The Experimental Study of Innovative Methods Regarding the Removal of Sm(III)
Abstract
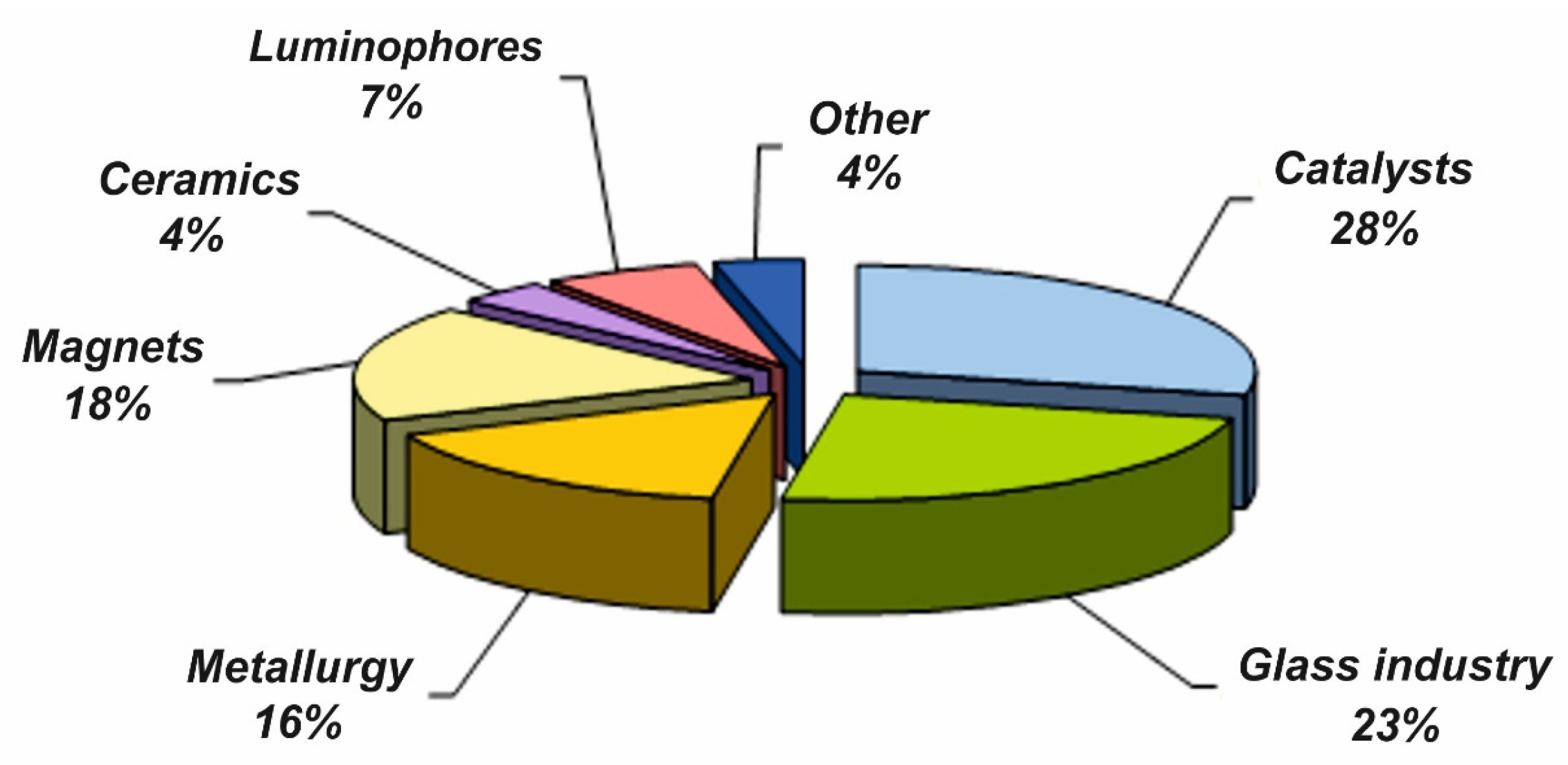
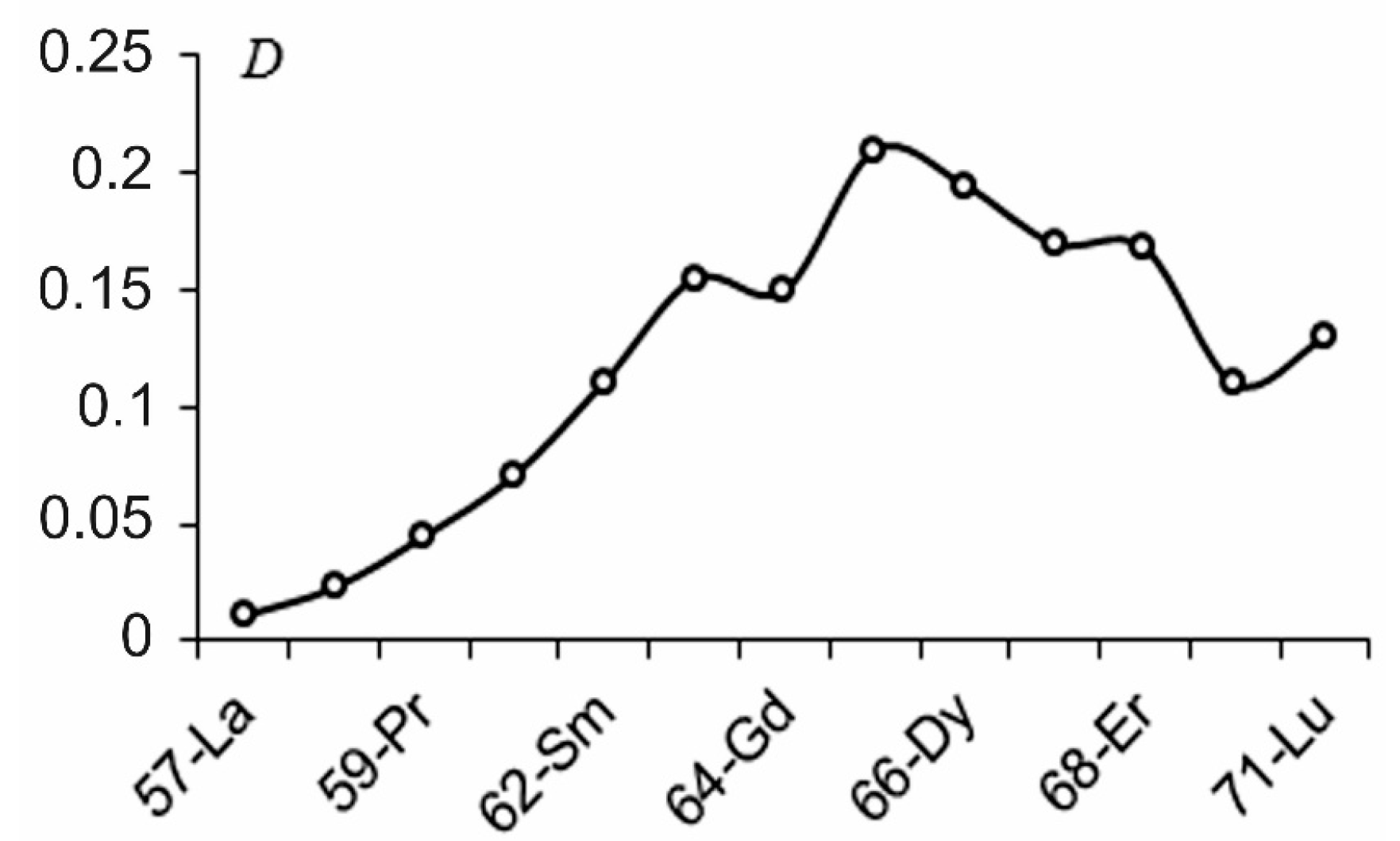
:1. Introduction
2. Materials and methods
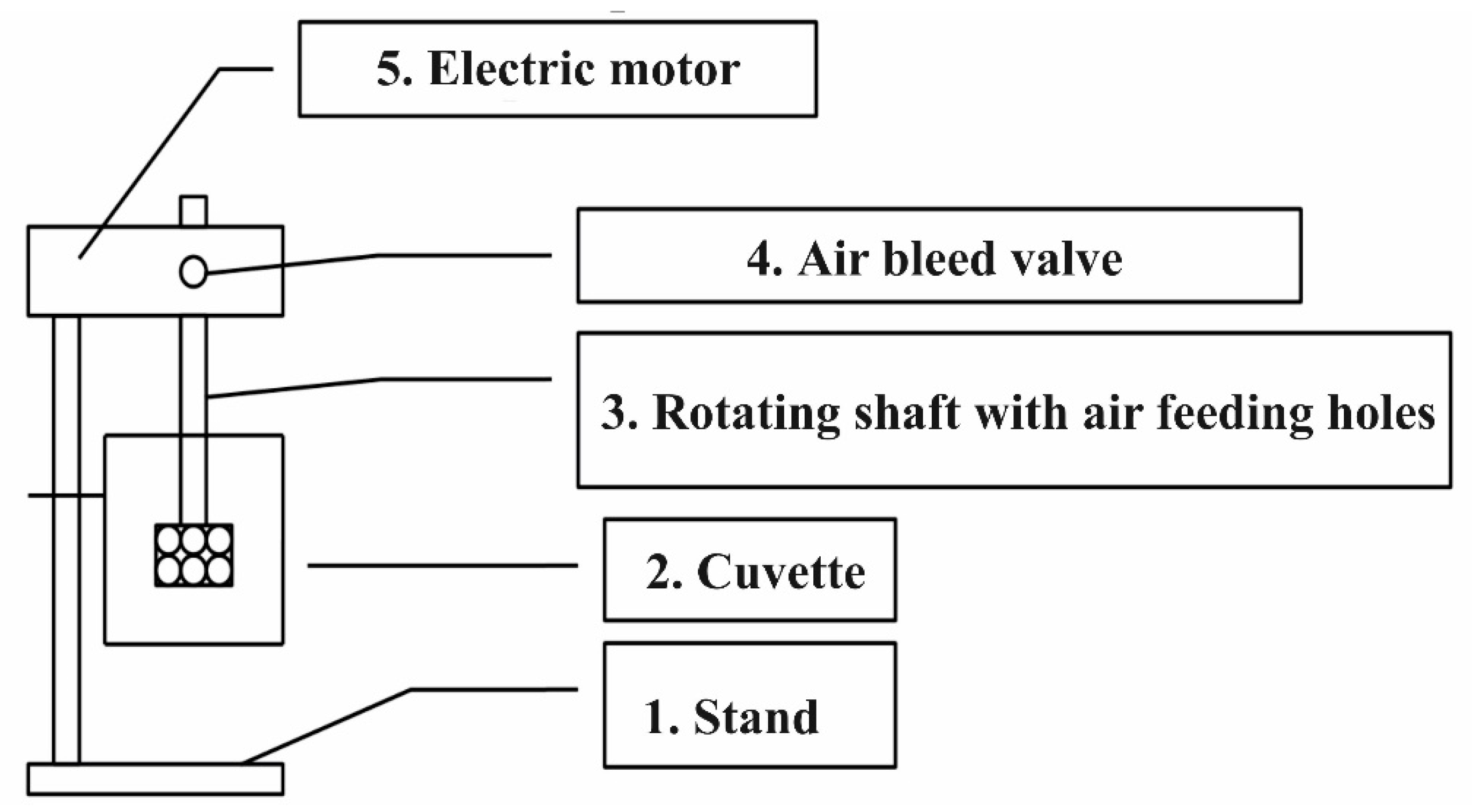
2.1. Ion Flotation
2.2. Solvent Sublation
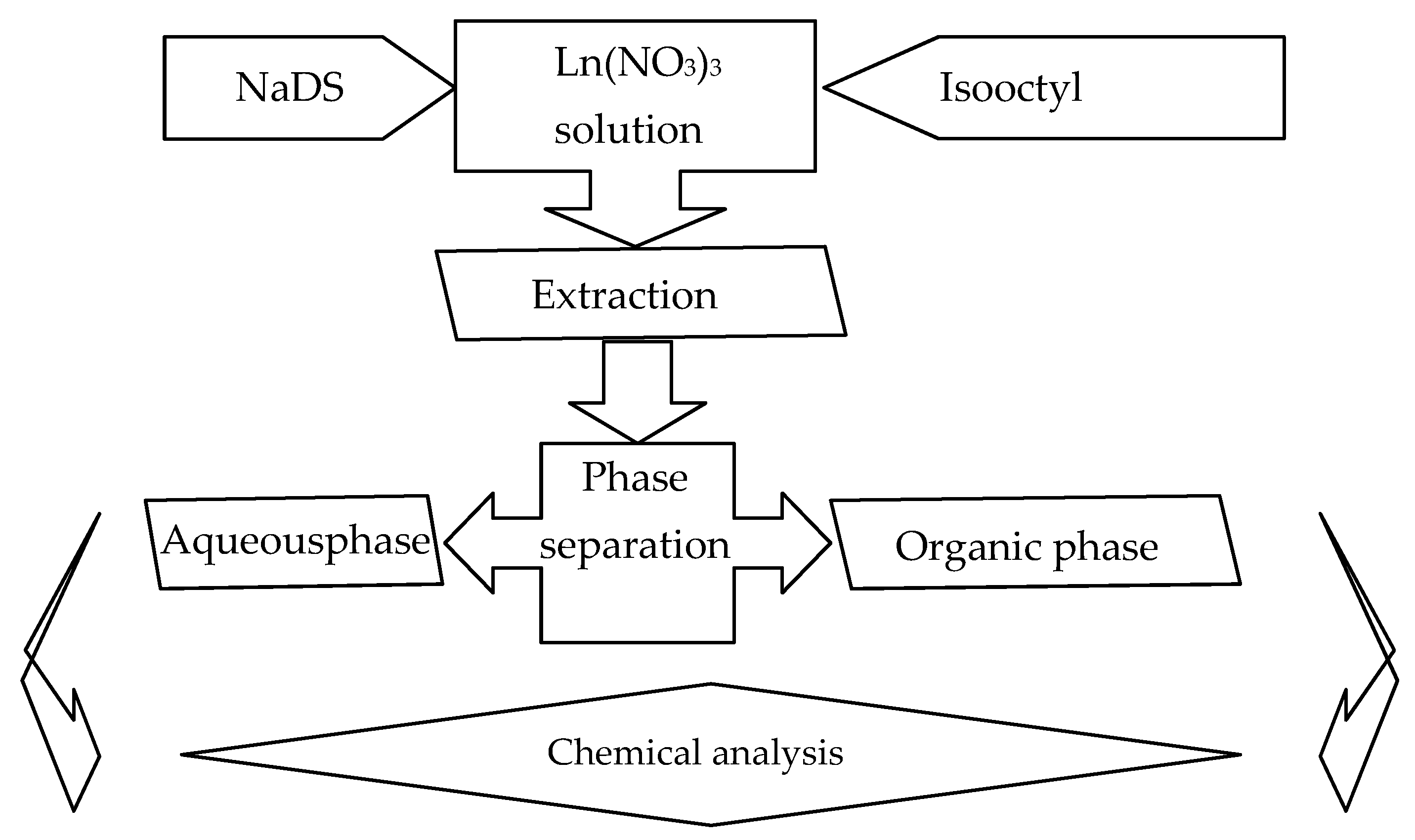
2.3. Extraction
3. Results and Discussion
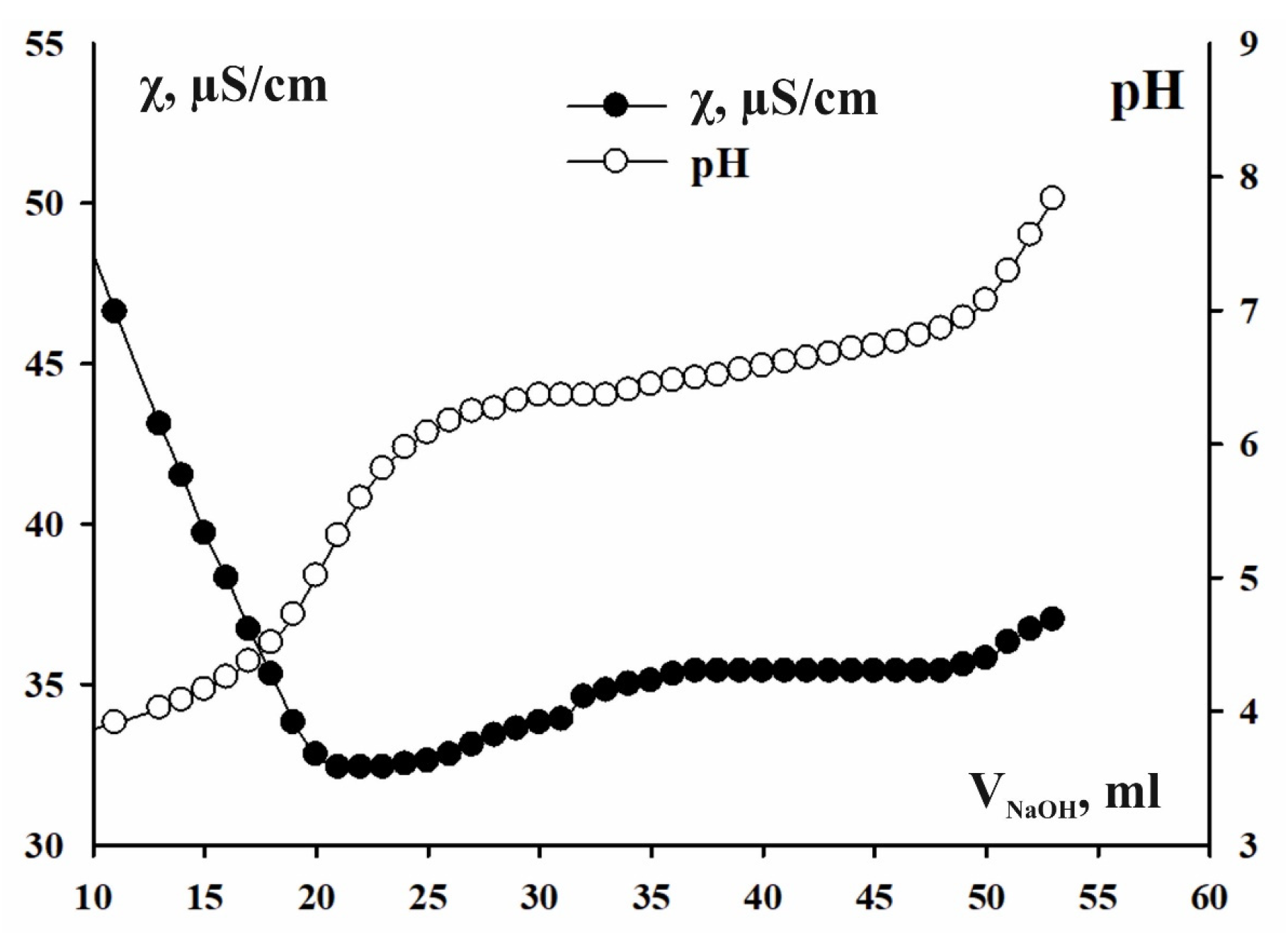
3.1. Results of the Ion Flotation Method
3.2. Solvent Sublation Results
3.3. Extraction Results
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Lemlich, R. Adsorptive Bubble Separation Techniques; N.Y. Academic Press: New York, NY, USA, 1972; 344p. [Google Scholar]
- Matveeva, V.; Danilov, A.; Pashkevich, M. Treatment of multi-tonnage manganese-containing waste water using vermiculite. J. Ecol. Eng. 2018, 19, 156–162. [Google Scholar] [CrossRef]
- Kogan, B.I. Rare Metals. State and Prospects; Nauka: Moscow, Russia, 1978; 376p. [Google Scholar]
- Hidayah, N.N.; Abidin, S.Z. The evolution of mineral processing in extraction of rare earth elements using liquid-liquid extraction: A review. Miner. Eng. 2018, 121, 146–157. [Google Scholar] [CrossRef]
- Goman, I.V.; Varlakova, E.A. Teaching communication skills in a foreign language to students of oil and gas specialization. Int. Multidiscip. Sci. GeoConf. Surv. Geol. Min. Ecol. Manag. SGEM 2019, 19, 295–300. [Google Scholar] [CrossRef]
- Goman, I.V. Teaching writing skills in the foreign language to future petroleum engineers specializing in oil and gas development and operation. Int. Multidiscip. Sci. GeoConf. Surv. Geol. Min. Ecol. Manag. SGEM 2017, 17, 195–202. [Google Scholar] [CrossRef]
- Lokshin, E.P.; Kalinnikov, V.T.; Tareeva, O.A. The removal of rare-earth elements from Khibini apatite concentrate conversion products, notably middling products and technogenic wastes. Tcvetnye Metally 2012, 3, 75–80. [Google Scholar]
- Voigt, W. Chemistry of salts in aqueous solutions: Applications, experiments, and theory. Pure Appl. Chem. 2011, 83, 1015–1030. [Google Scholar] [CrossRef]
- Medyanik, N.L.; Chanturia, V.A.; Shadrunova, I.V. Quantum-chemical method for selection of a collecting agent to recover zinc and copper (II) cation sin flotation of mine waste waters. J. Min. Sci. 2012, 48, 167–176. [Google Scholar] [CrossRef]
- Ramírez-Serrano, B.; Coello-Velazquez, A.L.; Bernardo, A.; Afif, E.; Menendez-Aguado, J.M. Recovery of copper-ion by flotation with potassium amylxanthate. Rev. Metal. 2012, 48, 254–263. [Google Scholar]
- Kazanskaya, L.F.; Smirnova, O.M.; Palomo, Á.; Pidal, I.M.; Romana, M. Supersulfated cement applied to produce lightweight concrete. Materials 2021, 14, 403. [Google Scholar] [CrossRef]
- Romashev, A.; He, D.; Aleksandrova, T.; Nikolaeva, N. Technological typomorphic associations in caustobiolithes and methods of their extraction. Metals 2021, 11, 121. [Google Scholar] [CrossRef]
- Bazhin, V.Y.; Aleksandrova, T.A.; Kotova, E.L.; Suslov, A.P. A modern view of anomalies in the metal groups of the periodic system of D.I. Mendeleev. J. Min. Inst. 2019, 239, 520–527. [Google Scholar] [CrossRef]
- Kurdiumov, V.R.; Timofeev, K.L.; Maltsev, G.I.; Lebed, A.B. Sorption of nickel (II) and manganese (II) ions from aqueous solutions. J. Min. Inst. 2020, 242, 209–217. [Google Scholar] [CrossRef]
- Storozhenko, D.; Dryuchko, O.; Jesionowski, T.; Ivanytska, I. Some Physicochemical Aspects of the Preparatory Stages in the Formation of Self-cleaning Photocatalytic Active Coatings for Building Construction Materials. Lect. Notes Civ. Eng. 2020, 73, 285–301. [Google Scholar] [CrossRef]
- Nikolaev, A.I.; Krivovichev, S.V. Kola Peninsula in solving problems of national arctic materials science. IOP Conf. Ser. Mater. Sci. Eng. 2019, 696, 012019. [Google Scholar] [CrossRef] [Green Version]
- Sebba, F. Ion flotation. Metallurgiya 1965, 17, 1062–1063. [Google Scholar] [CrossRef]
- Karger, B.L.; Devivo, D.G. General survey of adsorptive bubble separation processes. Sep. Sci. Technol. 1968, 3, 393–424. [Google Scholar] [CrossRef]
- Lobacheva, O.; Dzhevaga, N. Rare-earth elements recovery on the example of Europium (III) from lean technogenic raw materials. J. Ecol. Eng. 2017, 18, 122–126. [Google Scholar] [CrossRef]
- Savin, S.B. Arsenazo III. M: Atomizdat. Publ. 1966, 256. [Google Scholar]
- Timofeev, S.V.; Materova, V.A.; Arkhangelskiy, L.K. Electrode behaviour of anion-selective membranes. Vestn. LGU Phys. Chem. Ser. 1978, 16, 139–141. [Google Scholar]
- Hoseinian, F.S.; Rezai, B.; Kowsari, E.; Chinnappan, A.; Ramakrishna, S. Synthesis and characterization of a novel nanocollector for the removal of nickel ions from synthetic wastewater using ion flotation. Sep. Purif. Technol. 2020, 240, 116639. [Google Scholar] [CrossRef]
- Grieves, R.B.; Charewicz, W.R. Ion and colloid flotation of Ni, Co and Pt. Sep. Sci. 1975, 10, 77–92. [Google Scholar]
- Voronin, N.N.; Demidov, V.V.; Cherkasov, A.V.; Antonova, I.P. Froth solvent sublation of heavy metals from solutions. Zhurnal Prikladnoi Khimii 1992, 65, 2005–2012. [Google Scholar]
- Alves, R.C.; Nascimento, M.; Paulino, J.F.; Afonso, J.C. Selection of a hydrometallurgical process for rare earths extraction from a Brazilian monazite. Hydrometallurgy 2021, 200, 105556. [Google Scholar] [CrossRef]
- Valsaraj, K.T.; Thoma, G.J.; Thibodeaux, L.J. Nonfoaming adsorptive bubble separation processes. Sep. Technol. 1991, 1, 234–244. [Google Scholar] [CrossRef]
- Bryson, G.B.; Valsaraj, K.T. Solvent sublation for waste minimization in process water stream—A pilot scale study. J. Hazard. Mater. 2001, 82, 65–75. [Google Scholar] [CrossRef]
- Lu, Y.; Zhu, X. Solvent sublation: Theory and application. Sep. Purif. Method. 2001, 30, 157–189. [Google Scholar] [CrossRef]
- Chang, L.; Cao, Y.; Fan, G.; Li, C.; Peng, W. A review of the applications of ion floatation: Wastewater treatment, mineral beneficiation and hydrometallurgy. RSC Adv. 2019, 9, 20226–20239. [Google Scholar] [CrossRef]
- Singh, D.K.; Singh, H.; Mathur, J.N. ElsevierExtraction of rare earths and yttrium with high molecular weight carboxylic acids. Hydrometallurgy 2006, 81, 174–181. [Google Scholar] [CrossRef]
- Mikhailichenko, A.I. Rare-earth metals. M: Metallurgy 1987, 232. [Google Scholar]
- Lidin, R.A.; Andreeva, A.A.; Molochko, A.V. Handbook. Constants of Inorganic Substances; Dvora Publ: Moscow, Russia, 1995. [Google Scholar]
- Outo Kumpu Research OY; HSC Chemistry: Moscow, Russia, 2006; version 4.1.
- Ravdel., A.A.; Ponomareva, A.M. Quick Reference to Physical and Chemical Quantities, 10th ed.; Ivan Fedorov: St. Petersburg, FL, USA, 2003. [Google Scholar]
- Dzhevaga, N.V.; Lobacheva, O.L. Analysis of the mixture of rare earth elements by atomic spectroscopy. J. Phys. Conf. Ser. 2019, 1384, 012008. [Google Scholar] [CrossRef]
- Gu, X.; Tan, H.; He, X.; Smirnova, O.; Zhang, J.; Luo, Z. Utilization of Carbide Slag by Wet Grinding as an Accelerator in Calcium Sulfoaluminate Cement. Materials 2020, 13, 4526. [Google Scholar] [CrossRef] [PubMed]
- Artykova, A.V.; Melamud, V.S.; Boduen, A.Y.; Bulaev, A.G. Possibility of environment-friendly hydrometallurgical treatment of copper-zinc concentrate containing arsenic. IOP Conf. Ser. Earth Environ. Sci. 2021, 666, 032062. [Google Scholar] [CrossRef]
- Tatiana, L.; Igor, F.; Lutskiy, D. Investigation of the effect of the halide ion adding on extraction of rare earth ions from nitrate media applying naphthenic acid. ARPN J. Eng. App. Sci. 2020, 15, 2129–2134. [Google Scholar]
- Battsengel, A.; Batnasan, A.; Narankhuu, A.; Haga, K.; Watanabe, Y.; Shibayama, A. Recovery of light and heavy rare earth elements from apatite ore using sulphuric acid leaching, solvent extraction and precipitation. Hydrometallurgy 2018, 179, 100–109. [Google Scholar] [CrossRef]
- Cen, P.; Tyumentsev, M.S.; Spahiu, K.; Foreman, M. Metal extraction from a deep eutectic solvent, an insight into activities. Phys. Chem. Chem. Phys. 2020, 22, 11012–11024. [Google Scholar] [CrossRef]
- Chelgani, S.C.; Rudolph, M.; Leistner, T.; Gutzmer, J.; Peuker, U.A. A review of rare earth minerals flotation: Monazite and xenotime. Int. J. Min. Sci. Technol. 2015, 25, 877–883. [Google Scholar] [CrossRef]
| pH | [Sm3+]aq·104 mol/L | [Sm3+]org·103 mol/L | Kp |
|---|---|---|---|
| 1.8 | 5.64 | 1.79 | 3.2 |
| 3.0 | 2.36 | 2.15 | 9.1 |
| 3.5 | 1.39 | 2.24 | 16.2 |
| 4.0 | 1.30 | 2.31 | 17.7 |
| 4.9 | 1.71 | 2.00 | 11.7 |
| 5.4 | 1.83 | 2.18 | 11.9 |
| 6.0 | 1.31 | 2.13 | 16.2 |
| 6.7 | 0.23 | 3.03 | 130.4 |
| 6.9 | 0.25 | 3.27 | 128.9 |
| 7.5 | 0.28 | 3.56 | 128.6 |
| 7.9 | 0.30 | 3.91 | 128.7 |
| 9.5 | 0.30 | 3.91 | 129.5 |
| Compound | kJ·mol−1 | kJ·mol−1 | L (Kn) | pHhydr (pHcompl) |
|---|---|---|---|---|
| Sm(OH)3 | −1280.16 ± 3.63 | 146.33 ± 1.31 | (2.3 ± 1.6) 10−26 | 6.49 |
| Sm(OH)2+ | −866.49 ± 3.60 | −47.36 ± 1.21 | (5.0 ± 3.1) 10−9 | 5.31 |
| t, min. | pH = 5.1 | pH = 6.1 | pH = 6.9 | pH = 8.0 | pH = 9.9 |
|---|---|---|---|---|---|
| Caq, mol/L (Sm+3 Initial Concentration) | |||||
| 0 | 0.001 | 0.001 | 0.001 | 0.001 | 0.001 |
| C·104, mol/L | |||||
| 5 | 7.81 | 7.39 | 6.49 | 0.79 | 7.25 |
| 15 | 7.53 | 6.65 | 4.30 | 0.61 | 5.83 |
| 30 | 7.06 | 6.48 | 2.53 | 0.19 | 3.85 |
| 120 | 6.61 | 6.46 | 2.14 | 0.18 | 0.65 |
| pH | Corg·102 | Caq·104 | Kp | α% |
|---|---|---|---|---|
| 5.1 | 1.35 | 6.60 | 20.5 | 33.9 |
| 6.1 | 1.41 | 6.30 | 21.9 | 35.4 |
| 6.9 | 3.14 | 2.10 | 146.3 | 78.5 |
| 8.0 | 3.92 | 0.17 | 2222.3 | 98.2 |
| 9.9 | 3.74 | 0.65 | 576.9 | 93.5 |
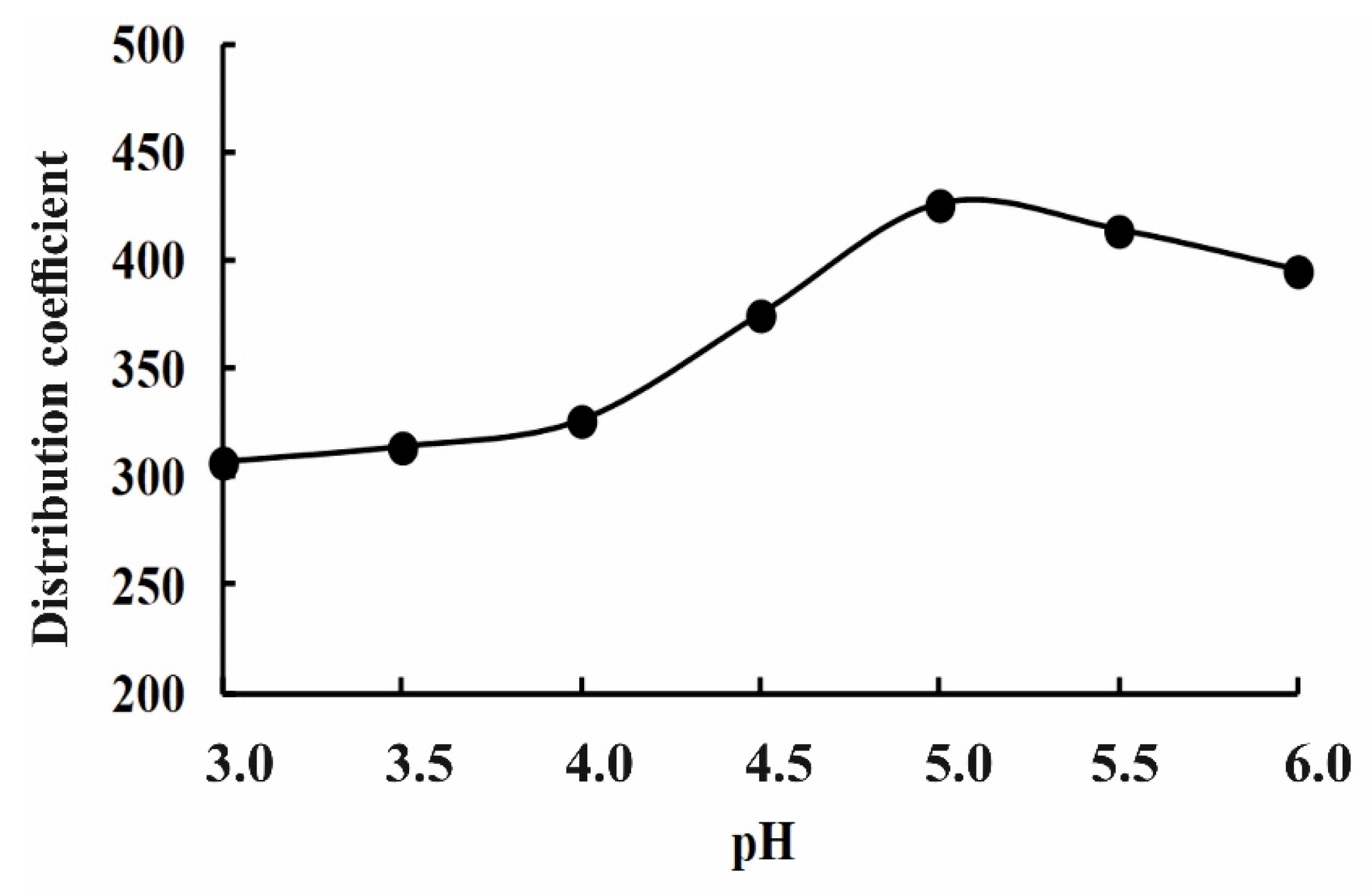
| Sm3+, mol/L | [Sm3+]aq103, mol/L | [Sm3+]org, mol/L | Kdistr | [DS−]aq103, mol/L |
|---|---|---|---|---|
| 0.1 | 16.48 | 4.02 | 243.6 | 0.57 |
| 0.01 | 1.36 | 0.42 | 306.8 | 0.68 |
| 0.001 | 0.13 | 0.04 | 326.1 | 0.76 |
| ΔSGo298, kJ/mol | TBP | TABAN | HNaft | Hol | NaDS |
|---|---|---|---|---|---|
| Ce | −4.1 ± 0.2 | −7.9 ± 0.5 | −27.6 ± 0.4 | −33.1 ± 0.4 | - |
| Sm | - | - | −22.2 ± 0.5 | −35.8 ± 0.5 | −70.0 ± 0.5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lobacheva, O.L.; Dzhevaga, N.V. The Experimental Study of Innovative Methods Regarding the Removal of Sm(III). Appl. Sci. 2021, 11, 7726. https://doi.org/10.3390/app11167726
Lobacheva OL, Dzhevaga NV. The Experimental Study of Innovative Methods Regarding the Removal of Sm(III). Applied Sciences. 2021; 11(16):7726. https://doi.org/10.3390/app11167726
Chicago/Turabian StyleLobacheva, Olga Leonidovna, and Natalia Vladimirovna Dzhevaga. 2021. "The Experimental Study of Innovative Methods Regarding the Removal of Sm(III)" Applied Sciences 11, no. 16: 7726. https://doi.org/10.3390/app11167726





