Evaluating the Physicochemical Properties of Some Kosovo’s and Imported Honey Samples
Abstract
:1. Introduction
2. Materials and Methods
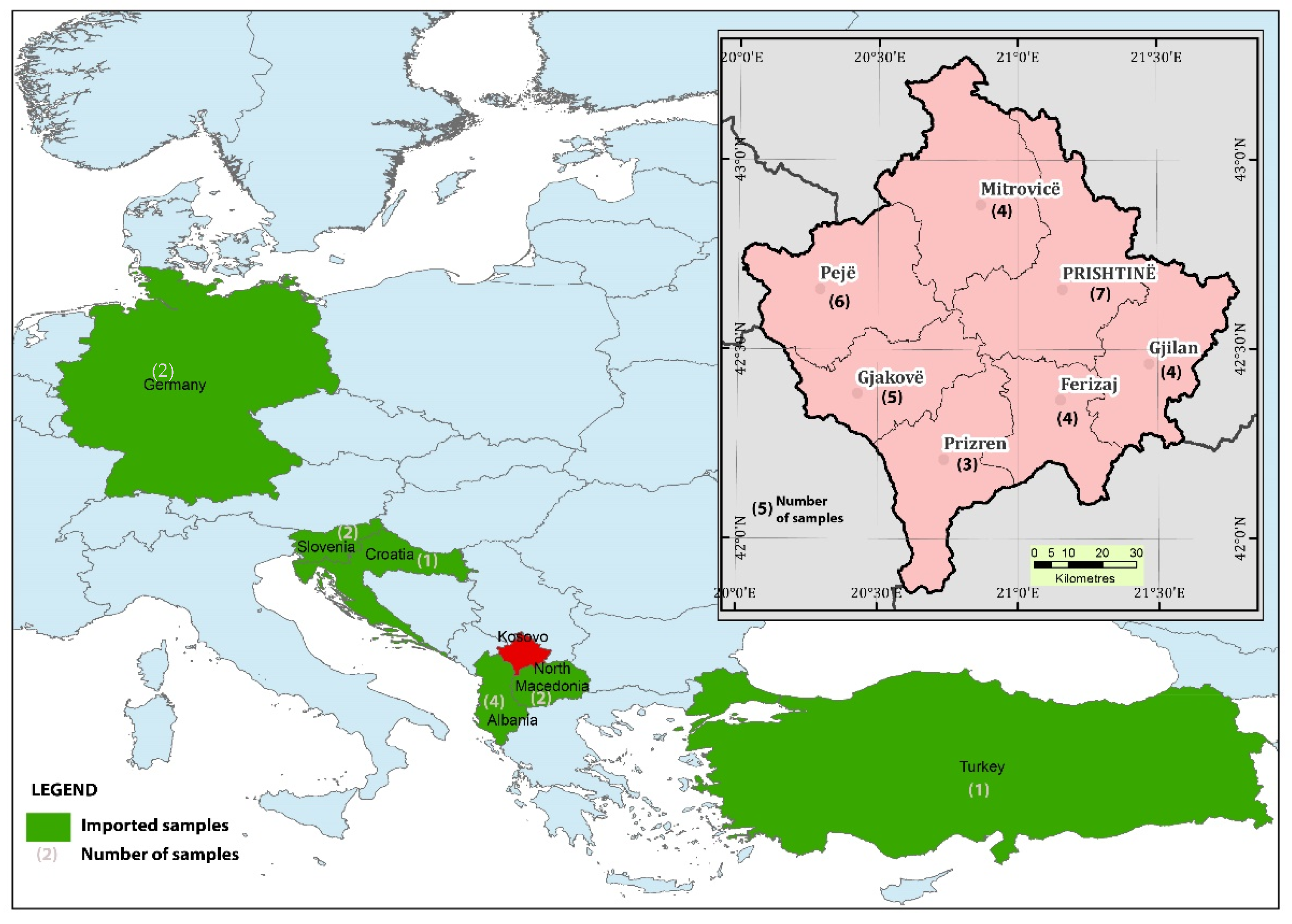
2.1. Samples
2.2. Samples from Kosovo
2.3. Samples from Imports
2.4. Determination of Moisture Content
2.5. Determination of pH, Electric Conductivity, and Free Acidity
2.6. Determination of Hydroxymethylfurfural (HMF)
2.7. Determination of Proteins
2.8. Determination of Soluble Solids
2.9. Determination of Ash
2.10. Statistical Analyses
3. Results and Discussion
3.1. Honey Samples from Kosovo and Imports
3.2. Moisture Content
3.3. pH
3.4. Electrical Conductivity
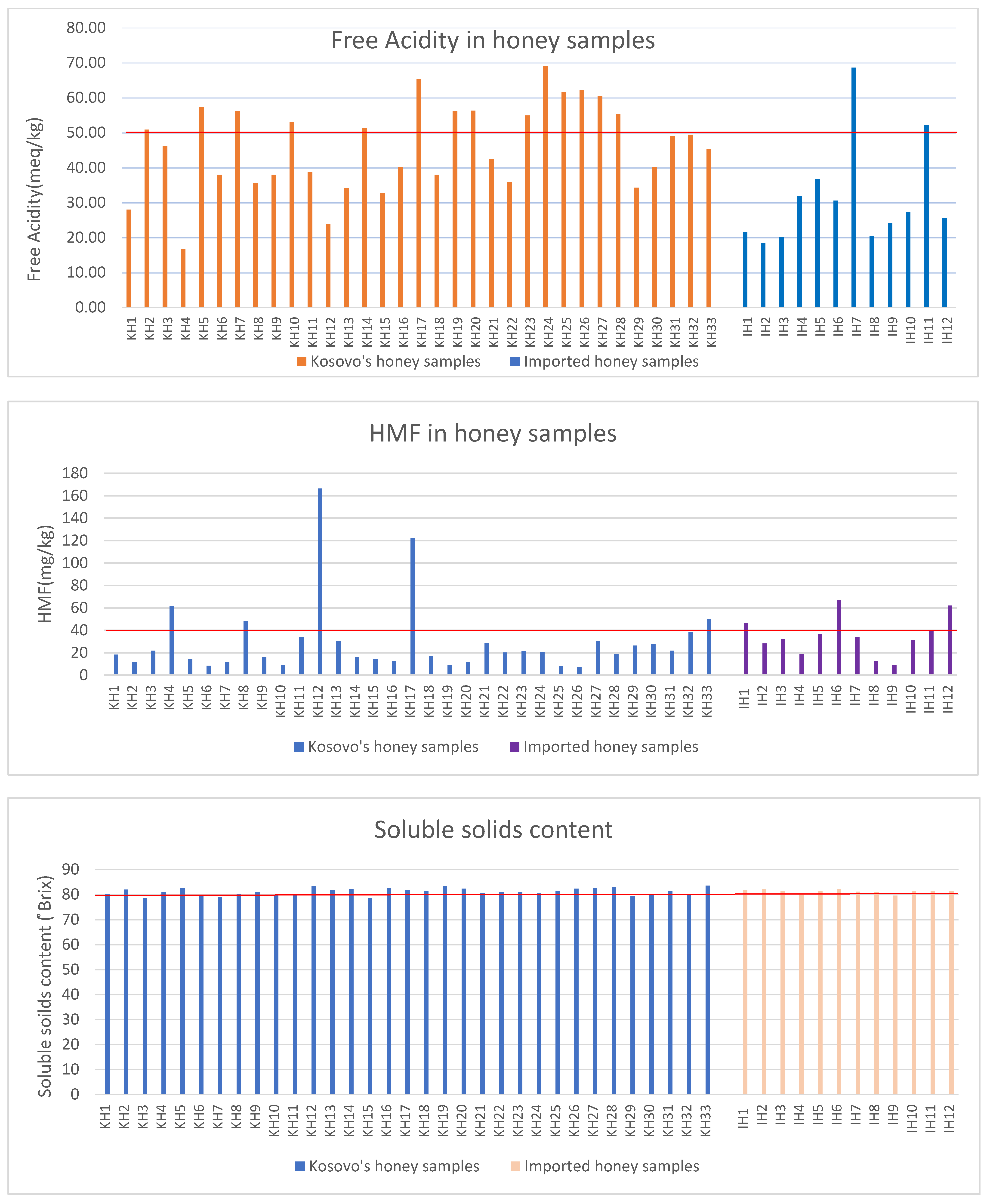
3.5. Free Acidity
3.6. Hydroxymethylfurfural (HMF)
3.7. Protein
3.8. Soluble Solids
3.9. Ash
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Saxena, S.; Gautam, S.; Sharma, A. Physical, biochemical and antioxidant properties of some Indian honeys. Food Chem. 2010, 118, 391–397. [Google Scholar] [CrossRef]
- Krell, R. Value-Added Products from Beekeeping; FAO Agricultural Services Bulletin No. 124; Food and Agriculture Organization of the United Nations: Rome, Italy, 1996. [Google Scholar]
- Blasa, M.; Candiracci, M.; Accorsi, A.; Piacentini, M.P.; Albertini, M.C.; Piatti, E. Raw Millefiori honey is packed full of antioxidants. Food Chem. 2006, 97, 217–222. [Google Scholar] [CrossRef]
- Bilandžić, N.; Dokić, M.; Sedak, M.; Kolanović, B.S.; Varenina, I.; Končurat, A.; Rudan, N. Determination of trace elements in Croatian floral honey originating from different regions. Food Chem. 2011, 128, 1160–1164. [Google Scholar] [CrossRef]
- Guler, A.; Bakan, A.; Nisbet, C.; Yavuz, O. Determination of important biochemical properties of honey to discriminate pure and adulterated honey with sucrose (Saccharum officinarum L.) syrup. Food Chem. 2007, 105, 1119–1125. [Google Scholar] [CrossRef]
- Bogdanov, S.; Martin, P.; Lüllmann, C. Harmonized methods of the European Honey Commission. Apidologie 1997, 1–59. Available online: https://agris.fao.org/agris-search/search.do?recordID=FR1998002973 (accessed on 15 November 2021).
- Crane, E. Honey: A Comprehensive Survey; Heinemann (for) the Bee Research Association: London, UK, 1975; p. 608. [Google Scholar]
- Zappi, A. Development and application of a database of food ingredient fraud and economically motivated adulteration from 1980 to 2010. Eur. food Res. Technol. A 2018, 244, 118–126. [Google Scholar] [CrossRef]
- Codex Alimentarius Commission. Draft Revised Standard for Standard for Honey (at Step 10 of the Codex Procedure) Alinorm; Codex Alimentarius Commission: Rome, Italy, 2001; pp. 19–26. [Google Scholar]
- The Council of The Europian Union Council Directive 2001/110/. Off. J. Eur. Communities 2001, L10, 47–52.
- Escuredo, O.; Míguez, M.; Fernández-González, M.; Carmen Seijo, M.C. Nutritional value and antioxidant activity of honeys produced in a European Atlantic area. Food Chem. 2013, 138, 851–856. [Google Scholar] [CrossRef]
- Karabagias, I.K.; Badeka, A.; Kontakos, S.; Karabournioti, S.; Kontominas, M.G. Characterisation and classification of Greek pine honeys according to their geographical origin based on volatiles, physicochemical parameters and chemometrics. Food Chem. 2014, 146, 548–557. [Google Scholar] [CrossRef]
- Suárez-Luque, S.; Mato, I.; Huidobro, J.F.; Simal-Lozano, J.; Sancho, M.T. Rapid determination of minority organic acids in honey by high-performance liquid chromatography. J. Chromatogr. A 2002, 955, 207–214. [Google Scholar] [CrossRef]
- Won, S.R.; Lee, D.C.; Ko, S.H.; Kim, J.W.; Rhee, H.I. Honey major protein characterization and its application to adulteration detection. Food Res. Int. 2008, 41, 952–956. [Google Scholar] [CrossRef]
- Albu, A.; Radu-Rusu, C.G.; Pop, I.M.; Frunza, G.; Nacu, G. Quality assessment of raw honey issued from Eastern Romania. Agriculture 2021, 11, 247. [Google Scholar] [CrossRef]
- Feás, X.; Pires, J.; Estevinho, M.L.; Iglesias, A.; de Araujo, J.P.P. Palynological and physicochemical data characterisation of honeys produced in the Entre-Douro e Minho region of Portugal. Int. J. Food Sci. Technol. 2010, 45, 1255–1262. [Google Scholar] [CrossRef]
- Pita-Calvo, C.; Guerra-Rodríguez, M.E.; Vázquez, M. Analytical methods used in the quality control of honey. J. Agric. Food Chem. 2017, 65, 690–703. [Google Scholar] [CrossRef] [PubMed]
- Flajak, I.; Primorac, L.; Bilić, B.; Novak, M.; Cvijetić Stokanović, M.; Kenjerić, D. Evaluation of 5-(Hydroxymethyl) Furan-2-Carbaldehyde (Hmf) Evaluation of 5- (Hydroxymethyl) Furan-2-Carbaldehyde (Hmf) Content in Honey: Comparison of Chromatographic and Spectrophotometric Method. Technol. Acta 2016, 9, 37–41. [Google Scholar]
- Codex Alimentarius Commission. Draft Amended Standard for Standard for Honey. CXS 12–1981, Amended in 2019. pp. 1–9. Available online: https://www.fao.org/fao-who-codexalimentarius/sh-proxy/es/?lnk=1&url=https%253A%252F%252Fworkspace.fao.org%252Fsites%252Fcodex%252FStandards%252FCXS%2B12-1981%252FCXS_012e.pdf (accessed on 15 November 2021).
- Datsyuk, V.V. Peculiarities of KrF excimer vibrational relaxation in low-pressure Kr/F2 mixtures excited by a short pulse. Appl. Phys. B Photophysics Laser Chem. 1992, 55, 60–64. [Google Scholar] [CrossRef]
- Beekeeping Sector in Kosovo. Recura 2021. Available online: https://agroportal-ks.com/wp-content/uploads/2021/03/Beekeeping-Sector-in-Kosovo.pdf (accessed on 15 November 2021).
- Rysha, A.; Delaš, F. Sensory properties and chemical composition of Shar cheese from Kosovo. Mljekarstvo 2014, 64, 295–303. [Google Scholar] [CrossRef] [Green Version]
- Rysha, A.; Markov, K.; Frece, J.; Čvek, D.; Delaš, F. A survey of the microbiological quality of Sharri, a hard mountain cheese from Kosovo. Int. J. Dairy Technol. 2014, 67, 277–282. [Google Scholar] [CrossRef]
- Kastrati, G.; Paçarizi, M.; Sopaj, F.; Tašev, K.; Stafilov, T.; Mustafa, M.K. Investigation of concentration and distribution of elements in three environmental compartments in the region of Mitrovica, kosovo: Soil, honey and bee pollen. Int. J. Environ. Res. Public Health 2021, 18, 2269. [Google Scholar] [CrossRef]
- Dizman, S.; Hodolli, G.; Kadiri, S.; Aliu, H.; Makolli, S. Radioactivity in Kosovo honey samples. Polish J. Environ. Stud. 2020, 29, 1119–1127. [Google Scholar] [CrossRef]
- Ibrahimi, H.; Hajdari, A. Phenolic and flavonoid content, and antioxidant activity of honey from Kosovo. J. Apic. Res. 2020, 59, 452–457. [Google Scholar] [CrossRef]
- Baroni, M.V.; Arrua, C.; Nores, M.L.; Fayé, P.; Diaz, M.P.; Chiabtando, G.A.; Wuderlin, D.A. Composition of honey from Córdoba (Argentina): Assessment of North/South provenance by chemometrics. Food Chem. 2009, 114, 727–733. [Google Scholar] [CrossRef]
- Uršulin-Trstenjak, N.; Levanić, D.; Grabar, I.; Koldenjak, M.; Bošnir, J. Physico-Chemical Profiles of Croatian Honey with an Overview of Its Consumption. J. Appl. Health Sci. 2017, 3, 51–60. [Google Scholar] [CrossRef]
- Šarić, G.; Matković, D.; Hruškar, M.; Vahčić, N. Characterisation and classification of Croatian honey by physicochemical parameters. Food Technol. Biotechnol. 2008, 46, 355–367. [Google Scholar]
- Marghitas, L.A.; Dezmirean, D.S.; Pocol, C.B.; Ilea, M.; Bobis, O.; Gergen, I. The development of a biochemical profile of black locust honey by identifying biochemical determinants of its quality. Not. Bot. Hort I Agrobot. Cluj Napoca 2010, 38, 84–90. [Google Scholar]
- Persano Oddo, L.; Piro, R. Main European unifloral honeys: Descriptive sheets. Apidologie 2004, 35, S38–S81. [Google Scholar] [CrossRef]
- Kenjerić, D.; Mandić, M.L.; Primorac., L.; Bubola, D.; Perl, A. Flavonoid profile of Robinia honeys produced in Croatia. Food Chem. 2007, 102, 683–690. [Google Scholar] [CrossRef]
- Acquarone, C.; Buera, P.; Elizalde, B. Pattern of pH and electrical conductivity upon honey dilution as a complementary tool for discriminating geographical origin of honeys. Food Chem. 2007, 101, 695–703. [Google Scholar] [CrossRef]
- Cantarelli, M.A.; Pellerano, R.G.; Marchevsky, E.J.; Camiña, J.M. Quality of Honey from Argentina: Study of Chemical Composition and Trace Elements. J. Argent. Chem. Soc. 2008, 96, 33–41. [Google Scholar]
- Missio da Silva, P.; Gonzaga, L.V.; Biluca, F.C.; Schulz, M.; Vitali, L.; Micke, G.A.; Oliveira Costa, A.C.; Fett, R. Stability of Brazilian Apis mellifera L. honey during prolonged storage: Physicochemical parameters and bioactive compounds. LWT 2020, 129, 109521. [Google Scholar] [CrossRef]
- Zarei, M.; Fazlara, A.; Alijani, N. Evaluation of the changes in physicochemical and antioxidant properties of honey during storage. Funct. Foods Heal. Dis. 2019, 9, 593–605. [Google Scholar] [CrossRef]
- Sancho, M.T.; Muniategui, S.; Sánchez, M.P.; Huidobro, J.F.; Simal, J. Relationships between electrical conductivity and total and sulphated ash contents in Basque honeys. Apidologie 1991, 22, 487–494. [Google Scholar] [CrossRef] [Green Version]
- Bromberg, W.; Berrian, B. An approach to recidivism. Bull. Am. Acad. Psychiatry Law 1974, 2, 111–114. [Google Scholar] [PubMed]
- Živkov Baloš, M.; Popov, N.; Vidaković, S.; Ljubojević Pelić, D.; Pelić, M.; Mihaljev, Ž.; Jakšić, S. Electrical conductivity and acidity of honey. Arch. Vet. Med. 2018, 11, 91–101. [Google Scholar] [CrossRef]
- Kaškoniene, V.; Venskutonis, P.R.; Čeksteryte, V. Carbohydrate composition and electrical conductivity of different origin honeys from Lithuania. LWT 2010, 43, 801–807. [Google Scholar] [CrossRef]
- Mondragón-Cortez, P.; Ulloa, J.A.; Rosas-Ulloa, P.; Rodríguez-Rodríguez, R.; Resendiz Vizquez, J.A. Physicochemical characterization of honey from the West region of México. CyTA J. Food 2013, 11, 7–13. [Google Scholar] [CrossRef]
- Oddo, L.P.; Piazza, M.G.G.; Sabatini, A.G.G.; Accorti, M. Characterization of Unifloral Honeys. Apidologie 1995, 26, 453–465. [Google Scholar] [CrossRef] [Green Version]
- Atanassova, J.; Yurukova, L.; Lazarova, M. Pollen and Inorganic Characteristics of Bulgarian Unifloral Honeys. Czech J. Food Sci. 2012, 30, 520–526. [Google Scholar] [CrossRef] [Green Version]
- Mato, I.; Huidobro, J.F.; Simal-Lozano, J.; Sancho, M.T. Rapid determination of nonaromatic organic acids in honey by capillary zone electrophoresis with direct ultraviolet detection. J. Agric. Food Chem. 2006, 54, 1541–1550. [Google Scholar] [CrossRef]
- Kahraman, T.; Buyukunal, S.K.; Vural, A.; Altunatmaz, S.S. Physico-chemical properties in honey from different regions of Turkey. Food Chem. 2010, 123, 41–44. [Google Scholar] [CrossRef]
- Ozcan, M.; Arslan, D.; Ceylan, D.A. Effect of inverted saccharose on some properties of honey. Food Chem. 2006, 99, 24–29. [Google Scholar] [CrossRef]
- Gomes, S.; Dias, L.G.; Moreira, L.L.; Rodrigues, P.; Estevinho, L. Physicochemical, microbiological and antimicrobial properties of commercial honeys from Portugal. Food Chem. Toxicol. 2010, 48, 544–548. [Google Scholar] [CrossRef] [Green Version]
- Desissa, Y. Detection of the Electrical Conductivity and Acidity of Honey from Different Areas of Tepi. Food Sci. Technol. 2014, 2, 59–63. [Google Scholar]
- Seraglio, S.K.T.; Silva, B.; Bergamo, G.; Brugnerotto, P.; Gonzaga, L.V.; Fett, R.; Costa, A.C.O. An overview of physicochemical characteristics and health-promoting properties of honeydew honey. Food Res. Int. 2019, 119, 44–66. [Google Scholar] [CrossRef] [PubMed]
- Ajlouni, S.; Sujirapinyokul, P. Hydroxymethylfurfuraldehyde and amylase contents in Australian honey. Food Chem. 2010, 119, 1000–1005. [Google Scholar] [CrossRef]
- Cavia, M.M.; Fernández-Muiño, M.A.; Alonso-Torre, S.; Huidobro, J.F.; Sancho, M.T. Evolution of acidity of honeys from continental climates: Influence of induced granulation. Food Chem. 2007, 100, 1728–1733. [Google Scholar] [CrossRef]
- Mouhoubi-Tafinine, Z.; Ouchemoukh, S.; Bachir Bey, M.; Louaileche, H.; Tamendjari, A. Effect of storage on hydroxymethylfurfural (HMF) and color of some Algerian honey. Int. Food Res. J. 2018, 25, 1044–1050. [Google Scholar]
- Gidamis, A.B.; Chove, B.E.; Shayo, N.B.; Nnko, S.A.; Bangu, N.T. Quality evaluation of honey harvested from selected areas in Tanzania with special emphasis on hydroxymethyl furfural (HMF) levels. Plant. Foods Hum. Nutr. 2004, 59, 129–132. [Google Scholar] [CrossRef]
- Zappalà, M.; Fallico, B.; Arena, E.; Verzera, A. Methods for the determination of HMF in honey: A comparison. Food Control 2005, 16, 273–277. [Google Scholar] [CrossRef]
- Da Silva, P.M.; Gauche, C.; Gonzaga, L.V.; Costa, A.C.O.; Fett, R. Honey: Chemical composition, stability and authenticity. Food Chem. 2016, 196, 309–323. [Google Scholar] [CrossRef]
- Pauliuc, D.; Ciursă, P.; Ropciuc, S.; Dranca, F.; Oroian, M. Physicochemical Parameters Prediction and Authentication of Different Monofloral Honeys Based on FTIR Spectra. J. Food Compos. Anal. 2021, 102, 104021. [Google Scholar] [CrossRef]
- Chirsanova, A.; Capcanari, T.; Boistean, A.; Siminiuc, R. Physico-Chemical Profile of Four Types of Honey from the South of the Republic of Moldova. Food Nutr. Sci. 2021, 12, 874–888. [Google Scholar] [CrossRef]
- Krishnasree, V.; Ukkuru, P.M. Quality Analysis of Bee Honeys. Int. J. Curr. Microbiol. Appl. Sci. 2017, 6, 626–636. [Google Scholar] [CrossRef] [Green Version]
- Silva, L.R.; Videira, R.; Monteiro, A.P.; Valentão, P.; Andrade, P.B. Honey from Luso region (Portugal): Physicochemical characteristics and mineral contents. Microchem. J. 2009, 93, 73–77. [Google Scholar] [CrossRef]
- Suárez-Luque, S.; Mato, I.; Huidobro, J.F.; Simal-Lozano, J. Capillary zone electrophoresis method for the simultaneous determination of cations in honey. J. Chromatogr. A 2005, 1083, 193–198. [Google Scholar] [CrossRef] [PubMed]
- Mendes, E.; Brojo, P.E.; Ferreira, I.; Ferreira, M.A. Quality evaluation of Portuguese honey. Carbohydr. Polym. 1998, 37, 219–223. [Google Scholar] [CrossRef]
- Al, M.L.; Daniel, D.; Moise, A.; Bobis, O.; Laslo, L.; Bogdanov, S. Physico-Chemical and Bioactive Properties of Different Floral Origin Honeys from Romania. Food Chem. 2009, 112, 863–867. [Google Scholar] [CrossRef]
| Directive 2001/110 EU | ||||
|---|---|---|---|---|
| Composition Criteria | Blossom Honey | Honeydew Honey * | ||
| General | Exception | General | Revised CODEX 2001 | |
| Moisture % | <20 | Calluna and baker’s honey < 23 Baker’s from Calluna < 25 | <20 | The same No indication for baker’s honey |
| Fructose + glucose %Sucrose % | >60 <5 | Robina, Medicogo, Banksia, Hedysarum, Eucaliptus, Eucryphiaspp, and Citrus <10 Lavandula & Borogo < 15 | >45 <5 | The same The same |
| Water-insoluble% | <0.1 | Pressed honey < 0.5 | <0.1 | The same |
| Electrical conductivity mS. Cm−¹ | <0.8 | Chestnut, Arbutus, Erica, Eucolyptus, Tilia, Calluna, Manuka and Melaleuca | <0.8 | The same |
| Free acid meq. kg−¹ | <50 | Baker’s honey < 50 | <50 | The same |
| Diastase activity DN ** | >8 | Baker’s honey and honey with low natural enzyme content > 8 when HMF is less than 15 mg.kg−¹ | >8 | Honeys with low natural enzyme cintent > 3 DN |
| HMF mg.kg−¹ ** | <50 | baker’s honey Honeys of tropical climate and blenda of these honey < 80 | <40 | Honeys with low natural enzyme vontent > 3 DN. Honeys of tropical climate and blends: <80 |
| Sample | Type of Honey | Moisture (%) | pH | EC (mS/cm) | Free Acidity (meq/kg) | HMF (mg/kg) | Protein (%) | Soluble Solids Content °Brix | Ash (%) |
|---|---|---|---|---|---|---|---|---|---|
| KH 1 | Flower | 17.76 | 4.07 | 0.25 | 28.00 | 18.31 | 0.30 | 80.20 | 0.15 |
| KH 2 | Forest | 15.17 | 3.90 | 0.58 | 50.90 ** | 11.26 | 0.38 | 81.90 | 0.57 |
| KH 3 | Acacia | 18.63 | 3.63 | 0.24 | 46.20 | 21.74 | 0.28 | 78.60 *** | 0.14 |
| KH 4 | Forest | 16.80 | 3.76 | 0.12 | 16.60 | 61.33 * | 0.20 | 81.00 | 0.01 |
| KH 5 | Mixed | 16.47 | 3.94 | 0.46 | 57.20 ** | 14.00 | 0.39 | 82.50 | 0.20 |
| KH 6 | Flower | 19.00 | 3.60 | 0.22 | 38.00 | 8.33 | 0.32 | 79.70 *** | 0.06 |
| KH 7 | Forest | 18.80 | 3.94 | 0.50 | 56.20 ** | 11.50 | 0.40 | 78.80 *** | 0.22 |
| KH 8 | Acacia | 16.50 | 4.14 | 0.40 | 35.60 | 48.52 * | 0.23 | 80.20 | 0.41 |
| KH 9 | Forest | 16.80 | 4.49 | 0.65 | 38.00 | 15.93 | 0.31 | 81.00 | 0.59 |
| KH 10 | Flower | 18.45 | 3.72 | 0.25 | 53.00 ** | 9.22 | 0.41 | 79.70 *** | 0.17 |
| KH 11 | Flower | 17.60 | 3.56 | 0.21 | 38.70 | 34.21 | 0.26 | 79.60 *** | 0.02 |
| KH 12 | Mixed | 14.50 | 3.80 | 0.11 | 23.90 | 166.4 * | 0.22 | 83.20 | 0.07 |
| KH 13 | Mixed | 15.60 | 3.81 | 0.41 | 34.20 | 30.24 | 0.33 | 81.60 | 0.16 |
| KH 14 | Flower | 15.10 | 4.15 | 0.69 | 51.40 ** | 16.10 | 1.26 | 82.00 | 0.75 |
| KH 15 | Forest | 18.30 | 3.58 | 0.18 | 32.70 | 14.62 | 0.28 | 78.60 *** | 0.39 |
| KH 16 | Mixed | 14.90 | 3.59 | 0.17 | 40.20 | 12.54 | 0.27 | 82.70 | 0.08 |
| KH 17 | Acacia | 15.56 | 4.22 | 0.41 | 65.20 ** | 122.34 * | 0.62 | 81.80 | 0.76 |
| KH 18 | Forest | 15.46 | 3.74 | 0.24 | 38.00 | 17.30 | 0.28 | 81.40 | 0.16 |
| KH 19 | Forest | 15.26 | 4.20 | 0.52 | 56.10 ** | 8.67 | 0.43 | 83.20 | 0.26 |
| KH 20 | Acacia | 14.60 | 3.81 | 0.31 | 56.30 ** | 11.42 | 0.43 | 82.30 | 0.14 |
| KH 21 | Flower | 16.70 | 3.87 | 0.63 | 42.50 | 28.74 | 0.40 | 80.40 | 0.06 |
| KH 22 | Flower | 16.80 | 4.11 | 0.34 | 35.90 | 20.07 | 0.40 | 81.00 | 0.13 |
| KH 23 | Forest | 16.67 | 3.99 | 0.44 | 54.90 ** | 21.40 | 0.28 | 80.90 | 0.15 |
| KH 24 | Mixed | 17.63 | 3.55 | 0.31 | 69.00 ** | 20.50 | 1.90 | 80.30 | 0.07 |
| KH 25 | Forest | 15.95 | 4.34 | 0.73 | 61.50 ** | 8.12 | 0.40 | 81.50 | 0.42 |
| KH 26 | Mixed | 15.80 | 3.77 | 0.39 | 62.10 ** | 7.41 | 0.53 | 82.30 | 0.10 |
| KH 27 | Mixed | 15.60 | 4.11 | 0.75 | 60.50 ** | 30.10 | 0.75 | 82.50 | 0.41 |
| KH 28 | Flower | 15.32 | 4.02 | 0.63 | 55.40 ** | 18.57 | 0.52 | 82.90 | 0.21 |
| KH 29 | Chestnut | 18.50 | 4.34 | 0.57 | 34.30 | 26.32 | 0.29 | 79.20 *** | 0.07 |
| KH 30 | Flower | 17.35 | 3.92 | 0.36 | 40.20 | 28.04 | 0.31 | 80.00 | 0.23 |
| KH 31 | Flower | 17.44 | 3.52 | 0.26 | 49.00 | 21.79 | 0.66 | 81.40 | 0.26 |
| KH 32 | Mixed | 16.63 | 3.55 | 0.30 | 49.40 | 38.04 | 0.38 | 80.10 | 0.10 |
| KH 33 | Flower | 14.00 | 4.05 | 0.29 | 45.40 | 49.88 * | 0.28 | 83.50 | 0.45 |
| Sample | Type of Honey | Moisture (%) | pH | EC (mS/cm) | Free Acidity (meq/kg) | HMF (mg/kg) | Protein (%) | Soluble Solids Content °Brix | Ash (%) |
|---|---|---|---|---|---|---|---|---|---|
| IH 1 | Acacia | 17.36 | 3.91 | 0.07 | 21.50 | 46.21 * | 0.21 | 81.70 | 0.06 |
| IH 2 | Forest | 16.05 | 4.32 | 0.60 | 18.40 | 28.25 | 0.48 | 82.00 | 0.53 |
| IH 3 | Acacia | 16.84 | 4.05 | 0.10 | 20.20 | 31.81 | 0.13 | 81.30 | 0.05 |
| IH 4 | Flower | 17.26 | 3.65 | 0.09 | 31.80 | 18.37 | 0.01 | 80.20 | 0.01 |
| IH 5 | Acacia | 16.85 | 3.88 | 0.11 | 36.80 | 36.61 | 0.37 | 81.20 | 0.01 |
| IH 6 | Acacia | 15.23 | 3.92 | 0.13 | 30.60 | 67.25 * | 0.19 | 82.20 | 0.02 |
| IH 7 | Flower | 16.00 | 3.96 | 0.10 | 68.60 ** | 33.72 | 0.11 | 81.10 | 0.03 |
| IH 8 | Chestnut | 17.00 | 5.02 | 1.02 | 20.50 | 12.33 | 1.16 | 80.90 | 0.60 |
| IH 9 | Flower | 17.45 | 3.83 | 0.16 | 24.20 | 9.20 | 0.20 | 79.50 *** | 0.01 |
| IH 10 | Flower | 16.38 | 3.83 | 0.14 | 27.40 | 31.16 | 7.68 | 81.40 | 0.07 |
| IH 11 | Chestnut | 16.54 | 4.17 | 0.51 | 52.30 ** | 40.25 * | 1.41 | 81.30 | 0.53 |
| IH 12 | Forest | 16.30 | 3.51 | 0.08 | 25.50 | 62.00 * | 0.13 | 81.40 | 0.01 |
| Total Samples (n = 45) | Moisture (%) | ph | EC (mS/cm) | Free Acidity (meq/kg) | HMF (mg/kg) | Protein (%) | Soluble Solids Content °Brix (%) | Ash (%) |
|---|---|---|---|---|---|---|---|---|
| Mean ± SD (min−max) | Mean ± SD (min−max) | Mean ± SD (min−max) | Mean ± SD (min−max) | Mean ± SD (min−max) | Mean ± SD (min−max) | Mean ± SD (min−max) | Mean ± SD (min−max) | |
| * KH Samples (n = 33) | 16.53 ± 1.35 (14.00−19.00) | 3.90 ± 0.25 (3.32−4.02) | 0.39 ± 0.18 (0.12−0.75) | 45.95 ± 12.30 (23.90−69.00) | 29.48 ± 32.26 (7.41−166.43) | 0.44 ± 0.32 (0.20−1.90) | 81.09 ± 1.37 (78.60−83.50) | 0.24 ± 0.20 (0.01−0.76) |
| ** IH samples (n = 12) | 16.60 ± 0.60 (15.23−17.45) | 4.00 ± 0.36 (3.51−5.02) | 0.26 ± 0.28 (0.07−1.02) | 31.48 ± 14.33 (18.40−68.60) | 34.76 ± 16.96 (9.20−67.25) | 1.00 ± 2.05 (0.01−7.68) | 81.18 ± 0.70 (79.50−82.20) | 0.16 ± 0.02 (0.01−0.60) |
| >p > 0.05 | p > 0.05 | p < 0.05 | p < 0.05 | p > 0.05 | p > 0.05 | p > 0.05 | p > 0.05 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rysha, A.; Kastrati, G.; Biber, L.; Sadiku, V.; Rysha, A.; Zogaj, F.; Kabashi-Kastrati, E. Evaluating the Physicochemical Properties of Some Kosovo’s and Imported Honey Samples. Appl. Sci. 2022, 12, 629. https://doi.org/10.3390/app12020629
Rysha A, Kastrati G, Biber L, Sadiku V, Rysha A, Zogaj F, Kabashi-Kastrati E. Evaluating the Physicochemical Properties of Some Kosovo’s and Imported Honey Samples. Applied Sciences. 2022; 12(2):629. https://doi.org/10.3390/app12020629
Chicago/Turabian StyleRysha, Agim, Granit Kastrati, Lejla Biber, Valon Sadiku, Aurorë Rysha, Florjana Zogaj, and Edona Kabashi-Kastrati. 2022. "Evaluating the Physicochemical Properties of Some Kosovo’s and Imported Honey Samples" Applied Sciences 12, no. 2: 629. https://doi.org/10.3390/app12020629
APA StyleRysha, A., Kastrati, G., Biber, L., Sadiku, V., Rysha, A., Zogaj, F., & Kabashi-Kastrati, E. (2022). Evaluating the Physicochemical Properties of Some Kosovo’s and Imported Honey Samples. Applied Sciences, 12(2), 629. https://doi.org/10.3390/app12020629







