The Effect of Polyethylene Film and Polypropylene Non-Woven Fabric Cover on Cobs Parameters and Nutritional Value of Two Sweet Maize (Zea mays L. var. saccharata Bailey) Hybrids
Abstract
:1. Introduction
2. Materials and Methods
2.1. Field Experiment
- Signet F1 (very early);
- Rustler F1 (medium early).
- Colorless perforated polyethylene foil (perforation of 100 holes per 1 m2, each with a diameter of 10 mm) of 0.2 mm thickness (PE);
- Red perforated polyethylene foil (PER);
- Green perforated polyethylene foil (PEG);
- Polypropylene non-woven fabric (with weight of 17 g m−2) (PP);
- Control—maize cultivation without cover (K).
2.2. Analysis of Soluble Sugar Content in Maize Grain
2.3. Analysis of Lutein and Zeaxanthin Content in Maize Grain
2.4. Statistical Analysis
3. Results
3.1. Weather Conditions and Vegetation Characteristic
3.2. Cobs Yield and Morphological Parameters
3.3. Sugar Content in Maize Kernels
3.4. Carotenoids Content in Maize Kernels
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kwabiah, A.B. Growth and yield of sweet corn (Zea mays L.) cultivars in response to planting date and plastic mulch in a short-season environment. Sci. Hortic. 2004, 102, 147–166. [Google Scholar] [CrossRef]
- Anioł, M.; Sowiński, J.; Adamczyk, J.; Baraszkiewicz, M.; Potaniec, B.; Zieliński, P. Analiza zawartości zeaksantyny i luteiny w ziarnie form kukurydzy oraz wybranych odmianach dostępnych na rynku polskim. Przemysł Chem. 2012, 91, 666–670. [Google Scholar]
- Smorowska, A.; Żołnierczyk, A.; Nawirska Olszanska, A.; Sowiński, J.; Szumny, A. Nutritional Properties and In Vitro Antidiabetic Activities of Blue and Yellow Corn Extracts: A Comparative Study. J. Food Qual. 2021, 2021, 8813613. [Google Scholar] [CrossRef]
- Panchal, B.H.; Patel, V.K.; Khimani, R.A. Influence of Pre Harvest Factor on Post-Harvest Quality of Green Sweet Corn at Ambient Condition (Zea mays convar. saccharata. rugosa) Cultivar, Madhuri. Int. J. Curr. Microbiol. Appl. Sci. 2017, 6, 30–38. [Google Scholar] [CrossRef]
- Khan, A.A.; Hussain, A.; Ganai, M.A.; Sofi, N.R.; Hussain, S.T. Yield, nutrient uptake and quality of sweet corn as influenced by transplanting dates and nitrogen levels. J. Pharmacogn. Phytochem. 2018, 7, 3567–3571. [Google Scholar]
- Szymanek, M.; Tanaśa, W.; Kassar, F.H. Kernel carbohydrates concentration in sugary-1, sugary enhanced and shrunken sweet corn kernels. Agric. Agric. Sci. Procedia 2015, 7, 260–264. [Google Scholar] [CrossRef] [Green Version]
- Zhang, X.; von Mogela KJ, H.; Lora, V.S.; Hirschc, C.N.; De Vriesa, B.; Kaepplera, H.F.; Tracya, W.F.; Kaepplera, S.M. Maize sugary enhancer1 (se1) is a gene affecting endosperm starch metabolism. Proc. Natl. Acad. Sci. USA 2019, 116, 20776–20785. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aman, R.; Biehl, J.; Carle, R.; Conrad, J.; Beifuss, U.; Schieber, A. Application of HPLC coupled with DAD, APcI-MS and NMR to the analysis of lutein and zeaxanthin stereoisomers in thermally processed vegetables. Food Chem. 2005, 92, 753–763. [Google Scholar] [CrossRef]
- Michaud, D.S.; Feskanich, D.; Rimm, E.B.; Colditz, G.A.; Speizer, F.E.; Willett, W.C.; Giovannucci, E. Intake of specific carotenoids and risk of lung cancer in 2 prospective US cohorts. Am. J. Clin. Nutr. 2000, 72, 990–997. [Google Scholar] [CrossRef] [Green Version]
- Slattery, M.L.; Benson, J.; Curtin, K.; Ma, K.N.; Schaeffer, D.; Potter, J.D. Carotenoids and colon cancer. Am. J. Clin. Nutr. 2000, 71, 575–582. [Google Scholar] [CrossRef] [Green Version]
- Snodderly, D.M. Evidence for protection against age-related macular degeneration by carotenoids and antioxidant vitamins. Am. J. Clin. Nutr. 1995, 62, 1448. [Google Scholar] [CrossRef]
- Ibrahim, K.E.; Juvik, J.A. Feasibility for improving phytonutrient content in vegetable crops using conventional breeding strategies: Case study with carotenoids and tocopherols in sweet corn and broccoli. J. Agric. Food Chem. 2009, 57, 4636–4644. [Google Scholar] [CrossRef] [PubMed]
- Aman, R.; Carle, R.; Conrad, J.; Beifuss, U.; Schieber, A. Isolation of carotenoids from plant materials and dietary supplements by high-speed counter-current chromatography. J. Chromatogr. A 2005, 1074, 99–105. [Google Scholar] [CrossRef] [PubMed]
- Holden, J.M.; Eldridge, A.L.; Beecher, G.R.; Buzzard, I.M.; Bhagwat, S.; Davis, C.S.; Douglass, L.W.; Gebhardt, S.; Haytowitz, D.; Schakel, S. Carotenoid content of U.S. foods: An update of the database. J. Food Compos. Anal. 1999, 12, 169–196. [Google Scholar] [CrossRef] [Green Version]
- Sommerburg, O.; Keunen, J.E.E.; Bird, A.C.; van Kuijk, F.J.G.M. Fruits and vegetables that are sources for lutein and zeaxanthin: Macular pigment in human eyes. Br. J. Ophthalmol. 1998, 82, 907–910. [Google Scholar] [CrossRef] [PubMed]
- Gallon, C.Z.; Fuller, S.C.; Fanning, K.J.; Smyth, H.E.; Pun, S.; Martin, I.F.; O’Hare, T.J. Increase in beta -ionone, a carotenoid-derived volatile in zeaxanthin-biofortified sweet corn. J. Agric. Food Chem. 2013, 61, 7181–7187. [Google Scholar] [CrossRef]
- Kopsell, D.A.; Armel, G.R.; Mueller, T.C.; Sams, C.E.; Deyton, D.E.; McElroy, J.S.; Kopsell, D.E. Increase in nutritionally important sweet corn kernel carotenoids following mesotrione and atrazine applications. J. Agric. Food Chem. 2009, 57, 6362–6368. [Google Scholar] [CrossRef]
- Kopsell, D.A.; Armel, G.R.; Abney, K.R.; Vargas, J.J.; Brosnan, J.T.; Kopsell, D.E. Leaf tissue pigments and chlorophyll fluorescence parameters vary among sweet corn genotypes of differential herbicide sensitivity. Pestic. Biochem. Physiol. 2011, 99, 194–199. [Google Scholar] [CrossRef]
- Aguyoh, J.; Taber, H.G.; Lawson, V. Maturity of fresh market sweet corn with direct seeded plants, transplants, clear plastic mulch, and row cover combinations. HortTechnology 1999, 9, 420–425. [Google Scholar] [CrossRef]
- Öktem, A.; Öktem, A.G.; Coşkun, Y. Determination of sowing dates of sweet corn (Zea mays L. saccharate Sturt.) under Sanliurfa conditions. Turk. J. Agric. For. 2004, 28, 83–91. [Google Scholar]
- Nagy, J. Effect of sowing date on the yield and quality of maize hybrids with different growing seasons. Acta Agron. Hung. 2009, 57, 389–399. [Google Scholar] [CrossRef]
- Ahmad, I.; Khaliq, T.; Ahmad, A.; Basra, S.M.A.; Hasnain, Z.; Ali, A. Effect of seed priming with ascorbic acid, salicylic acid and hydrogen peroxide on emergence, vigor and antioxidant activities of maize. Afr. J. Biotech 2012, 11, 1127–1132. [Google Scholar]
- Slezak, K.; Orosz, F.; Osz, A. Enhancing earliness of sweet corn by using transplants and plastic row covers. Kertgazdasag Hortic. 2006, 38, 14–19. [Google Scholar]
- Adamczewska-Sowińska, K.; Sowiński, J. Reaction of Sweet Maize to the Use of Polyethylene Film and Polypropylene Non-Woven Fabric in the Initial Growth Phase. Agronomy 2020, 10, 141. [Google Scholar] [CrossRef] [Green Version]
- Ghimire, S.; Scheenstra, E.; Miles, C.A. Soil-biodegradable Mulches for Growth, Yield, and Quality of Sweet Corn in a Mediterranean-type Climate. HortScience 2020, 55, 317–325. [Google Scholar] [CrossRef] [Green Version]
- Waterer, D. Evaluation of biodegradable mulches for production of warm-season vegetable crops. Can. J. Plant Sci. 2010, 90, 737–743. [Google Scholar] [CrossRef] [Green Version]
- Zandstra, J.; Squire, R.; Westervelt, S.; Baker, C. Evaluation of Biodegradable Mulches in Fresh Market Sweet Corn, Pepper Production. 2007. Available online: http://www.ridgetownc.uoguelph.ca/research/documents/zandstra_degradable_mulch_2007.pdf (accessed on 25 January 2021).
- Abd El-Hamed, K.E.; Elwan, M.W.M.; Shaban, W.I. Enhanced sweet corn propagation: Studies on transplanting feasibility and seed priming. Veg. Crop. Res. Bull. 2011, 75, 31–50. [Google Scholar] [CrossRef]
- Knudsen, K.E.B.; Li, B.W. Determination of oligosaccharides in protein-rich feedstuffs by gas-liquid chromatography and high performance liquid chromatography. J. Agric. Food Chem. 1991, 39, 689–694. [Google Scholar] [CrossRef]
- Panfili, G.; Fratianni, A.; Irano, M. Normal phase high-performance liquid chromatography method for the determination of tocopherols and tocotrienols in cereals. J. Agric. Food Chem. 2003, 51, 3940–3944. [Google Scholar] [CrossRef]
- Franco, A.A.N.; Vidigal Filho, P.S.; Scapim, C.A.; Okumura, R.S.; Marques, O.J.; Numoto, A.Y. Effect of sowing time on the growth and yield of sweet corn (Zea mays L.) cultivated during fall-winter period in Subtropical climate. Aust. J. Crop Sci. 2016, 10, 831–841. [Google Scholar] [CrossRef]
- Wang, Y.; Xie, Z.; Malhi, S.S.; Vera, C.L.; Zhang, Y.; Wang, J. Effects of rainfall harvesting and mulching technologies on water use efficiency and crop yield in the semi-arid Loess Plateau, China. Agric. Water Manag. 2009, 96, 374–382. [Google Scholar] [CrossRef]
- Liu, Y.; Yang, S.J.; Li, S.Q.; Chen, X.P.; Chen, F. Growth and development of maize (Zea mays L.) in response to different field water management practices: Resource capture and use efficiency. Agric. For. Meteorol. 2010, 150, 606–613. [Google Scholar]
- Kara, B.; Atar, B. Effects of mulch practices on fresh ear yield and yield components of sweet corn. Turk. J. Agric. For. 2013, 37, 281–287. [Google Scholar]
- Kebede, H.; Sui, R.; Fisher, D.K.; Reddy, K.N.; Bellaloui, N.; Molin, W.T. Corn yield response to reduced water use at different growth stages. Agric. Sci. 2014, 5, 1305–1315. [Google Scholar] [CrossRef] [Green Version]
- Sbrussi, C.A.G.; Zucareli, C. Germination of corn seeds with different levels of vigour in response to differents temperatures. Ciências Agrárias Londrina 2014, 35, 215–226. [Google Scholar] [CrossRef] [Green Version]
- Stall, W.M.; Waters, L.; Davis, D.W.; Rosen, C.; Clough, G.H. National Corn Handbook: Sweet Corn Production. Purdue University, West Lafayette. 2019. Available online: https://www.extension.purdue.edu/extmedia/NCH/NCH-43.html (accessed on 25 January 2021).
- Xu, J.; Li, C.; Liu, H.; Zhou, P.; Tao, Z.; Wang, P.; Meng, Q.; Zhao, M. The Effects of Plastic Film Mulching on Maize Growth and Water Use in Dry and Rainy Years in Northeast China. PLoS ONE 2015, 10, e0125781. [Google Scholar] [CrossRef]
- Zhang, P.; Wei, T.; Cai, T.; Ali, S.; Han, Q.; Ren, X.; Jia, Z. Plastic-film mulching for enhanced water-use efficiency and economic returns from maize fields in semiarid China. Front. Plant Sci. 2017, 8, 512. [Google Scholar] [CrossRef] [Green Version]
- Lamont, W.J. Plastics: Modifying the Microclimate for the Production of Vegetable Crops. HortTechnology 2005, 15, 477–481. [Google Scholar] [CrossRef]
- Liu, P.; Hu, C.H.; Dong, S.T.; Wang, K.J. The comparison of sugar components in the developing grains of sweet corn and normal corn. Agric. Sci. China 2003, 2, 258–264. [Google Scholar]
- Kumari, J.; Gadag, R.N.; Jha, G.K. Heritability and correlation studies in sweet corn for quality traits, field emergence and grain yield. MNL 2006, 80, 18–19. [Google Scholar]
- Cao, D.D.; Hu, J.; Huang, X.X.; Wang, X.J.; Guan, Y.J.; Wang, Z.F. Relationship between changes of kernel nutritive components and seed vigor during development stages of F1 seeds of sh2 sweet corn. J. Zhejiang Univ. Sci. B 2008, 9, 964–968. [Google Scholar] [CrossRef]
- Xu, J.G.; Hu, Q.P.; Wang, X.D.; Luo, J.Y.; Liu, Y.; Tain, C.R. Changes in the main nutrients, phytochemicals and antioxidant activity in yellow corn grain during maturation. J. Agric. Food Chem. 2010, 58, 5751–5756. [Google Scholar] [CrossRef]
- Pairochteerakul, P.; Jothityangkoon, D.; Ketthaisong, D.; Simla, S.; Lertrat, K.; Suriharn, B. Seed Germination in Relation to Total Sugar and Starch in Endosperm Mutant of Sweet Corn Genotypes. Agronomy 2018, 8, 299. [Google Scholar] [CrossRef] [Green Version]
- O’Hare, T.J.; Fanning, K.J.; Martin, I.F. Zeaxanthin biofortification of sweetcorn and factors affecting zeaxanthin accumulation and colour change. Arch. Biochem. Biophys. 2015, 572, 184–187. [Google Scholar] [CrossRef] [PubMed]
- De Oliveira, G.P.R.; Rodriguez-Amaya, D.B. Processed and prepared corn products as sources of lutein and zeaxanthin: Compositional variation in the food chain. J. Food Sci. 2007, 72, 79–85. [Google Scholar] [CrossRef] [PubMed]
- Baseggio, M.; Murray, M.; Magallanes-Lundback, M.; Kaczmar, N.; Chamness, J.; Buckler, E.S.; Smith, M.E.; Della Penna, D.; Tracy, W.F.; Gore, M.A. Natural variation for carotenoids in fresh kernels is controlled by uncommon variants in sweet corn. Plant Genome 2020, 13, e20008. [Google Scholar] [CrossRef] [Green Version]
- Calvo-Brenes, P.; Fanning, K.; O’Hare, T. Does kernel position on the cob affect zeaxanthin, lutein and total carotenoid contents or quality parameters, in zeaxanthin-biofortified sweet-corn? Food Chem. 2019, 277, 490–495. [Google Scholar] [CrossRef]
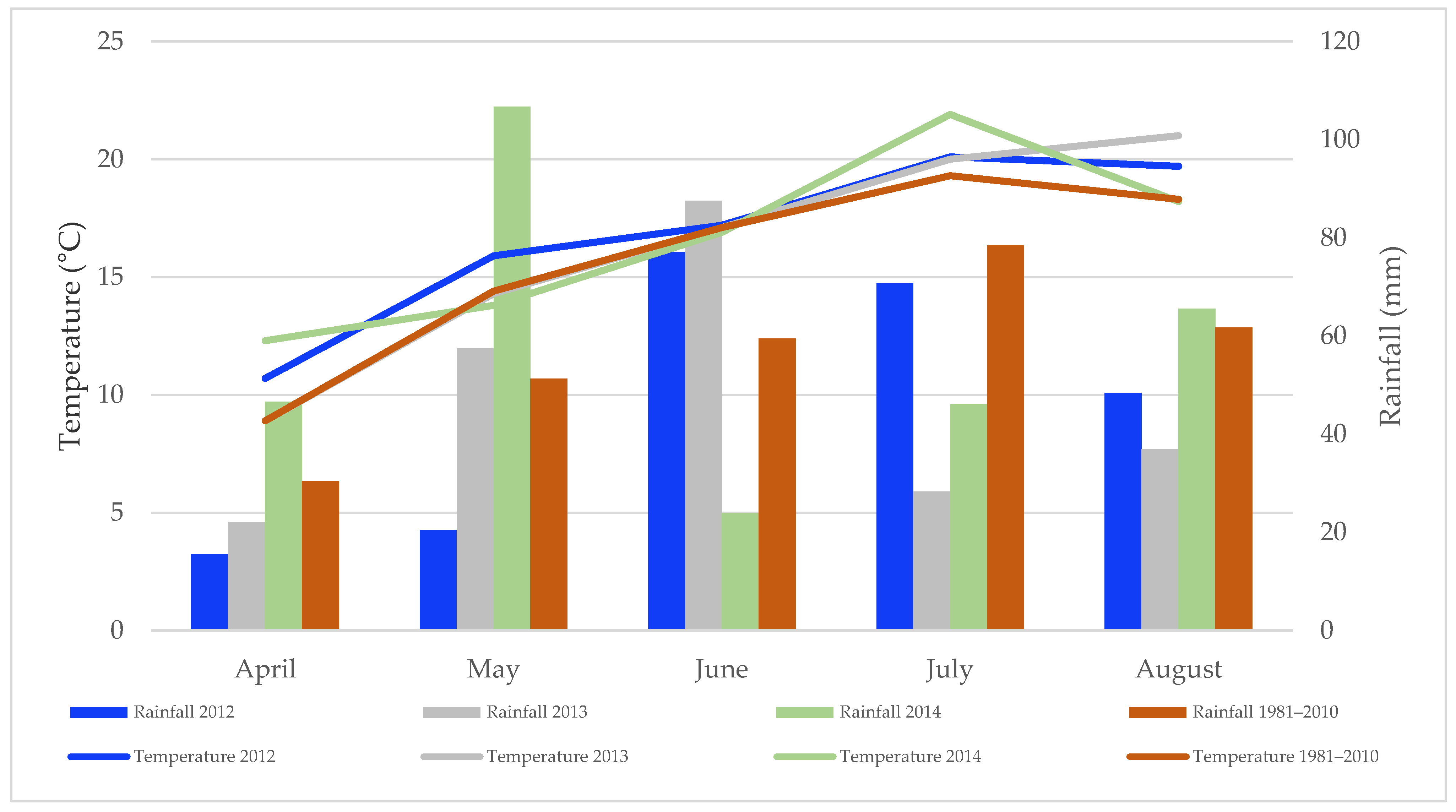
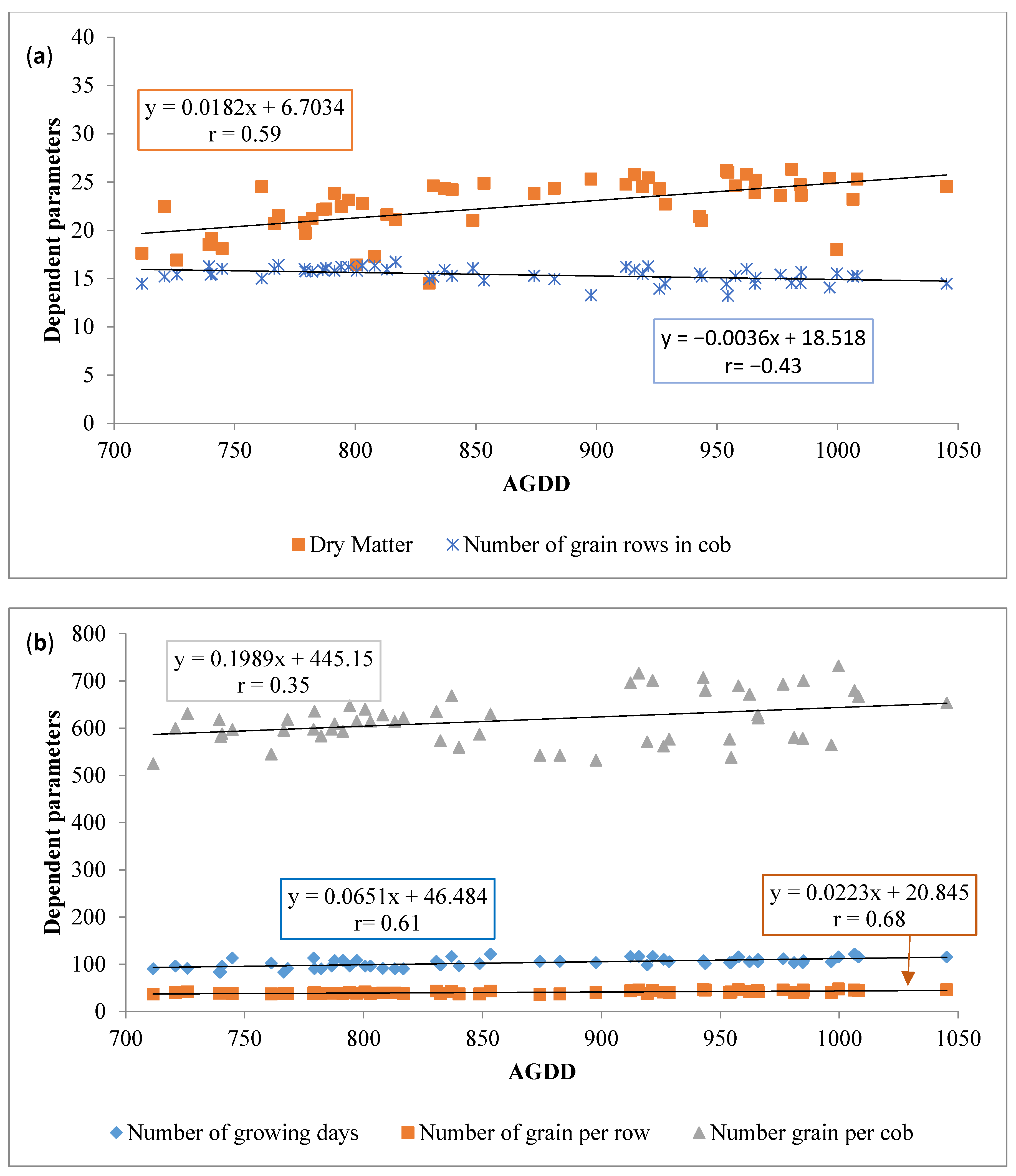

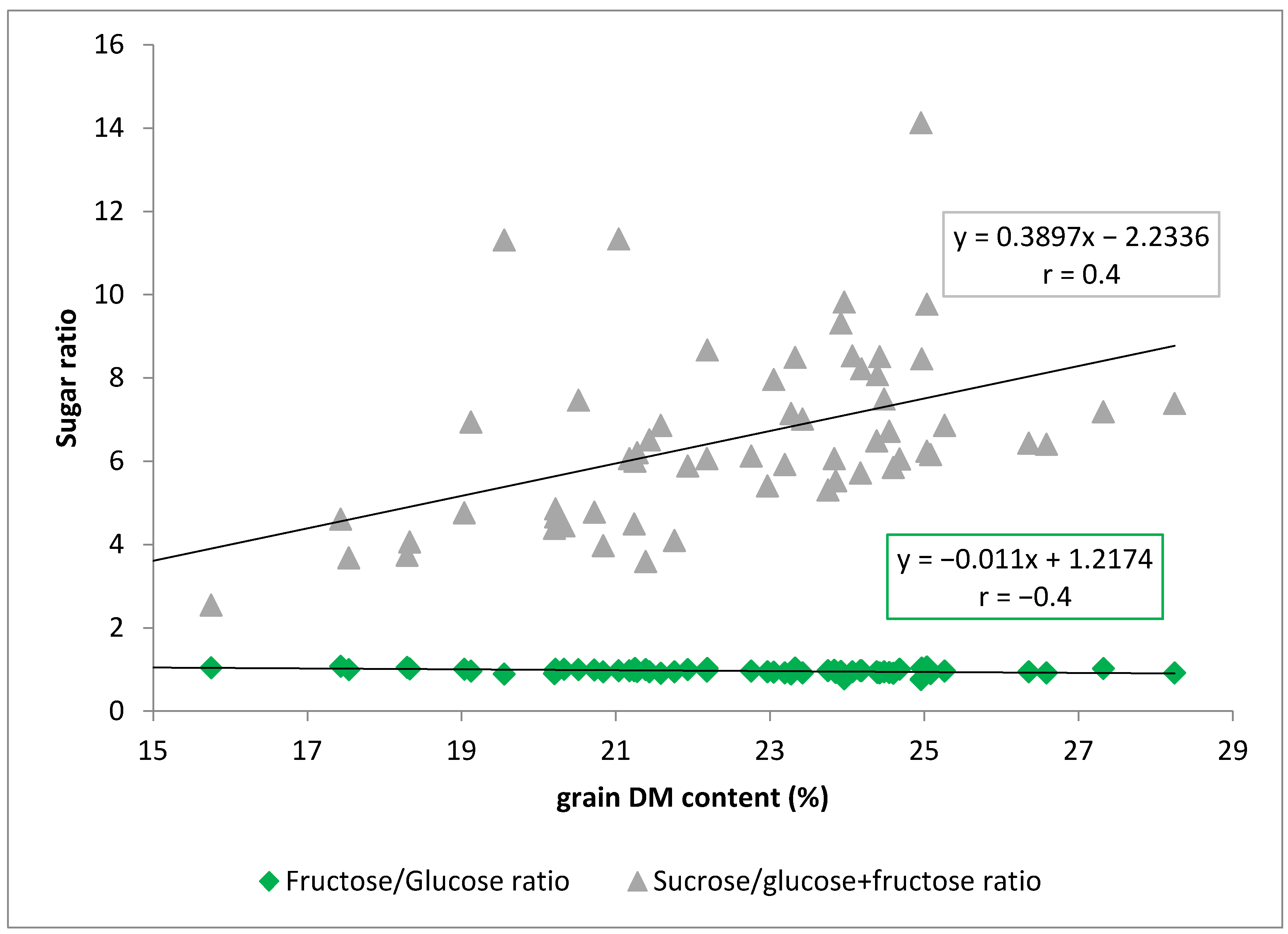

| Treatments | AGDD | Growing Days | Grain DM (%) Content |
|---|---|---|---|
| Average for hybrids | |||
| Signet F1 | 792.9 ± 49.2 | 97.0 ± 8.5 | 21.1 ± 2.8 |
| Rustler F1 | 952.9 ± 47.6 | 109.9 ± 6.2 | 24.2 ± 1.9 |
| Average for sowing term | |||
| I | 874.6 ± 91.7 | 107.4 ± 9.4 | 22.7 ± 2.8 |
| II | 854.8 ± 96.7 | 97.4 ± 7.5 | 22.2 ± 3.0 |
| Average for cover type | |||
| PE | 895.2 ± 85.6 | 102.2 ± 8.5 | 22.9 ± 2.5 |
| PER | 884.4 ± 87.3 | 102.5 ± 10.3 | 23.9 ± 1.8 |
| PEG | 871.6 ± 101.9 | 101.8 ± 10.6 | 22.5 ± 2.6 |
| PP | 831.4 ± 95.4 | 101.9 ± 10.9 | 21.7 ± 3.0 |
| K | 845.6 ± 99.2 | 105.9 ± 10.3 | 21.4 ± 3.9 |
| Sowing Term (St) | Cover Type (Ct) | Number of Rows in Cob | Number of Grain in Row | Number of Grain Per Cob | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Signet F1 | Rustler F1 | Mean | Signet F1 | Rustler F1 | Mean | Signet F1 | Rustler F1 | Mean | |||
| I | PE | 16.07 | 15.58 | 15.82 | 37.64 | 43.44 | 40.54 | 605.3 | 675.6 | 640.4 | |
| PER | 15.82 | 15.42 | 15.62 | 37.09 | 43.29 | 40.19 | 585.8 | 668.5 | 627.1 | ||
| PEG | 15.69 | 15.27 | 15.48 | 36.87 | 43.40 | 40.13 | 578.2 | 664.0 | 621.1 | ||
| PP | 15.58 | 15.20 | 15.39 | 38.04 | 42.38 | 40.21 | 592.9 | 644.9 | 618.9 | ||
| control | 15.13 | 14.64 | 14.89 | 38.69 | 42.53 | 40.61 | 585.6 | 623.8 | 604.7 | ||
| mean | 15.66 | 15.22 | 15.44 | 37.67 | 43.01 | 40.34 | 589.6 | 655.4 | 622.5 | ||
| II | PE | 16.04 | 14.87 | 15.57 | 37.69 | 42.30 | 39.53 | 604.6 | 629.2 | 614.4 | |
| PER | 15.82 | 14.50 | 15.29 | 37.93 | 41.67 | 39.43 | 600.8 | 604.0 | 602.1 | ||
| PEG | 15.89 | 14.43 | 15.31 | 37.80 | 42.73 | 39.77 | 599.4 | 619.4 | 607.4 | ||
| PP | 15.71 | 14.97 | 15.41 | 38.96 | 42.80 | 40.49 | 612.3 | 642.0 | 624.2 | ||
| control | 15.40 | 14.33 | 14.97 | 39.22 | 42.57 | 40.56 | 604.5 | 612.6 | 607.8 | ||
| mean | 15.77 | 14.62 | 15.31 | 38.32 | 42.41 | 39.96 | 604.3 | 621.5 | 611.2 | ||
| Mean | PE | 16.06 | 15.29 | 15.71 | 37.67 | 42.99 | 40.08 | 604.9 | 657.0 | 628.6 | |
| PER | 15.82 | 15.05 | 15.47 | 37.51 | 42.64 | 39.84 | 593.3 | 642.7 | 615.7 | ||
| PEG | 15.79 | 14.93 | 15.40 | 37.33 | 43.13 | 39.97 | 588.8 | 646.1 | 614.9 | ||
| PP | 15.64 | 15.11 | 15.40 | 38.50 | 42.55 | 40.34 | 602.6 | 643.8 | 621.3 | ||
| control | 15.27 | 14.52 | 14.93 | 38.96 | 42.55 | 40.59 | 595.1 | 619.3 | 606.1 | ||
| Mean from hybrids (Hy) | 15.72 | 14.98 | 37.99 | 42.77 | 596.9 | 641.8 | |||||
| LSDα = 0.05 for: Hy | 0.25 | 0.72 | 14.7 | ||||||||
| St | n.s. | n.s. | n.s. | ||||||||
| Ct | 0.38 | n.s. | n.s. | ||||||||
| Hy × Ct | n.s. | n.s. | 18.7 | ||||||||
| Hy × St | n.s. | 1.11 | n.s. | ||||||||
| St × Ct | n.s. | n.s. | n.s. | ||||||||
| Hy × St × Ct | n.s. | n.s. | n.s | ||||||||
| Sowing Term (St) | Cover Type (Ct) | Cobs Yield | Percentage of Marketable Cob Yield | ||||
|---|---|---|---|---|---|---|---|
| Signet F1 | Rustler F1 | Mean | Signet F1 | Rustler F1 | Mean | ||
| I | PE | 166.8 | 226.8 | 196.8 | 61.19 | 78.50 | 69.85 |
| PER | 145.0 | 195.8 | 170.4 | 50.39 | 82.53 | 66.46 | |
| PEG | 125.7 | 199.6 | 162.6 | 56.91 | 76.61 | 66.76 | |
| PP | 157.5 | 176.6 | 167.0 | 65.04 | 84.37 | 74.71 | |
| control | 143.9 | 183.2 | 163.6 | 55.96 | 77.63 | 66.80 | |
| mean | 147.8 | 196.4 | 172.1 | 57.90 | 79.93 | 68.91 | |
| II | PE | 227.0 | 209.0 | 218.0 | 75.36 | 80.13 | 77.75 |
| PER | 164.2 | 221.7 | 192.9 | 74.47 | 88.69 | 81.58 | |
| PEG | 176.3 | 227.6 | 202.0 | 70.95 | 74.95 | 72.95 | |
| PP | 170.2 | 200.7 | 185.5 | 69.48 | 88.19 | 78.84 | |
| control | 139.7 | 179.3 | 159.5 | 79.12 | 72.76 | 75.94 | |
| mean | 175.5 | 207.7 | 191.6 | 73.88 | 80.94 | 77.41 | |
| mean | PE | 196.9 | 217.9 | 207.4 | 68.27 | 79.32 | 73.80 |
| PER | 154.6 | 208.8 | 181.7 | 82.43 | 85.61 | 84.02 | |
| PEG | 151.0 | 213.6 | 182.3 | 63.93 | 75.78 | 69.86 | |
| PP | 163.8 | 188.7 | 176.3 | 67.26 | 86.28 | 76.77 | |
| control | 141.8 | 181.3 | 161.6 | 67.54 | 75.20 | 71.37 | |
| Mean from hybrids (Hy) | 162.6 | 202.1 | - | 65.89 | 80.44 | - | |
| LSDα = 0.05 for: Hy | 12 | n.a. | |||||
| St | 11 | n.a. | |||||
| Ct | 11 | n.a. | |||||
| Hy × Ct | 18 | n.a. | |||||
| Hy × St | n.s. | n.a. | |||||
| St × Ct | 17 | n.a. | |||||
| Hy × St × Ct | 21 | n.a. | |||||
| Sowing Term (St) | Cover Type (Ct) | Sucrose | Glucose | Fructose | Maltose | Sugar Total | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Signet F1 | Rustler F1 | Mean | Signet F1 | Rustler F1 | Mean | Signet F1 | Rustler F1 | Mean | Signet F1 | Rustler F1 | Mean | Signet F1 | Rustler F1 | Mean | ||
| I | PE | 7.07 | 6.05 | 6.56 | 0.65 | 0.47 | 0.56 | 0.62 | 0.44 | 0.53 | 0.05 | 0.02 | 0.03 | 8.39 | 6.97 | 7.68 |
| PER | 6.54 | 6.14 | 6.34 | 0.66 | 0.42 | 0.54 | 0.63 | 0.40 | 0.52 | 0.04 | 0.03 | 0.03 | 7.86 | 6.99 | 7.43 | |
| PEG | 6.40 | 6.49 | 6.44 | 0.79 | 0.42 | 0.60 | 0.78 | 0.40 | 0.59 | 0.05 | 0.03 | 0.04 | 8.02 | 7.34 | 7.68 | |
| PP | 6.85 | 6.16 | 6.51 | 0.63 | 0.46 | 0.54 | 0.62 | 0.44 | 0.53 | 0.04 | 0.02 | 0.03 | 8.14 | 7.09 | 7.62 | |
| control | 4.87 | 6.48 | 5.67 | 0.76 | 0.49 | 0.62 | 0.82 | 0.48 | 0.65 | 0.03 | 0.03 | 0.03 | 6.47 | 7.47 | 6.97 | |
| mean | 6.35 | 6.26 | 6.30 | 0.70 | 0.45 | 0.57 | 0.69 | 0.43 | 0.56 | 0.04 | 0.03 | 0.03 | 7.78 | 7.17 | 7.47 | |
| II | PE | 6.37 | 6.16 | 6.26 | 0.46 | 0.43 | 0.45 | 0.45 | 0.41 | 0.43 | 0.04 | 0.03 | 0.03 | 7.32 | 7.04 | 7.18 |
| PER | 6.54 | 6.29 | 6.42 | 0.66 | 0.36 | 0.51 | 0.66 | 0.33 | 0.50 | 0.04 | 0.03 | 0.04 | 7.90 | 7.01 | 7.46 | |
| PEG | 5.73 | 6.12 | 5.92 | 0.65 | 0.39 | 0.52 | 0.67 | 0.37 | 0.52 | 0.03 | 0.03 | 0.03 | 7.07 | 6.91 | 6.99 | |
| PP | 5.83 | 6.34 | 6.09 | 0.51 | 0.43 | 0.47 | 0.51 | 0.40 | 0.45 | 0.02 | 0.03 | 0.02 | 6.87 | 7.19 | 7.03 | |
| control | 7.19 | 6.73 | 6.96 | 0.61 | 0.52 | 0.57 | 0.58 | 0.50 | 0.54 | 0.05 | 0.03 | 0.04 | 8.44 | 7.78 | 8.11 | |
| mean | 6.33 | 6.33 | 6.33 | 0.58 | 0.43 | 0.50 | 0.57 | 0.40 | 0.49 | 0.04 | 0.03 | 0.03 | 7.52 | 7.19 | 7.35 | |
| mean | PE | 6.72 | 6.10 | 6.41 | 0.56 | 0.45 | 0.50 | 0.53 | 0.43 | 0.48 | 0.04 | 0.02 | 0.03 | 7.85 | 7.00 | 7,43 |
| PER | 6.54 | 6.21 | 6.38 | 0.66 | 0.39 | 0.52 | 0.64 | 0.37 | 0.51 | 0.04 | 0.03 | 0.04 | 7.88 | 7.00 | 7.44 | |
| PEG | 6.06 | 6.31 | 6.18 | 0.72 | 0.40 | 0.56 | 0.72 | 0.38 | 0.55 | 0.04 | 0.03 | 0.03 | 7.54 | 7.12 | 7.33 | |
| PP | 6.34 | 6.25 | 6.30 | 0.57 | 0.45 | 0.51 | 0.56 | 0.42 | 0.49 | 0.03 | 0.02 | 0.03 | 7.51 | 7.14 | 7.32 | |
| control | 6.03 | 6.60 | 6.32 | 0.69 | 0.51 | 0.60 | 0.70 | 0.49 | 0.59 | 0.04 | 0.03 | 0.03 | 7.46 | 7.63 | 7.54 | |
| Mean from hybrids (Hy) | 6.34 | 6.30 | - | 0.64 | 0.44 | - | 0.63 | 0.42 | - | 0.04 | 0.03 | - | 7.65 | 7.18 | - | |
| LSDα = 0.05 for: Hy | n.s. | 0.06 | 0.06 | 0.01 | 0.35 | |||||||||||
| St | n.s | n.s. | n.s. | n.s. | n.s. | |||||||||||
| Ct | n.s | n.s. | n.s. | n.s. | n.s. | |||||||||||
| Hy × Ct | n.s. | n.s. | n.s. | n.s. | n.s. | |||||||||||
| St × Ct | 0.69 | n.s. | n.s. | n.s. | 0.77 | |||||||||||
| Hy × St × Ct | 0.83 | n.s. | n.s. | 0.02 | 0.90 | |||||||||||
| Sowing Term (St) | Cover Type (Ct) | Lutein | Zeaxanthin | Lutein + Zeaxanthin | Lutein/Zeaxanthin Ratio | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Signet F1 | Rustler F1 | Mean | Signet F1 | Rustler F1 | Mean | Signet F1 | Rustler F1 | Mean | Signet F1 | Rustler F1 | Mean | |||
| I | PE | 5.2 | 6.0 | 6.8 | 2.4 | 2.9 | 3.3 | 7.6 | 9.0 | 10.1 | 2.2 | 2.1 | 2.1 | |
| PER | 4.5 | 7.5 | 5.4 | 2.1 | 3.1 | 2.6 | 6.6 | 10.6 | 8.0 | 2.1 | 2.4 | 2.1 | ||
| PEG | 5.5 | 8.1 | 5.9 | 2.8 | 3.8 | 2.8 | 8.2 | 11.9 | 8.7 | 2.0 | 2.1 | 2.2 | ||
| PP | 5.7 | 5.6 | 5.7 | 2.8 | 2.8 | 2.8 | 8.5 | 8.5 | 8.5 | 2.1 | 2.0 | 2.0 | ||
| control | 3.5 | 7.4 | 5.6 | 1.7 | 3.5 | 2.7 | 5.2 | 10.9 | 8.3 | 2.0 | 2.1 | 2.1 | ||
| mean | 4.9 | 6.9 | 5.9 | 2.4 | 3.2 | 2.8 | 7.2 | 10.2 | 8.7 | 2.1 | 2.2 | 2.1 | ||
| II | PE | 5.1 | 6.5 | 6.9 | 2.7 | 3.2 | 3.5 | 7.8 | 9.7 | 10.4 | 2.0 | 2.1 | 2.0 | |
| PER | 3.8 | 8.0 | 5.8 | 1.7 | 3.9 | 2.9 | 5.5 | 11.9 | 8.8 | 2.3 | 2.1 | 2.0 | ||
| PEG | 5.1 | 7.6 | 6.3 | 2.4 | 3.7 | 3.0 | 7.4 | 11.3 | 9.4 | 2.1 | 2.1 | 2.1 | ||
| PP | 6.4 | 7.5 | 5.5 | 3.2 | 3.8 | 2.6 | 9.6 | 11.2 | 8.1 | 2.0 | 2.0 | 2.1 | ||
| control | 4.8 | 5.8 | 6.0 | 2.1 | 3.0 | 2.6 | 6.9 | 8.8 | 8.6 | 2.3 | 2.0 | 2.3 | ||
| mean | 5.0 | 7.0 | 6.1 | 2.4 | 3.5 | 3.0 | 7.4 | 10.5 | 9.0 | 2.1 | 2.0 | 2.1 | ||
| mean | PE | 5.2 | 6.3 | 5.7 | 2.5 | 3.0 | 2.8 | 7.7 | 9.3 | 8.5 | 2.1 | 2.1 | 2.1 | |
| PER | 4.2 | 7.7 | 5.9 | 1.9 | 3.5 | 2.7 | 6.0 | 11.2 | 8.6 | 2.2 | 2.3 | 2.2 | ||
| PEG | 5.3 | 7.8 | 6.6 | 2.6 | 3.7 | 3.2 | 7.8 | 11.6 | 9.7 | 2.1 | 2.1 | 2.1 | ||
| PP | 6.0 | 6.6 | 6.3 | 3.0 | 3.3 | 3.2 | 9.0 | 9.9 | 9.5 | 2.0 | 2.0 | 2.0 | ||
| control | 4.1 | 6.5 | 5.4 | 1.9 | 3.2 | 2.6 | 6.0 | 9.7 | 8.0 | 2.2 | 2.0 | 2.1 | ||
| Mean from hybrids (Hy) | 5.0 | 7.0 | 2.4 | 3.4 | 7.3 | 10.3 | 2.1 | 2.1 | ||||||
| LSDα = 0.05 for: Hy | 0.6 | 0.3 | 0.9 | n.s | ||||||||||
| St | n.s | n.s | n.s | n.s | ||||||||||
| Ct | n.s | n.s | n.s | n.s | ||||||||||
| Hy × Ct | 1.1 | 0.6 | 1.7 | n.s. | ||||||||||
| St × Ct | n.s. | n.s. | n.s. | n.s. | ||||||||||
| Hy × St × Ct | 1.5 | n.s. | n.s. | 0.3 | ||||||||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Adamczewska-Sowińska, K.; Sowiński, J.; Anioł, M.; Ochodzki, P.; Warzecha, R. The Effect of Polyethylene Film and Polypropylene Non-Woven Fabric Cover on Cobs Parameters and Nutritional Value of Two Sweet Maize (Zea mays L. var. saccharata Bailey) Hybrids. Agronomy 2021, 11, 539. https://doi.org/10.3390/agronomy11030539
Adamczewska-Sowińska K, Sowiński J, Anioł M, Ochodzki P, Warzecha R. The Effect of Polyethylene Film and Polypropylene Non-Woven Fabric Cover on Cobs Parameters and Nutritional Value of Two Sweet Maize (Zea mays L. var. saccharata Bailey) Hybrids. Agronomy. 2021; 11(3):539. https://doi.org/10.3390/agronomy11030539
Chicago/Turabian StyleAdamczewska-Sowińska, Katarzyna, Józef Sowiński, Mirosław Anioł, Piotr Ochodzki, and Roman Warzecha. 2021. "The Effect of Polyethylene Film and Polypropylene Non-Woven Fabric Cover on Cobs Parameters and Nutritional Value of Two Sweet Maize (Zea mays L. var. saccharata Bailey) Hybrids" Agronomy 11, no. 3: 539. https://doi.org/10.3390/agronomy11030539






