Effects of Captivity on the Morphology of the Insertion Sites of the Palmar Radiocarpal Ligaments in Hominoid Primates
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Osteological Samples
2.2. 3D GM Analysis
3. Results
4. Discussion
5. Conclusions
6. Limitations and Future Directions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Albrecht, G.H. Collections of nonhuman primate skeletal materials in the United States and Canada. Am. J. Phys. Anthropol. 1982, 57, 77–97. [Google Scholar] [CrossRef]
- Lewton, K.L. The effects of captive versus wild rearing environments on long bone articular surfaces in common chimpanzees (Pan troglodytes). PeerJ 2017, 5, e3668. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Edes, A.N.; Wolfe, B.A.; Crews, D.E. The first multi-zoo application of an allostatic load index to western lowland gorillas (Gorilla gorilla gorilla). Gen. Comp. Endocrinol. 2018, 266, 135–149. [Google Scholar] [CrossRef] [PubMed]
- Martín-Serra, A.; Figueirido, B.; Palmqvist, P. In the pursuit of the predatory behavior of Borophagines (Mammalia, Carnivora, Canidae): Inferences from forelimb morphology. J. Mammal. Evol. 2016, 23, 237–249. [Google Scholar] [CrossRef]
- Fabre, A.C.; Goswami, A.; Peigné, S.; Cornette, R. Morphological integration in the forelimb of musteloid carnivorans. J. Anat. 2014, 225, 19–30. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martín-Serra, A.; Figueirido, B.; Palmqvist, P. A three-dimensional analysis of morphological evolution and locomotor performance of the carnivoran forelimb. BMC Evol. Biol. 2014, 14, 129. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Isler, K.; Thorpe, S.K.S. Gait parameters in vertical climbing of captive, rehabilitant and wild Sumatran orangutans (Pongo pygmaeus abelii). J. Exp. Biol. 2003, 206, 4081–4096. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fourie, N.H.; Brown, J.L.; Jolly, C.J.; Phillips-Conroy, J.E.; Rogers, J.; Bernstein, R.M. Sources of variation in hair cortisol in wild and captive non-human primates. Zoology 2016, 119, 119–125. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Canington, S.L.; Sylvester, A.D.; Burgess, M.L.; Junno, J.A.; Ruff, C.B. Long bone diaphyseal shape follows different ontogenetic trajectories in captive and wild gorillas. Am. J. Phys. Anthropol. 2018, 167, 366–376. [Google Scholar] [CrossRef] [PubMed]
- Cofran, Z. Brain size growth in wild and captive chimpanzees (Pan troglodytes). Am. J. Primatol. 2018, 80, e22876. [Google Scholar] [CrossRef] [PubMed]
- Doran, D.M.; Hunt, K.D. The comparative locomotor behavior of chimpanzees and bonobos: Species and habitat differences. In Chimpanzee Cultures; Wrangham, R.W., McGrew, W.C., De Waal, F., Heltne, P.G., Eds.; Harvard University Press: Cambridge, UK, 1994; pp. 93–108. [Google Scholar]
- Hosey, G.R. How does the zoo environment affect the behaviour of captive primates? Appl. Anim. Behav. Sci. 2005, 90, 107–129. [Google Scholar] [CrossRef]
- Traylor-Holzer, K.; Fritz, P. Utilization of space by adult and juvenile groups of captive chimpanzees (Pan troglodytes). Zoo. Biol. 1985, 4, 115–127. [Google Scholar] [CrossRef]
- Bettinger, T.; Wallis, J.; Carter, C. Spatial selection in captive adult female chimpanzees. Zoo. Biol. 1994, 13, 167–176. [Google Scholar] [CrossRef]
- Goff, C.; Howell, S.M.; Fritz, J.; Nankivell, B. Space use and proximity of captive chimpanzee (Pan troglodytes) mother/offspring pairs. Zoo. Biol. 1994, 13, 61–68. [Google Scholar] [CrossRef]
- Hebert, P.L.; Bard, K. Orangutan use of vertical space in an innovative habitat. Zoo. Biol. 2000, 19, 239–251. [Google Scholar] [CrossRef]
- Stoinski, T.S.; Hoff, M.P.; Maple, T.L. Habitat use and structural preferences of captive western lowland gorillas (Gorilla gorilla gorilla): Effects of environmental and social variables. Int. J. Primatol. 2001, 22, 431–448. [Google Scholar] [CrossRef]
- Stoinski, T.S.; Hoff, M.P.; Lukas, K.E.; Maple, T.L. A preliminary behavioral comparison of two captive all-male gorilla groups. Zoo. Biol. 2001, 20, 27–40. [Google Scholar] [CrossRef] [PubMed]
- Fàbregas, M.C.; Guillén-Salazar, F.; Garcés-Narro, C. Do naturalistic enclosures provide suitable environments for zoo animals? Zoo. Biol. 2012, 31, 362–373. [Google Scholar] [CrossRef]
- Hemsworth, P.H.; Edwards, L.E. Natural behaviours, their drivers and their implications for laying hen welfare. Anim. Prod. Sci. 2020. [Google Scholar] [CrossRef]
- Kiley-Worthington, M. Ecological, ethological, and ethically sound environments for animals: Toward symbiosis. J. Agric. Ethics. 1989, 2, 323–347. [Google Scholar] [CrossRef]
- Hughes, B.O.; Duncan, I.J.H. The notion of ethological ‘need’, models of motivation and animal welfare. Anim. Behav. 1988, 36, 1696–1707. [Google Scholar] [CrossRef]
- Young, R.J. Environmental Enrichment for Captive Animals; Blackwell Science: Oxford, UK, 2003. [Google Scholar]
- Mellen, J.; MacPhee, M.S. Philosophy of environmental enrichment: Past, present, and future. Zoo. Biol. 2001, 20, 211–226. [Google Scholar] [CrossRef]
- Schapiro, S.J. Handbook of Primate Behavioral Management; CRC Press: Boca Raton, FL, USA, 2020. [Google Scholar]
- Ogden, J.J.; Lindburg, D.G.; Maple, T.L. Preference for structural environmental features in captive lowland gorillas (Gorilla gorilla gorilla). Zoo. Biol. 1993, 12, 381–395. [Google Scholar] [CrossRef]
- Ross, S.R.; Lukas, K.E. Use of space in a non-naturalistic environment by chimpanzees (Pan troglodytes) and lowland gorillas (Gorilla gorilla gorilla). Appl. Anim. Behav. Sci. 2006, 96, 143–152. [Google Scholar] [CrossRef]
- Ogden, J.J.; Finlay, T.W.; Maple, T.L. Gorilla adaptation to naturalistic environments. Zoo. Biol. 1990, 9, 107–121. [Google Scholar] [CrossRef]
- Bloomsmith, M.A.; Marr, M.J.; Maple, T.L. Addressing nonhuman primate behavioral problems through the application of operant conditioning: Is the human treatment approach a useful model? Appl. Anim. Behav. Sci. 2007, 2, 205–222. [Google Scholar] [CrossRef]
- Videan, E.N.; McGrew, W.C. Body weight and bipedality in captive chimpanzees (Pan troglodytes). Lab. Prim. News. 2002, 41, 1–3. [Google Scholar]
- Videan, E.N.; Fritz, J.; Murphy, J. Development of guidelines for assessing obesity in captive chimpanzees (Pan troglodytes). Zoo. Biol. 2007, 26, 93–104. [Google Scholar] [CrossRef] [PubMed]
- Brüne, M.; Brüne-Cohrs, U.; McGrew, W.; Preuschoft, S. Psychopathology in great apes: Concepts, treatment options and possible homologies to human psychiatric disorders. Neurosci. Biobehav. Rev. 2006, 30, 1246–1259. [Google Scholar] [CrossRef]
- Anderson, J.R.; Ang, M.Y.L.; Lock, L.C.; Weiche, I. Nesting, sleeping, and nighttime behaviors in wild and captive great apes. Primates 2019, 60, 321–332. [Google Scholar] [CrossRef]
- Hartstone-Rose, A.; Selvey, H.; Villari, J.R.; Atwell, M.; Schmidt, T.; Sueur, C. The three-dimensional morphological effects of captivity. PLoS ONE 2014, 9, e113437. [Google Scholar] [CrossRef] [PubMed]
- Bello-Hellegouarch, G.; Potau, J.M.; Arias-Martorell, J.; Pastor, J.F.; Pérez-Pérez, A. Morphological effects of captivity: A geometric morphometric analysis of the dorsal side of the scapula in captive-bred and wild caught hominoidea. Am. J. Phys. Anthropol. 2013, 152, 306–310. [Google Scholar] [CrossRef] [PubMed]
- Turner, T.R.; Cramer, J.D.; Nisbett, A.; Gray, J.P. A comparison of adult body size between captive and wild vervet monkeys (Chlorocebus aethiops sabaeus) on the island of St. Kitts. Primates 2016, 57, 211–220. [Google Scholar] [CrossRef] [Green Version]
- Apergis, E. Wirst Anatomy. In Fracture-Dislocations of the Wrist; Springer: Milano, Italy, 2013; pp. 7–41. [Google Scholar] [CrossRef]
- Casado, A.; Punsola, V.; Gómez, M.; De Diego, M.; Barbosa, M.; De Paz, F.J.; Pastor, J.F.; Potau, J.M. Three-dimensional geometric morphometric analysis of the distal radius insertion sites of the palmar radiocarpal ligaments in hominoid primates. Am. J. Phys. Anthropol. 2019, 170, 24–36. [Google Scholar] [CrossRef]
- Bucchi, A.; Luengo, J.; Del Bove, A.; Lorenzo, C. Insertion sites in manual proximal phalanges of African apes and modern humans. Am. J. Phys. Anthropol. 2020, 173, 556–567. [Google Scholar] [CrossRef] [PubMed]
- Cignoni, P.; Callieri, M.; Corsini, M.; Dellepiane, M.; Ganovelli, F.; Ranzuglia, G. MeshLab: An open-source mesh processing tool. In Proceedings of the Sixth Eurographics Italian Chapter Conference, Salerno, Italy, 2–4 July 2008; pp. 129–136. [Google Scholar]
- Wiley, D.F. IDAV Landmark Editor, 3rd ed.; University of California: Davis, CA, USA, 2006. [Google Scholar]
- Klingenberg, C.P. MorphoJ: An integrated software package for geometric morphometrics. Mol. Ecol. Resour. 2011, 11, 353–357. [Google Scholar] [CrossRef] [PubMed]
- Bookstein, F.L. Morphometric Tools for Landmark Data: Geometry and Biology; Cambridge University Press: Cambridge, UK, 1991. [Google Scholar]
- O’Higgins, P. The study of morphological variation in the hominid fossil record: Biology, landmarks and geometry. J. Anat. 2000, 197, 103–120. [Google Scholar] [CrossRef] [PubMed]
- Zelditch, M.L.; Swiderski, D.L.; Sheets, H.D.; Fink, W.L. Geometric Morphometrics for Biologists: A Primer; Academic Press: Cambridge, UK, 2004. [Google Scholar]
- Remis, M.J. The gorilla paradox: The effects of body size and habitat on the positional behavior of lowland and mountain gorillas. In Primate Locomotion; Strasser, E., Ed.; Plenum Press: New York, NY, USA, 1998; pp. 95–106. [Google Scholar]
- Orr, C.M. Locomotor hand postures, carpal kinematics during wrist extension, and associated morphology in anthropoid primates. Anat. Rec. 2017, 300, 382–401. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thorpe, S.K.S.; Crompton, R.H. Origin of human bipedalism as an adaptation for locomotion on flexible branches. Science 2007, 316, 1328–1331. [Google Scholar] [CrossRef]
- Sarmiento, E.E. Anatomy of the hominoid wrist joint: Its evolutionary and functional implications. Int. J. Primatol. 1988, 9, 281–345. [Google Scholar] [CrossRef]
- Richmond, B.G.; Begun, D.R.; Strait, D.S. Origin of human bipedalism: The knuckle-walking hypothesis revisited. Yearb. Phys. Anthropol. 2001, 44, 70–105. [Google Scholar] [CrossRef]
- Kivell, T.L.; Barros, A.P.; Smaers, J.B. Different evolutionary pathways underlie the morphology of wrist bones in hominoids. BMC Evol. Biol. 2013, 13, 1–12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Heinrich, R.E.; Rose, M.D.; Leakey, R.E.; Walker, A.C. Hominid radius from the Middle Pliocene of Lake Turkana, Kenya. Am. J. Phys. Anthropol. 1993, 92, 139–148. [Google Scholar] [CrossRef] [PubMed]
- Doran, D.M. Comparison of instantaneous and locomotor bout sampling methods: A case study of adult male chimpanzee locomotor behavior and substrate use. Am. J. Phys. Anthropol. 1992, 89, 85–99. [Google Scholar] [CrossRef]
- Hunt, K.D. Positional behavior of Pan troglodytes in the Mahale mountains and Gombe Stream National Parks, Tanzania. Am. J. Phys. Anthropol. 1992, 87, 83–105. [Google Scholar] [CrossRef] [PubMed]
- Remis, M.J. Nesting behavior of lowland gorillas in the Dzanga-Sangha Reserve. Central African Republic: Implications for population estimates and understandings of group dynamics. Tropics 1993, 2, 245–255. [Google Scholar] [CrossRef] [Green Version]
- Lukas, K.E.; Stoinski, T.S.; Snyder, R.; Bexell, S.; Burks, K. Nest building in captive Gorilla gorilla gorilla. Int. J. Primatol. 2003, 24, 103–124. [Google Scholar] [CrossRef]
- Sarringhaus, L.A.; Maclatchy, L.M.; Mitani, J.C. Locomotor and postural development of wild chimpanzees. J. Hum. Evol. 2014, 66, 29–38. [Google Scholar] [CrossRef] [PubMed]
- Sarringhaus, L.A.; MacLatchy, L.M.; Mitani, J.C. Long bone cross-sectional properties reflect changes in locomotor behavior in developing chimpanzees. Am. J. Phys. Anthropol. 2016, 160, 16–29. [Google Scholar] [CrossRef]
- Nadler, R.; Braggio, J. Sex and species differences in captive-reared juvenile chimpanzees and orangutans. J. Hum. Evol. 1974, 3, 541–550. [Google Scholar] [CrossRef]
- Schwandt, M.L. The Ontogeny of Positional Behavior in Captive Chimpanzees (Pan Troglodytes). Ph.D. Thesis, Arizona State University, Phoenix, AZ, USA, 2002. [Google Scholar]
- Lieberman, D.E.; Devlin, M.J.; Pearson, O.M. Articular area responses to mechanical loading: Effects of exercise, age, and skeletal location. Am. J. Phys. Anthropol. 2001, 116, 266–277. [Google Scholar] [CrossRef]
- Wrangham, R.W. Feeding behaviour of chimpanzees in Gombe National Park, Tanzania. In Primate Ecology: Studies Of Feeding And Ranging Behaviour In Lemurs, Monkeys and Apes; Clutton-Brock, T.H., Ed.; Academic Press: New York, NY, USA, 2002; pp. 504–538. [Google Scholar]
- Pruetz, J.D.E.; McGrew, W.C. What does a chimpanzee need? Using natural behavior to guide the care and management of captive populations. In Special Topics in Primatology; Brent, L., Ed.; ASP: San Antonio, TX, USA, 2001; pp. 17–37. [Google Scholar]
- Llorente, M.; Riba, D.; Ballesta, S.; Feliu, O.; Rostán, C. Rehabilitation and socialization of chimpanzees (Pan troglodytes) used for entertainment and as pets: An 8-year study at Fundació Mona. Int. J. Primatol. 2015, 36, 605–624. [Google Scholar] [CrossRef]
- Cant, J.G.H. Positional behavior of female Bornean orangutans (Pongo pygmaeus). Am. J. Primatol. 1987, 12, 71–90. [Google Scholar] [CrossRef] [PubMed]
- Thorpe, S.K.S.; Crompton, R.H. Locomotor ecology of wild orangutans (Pongo pygmaeus abelii) in the Gunung Leuser Ecosystem, Sumatra, Indonesia: A multivariate analysis using log-linear modelling. Am. J. Phys. Anthropol. 2005, 127, 58–78. [Google Scholar] [CrossRef] [PubMed]
- Cant, J.G.H. Positional behaviour and body size of arboreal primates: A theoretical framework for field studies and an illustration of its application. Am. J. Phys. Anthropol. 1992, 88, 273–283. [Google Scholar] [CrossRef]
- Novak, M.A.; Suomi, S.J. Psychological well-being of primates in captivity. Am. Psychol. 1988, 43, 765–773. [Google Scholar] [CrossRef] [PubMed]
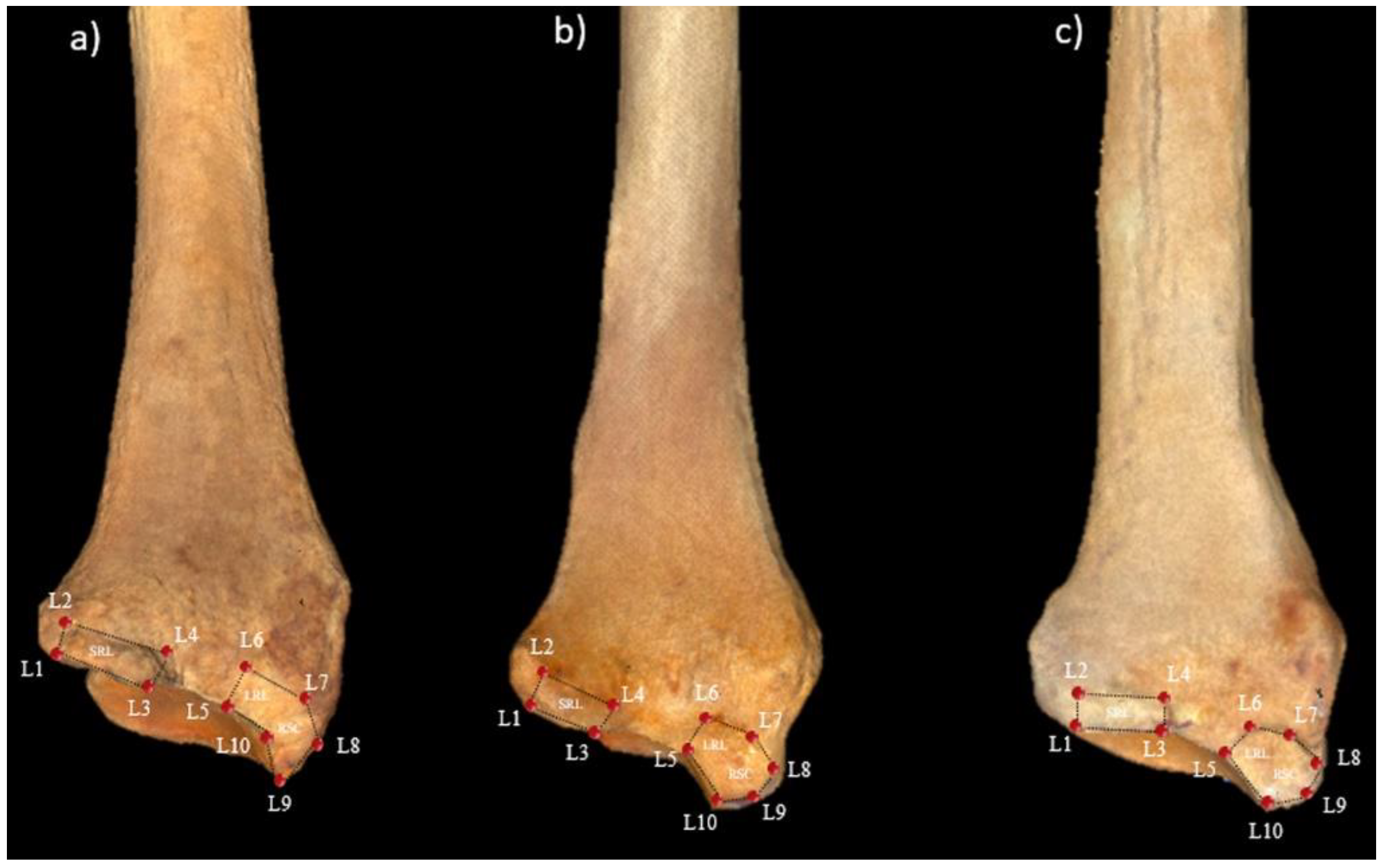


| Species | n | Sex | Origin |
|---|---|---|---|
| Gorilla gorilla (Wild) | 31 | M = 17/F = 14 | Equatorial Guinea, Cameroon, Gabon, French Cameroon |
| Gorilla gorilla (Captive) | 12 | M = 6/F = 6 | Madrid Zoo, Loro Parque de Tenerife, Fuengirola Zoo, Bioparc de Valencia, Barcelona Zoo |
| Pan troglodytes (Wild) | 25 | M = 11/F = 13/I = 1 | Equatorial Guinea, Liberia, French Cameroon |
| Pan troglodytes (Captive) | 26 | M = 15/F = 11 | Valladolid Valwo Zoo, Fuengirola Zoo, Madrid Zoo, Barcelona Zoo |
| Pongo pygmaeus (Wild) | 15 | M = 8/F = 7 | Sumatra, Borneo |
| Pongo pygmaeus (Captive) | 9 | M = 2/F = 7 | Santillana del Mar Zoo, Fuengirola Zoo, Madrid Zoo, Barcelona Zoo |
| TOTAL | 118 | Wild = 71 Captive= 47 |
| Species | Procrustes Distances | Mahalanobis Distances |
|---|---|---|
| Wild vs. Captive Gorilla gorilla | 0.09 (p < 0.0001) | 4.03 (p = 0.01) |
| Wild vs. Captive Pan troglodytes | 0.08 (p < 0.0001) | 2.70 (p = 0.01) |
| Wild vs. Captive Pongo pygmaeus | 0.08 (p = 0.006) | 33.93 (p = 0.07) |
| Discriminant Functions | After Cross-Validation | Decrease in Correct Classification | |
|---|---|---|---|
| Wild vs. Captive Gorilla gorilla | 96.66% | 63.58% | 33.08% |
| Wild vs. Captive Pan troglodytes | 94.16% | 64.69% | 29.47% |
| Wild vs. Captive Pongo pygmaeus | 100% | 69.99% | 30.01% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Casado, A.; Avià, Y.; Llorente, M.; Riba, D.; Pastor, J.F.; Potau, J.M. Effects of Captivity on the Morphology of the Insertion Sites of the Palmar Radiocarpal Ligaments in Hominoid Primates. Animals 2021, 11, 1856. https://doi.org/10.3390/ani11071856
Casado A, Avià Y, Llorente M, Riba D, Pastor JF, Potau JM. Effects of Captivity on the Morphology of the Insertion Sites of the Palmar Radiocarpal Ligaments in Hominoid Primates. Animals. 2021; 11(7):1856. https://doi.org/10.3390/ani11071856
Chicago/Turabian StyleCasado, Aroa, Yasmina Avià, Miquel Llorente, David Riba, Juan Francisco Pastor, and Josep Maria Potau. 2021. "Effects of Captivity on the Morphology of the Insertion Sites of the Palmar Radiocarpal Ligaments in Hominoid Primates" Animals 11, no. 7: 1856. https://doi.org/10.3390/ani11071856








