.D. Innovative plasma generation in flexible biopsy channels for inner-tube decontamination and medical applications. Plasma Process Polym. 2012, 9, 67–76. [Google Scholar] [CrossRef]
- Kim, S.W.; Park, H.S.; Kim, H.J. 100 KW Steam Plasma Process for Treatment of PCBS (Polychlorinated Biphenyls) Waste. Vacuum 2003, 70, 59–66. [Google Scholar] [CrossRef]
- Cai, X.W.; Du, C.M. Thermal Plasma Treatment of Medical Waste. Plasma Chem. Plasma Process. 2020, 41, 1–46. [Google Scholar] [CrossRef]
- Dors, M.; Kurzyńska, D. Tar Removal by Nanosecond Pulsed Dielectric Barrier Discharge. Appl. Sci. 2020, 10, 991. [Google Scholar] [CrossRef]
- Łukasz, S. (Ed.) Plazmowe technologie w zagospodarowaniu odpadów przemysłowych i komunalnych/Plasma technologies in the management of industrial and municipal waste. In Współczesne Problemy Ochrony Środowiska i Energetyki/Contemporary Problems of Environmental Protection and Energy, Collective Work; Wydawnictwo Politechniki Śląskiej: Gliwice, Poland, 2020; p. 415. ISBN 978-83-950087-8-8. (In Polish) [Google Scholar]
- Baboo, R.A. Study on plasma chemistry for human health & waste management. Int. J. Res. Anal. 2018, 5, 194–197, e-ISSN 2348-1269. [Google Scholar]
- Holub, M.; Brandenburg, R.; Grosch, H.; Weinmann, S.; Hansel, B. Plasma supported odour removal from waste air in water treatment plants: An industrial case study. Aerosol Air Qual. Res. 2014, 14, 697–707. [Google Scholar] [CrossRef]
- Bourke, P.; Ziuzina, D.; Boehm, D.; Cullen, P.J.; Keener, K. The potential of cold plasma for safe and sustainable food production. Trends Biotechnol. 2018, 36, 615–626. [Google Scholar] [CrossRef] [PubMed]
- Misra, N.N.; Schlüter, O.; Cullen, P.J. Cold Plasma in Food and Agriculture: Fundamentals and Applications; Elsevier: London, UK, 2016; 361p, ISBN 9780128014899. [Google Scholar]
- Abdi, S.; Dorranian, D.; Mohammadi, K. Effect of oxygen on decontamination of cumin seeds by atmospheric pressure dielectric barrier discharge plasma. Plasma Med. 2016, 6, 339–347. [Google Scholar] [CrossRef]
- Filatova, I.; Azharonok, V.; Lushkevich, V.; Zhukovsky, A.; Gadzhieva, G.; Spasic, K.; Zickovic, S.; Puac, N.; Lazovic, S.; Malovic, G.; et al. Plasma seeds treatment as a promising technique for seed germination improvement. In Proceedings of the 31st International Conference on Phenomena in Ionized Gases, Granada, Spain, 14–19 July 2013. [Google Scholar]
- Henselova, M.; SlovakovA, L.; Martinka, M.; Zahoranova, A. Growth, anatomy and enzyme activity changes in maize roots induced by treatment of seeds with low-temperature plasma. Biologia 2012, 67, 490–497. [Google Scholar] [CrossRef]
- Tong, J.; He, R.; Zhang, X.; Zhan, R.; Chen, W.; Yang, S. Effects of atmospheric pressure air plasma pretreatment on the seed germination and early growth of Andrographis paniculata. Plasma Sci. Technol. 2014, 16, 260. [Google Scholar] [CrossRef]
- Kordas, L.; Pusz, W.; Czapka, T.; Kacprzyk, R. The Effect of Low-Temperature Plasma on Fungus Colonization of Winter Wheat Grain and Seed Quality. Pol. J. Environ. Stud. 2015, 4, 433–438. [Google Scholar]
- Lotfy, K.; Al-Harbi, N.A.; El-Raheem, H.A. Cold atmospheric pressure nitrogen plasma jet for enhancement germination of wheat seeds. Plasma Chem. Plasma Process. 2019, 39, 897–912. [Google Scholar] [CrossRef]
- Nishioka, T.; Takai, Y.; Kawaradani, M.; Okada, K.; Tanimoto, H.; Misawa, T.; Kusakari, S. Seed disinfection effect of atmospheric pressure plasma and low-pressure plasma on Rhizoctonia solani. Biocontrol Sci. 2014, 19, 99–102. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Park, Y.; Oh, K.S.; Oh, J.; Seok, D.C.; Kim, S.B.; Yoo, S.J.; Lee, M.J. The biological effects of surface dielectric barrier discharge on seed germination and plant growth with barley. Plasma Process. Polym. 2018, 15, 1600056. [Google Scholar] [CrossRef]
- Sera, B.; Sery, M. Non-thermal plasma treatment as a new biotechnology in relation to seeds, dry fruits, and grains. Plasma Sci. Technol. 2018, 20, 044012. [Google Scholar] [CrossRef]
- Sivachandiran, L.; Khacef, A. Enhanced seed germination and plant growth by atmospheric pressure cold air plasma: Combined effect of seed and water treatment. RSC Adv. 2017, 7, 1822–1832. [Google Scholar] [CrossRef]
- Thirumdas, R. Exploitation of cold plasma technology for enhancement of seed germination. Agri. Res. Technol. 2018, 13, 1–4. [Google Scholar] [CrossRef]
- Ruan, R.R.; Lei, H.; Chen, P.L.; Deng, S.; Lin, X.; Li, Y.; Wilcke, W.; Fulcher, G. Ozone-Aided Corn Steeping Process. Cereal Chem. 2004, 81, 182–187. [Google Scholar] [CrossRef]
- Chen, Z.; Garcia, G.; Arumugaswami, V.; Wirz, R.E. Cold atmospheric plasma for SARS-CoV-2 inactivation. Phys. Fluids 2020, 32, 111702. [Google Scholar] [CrossRef]
- Muszański, R. Dlaczego OWWO? (Why OWWO?—Highly ozonated degassed water). Wodociągi Pol. Pol. Water Control. 2020, 12, 44–46. (In Polish) [Google Scholar]
- Petitpas, G.; Rollier, J.D.; Darmon, A.; Gonzalez-Aguilar, J.; Metkemeijer, R.; Fulcheri, L. A comparative study of non-thermal plasma assisted reforming technologies. Int. J. Hydrog. Energy 2007, 32, 2848–2867. [Google Scholar] [CrossRef]
- Sobiecka, E.; Kołacinski, Z.; Rincon, J.M.; Szymanski, L.; Olejnik, T.P. Coloured sintered glass-ceramics from hospital incineration fly ash. Mater. Lett. 2019, 252, 35–37. [Google Scholar] [CrossRef]
- Sobiecka, E.; Szymanski, L. Thermal Plasma Vitrification Process as The Effective Technology for Fly Ash and Chromium Rich Sewage Sludge Utilization. J. Chem. Technol. Biotechnol. 2013, 89, 1115–1117. [Google Scholar] [CrossRef]
- Wystalska, K.; Sobik-Szoltysek, J. Sludge from tannery industries. In Industrial and Municipal Sludge, 1st ed.; Prasad, M.N.V., de Campos Favas, P.J., Vithanage, M., Mohan, S.V., Eds.; Butterworth-Heinemann: Oxford, UK, 2019; pp. 31–46. [Google Scholar] [CrossRef]
- Thirumdas, R.; Kothakota, A.; Annapure, U.; Siliveru, K.; Blundell, R.; Gatt, R.; Valdramidis, V.P. Plasma activated water (PAW): Chemistry, physico-chemical properties, applications in food and agriculture. Trends Food Sci. Technol. 2018, 77, 21–31. [Google Scholar] [CrossRef]
- Malik, M.A.; Ghaffar, A.; Malik, S.A. Water purification by electrical discharges. Plasma Sources Sci. Technol. 2001, 10, 82–91. [Google Scholar] [CrossRef]
- Jiang, H.J.; Chen, N.; Shen, Z.Q.; Yin, J.; Qiu, Z.G.; Miao, J.; Yang, Z.W.; Shi, D.Y.; Wang, H.R.; Wang, X.W.; et al. Inactivation of Poliovirus by Ozone and the Impact of Ozone on the Viral Genome. Biomed. Environ. Sci. 2019, 32, 324–333. [Google Scholar] [CrossRef]
- Bruggeman, P.J.; Kushner, M.J.; Locke, B.R.; Gardeniers, J.G.E.; Graham, W.G.; Graves, D.B.; Hofman-Caris, R.C.H.M.; Maric, D.; Reid, J.P.; Ceriani, E.; et al. Plasma-liquid interactions: A review and roadmap. Plasma Sources Sci. Technol. 2016, 25, 053002. [Google Scholar] [CrossRef]
- Stryczewska, H.D.; Ebihara, K.; Mitsugi, F.; Aoqui, S.; Muszański, R. Plasma Agriculture in Europe. Review of research on agricultural applications of non-thermal plasma. In Proceedings of the First International Conference on Hybrid Agriculture (Hybrid 2016), Kumamoto, Japan, 21–24 October 2016; Sojo University: Kumamoto, Japan, 2016. [Google Scholar]
- Joanna, P. Electrical Discharges in Humid Environments. Generators, Effects, Application; Lublin University of Technology: Lublin, Poland, 2013; ISBN 978-83-63569-37-2. [Google Scholar]
- Ebihara, K.; Mitsugi, F.; Ikegami, T.; Yamashita, Y.; Hashimoto, Y.; Yamashita, T.; Kanazawa, S.; Stryczewska, H.D.; Pawlat, J.; Teii, S.; et al. Sterilization Characteristics of Ozone-mist Spray for Chemical Free Agriculture. Int. J. Plasma Environ. Sci. 2016, 10, 11–15. [Google Scholar] [CrossRef]
- Volkering, F.A.; Breure, M.; Rulkens, W.H. Microbiological aspects of surfactant use for biological soil remediation. Biodegradation 1998, 8, 401–417. [Google Scholar] [CrossRef]
- Lim, H.; Choi, H.; Hwang, T.; Kang, J. Characterization of ozone decomposition in a soil slurry: Kinetics and mechanism. Water Res. 2002, 36, 219–229. [Google Scholar] [CrossRef]
- Alcantara-Garduno, M.; Okuda, T.; Tsai, T.; Nishijima, W.; Okada, M. Experimental and mathematical evaluation of trichloroethylene removal from saturated soil using acetic acid with saturated ozone. Sep. Purif. Technol. 2008, 60, 299–307. [Google Scholar] [CrossRef]
- Hsu, M.; Masten, S. The kinetics of the reaction of ozone with phenanthrene in unsaturated soils. Environ. Eng. Sci. 1997, 14, 207–218. [Google Scholar] [CrossRef]
- Barraud, N.; Hassett, D.; Hwang, S.; Rice, S.; Kjelleberg, S.; Webb, J. Involvement of Nitric Oxide in Biofilm Dispersal of Pseudomonas aeruginosa. J. Bacteriol. 2006, 188, 7344–7353. [Google Scholar] [CrossRef]
- Mitsugi, F.; Ebihara, K.; Horibe, N.; Aoqui, S.; Nagahama, K. Practical Soil Treatment in a Greenhouse Using Surface Barrier Discharge Ozone Generator. IEEE Trans. Plasma Sci. 2017, 45, 3082–3088. [Google Scholar] [CrossRef]
- Pauzaite, G.; Malakauskiene, A.; Nauciene, Z.; Zukiene, R.; Filatova, I.; Lyushkevich, V.; Azarko, I.; Mildaziene, V. Changes in Norway spruce germination and growth induced by pre sowing seed treatment with cold plasma and electromagnetic field: Short-term versus long-term effects. Plasma Process Polym. 2018, 15, e1700068. [Google Scholar] [CrossRef]
- Randeniya, L.K.; de Groot, G.J.J.B. Non-Thermal Plasma Treatment of Agricultural Seeds for Stimulation of Germination, Removal of Surface Contamination and Other Benefits: A Review. Plasma Process Polym. 2015, 12, 608–623. [Google Scholar] [CrossRef]
- Da Silva, J.A.T.; Dobránszki, J. Magnetic fields: How is plant growth and development impacted? Protoplasma 2016, 253, 231–248. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Jiang, J.F.; Li, J.G.; Shen, M.C.; He, X.; Shao, H.L.; Dong, Y.H. Effects of cold plasma treatment on seed germination and seedling growth of soybean. Sci. Rep. 2014, 4, 5859. [Google Scholar] [CrossRef]
- Hosseini, S.I.; Mohsenimehr, S.; Hadian, J.; Ghorbanpour, M.; Shokri, B. Physico-chemical induced modification of seed germination and early development in artichoke (Cynara scolymus L.) using low energy plasma technology. Phys. Plasmas. 2018, 25, 013525. [Google Scholar] [CrossRef]
- Filatova, I.; Lyushkevich, V.; Goncharik, S.; Zhukovsky, A.; Krupenko, N.; Kalatskaja, J. The effect of low-pressure plasma treatment of seeds on the plant resistance to pathogens and crop yields. J. Phys. D Appl. Phys. 2020, 53, 244001. [Google Scholar] [CrossRef]
- Jiang, J.F.; Lu, Y.F.; Li, J.G.; Li, L.; He, X.; Shao, H.L.; Dong, Y.H. Effect of Seed Treatment by Cold Plasma on the Resistance of Tomato to Ralstonia solanacearum (Bacterial Wilt). PLoS ONE 2014, 9, e97753. [Google Scholar] [CrossRef]
- Mildaziene, V.; Pauzaite, G.; Malakauskiene, A.; Zukiene, R.; Nauciene, Z.; Filatova, I.; Azharonok, V.; Lyushkevich, V. Response of perennial woody plants to seed treatment by electromagnetic field and low-temperature plasma. Bioelectromagnetics 2016, 37, 536–548. [Google Scholar] [CrossRef] [PubMed]
- Ivankov, A.; Nauciene, Z.; Zukiene, R.; Degutyte-Fomins, L.; Malakauskiene, A.; Kraujalis, P.; Venskutonis, P.R.; Filatova, I.; Lyushkevich, V.; Mildaziene, V. Changes in Growth and Production of Non-Psychotropic Cannabinoids Induced by Pre-Sowing Treatment of Hemp Seeds with Cold Plasma, Vacuum and Electromagnetic Field. Appl. Sci. 2020, 10, 8519. [Google Scholar] [CrossRef]
- Boehm, D.; Canal, C. Application of Plasma Technology in Bioscience and Biomedicine. Appl. Sci. 2021, 11, 7203. [Google Scholar] [CrossRef]
- Rezaei, F.; Vanraes, P.; Nikiforov, A.; Morent, R.; De Geyter, N. Applications of Plasma-Liquid Systems: A Review. Materials 2019, 12, 2751. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Choi, K.H.; Kim, Y.; Park, B.J.; Cho, G. Wearable Plasma Pads for Biomedical Applications. Appl. Sci. 2021, 17, 1308. [Google Scholar] [CrossRef]
- Zhou, R.W.; Zhou, R.S.; Wang, P.Y.; Xian, Y.B.; Mai-Prochnow, A.; Lu, X.P.; Cullen, P.J.; Ostrikov, K.; Bazaka, K. Plasma-activated water: Generation, origin of reactive species and biological applications. J. Phys. D Appl. Phys. 2020, 53, 303001. [Google Scholar] [CrossRef]
- Laroussi, M. Sterilization of contaminated matter with an atmospheric pressure plasma. IEEE Trans. Plasma Sci. 1996, 24, 1188–1191. [Google Scholar] [CrossRef]
- Laroussi, M. Sterilization of Liquids Using a Plasma Glow Discharge. U.S. Patent 5,876,663, 2 March 1999. [Google Scholar]
- Laroussi, M. Cold Plasma in Medicine and Healthcare: The New Frontier in Low Temperature Plasma Applications. Front. Phys. 2020, 8, 74. [Google Scholar] [CrossRef]
- Boudam, M.K.; Moisan, M. Synergy effect of heat and UV photons on bacterial-spore inactivation in an N2-O2 plasma-afterglow sterilizer. J. Phys. D Appl. Phys. 2010, 43, 295202. [Google Scholar] [CrossRef]
- Isbary, G.; Morfill, G.; Schmidt, H.U.; Georgi, M.; Ramrath, K.; Heinlin, J.; Karrer, S.; Landthaler, M.; Shimizu, T.; Steffes, B.; et al. A first prospective randomized controlled trial to decrease bacterial load using cold atmospheric argon plasma on chronic wounds in patients. Br. J. Dermatol. 2010, 163, 78–82. [Google Scholar] [CrossRef] [PubMed]
- Vandamme, M.; Robert, E.; Pesnel, S.; Barbosa, E.; Dozias, S.; Sobilo, J.; Lerondel, S.; Le Pape, A.; Pouvesle, J.M. Antitumor effect of plasma treatment on U87 glioma xenografts: Preliminary results. Plasma Process Polym. 2010, 7, 264–273. [Google Scholar] [CrossRef]
- Laroussi, M. From killing bacteria to destroying cancer cells: Twenty years of plasma medicine. Plasma Process Polym. 2014, 11, 1138–1141. [Google Scholar] [CrossRef]
- Delben, J.A.; Zago, C.E.; Tyhovych, N.; Duarte, S.; Vergani, C.E. Effect of atmospheric pressure cold plasma on pathogenic oral biofilms and in vitro reconstituted oral epithelium. PLoS ONE 2016, 11, e0155427. [Google Scholar] [CrossRef]
- Carreiro, A.F.; Delben, J.A.; Guedes, S.; Silveira, E.J.; Janal, M.N.; Vergani, C.E.; Pushalkar, S.; Duarte, S. Low-temperature plasma on peri-implant-related biofilm and gingival tissue. J. Periodontol. 2019, 90, 507–515. [Google Scholar] [CrossRef] [PubMed]
- Daeschlein, G.; Napp, M.; Lutze, S.; Arnold, A.; von Podewils, S.; Guembel, D.; Jünger, M. Skin and wound decontamination of multidrug-resistant bacteria by cold atmospheric plasma coagulation. Dtsch. Dermatol Ges. 2015, 13, 143–150. [Google Scholar] [CrossRef]
- Shi, X.M.; Xu, G.M.; Zhang, G.J.; Liu, J.R.; Wu, Y.M.; Gao, L.G.; Yang, Y.; Chang, Z.S.; Yao, C.W. Low-temperature plasma promotes fibroblast proliferation in wound healing by ROS-activated NF-κB signaling pathway. Curr. Med. Sci. 2018, 38, 107–114. [Google Scholar] [CrossRef]
- Bunz, O.; Mese, K.; Funk, C.; Wulf, M.; Bailer, S.M.; Piwowarczyk, A.; Ehrhardt, A. Cold atmospheric plasma as antiviral therapy—Effect on human herpes simplex virus type 1. J. Gen. Virol. 2020, 101, 208–215. [Google Scholar] [CrossRef] [PubMed]
- Duarte, S.; Panariello, B.H.D. Comprehensive biomedical applications of low temperature plasmas. Arch. Biochem. Biophys. 2020, 693, 108560. [Google Scholar] [CrossRef]
- Bourke, P.; Ziuzina, D.; Han, L.; Cullen, P.J.; Gilmore, B.F. Microbiological interactions with cold plasma. J. Appl. Microbiol. 2017, 123, 308–324. [Google Scholar] [CrossRef]
- Niedzwiedz, I.; Wasko, A.; Pawlat, J.; Polak-Berecka, M. The State of Research on Antimicrobial Activity of Cold Plasma. Pol. J. Microbiol. 2019, 68, 153–164. [Google Scholar] [CrossRef]
- Yasuda, H.; Miura, T.; Kurita, H.; Takashima, K.; Mizuno, A. Biological evaluation of DNA damage in bacteriophages inactivated by atmospheric pressure cold plasma. Plasma Process Polym. 2010, 7, 301–308. [Google Scholar] [CrossRef]
- Terrier, O.; Essere, B.; Yver, M.; Barthelemy, M.; Bouscambert-Duchamp, M.; Kurtz, P.; VanMechelen, D.; Morfin, F.; Billaud, G.; Ferraris, O.; et al. Cold oxygen plasma technology efficiency against different airborne respiratory viruses. J. Clin. Virol. 2009, 45, 119–124. [Google Scholar] [CrossRef] [PubMed]
- Zimmermann, J.L.; Dumler, K.; Shimizu, T.; Morfill, G.E.; Wolf, A.; Boxhammer, V.; Schlegel, J.; Gansbacher, B.; Anton, M. Effects of cold atmospheric plasmas on adenoviruses in solution. J. Phys. D Appl. Phys. 2011, 44, 505201. [Google Scholar] [CrossRef]
- Klampfl, T.G.; Isbary, G.; Shimizu, T.; Li, Y.F.; Zimmermann, J.L.; Stolz, W.; Schlegel, J.; Morfill, G.E.; Schmidt, H.U. Cold atmospheric air plasma sterilization against spores and other microorganisms of clinical interest. Appl. Environ. Microbiol. 2012, 78, 5077–5082. [Google Scholar] [CrossRef] [PubMed]
- Liao, X.; Liu, D.; Xiang, Q.; Ahn, J.; Chen, S.; Ye, X.; Ding, T. Inactivation mechanisms of non-thermal plasma on microbes: A review. Food Control. 2017, 75, 83–91. [Google Scholar] [CrossRef]
- Colagar, A.H.; Memariani, H.; Sohbatzadeh, F.; Omran, A.V. Nonthermal atmospheric argon plasma jet effects on Escherichia coli biomacromolecules. Appl. Biochem. Biotechnol. 2013, 171, 1617–1629. [Google Scholar] [CrossRef] [PubMed]
- Ziuzina, D.; Boehm, D.; Patil, S.; Cullen, P.J.; Bourke, P. Cold Plasma Inactivation of Bacterial Biofilms and Reduction of Quorum Sensing Regulated Virulence Factors. PLoS ONE 2015, 10, e0138209. [Google Scholar] [CrossRef]
- Alkawareek, M.Y.; Algwari, Q.T.; Gorman, S.P.; Graham, W.G.; O’Connell, D.; Gilmore, B.F. Application of atmospheric pressure nonthermal plasma for the in vitro eradication of bacterial biofilms. Fems. Immunol. Med. Microbiol. 2012, 65, SI381–SI384. [Google Scholar] [CrossRef] [PubMed]
- Patange, A.; Boehm, D.; Ziuzina, D.; Cullen, P.J.; Gilmore, B.; Bourke, P. High voltage atmospheric cold air plasma control of bacterial biofilms on fresh produce. Int. J. Food Microbiol. 2019, 293, 137–145. [Google Scholar] [CrossRef]
- Boxhammer, V.; Li, Y.F.; Köritzer, J.; Shimizu, T.; Maisch, T.; Thomas, H.M.; Schlegel, J.; Morfill, G.E.; Zimmermann, J.L. Investigation of the mutagenic potential of cold atmospheric plasma at bactericidal dosages. Mutat. Res./Genet. Toxicol. Environ. Mutagenes. 2013, 753, 23–28. [Google Scholar] [CrossRef] [PubMed]
- Nishime, T.M.C.; Borges, A.C.; Koga-Ito, C.Y.; Machida, M.; Hein, L.R.O.; Kostov, K.G. Non-thermal atmospheric pressure plasma jet applied to inactivation of different microorganisms. Surf. Coat. Technol. 2017, 312, 19–24. [Google Scholar] [CrossRef]
- Colonna, W.; Wan, Z.; Pankaj, S.K.; Keener, K.M. High-voltage atmospheric cold plasma treatment of yeast for spoilage prevention. Plasma Med. 2017, 7, 97–107. [Google Scholar] [CrossRef]
- Tolouie, H.; Mohammadifar, M.A.; Ghomi, H.; Hashemi, M. Cold atmospheric plasma manipulation of proteins in food systems. Crit. Rev. Food Sci. Nutr. 2018, 58, 2583–2597. [Google Scholar] [CrossRef]
- Tolouie, H.; Mohammadifar, M.A.; Ghomi, H.; Yaghoubi, A.S.; Hashemi, M. The impact of atmospheric cold plasma treatment on inactivation of lipase and lipoxygenase of wheat germs. Innov. Food Sci. Emerg. Technol. 2018, 47, 346–352. [Google Scholar] [CrossRef]
- Misra, N.N.; Pankaj, S.K.; Segat, A.; Ishikawa, K. Cold plasma interactions with enzymes in foods and model systems. Trends Food Sci. Technol. 2016, 55, 39–47. [Google Scholar] [CrossRef]
- Dubey, S.K.; Parab, S.; Amit, A.; Agrawal, M.; Achalla, V.P.K.; Pal, U.N.; Pandey, M.M.; Kesharwani, P. Cold atmospheric plasma therapy in wound healing. Process Biochem. 2022, 112, 112–123. [Google Scholar] [CrossRef]
- Graves, D.B. Mechanisms of plasma medicine: Coupling plasma physics, biochemistry, and biology. IEEE Trans. Radiat. Plasma Med. Sci. 2017, 1, 281–292. [Google Scholar] [CrossRef]
- Gorain, B.; Choudhury, H.; Pandey, M.; Kesharwani, P.; Abeer, M.M.; Tekade, R.K.; Hussain, Z. Carbon nanotube scaffolds as emerging nanoplatform for myocardial tissue regeneration: A review of recent developments and therapeutic implications. Biomed. Pharmacother. 2018, 104, 496–508. [Google Scholar] [CrossRef] [PubMed]
- Cheng, K.Y.; Lin, Z.H.; Cheng, Y.P.; Chiu, H.Y.; Yeh, N.L.; Wu, T.K.; Wu, J.S. Wound healing in streptozotocin-induced diabetic rats using atmospheric-pressure argon plasma jet. Sci. Rep. 2018, 8, 12214. [Google Scholar] [CrossRef] [PubMed]
- Nasruddin, Y.; Nakajima, K.; Mukai, H.S.E.; Nur Rahayu, M.; Ishijima, T.; Enomoto, H.; Uesugi, Y.; Sugama, J.; Nakatani, T. Cold plasma on full-thickness cutaneous wound accelerates healing through promoting inflammation, re-epithelialization and wound contraction. Clin. Plasma Med. 2014, 2, 28–35. [Google Scholar] [CrossRef]
- Dunnill, C.; Patton, T.; Brennan, J.; Barrett, J.; Dryden, M.; Cooke, J.; Leaper, D.; Georgopoulos, N.T. Reactive oxygen species (ROS) and wound healing: The functional role of ROS and emerging ROS-modulating technologies for augmentation of the healing process. Int. Wound J. 2017, 14, 89–96. [Google Scholar] [CrossRef]
- Eming, S.A.; Martin, P.; Tomic-Canic, M. Wound repair and regeneration: Mechanisms, signaling, and translation. Sci. Transl. Med. 2014, 6, 265sr6. [Google Scholar] [CrossRef] [PubMed]
- Szili, E.J.; Hong, S.H.; Oh, J.S.; Gaur, N.; Short, R.D. Tracking the penetration of plasma reactive species in tissue models. Trends Biotechnol. 2018, 36, 594–602. [Google Scholar] [CrossRef] [PubMed]
- Agren, M.S.; Taplin, C.J.; Woessner, J.F.; Eaglstein, W.H.; Mertz, P.M. Collagenase in wound healing: Effect of wound age and type. J. Investig. Dermatol. 1992, 99, 709–714. [Google Scholar] [CrossRef] [PubMed]
- Zehtabchi, S.; Tan, A.; Yadav, K.; Badawy, A.; Lucchesi, M. The impact of wound age on the infection rate of simple lacerations repaired in the emergency department. Injury 2012, 43, 1793–1798. [Google Scholar] [CrossRef] [PubMed]
- Beyene, R.T.; Derryberry, S.L.; Barbul, A. The effect of comorbidities on wound healing. Surg. Clin. 2020, 100, 695–705. [Google Scholar] [CrossRef]
- Nahid, M.A.; Griffin, J.M.; Lustik, M.B.; Hayes, J.J.; Fong, K.S.K.; Horseman, T.S.; Menguito, M.; Snesrud, E.C.; Barnhill, J.C.; Washington, M.A. A longitudinal evaluation of the bacterial pathogens colonizing chronic non-healing wound sites at a United States military treatment facility in the pacific region. Infect. Drug Resist. 2021, 14, 1–10. [Google Scholar] [CrossRef]
- Hirst, A.M.; Frame, F.M.; Arya, M.; Maitland, N.J.; O’Connell, D. Low temperature plasmas as emerging cancer therapeutics: The state of play and thoughts for the future. Tumor Biol. 2016, 37, 7021–7031. [Google Scholar] [CrossRef] [PubMed]
- Liang, K.; Liu, F.; Fan, J.; Sun, D.L.; Liu, C.; Lyon, C.J.; Bernard, D.W.; Li, Y.; Yokoi, K.; Katz, M.H.; et al. Nanoplasmonic quantification of tumour-derived extracellular vesicles in plasma microsamples for diagnosis and treatment monitoring. Nat. Biomed. Eng. 2017, 1, 0021. [Google Scholar] [CrossRef]
- Wang, Z.J.; Cheng, Y.; An, T.T.; Gao, H.J.; Wang, K.; Zhou, Q.; Hu, Y.P.; Song, Y.; Ding, C.M.; Peng, F.; et al. Detection of EGFR mutations in plasma circulating tumour DNA as a selection criterion for first-line gefitinib treatment in patients with advanced lung adenocarcinoma (BENEFIT): A phase 2, single-arm, multicentre clinical trial. Lancet Resp. Med. 2018, 6, 681–690. [Google Scholar] [CrossRef]
- Iijima, Y.; Hirotsu, Y.; Amemiya, K.; Ooka, Y.; Mochizuki, H.; Oyama, T.; Nakagomi, T.; Uchida, Y.; Kobayashi, Y.; Tsutsui, T.; et al. Very early response of circulating tumour-derived DNA in plasma predicts efficacy of nivolumab treatment in patients with non-small cell lung cancer. Eur. J. Cancer 2017, 86, 349–357. [Google Scholar] [CrossRef] [PubMed]
- Privat-Maldonado, A.; Bengtson, C.; Razzokov, J.; Smits, E.; Bogaerts, A. Modifying the Tumour Microenvironment: Challenges and Future Perspectives for Anticancer Plasma Treatments. Cancers 2019, 11, 1920. [Google Scholar] [CrossRef]
- Hirst, A.M.; Simms, M.S.; Mann, V.M.; Maitland, N.J.; O’Connell, D.; Frame, F.M. Low-temperature plasma treatment induces DNA damage leading to necrotic cell death in primary prostate epithelial cells. Br. J. Cancer. 2015, 112, 1536–1545. [Google Scholar] [CrossRef] [PubMed]
- Choi, E.H.; Uhm, H.S.; Kaushik, N.K. Plasma bioscience and its application to medicine. AAPPS Bull. 2021, 31, 10. [Google Scholar] [CrossRef]
- Kaushik, N.K.; Uhm, H.S.; Choi, E.H. Micronucleus formation induced by dielectric barrier discharge plasma exposure in brain cancer cells. Appl. Phys. Lett. 2012, 100, 084102. [Google Scholar] [CrossRef]
- Panngom, K.; Baik, K.Y.; Ryu, Y.H.; Uhm, H.S.; Choi, E.H. Differential responses of cancer cell lines to non-thermal plasma from dielectric barrier discharge. Curr. Appl. Phys. 2013, 13, S6–S11. [Google Scholar] [CrossRef]
- Lata, S.; Chakravorty, S.; Mitra, T.; Pradhan, P.K.; Mohanty, S.; Patel, P.; Jha, E.; Panda, P.K.; Verma, S.K.; Suar, M. Aurora Borealis in dentistry: The applications of cold plasma in biomedicine. Mater. Today Bio 2022, 13, 100200. [Google Scholar] [CrossRef] [PubMed]
- Ranjan, R.; Krishnamraju, P.V.; Shankar, T.; Gowd, S. Nonthermal plasma in dentistry: An update. J. Int. Soc. Prev. Community Dent. 2017, 7, 71–75. [Google Scholar] [CrossRef]
- Cha, S.; Park, Y.S. Plasma in dentistry. Clin. Plasma Med. 2014, 2, 4–10. [Google Scholar] [CrossRef]
- Gherardi, M.; Tonini, R.; Colombo, V. Plasma in dentistry: Brief history and current status. Trends Biotechnol. 2018, 36, 583–585. [Google Scholar] [CrossRef]
- Borges, A.C.; Kostov, K.G.; Pessoa, R.S.; de Abreu, G.M.A.; Lima, G.D.G.; Figueira, L.W.; Koga-Ito, C.Y. Applications of Cold Atmospheric Pressure Plasma in Dentistry. Appl. Sci. 2021, 11, 1975. [Google Scholar] [CrossRef]
- Sung, S.J.; Huh, J.B.; Yun, M.J.; Chang, B.M.W.; Jeong, C.M.; Jeon, Y.C. Sterilization effect of atmospheric pressure non-thermal air plasma on dental instruments. J. Adv. Prosthodont. 2013, 5, 2–8. [Google Scholar] [CrossRef]
- Sladek, R.E.J.; Stoffels, E.; Walraven, R.; Tielbeek, P.J.A.; Koolhoven, R.A. Plasma treatment of dental cavities: A feasibility study. IEEE Trans. Plasma Sci. 2004, 32, 1540–1543. [Google Scholar] [CrossRef]
- Vandana, B.L. From distant stars to dental chairs: An update on plasma needle. Int. J. Dent. Sci. Res. 2014, 2, 19–20. [Google Scholar] [CrossRef]
- Li, Y.; Sun, K.; Ye, G.; Liang, Y.; Pan, H.; Wang, G.; Zhao, Y.; Pan, J.; Zhang, J.; Fang, J. Evaluation of cold plasma treatment and safety in disinfecting 3-week root canal Enterococcus faecalis biofilm in vitro. J. Endod. 2015, 41, 1325–1330. [Google Scholar] [CrossRef] [PubMed]
- Pan, J.; Sun, K.; Liang, Y.; Sun, P.; Yang, X.; Wang, J.; Zhang, J.; Zhu, W.; Fang, J.; Becker, K.H. Cold plasma therapy of a tooth root canal infected with enterococcus faecalis biofilms in vitro. J. Endod. 2013, 39, 105–110. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.W.; Nam, S.H.; Mohamed, A.A.H.; Kim, G.C.; Lee, J.K. Atmospheric pressure plasma jet composed of three electrodes: Application to tooth bleaching. Plasma Process. Polym. 2010, 7, 274–280. [Google Scholar] [CrossRef]
- Park, J.K.; Nam, S.H.; Kwon, H.C.; Mohamed, A.A.H.; Lee, J.K.; Kim, G.C. Feasibility of nonthermal atmospheric pressure plasma for intracoronal bleaching. Int. Endod. J. 2011, 44, 170–175. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.S.; Koo, I.G.; Choi, M.Y.; Jung, J.C.; Eldali, F.; Lee, J.K.; Collins, G.J. Correlated electrical and optical studies of hybrid argon gas-water plasmas and their application to tooth whitening. Plasma Process. Polym. 2012, 9, 339–345. [Google Scholar] [CrossRef]
- Rapuano, B.E.; Singh, H.; Boskey, A.L.; Doty, S.B.; MacDonald, D.E. Heat and radiofrequency plasma glow discharge pretreatment of a titanium alloy: Evidence for enhanced osteoinductive properties. J. Cell. Biochem. 2013, 114, 1917–1927. [Google Scholar] [CrossRef]
- Giro, G.; Tovar, N.; Witek, L.; Marin, C.; Silva, N.R.F.; Bonfante, E.A.; Coelho, P.G. Osseointegration assessment of chairside argon-based nonthermal plasma-treated Ca-P coated dental implants. J. Biomed. Mater. Res. 2013, 101A, 98–103. [Google Scholar] [CrossRef]
- Koban, I.; Duske, K.; Jablonowski, L.; Schroder, K.; Nebe, B.; Sietmann, R.; Weltmann, K.D.; Hubner, N.O.; Kramer, A.; Kocher, T. Atmospheric plasma enhances wettability and osteoblast spreading on dentin in vitro: Proof-of-principle. Plasma Process. Polym. 2011, 8, 975–982. [Google Scholar] [CrossRef]
- Miletic, M.; Mojsilovic, S.; Okicorevic, I.; Maletic, D.; Puac, N.; Lazovic, S.; Malovic, G.; Milenkovic, P.; Lj Petrovic, Z.; Bugarski, D. Effects of non-thermal atmospheric plasma on human periodontal ligament mesenchymal stem cells. J. Phys. D Appl. Phys. 2013, 46, 345401. [Google Scholar] [CrossRef]
- Nima, G.; Harth-Chu, E.; Hiers, R.D.; Pecorari, V.G.A.; Dyer, D.W.; Khajotia, S.S.; Giannini, M.; Florez, F.L.E. Antibacterial efficacy of non-thermal atmospheric plasma against Streptococcus mutans biofilm grown on the surfaces of restorative resin composites. Sci. Rep. 2021, 11, 23800. [Google Scholar] [CrossRef] [PubMed]
- Makkar, H.; Verma, S.K.; Panda, P.K.; Pramanik, N.; Jha, E.; Suar, M. Molecular insight to size and dose-dependent cellular toxicity exhibited by a green synthesized bioceramic nanohybrid with macrophages for dental applications. Toxicol. Res. 2018, 7, 959–969. [Google Scholar] [CrossRef] [PubMed]
- Yamazaki, H.; Ohshima, T.; Tsubota, Y.; Yamaguchi, H.; Jayawardena, J.A.; Nishimura, Y. Microbicidal activities of low frequency atmospheric pressure plasma jets on oral pathogens. Dent. Mater. J. 2011, 30, 384–391. [Google Scholar] [CrossRef]
- Jiang, C.; Chen, M.T.; Gorur, A.; Schaudinn, C.; Jaramillo, D.E.; Costerton, J.W.; Sedghizadeh, P.P.; Vernier, P.T.; Gundersen, M.A. Nanosecond pulsed plasma dental probe. Plasma Process. Polym. 2009, 6, 479–483. [Google Scholar] [CrossRef]
- Armand, A.; Khani, M.; Asnaashari, M.; AliAhmadi, A.; Shokri, B. Comparison study of root canal disinfection by cold plasma jet and photodynamic therapy. Photodiagn. Photodyn. Ther. 2019, 26, 327–333. [Google Scholar] [CrossRef]
- Dong, X.; Chen, M.; Wang, Y.; Yu, Q. A mechanistic study of plasma treatment effects on demineralized dentin surfaces for improved adhesive/dentin interface bonding. Clin. Plasma Med. 2014, 2, 11–16. [Google Scholar] [CrossRef][Green Version]
- Yavirach, P.; Chaijareenont, P.; Boonyawan, D.; Pattamapun, K.; Tunma, S.; Takahashi, H.; Arksornnukit, M. Effects of plasma treatment on the shear bond strength between fiber reinforced composite posts and resin composite for core build-up. Dent. Mater. J. 2009, 28, 686–692. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Guo, J.; Zhou, X.; Liu, Z.; Wang, C.; Wang, K.; Zhang, J.; Wang, Z. A novel cold atmospheric pressure air plasma jet for peri-implantitis treatment: An in vitro study. Dent. Mater. J. 2018, 37, 157–166. [Google Scholar] [CrossRef] [PubMed]
- Koban, I.; Holtfreter, B.; Hübner, N.O.; Matthes, R.; Sietmann, R.; Kindel, E.; Weltmann, K.D.; Welk, A.; Kramer, A.; Kocher, T. Antimicrobial efficacy of nonthermal plasma in comparison to chlorhexidine against dental biofilms on titanium discs in vitro—Proof of principle experiment. J. Clin. Periodontol. 2011, 38, 956–965. [Google Scholar] [CrossRef] [PubMed]
- Cold Plasma Is a Hot New Tool in Veterinary Medicine | Tufts Now. Available online: https://now.tufts.edu/articles/cold-plasma-hot-new-tool-veterinary-medicine (accessed on 14 April 2022).
- Lee, J.; Moon, H.; Ku, B.; Lee, K.; Hwang, C.Y.; Baek, S.J. Anticancer Effects of Cold Atmospheric Plasma in Canine Osteosarcoma Cells. Int. J. Mol. Sci. 2020, 21, 4556. [Google Scholar] [CrossRef]
- Van den Broek, A.; Miller, W.; Griffin, C.; Campbell, K. Muller and Kirk’s Small Animal Dermatology. Vet. Dermatol. 2013, 24, 559. [Google Scholar] [CrossRef]
- Bourely, C.; Cazeau, G.; Jarrige, N.; Leblond, A.; Madec, J.Y.; Haenni, M.; Gay, E. Antimicrobial resistance patterns of bacteria isolated from dogs with otitis. Epidemiol. Infect. 2019, 147, e121. [Google Scholar] [CrossRef] [PubMed]
- Jin, H.J.; Hwang, C.Y.; Kang, J.H.; Baek, S.J.; Hyun, J.E. In Vitro antimicrobial activity of cold atmospheric microwave plasma against bacteria causing canine skin and ear infections. Vet. Dermatol. 2021, 32, 462-e126. [Google Scholar] [CrossRef] [PubMed]
- Nitsch, A.; Napiletzki, K.; Stope, M.B. Non-invasive physical plasma (NIPP) treatment of a hedgehog with head injury: A novel therapy in veterinary medicine. Vet. Rec. Case Rep. 2022, e343. [Google Scholar] [CrossRef]
- Nolff, M.C.; Winter, S.; Reese, S.; Meyer-Lindenberg, A. Comparison of polyhexanide, cold atmospheric plasma and saline in the treatment of canine bite wounds. J. Small Anim. Pract. 2019, 60, 348–355. [Google Scholar] [CrossRef] [PubMed]
- Martines, E.; Brun, P.; Cavazzana, R.; Cordaro, L.; Zuin, M.; Martinello, T.; Gomiero, C.; Perazzi, A.; Melotti, L.; Maccatrozzo, L.; et al. Wound healing improvement in large animals using an indirect helium plasma treatment. Clin Plasma Med. 2020, 17–18, 100095. [Google Scholar] [CrossRef]
- Martines, E.; Zuin, M.; Cavazzana, R.; Gazza, E.; Serianni, G.; Spagnolo, S.; Spolaore, M.; Leonardi, A.; Deligianni, V.; Brun, P.; et al. A novel plasma source for sterilization of living tissues. New J. Phys. 2009, 11, 115014. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).