Cold-Shock Test Is a Practical Method for Selecting Boar Ejaculates Yielding Appropriate Seminal Plasma for Post-Thawing Supplementation
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Experimental Design
2.2.1. Experiment 1: Cold-Shock Resistance Test (CST), Boar Classification, and Seminal Plasma (SP) Preparation
2.2.2. Experiment 2: Effects of SPr and SPs on Thawed Boar Semen
2.3. Semen Samples
2.4. Assessment of Sperm Motility by CASA (Computer-Assisted Sperm Analysis)
2.5. Assessment of Sperm Physiology by Flow Cytometry
2.5.1. Sperm Viability and Acrosomal Integrity
2.5.2. Apoptosis-Like Features, Membrane Fluidity, and Mitochondrial Activity
2.5.3. Osmotic Resistance Test
2.5.4. Flow Cytometry Analysis of Sperm Physiology
2.5.5. Sperm Chromatin Assessment
2.6. Data Analysis
3. Results
3.1. Cold-Shock Resistance Test
3.2. Testing the Effect of Seminal Plasma (SP) from Males with a High or Low Resistance to the Cold-Shock on Thawed Semen
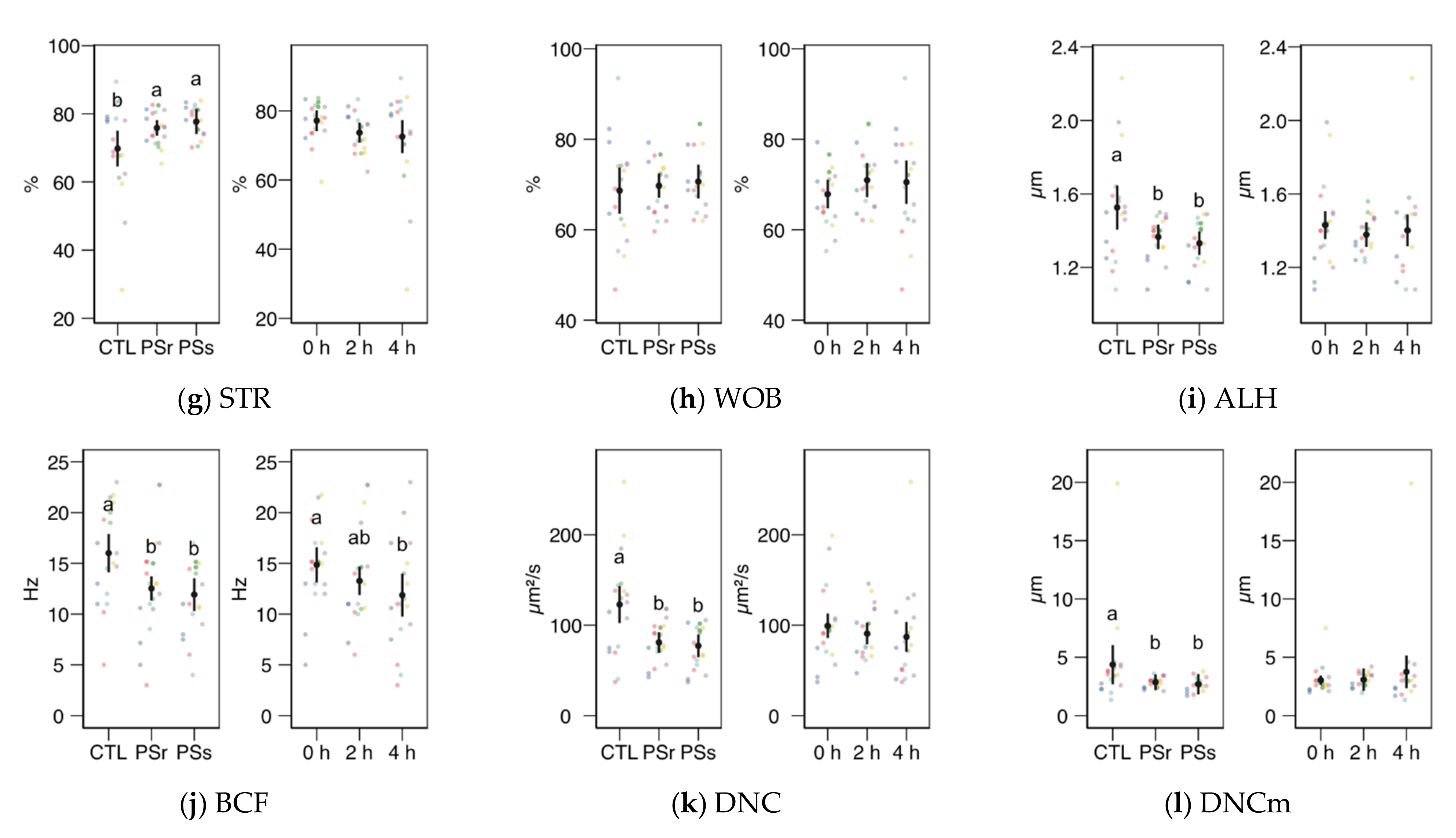
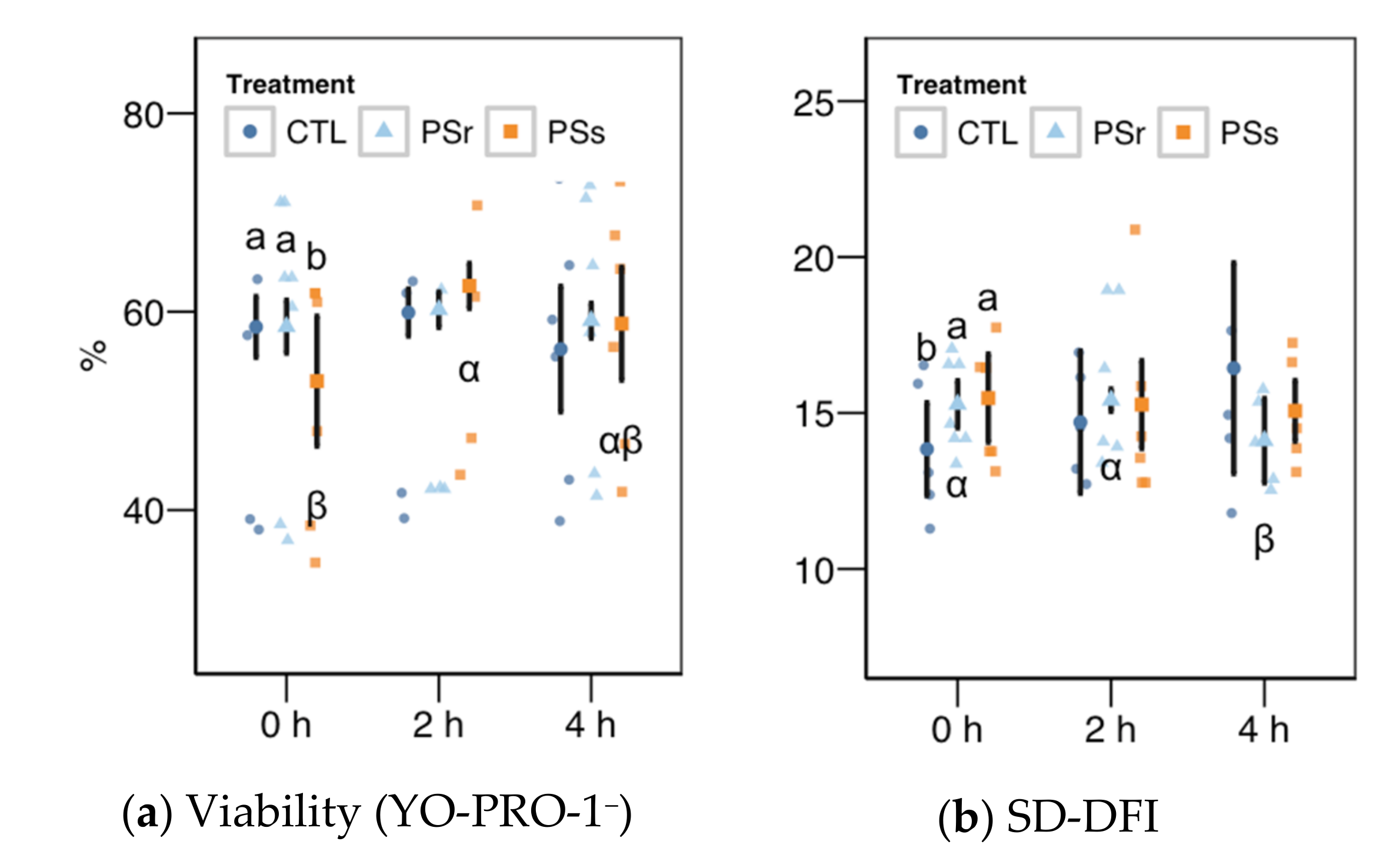
3.2.1. Sperm Motility
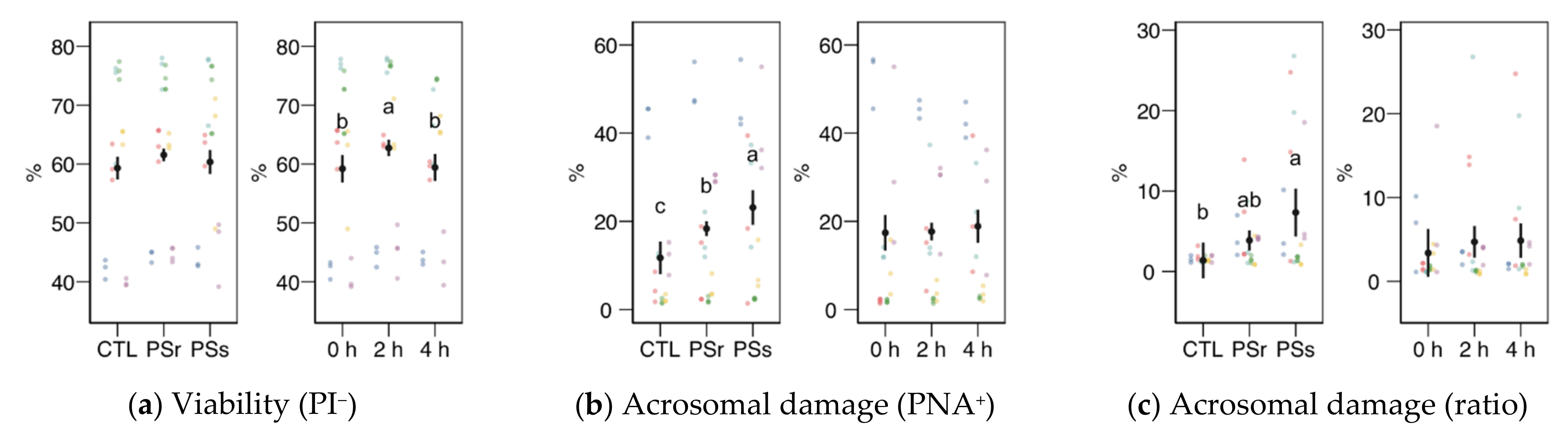
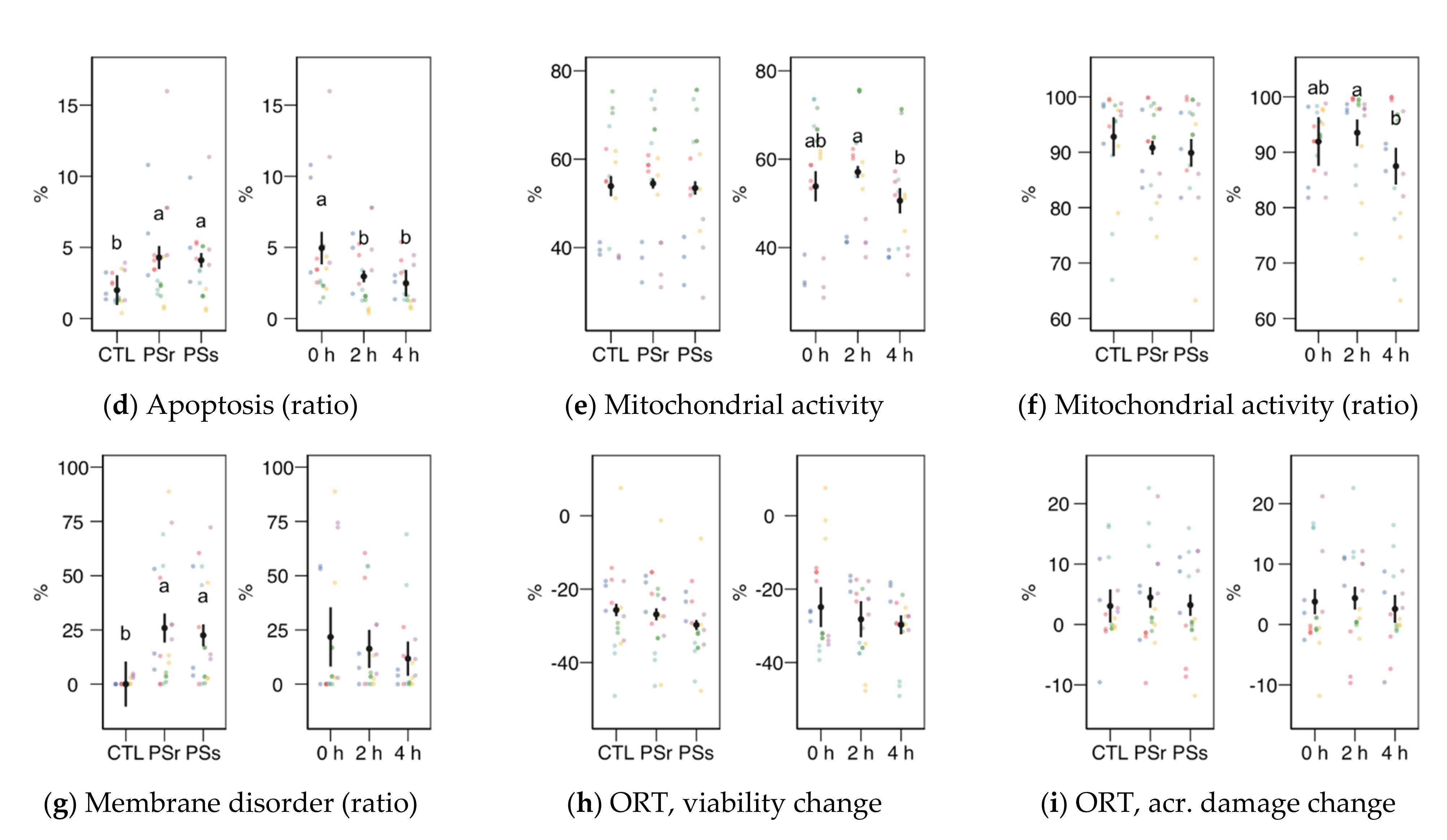
3.2.2. Sperm Physiology
3.2.3. Osmotic Resistance Test
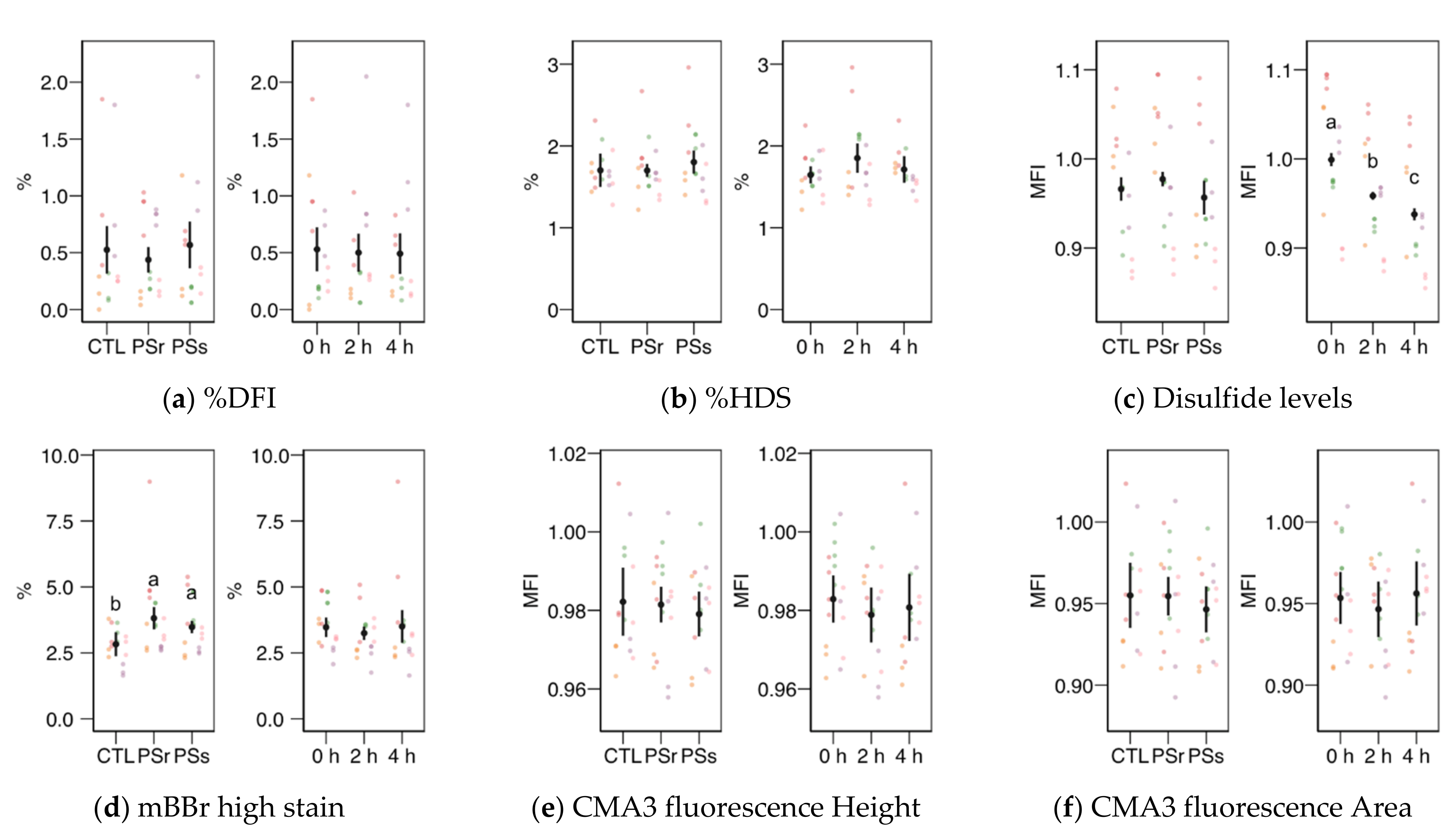
3.2.4. Sperm Chromatin Structure
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Roca, J.; Parrilla, I.; Bolarin, A.; Martinez, E.A.; Rodriguez-Martinez, H. Will AI in Pigs Become More Efficient? Theriogenology 2016, 86, 187–193. [Google Scholar] [CrossRef]
- Waberski, D.; Riesenbeck, A.; Schulze, M.; Weitze, K.F.; Johnson, L. Application of Preserved Boar Semen for Artificial Insemination: Past, Present and Future Challenges. Theriogenology 2019, 137, 2–7. [Google Scholar] [CrossRef] [PubMed]
- Yeste, M. State-of-the-Art of Boar Sperm Preservation in Liquid and Frozen State. Anim. Reprod. 2017, 14, 69–81. [Google Scholar] [CrossRef]
- Bailey, J.L.; Lessard, C.; Jacques, J.; Brèque, C.; Dobrinski, I.; Zeng, W.; Galantino-Homer, H.L. Cryopreservation of Boar Semen and Its Future Importance to the Industry. Theriogenology 2008, 70, 1251–1259. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Gago, R.; Álvarez-Rodríguez, M.; Alonso, M.E.; Ramiro González, J.; Alegre, B.; Domínguez, J.C.; Martínez-Pastor, F. Thawing Boar Semen in the Presence of Seminal Plasma Improves Motility, Modifies Subpopulation Patterns and Reduces Chromatin Alterations. Reprod. Fertil. Dev. 2017, 29, 1576–1584. [Google Scholar] [CrossRef]
- Roca, J.; Hernández, M.; Carvajal, G.; Vázquez, J.M.; Martínez, E.A. Factors Influencing Boar Sperm Cryosurvival. J. Anim. Sci. 2006, 84, 2692–2699. [Google Scholar] [CrossRef] [PubMed]
- Johnson, L.A.; Weitze, K.F.; Fiser, P.; Maxwell, W.M.C. Storage of Boar Semen. Anim. Reprod. Sci. 2000, 62, 143–172. [Google Scholar] [CrossRef]
- Vadnais, M.L.; Althouse, G.C. Characterization of Capacitation, Cryoinjury, and the Role of Seminal Plasma in Porcine Sperm. Theriogenology 2011, 76, 1508–1516. [Google Scholar] [CrossRef]
- Evenson, D.P. Sperm Chromatin Structure Assay (SCSA®): 30 Years of Experience with the SCSA®. In Sperm Chromatin; Zini, A., Agarwal, A., Eds.; Springer New York: New York, NY, USA, 2011; pp. 125–149. ISBN 978-1-4419-1781-2. [Google Scholar]
- Radomil, L.; Pettitt, M.J.; Merkies, K.M.; Hickey, K.D.; Buhr, M.M. Stress and Dietary Factors Modify Boar Sperm for Processing. Reprod. Domest. Anim. Zuchthyg. 2011, 46 (Suppl. S2), 39–44. [Google Scholar] [CrossRef] [PubMed]
- Holt, W.V.; Medrano, A.; Thurston, L.M.; Watson, P.F. The Significance of Cooling Rates and Animal Variability for Boar Sperm Cryopreservation: Insights from the Cryomicroscope. Theriogenology 2005, 63, 370–382. [Google Scholar] [CrossRef]
- Okazaki, T.; Abe, S.; Yoshida, S.; Shimada, M. Seminal Plasma Damages Sperm during Cryopreservation, but Its Presence during Thawing Improves Semen Quality and Conception Rates in Boars with Poor Post-Thaw Semen Quality. Theriogenology 2009, 71, 491–498. [Google Scholar] [CrossRef]
- Yeste, M. Sperm Cryopreservation Update: Cryodamage, Markers, and Factors Affecting the Sperm Freezability in Pigs. Theriogenology 2016, 85, 47–64. [Google Scholar] [CrossRef]
- Bromfeld, J.J. A Role for Seminal Plasma in Modulating Pregnancy Outcomes in Domestic Species. Reproduction 2016, 152, R223–R232. [Google Scholar] [CrossRef] [PubMed]
- Vicens, A.; Lüke, L.; Roldan, E.R.S. Proteins Involved in Motility and Sperm-Egg Interaction Evolve More Rapidly in Mouse Spermatozoa. PLoS ONE 2014, 9, e91302. [Google Scholar] [CrossRef]
- Maxwell, W.M.C.; Johnson, L.A. Physiology of Spermatozoa at High Dilution Rates: The Influence of Seminal Plasma. Theriogenology 1999, 52, 1353–1362. [Google Scholar] [CrossRef]
- Martinez, C.A.; Cambra, J.M.; Parrilla, I.; Roca, J.; Ferreira-Dias, G.; Pallares, F.J.; Lucas, X.; Vazquez, J.M.; Martinez, E.A.; Gil, M.A.; et al. Seminal Plasma Modifies the Transcriptional Pattern of the Endometrium and Advances Embryo Development in Pigs. Front. Vet. Sci. 2019, 6, 465. [Google Scholar] [CrossRef] [PubMed]
- Álvarez-Rodríguez, M.; Martinez, C.A.; Wright, D.; Rodríguez-Martinez, H. The Role of Semen and Seminal Plasma in Inducing Large-Scale Genomic Changes in the Female Porcine Peri-Ovulatory Tract. Sci. Rep. 2020, 10, 5061. [Google Scholar] [CrossRef] [PubMed]
- O’Leary, S.; Jasper, M.J.; Warnes, G.M.; Armstrong, D.T.; Robertson, S.A. Seminal Plasma Regulates Endometrial Cytokine Expression, Leukocyte Recruitment and Embryo Development in the Pig. Reproduction 2004, 128, 237–247. [Google Scholar] [CrossRef]
- Martinez, C.A.; Cambra, J.M.; Nohalez, A.; Parrilla, I.; Roca, J.; Ferreira-Dias, G.; Lucas, X.; Martinez, E.A.; Gil, M.A.; Rodriguez-Martinez, H.; et al. Pre-AI Intrauterine Seminal Plasma Infusions Advance Embryo Development by up-Regulating PI3K/AKT and MAPK/ERK Signaling Pathways? Theriogenology 2019, 137, 136–137. [Google Scholar] [CrossRef]
- Manjunath, P.; Bergeron, A.; Lefebvre, J.; Fan, J. Seminal Plasma Proteins: Functions and Interaction with Protective Agents during Semen Preservation. Soc. Reprod. Fertil. Suppl. 2007, 65, 217–228. [Google Scholar] [PubMed]
- Leahy, T.; Gadella, B. Capacitation and Capacitation-like Sperm Surface Changes Induced by Handling Boar Semen. Reprod. Domest. Anim. Zuchthyg. 2011, 46 (Suppl. S2), 7–13. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Gago, R.; Domínguez, J.C.; Martínez-Pastor, F. Seminal Plasma Applied Post-Thawing Affects Boar Sperm Physiology: A Flow Cytometry Study. Theriogenology 2013, 80, 400–410. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Martínez, H.; Kvist, U.; Ernerudh, J.; Sanz, L.; Calvete, J.J. Seminal Plasma Proteins: What Role Do They Play? Am. J. Reprod. Immunol. 2011, 66, 11–22. [Google Scholar] [CrossRef]
- Pavaneli, A.P.P.; Recuero, S.; Chaves, B.R.; Garcia-Bonavila, E.; Llavanera, M.; Pinart, E.; Bonet, S.; De Andrade, A.F.C.; Yeste, M. The Presence of Seminal Plasma during Liquid Storage of Pig Spermatozoa at 17 °C Modulates Their Ability to Elicit In Vitro Capacitation and Trigger Acrosomal Exocytosis. Int. J. Mol. Sci. 2020, 21, 4520. [Google Scholar] [CrossRef]
- Garcia, J.C.; Dominguez, J.C.; Pena, F.J.; Alegre, B.; Gonzalez, R.; Castro, M.J.; Habing, G.G.; Kirkwood, R.N. Thawing Boar Semen in the Presence of Seminal Plasma: Effects on Sperm Quality and Fertility. Anim. Reprod. Sci. 2010, 119, 160–165. [Google Scholar] [CrossRef]
- Recuero, S.; Fernandez-Fuertes, B.; Bonet, S.; Barranco, I.; Yeste, M. Potential of Seminal Plasma to Improve the Fertility of Frozen-Thawed Boar Spermatozoa. Theriogenology 2019, 137, 36–42. [Google Scholar] [CrossRef]
- Okazaki, T.; Akiyoshi, T.; Kan, M.; Mori, M.; Teshima, H.; Shimada, M. Artificial Insemination With Seminal Plasma Improves the Reproductive Performance of Frozen-Thawed Boar Epididymal Spermatozoa. J. Androl. 2012, 33, 990–998. [Google Scholar] [CrossRef] [PubMed]
- Caballero, I.; Vazquez, J.M.; Centurión, F.; Rodríguez-Martinez, H.; Parrilla, I.; Roca, J.; Cuello, C.; Martinez, E.A. Comparative Effects of Autologous and Homologous Seminal Plasma on the Viability of Largely Extended Boar Spermatozoa. Reprod. Domest. Anim. Zuchthyg. 2004, 39, 370–375. [Google Scholar] [CrossRef] [PubMed]
- Alkmin, D.V.; Perez-Patiño, C.; Barranco, I.; Parrilla, I.; Vazquez, J.M.; Martinez, E.A.; Rodriguez-Martinez, H.; Roca, J. Boar Sperm Cryosurvival Is Better after Exposure to Seminal Plasma from Selected Fractions than to Those from Entire Ejaculate. Cryobiology 2014, 69, 203–210. [Google Scholar] [CrossRef]
- Hernández, M.; Roca, J.; Calvete, J.J.; Sanz, L.; Muiño-Blanco, T.; Cebrián-Pérez, J.A.; Vázquez, J.M.; Martínez, E.A. Cryosurvival and in Vitro Fertilizing Capacity Postthaw Is Improved When Boar Spermatozoa Are Frozen in the Presence of Seminal Plasma from Good Freezer Boars. J. Androl. 2007, 28, 689–697. [Google Scholar] [CrossRef]
- Eriksson, B.M.; Rodriguez-Martinez, H. Effect of Freezing and Thawing Rates on the Post-Thaw Viability of Boar Spermatozoa Frozen in FlatPacks and Maxi-Straws. Anim. Reprod. Sci. 2000, 63, 205–220. [Google Scholar] [CrossRef]
- Yeste, M.; Briz, M.; Pinart, E.; Sancho, S.; Bussalleu, E.; Bonet, S. The Osmotic Tolerance of Boar Spermatozoa and Its Usefulness as Sperm Quality Parameter. Anim. Reprod. Sci. 2010, 119, 265–274. [Google Scholar] [CrossRef] [PubMed]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2020; Available online: http://www.R-project.org/ (accessed on 17 March 2021).
- Martin, M.; Peter, R.; Anja, S.; Mia, H. Cluster: Cluster Analysis Basics and Extensions. R Package. Available online: https://cran.r-project.org/web/packages/cluster/citation.html (accessed on 17 March 2021).
- Torres, M.A.; Ravagnani, G.M.; Leal, D.F.; Martins, S.M.M.K.; Muro, B.B.D.; Meirelles, F.V.; Papa, F.O.; Dell’aqua Junior, J.A.; Alvarenga, M.A.; Moretti, A.S.; et al. Seminal Plasma Arising from the Whole Boar Sperm-Rich Fraction Increases the Stability of Sperm Membrane after Thawing. J. Anim. Sci. 2016, 94, 1906–1912. [Google Scholar] [CrossRef]
- Ledesma, A.; Fernández-Alegre, E.; Cano, A.; Hozbor, F.; Martínez-Pastor, F.; Cesari, A. Seminal Plasma Proteins Interacting with Sperm Surface Revert Capacitation Indicators in Frozen-Thawed Ram Sperm. Anim. Reprod. Sci. 2016, 173, 35–41. [Google Scholar] [CrossRef]
- Samanta, L.; Parida, R.; Dias, T.R.; Agarwal, A. The Enigmatic Seminal Plasma: A Proteomics Insight from Ejaculation to Fertilization. Reprod. Biol. Endocrinol. 2018, 16, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Gadella, B.M.; Boerke, A. An Update on Post-Ejaculatory Remodeling of the Sperm Surface before Mammalian Fertilization. Theriogenology 2016, 85, 113–124. [Google Scholar] [CrossRef]
- Caballero, I.; Vazquez, J.M.; García, E.M.; Parrilla, I.; Roca, J.; Calvete, J.J.; Sanz, L.; Martínez, E.A. Major Proteins of Boar Seminal Plasma as a Tool for Biotechnological Preservation of Spermatozoa. Theriogenology 2008, 70, 1352–1355. [Google Scholar] [CrossRef]
- Vilagran, I.; Yeste, M.; Sancho, S.; Castillo, J.; Oliva, R.; Bonet, S. Comparative Analysis of Boar Seminal Plasma Proteome from Different Freezability Ejaculates and Identification of Fibronectin 1 as Sperm Freezability Marker. Andrology 2015, 3, 345–356. [Google Scholar] [CrossRef]
- Wysocki, P.; Orzołek, A.; Strzezek, J.; Koziorowska-Gilun, M.; Zasiadczyk, Ł.; Kordan, W. The Activity of N-Acetyl-β-Hexosaminidase in Boar Seminal Plasma Is Linked with Semen Quality and Its Suitability for Cryopreservation. Theriogenology 2015, 83, 1194–1202. [Google Scholar] [CrossRef]
- Parrilla, I.; del Olmo, D.; Sijses, L.; Martinez-Alborcia, M.J.; Cuello, C.; Vazquez, J.M.; Martinez, E.A.; Roca, J. Differences in the Ability of Spermatozoa from Individual Boar Ejaculates to Withstand Different Semen-Processing Techniques. Anim. Reprod. Sci. 2012, 132, 66–73. [Google Scholar] [CrossRef] [PubMed]
- Waterhouse, K.E.; Hofmo, P.O.; Tverdal, A.; Miller, R.R. Within and between Breed Differences in Freezing Tolerance and Plasma Membrane Fatty Acid Composition of Boar Sperm. Reproduction 2006, 131, 887–894. [Google Scholar] [CrossRef] [PubMed]
- Thurston, L.M.; Siggins, K.; Mileham, A.J.; Watson, P.F.; Holt, W.V. Identification of Amplified Restriction Fragment Length Polymorphism Markers Linked to Genes Controlling Boar Sperm Viability Following Cryopreservation1. Biol. Reprod. 2002, 66, 545–554. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Roca, J.; Pérez-Patiño, C.; Barranco, I.; Martinez, E.A.; Rodriguez-Martinez, H.; Parrilla, I. Is Boar Sperm Freezability More Intrinsically Linked to Spermatozoa than to the Surrounding Seminal Plasma? Anim. Reprod. Sci. 2018, 195, 30–37. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Martínez, H.; Kvist, U.; Saravia, F.; Wallgren, M.; Johannisson, A.; Sanz, L.; Peña, F.J.; Martínez, E.A.; Roca, J.; Vázquez, J.M.; et al. The Physiological Roles of the Boar Ejaculate. Soc. Reprod. Fertil. Suppl. 2009, 66, 1–21. [Google Scholar] [CrossRef]
- Sharkey, D.J.; Macpherson, A.M.; Tremellen, K.P.; Robertson, S.A. Seminal Plasma Differentially Regulates Inflammatory Cytokine Gene Expression in Human Cervical and Vaginal Epithelial Cells. Mol. Hum. Reprod. 2007, 13, 491–501. [Google Scholar] [CrossRef]
- Tsikis, G.; Reynaud, K.; Ferchaud, S.; Druart, X. Seminal Plasma Differentially Alters the Resistance of Dog, Ram and Boar Spermatozoa to Hypotonic Stress. Anim. Reprod. Sci. 2018, 193, 1–8. [Google Scholar] [CrossRef]
- Caballero, I.; Vazquez, J.M.; Mayor, G.M.; Almiñana, C.; Calvete, J.J.; Sanz, L.; Roca, J.; Martinez, E.A. PSP-I/PSP-II Spermadhesin Exert a Decapacitation Effect on Highly Extended Boar Spermatozoa. Int. J. Androl. 2009, 32, 505–513. [Google Scholar] [CrossRef]
- Caballero, I.; Vazquez, J.M.; Gil, M.A.; Calvete, J.J.; Roca, J.; Sanz, L.; Parrilla, I.; Garcia, E.M.; Rodriguez-Martinez, H.; Martinez, E.A. Does Seminal Plasma PSP-I/PSP-II Spermadhesin Modulate the Ability of Boar Spermatozoa to Penetrate Homologous Oocytes in Vitro? J. Androl. 2004, 25, 1004–1012. [Google Scholar] [CrossRef]
- Satake, N.; Elliott, R.M.A.; Watson, P.F.; Holt, W.V. Sperm Selection and Competition in Pigs May Be Mediated by the Differential Motility Activation and Suppression of Sperm Subpopulations within the Oviduct. J. Exp. Biol. 2006, 209, 1560–1572. [Google Scholar] [CrossRef] [PubMed]
- Didion, B.A.; Kasperson, K.M.; Wixon, R.L.; Evenson, D.P. Boar Fertility and Sperm Chromatin Structure Status: A Retrospective Report. J. Androl. 2009, 30, 655–660. [Google Scholar] [CrossRef] [PubMed]
- Roca, J.; Broekhuijse, M.; Parrilla, I.; Rodriguez-Martinez, H.; Martinez, E.A.; Bolarin, A. Boar Differences In Artificial Insemination Outcomes: Can They Be Minimized? Reprod. Domest. Anim. Zuchthyg. 2015, 50 (Suppl. S2), 48–55. [Google Scholar] [CrossRef] [PubMed]
- Betarelli, R.P.; Rocco, M.; Yeste, M.; Fernández-Novell, J.M.; Placci, A.; Azevedo Pereira, B.; Castillo-Martín, M.; Estrada, E.; Peña, A.; Zangeronimo, M.G.; et al. The Achievement of Boar Sperm in Vitro Capacitation Is Related to an Increase of Disrupted Disulphide Bonds and Intracellular Reactive Oxygen Species Levels. Andrology 2018, 6, 781–797. [Google Scholar] [CrossRef] [PubMed]
- Parrilla, I.; Perez-Patiño, C.; Li, J.; Barranco, I.; Padilla, L.; Rodriguez-Martinez, H.; Martinez, E.A.; Roca, J. Boar Semen Proteomics and Sperm Preservation. Theriogenology 2019, 137, 23–29. [Google Scholar] [CrossRef] [PubMed]
- Höfner, L.; Luther, A.-M.; Palladini, A.; Fröhlich, T.; Waberski, D. Tolerance of Stored Boar Spermatozoa to Autologous Seminal Plasma: A Proteomic and Lipidomic Approach. Int. J. Mol. Sci. 2020, 21, 6474. [Google Scholar] [CrossRef] [PubMed]
- Ramón, M.; Martínez-Pastor, F.; García-Álvarez, O.; Maroto-Morales, A.; Soler, A.J.; Jiménez-Rabadán, P.; Fernández-Santos, M.R.; Bernabéu, R.; Garde, J.J. Taking Advantage of the Use of Supervised Learning Methods for Characterization of Sperm Population Structure Related with Freezability in the Iberian Red Deer. Theriogenology 2012, 77, 1661–1672. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.-T.; Liu, Y.; Liang, H.-L.; Xu, Q.-Q.; Liu, Z.-H.; Weng, X.-G. Metabolomic Differences of Seminal Plasma between Boars with High and Low Average Conception Rates after Artificial Insemination. Reprod. Domest. Anim. Zuchthyg. 2021, 56, 161–171. [Google Scholar] [CrossRef]
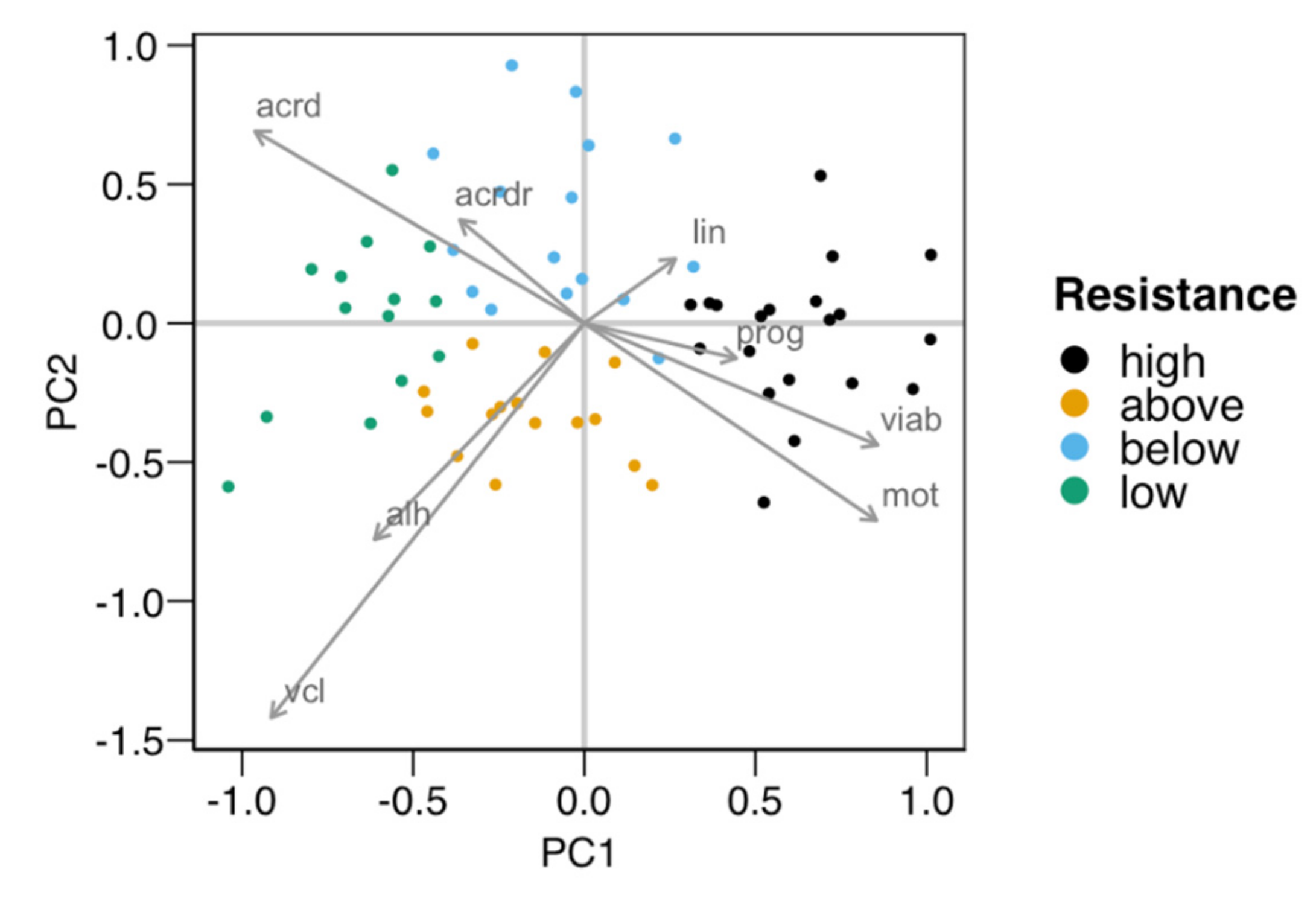

| Variable | High | Above | Below | Low |
|---|---|---|---|---|
| Number of boars in the cluster | 20 | 15 | 16 | 14 |
| Viability | 63.1 ± 14.9 | 36.1 ± 9.8 | 28.8 ± 15 | 14.7 ± 7.6 |
| Damaged acrosomes | 23.5 ± 12.5 | 55 ± 18.7 | 66.1 ± 18.5 | 80.6 ± 9.9 |
| Damaged acrosomes (ratio viable) | 6 ± 3.2 | 7.5 ± 5.2 | 16.5 ± 15 | 23.9 ± 13.4 |
| Total motility | 61.4 ± 20 | 43.3 ± 14.5 | 18.5 ± 8.9 | 13.8 ± 9.5 |
| Progressive motility | 17 ± 7.7 | 8.3 ± 5 | 5 ± 2.8 | 2.3 ± 2 |
| VCL | 75.5 ± 17 | 132.6 ± 13.4 | 80.1 ± 18.9 | 131.8 ± 37.2 |
| LIN | 44.2 ± 10.7 | 30.8 ± 9.8 | 41.7 ± 13.3 | 27 ± 8.1 |
| ALH | 1.6 ± 0.3 | 2.7 ± 0.4 | 1.8 ± 0.3 | 2.9 ± 0.9 |
| Variable | PC1 | PC2 |
|---|---|---|
| Viability | 0.428 | −0.219 |
| Damaged acrosomes | −0.481 | 0.346 |
| Damaged acrosomes (ratio viable) | −0.182 | 0.186 |
| Total motility | 0.427 | −0.355 |
| Progressive motility | 0.222 | −0.063 |
| VCL | −0.457 | −0.71 |
| LIN | 0.133 | 0.116 |
| ALH | −0.306 | −0.389 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lacalle, E.; Núñez, A.; Fernández-Alegre, E.; Crespo-Félez, I.; Domínguez, J.C.; Alonso, M.E.; González-Urdiales, R.; Martínez-Pastor, F. Cold-Shock Test Is a Practical Method for Selecting Boar Ejaculates Yielding Appropriate Seminal Plasma for Post-Thawing Supplementation. Animals 2021, 11, 871. https://doi.org/10.3390/ani11030871
Lacalle E, Núñez A, Fernández-Alegre E, Crespo-Félez I, Domínguez JC, Alonso ME, González-Urdiales R, Martínez-Pastor F. Cold-Shock Test Is a Practical Method for Selecting Boar Ejaculates Yielding Appropriate Seminal Plasma for Post-Thawing Supplementation. Animals. 2021; 11(3):871. https://doi.org/10.3390/ani11030871
Chicago/Turabian StyleLacalle, Estíbaliz, Andrea Núñez, Estela Fernández-Alegre, Itxaso Crespo-Félez, Juan Carlos Domínguez, Marta Elena Alonso, Raúl González-Urdiales, and Felipe Martínez-Pastor. 2021. "Cold-Shock Test Is a Practical Method for Selecting Boar Ejaculates Yielding Appropriate Seminal Plasma for Post-Thawing Supplementation" Animals 11, no. 3: 871. https://doi.org/10.3390/ani11030871
APA StyleLacalle, E., Núñez, A., Fernández-Alegre, E., Crespo-Félez, I., Domínguez, J. C., Alonso, M. E., González-Urdiales, R., & Martínez-Pastor, F. (2021). Cold-Shock Test Is a Practical Method for Selecting Boar Ejaculates Yielding Appropriate Seminal Plasma for Post-Thawing Supplementation. Animals, 11(3), 871. https://doi.org/10.3390/ani11030871







