Modulation of OMV Production by the Lysis Module of the DLP12 Defective Prophage of Escherichia coli K12
Abstract
:1. Introduction
2. Materials and Methods
2.1. Bacterial Strains and General Growth Conditions
2.2. Plasmid Construction
2.3. General Procedures
2.4. Biosensor Assay
2.5. OMV Purification
2.6. SDS-PAGE and Immunoblot Analysis
2.7. DiO Staining
2.8. Statistical Analyses
| E. coli Strain | Relevant Characteristics | Source/Reference |
|---|---|---|
| DH10b | E. coli K12 | [40] |
| MG1655 | E. coli K12, F-, λ-, ilvG-, rfb-50, rph-1 | [41] |
| ULS153 | ΔlacZYA derivative of MG1655 | [30] |
| ULS153 ΔDlm | ULS153 mutant defective in DLP12 operon | This study |
| ULS153 ΔdegP | ULS153 mutant defective in degP gene | This study |
| ULS153 ΔS | ULS153 mutant defective in essD gene | This study |
| ULS153 ΔR | ULS153 mutant defective in ybcS gene | This study |
| ULS153 ΔRz | ULS153 mutant defective in rzpD/rzoD genes | This study |
| ULS153 ΔSR | ULS153 mutant defective in essD and ybcS genes | This study |
| ULS153 ΔRRz | ULS153 mutant defective in ybcS and rzpD/rzoD genes | This study |
| ULS153 ΔSRz | ULS153 mutant defective in essD and rzpD/rzoD genes | This study |
| Plasmid Name | Relevant Characteristics | Source/Reference |
| pACYC184 | Low/Medium copy number cloning vector | [40] |
| pAC2.10 | pACYC184 derivate carrying the DLP12 operon | This study |
| pKD46 | Red recombinase expression plasmid | [31] |
| pKD3 | Template plasmid carrying a CmR gene with FLP recognition target sequence | [31] |
| pCP20 | Temperature sensitive replicon carrying the yeast Flp recombinase gene | [31] |
| pAG4 | High copy number plasmid carrying the lux operon | [33] |
| pAGPDlm | pAG4 derivate carrying the DLP12 promoter region of MG1655 strain upstream lux operon | This study |
| pAGPlac | pAG4 derivate carrying the lac promoter region of MG1655 strain upstream lux operon | This study |
3. Results
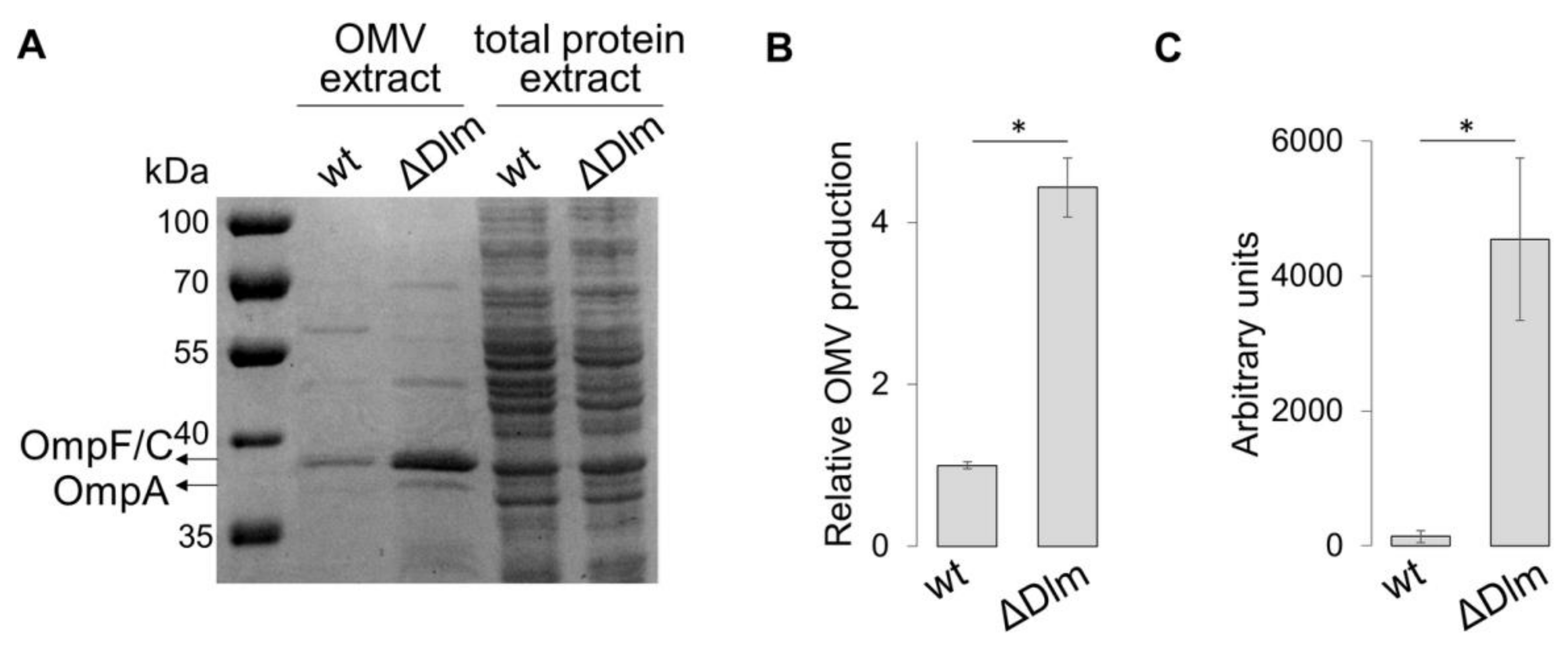
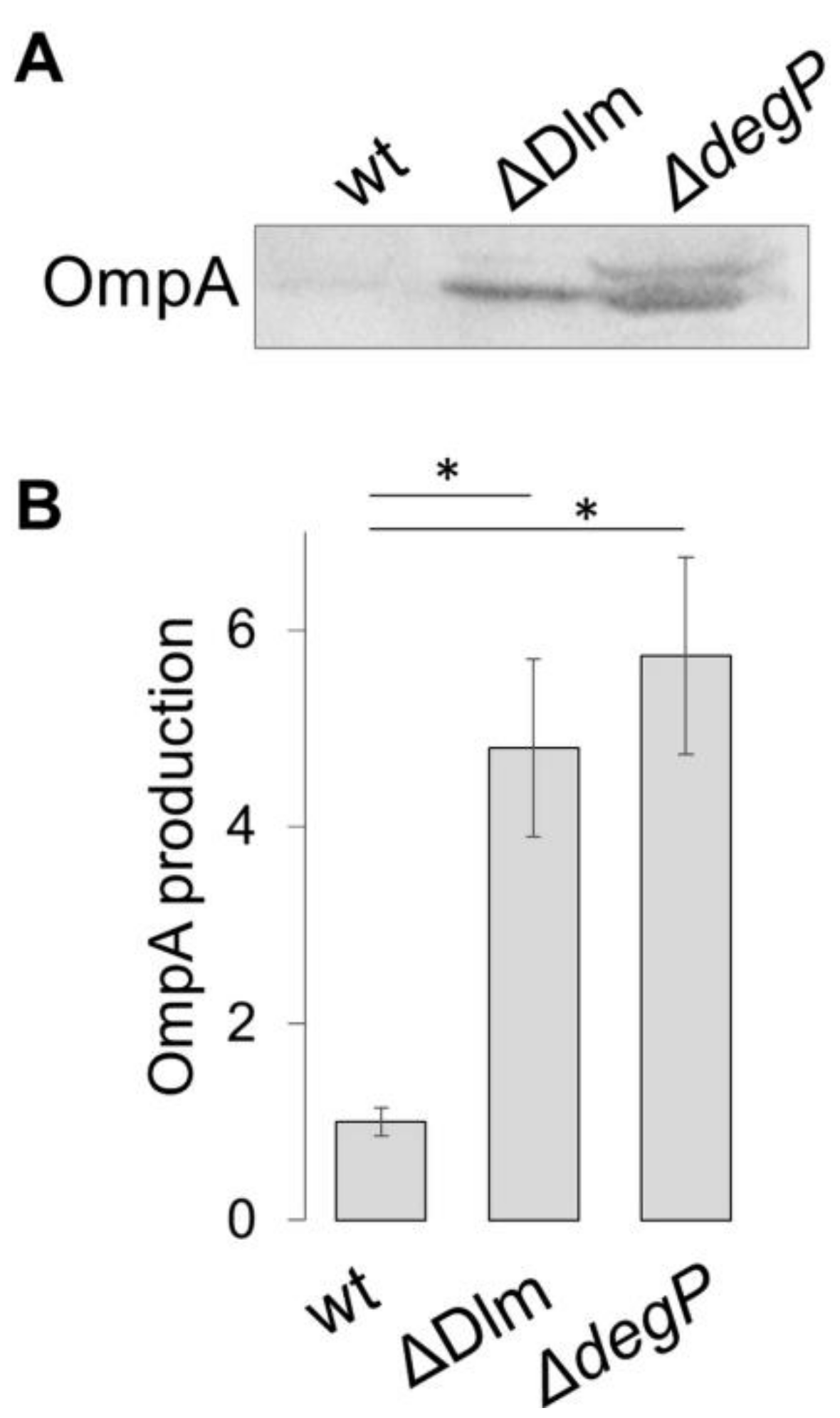
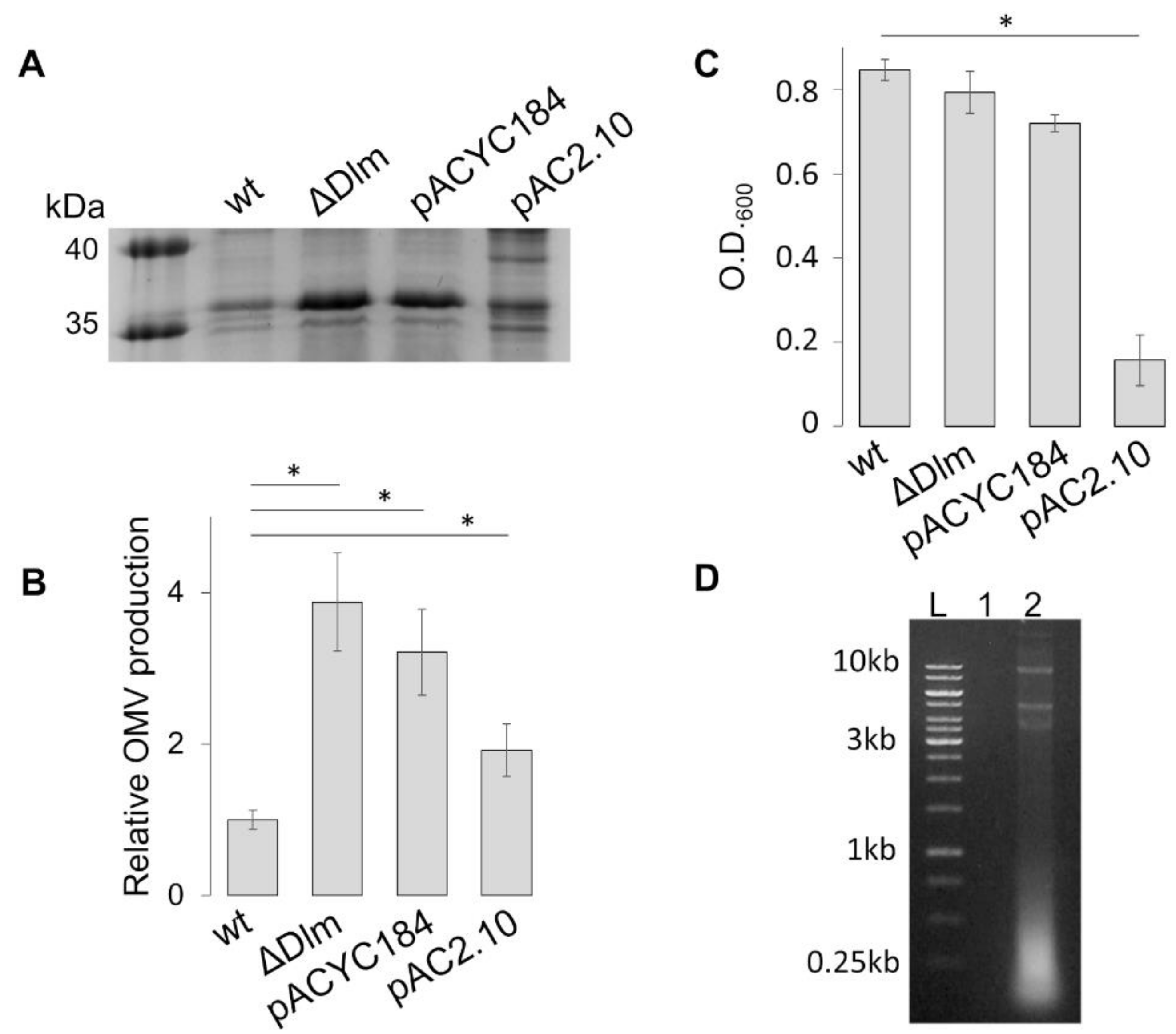
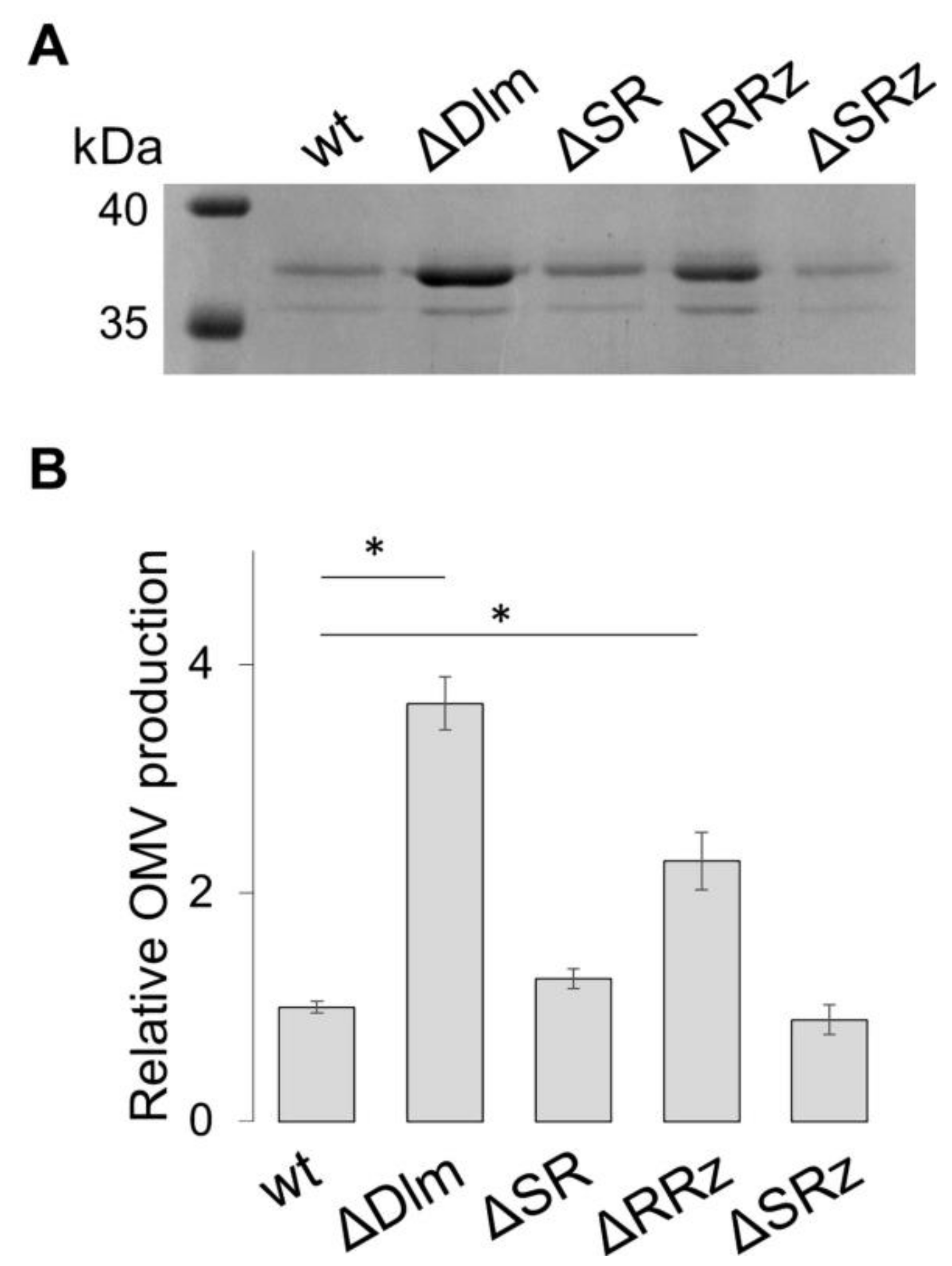
3.1. The SRRz/Rz1 Operon Regulates OMV Production
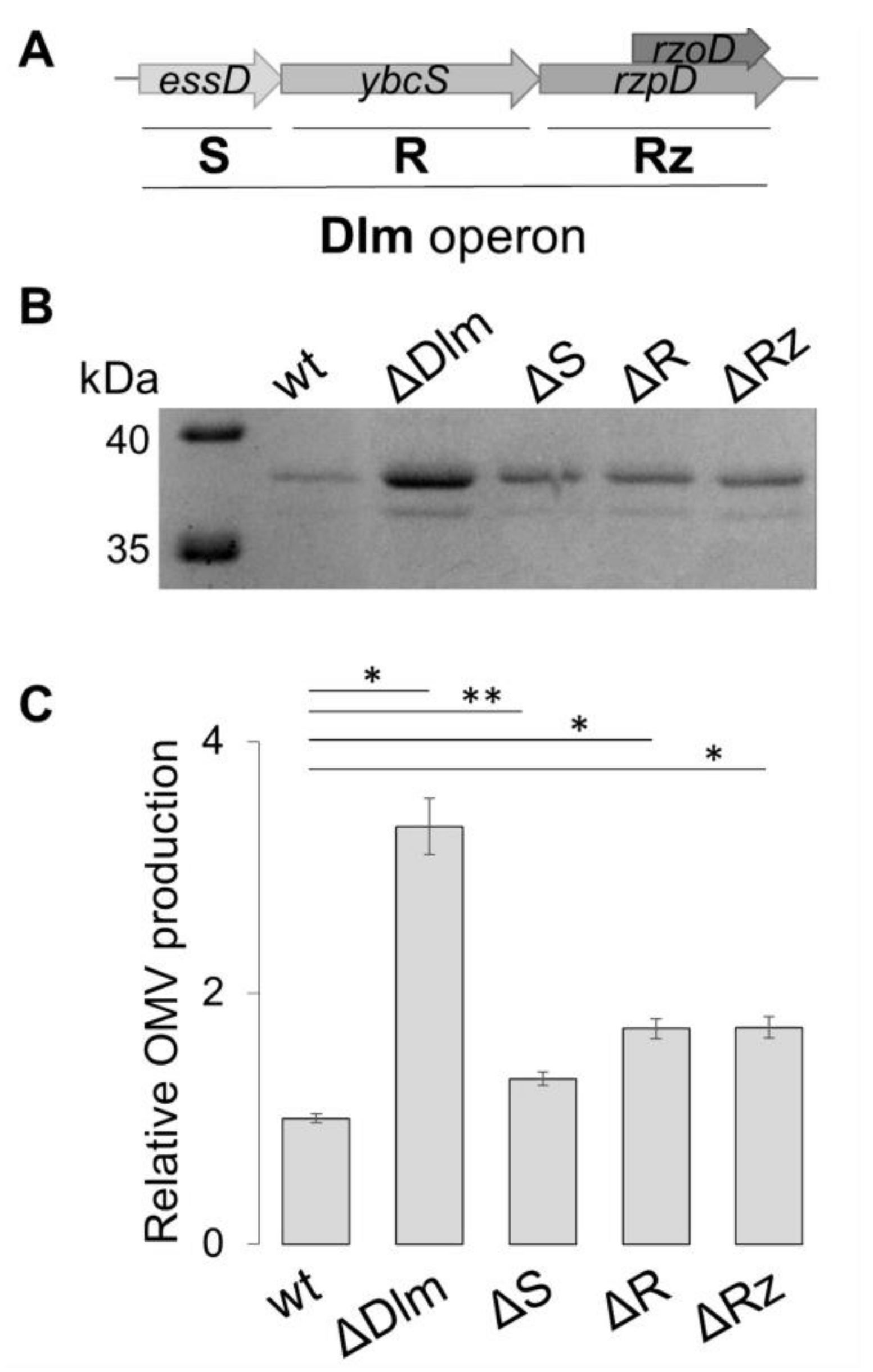
3.2. Defining the Role of the Single Genes of the SRRz/Rz1 Operon
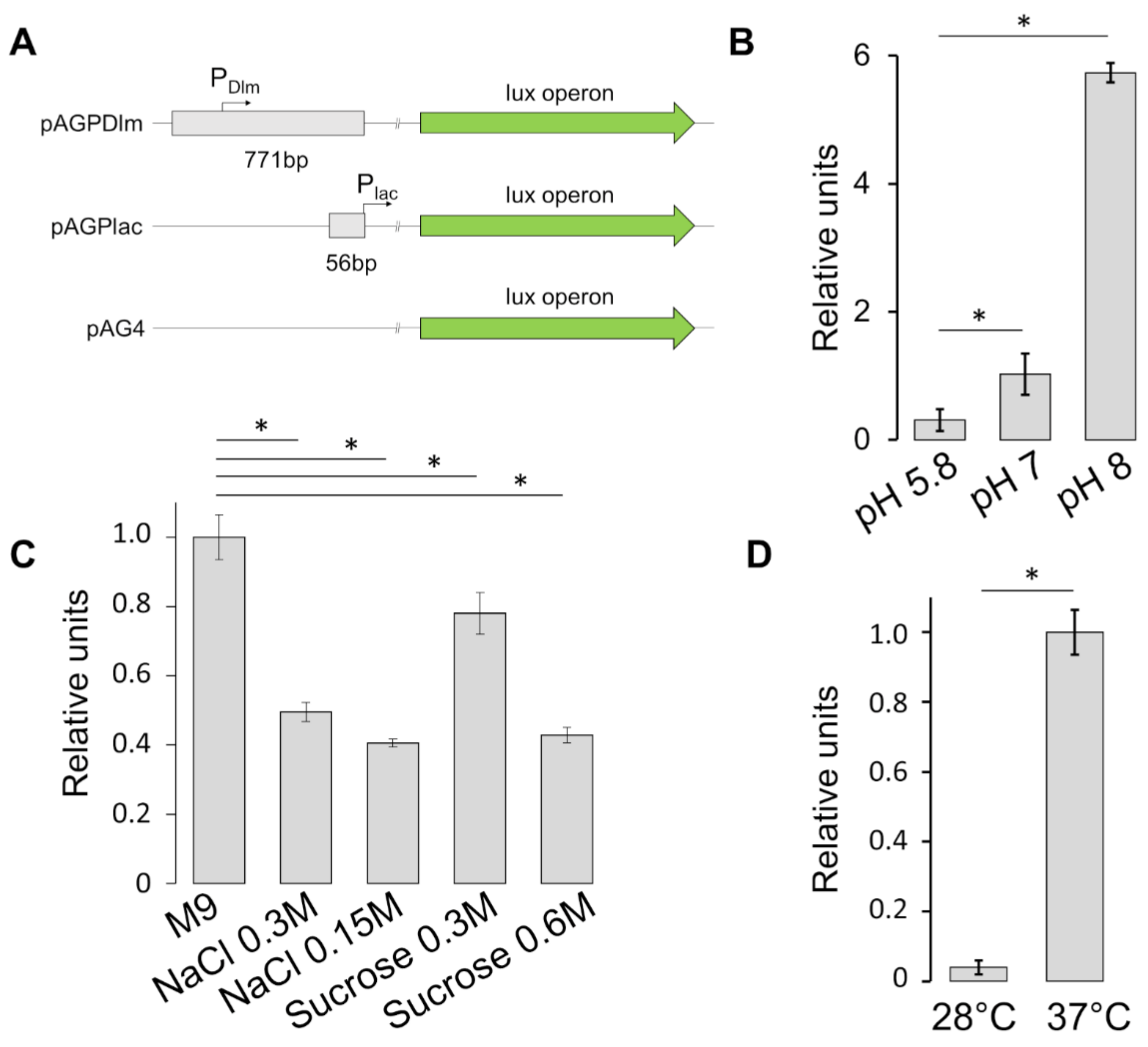
3.3. Stress Factors Modulate the Expression of SRRz/Rz1 Operon
4. Discussion
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Zhou, L.; Srisatjaluk, R.; Justus, D.; Doyle, R. On the origin of membrane vesicles in Gram-negative bacteria. FEMS Microbiol. Lett. 1998, 163, 223–228. [Google Scholar] [CrossRef]
- Beveridge, T.J. Structures of gram-negative cell walls and their derived membrane vesicles. J. Bacteriol. 1999, 181, 4725–4733. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kulp, A.; Kuehn, M.J. Biological Functions and biogenesis of secreted bacterial outer membrane vesicles. Annu. Rev. Microbiol. 2010, 64, 163–184. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schwechheimer, C.; Kuehn, M.J. Outer-membrane vesicles from Gram-negative bacteria: Biogenesis and functions. Nat. Rev. Microbiol. 2015, 13, 605–619. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Toyofuku, M.; Nomura, N.; Eberl, L. Types and origins of bacterial membrane vesicles. Nat. Rev. Microbiol. 2019, 17, 13–24. [Google Scholar] [CrossRef]
- Lee, J.; Kim, O.Y.; Gho, Y.S. Proteomic profiling of Gram-negative bacterial outer membrane vesicles: Current perspectives. Proteom. Clin. Appl. 2016, 10, 897–909. [Google Scholar] [CrossRef] [PubMed]
- Ellis, T.N.; Kuehn, M.J. Virulence and Immunomodulatory Roles of Bacterial Outer Membrane Vesicles. Microbiol. Mol. Biol. Rev. 2010, 74, 81–94. [Google Scholar] [CrossRef] [Green Version]
- Pathirana, R.D.; Kaparakis-Liaskos, M. Bacterial membrane vesicles: Biogenesis, immune regulation and pathogenesis. Cell. Microbiol. 2016, 18, 1518–1524. [Google Scholar] [CrossRef] [Green Version]
- Anand, D.; Chaudhuri, A. Bacterial outer membrane vesicles: New insights and applications. Mol. Membr. Biol. 2016, 33, 125–137. [Google Scholar] [CrossRef] [Green Version]
- Manning, A.J.; Kuehn, M.J. Contribution of bacterial outer membrane vesicles to innate bacterial defense. BMC Microbiol. 2011, 11. [Google Scholar] [CrossRef] [Green Version]
- Jan, A.T. Outer Membrane Vesicles (OMVs) of gram-negative bacteria: A perspective update. Front. Microbiol. 2017, 8. [Google Scholar] [CrossRef]
- Rueter, C.; Bielaszewska, M. Secretion and Delivery of Intestinal Pathogenic Escherichia coli Virulence Factors via Outer Membrane Vesicles. Front. Cell. Infect. Microbiol. 2020, 10. [Google Scholar] [CrossRef]
- Schwechheimer, C.; Sullivan, C.J.; Kuehn, M.J. Envelope control of outer membrane vesicle production in Gram-negative bacteria. Biochemistry 2013, 52, 3031–3040. [Google Scholar] [CrossRef]
- Schwechheimer, C.; Kulp, A.; Kuehn, M.J. Modulation of bacterial outer membrane vesicle production by envelope structure and content. BMC Microbiol. 2014, 14. [Google Scholar] [CrossRef] [Green Version]
- McBroom, A.J.; Kuehn, M.J. Release of outer membrane vesicles by Gram-negative bacteria is a novel envelope stress response. Mol. Microbiol. 2007, 63, 545–558. [Google Scholar] [CrossRef] [PubMed]
- Lima, S.; Guo, M.S.; Chaba, R.; Gross, C.A.; Sauer, R.T. Dual molecular signals mediate the bacterial response to outer-membrane stress. Science 2013, 340, 837–841. [Google Scholar] [CrossRef] [Green Version]
- Leuzzi, A.; Grossi, M.; Di Martino, M.L.; Pasqua, M.; Micheli, G.; Colonna, B.; Prosseda, G. Role of the SRRz/Rz1 lambdoid lysis cassette in the pathoadaptive evolution of Shigella. Int. J. Med. Microbiol. 2017, 307, 268–275. [Google Scholar] [CrossRef]
- Lindsey, D.F.; Mullin, D.A.; Walker, J.R. Characterization of the cryptic lambdoid prophage DLP12 of Escherichia coli and overlap of the DLP12 integrase gene with the tRNA gene argU. J. Bacteriol. 1989, 171, 6197–6205. [Google Scholar] [CrossRef] [Green Version]
- Toba, F.A.; Thompson, M.G.; Campbell, B.R.; Junker, L.M.; Rueggeberg, K.G.; Hay, A.G. Role of DLP12 lysis genes in Escherichia coli biofilm formation. Microbiology 2011, 157, 1640–1650. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pupo, G.M.; Lan, R.; Reeves, P.R. Multiple independent origins of Shigella clones of Escherichia coli and convergent evolution of many of their characteristics. Proc. Natl. Acad. Sci. USA 2000, 97, 10567–10572. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pasqua, M.; Michelacci, V.; Di Martino, M.L.; Tozzoli, R.; Grossi, M.; Colonna, B.; Morabito, S.; Prosseda, G. The Intriguing Evolutionary Journey of Enteroinvasive E. coli (EIEC) toward Pathogenicity. Front. Microbiol. 2017, 8. [Google Scholar] [CrossRef] [Green Version]
- Prosseda, G.; Di Martino, M.L.; Campilongo, R.; Fioravanti, R.; Micheli, G.; Casalino, M.; Colonna, B. Shedding of genes that interfere with the pathogenic lifestyle: The Shigella model. Res. Microbiol. 2012, 163, 399–406. [Google Scholar] [CrossRef]
- Campilongo, R.; Di Martino, M.L.; Marcocci, L.; Pietrangeli, P.; Leuzzi, A.; Grossi, M.; Casalino, M.; Nicoletti, M.; Micheli, G.; Colonna, B.; et al. Molecular and functional profiling of the polyamine content in enteroinvasive E. coli: Looking into the gap between commensal E. coli and harmful Shigella. PLoS ONE 2014, 9, e106589. [Google Scholar] [CrossRef]
- Bliven, K.A.; Maurelli, A.T. Antivirulence genes: Insights into pathogen evolution through gene loss. Infect. Immun. 2012, 80, 4061–4070. [Google Scholar] [CrossRef] [Green Version]
- Campbell, A. Comparative molecular biology of lambdoid phages. Annu. Rev. Microbiol. 1994, 48, 193–222. [Google Scholar] [CrossRef]
- Saier, M.H.; Reddy, B.L. Holins in bacteria, eukaryotes, and archaea: Multifunctional xenologues with potential biotechnological and biomedical applications. J. Bacteriol. 2015, 197, 7–17. [Google Scholar] [CrossRef] [Green Version]
- Rueggeberg, K.G.; Toba, F.A.; Thompson, M.G.; Campbell, B.R.; Hay, A.G. A Q-like transcription factor regulates biofilm development in Escherichia coli by controlling expression of the DLP12 lysis cassette. Microbiology 2013, 159, 691–700. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rueggeberg, K.G.; Toba, F.A.; Bird, J.G.; Franck, N.; Thompson, M.G.; Hay, A.G. The lysis cassette of DLP12 defective prophage is regulated by RpoE. Microbiology 2015, 161, 1683–1693. [Google Scholar] [CrossRef] [PubMed]
- García-Contreras, R.; Zhang, X.S.; Kim, Y.; Wood, T.K. Protein translation and cell death: The role of rare tRNAs in biofilm formation and in activating dormant phage killer genes. PLoS ONE 2008, 3, e2394. [Google Scholar] [CrossRef]
- Leuzzi, A.; Di Martino, M.L.; Campilongo, R.; Falconi, M.; Barbagallo, M.; Marcocci, L.; Pietrangeli, P.; Casalino, M.; Grossi, M.; Micheli, G.; et al. Multifactor regulation of the MdtJI polyamine transporter in Shigella. PLoS ONE 2015, 10, e0136744. [Google Scholar] [CrossRef]
- Datsenko, K.A.; Wanner, B.L. One-step inactivation of chromosomal genes in Escherichia coli K-12 using PCR products. Proc. Natl. Acad. Sci. USA 2000, 97, 6640–6645. [Google Scholar] [CrossRef] [Green Version]
- Baba, T.; Ara, T.; Hasegawa, M.; Takai, Y.; Okumura, Y.; Baba, M.; Datsenko, K.A.; Tomita, M.; Wanner, B.L.; Mori, H.; et al. Construction of Escherichia coli K-12 in-frame, single-gene knockout mutants: The Keio collection. Mol. Syst. Biol. 2006. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Glassing, A.; Lewis, T.A. An improved Tn7-lux reporter for broad host range, chromosomally-integrated promoter fusions in Gram-negative bacteria. J. Microbiol. Methods 2015, 118, 75–77. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fanelli, G.; Pasqua, M.; Colonna, B.; Prosseda, G.; Grossi, M. Expression Profile of Multidrug Resistance Efflux Pumps During Intracellular Life of Adherent-Invasive Escherichia coli Strain Lf82. Front. Microbiol. 2020, 11. [Google Scholar] [CrossRef]
- Pasqua, M.; Grossi, M.; Scinicariello, S.; Aussel, L.; Barras, F.; Colonna, B.; Prosseda, G. The MFS efflux pump EmrKY contributes to the survival of Shigella within macrophages. Sci. Rep. 2019, 9. [Google Scholar] [CrossRef] [Green Version]
- Henry, T.; Pommier, S.; Journet, L.; Bernadac, A.; Gorvel, J.P.; Lloubès, R. Improved methods for producing outer membrane vesicles in Gram-negative bacteria. Res. Microbiol. 2004, 155, 437–446. [Google Scholar] [CrossRef]
- Ambrosi, C.; Pompili, M.; Scribano, D.; Zagaglia, C.; Ripa, S.; Nicoletti, M. Outer Membrane Protein A (OmpA): A New Player in Shigella flexneri Protrusion Formation and Inter-Cellular Spreading. PLoS ONE 2012, 7, e49625. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Di Martino, M.L.; Romilly, C.; Wagner, E.G.H.; Colonna, B.; Prosseda, G. One gene and two proteins: A leaderless mRNA supports the translation of a shorter form of the Shigella VirF regulator. mBio 2016, 7. [Google Scholar] [CrossRef] [Green Version]
- Parker, H.; Chitcholtan, K.; Hampton, M.B.; Keenan, J.I. Uptake of Helicobacter pylori outer membrane vesicles by gastric epithelial cells. Infect. Immun. 2010, 78, 5054–5061. [Google Scholar] [CrossRef] [Green Version]
- Sambrock, J.; Russel, D.W. Molecular Cloning: A Laboratory Manual, 3rd ed.; Cold Spring Harbor Laboratory Press: New York, NY, USA, 2001; ISBN 978-087969577-4. [Google Scholar]
- Blattner, F.R.; Plunkett, G.; Bloch, C.A.; Perna, N.T.; Burland, V.; Riley, M.; Collado-Vides, J.; Glasner, J.D.; Rode, C.K.; Mayhew, G.F.; et al. The complete genome sequence of Escherichia coli K-12. Science 1997, 277, 1453–1462. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Spiess, C.; Beil, A.; Ehrmann, M. A temperature-dependent switch from chaperone to protease in a widely conserved heat shock protein. Cell 1999, 97, 339–347. [Google Scholar] [CrossRef] [Green Version]
- Ojima, Y.; Yamaguchi, K.; Taya, M. Quantitative Evaluation of Recombinant Protein Packaged into Outer Membrane Vesicles of Escherichia coli Cells. Biotechnol. Prog. 2018, 34, 51–57. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cheung, C.; Lee, J.; Lee, J.; Shevchuk, O. The Effect of Ionic (NaCl) and Non-ionic (Sucrose) Osmotic Stress on the Expression of β-galactosidase in Wild Type E. coli BW25993 and in the Isogenic BW25993ΔlacI Mutant. J. Exp. Microbiol. Immunol. 2009, 13, 1–6. [Google Scholar]
- Brüssow, H.; Hendrix, R.W. Phage Genomics: Small is beautiful. Cell 2002, 108, 13–16. [Google Scholar] [CrossRef] [Green Version]
- Touchon, M.; Moura de Sousa, J.A.; Rocha, E.P. Embracing the enemy: The diversification of microbial gene repertoires by phage-mediated horizontal gene transfer. Curr. Opin. Microbiol. 2017, 38, 66–73. [Google Scholar] [CrossRef] [PubMed]
- Casjens, S. Prophages and bacterial genomics: What have we learned so far? Mol. Microbiol. 2003, 49, 277–300. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Kim, Y.; Ma, Q.; Hong, S.H.; Pokusaeva, K.; Sturino, J.M.; Wood, T.K. Cryptic prophages help bacteria cope with adverse environments. Nat. Commun. 2010, 1. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bobay, L.M.; Touchon, M.; Rocha, E.P.C. Pervasive domestication of defective prophages by bacteria. Proc. Natl. Acad. Sci. USA 2014, 111, 12127–12132. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vollmer, W.; Bertsche, U. Murein (peptidoglycan) structure, architecture and biosynthesis in Escherichia coli. Biochim. Biophys. Acta Biomembr. 2008, 1778, 1714–1734. [Google Scholar] [CrossRef] [Green Version]
- Young, R. Phage lysis: Three steps, three choices, one outcome. J. Microbiol. 2014, 52, 243–258. [Google Scholar] [CrossRef]
- Sharma-Kuinkel, B.K.; Mann, E.E.; Ahn, J.S.; Kuechenmeister, L.J.; Dunman, P.M.; Bayles, K.W. The Staphylococcus aureus LytSR two-component regulatory system affects biofilm formation. J. Bacteriol. 2009, 191, 4767–4775. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mehner-Breitfeld, D.; Rathmann, C.; Riedel, T.; Just, I.; Gerhard, R.; Overmann, J.; Brüser, T. Evidence for an adaptation of a phage-derived holin/endolysin system to toxin transport in Clostridioides difficile. Front. Microbiol. 2018, 9. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pasqua, M.; Zennaro, A.; Trirocco, R.; Fanelli, G.; Micheli, G.; Grossi, M.; Colonna, B.; Prosseda, G. Modulation of OMV Production by the Lysis Module of the DLP12 Defective Prophage of Escherichia coli K12. Microorganisms 2021, 9, 369. https://doi.org/10.3390/microorganisms9020369
Pasqua M, Zennaro A, Trirocco R, Fanelli G, Micheli G, Grossi M, Colonna B, Prosseda G. Modulation of OMV Production by the Lysis Module of the DLP12 Defective Prophage of Escherichia coli K12. Microorganisms. 2021; 9(2):369. https://doi.org/10.3390/microorganisms9020369
Chicago/Turabian StylePasqua, Martina, Alessandro Zennaro, Rita Trirocco, Giulia Fanelli, Gioacchino Micheli, Milena Grossi, Bianca Colonna, and Gianni Prosseda. 2021. "Modulation of OMV Production by the Lysis Module of the DLP12 Defective Prophage of Escherichia coli K12" Microorganisms 9, no. 2: 369. https://doi.org/10.3390/microorganisms9020369







